Effectiveness of Tannin Removal by Alkaline Pretreatment on Sorghum Ethanol Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Tannin Removal Procedure
2.2.2. Mash Liquefaction
2.2.3. Fermentations with No Cellulase Added
2.2.4. Fermentations with Added Cellulase
2.3. Analytical Methods
2.3.1. Tannin Analysis
2.3.2. Starch Determination
2.3.3. Ethanol Determination
2.3.4. Carbon Dioxide Determination
2.3.5. Statistical Analysis
3. Results and Discussion
3.1. Alkaline Treatment for Tannin Removal
3.2. Fermentations with No Cellulase Added
3.3. Fermentations with Added Cellulase
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Calculation of Theoretical Ethanol Production from Starch
References
- Solomon, B.D.; Barnes, J.R.; Halvorsen, K.E. Grain and cellulosic ethanol: History, economics, and energy policy. Biomass Bioenergy 2007, 31, 416–425. [Google Scholar] [CrossRef]
- Balat, M.; Balat, H.; Oz, C. Progress in bioethanol processing. Prog. Energy Combust. Sci. 2008, 34, 551–573. [Google Scholar] [CrossRef]
- Ahmetović, E.; Martín, M.; Grossmann, I.E. Optimization of Energy and Water Consumption in Corn–based Ethanol Plants. Ind. Eng. Chem. Res. 2010, 49, 7972–7982. [Google Scholar] [CrossRef]
- Crago, C.L.; Khanna, M.; Barton, J.; Giuliani, E.; Amaral, W. Competitiveness of Brazilian sugarcane ethanol compared to US corn ethanol. Energy Policy 2010, 38, 7404–7415. [Google Scholar] [CrossRef] [Green Version]
- Drapcho, C.M.; Nghiem, N.P.; Walker, T.H. Biofuels Engineering Process Technology; McGraw Hill professional; McGraw-Hill Education: New York, NY, USA, 2007; ISBN 9780071509923. [Google Scholar]
- Taylor, J.R.N.; Schober, T.J.; Bean, S.R. Novel food and non-food uses for sorghum and millets. J. Cereal Sci. 2006, 44, 252–271. [Google Scholar] [CrossRef]
- U.S. Grain Council. 2016/2017 Corn Harvest Quality Report and 2016/2017 Sorghum Harvest Quality Report; U.S. Grain Council: Washington, DC, USA, 2016. [Google Scholar]
- Beringer, J.; Bing, J.; Wei, C.; Coram, T.; Curtis, T.; Jim, J.; Elikana Anami, S.; Gabor, G.; Nigel, N.; Jones, H. Biotechnology of Major Cereals; CABI: Wallingford, UK, 2016; ISBN 9781780645193. [Google Scholar]
- Donke, A.; Nogueira, A.; Matai, P.; Kulay, L. Environmental and Energy Performance of Ethanol Production from the Integration of Sugarcane, Corn, and Grain Sorghum in a Multipurpose Plant. Resources 2016, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Chuck-Hernandez, C.; Peralta-Contreras, M.; Bando-Carranza, G.; Vera-Garcia, M.; Gaxiola-Cuevas, N.; Tamayo-Limon, R.; Cardenas-Torres, F.; Perez-Carrillo, E.; Serna-Saldivar, S.O. Bioconversion into ethanol of decorticated red sorghum (Sorghum bicolor L. Moench) supplemented with its phenolic extract or spent bran. Biotechnol. Lett. 2012, 34, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, C.A.; Maeda, R.N.; Betancur, G.J.V.; Pereira, N. Ethanol production from sorghum grains [Sorghum bicolor (L.) moench]: Evaluation of the enzymatic hydrolysis and the hydrolysate fermentability. Braz. J. Chem. Eng. 2011, 28, 597–604. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Bean, S.; McLaren, J.; Seib, P.; Madl, R.; Tuinstra, M.; Shi, Y.; Lenz, M.; Wu, X.; Zhao, R. Grain sorghum is a viable feedstock for ethanol production. J. Ind. Microbiol. Biotechnol. 2008, 35, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Johnston, D.J.; Moreau, R.A. A Comparison Between Corn andGrain SorghumFermentation Rates, Distillers Dried Grains with Solubles Composition, and Lipid Profiles. Bioresour. Technol. 2017, 226, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, W.D.; Rogler, J.C.; Featherston, W.R. Effect of tannin extraction on the performance of chicks fed bird resistant sorghum grain diets. Poult. Sci. 1974, 53, 714–720. [Google Scholar] [CrossRef]
- Top, S.M.; Preston, C.M.; Dukes, J.S.; Tharayil, N. Climate Influences the Content and Chemical Composition of Foliar Tannins in Green and Senesced Tissues of Quercus rubra. Front. Plant Sci. 2017, 8, 432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awika, J.M.; Rooney, L.W. Sorghum phytochemicals and their potential impact on human health. Phytochemistry 2004, 65, 1199–1221. [Google Scholar] [CrossRef] [PubMed]
- Nkomba, E.Y.; van Rensburg, E.; Chimphango, A.F.A.; Görgens, J.F. The influence of sorghum grain decortication on bioethanol production and quality of the distillers’ dried grains with solubles using cold and conventional warm starch processing. Bioresour. Technol. 2016, 203, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Wu, X.; Faubion, J.; Bean, S.; Cai, L.; Shi, Y.-C.; Sun, X.; Wang, D. Ethanol-Production Performance of Ozone-Treated Tannin Grain Sorghum Flour. Cereal Chem. 2012, 89, 30–37. [Google Scholar] [CrossRef]


| Experiment | Mean Ethanol (% w/v) at 96 h Fermentation Time |
|---|---|
| 100% Corn | 9.39 ± 0.14 |
| 100% Treated sorghum | 9.39 ± 0.26 |
| 100% Untreated sorghum | 8.02 ± 0.15 |
| Treatment | Theoretical Ethanol Production (mL) | Ethanol Produced (mL) | Theoretical Yield (%) |
|---|---|---|---|
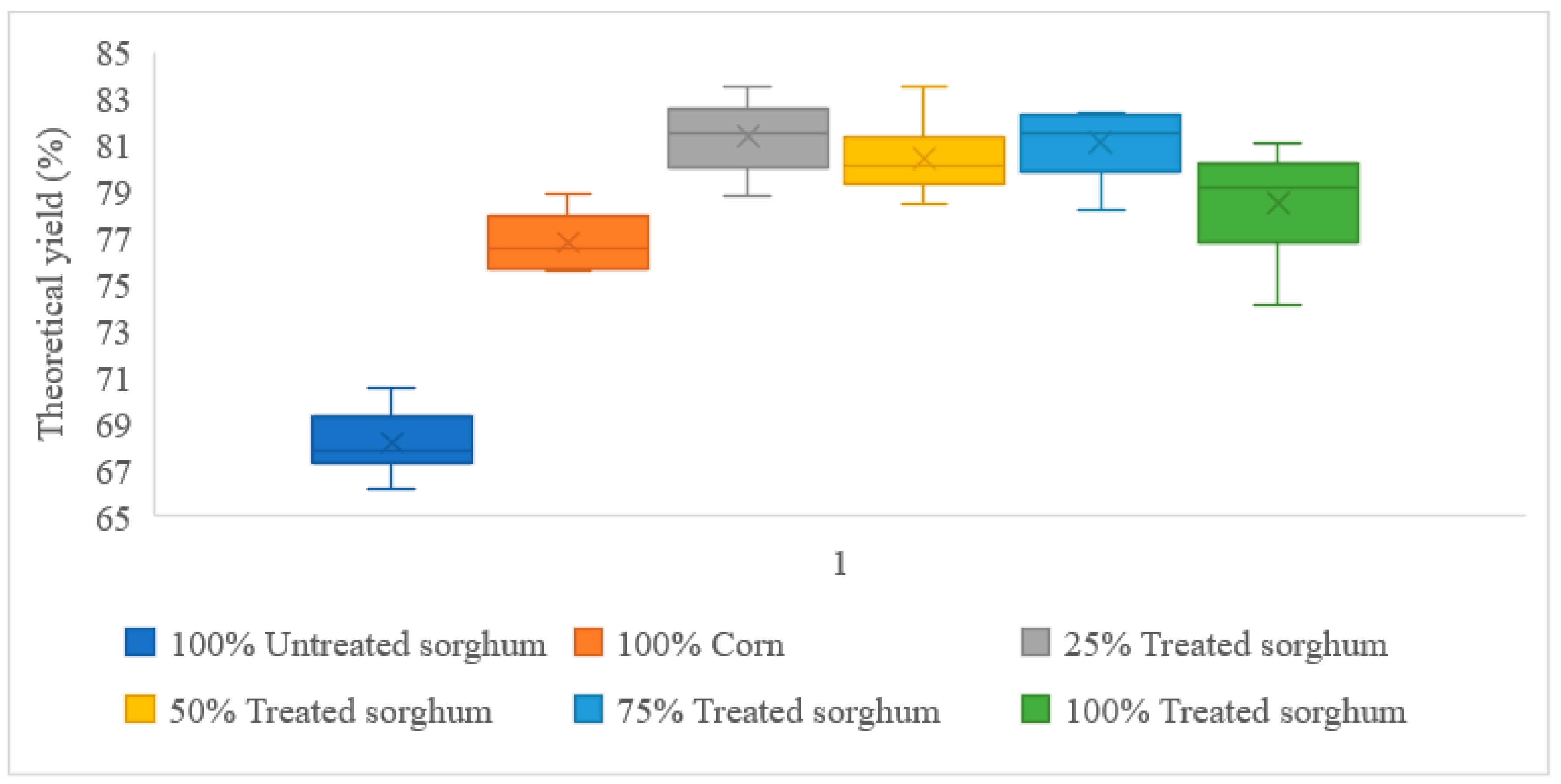
| 100% Corn | 12.83 | 9.85 | 76.79% |
| 100% Treated sorghum | 12.56 | 9.85 | 78.48% |
| 100% Untreated sorghum | 12.13 | 8.26 | 68.15% |
| Experiment | Mean Ethanol (% w/v) at 96 h Fermentation Time |
|---|---|
| 75% Corn + 25% Treated sorghum | 9.83 ± 0.18 |
| 50% Corn + 50% Treated sorghum | 9.68 ± 0.17 |
| 25% Corn + 75% Treated sorghum | 9.71 ± 0.18 |
| Treatment | Theoretical Ethanol Production (mL) | Ethanol Produced (mL) | Theoretical Yield (%) |
|---|---|---|---|
| 25% Treated sorghum + 75% Corn | 127.60 | 103.78 | 81.33% |
| 50% Treated sorghum + 50% Corn | 126.93 | 102.05 | 80.40% |
| 75% Treated sorghum + 25% Corn | 126.26 | 102.35 | 81.07% |
| Experiment | Mean Ethanol (% w/v) at 96 h Fermentation Time |
|---|---|
| 100% Corn | 11.03 ± 0.13 |
| 100% Treated sorghum | 11.29 ± 0.21 |
| 100% Untreated sorghum | 8.77 ± 0.18 |
| Treatment | Theoretical Ethanol Production (mL) | Ethanol Produced (mL) | Theoretical Yield (%) |
|---|---|---|---|
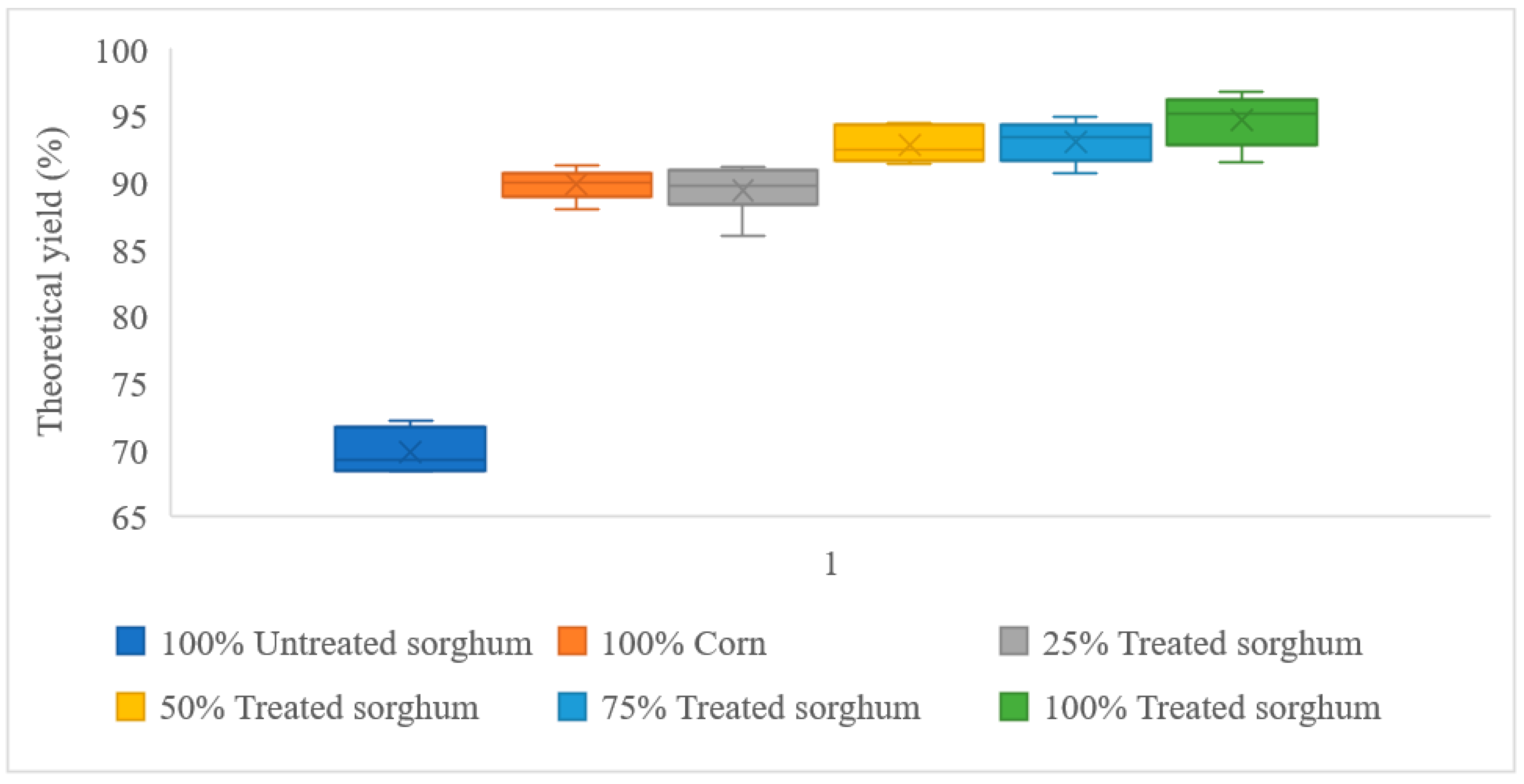
| 100% Corn | 131.87 | 118.43 | 89.81% |
| 100% Treated sorghum | 128.64 | 121.70 | 94.61% |
| 100% Untreated sorghum | 130.61 | 91.13 | 69.77% |
| Experiment | Mean Ethanol (% w/v) at 96 h Fermentation Time |
|---|---|
| 25% Treated sorghum + 75% Corn | 10.93 ± 0.22 |
| 50% Treated sorghum + 50% Corn | 11.22 ± 0.15 |
| 75% Treated sorghum + 25% Corn | 11.19 ± 0.19 |
| Treatment | Theoretical Ethanol Production (mL) | Ethanol Produced (mL) | Theoretical Yield (%) |
|---|---|---|---|
| 25% Treated sorghum + 75% Corn | 131.06 | 117.15 | 89.38% |
| 50% Treated sorghum + 50% Corn | 130.25 | 120.82 | 92.76% |
| 75% Treated sorghum + 25% Corn | 129.44 | 120.45 | 93.05% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foglia, F.; Drapcho, C.; Nghiem, J. Effectiveness of Tannin Removal by Alkaline Pretreatment on Sorghum Ethanol Production. Fermentation 2022, 8, 274. https://doi.org/10.3390/fermentation8060274
Foglia F, Drapcho C, Nghiem J. Effectiveness of Tannin Removal by Alkaline Pretreatment on Sorghum Ethanol Production. Fermentation. 2022; 8(6):274. https://doi.org/10.3390/fermentation8060274
Chicago/Turabian StyleFoglia, Franco, Caye Drapcho, and John Nghiem. 2022. "Effectiveness of Tannin Removal by Alkaline Pretreatment on Sorghum Ethanol Production" Fermentation 8, no. 6: 274. https://doi.org/10.3390/fermentation8060274
APA StyleFoglia, F., Drapcho, C., & Nghiem, J. (2022). Effectiveness of Tannin Removal by Alkaline Pretreatment on Sorghum Ethanol Production. Fermentation, 8(6), 274. https://doi.org/10.3390/fermentation8060274






