Effect of Pretreatment by Freeze Vacuum Drying on Solid-State Anaerobic Digestion of Corn Straw
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sources of Corn Straw and Inoculum
2.2. Freeze Vacuum Drying Pretreatment
2.3. Experimental Design
2.4. Analytical Methods
2.5. Kinetic Model
3. Results and Discussion
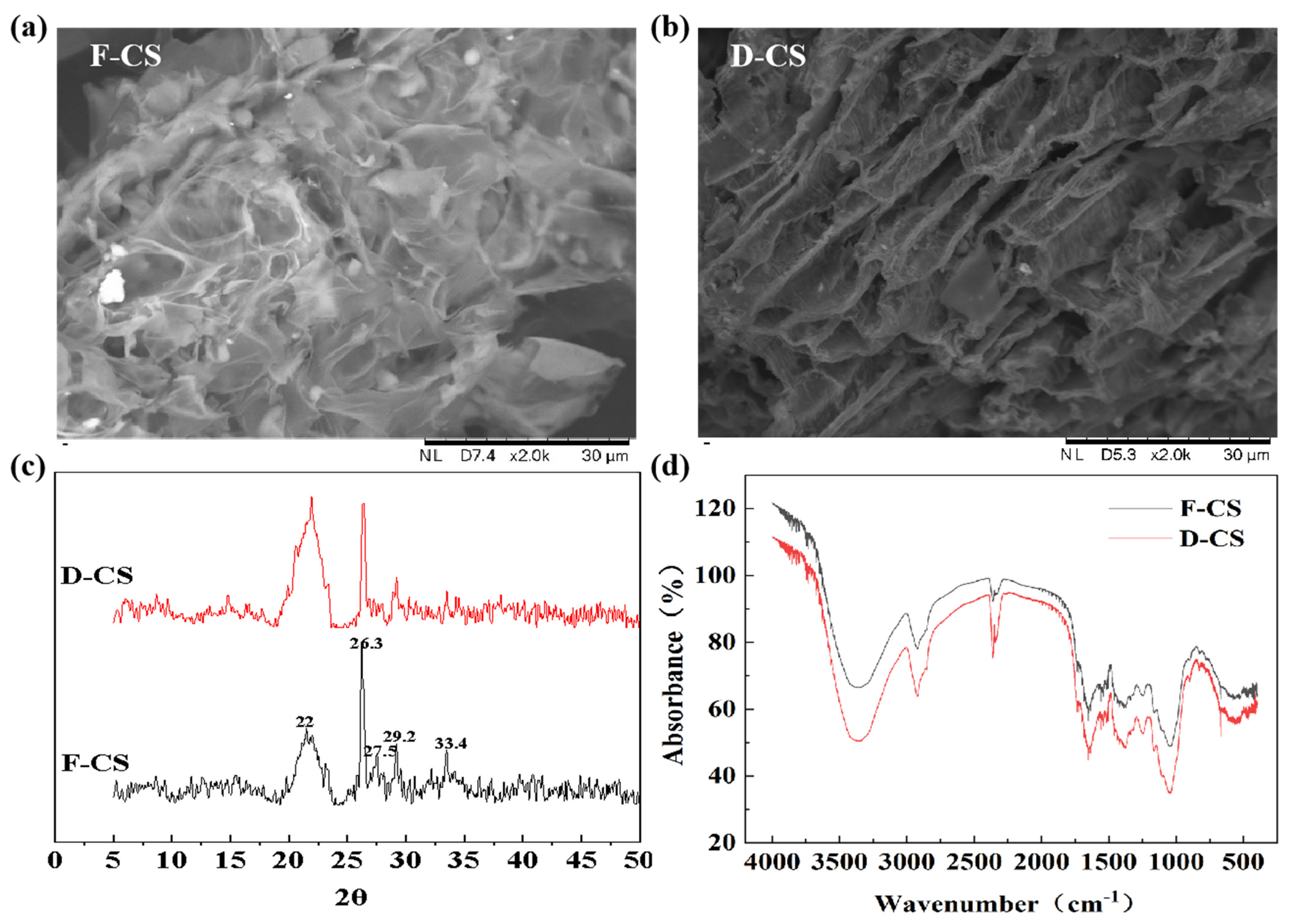
3.1. Variations in Surface Structure and Compositions during FVD Pretreatment
3.1.1. Surface Structure Analysis
3.1.2. Nutrient and Elemental Analyses of F-CS, D-CS, and AD Digestate
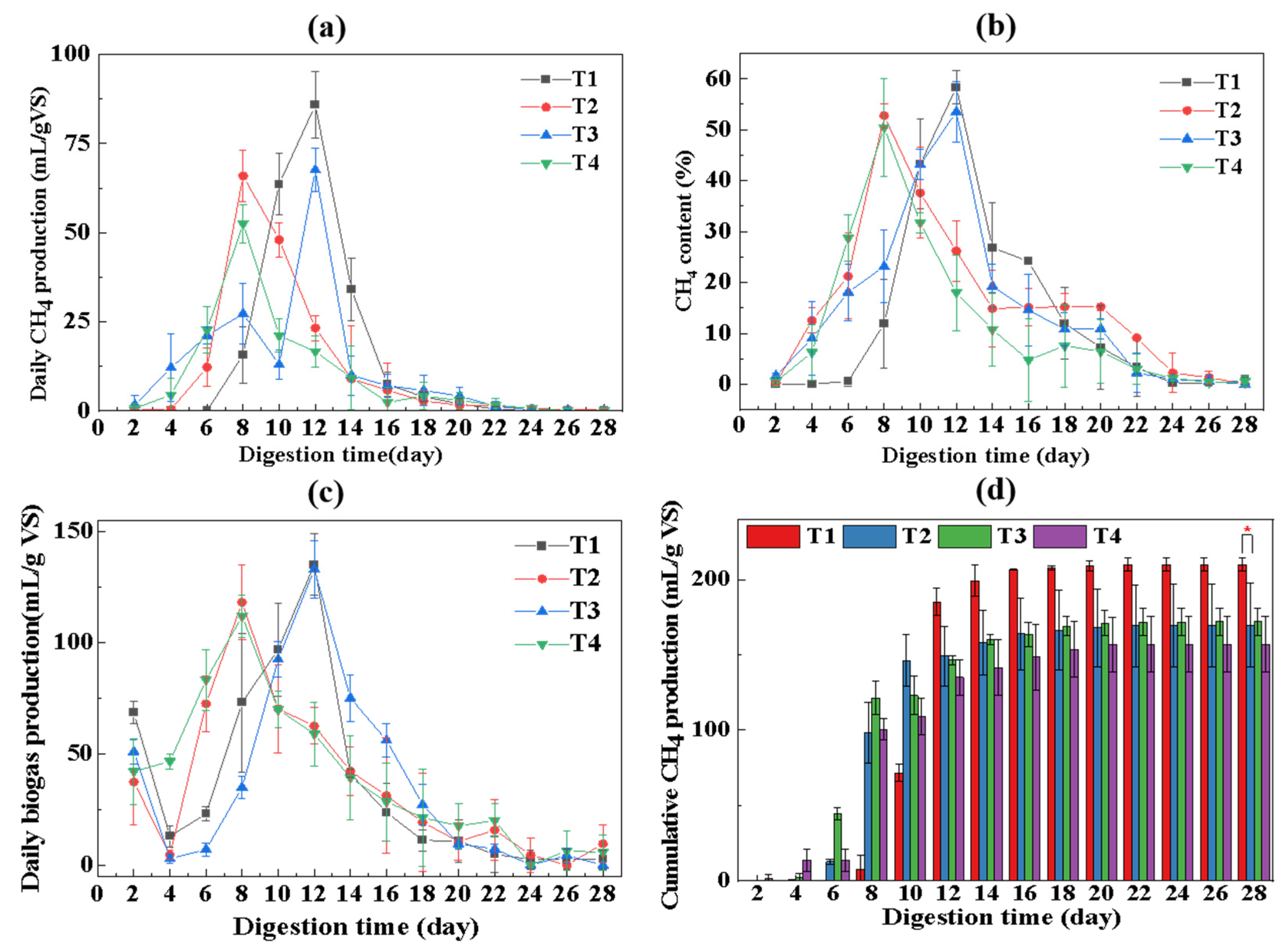
3.2. Methane Production during AD of F-CS and D-CS
3.2.1. Daily and Cumulative Methane Production
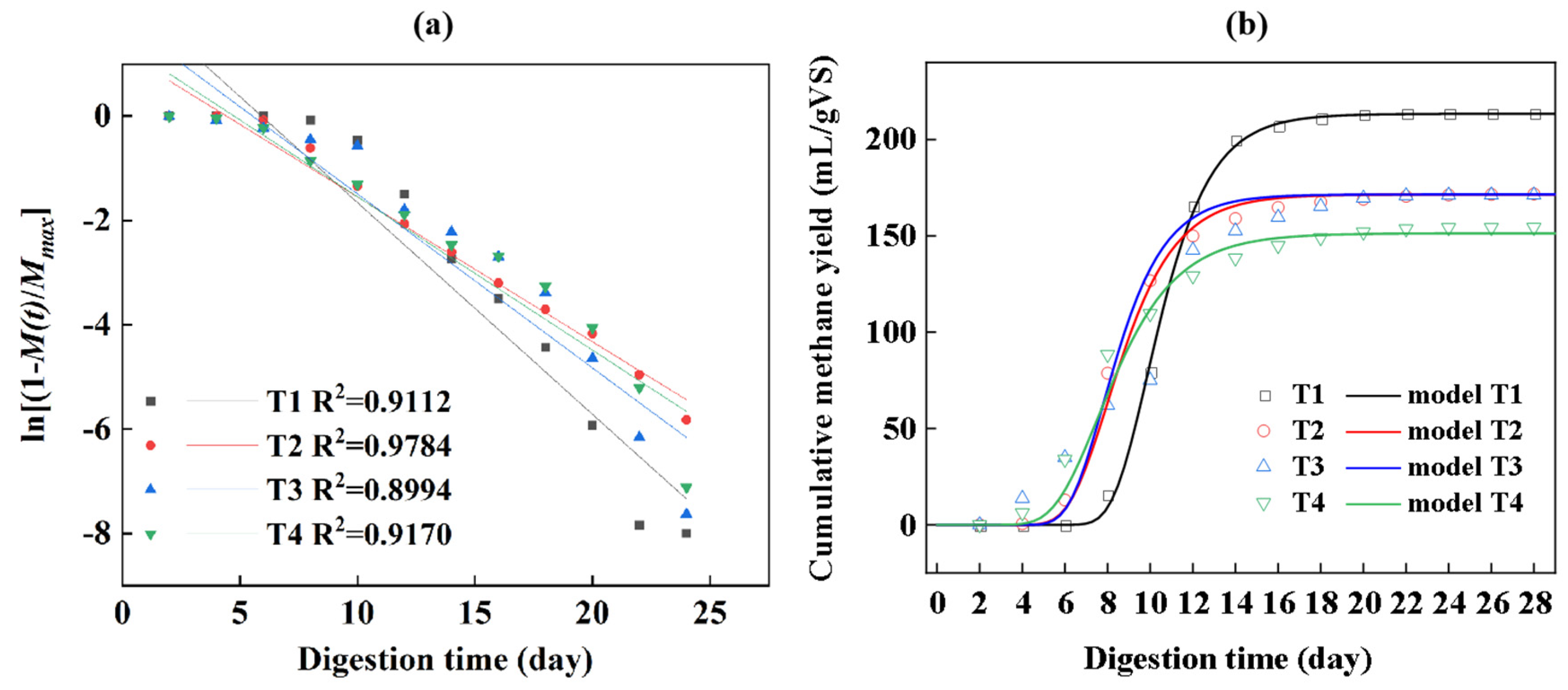
3.2.2. Kinetic Model
3.3. Variation of Intermediate Metabolism during AD of F-CS and D-CS
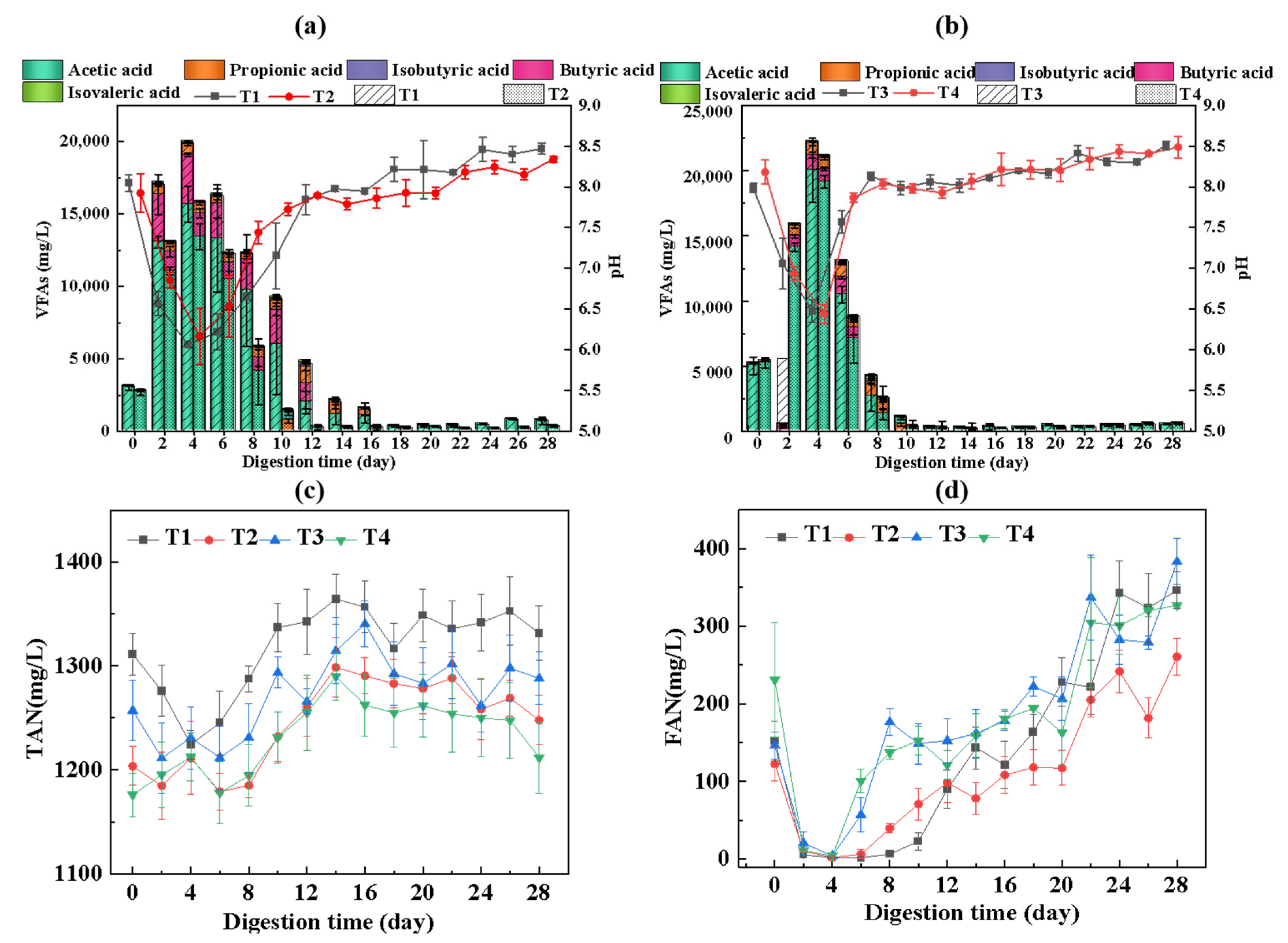
3.3.1. VFA Production
3.3.2. Variation of TAN and FAN
3.3.3. D-EEM Fluorescence Spectra Analysis
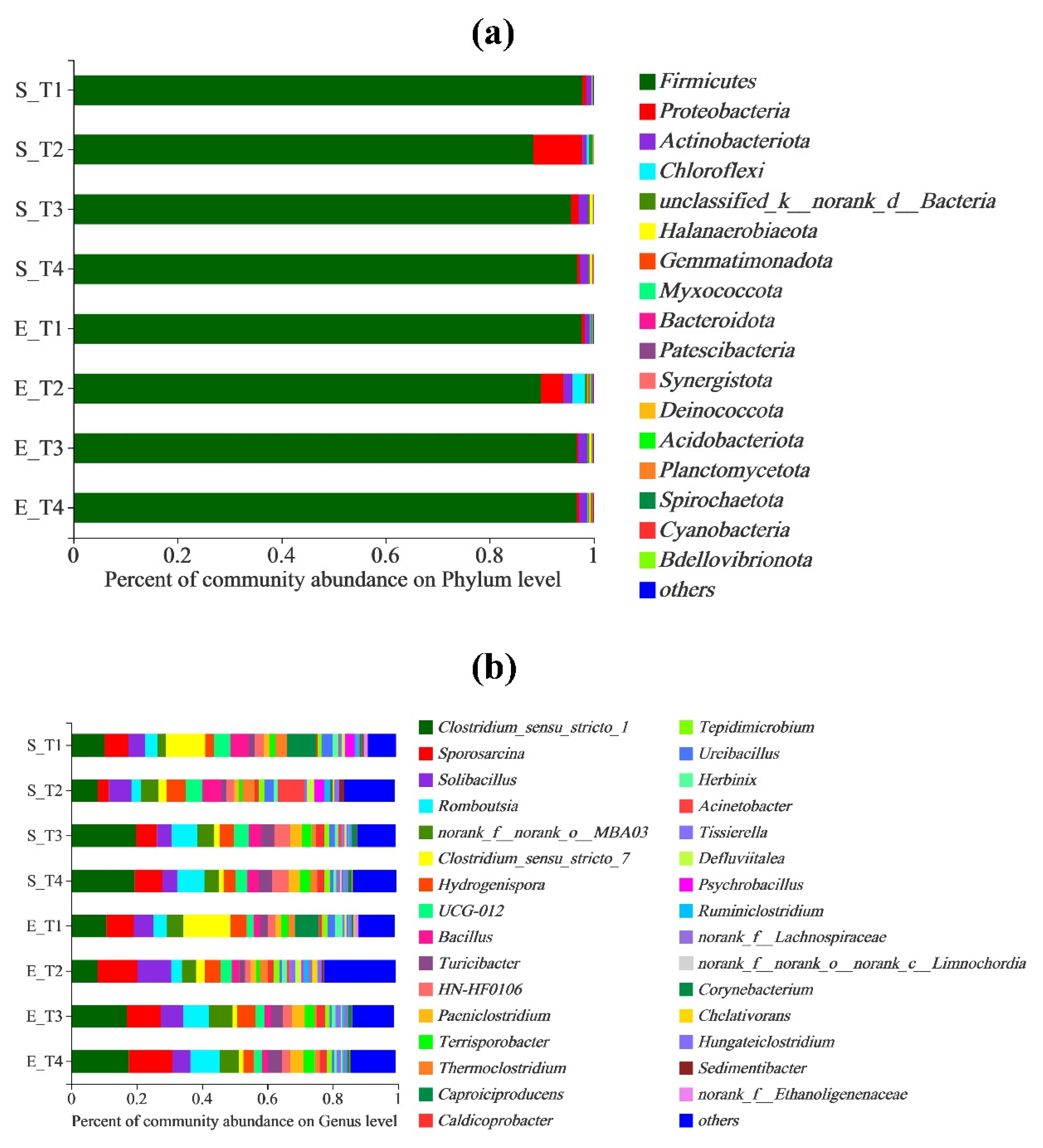
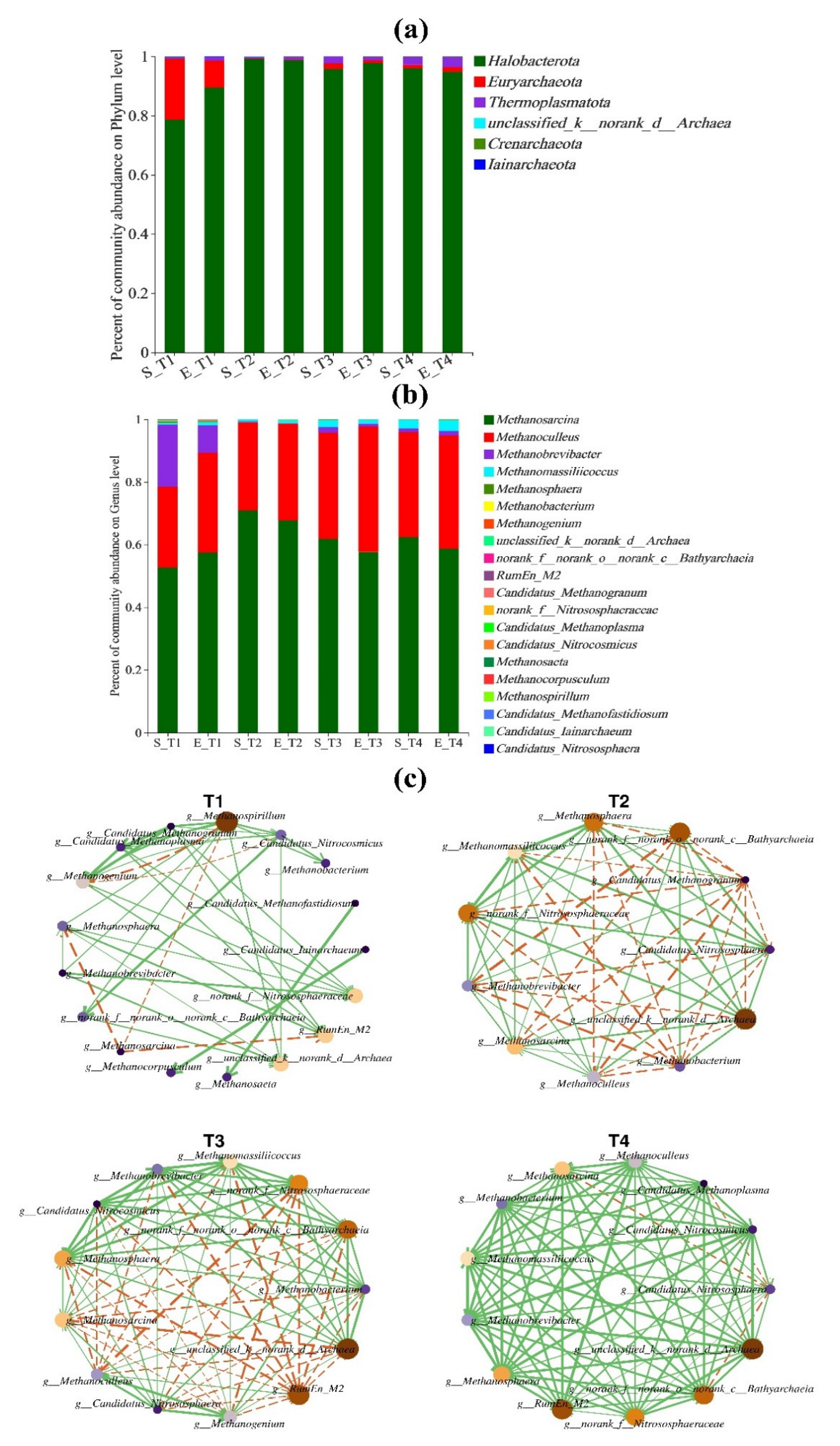
3.4. Microbial Community Analysis
3.4.1. Analysis of Bacterial Community
3.4.2. Analysis of Archaeal Community
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, W.; Habiba, K.; Zhe, Z.; Zhang, R.; Liu, G.; Chang, C.; Eva, T. Methane production through anaerobic digestion: Participation and digestion characteristics of cellulose, hemicellulose and lignin. Appl. Energy 2018, 226, 1219–1228. [Google Scholar] [CrossRef]
- Romero-Güiza, M.S.; Wahid, R.; Hernández, V.; Møller, H.; Fernández, B. Improvement of wheat straw anaerobic digestion through alkali pre-treatment: Carbohydrates bioavailability evaluation and economic feasibility. Sci. Total Environ. 2017, 595, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, C.; Zhang, R.; He, Y.; Wang, W.; Liu, G. Pretreatment of Corn Stover for Methane Production with the Combination of Potassium Hydroxide and Calcium Hydroxide. Energy Fuel. 2015, 29, 2082561664. [Google Scholar] [CrossRef]
- Yfw, A.; Hry, A.; Acw, B.; Xjl, A. Anaerobic co-digestion of cattle manure and liquid fraction of digestate (LFD) pretreated corn stover: Pretreatment process optimization and evolution of microbial community structure. Bioresour. Technol. 2020, 296, 122282. [Google Scholar]
- Ji, J.; Zhang, J.; Yang, L.; He, Y.; Zhang, R.; Liu, G.; Chen, C. Impact of co-pretreatment of calcium hydroxide and steam explosion on anaerobic digestion efficiency with corn stover. Environ. Technol. 2016, 38, 1465–1473. [Google Scholar] [CrossRef]
- Ma, S.; Wang, H.; Li, L.; Gu, X.; Zhu, W. Enhanced biomethane production from corn straw by a novel anaerobic digestion strategy with mechanochemical pretreatment. Renew. Sustain. Energy Rev. 2021, 146, 111099. [Google Scholar] [CrossRef]
- You, Z.; Pan, S.Y.; Sun, N.; Kim, H.; Chiang, P.C. Enhanced corn-stover fermentation for biogas production by NaOH pretreatment with CaO additive and ultrasound. J. Clean. Prod. 2019, 238, 117813. [Google Scholar] [CrossRef]
- Rouches, E.; Escudie, R.; Latrille, E.; Carrere, H. Solid-state anaerobic digestion of wheat straw: Impact of S/I ratio and pilot-scale fungal pretreatment. Waste Manag. 2019, 85, 464–476. [Google Scholar] [CrossRef]
- Wu, Y.; Song, K. Anaerobic co-digestion of waste activated sludge and fish waste: Methane production performance and mechanism analysis. J. Clean. Prod. 2020, 279, 123678. [Google Scholar] [CrossRef]
- Yu, J.; Zhao, Y.; Liu, B.; Zhao, Y.; Wu, J.; Yuan, X.; Zhu, W.; Cui, Z. Accelerated acidification by inoculation with a microbial consortia in a complex open environment. Bioresour. Technol. 2016, 216, 294–301. [Google Scholar] [CrossRef]
- Pakarinen, O.; Lehtomki, A.; Rissanen, S.; Rintala, J. Storing energy crops for methane production: Effects of solids content and biological additive. Bioresour. Technol. 2008, 99, 7074–7082. [Google Scholar] [CrossRef] [PubMed]
- Tabacco, E.; Righi, F.; Quarantelli, A.; Borreani, G. Dry matter and nutritional losses during aerobic deterioration of corn and sorghum silages as influenced by different lactic acid bacteria inocula. J. Dairy Sci. 2011, 94, 1409–1419. [Google Scholar] [CrossRef] [PubMed]
- Harguindeguy, M.; Fissore, D. On the effects of freeze-drying processes on the nutritional properties of foodstuff: A review. Dry. Technol. 2019, 38, 846–868. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, J.; Zhao, X.; Zheng, Z.; Cai, Y.; Hu, Y.; Cui, Z.; Wang, X. The macro- and micro-prospects of the energy potential of the anaerobic digestion of corn straw under different storage conditions. Bioresour. Technol. Rep. 2019, 7, 100189. [Google Scholar] [CrossRef]
- Rooni, V.; Raud, M.; Kikas, T. The Freezing Pre-Treatment of Lignocellulosic Material: A Cheap Alternative for Nordic Countries. Energy 2017, 139, 1–7. [Google Scholar] [CrossRef]
- Qi, N.; Hu, X.; Xin, X.; Ye, S.; Zhao, X. Mechanisms of biohydrogen recovery enhancement from peanut shell by C. guangxiense: Temperature pretreatment ranges from -80 to 100 °C. Bioresour. Technol. 2020, 304, 123026. [Google Scholar] [CrossRef]
- Rui, X.; Yang, Z.H.; Wang, Q.P.; Yang, B.; Fan, C.Z. Rapid startup of thermophilic anaerobic digester to remove tetracycline and sulfonamides resistance genes from sewage sludge. Sci. Total Environ. 2017, 612, 788. [Google Scholar]
- Boulanger, A.; Pinet, E.; Bouix, M.; Bouchez, T.; Mansour, A.A. Effect of inoculum to substrate ratio (I/S) on municipal solid waste anaerobic degradation kinetics and potential. Waste Manag. 2012, 32, 2258–2265. [Google Scholar] [CrossRef]
- Benbelkacem, H.; Bollon, J.; Bayard, R.; Escudié, R.; Buffière, P. Towards optimization of the total solid content in high-solid (dry) municipal solid waste digestion. Chem. Eng. J. 2015, 273, 261–267. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templaton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; Technical Report NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2010. [Google Scholar]
- Casado, A.G.; Hernández, E.; Espinosa, P.; VíLchez, J.L. Determination of total fatty acids (C8–C22) in sludges by gas chromatography–mass spectrometry. J. Chromatogr. A 1998, 826, 49–56. [Google Scholar] [CrossRef]
- Siles, J.A.; Brekelmans, J.; Martín, M.A.; Chica, A.F.; Martín, A. Impact of ammonia and sulphate concentration on thermophilic anaerobic digestion. Bioresour. Technol. 2010, 101, 9040–9048. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.S.; Singh, S.P.; Singh, A.K.; Sodha, M.S. Bioenergy conversion studies of the organic fraction of MSW: Assessment of ultimate bioenergy production potential of municipal garbage. Appl. Energy 2000, 66, 75–87. [Google Scholar] [CrossRef]
- Kafle, G.K.; Sang, H.K. Anaerobic treatment of apple waste with swine manure for biogas production: Batch and continuous operation. Appl. Energy 2013, 103, 61–72. [Google Scholar] [CrossRef]
- Kafle, G.K.; Sang, H.K.; Sung, K.I. Ensiling of fish industry waste for biogas production: A lab scale evaluation of biochemical methane potential (BMP) and kinetic. Bioresour. Technol. 2013, 127, 326–336. [Google Scholar] [CrossRef]
- Xie, S.; Wu, G.; Lawlor, P.G.; Frost, J.P.; Zhan, X. Methane production from anaerobic co-digestion of the separated solid fraction of pig manure with dried grass silage. Bioresour. Technol. 2012, 104, 289–297. [Google Scholar] [CrossRef]
- Cs, A.; Fang, L.B.; Zs, A.; Jing, W.B.; Yl, C.; Yu, P.A.; Tao, S.A.; Ll, A. Feasibility of dry anaerobic digestion of beer lees for methane production and biochar enhanced performance at mesophilic and thermophilic temperature. Bioresour. Technol. 2019, 276, 65–73. [Google Scholar]
- Menardo, S.; Balsari, P.; Tabacco, E.; Borreani, G. Effect of Conservation Time and the Addition of Lactic Acid Bacteria on the Biogas and Methane Production of Corn Stalk Silage. Bioenerg. Res. 2015, 8, 1810–1823. [Google Scholar] [CrossRef]
- Miryahyaei, S.; Das, T.; Othman, M.; Batstone, D.; Eshtiaghi, N. Anaerobic co-digestion of sewage sludge with cellulose, protein, and lipids: Role of rheology and digestibility. Sci. Total Environ. 2020, 731, 139214. [Google Scholar] [CrossRef]
- Shi, X.S.; Yuan, X.Z.; Wang, Y.P.; Zeng, S.J.; Qiu, Y.L.; Guo, R.B.; Wang, L.S. Modeling of the methane production and pH value during the anaerobic co-digestion of dairy manure and spent mushroom substrate. Chem. Eng. J. 2014, 244, 258–263. [Google Scholar] [CrossRef]
- Jing, Z.A.; Yu, L.A.; Gjwea, B. Effect of bioaugmentation combined with activated charcoal on the mitigation of volatile fatty acids inhibition during anaerobic digestion. Chem. Eng. J. 2021, 428, 131015. [Google Scholar]
- Semenov, G.V.; Krasnova, I.S.; Khvylia, S.I.; Balabolin, D.N. Freezing and freeze-drying of strawberries with an additional effect of micro-vibrations. J. Food Sci. Technol. 2020, 58, 3192–3198. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Huang, Y.; Xu, Q.; Liu, X.; Yang, Q.; Li, X. Free ammonia aids ultrasound pretreatment to enhance short-chain fatty acids production from waste activated sludge. Bioresour. Technol. 2018, 275, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Athanasoulia, E.; Melidis, P.; Aivasidis, A. Optimization of biogas production from waste activated sludge through serial digestion. Renew Energy. 2012, 47, 147–151. [Google Scholar] [CrossRef]
- Meng, S.A.; Bing, L.B.; Ky, A.; Nth, C.; Rg, D.; Mt, A.; Hy, A. Effects of low pH conditions on decay of methanogenic biomass—ScienceDirect. Water Res. 2020, 179, 115883. [Google Scholar]
- Gc, A.; Gzb, C.; Mza, D.; Nan, L.; Rwa, D.; Cl, E.; Jla, D.; Xp, A. Syntrophic butyrate-oxidizing methanogenesis promoted by anthraquinone-2-sulfonate and cysteine: Distinct tendencies towards the enrichment of methanogens and syntrophic fatty-acid oxidizing bacteria. Bioresour. Technol. 2021, 332, 125074. [Google Scholar]
- Barredo, M.S.; Evison, L.M. Effect of propionate toxicity on methanogen-enriched sludge, Methanobrevibacter smithii, and Methanospirillum hungatii at different pH values. Appl. Environ. Microbiol. 1991, 57, 1764. [Google Scholar] [CrossRef] [Green Version]
- Dhaked, R.K.; Waghmare, C.K.; Alam, S.I.; Kamboj, D.V.; Singh, L. Effect of propionate toxicity on methanogenesis of night soil at phychrophilic temperature. Bioresour. Technol. 2003, 87, 299–303. [Google Scholar] [CrossRef]
- Gourdon, R.; Vermande, P. Effects of propionic acid concentration on anaerobic digestion of pig manure. Biomass 1987, 13, 1–12. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, T.; Si, B.; Watson, J.; Zhang, Y. Accelerating anaerobic digestion for methane production: Potential role of direct interspecies electron transfer. Renew. Sustain. Energy Rev. 2021, 145, 111069. [Google Scholar] [CrossRef]
- Salama, E.S.; Saha, S.; Kurade, M.B.; Dev, S.; Jeon, B.H. Recent trends in anaerobic co-digestion of lipidic-waste: Fat, oil, and grease (FOG) for enhanced biomethanation. Prog. Energy Combust. 2019, 70, 22–42. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, H.; Zhang, P.; Cai, Y.; Ding, Y. Transformation of bacterial community structure in rumen liquid anaerobic digestion of rice straw. Environ. Pollut. 2020, 269, 116130. [Google Scholar] [CrossRef] [PubMed]
- Kainthola, J.; Kalamdhad, A.S.; Goud, V.V. Enhanced methane production from anaerobic co-digestion of rice straw and hydrilla verticillata and its kinetic analysis. Biomass Bioenergy 2019, 125, 8–16. [Google Scholar] [CrossRef]
- Poirier, S.; Quéméner, E.D.; Madigou, C.; Bouchez, T.; Chapleur, O. Anaerobic digestion of biowaste under extreme ammonia concentration: Identification of key microbial phylotypes. Bioresour. Technol. 2016, 207, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Artif. Intell. 2006, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.A.; Hua, Z.; Yi, Z.C.; Sffa, B. Improving anaerobic digestion of corn straw by using solid-state urea pretreatment. Chemosphere 2022, 293, 133559. [Google Scholar]
- Steinhaus, B.; Garcia, M.L.; Shen, A.Q.; Angenent, L.T. A Portable Anaerobic Microbioreactor Reveals Optimum Growth Conditions for the Methanogen Methanosaeta concilii. Appl. Environ. Microbiol. 2007, 73, 1653. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Bian, C.; Zhu, N.; Shen, Y.; Yuan, H. Enhancement of methane production from anaerobic digestion of waste activated sludge with choline supplement. Energy 2019, 173, 1021–1029. [Google Scholar] [CrossRef]
- Jiang, C.; Peces, M.; Andersen, M.H.; Kucheryavskiy, S.; Nierychlo, M.; Yashiro, E.; Andersen, A.; Kirkegaard, R.H.; Hao, L.; Høgh, J.; et al. Characterizing the growing microorganisms at species level in 46 anaerobic digesters at Danish wastewater treatment plants: A six-year survey on microbial community structure and key drivers. Water Res. 2021, 193, 116871. [Google Scholar] [CrossRef]
- Xu, Y.; Geng, H.; Chen, R.; Liu, R.; Dai, X. Enhancing methanogenic fermentation of waste activated sludge via isoelectric-point pretreatment: Insights from interfacial thermodynamics, electron transfer and microbial community. Water Res. 2021, 197, 117072. [Google Scholar] [CrossRef]
- Zamanzadeh, M.; Hagen, L.H.; Svensson, K.; Linjordet, R.; Horn, S.J. Anaerobic digestion of food waste—Effect of recirculation and temperature on performance and microbiology. Water Res. 2016, 96, 246–254. [Google Scholar] [CrossRef]
- Li, Y.; Tang, Y.; Xiong, P.; Zhang, M.; Deng, Q.; Liang, D.; Zhang, Y. High-efficiency methanogenesis via kitchen wastes served as ethanol source to establish direct interspecies electron transfer during anaerobic Co-digestion with waste activated sludge. Water Res. 2020, 176, 115763. [Google Scholar] [CrossRef] [PubMed]
- Wainaina, S.; Awasthi, M.K.; Horvath, I.S.; Taherzadeh, M.J. Anaerobic digestion of food waste to volatile fatty acids and hydrogen at high organic loading rates in immersed membrane bioreactors. Renew. Energy 2020, 152, 1140–1148. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, J.; Liu, J.; Yang, H.; Gao, L.; Yuan, X.; Wang, X. Material and microbial changes during corn stalk silage and their effects on methane fermentation. Bioresour. Technol. 2016, 222, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Lang, K.; Schuldes, J.; Klingl, A.; Poehlein, A.; Daniel, R.; Brune, A. New mode of energy metabolism in the seventh order of methanogens as revealed by comparative genome analysis of “Candidatus Methanoplasma termitum”. Appl. Environ. Microbiol. 2015, 81, 1338. [Google Scholar] [CrossRef] [Green Version]
- Qian, P.; Xiao, L.; Ming, Z.; Li, Y. Effect of CaO addition on anaerobic digestion of waste activated sludge at different temperatures and the promotion of valuable carbon source production under ambient condition. Bioresour. Technol. 2018, 265, 247. [Google Scholar]
- Liu, L.; Tong, Z.; Wan, H.; Chen, Y.; Ren, G. Anaerobic co-digestion of animal manure and wheat straw for optimized biogas production by the addition of magnetite and zeolite. Energy Convers. Manag. 2015, 97, 132–139. [Google Scholar] [CrossRef]
- Nobu, M.K.; Narihiro, T.; Kuroda, K.; Mei, R.; Liu, W.T. Chasing the elusive Euryarchaeota class WSA2: Genomes reveal a uniquely fastidious methyl-reducing methanogen. ISME J. 2016, 10, 2478–2487. [Google Scholar] [CrossRef]
- Ni, B.J.; Batstone, D.; Zhao, B.H.; Yu, H.Q. Microbial Internal Storage Alters the Carbon Transformation in Dynamic Anaerobic Fermentation. Environ. Sci. Technol. 2015, 49, 9159–9167. [Google Scholar] [CrossRef]
- Ki, B.M.; Kim, Y.M.; Jeon, J.M.; Ryu, H.W.; Cho, K.S. Characterization of odor emissions and microbial community structure during degradation of pig carcasses using the soil burial-composting method. Waste Manag. 2018, 77, 30–42. [Google Scholar] [CrossRef]
- Chen, H.; Rao, Y.; Cao, L.; Shi, Y.; Hao, S.; Luo, G.; Zhang, S. Hydrothermal conversion of sewage sludge: Focusing on the characterization of liquid products and their methane yields. Chem. Eng. J. 2019, 357, 367–375. [Google Scholar] [CrossRef]
- Wandera, S.M.; Westerholm, M.; Qiao, W.; Yin, D.; Jiang, M.; Dong, R. The correlation of methanogenic communities’ dynamics and process performance of anaerobic digestion of thermal hydrolyzed sludge at short hydraulic retention times. Bioresour. Technol. 2019, 272, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Solli, L.; Velsrud, O.H.; Horn, S.; Rike, A. A metagenomic study of the microbial communities in four parallel biogas reactors. Biotechnol. Biofuels 2014, 7, 146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gao, P.; Cheng, J.; Liu, G.; Zhang, X.; Feng, Y. Enhancing anaerobic digestion and methane production of tetracycline wastewater in EGSB reactor with GAC/NZVI mediator. Water Res. A J. Int. Water Assoc. 2018, 136, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.C.; Xu, Y.Q.; Lu, Y.H. Microbial mechanisms of methane production and oxidation in terrestrial ecosystems. Acta Ecol. Sin. 2015, 35, 6592–6603. [Google Scholar]
- Park, J.G.; Lee, B.; Park, H.R.; Jun, H.B. Long-term evaluation of methane production in a bio-electrochemical anaerobic digestion reactor according to the organic loading rate—ScienceDirect. Bioresour. Technol. 2019, 273, 478–486. [Google Scholar] [CrossRef]
- Cengel, A.Y.; Boles, A.M. Thermodynamics: An Engineering Approach, 9th ed.; McGraw-Hill: New York, NY, USA, 2019; ISBN 9781259822674. [Google Scholar]
- GE POWER. 2016. Available online: https://www.gepower.com/content/dam/gepower-pgdp/global/en_US/documents/product/Reciprocating%20E-ngines/Jenbacher/Type%202/jenbacher-type-2-fs-en-metric-2016.pdf (accessed on 25 January 2018).
- GERONIMO 2 BIOGAS. Available online: http://www.cea.org.cy/TOPICS/Renewable%20Energy/2013/Biogas_Plants_Europe_GeronimoII.pdf (accessed on 25 January 2018).
- Pitts, D.; Sissom, L.E. Schaum’s Outline of Heat Transfer, 2nd ed.; McGraw-Hill: New York, NY, USA, 2011. [Google Scholar]

| Type | Treatment Groups | S/I Ratio | Substrate |
|---|---|---|---|
| High S/I ratio system | T1 | 4 | F-CS |
| T2 | D-CS | ||
| Low S/I ratio system | T3 | 1 | F-CS |
| T4 | D-CS |
| Parameters | F-CS | D-CS | Inoculum |
|---|---|---|---|
| TS (%) | 94.04 ± 0.40 | 99.11 ± 0.79 | 7.07 ± 0.02 |
| VS ((% of TS)) | 86.42 ± 1.11 | 85.34 ± 0.48 | 5.27 ± 0.08 |
| C (% of TS) | 38.27 ± 7.73 | 38.03 ± 5.90 | 34.90 ± 5.41 |
| N (% of TS) | 2.25 ± 0.71 | 1.82 ± 0.26 | 2.11 ± 0.72 |
| C/N (% of TS) | 16.98 ± 1.52 | 20.89 ± 1.34 | 16.55 ± 2.30 |
| Cellulose (% of TS) | 37.49 ± 1.52 | 34.62 ± 0.21 | 24.70 ± 0.98 |
| Hemicellulose (% of TS) | 31.22 ± 1.67 | 26.25 ± 0.51 | 21.52 ± 1.23 |
| Lignin (% of TS) | 16.88 ± 0.22 | 19.03 ± 0.32 | 5.39 ± 1.92 |
| Crude protein (% of TS) | 19.63 ± 0.37 | 12.36 ± 0.15 | 13.92 ± 0.30 |
| Degradation Ratio of Cellulose (%) | Degradation Ratio of Hemicellulose (%) | Degradation Ratio of Lignin (%) | Initial Crude Protein (%) | End Crude Protein (%) | |
|---|---|---|---|---|---|
| T1 | 30.01 ± 0.27 | 27.02 ± 1.59 | −1.34 ± 0.10 | 11.96 ± 1.60 | 18.13 ± 0.50 |
| T2 | 29.78 ± 1.32 | 23.64 ± 1.74 | −0.95 ± 005 | 11.20 ± 2.62 | 17.18 ± 2.01 |
| T3 | 44.62 ± 0.63 | 13.72 ± 1.56 | 1.45 ± 0.36 | 12.14 ± 0.57 | 15.91 ± 0.69 |
| T4 | 45.97 ± 2.31 | 15.68 ± 1.89 | 0.37 ± 0.31 | 11.86 ± 1.92 | 16.59 ± 0.62 |
| Model | Parameters | T1 | T2 | T3 | T4 |
|---|---|---|---|---|---|
| First order kinetic model | correlation coefficient R2 | 0.9300 | 0.9776 | 0.8994 | 0.9299 |
| K (1/day) | 0.41 | 0.29 | 0.33 | 0.31 | |
| Modified Gompertz model | correlation coefficient R2 | 0.9993 | 0.9941 | 0.9428 | 0.9884 |
| Rmax (mL/gVS-d) | 47.9 | 35.3 | 33.7 | 26.2 | |
| λ (d) | 8.2 | 6.2 | 6.2 | 5.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Huang, J.; Yao, Y.; Wang, M.; Li, A. Effect of Pretreatment by Freeze Vacuum Drying on Solid-State Anaerobic Digestion of Corn Straw. Fermentation 2022, 8, 259. https://doi.org/10.3390/fermentation8060259
Liu Z, Huang J, Yao Y, Wang M, Li A. Effect of Pretreatment by Freeze Vacuum Drying on Solid-State Anaerobic Digestion of Corn Straw. Fermentation. 2022; 8(6):259. https://doi.org/10.3390/fermentation8060259
Chicago/Turabian StyleLiu, Zhen, Jinzhi Huang, Yiqing Yao, Mengyi Wang, and Anjie Li. 2022. "Effect of Pretreatment by Freeze Vacuum Drying on Solid-State Anaerobic Digestion of Corn Straw" Fermentation 8, no. 6: 259. https://doi.org/10.3390/fermentation8060259





