Abstract
Broussonetia papyrifera L. (paper mulberry) is an alternative woody plant, which can used to replace part of the protein feed for ruminants. Ensiling is an effective way to preserve fresh pasture and to solve the problem of stable storage and feed conversion of paper mulberry in the rapid growth period. However, low dry matter (DM), water-soluble carbohydrate, and lactic acid bacteria (LAB) reduce the quality of paper mulberry silage. This study assesses the influence of wilting time (0 h and 3.5 h; lighting: 3.43 × 104 Lux) and three additives (Enterococcus durans, CL; cellulase, CE; and formic acid, FA) on the fermentation quality, aerobic stability, and bacterial community of whole plant B. papyrifera silage. The whole plant B. papyrifera sample was mowed and wilted for 0 h and 3.5 h, and then had CL, CE, or FA added, followed by 60 days of ensiling. The results show all silage samples had high fermentation quality with pH below 4.2, ammonia-nitrogen below 100 g/kg DM, and no detectable butyric acid. The additives protected the DM and the crude protein from protease activity (p < 0.05), and CL was the most effective among them. Furthermore, wilting time influenced the silage’s bacterial communities, but overall, CL treatment had the greatest impact on bacterial communities. Wilting time and formic acid treatment significantly improved aerobic stability (p < 0.05). Enterococcus was positively correlated with lactic acid (LA), while negatively correlated with LA and Weissella (p < 0.001). Enterococcus was identified as the main driver of the whole plant paper mulberry ensiling process in the present study. In conclusion, compared to other additives, LAB is the most effective and economical to improve the fermentation quality and reduce the protein degradation of whole plant paper mulberry silage. Our findings provide a theoretical basis to improve the quality and production of paper mulberry silage.
1. Introduction
Broussonetia papyrifera L. (paper mulberry), a member of the Moraceae family, is a deciduous tree native to eastern Asia commonly used in papermaking and used as a new forage resource to supplement ruminant feed [1]. Since 2015, it has been planted widely in China as a part of the development-oriented poverty program for a high yield of 120 tonnes per hectare and a high crude protein (CP) content of 160 g/kg dry matter (DM) [2]. Moreover, paper mulberry has been used to increase the antioxidant capacity and anti-inflammatory activity of animals since 2020 when the Chinese government banned antibiotic supplements in animal feed [3]. However, the multi-cutting nature of paper mulberry and the rapid growth period (July to September) that occurs every year, resulting in huge biomass accumulation, brings unprecedented pressure on stable storage and feed conversion [4].
Ensiling is an effective way to preserve fresh pasture and to solve the problem of seasonal balance of forage for ruminants [5]. Previous studies concluded that low fermentation quality of paper mulberry silage was obtained due to its low DM, lower water-soluble carbohydrate (WSC) content, and relatively lower count of lactic acid bacteria (LAB) [6,7,8], which is similar to forage legumes such as alfalfa. Recent studies have shown that wilting and the use of additives reduces the problems mentioned regarding silage production. Studies have also demonstrated wilting as an effective method to enhance silage quality as high-moisture materials can improve the buffering capacity and eased butyric acid (BA) fermentation [9,10,11]. Additives like LAB and cellulase were used to decrease the pH and ammonia-nitrogen (NH3-N) concentration in alfalfa and paper mulberry silage, respectively [7,12,13,14]. Furthermore, formic acid has been added to silage as an inhibitor of spoilage bacteria. FA causes direct acidification and promotes LAB growth [5]. Silage is the product of an anaerobic fermentation process driven by lactic acid (LA)-producing bacteria (e.g., LAB), and microbial diversity and community structure are very important for the process of ensiling. Therefore, numerous studies have focused on reporting the widespread use of Lactobacillus species as an inoculant to regulate the process of silage fermentation and applying bacterial community analysis to understand the role of micro-organisms while monitoring silage quality. By high-throughput sequencing, Chen et al. found that LAB inoculation reconstructed and improved the bacterial community in round bale oat silage [15]. Dong et al. revealed the bacterial community dynamics during the ensiling of paper mulberry with perennial ryegrass [6]. Du et al. explored the microbial network and fermentation characteristics of woody silage prepared with exogenous carbohydrate additives [14]. In particular, the study by Bai et al. showed that silage with Lactobacillus cocci had better fermentation quality and higher feeding value than other silages, such as Leuconostocs, Pediococcus, Lactococci, and Enterococci, although they had lower tolerance acceptability [16]. However, little is known about the bacterial community of paper mulberry silage with additives and wilting time, especially the cocci LAB inoculate.
Furthermore, to the best of our knowledge, most of the current research on paper mulberry silage has focused on tender stems and leaves with short planting years and higher nutritional value [4,6,7,8,14]. The remaining stalks may cause waste of raw material of feedstuff, and the feasibility of whether whole-plant paper mulberry with a long planting age (more than 2 years) can be successfully prepared as silage has not been trialed. The suitable moisture content of silage is generally 65–75%, and wilting is used to regulate the moisture content of silage materials to avoid or reduce the impact of unfavorable factors for silage fermentation [4]. As mentioned above, additives are often used to influence the fermentation process, thereby improving the quality of silage. LAB and cellulase play important roles in improving silage fermentation by increasing the number of beneficial micro-organisms and breaking down cellulose to produce soluble carbohydrates, respectively [17]. Formic acid is used as a silage additive due to its ability to inhibit the growth of Clostridium and Escherichia coli without inhibiting the growth of Lactobacillus [5]. Would these different types of additives still improve silage fermentation quality despite the relatively low nutrient levels of the whole paper mulberry, and which one is the most effective? Therefore, this study investigated the effects of wilting time (0 h and 3.5 h) and three additives, LAB, cellulase, and formic acid, on the fermentation characteristics, bacterial community, and aerobic stability of whole-plant paper mulberry silage after 2 years of planting. This study provides detailed information about ensiling whole-plant paper mulberry to maintain high quality.
2. Materials and Methods
2.1. Raw Material and Silage Preparation
The raw material for this experiment was whole paper mulberry planted in the third year of planting (mown 4–5 times a year, material from the second crop was used in this study). No fertilizers or pesticides were used during planting. Paper mulberry was harvested as a whole plant (plant height 1.6–2.0 m, cutting stubble height 20–30 cm) on the 14th of June 2019 from Changshun County, Guizhou Province, and in situ wilted for 0 h and 3.5 h (lighting: 3.43 × 104 lux). The detailed weather conditions and complex geographic environment of the region are described in previous studies by Du et al. [4]. The whole plant raw materials (including stems, main stems, twigs and leaves) were chopped into an approximate length of 2.5 cm with a manual forage chopper (92-2S, Sida Agri-Machine Co., Ltd., Luoyang, China). The chemical composition of unensiled paper mulberry is shown in Table 1.

Table 1.
Chemical composition and microbial population of B. papyrifera before ensiling.
This experiment was performed with different ensiling treatments as a 2 × 4 factorial in completely randomized design (CRD) with three replication runs. The experiment was run with two factors, where A factors were un-wilt (U) and wilt (W) and Factor B consisted of four materials treated with (i) no additive (CK), (ii) add 1.0 × 1010 CFU/kg of Enterococcus durans (CL) at 0.02 g/kg FM of silage, (iii) add cellulase (CE) at 1 g/kg of silage, and (iv) add formic acid (FA) at 4 mL/kg of silage. All additives were added into the silage material based on fresh matter. Enterococcus durans was purchased from Belefeng Co., Guiyang, China; cellulase and formic acid were purchased from Wela Co., Ltd. Guiyang, China. Later, 10 mL/kg distilled water was added to CK, CL, and CE, while 6 mL/kg distilled water was added to FA to ensure the same moisture content under the same wilting time.
The material was compacted in 30 × 40 cm PE bags and then vacuumed; each bag contained about 500 g of fresh matter. Triplicate bags were prepared for each treatment and ensiled for 60 days at room temperature (16 °C–22 °C). After blending, the mixture was placed into a polyvinyl chloride vacuum sealing bag, followed by vacuum pumping and sealing using a vacuum gauge. The bag was unsealed after storage in a laboratory away from light for 60 d, and the indexes were measured.
2.2. Analysis of Chemical Composition, Fermentation Quality and Aerobic Stability
After fermentation was completed, bags were opened to evaluate the chemical composition and fermentation quality. DM content of silage samples was determined by drying the samples at 65 °C for 48 h to a constant mass, and then grinding with an electric mill to pass through a 1 mm screen. The CP content was measured using a Kjeldahl nitrogen analyzer (Kjeltec 8400; FOSS, Hilleroed, Denmark) according to method 990.03 of Association of Official Analytical Chemists (AOAC) [18]. The WSC was determined by colorimetric after-reaction with an anthrone reagent [19]. The neutral detergent fiber (NDF) and acid detergent fiber (ADF) were analyzed using an ANKOM fiber analyzer (ANKOM220; Macedon, NY, USA) [20].
To measure fermentation quality, 20 g of each treatment was blended with 180 mL deionized water in the juicer, then extracted at 4 °C for 12 h. The extracts were filtered through qualitative filter paper, filtrates were used for measuring the pH, LA, acetic acid (AA), propionic acid (PA), BA, and NH3-N. The pH of the FTMR was measured using a pH meter (PHS-3C; Yidian Co., Shanghai, China). Organic acids were determined using a high performance liquid chromatography (Agilent 1260LC, Santa Clara, CA, USA, with 250 mm × 4.6 mm (Particle Size 5 µm)) column, TC-C18, Agilent Co., Santa Clara, CA, USA; condition: column temperature, 50 °C; the first mobile phase, pure methanol; the second mobile phase, 0.01 mol/L KH2PO4; pH, 2.7; sample injection volume, 10μL; flow rate, 0.7 mL/min; standard LA, L118492; standard AA, A298827; standard PA, P110446; and standard BA, B110438 (Aladdin Co., Shanghai, China). The NH3-N concentration was determined using the phenol-hypochlorite reaction method [21]. The aerobic stability was tested using the method followed by Liu et al. [22]; aerobic stability was defined as the time taken to increase the sample temperature by 2 °C above the ambient temperature.
2.3. Bacterial Diversity Analysis
The procedures for DNA extraction and PCR amplification followed those used by Bai et al. [23]. The DNA samples were sequenced at the Genedenovo Biotechnology Company using paired-end sequencing with an Illumina MiSeq PE300 platform. To get high-quality sequencing, the barcode and primers were discharged, and then Mothur (v.1.34.4) was used to discharge the sequences less than 200 bp with a maximum homopurine value greater than 10 following the mothur standard operating procedures as previously described [24]. Remaining sequences were checked for chimeras in the de novo mode using USEARCH 8.0 (Berkeley, CA, USA). After the filtering process, the clean tag remained for downstream analysis. The operational taxonomic units (OTUs) at 97% similarity level were clustered using QIIME (v1.8.0) (Boulder, CO, USA). The OTUs file was used to calculate rarefaction (R (v.22)) and alpha diversity (Mothur (v1.34.4)). The weighted UniFrac distance matrix was employed to calculate the β diversity, and principal co-ordinates analysis (PCoA) was performed at 3% dis-similarity level. Redundancy analysis (RDA) was performed using the R language vegan package (version 2.5.3). Spearman correlation coefficients between fermentation characteristics and microbes were analyzed using the R language psych package (version 1.8.4).
2.4. Statistical Analysis
The statistical analysis of two-way ANOVA was performed using R (v.22). Duncan’s HSD test was employed to determines the differences in the treatment means, significance was set at p < 0.05, and the data were expressed as mean and accompanied by standard error of the mean (SEM). The bacterial relative abundance, principal PCoA, and ANOSIM analysis were analyzed using a free online platform of OmicShare tools (http://www.omicshare.com/tools, accessed on 10 June 2020).
Data concerning the chemical composition and fermentation characteristics were subjected to two-way ANOVA with a fully randomized design, with wilting or not (Wi) and additive (Ad) (2 × 4) as the main variables. If the difference between groups was found to be significant, Duncan’s range test was applied. The applied mathematical model was as follows:
Yij = µ + Ai + Bj + (AB)ij + eijl.
Yij: observation applying the i th wilt with j th inclusion different additions; µ: overall average; Ai: effect of i th wilt; Bj: effect of j th inclusion different additions; (AB)ij: interaction effect of i th wilt × j th inclusion different additions; and eijl: error associated with each observation.
3. Results
3.1. Chemical Composition of Whole Plant B. papyrifera Silage
Table 2 shows the chemical composition of ensiled paper mulberry with different additives. After 60 days of ensiling, additives significantly affected the contents of DM, CP, NDF, ADF, and WSC (p < 0.05). Treatments with additives had higher DM and CP than that in the CK group, while NDF and ADF in the CK groups were higher than in the CE and FA groups (p < 0.01). The contents of WSC in the CL group were lower than other groups (p < 0.001). Treatments wilted for 3.5 h had higher DM, ADF, and WSC contents than those that were unwilted (p < 0.05). Wilting time did not affect CP and NDF content within different additives (p < 0.05). The interaction between wilting time and additives showed a significant effect on the DM and WSC content (p < 0.05), but had no significant effects on DM, NDF, or ADF content (p > 0.05).

Table 2.
The chemical positions of B. papyrifera silage with additives.
3.2. Fermentation Profile and Aerobic Stability of Whole Plant B. papyrifera Silages
Table 3 and Figure 1 show the fermentation quality and aerobic stability of paper mulberry silage after 60 days of ensiling, respectively. Additives significantly influenced the silage pH, LA, AA, NH3-N, and aerobic stability (p < 0.05), while wilting time did not affect NH3-N content (p < 0.05). U-FA had the lowest pH value when compared to other treatments (p < 0.001). Higher LA and LA/AA were found in the CL group, while higher AA and PA were observed in the FA group (p < 0.05). The CK group had higher PA and NH3-N contents than other groups (p < 0.05). Additives and wilting time showed a significant interaction effect on LA and PA content (p < 0.05), but had no significant effects on pH, AA, and NH3-N content (p > 0.05). BA was not detected in any treatments.

Table 3.
The fermentation characteristics of B. papyrifera silage with additives.
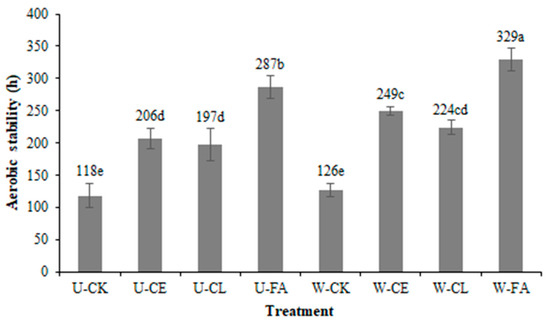
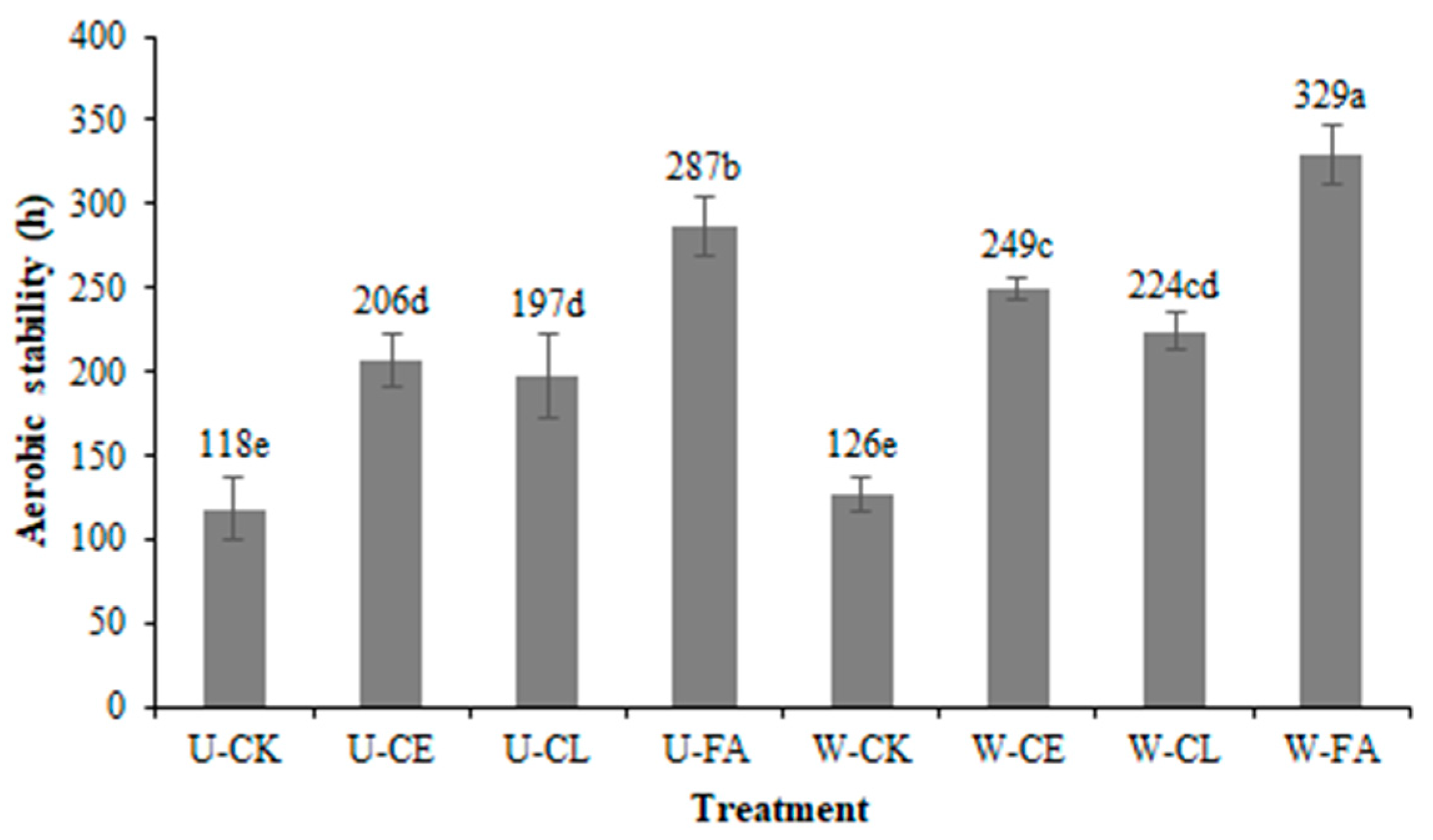
Figure 1.
Effect of additives on the aerobic stability (h) of paper mulberry silage after 60 days of ensiling (SEM = 14.47, p < 0.001). Vertical bars are the standard errors of the means, bars with different letters differ (p < 0.05). a–e: Values shown in different letters in the same column are statistically important (p < 0.05). Treatment: U, wilt 0 h; W, wilt 3.5 h; CK, control (no additives); CL, Enterococcus durans; CE, cellulase; and FA, formic acid.
As shown in Figure 1, the FA group reduced the silage spoilage upon exposure to air, with maximum aerobic stability among the different additives under both unwilted (287 h) and wilted for 3.5 h (329 h, p < 0.05). On the contrary, the CK group quickly deteriorated (117.83 h and 126.00 h; p < 0.05). From the point of view of aerobic stabilization time, wilting treatment has a longer aerobic stabilization time than non-wilting treatment (p < 0.05).
3.3. Analysis of Bacterial Diversity and Community of Whole Plant B. papyrifera Silages
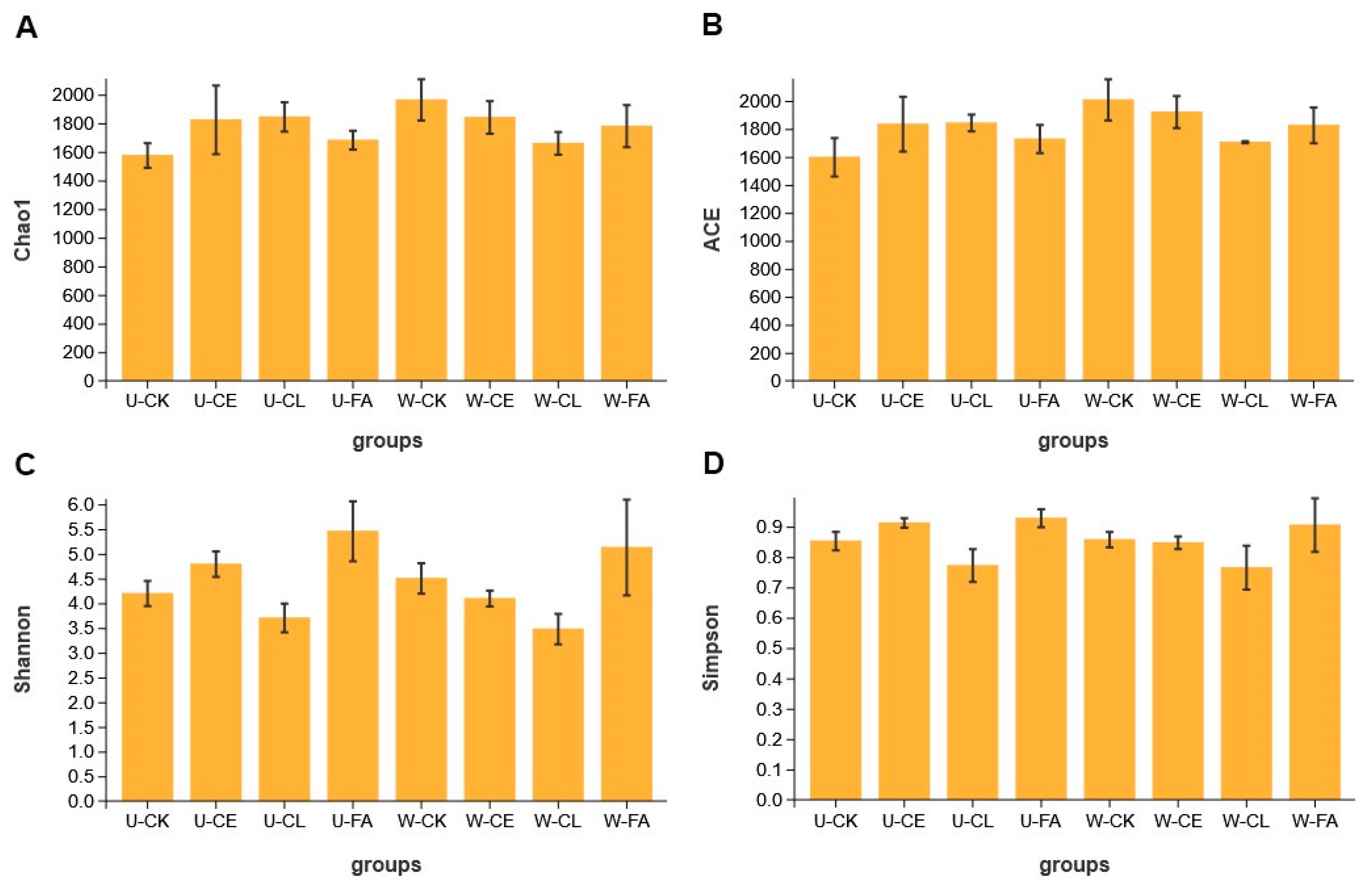
Alpha diversity indexes of bacterial diversity for paper mulberry silage are shown in Figure 2. Except in the CL group, the ACE and Chao indexes in wilted treatments were higher than those in unwilted treatments, indicating higher bacterial species richness of paper mulberry silage under wilting. The Shannon and Simpson indexes in unwilted treatments were higher than in wilted treatments except in the CK group, indicating that wilting will reduce the bacterial diversity of silage under the condition of added additives. The lowest Shannon and Simpson indexes were observed in the CL group, indicating that the addition of Enterococcus durans would reduce the bacterial diversity of paper mulberry silage.
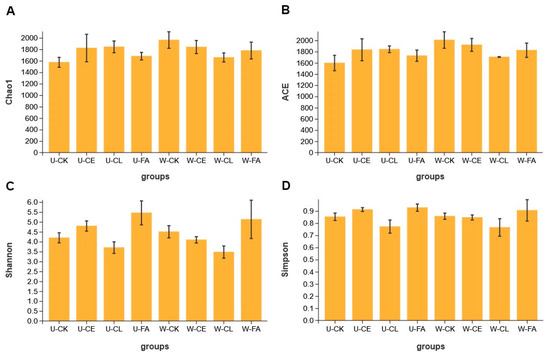
Figure 2.
Alpha diversity of bacterial diversity at the Chao1 index (A), ACE (abundance-based coverage estimator) index (B), Shannon index (C), and Simpson index (D). U, wilt 0 h; W, wilt 3.5 h; CK, control (no additives); CL, Enterococcus durans; CE, cellulase; and FA, formic acid.
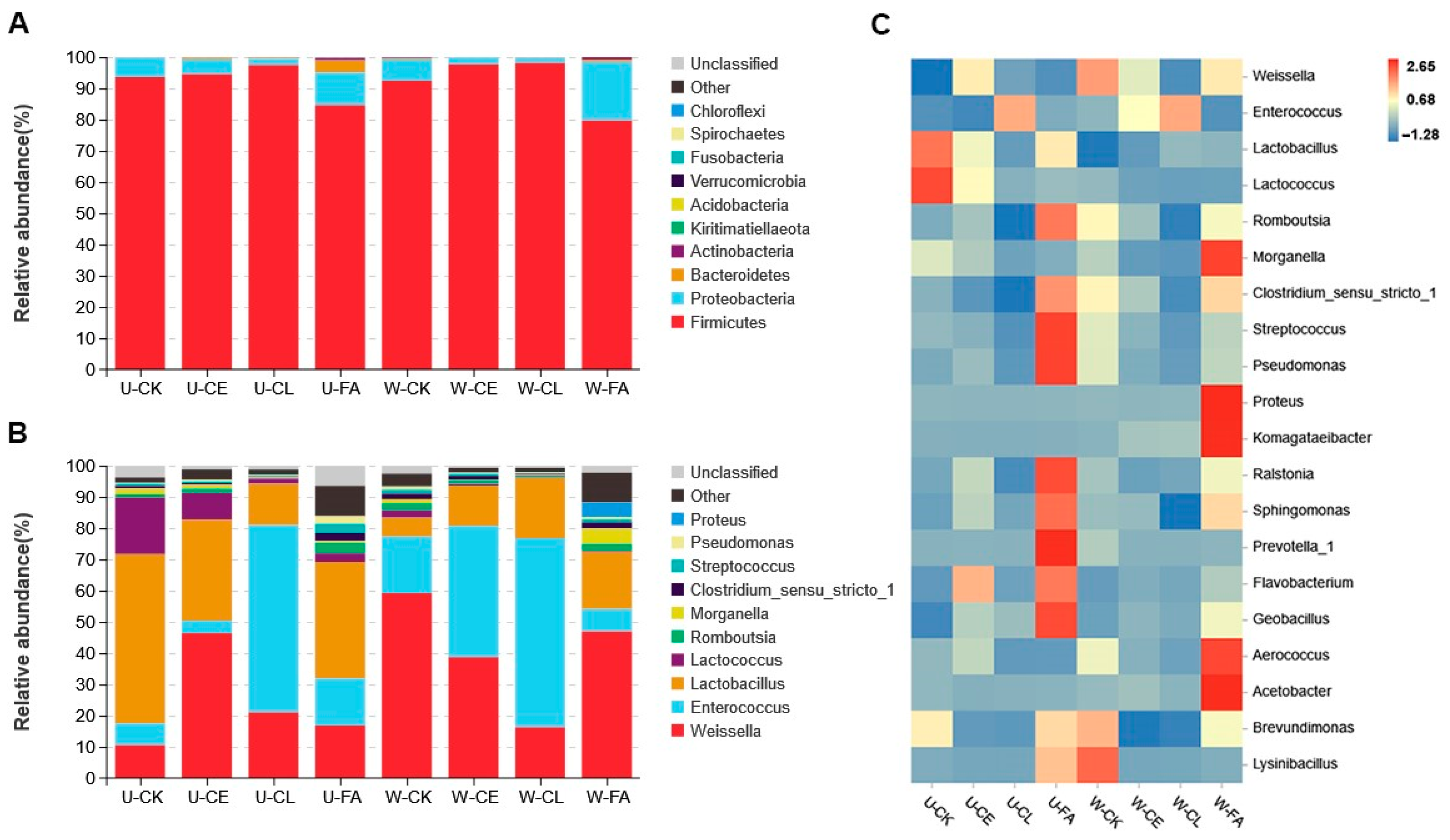
The bacterial communities at the phylum level and genus level of the silage samples are shown in Figure 3. As shown in Figure 3A, at the end of ensiling, Firmicutes were the predominant phylum (75.76–98.16%). As shown in Figure 3B,C, Enterococcus, Lactobacillus, and Weissella were the dominant bacteria in all treatments. FA at both wilting times showed an increase in Proteobacteria compared with CK. Lactobacillus (54.22%) was the main bacteria in U-CK silage at the genus level; however, as wilting was prolonged, Weissella was predominant (59.18%). Compared to U-CE, the relative abundance of Enterococcus increased from 4.00% to 42.01%, while Lactobacillus decreased from 32.37% to 12.87% in W-CE. The dominant LAB identified in U-CL and W-CL were Enterococcus (59.89% and 60.54%), Weissella (21.04% and 16.27%), and Lactobacillus (13.22% and 19.30%).
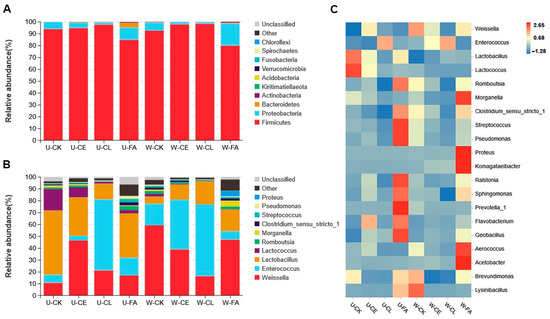
Figure 3.
Relative abundances of bacterial communities at the levels of phylum (A) and genera (B). Correlation analysis of the bacterial genus with the different treatments (C). U, wilt 0 h; W, wilt 3.5 h; CK, control (no additives); CL, Enterococcus durans; CE, cellulase; and FA, formic acid.
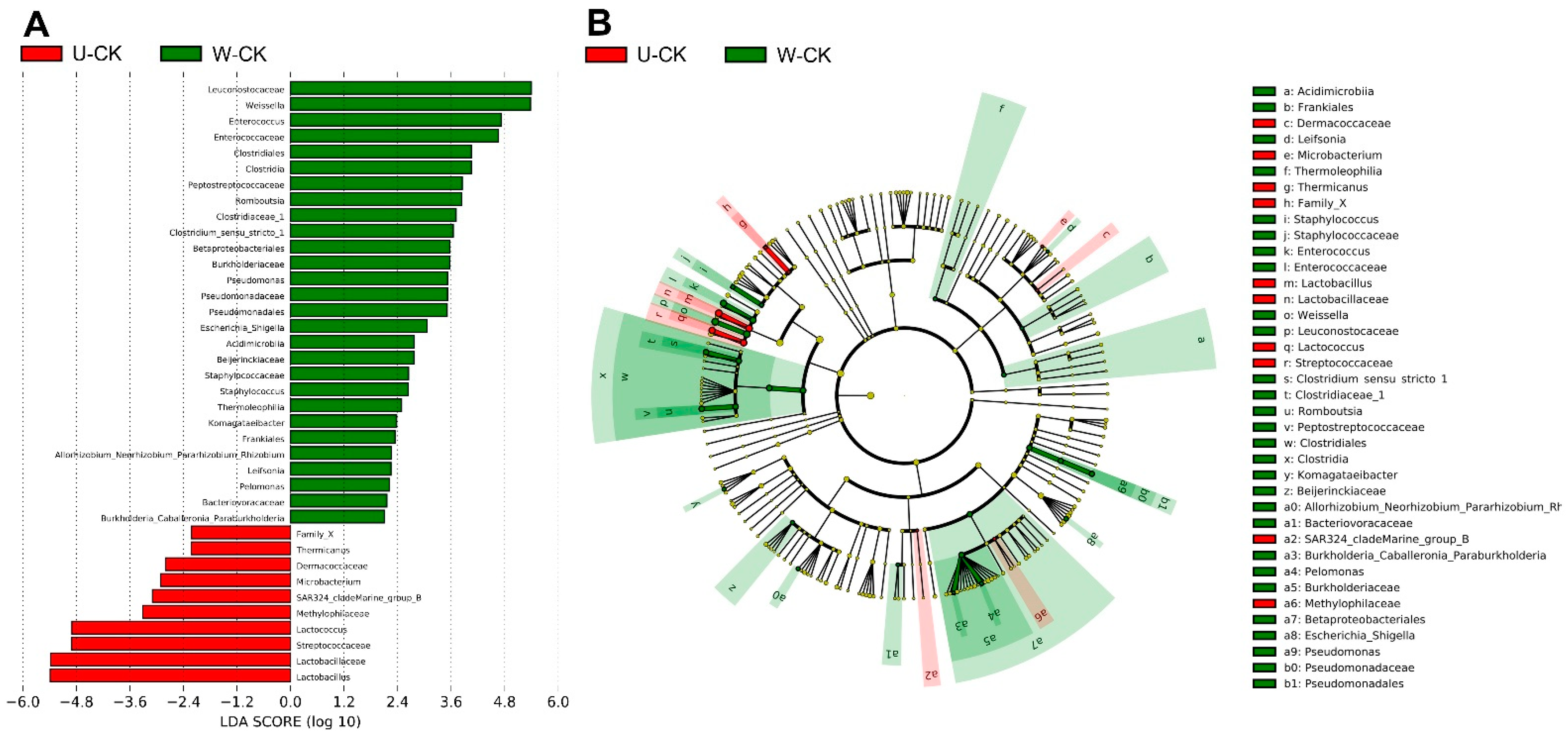
The comparison of microbial variations between unwilted and wilted is shown in Figure 4, unwilted and wilted had a significant effect on the bacterial flora. Lactobacillus and Lactococcus, which were most abundant in unwilted silage, and Enterococcus, Weissella, Romboutsia, Clostridium_sensu_stricto_1, and Pseudomonas, which were most abundant in wilted silage, were the dominant genera that contributed to the differences between wilted and unwilted silage.
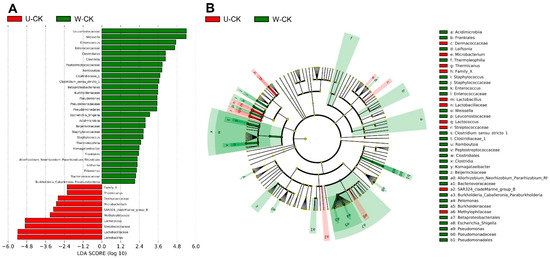
Figure 4.
Comparison of microbial variations between U-CK and W-CK, using the LEfSe online tool. (A) Histogram of the LDA scores for differentially abundant features between U-CK and W-CK. U, wilt 0 h; W, wilt 3.5 h; and CK, control (no additives). The threshold on the logarithmic LDA score for discriminative features was set to 2.0. (B) Cladogram for taxonomic representation of significant differences. Differences are represented in the color of the most abundant taxa.
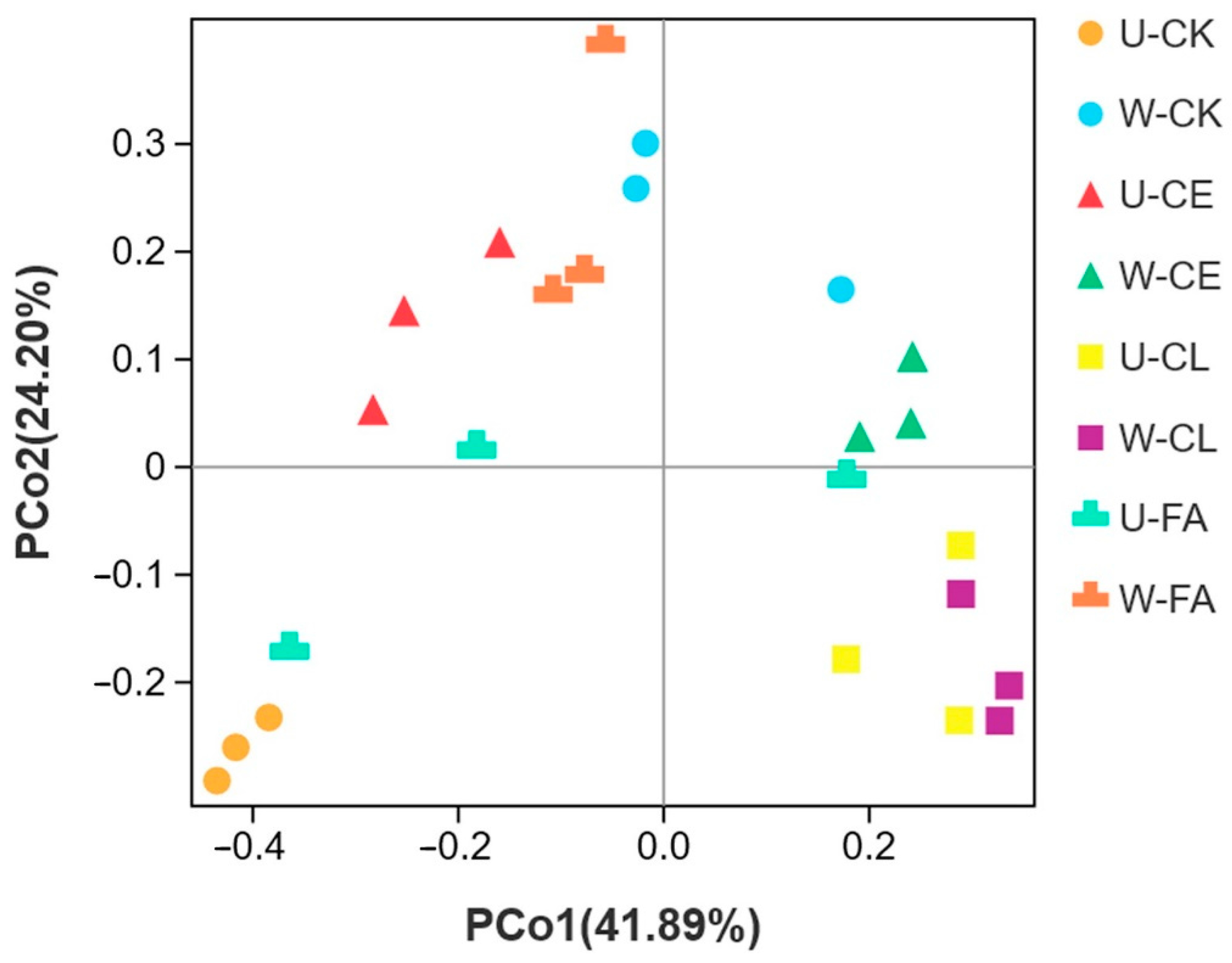
PCoA and ANOSIM were performed to determine the bacterial community structure variance of paper mulberry silage after ensiling for 60 days. As shown in Figure 5, principal component 1 (PCo1) and principal component 2 (PCo2) explained 41.89% and 24.20% of the total variance, respectively. Distinct clusters were observed for unwilted and wilted for 3.5 h of all additives, except the CL group.
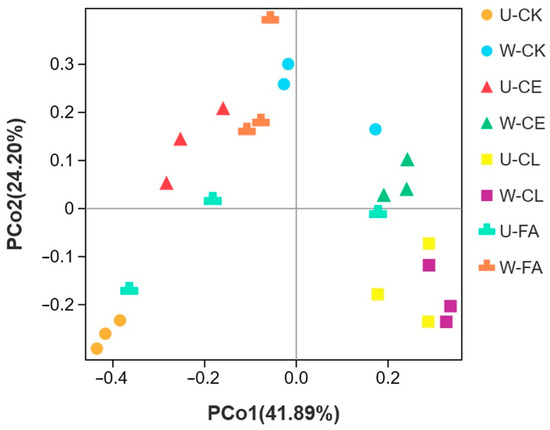
Figure 5.
Principal co-ordinates analysis (PCoA) and ANOSIM analysis of B. papyrifera silages. U, wilt 0 h; W, wilt 3.5 h; CK, control (no additives); CL, Enterococcus durans; CE, cellulase; and FA, formic acid. The distance between treatments represents the degree of bacterial community difference, and the closer the distance, the more similar the difference.
3.4. Correlation Analysis of the Bacterial Community and Fermentation Characteristics
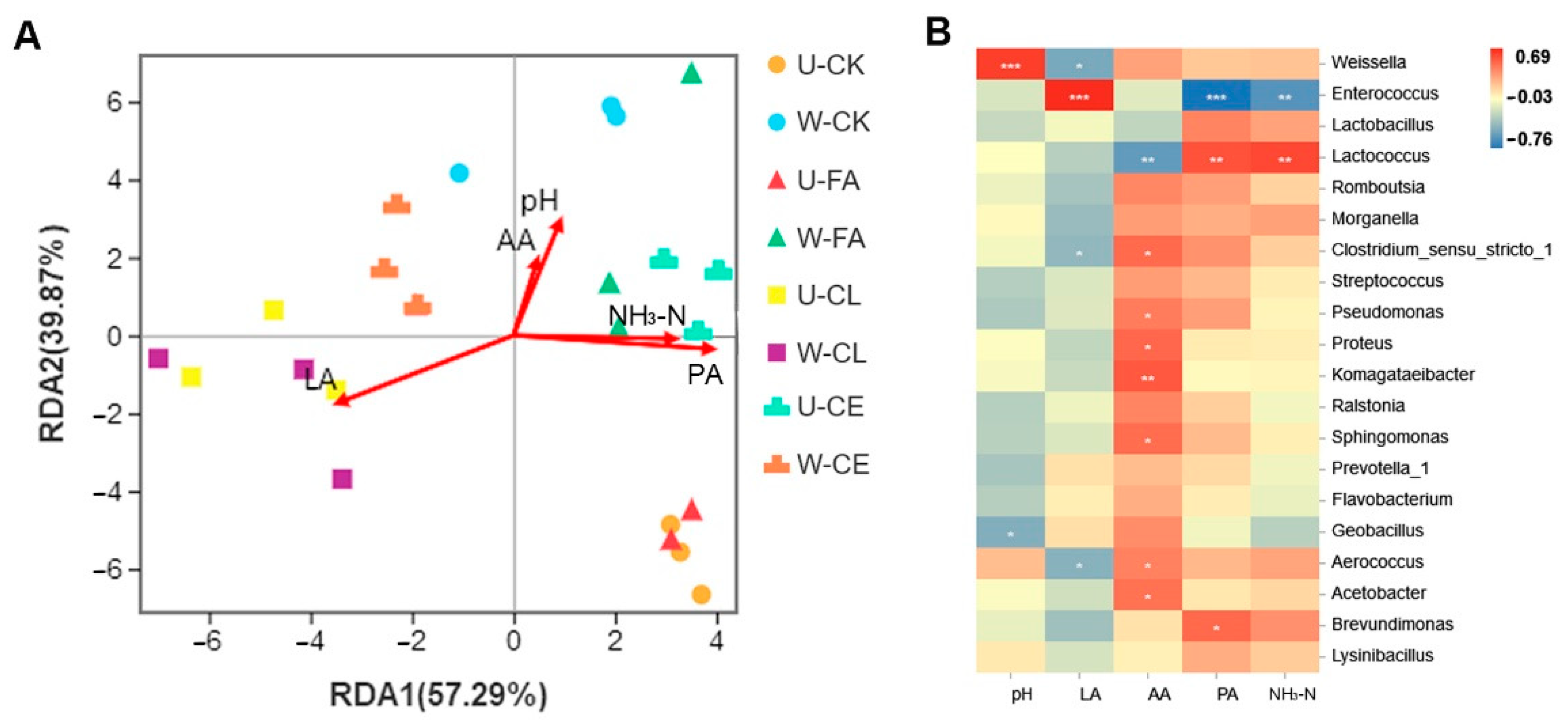
To clarify the main drivers in different fermentation processes, distance-based redundancy analysis (db-RDA) and correlation networks between microbiota and driving factors were performed as shown in Figure 6. Results of db-RDA analyses showed that the explanatory rate of chemical compositions and fermentation characteristics of samples on the distribution of microbial communities was 97.16% (see Figure 6A). This indicated that physicochemical indexes had an important impact on microbial succession change. Furthermore, PA, LA, and NH3-N had strong correlations with microbial community (p < 0.05). Among them, LA in the CL group was positively correlated with microbial community. The LA content was the main driving factor for microbial succession in this stage. AA, NH3-N, PA, and pH were positively correlated with the microbial community of the other treatment groups. To clarify the relationships among specific genera and driving forces, we analyzed their correlation via a Pearson correlation coefficient (p < 0.05; Figure 6B). Figure 6B shows that the genera Enterococcus was positively correlated with LA. The result indicated that there was a certain correlation between LA and the dominant bacteria in the CL group. PA and NH3-N were positively correlated with Lactococcus and negatively correlated with Enterococcus. This indicated that the above fermentation characteristics had an important impact on the dominant micro-organism succession in paper mulberry silage. We also found that AA was negatively correlated with Lactococcus, LA was negatively correlated with Weissella, and pH was positively correlated with Weissella.
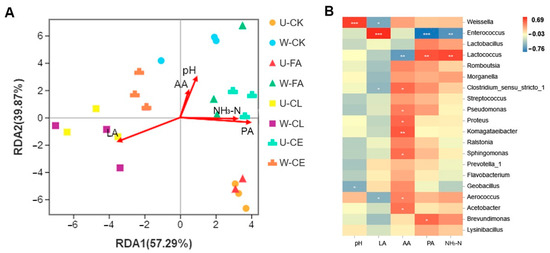
Figure 6.
Correlation between different treatment groups and between community composition. (A) Distance-based-redundancy analysis (RDA)-forwsel analysis of microbial community composition and fermentation characteristics. (B) Relationships of fermentation characteristics with silage bacterial community at the genus level. Positive correlations are shown in red, and negative correlations are shown in blue. “*, **, and ***” represent p < 0.05, p < 0.01, and p < 0.001, respectively. U, wilt 0 h; W, wilt 3.5 h; CK, control (no additives); CL, Enterococcus durans; CE, cellulase; FA, formic acid; LA, lactic acid; AA, acetic acid; PA, propanoic acid; and NH3-N, ammonia-N.
4. Discussion
In this study, the DM content of silage wilted for 3.5 h was higher than that unwilted due to moisture loss. The DM and CP contents in the CK group were lower than those in the additive groups, which might be due to the additives promoting fermentation quality and reducing nutrient loss of silages [25,26,27]. Cellulase can decompose insoluble carbohydrates such as plant cellulose and lignin into soluble carbohydrates, providing more sufficient fermentation substrates for LAB. After formic acid is added, the pH is directly lowered, which provides a suitable fermentation environment for LAB. However, the addition of Enterococcus durans directly changed the microbial community and increased lactic acid production, thus effectively inhibiting the growth of aerobic bacteria and reducing their fermentation of nutrients. The structural carbohydrates of silage, such as NDF and ADF (especially NDF), are important indicators of rumen digestion. A high proportion of NDF made of cellulose, hemicellulose, and lignin causes difficulty in digestion; therefore, the NDF content is negatively correlated with the energy content. Lower NDF levels may ease rumen digestion [28]. Researchers have reported that LAB reduces the cell wall fraction of silage [29,30]. Unexpectedly, the CL inoculation group in this study increased NDF content compared with the CK group, probably because the LAB played no role in cell wall hydrolysis [31]. Meanwhile, the WSC content of silage is a key indicator of microbial activity, as WSC serves as a direct source of energy and carbon for microbes [32,33]. Among the different additives used in the study, the FA group preserved more WSC, indicating inhibition of microbial growth. Compared with the FA group, the CL group increased the bacterial count and thoroughly utilized WSC. WSC of W-CL was higher than that of U-CL, probably because low moisture restricted LAB activity and decreased the energy demand [34,35].
A silage pH less than 4.2 indicates good fermentation. In this study, the silage pH was less than 4.2, which is better than that reported by Si et al. [1]. This may be attributed to the suitable moisture content of the raw materials in this study, sufficient WSC that can be used as LAB fermentation substrates, and the extremely abundant LAB populations attached to the whole plant paper mulberry. Meanwhile, as wilting was prolonged, the silage LA content decreased, which indicates that low moisture restricted LAB activity [34,35]. In temperate regions, homofermentative species lead to ensiling [36]. The high LA/AA ratios of all the treatments indicate that homofermentative LAB led to the ensiling of paper mulberry. For silage with high CP content, efficient N utilization and preservation are important. The NH3-N content is an important index that indicates N utilization. Ruminates hardly absorb NH3-N, and well-fermented silage should contain less than 100 g/kg NH3-N [37]. Under the two wilting times of this study, NH3-N content of the CK group (97.49 and 94.99 g/kg for the U-CK and W-CK groups, respectively) was higher than the other additive groups (p < 0.05), and the CP content of silage with different additives (CL, CE, and FA) was more than that of the CK group and similar to alfalfa silage [38]. The additives protected the silage proteins from proteolysis, consistent with He et al. [39] and Kaewpila et al. [40]. Additives influenced silage proteolysis through different pathways. CL and FA rapidly decreased the pH of silage and inhibited the multiplication of micro-organisms which cause proteolysis [39,41] and degrade native plant proteins to non-protein N [42]. Meanwhile, CE degraded cellulose to monosaccharides and generated more substrates for LAB fermentation [43]. CE improved to the silage CP content for its protein enzyme essence. Our study also found that higher LA content was related to less proteolysis. This may be because the dominant genus, Enterococcus, produced more LA at the end of the fermentation, thus lowering the pH, and the higher acidic environment inhibited the activity of the unfavorable genus proteolysis; this result is similar to Bai et al. [23].
Wilting in this study improved the aerobic stability of silage. Tao et al. [38] reported that prolonged wilting promoted the aerobic stability of alfalfa silage. Upon exposure to air, lactate-assimilating yeasts cause silage spoilage via LA oxidation [44]. The oxidation probably reduced the aerobic stability of the CL group compared with the other additive groups. The CK group silages were more consumed by yeasts and other aerobic microbes due to insufficient inhibitors [45,46]. Yuan et al. [47] reported that FA directly inhibited the growth of yeasts in silage. Few other scientists have reported that an increase in AA content would enhance aerobic stability via yeast growth inhibition [48]. In our study, silage with W-FA showed the highest AA content (p < 0.05), proving it to be the best treatment to improve aerobic stability. The homofermentative LAB species promote yeast growth and reduce aerobic stability, while the dominant heterofermentative LAB species improve aerobic stability [49]. Thus, CL showed lower aerobic stability than CE and FA.
Lactobacillus and Pediococcus were dominant in the unwilted group, while Lactobacillus was dominant in the wilted group. In the silage of fresh forage soybean, Lactobacillus and Weissella were identified as the main microbes [50]. In the silage with CE, Enterococcus multiplied with time, as it alone might have used the degradation products as substrate [5]. Moreover, Enterococcus, which generates lactate during fermentation [51], probably reduced silage pH after being wilted for 3.5 h. The bacterial communities of silage with FA were more similar between the two wilting times than the CK and CE groups (Figure 3 and Figure 4), probably because FA sharply decreased silage pH and inhibited microbial activity [5]. In the CL group, the bacterial communities were slightly different between the two wilting times. Enterococcus finally inhibited the growth of Weissella, Lactobacillus, and Lactococcus. The treatment of corn silage with E. faecium resulted in a higher LA content and a lower NH3-N rate [52]. Delpech et al. [53] found that E. faecalis strain CECCT7121 isolated from corn silage had antibacterial activity against gram–positive pathogens. Kuley et al. [54] confirmed E. gallinarum as a safe LAB for fermentative foods. Our study also shows that the inoculant E. durans has the characteristics suitable for ensiling. It dominated the fermentation and limited microbial growth. Thus, wilting for different times changed the microbial composition, except in silage with LAB [54,55]. Previous studies have already indicated that Weissella, Pediococcus, and Lactococcus generally initiate silage fermentation; subsequently, the low pH makes Lactobacillus dominate the fermentation process [56,57].
Previous studies always use redundancy analysis (RDA) to evaluate the influence of fermentation characteristics on microbial communities [58,59]. Results in the present study showed that the micro-organisms were positively related to LA content and were the main driving factor for microbial succession in this stage, which is consistent with Guo et al. [7]. Enterococcus was positively correlated with LA, while a negative correlation between LA and Weissella was found in this study. Results were different from those of Sun et al. [60] and Bai et al. [61], probably due to Enterococcus playing a dominant role in the fermentation process of paper mulberry silage rather than Lactobacillus or Weissella as in the present study.
5. Conclusions
The addition of CL and CE improved the fermentation quality of whole plant paper mulberry silage in different ways, and both changed the bacterial community structure, lowered the pH, and increased LA production. FA and wilting generally improve the aerobic stability of paper mulberry silage. There are more bacterial genera that are not conducive to silage fermentation, such as Clostridium_sensu_stricto_1 and Pseudomonas. Hence, additives should be used in the preparation of paper mulberry silage. In the present study, Enterococcus was identified as the main driver in the paper mulberry ensiling process. The utilization of LAB additive is most effective and economical to improve the fermentation quality and reduce the protein degradation of paper mulberry silage, and Enterococcus is one of the choices. Further studies are advised using the omics approaches that will reveal the mechanisms during ensiling of whole plant paper mulberry.
Author Contributions
Conceptualization, J.H. and C.C.; methodology, W.-T.S.; software, W.-T.S.; validation, C.-R.W., M.-Z.Z. and G.-H.X.; formal analysis, W.-T.S.; investigation, W.-T.S.; resources, J.H.; data curation, C.-R.W.; writing—original draft preparation, W.-T.S.; writing—review and editing, J.H.; visualization, M.-Z.Z.; supervision, Y.-L.Z.; project administration, C.C.; funding acquisition, C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Scientific Research Cultivation Project of Guizhou University ([2020]17), Science and Technology Support Project of Guizhou Province ([2020]1Y046), Major Special Project of Science and Technology of Guizhou Province ([2020]3009-2), and Guizhou Talent Base of Grassland Ecological Animal Husbandry (RCJD2018-13).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the first author. The data are not publicly available due to restrictions by the research group.
Acknowledgments
The authors gratefully acknowledge the support of Shu-xin Ge for the guidance on the SAS software.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Si, B.W.; Tao, H.; Zhang, X.L.; Guo, J.P.; Cui, K.; Tu, Y.; Diao, Q.Y. Effect of Broussonetia papyrifera L. (paper mulberry) silage on dry matter intake, milk composition, antioxidant capacity and milk fatty acid profile in dairy cows. Asian-Australas. J. Anim. Sci. 2018, 31, 1259–1266. [Google Scholar] [CrossRef] [Green Version]
- Tao, H.; Si, B.W.; Xu, W.C.; Tu, Y.; Diao, Q.Y. Effect of Broussonetia papyrifera L. silage on blood biochemical parameters, growth performance, meat amino acids and fatty acids compositions in beef cattle. Asian-Australas. J. Anim. Sci. 2019, 33, 732–741. [Google Scholar] [CrossRef]
- Hao, Y.Y.; Huang, S.; Si, J.F.; Zhang, J.; Gaowa, N.; Sun, X.G.; Lv, J.Y.; Liu, G.K.; He, Y.Q.; Wang, W.; et al. Effects of paper mulberry silage on the milk production, apparent digestibility, antioxidant capacity, and fecal bacteria composition in Holstein dairy cows. Animals 2020, 10, 1152. [Google Scholar] [CrossRef]
- Du, Z.; Sun, L.; Chen, C.; Lin, J.; Yang, F.; Cai, Y. Exploring microbial community structure and metabolic gene clusters during silage fermentation of paper mulberry, a high-protein woody plant. Anim. Feed Sci. Technol. 2020, 275, 114766. [Google Scholar] [CrossRef]
- Muck, R.E.; Nadeau, E.M.G.; McAllister, T.A.; Contreras-Govea, F.E.; Santos, M.C.; Kung, L., Jr. Silage review: Recent advances and future uses of silage additives. J. Dairy Sci. 2018, 101, 3980–4000. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.F.; Zhang, H.S.; Gao, Y.H.; Diao, Q.Y. Dynamic profiles of fermentation characteristics and bacterial community composition of Broussonetia papyrifera ensiled with perennial ryegrass. Bioresour. Technol. 2020, 310, 123396. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.N.; Wang, X.K.; Lin, Y.L.; Yang, X.P.; Ni, K.K.; Yang, F.Y. Microorganisms that are critical for the fermentation quality of paper mulberry silage. Food Energy Secur. 2021, 10, e304. [Google Scholar] [CrossRef]
- He, Q.; Zhou, W.; Chen, X.Y.; Zhang, Q. Chemical and bacterial composition of Broussonetia papyrifera leaves ensiled at two ensiling densities with or without Lactobacillus Plantarum. J. Clean. Prod. 2021, 329, 129792. [Google Scholar] [CrossRef]
- Nishino, N.; Li, Y.; Wang, C.; Parvin, S. Effects of wilting and molasses addition on fermentation and bacterial community in guinea grass silage. Lett. Appl. Microbiol. 2012, 54, 175–181. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Zhou, W.; Yang, F.Y.; Chen, X.Y.; Zhang, Q. Effects of wilting and Lactobacillus plantarum addition on the fermentation quality and microbial community of moringa oleifera leaf silage. Front. Microbiol. 2018, 9, 1817. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; He, L.W.; Xing, Y.Q.; Zheng, Y.T.; Zhou, W.; Pian, R.Q.; Yang, F.Y.; Chen, X.Y.; Zhang, Q. Dynamics of bacterial community and fermentation quality during ensiling of wilted and unwilted moringa oleifera leaf silage with or without lactic acid bacterial inoculants. Msphere 2019, 4, e00341-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, R.N.; Ni, K.K.; Wang, T.W.; Yang, X.P.; Zhang, J.; Liu, Y.Y.; Shi, W.X.; Yan, L.; Jie, C.; Zhong, J. Effects of ferulic acid esterase-producing Lactobacillus fermentum and cellulase additives on the fermentation quality and microbial community of alfalfa silage. PeerJ 2019, 7, e7712. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.L.; Yuan, X.J.; Li, J.F.; Dong, Z.H.; Shao, T. Dynamics of microbial community and fermentation quality during ensiling of sterile and nonsterile alfalfa with or without Lactobacillus plantarum inoculant. Bioresour. Technol. 2019, 275, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Sun, L.; Lin, Y.; Yang, F.; Cai, Y. The use of PacBio SMRT technology to explore the microbial network and fermentation characteristics of woody silage prepared with exogenous carbohydrate additives. J. Appl. Microbiol. 2021, 131, 2193–2211. [Google Scholar] [CrossRef]
- Chen, L.Y.; Bai, S.Q.; You, M.H.; Xiao, B.X.; Li, P.; Cai, Y.M. Effect of a low temperature tolerant lactic acid bacteria inoculant on the fermentation quality and bacterial community of oat round bale silage. Anim. Feed Sci. Technol. 2020, 269, 114669. [Google Scholar] [CrossRef]
- Bai, J.; Ding, Z.T.; Ke, W.C.; Xu, D.M.; Wang, M.S.; Huang, W.K.; Zhang, Y.X.; Liu, F.; Guo, X.S. Different lactic acid bacteria and their combinations regulated the fermentation process of ensiled alfalfa: Ensiling characteristics, dynamics of bacterial community and their functional shifts. Microb. Biotechnol. 2021, 14, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.M.; Du, Z.M.; Yamasaki, S.; Nguluve, D.; Tinga, B.; Macome, F.; Oya, T. Community of natural lactic acid bacteria and silage fermentation of corn stover and sugarcane tops in Africa. Asian-Australas. J. Anim. Sci. 2020, 33, 1252–1264. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 19th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Turula, V.E.; Gore, T.; Singh, S.; Arumugham, R.G. Automation of the anthrone assay for carbohydrate concentration determinations. Anal. Chem. 2010, 82, 1786–1792. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Broderick, G.A.; Kang, J.H. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef]
- Liu, Q.H.; Lindow, S.E.; Zhang, J.G. Lactobacillus parafarraginis ZH1 producing anti-yeast substances to improve the aerobic stability of silage. Anim. Sci. J. 2018, 89, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Xu, D.M.; Xie, D.M.; Wang, M.S.; Li, Z.Q.; Guo, X.S. Effects of antibacterial peptide-producing Bacillus subtilis and Lactobacillus buchneri on fermentation, aerobic stability, and microbial community of alfalfa silage. Bioresour. Technol. 2020, 315, 123881. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [Green Version]
- Yitbarek, M.B.; Tamir, B. Silage Additives: Review. Open J. Appl. Sci. 2014, 4, 258–274. [Google Scholar] [CrossRef] [Green Version]
- Irawan, A.; Sofyan, A.; Ridwan, R.; Hassim, H.A.; Respati, A.N.; Wardani, W.W.; Sadarman; Astuti, W.D.; Jayanegara, A. Effects of different lactic acid bacteria groups and fibrolytic enzymes as additives on silage quality: A meta-analysis. Bioresour. Technol. Rep. 2021, 14, 100654. [Google Scholar] [CrossRef]
- Zong, C.; Wu, Q.F.; Wu, A.L.; Chen, S.F.; Dong, D.; Zhao, J.; Shao, T.; Liu, Q.H. Exploring the diversity mechanism of fatty acids and the loss mechanisms of polyunsaturated fatty acids and fat-soluble vitamins in alfalfa silage using different additives. Anim. Feed Sci. Technol. 2021, 280, 115044. [Google Scholar] [CrossRef]
- Jang, S.Y.; Kim, E.K.; Park, J.H.; Oh, M.R.; Tang, Y.J.; Ding, Y.L.; Seong, H.J.; Kim, W.H.; Yun, Y.S.; Moon, S.H. Effects of physically effective neutral detergent fiber content on dry matter intake, digestibility, and chewing activity in Korean native goats (Capra hircus coreanae) fed with total mixed ration. Asian-Australas. J. Anim. Sci. 2017, 30, 1405–1409. [Google Scholar] [CrossRef] [Green Version]
- Moselhy, M.A.; Borba, J.P.; Borba, A.E.S. Improving the nutritive value, in vitro digestibility and aerobic stability of Hedychium gardnerianum silage through application of additives at ensiling time. Anim. Feed Sci. Technol. 2015, 206, 8–18. [Google Scholar] [CrossRef]
- Li, M.; Zhou, H.L.; Zi, X.J.; Cai, Y.M. Silage fermentation and ruminal degradation of stylo prepared with lactic acid bacteria and cellulase. Anim. Sci. J. 2017, 88, 1531–1537. [Google Scholar] [CrossRef]
- He, L.W.; Zhou, W.; Wang, Y.; Wang, C.; Chen, X.Y.; Zhang, Q. Effect of applying lactic acid bacteria and cellulase on the fermentation quality, nutritive value, tannins profile and in vitro digestibility of Neolamarckia cadamba leaves silage. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1429–1436. [Google Scholar] [CrossRef]
- Kaewpila, C.; Gunun, P.; Kesorn, P.; Subepang, S.; Thip-uten, S.; Cai, Y.M.; Pholsen, S.; Cherdthong, A.; Khota, W. Improving ensiling characteristics by adding lactic acid bacteria modifies in vitro digestibility and methane production of forage-sorghum mixture silage. Sci. Rep. 2021, 11, 1968. [Google Scholar] [CrossRef] [PubMed]
- Kaewpila, C.; Khota, W.; Gunun, P.; Kesorn, P.; Kimprasit, T.; Sarnklong, C.; Cherdthong, A. Characterization of green manure sunn hemp crop silage prepared with additives: Aerobic instability, nitrogen value, and in vitro rumen methane production. Fermentation 2022, 8, 104. [Google Scholar] [CrossRef]
- da Silva, E.B.; Smith, M.L.; Savage, R.M.; Polukis, S.A.; Drouin, P.; Kung, L., Jr. Effects of Lactobacillus hilgardii 4785 and Lactobacillus buchneri 40788 on the bacterial community, fermentation and aerobic stability of high-moisture corn silage. J. Appl. Microbiol. 2021, 130, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Luo, Y.N.; Bao, J.Z.; Luo, Y.; Yu, Z. Additives affect the distribution of metabolic profile, microbial communities and antibiotic resistance genes in high-moisture sweet corn kernel silage. Bioresour. Technol. 2020, 315, 123821. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Drouin, P.; Lafreniere, C. Effect of temperature (5–25 °C) on epiphytic lactic acid bacteria populations and fermentation of whole-plant corn silage. J. Appl. Microbiol. 2016, 121, 657–671. [Google Scholar] [CrossRef] [PubMed]
- McDonald, P.; Henderson, A.R.; Heron, A.J.E. The Biochemistry of Silage, 2nd ed.; Chalcombe Publications: Marlow, UK, 1991. [Google Scholar]
- Tao, L.; Zhou, H.; Zhang, N.F.; Si, B.W.; Tu, Y.; Ma, T.; Diao, Q.Y. Effects of different source additives and wilt conditions on the pH value, aerobic stability, and carbohydrate and protein fractions of alfalfa silage. Anim. Sci. J. 2017, 88, 99–106. [Google Scholar] [CrossRef] [Green Version]
- He, L.W.; Wang, C.; Xing, Y.Q.; Zhou, W.; Pian, R.Q.; Chen, X.Y.; Zhang, Q. Ensiling characteristics, proteolysis and bacterial community of high-moisture corn stalk and stylo silage prepared with Bauhinia variegate flower. Bioresour. Technol. 2020, 296, 122336. [Google Scholar] [CrossRef] [PubMed]
- Kaewpila, C.; Khota, W.; Gunun, P.; Kesorn, P.; Cherdthong, A. Strategic addition of different additives to improve silage fermentation, aerobic stability and in vitro digestibility of Napier grasses at late maturity stage. Agriculture 2020, 10, 262. [Google Scholar] [CrossRef]
- Yan, Y.H.; Li, X.M.; Guan, H.; Huang, L.K.; Ma, X.; Peng, Y.; Li, Z.; Nie, G.; Zhou, J.Q.; Yang, W.Y.; et al. Microbial community and fermentation characteristic of Italian ryegrass silage prepared with corn stover and lactic acid bacteria. Bioresour. Technol. 2019, 279, 166–173. [Google Scholar] [CrossRef]
- Li, P.; Ji, S.R.; Hou, C.; Tang, H.Y.; Wang, Q.; Shen, Y.X. Effects of chemical additives on the fermentation quality and N distribution of alfalfa silage in south of China. Anim. Sci. J. 2016, 87, 1472–1479. [Google Scholar] [CrossRef]
- Li, M.; Zi, X.J.; Zhou, H.L.; Lv, R.L.; Tang, J.; Cai, Y.M. Silage fermentation and ruminal degradation of cassava foliage prepared with microbial additive. AMB Express. 2019, 9, 180. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.B.; Savage, R.M.; Biddle, A.S.; Polukis, S.A.; Smith, M.L.; Kung, L. Effects of a chemical additive on the fermentation, microbial communities, and aerobic stability of corn silage with or without air stress during storage. J. Anim. Sci. 2020, 98, skaa246. [Google Scholar] [CrossRef]
- Li, F.H.; Ding, Z.T.; Adesogan, A.T.; Ke, W.C.; Jiang, Y.; Bai, J.; Mudassar, S.; Zhang, Y.X.; Huang, W.K.; Guo, X.S. Effects of class iia bacteriocin-producing Lactobacillus species on fermentation quality and aerobic stability of alfalfa silage. Animals 2020, 10, 1575. [Google Scholar] [CrossRef]
- Mu, L.; Xie, Z.; Hu, L.X.; Chen, G.H.; Zhang, Z.F. Cellulase interacts with Lactobacillus plantarum to affect chemical composition, bacterial communities, and aerobic stability in mixed silage of high-moisture amaranth and rice straw. Bioresour. Technol. 2020, 315, 123772. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wen, A.; Dong, Z.; Desta, S.T.; Shao, T. Effects of formic acid and potassium diformate on the fermentation quality, chemical composition and aerobic stability of alfalfa silage. Grass Forage Sci. 2017, 72, 833–839. [Google Scholar] [CrossRef]
- Filya, I. The effect of Lactobacillus buchneri and Lactobacillus plantarum on the fermentation, aerobic Stability, and ruminal degradability of low dry matter corn and sorghum silages. J. Dairy Sci. 2003, 86, 3575–3581. [Google Scholar] [CrossRef] [Green Version]
- Ferrero, F.; Prencipe, S.; Spadaro, D.; Gullino, M.L.; Cavallarin, L.; Piano, S.; Tabacco, E.; Borreani, G. Increase in aflatoxins due to Aspergillus section Flavi multiplication during the aerobic deterioration of corn silage treated with different bacteria inocula. J. Dairy Sci. 2019, 102, 1176–1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, K.K.; Zhao, J.Y.; Zhu, B.G.; Su, R.N.; Pan, Y.; Ma, J.K.; Zhou, G.A.; Tao, Y.; Liu, X.R.; Zhong, J. Assessing the fermentation quality and microbial community of the mixed silage of forage soybean with crop corn or sorghum. Bioresour. Technol. 2018, 265, 563–567. [Google Scholar] [CrossRef]
- Liu, W.; Wang, J.; Zhang, H.Y.; Yang, Y.C.; Kang, R.X.; Bai, P.; Fu, H.; Chen, L.R.; Gao, Y.P.; Tan, E.K. Symbiotic bacteria attenuate Drosophila oviposition repellence to alkaline through acidification. Insect Sci. 2020, 28, 403–414. [Google Scholar] [CrossRef]
- Li, J.F.; Yuan, X.J.; Dong, Z.H.; Mugabe, W.; Shao, T. The effects of fibrolytic enzymes, cellulolytic fungi and bacteria on the fermentation characteristics, structural carbohydrates degradation, and enzymatic conversion yields of Pennisetum sinese silage. Bioresour. Technol. 2018, 264, 123–130. [Google Scholar] [CrossRef]
- Delpech, G.; Hebert, E.M.; Sparo, M.; Saavedra, L. Draft genome sequence of Enterococcus faecalis strain CECT7121, a corn silage isolate with antibacterial activity against gram-positive pathogens. Microbiol. Resour. Announc. 2019, 8, e00245-19. [Google Scholar] [CrossRef] [Green Version]
- Kuley, E.; Ozyurt, G.; Ozogul, I.; Boga, M.; Akyol, I.; Rocha, J.M.; Ozogul, F. The role of selected lactic acid bacteria on organic acid accumulation during wet and spray-dried fish-based silages. Contributions to the winning combination of microbial food safety and environmental sustainability. Microorganisms 2020, 8, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitiku, A.A.; Andeta, A.F.; Borremans, A.; Lievens, B.; Bossaert, S.; Crauwels, S.; Aernouts, B.; Kechero, Y.; Van Campenhout, L. Silage making of maize stover and banana pseudostem under South Ethiopian conditions: Evolution of pH, dry matter and microbiological profile. Microb. Biotechnol. 2020, 13, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.R.; Li, X.L.; Guan, H.; Yang, W.Y.; Liu, W.G.; Liu, J.; Du, Z.C.; Li, X.M.; Xiao, Q.Y.; Wang, X.C.; et al. Dynamic microbial diversity and fermentation quality of the mixed silage of corn and soybean grown in strip intercropping system. Bioresour. Technol. 2020, 313, 123655. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.J.; Bolsen, K.K.; Brent, B.E.; Fung, D.Y.C. Epiphytic lactic acid bacteria succession during the pre-ensiling and ensiling periods of alfalfa and maize. J. Appl. Bacteriol. 1992, 73, 375–387. [Google Scholar] [CrossRef]
- Cai, Y.M.; Benno, Y.; Ogawa, M.; Ohmomo, S.; Kumai, S.; Nakase, T. Influence of Lactobacillus spp. from an Inoculant and of Weissella and Leuconostoc spp. from forage crops on silage fermentation. Appl. Environ. Microbiol. 1998, 64, 2982–2987. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.R.; Zhao, J.; Dong, Z.H.; Li, J.F.; Kaka, N.A.; Shao, T. Sequencing and microbiota transplantation to determine the role of microbiota on the fermentation type of oat silage. Bioresour. Technol. 2020, 309, 123371. [Google Scholar] [CrossRef]
- Sun, L.; Bai, C.S.; Xu, H.W.; Na, N.; Jiang, Y.; Yin, G.M.; Liu, S.B.; Xue, Y.L. Succession of bacterial community during the initial aerobic, intense fermentation, and stable phases of whole-plant corn silages treated with lactic acid bacteria suspensions prepared from other silages. Front. Microbiol. 2021, 12, 591. [Google Scholar] [CrossRef]
- Bai, J.; Ding, Z.T.; Su, R.N.; Wang, M.S.; Cheng, M.Y.; Xie, D.M.; Guo, X.S. Storage temperature is more effective than lactic acid bacteria inoculations in manipulating fermentation and bacterial community diversity, co-occurrence and functionality of the whole-plant corn silage. Microbiol. Spectr. 2022, 10, e00101-22. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).