Abstract
With the increasing demand for the biodegradable polymer material polylactic acid and its advantage of being metabolized by the human body, L-lactic acid (L-LA) is becoming increasingly attractive in environmental protection and food industry applications. However, the supply of L-LA is not satisfied, and the price is still high. Compared to enzymatic and chemical synthesis methods, L-LA production by microbial fermentation has the advantages of low cost, large yield, simple operation, and environmental protection. This review summarizes the advances in engineering microbial cell factories to produce L-LA. First, the synthetic pathways and microorganisms for L-LA production are outlined. Then, the metabolic engineering strategies for constructing cell factories to overproduce L-LA are summarized and fermentation modes for L-LA production are also given. Finally, the challenges and prospects of the microbial production of L-LA are discussed. This review provides theoretical guidance for researchers engaged in L-LA production.
1. Introduction
Lactic acid (Lac; CH3CHCOOH), one of the three major organic acids, exists in nature in three forms due to its optical isomerism: D-Lac, L-Lac, and DL-Lac [1]. Lac is widely used in food, medicine, cosmetics, tobacco and chemical industries [2]. Since humans and animals can only metabolize L-lac enzymes, D-lac cannot be absorbed. The excessive intake of D-lac or DL-lac will lead to the accumulation of D-lac in blood, which may cause fatigue, metabolic disorders, and even acidosis [3]. The World Health Organization advocates the use of L-lac as a food additive and oral medicine. L-Lac (L-LA) is used in the food industry as a sourness agent, preservative, and food fortifier [4]. L-LA also enhances human physiology and improves immunity in medicine [5]. In recent years, a variety of L-LA-derived products have been put on the market for the medical industry, such as surgical sutures, drug-controlled release preparations, and fracture internal fixation materials [6,7]. L-LA has also made important contributions to the field of environmental protection, as it can be used in the production of the green solvent L-methyl lactate, L-ethyl lactate, and the biodegradable plastic polylactic acid (PLA) [8,9]. With increasingly serious environmental problems, the demand for degradable plastics is increasing at a compound annual growth rate of 33% [10]. In addition, the precursors of PLA synthesis must be Lac monomers with high optical purity. Therefore, it is of great significance to improve the production capacity and reduce the production costs of L-LA.
The production strategies of L-LA include chemical synthesis, enzyme transformation, and microbial fermentation. The Monsanto Company of the United States first developed the chemical synthesis approach to synthesize L-LA using acetaldehyde and hydrocyanic acid as substrates [11]. However, this production pipeline generates serious pollution, high costs, and it is difficult to synthesize L-LA by a single configuration. Meanwhile, there are many inevitable by-products and residual harmful intermediates which can pose serious safety risks if consumed by humans. L-LA production by enzymatic catalysis takes pyruvate (Pyr) or 2-chloropropionic acid as a substrate, catalyzed by high-specific lactate dehydrogenase (LDH) or L-2-halogenate enzyme. In this way, single optical rotation L-LA could be obtained. However, due to the complex transformation conditions, enzymatic catalysis has the disadvantages of low yield and high costs, and therefore is rarely used in industrial applications [12]. Compared to chemical and enzymatic synthesis, L-LA bioproduction by microbial fermentation meets the needs of industrial mass production and reduces the production costs due to cheap biomass resources. Furthermore, L-LA production with high optical purity can be realized using engineered microorganisms. Therefore, microbial fermentation has become the main route for L-LA production.
At present, the global L-LA market demand is increasing by 10% annually, and the production of L-LA by microorganisms has become the mainstream. How to produce high-quality L-LA under the premise of environmental protection and economic growth remains a topic of concern. In recent years, many microbial fermentation methods for L-LA production have emerged, but a relevant review of these is lacking. This review thus aims to summarize the recent advances in L-LA bioproduction, mainly focusing on the synthetic pathways and microorganisms for L-LA production, metabolic engineering strategies for constructing cell factories to overproduce L-LA, and fermentation modes for L-LA production. This review also discusses the challenges for L-LA production and will hopefully guide biological engineers working in L-LA production and applications.
2. Synthetic Pathways and Microorganisms for L-LA Production
2.1. Biosynthetic Pathway of L-LA
Different microorganisms have different enzyme systems, which means that they have different Lac fermentation mechanisms. The L-LA anabolic pathway can be divided into four types as follows.
2.1.1. Homolactic Fermentation
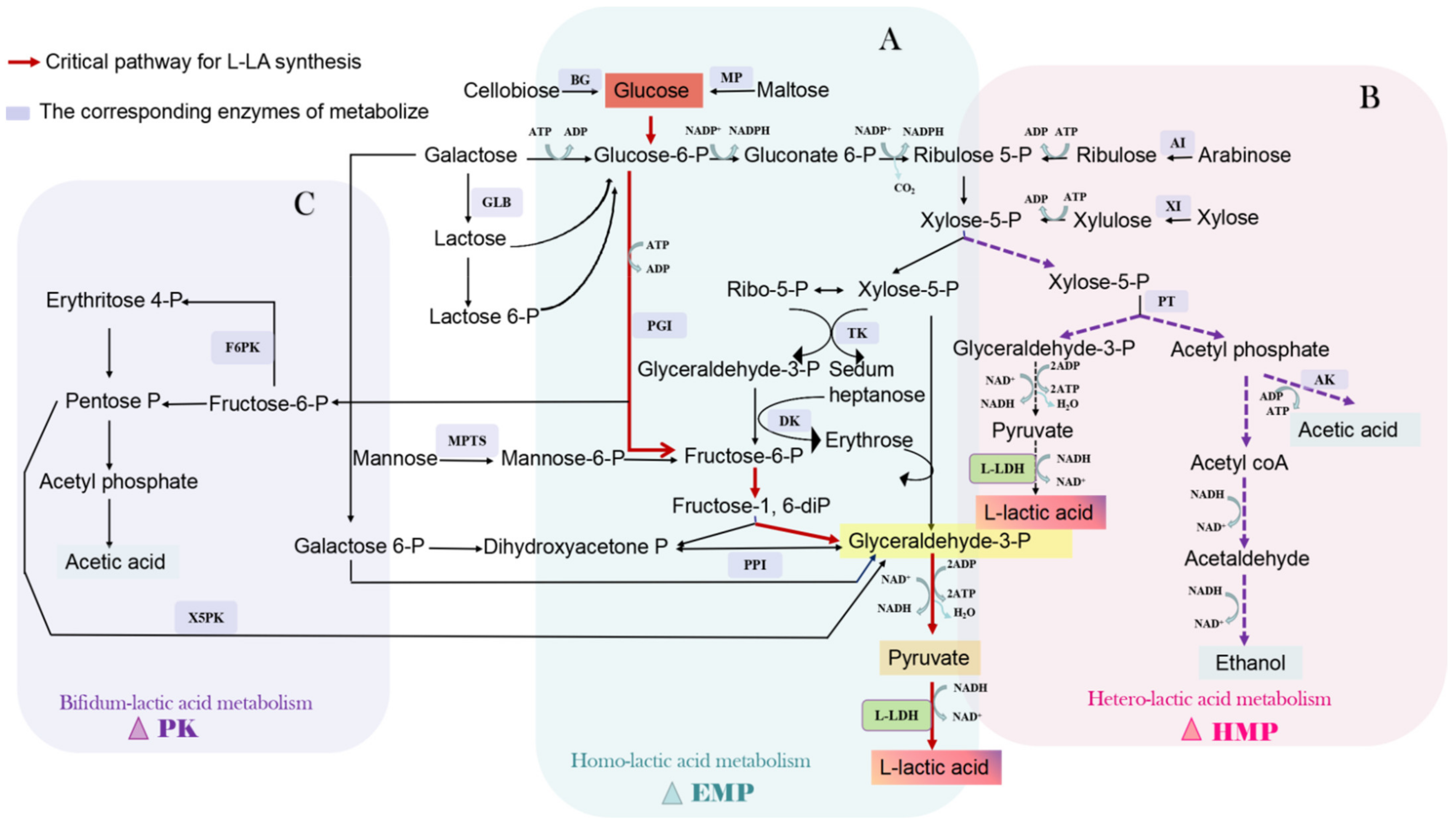
Homotype Lac fermentation refers to glucose (Glu) assimilation by microorganisms through the Embden–Meyerhof–Parnas (EMP) pathway (Figure 1A). Glu is degraded into Pyr through the EMP pathway, and Pyr is reduced to Lac under the catalysis of LDH. Then, 1 mol Glu can generate 2 mol Lac, and its theoretical conversion rate is 100%. However, owing to a series of other physiological activities in microorganisms during fermentation, the actual conversion rate of homotype fermentation is considered >80% [11]. Streptococcus, Diplococcus, Small coccus and a part of Lactobacillus all belong to this fermentation type. The general reaction formula is as follows:
C6H12O6 + 2 ADP + 2 Pi → 2 CH3CH(OH)COOH + 2 ATP
Figure 1.
Biosynthesis pathway of L-LA. BG, β-1,4-glucanase; MP, maltose phosphorylase; GLB, β-galactoenzyme; AI, arabinose isomerase; XI, xylose isomerase; TK, transketolase; DK, dihydroxyacetone kinase; PT, phosphotransferase; AK, acetate kinase; PPI, propanose phosphate isomerase; PGI, phosphoglucose isomerase; MPTS, mannose phosphotransferase system; F6PK, F6P ketolyase; X5PK, xylukelose 5-phosphate ketolyase.
2.1.2. Heterolactic Fermentation
Some Lac bacteria (LAB) can synthesize Lac via the hexose monophosphate pathway (Figure 1B), namely heterolactic fermentation. These LAB can decompose Glu into phosphate-5-ribulose, which is converted into phosphate-5-xylulose (X5P) by epimerase and catalyzed by phosphoketolase (PK) to generate glyceraldehyde-3-phosphate (G3P) and acetyl phosphate (AP). AP is further reduced to ethanol (ET) and phosphoric acid. G3P is reduced to Lac after a series of reactions, with the accompanied generation of ET, CO2, and ATP, so the actual conversion rate of heterolactic fermentation is only 50%. The strains that undergo heterolactic fermentation include Candida intestinalis, Lactobacillus brevis, and Bifidobacteria. The general reaction formula is as follows:
C6H12O6 + ADP + Pi → CH3CH(OH)COOH + CH3CH2OH + CO2 + ATP
2.1.3. Bifidobacterium Fermentation
Bifidobacterium cannot utilize Glu via the EMP pathway because it lacks aldolase and Glu-6-phosphate dehydrogenase. Instead, Lac is produced through the PK pathway (Figure 1C), accompanied by acetic acid formation. There are two PKs involved in this pathway: (1) phospho-6-fructose ketolase, which decomposes fructose-6-phosphate (F6P) into acetic acid phosphate and erythritol-4-phosphate; and (2) phosphate-5-xylose ketolase, which decomposes X5P into G3P and AP. G3P forms Lac under the action of G3P and LDH with a conversion rate of 50%. The general reaction formula is as follows:
2 C6H12O6 → 2 CH3CH(OH)COOH + 3 CH3OOH
2.2. Strains for L-LA Production
The natural producers of L-LA mainly include Lactobacillus [13] (e.g., Lactobacillus rhamnosus, Lactobacillus delbrueckii, and Lactobacillus casei), Rhizopus [14,15,16] (Rhizopus nigricans, Rhizopus oryzae, Rhizopus chinensis, etc.), Streptococcus [17], and Bacillus [18]. The production of L-LA by Lactobacillus belongs to anaerobic fermentation or facultative anaerobic fermentation, which can greatly decrease the energy consumption and facilitate continuous fermentation to reduce the production cost. The actual conversion rate of Lactobacillus homotype fermentation is above 90%. Nevertheless, Lactobacillus are chemotrophic heterotrophic microorganisms with complex nutritional conditions and which require intricate nitrogen sources and increasing costs. General Lactobacillus does not produce amylase, and cannot directly use starch to produce L-LA, which needs saccharification treatment [19]. The nutritional requirements of the L-LA fermentation of Rhizopus oryzae are elementary, and the starch can be directly utilized without saccharification. Meanwhile, Rhizopus oryzae has the advantages of a low pH tolerance, a product with high optical purity, large bacteria size, and being convenient for purifying the product. Nonetheless, the yield and conversion rate of L-LA by Rhizopus oryzae were lower than those of lactic acid bacteria. In addition, Rhizopus oryzae requires ventilation and agitation during fermentation, thereby increasing the production costs [20]. Their fermentation conditions, LA titers, and advantages are summarized in Table 1. Among them, L. rhamnosus CGMCC No. 2183 could produce 235 g/L L-LA, the yield was 94.5–96.5%, and the optical purity was 98% [21]. Tsuneo et al. [22] used immobilized R. oryzae to produce L-LA, and the titer was 321 g/L.

Table 1.
Microbial strains producing L-LA.
High-throughput screening or adaptive evolution is usually required to obtain high-producing strains with high L-LA production, which are time-consuming and inefficient. With the advent of genetic engineering technology, microbial production is no longer limited by natural variation and screening, and genetic engineering can modify the metabolic network of the strain to efficiently increase L-LA production. The model microorganisms Escherichia coli and Saccharomyces cerevisiae are the most commonly used cell factories for L-LA production because of their clear genetic background, simple genetic manipulation methods, and easy high-density fermentation. Compared to E. coli, S. cerevisiae is more tolerant to low pH [36], making S. cerevisiae more suitable for organic acid production. Meanwhile, pH < 3.0 can effectively avoid bacterial contamination during the fermentation process. Therefore, S. cerevisiae has unique advantages in L-LA production. Colombié et al. [37] increased the L-LA titer to 50 g/L by integrating the LDH gene from Lactobacillus plantarum into the genome of S. cerevisiae. Novy et al. [38] integrated the pfLDH gene (encoding L-LA dehydrogenase) from Plasmodium falciparum into the genome of S. cerevisiae, yielding strain IBB14LA1-5. The L-LA productivity in strain IBB14LA1-5 could reach 1.8 g/L/h under microaerophilic conditions. Compared to S. cerevisiae, E. coli has the advantages of fast growth, simple nutritional requirements, and a high optical purity of Lac products [39]. Therefore, many studies have focused on engineering E. coli for the efficient biosynthesis of L-LA. By screening the LDH gene from different sources, modifying the metabolic network of L-LA, and optimizing the fermentation conditions, the L-LA titer in E. coli could reach 142.2 g/L [40].
In addition to the selection of strains, the study on strain improvement through mutation and the construction of L-LA high-yield strains by metabolic engineering has become a research hotspot, one that is mainly focused on the following aspects.
3. Metabolic Engineering Strategies for Improving L-LA Production
3.1. Mutation Breeding
Mutagenesis technologies can be physical (infrared ray, X-ray, gamma-ray, fast neutron, ion beam, laser, ultraviolet (UV) irradiation, and ultrasonic wave) or chemical (base analogs, alkylating agents, deamination, frameshifts mutagens, hydroxylating agents, and metal salts). Le et al. [41] screened an L-LA high-yield mutant YBQH2-14 using UV mutagenesis, whose L-LA titer reached 93 g/L and the sugar conversion rate reached 77.5%. Gu et al. [42] mutated the original strain R. oryzae PW352 using ion beam mutagenesis and obtained a mutant strain RE3303. Its L-LA titer increased by 48.5% compared to the original strain and reached 140 g/L, and the conversion rate was 86%. Xian et al. [43] used L. casei ZW-63A as the original strain and obtained the mutant strain CGMCC No. 8029 using UV mutagenesis and diethyl sulfate mutagenesis. The L-LA titer in strain CGMCC No. 8029 reached 140 g/L, and the optical purity of L-LA reached 98.83%. Jiang et al. [44] performed a highly efficient heavy ion mutagenesis technique to improve the L-LA titer of the strain Lactobacillus thermophilus SRZ50. Based on the microtiter plate screening method, the mutant strain A69 was screened, and A69 could synthesize 114.2 g/L L-LA at 96 h in fed-batch fermentation compared to the original strain which increased by 16.2%. In summary, high-throughput screening-assisted mutation breeding is an effective strategy for obtaining high-yield LA strains.
3.2. Strain Improvement by Metabolic Engineering
3.2.1. Expression of Exogenous L-LA Dehydrogenase
Because some strains lack the L-LA biosynthetic pathway, it is necessary to express L-LA dehydrogenase heterologously in their cells. Ishida et al. [45] found that bovine-derived LDH was more suitable for producing L-LA in S. cerevisiae and integrated four or six copies of exogenous LDH into the genome, respectively. Their results showed that the L-LA titer was positively correlated with the copy number of LDH, indicating that the expression level or catalysis efficiency of LDH was a bottleneck for the efficient synthesis of L-LA. Kong et al. [46] found that the LDHs from P. falciparum and Bacillus subtilis were more efficient for L-LA production in Kluyveromyces marxianus. By overexpressing the proton-coupled monocarboxylate transporter from S. cerevisiae, native 6-phosphofructokinase, and disrupting the native putative D-LDH, the L-LA titer in strain K. marxianus increased to 103 g/L. Moreover, with the development of synthetic biology tools and strategies, some gene expression elements or methods can be applied to increase the expression level and catalysis efficiency of LDH. Redden et al. [47] adapted the 655 bp natural promoter PGPD into 116 bp in yeast according to the structural composition of the promoter and combined it with the 47 bp minimum terminator assembly as an expression vector, which could reduce the load regulation amount on DNA by 80–90%. Flagfeldt et al. [48] characterized the expression level of the heterologous gene lacZ (encodes β-half-lactosidase) in 20 different integration sites of the S. cerevisiae genome. The expression levels of LacZ in different loci were significantly different. Reider et al. [49] employed clustered regularly interspaced short palindromic repeats (CRISPR)-CRISPR-associated protein 9 (Cas9) technology to build a cloning-free toolkit in S. cerevisiae, including 23 Cas9-sgRNA plasmids, 37 promoters with various strengths and temporal expression profiles, and 10 protein-localization, degradation, and solubility tags.
3.2.2. Pyr Metabolic Pathway
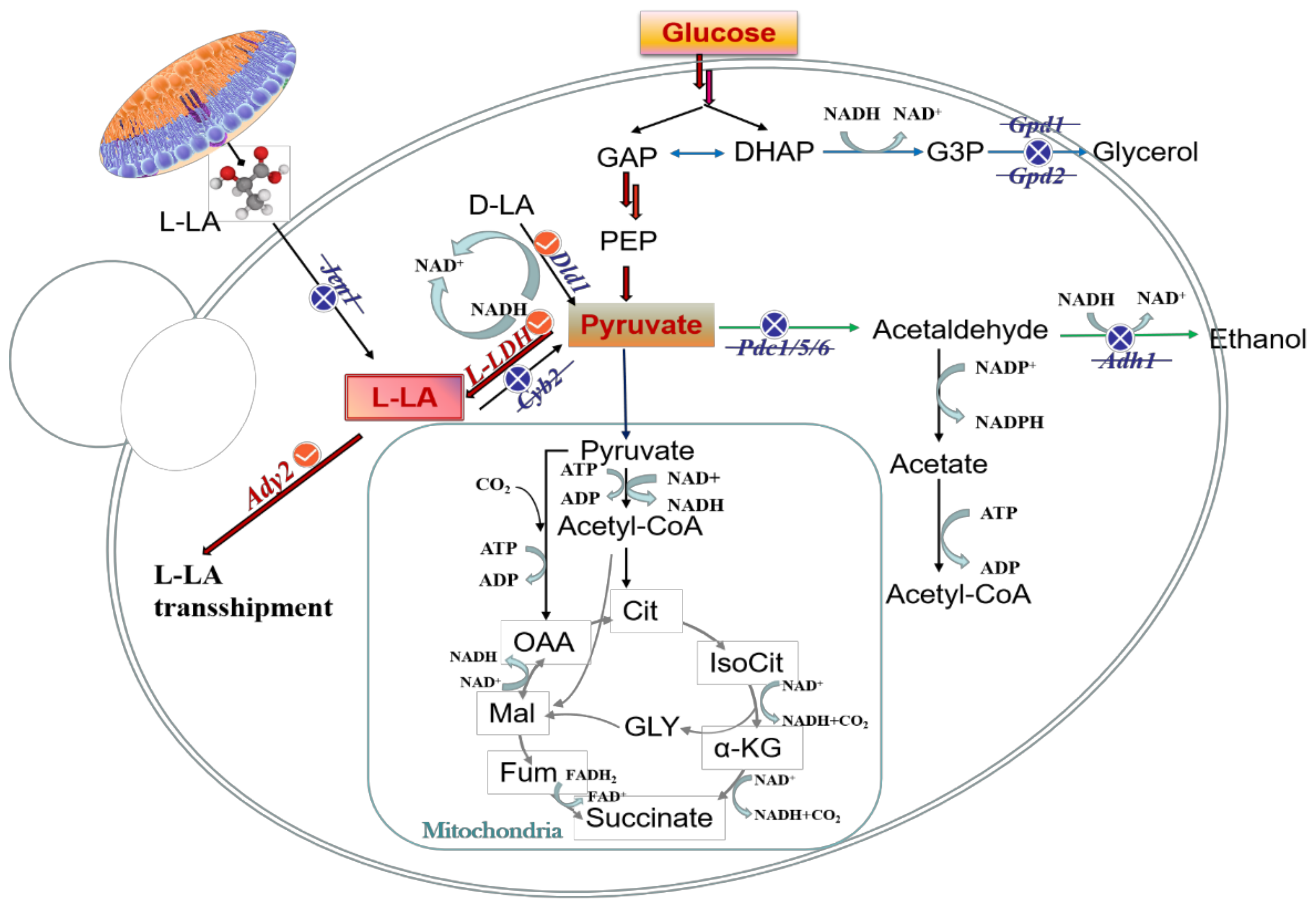
In general, engineered strains should choose the homotype fermentation pathway to produce L-LA owing to its theoretical conversion rate of 100%. Therefore, promoting the accumulation of the precursor Pyr is a critical step in L-LA synthesis. To increase the accumulation of Pyr, it is necessary to weaken the branching pathway of the EMP pathway and reduce the generation of by-products, including ethanol and glycerin (Figure 2).
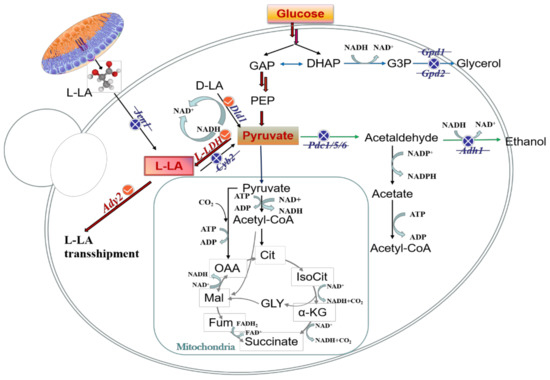
Figure 2.
Metabolic regulation strategies map of L-LA. The red line is the L-LA synthesis pathway. The blue line is the by-product glycerol synthesis pathway. The green line is the by-product ethanol synthesis pathway. The blue cross line indicates the blocking pathway. The red checkmark represents the overexpression pathway. GAP, phosphoglyceraldehyde; DHAP, dihydroxyacetone phosphate; Cit, citric acid; IsoCit, isocitrate; α-KG, α-ketoglutaric acid; Fum, fumaric acid; Mal, malic acid. Gpd1/2, glycerol-3-phosphate dehydrogenase 1/2; L-LDH, L-lactate dehydrogenase (exogenous gene); Cyb2, lactate dehydrogenase (cytochrome); Pdc1/5/6, indole pyruvate decarboxylase 1/5/6; ACS, acetyl-CoA synthetase; Jen1, carboxyl transporter Jen1p; Ady2, accumulation of dyads protein 2, transporter protein required for ammonia export and acetate uptake and resistance.
The key regulatory enzyme of ethanol synthesis is Pyr decarboxylase (Pdc). Pdc1, Pdc5, and Pdc6 are the most frequently disrupted enzymes in L-LA production. Nobuhiro et al. [50] knocked out Pdc1 and inserted double copies of LDH into the genome of S. cerevisiae, resulting in an L-LA titer increase to 55.6 g/L. They further knocked out Pdc5, the L-LA titer increased to 82.3 g/L, and the by-product ET was completely eliminated. Alcohol dehydrogenase 1 (Adh1) is the isoenzyme in S. cerevisiae ET fermentation. Kenro et al. [51] replaced Adh1 with LDH by homologous recombination in S. cerevisiae. A recombinant strain AF297C with four copies of LDH and Pdc1 and Adh1 deletions was further constructed. The L-LA titer of AF297C reached 74.1 g/L, and the production of the by-product ET was reduced to 7 g/L. Mazumdar et al. [52] engineered E. coli to produce L-LA by replacing the native D-lactate-specific dehydrogenase with Streptococcus bovis L-LDH. Blocking the ET bypass pathways knocked out the ADH gene adhA. Then, the native aerobic L-LDH lldD was blocked to prevent the consumption of L-lactate. The engineered strain produced 50 g/L L-LA from 56 g/L crude glycerol at a yield of 93% of the theoretical maximum and with high optical purity (99.9%). In anaerobic fermentation, S. cerevisiae will consume excessive NADH through the glycerol synthesis pathway to maintain the balance of intracellular cofactors, but the synthesis and accumulation of the by-product glycerol will lead to a decrease in the titer of the target product. To weaken competitive pathways and eliminate glycerol production, the glycerol triphosphate dehydrogenases Gpd1 and Gpd2 should be knocked out [53]. Table 2 summarizes the research status of L-LA production by genetically engineered strains and superiority strains in recent years.

Table 2.
Research status of L-LA production by genetically engineered strains.
3.2.3. Cofactor Engineering Strategies
In metabolic engineering, cofactor imbalance or insufficient supply is also a key factor limiting product synthesis. The cofactor supply was mainly improved by balancing the cofactor supply and adjusting cofactor specificity [74]. Reducing the ratio of NADH/NAD+ in the cytoplasm will facilitate the conversion of Glu into L-LA. Heux et al. [75] found that the expression of the NADH oxidase under the control of a yeast promoter led to large decreases in the intracellular NADH concentration (five-fold) and NADH/NAD+ ratio (six-fold). In addition, the ethanol, glycerol, succinate, and hydroxyglutarate yields were significantly reduced because of the lower NADH availability. Bhatt et al. [76] found that the mannitol added to the medium inhibited ethanol production by changing the ratio of NADH/NAD+, thus increasing L-LA production. Cytoplasmic acetyl-CoA is a precursor of many metabolites in S. cerevisiae. Weakening the Pdc activity of S. cerevisiae will affect the supply of cytoplasm acetyl-CoA and cell growth [77]. Therefore, compensating the intermediate metabolites of acetyl-CoA could improve bacterial growth and L-LA synthesis. Lian et al. [78] introduced the exogenous synthesis pathway of acetyl-CoA to increase the acetyl-CoA content in the cytoplasm.
3.2.4. Intracellular and Extracellular Transport of L-LA
With the metabolic production of microorganisms, L-LA will be accumulated in cells. Promoting acid efflux and inhibiting acid influx can alleviate the growth inhibition caused by intracellular L-LA, thereby improving the L-LA titer. Acetate transmembrane transporter ADY2 and carboxylic acid transporter protein homolog JEN1 were the two major transporters responsible for LA assimilation in S. cerevisiae. Pacheco et al. [79] found that overexpressing ADY2 and JEN1 in S. cerevisiae increased the L-LA titer by 15%. Kok et al. discovered two novel LA transporter mutants using laboratory evolution, which could enhance the growth ability of the strain in the medium using LA as the sole carbon source. In addition, they found that the mutation sites of these two mutants are both located in the protein ADY2 (C755G/Leu219Val and C655G/Ala252Gly). However, the intracellular transport function of JEN1 was also found. Paiva et al. [80] found that when JEN1 was knocked out, the strain could not absorb LA, indicating that JEN1 was involved in the intracellular transport of LA in S. cerevisiae. In addition, Wakamatsu et al. [81] also confirmed that JEN1 was involved in the intracellular transport of LA. In the presence of Glu, the constitutive expression of JEN1 and ADY2 led to higher external LA concentrations. However, Glu deficiency would lead to the consumption of LA. Andrade et al. [82] found that excessive Glu could rapidly reduce the activity of JEN1, resulting in an irreversible loss of activity. Therefore, Glu concentration has a certain effect on transporters. Presently, only JEN1 and ADY2 are known as LA transporters in S. cerevisiae. At present, ADY2 is responsible for the extracellular transfer of LA, and JEN1 may be responsible for both intracellular and extracellular transfer according to reports, but other transporters might also be involved. The transport mechanism of L-LA still needs to be studied further.
3.2.5. Genome Editing Tools
Although many traditional metabolic engineering techniques have been applied in the construction of L-LA high-yielding strains, the efficiency of these modification methods has been low, limiting the further improvement of the L-LA titer. In recent years, many genome editing methods have been developed to efficiently modify multiple target genes in the genome, including the Cre-LoxP method, polymerase chain reaction-mediated gene traceless knockout technology, zinc finger nuclease technology (ZFNs), transcription activator effector nuclease, and CRISPR-CAS system [83]. Amanda et al. [49] constructed a cloning-free toolkit based on CRISPR-cas9 technology which could address common obstacles in metabolic engineering including select chromosome integration site, promoter strength, and protein location. Liu et al. [84] constructed an endogenous subtype II-A CRISPR-Cas system-based genome interference plasmids to exert high-efficiency markerless gene deletion, gene integration, and point mutation in Pediococcus acidilactici. Using this method, they found that the depletion of the native plasmids would increase cell growth, and the integration of an L-LDH gene into the genome would enhance cell growth and L-LA production.
4. Utilization of Raw Materials and Renewable Resources
Using waste and cheap raw materials including whey, molasses, starchy raw materials, and cellulose raw materials to produce L-LA not only reduces the costs, but also solves problems of waste treatment and pollution
4.1. Whey
Whey is a by-product of the dairy products processing industry. A kilogram of cheese products can produce 9 kg of whey, which is rich in nutrients, such as protein, carbohydrates, inorganic salts, and vitamins, and can promote the growth of many microorganisms [85]. Four acid-tolerant Pedioccocus spp. strains, previously isolated from sourdough medium, were screened for their ability to produce enantioselective lactic acid from cheese whey [86]. The results showed that the maximum L-LA production was 47.0–51.2 g/L. Turner et al. [87] expressed a cellodextrin transporter CDT-1 and a β-glucosidase GH1-1 from Neurospora crassa in S. cerevisiae to produce lactose. An LDH from R. oryzae was further expressed in the strain. The final strain could produce 23.77 g/L L-LA.
4.2. Molasses
Molasses is a by-product of the sugar industry, and 100 tons of sugarcane can produce 3–4 tons of molasses, and the same weight of sugar beet can produce 4–6 tons of molasses [88]. The main component of molasses is sucrose, but it also contains some Glu, fructose, and other water-soluble organic and inorganic substances. Xu et al. [67] applied a cane molasses/carbon sources cofeeding method to efficient L-LA production from cane molasses. After medium optimization, 168.3 g/L L-LA was synthesized by strain Bacillus coagulans H-1. Liu [89] optimized the L-LA fermentation conditions of strain L. rhamnosus, and 84.2 g/L L-LA was produced from sweet potato residue. Nurkhamidah et al. [90] produced 19.68 g/L L-LA from molasses using strain L. delbrueckii and L. plantarum.
4.3. Starch
Starch, including corn, corncob, sorghum, brown rice, and sweet potato, is economical for L-LA production. Trakarnpaiboon et al. [91] isolated the thermotolerant strain Rhizopus microsporus DMKU 33, which could produce 84 g/L L-LA from liquefied cassava starch at pH 5.5 in 3 days. L-LA production was further increased to 105 to 118 g/L with a yield of 0.93 g/g and productivity of 1.25 g/L/h in fed-batch fermentation. In addition, R. oryzae MTCC 8784 could produce 15.5 g/L L-LA using 30 g/L starch [92].
4.4. Other Wastes
In recent years, waste yeast from breweries [93], kitchen waste [94], distiller’s grains [95], bran [96], bagasse [97], and straw [67] also have been used as raw materials for producing L-LA. Hu [98] analyzed the ability of B. coagulans LA204 to produce Lac from different straws and found that LA204 could effectively utilize Glu, xylose, and cellobiose generated from straw hydrolysis. Li et al. [99] reported a new strategy for the efficient production of optically pure L-LA from food waste (FW) at ambient temperature, i.e., by regulating key enzyme activity by sewage sludge supplement and intermittent alkaline fermentation. Ma et al. [100] investigated the effects of different lignocellulosic wastes on alleviating acidification in L-LA fermentation from FW. The results showed that pretreated spent mushroom substance was the best choice for FW cofermentation, and the maximum L-LA titer could reach 46.12 g/L.
To produce L-LA with cheap raw materials, mixed fermentation was also considered due to the complexity of substrate materials. As such, multiple substrates and even microbial interactions can also perform a promoting role and enhance fermentation efficiency. Mendes et al. discovered a complementary mechanism between Lactobacillus and S. cerevisiae in the mixed culture process. S. cerevisiae provided nutritional factors such as pyruvate, vitamins, and amino acids for Lactobacillus, while Lactobacillus provided an energy source for S. cerevisiae [101]. In the process of converting kitchen waste into L-LA, open fermentation at room temperature (without disinfection and adding cultured strains) could produce a certain amount of lactic acid. Sakai et al. [102] studied the effect of pH adjustment on open fermentation to obtain a higher L-LA yield. The results revealed that lactic acid fermentation could be well sustained at pH 7.0. They subsequently discovered that constant pH control shortened the fermentation time but reduced the optical purity of L-LA. The induction of bacillus thermophilus growth and the change of pH from swing control to constant control showed the best effect, and the highest L-LA yield reached 39.2 g/L [103]. Policastro et al. [104] screened L-LA in mixed cultures by suddenly changing pH, and they found that the production of LA depended on Bacillus sp., Cytobacillus sp. and Azospirillum sp. by mixed microbial culture was screened by the pH oscillation biofortification technique.
5. Fermentation Modes for L-LA Production
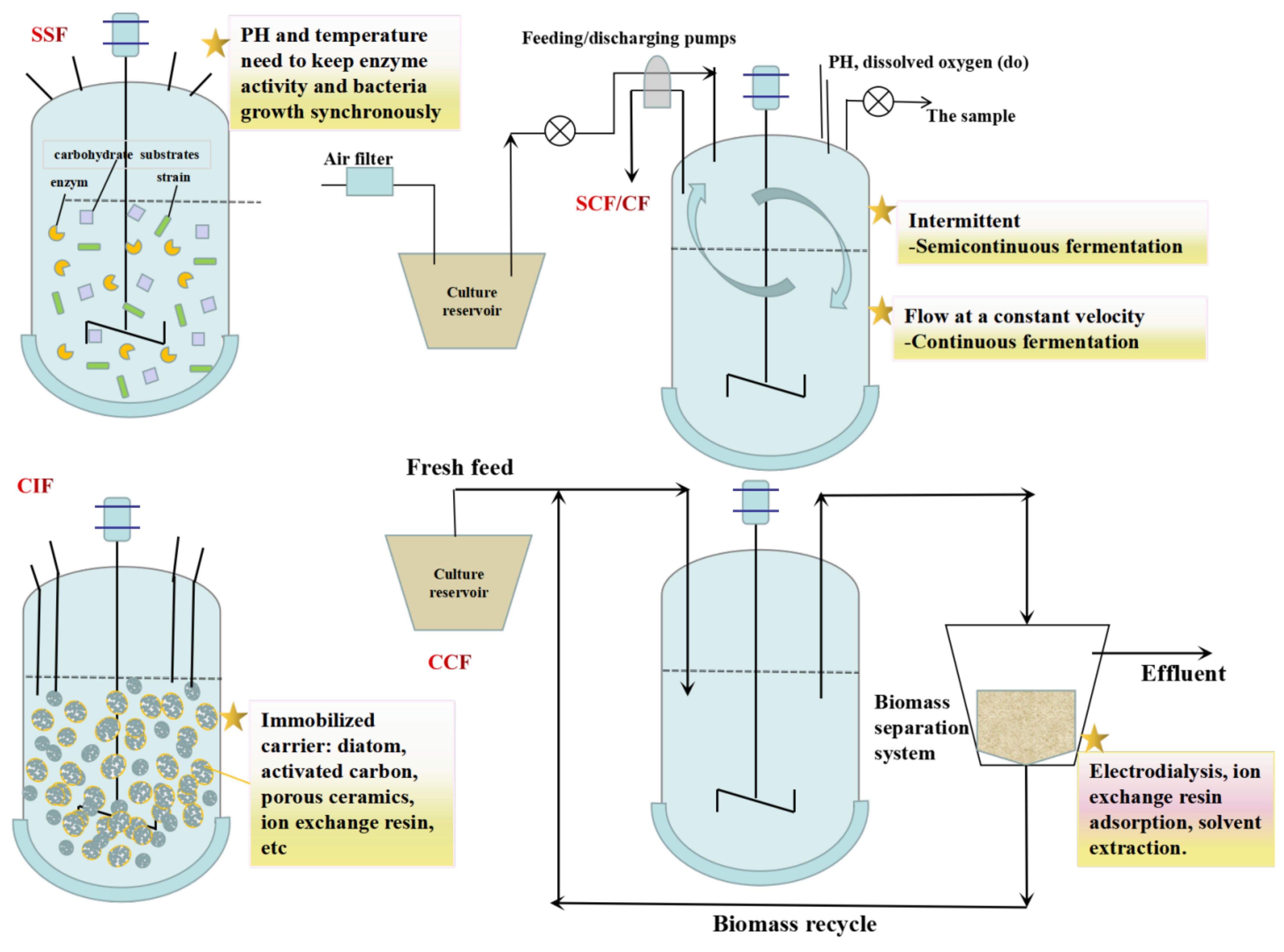
Optimizing the L-LA fermentation process is an important method of reducing production costs. Because the strains and raw materials used for fermentation are different, the process is also varied (Figure 3). Therefore, it is crucial to choose a suitable fermentation process. To reduce the production costs of L-LA, various cheap raw materials are often used as fermentation materials. Simultaneous saccharification fermentation (SSF) could save energy and prevent the adverse effects of a high sugar concentration on fermentation strains. SSF mainly aims to produce L-LA from cellulose and starch as raw materials. In addition, in the L-LA fermentation process, the pH reduction caused by L-LA accumulation will gradually inhibit the growth of the strain. The traditional fermentation process requires the addition of neutralizing agents, making it difficult to extract L-LA in downstream processing. The methods of semicontinuous fermentation (SCF), continuous fermentation (CF), cell immobilization fermentation (CIF), and cell circular fermentation (CCF) can remove L-LA from the fermented liquid in time during the fermentation process, thereby reducing product inhibition and improving the utilization rate of raw materials and the product yield.
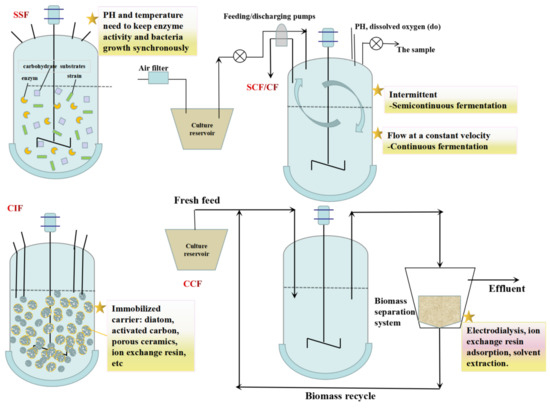
Figure 3.
Fermentation modes for L-LA production.
5.1. SSF
SSF is a process in which enzymes and strains are added to the bioreactor at the same time and the enzyme-catalyzed hydrolysis of carbohydrate substrates and microbial fermentation are coupled into one step. Compared to traditional fermentation, SSF has the advantages of shortening the production cycle, saving equipment investment, improving the yield, and reducing energy consumption [105]. In recent years, SSF has been gradually used in the waste utilization fermentation process, which is helpful in increasing the yield and productivity of LA [15]. Li et al. [55] integrated the cellulase enzyme production into L-LA fermentation from papermill sludge, and the L-LA titer reached 25 g/L.
5.2. SCF/CF
Based on batch fermentation, SCF is the release of the part of the feed liquid which periodically contains products in the fermentation process and replenishes the same amount of new feed liquid. CF is the continuous release of the same amount of fermentation liquid at a certain speed to replenish the new feed liquid. Using the SCF/CF mode to produce L-LA not only makes the cell obtain a steady stream of new nutrition in the fermentation process, but also dilutes the growth-inhibiting substances generated by bacteria to ensure the high-quality growth and metabolism of bacteria. Liu [106] fermented R. oryzae for 15 batches using the SCF mode, and the average productivity of L-LA was 3.05 g/(L h), which was higher than that of batch fermentation (1.32 g/(L h)). Zhao et al. [107] immobilized R. oryzae with corncob and continuously fermented L-LA for six batches, which was 16 h shorter than the fermentation of inoculated spores. In addition, the L-LA titer reached 33.2 g/L, and the sugar conversion rate increased by 12% compared to that of pure sugar fermentation. Luongo et al. [108] investigated the fermentation performance of two reactors operating in a repeated-batch mode for semicontinuous L-LA production. The maximum L-LA concentration reached 20.1 g/L.
5.3. CIF
CIF is when cell particles or biological particles are confined to specific carriers by embedding technology which can retain their high biological activity and improve the acid production efficiency. The CIF mode can reduce the acid inhibition of the product L-LA and recycle the bacteria. Adsorption, covalent bonding, cross-linking, and embedding are the common methods for preparing immobilized cells. Radosavljevic et al. [109] studied the immobilization of L. rhamnosus ATCC7469 in a poly(vinyl alcohol)/calcium alginate matrix using the freezing–thawing technique for application in Lac fermentation. In batch fermentation, the immobilized biocatalyst was superior to the free cell fermentation system (by 37.1%). The highest L-LA yield and volumetric productivity of 97.6% and 0.8 g/(L h), respectively, were attained in repeated-batch fermentation. During seven consecutive batch fermentations, the biocatalyst showed high mechanical and operational stability, reaching an overall productivity of 0.78 g/(L h). Zheng et al. [110] immobilized L. delbrueckii on sodium alginate gel beads to produce L-LA from cellulose hydrolysate and finally obtained 48.7 g/L L-LA, and the yield of L-LA/Glu reached 95.2%.
5.4. CCF
The CCF mode uses certain separation technologies to return fermented bacteria to the bioreactor for the further utilization and discharge the aged cells in time. At present, the developed separation methods include electrodialysis, ion exchange resin adsorption, solvent extraction, and membrane fermentation. Garrett et al. [66] used the Amberlite IRA-67 ion exchange resin for building an extractive fermentation system in the fed-batch fermentation. Compared to fed-batch fermentation without the extraction fermentation system, the L-LA titer of this method could be increased by 1.31 times. Danner et al. [111] coupled a biofilm reactor and electrodialysis technology to design the membrane bioreactor (MBR)-electrodialysis system, which was used for the CF of L-LA. The system accomplished cell circulation through ultrafiltration MBR and used the unipolar electrodialysis box to separate and purify L-LA in the fermentation broth. The remaining culture medium and fermentation substrate in the fermentation broth were reused in the fermentation tank. The L-LA titer by this system was capable of reaching 115 g/L, and this system could have run stable for a long time. After the system ran for >1000 h, there was still no cell penetration. In addition, this system has less wastewater discharge, low energy consumption, and good environmental benefits.
Nowadays, a variety of fermentation and extraction coupling technologies have emerged to overcome the shortcomings of traditional fermentation, such as product inhibition, high production costs, and the by-products produced by a neutralizer [80], representing the development directions of the L-LA fermentation industry in the future.
6. Conclusions
The microbial production of L-LA is a hot topic in global research at present. The construction of an efficient microbial cell factory for the sustainable production of L-LA is crucial for maintaining the sustainable development of the social economy. Recently, many microbial cell factories have been developed and fermentation progress have been made to efficiently synthesize L-LA. However, there are still many problems in the microbial production of L-LA, such as the imbalance of the metabolic fluxes between the cell growth and synthesis of L-LA, the high production costs in the fermentation process, and the adverse effects of a low pH caused by L-LA accumulation during fermentation.
To improve the synthesis efficiency of L-LA, we suggest that the two following aspects should be studied further. First, although enhancing Pyr accumulation can increase the L-LA titer, the production of by-products such as glycerol will also increase, resulting in the imbalance of a cofactor supply. Therefore, it is crucial to develop a global L-LA metabolism regulatory strategy through the genome-scale metabolic mode to balance the metabolic fluxes between the cell growth, cofactor supply, and L-LA synthesis. Although no metabolic model of L-LA production has been reported, there are some relevant models [112,113,114]. The same method can also be used as a mathematical tool for L-LA metabolism prediction and reactor optimization. Second, the L-LA accumulation will decrease the pH of the fermentation medium, further inhibiting bacterial growth. However, adding neutralizers will increase fermentation costs and introduce salt ion magazines. The high ion concentration will consume energy for cells and directly inhibit cell activity. Improving the acid resistance of cells may overcome this limitation, which can be achieved by adaptive evolution and genetic engineering [115,116].
In conclusion, microbial cell factory synthesis technology and gene dynamic regulation technology can be used to convert L-LA into low-value renewable resources for raw material production by reconstructing and engineering metabolic pathways to therefore develop the sustainable, green, and clean production of bulk chemical L-LA. The development prospects of the L-LA fermentation industry are vast, which will promote the upgrading of the food and medicine industry and represent a new horizon for the sustainable development of human society.
Author Contributions
Conceptualization, L.L.; validation, Y.L.; investigation, J.L.; resources, G.D.; writing—original draft preparation, T.L.; writing—review and editing, X.X. and X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Project of the National Natural Science Foundation of China (32100439), the Key Research and Development Program of China (2018YFA0900504), the Natural Science Foundation of Jiangsu Province (BK20210467), and the China Postdoctoral Science Foundation (2021M691283).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Douglas, R.D.; Arne, N.W. Metabolism of lactic acid in the intact rabbit. Am. J. Physiol. 1956, 184, 304–308. [Google Scholar]
- Nduko, J.M.; Matsumoto, K.; Ooi, T.; Taguchi, S. Enhanced production of poly(lactate-co-3-hydroxybutyrate) from xylose in engineered Escherichia coli overexpressing a galactitol transporter. Appl. Microbiol. Biotechnol. 2014, 98, 2453–2460. [Google Scholar] [CrossRef]
- Younes, M.; Aquilina, G.; Castle, L.; Engel, K.H.; Fowler, P.; Frutos Fernandez, M.J.; Furst, P.; Gurtler, R.; Gundert-Remy, U.; Husoy, T.; et al. Re-evaluation of acetic acid, lactic acid, citric acid, tartaric acid, mono- and diacetyltartaric acid, mixed acetic and tartaric acid esters of mono- and diglycerides of fatty acids (E 472a-f) as food additives. EFSA J. 2020, 18, e06032. [Google Scholar] [PubMed]
- Chen, L.; Song, Z.; Tan, S.Y.; Zhang, H.; Yuk, H.G. Application of bacteriocins produced from lactic acid bacteria for microbiological food safety. Curr. Top. Lact. Acid Bact. Probiotics 2020, 6, 1–8. [Google Scholar] [CrossRef]
- Zhang, G.M.; Wang, X.F.; Tan, H.; Lin, X.U.; Zhao, C. Lactic acid and saturated hydrogen saline could attenuate myocardial apoptosis by mimicking ischemic postconditioning. Chin. J. Evid.-Based Cardiovasc. Med. 2016, 3, 533–538. [Google Scholar]
- Venkatesan, K.B.; Karkhanis, S.S.; Matuana, L. Microcellular foaming of poly(lactic acid) branched with food-grade chain extenders. J. Appl. Polym. Sci. 2021, 138, 121–134. [Google Scholar] [CrossRef]
- Huang, Y.; Dapeng, L.I.; Zuo, H.; Shen, T.; Zou, J.D.L. Effect of plla/β-tcp abosorable plate by internal fixation on fracture healing of dog middle bitia. Clin. Med. Eng. 2011, 18, 15–18. [Google Scholar]
- Maki-Arvela, P.; Aho, A.; Murzin, D.Y. Heterogeneous catalytic synthesis of methyl lactate and lactic acid from sugars and their derivatives. ChemSusChem 2020, 13, 4833–4855. [Google Scholar] [CrossRef] [PubMed]
- Würmel, J.; Somers, K.P.; Simmie, J.M. Selections Reprinted from Mathematical Reviews. Bull. Am. Math. Soc. 2006, 43, 405–414. [Google Scholar]
- Yusoff, N.H.; Pal, K.; Narayanan, T.; de Souza, F.G. Recent trends on bioplastics synthesis and characterizations: Polylactic acid (PLA) incorporated with tapioca starch for packaging applications. J. Mol. Struct. 2021, 1232, 129954. [Google Scholar] [CrossRef]
- Fabio, A.C.M.; Eduardo, M.B.; José, M.S.; José, M.D.G.; Attilio, C.; Ricardo, P.D.S.O. Lactic acid properties, applications and production: A review. Trends Food Sci. Technol. 2013, 30, 70–83. [Google Scholar]
- Wasewar, K.L.; Pangarkar, V.G.; Bert, A.; Heesink, M.; Versteeg, G.F. Intensification of enzymatic conversion of glucose to lactic acid by reactive extraction. Chem. Eng. Sci. 2003, 15, 3385–3393. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Lactic acid production from lignocellulose-derived sugars using lactic acid bacteria: Overview and limits. J. Biotechnol. 2011, 156, 286–301. [Google Scholar] [CrossRef] [PubMed]
- Amini, Z.; Self, R.; Strong, J.; Speight, R.; Harrison, M.D. Valorization of sugarcane biorefinery residues using fungal biocatalysis. Biomass Convers. Biorefin. 2022, 12, 997–1011. [Google Scholar] [CrossRef]
- Chung, M.; Tan, I.S.; Foo, H.; Lam, M.K.; Lim, S. Potential of macroalgae-based biorefinery for lactic acid production from exergy aspect. Biomass Convers. Biorefin. 2021, 1, 1–31. [Google Scholar] [CrossRef]
- Shahri, S.Z.; Vahabzadeh, F.; Mogharei, A. Lactic acid production by loofah-immobilized Rhizopus oryzae through one-step fermentation process using starch substrate. Bioproc. Biosyst. Eng. 2020, 43, 333–345. [Google Scholar] [CrossRef]
- Razi, F.; Yuwono, S.D. Batch and Continuous Lactic Acid Production from Cassava by Streptococcus bovis. J. Rekayasa Kimia Lingkungan 2006, 5, 17–20. [Google Scholar]
- Peng, L.; Wang, L.; Che, C.; Yang, G.; Yu, B.; Ma, Y. Bacillus sp. strain P38: An efficient producer of L-lactate from cellulosic hydrolysate, with high tolerance for 2-furfural. Bioresour. Technol. 2013, 149, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Vuyst, L.D.; Leroy, F. Functional role of yeasts, lactic acid bacteria, and acetic acid bacteria in cocoa fermentation processes. FEMS Microbiol. Rev. 2020, 44, 432–453. [Google Scholar] [CrossRef]
- Ma, X.; Gao, M.; Yin, Z.; Zhu, W.; Wang, Q. Lactic acid and animal feeds production from sophora flavescens residues by Rhizopus oryzae fermentation. Process Biochem. 2020, 92, 401–408. [Google Scholar] [CrossRef]
- Xu, P.; Zhao, B.; Qin, J.Y. A Method for Producing L-lactic Acid and Its Special Lactobacillus rhamnosus. Patent CN/2007/10176057, 7 May 2008. Available online: https://d.wanfangdata.com.cn/patent/CN200710176057.7 (accessed on 23 May 2022).
- Tsuneo, Y.; Ryohsuke, T. Highly accumulative production of L(+)-lactate from glucose by crystallization fermentation with immobilized Rhizopus oryzae. J. Biosci. Bioeng. 2013, 115, 90–95. [Google Scholar]
- PMD Oliveira, S.L.P.C. Production of L(+) Lactic Acid by Lactobacillus casei Ke11: Fed Batch Fermentation Strategies. Fermentation 2021, 7, 151. [Google Scholar] [CrossRef]
- Wang, J.; Huang, J.; Laffend, H.; Jiang, S.L.S. Optimization of immobilized lactobacillus pentosus cell fermentation for lactic acid production. Bioresour. Bioprocess. 2020, 7, 1–14. [Google Scholar] [CrossRef]
- Cubas-Cano, E.; Gonzalez-Fernandez, C.; Tomas-Pejo, E. Evolutionary engineering of Lactobacillus pentosus improves lactic acid productivity from xylose-rich media at low pH. Bioresour. Technol. 2019, 288, 121540. [Google Scholar] [CrossRef] [PubMed]
- Liguori, R.; Soccol, C.R.; Vandenberghe, L.P.; Woiciechowski, A.L.; Ionata, E.; Marcolongo, L.; Faraco, V. Selection of the strain Lactobacillus acidophilus atcc 43121 and its application to brewers’ spent grain conversion into lactic acid. Biomed. Res. Int. 2015, 2015, 240231. [Google Scholar] [CrossRef]
- Kato, H.; Shiwa, Y.; Oshima, K.; Machii, M.; Araya-Kojima, T.; Zendo, T.; Shimizu-Kadota, M.; Hattori, M.; Sonomoto, K.; Yoshikawa, H. Complete genome sequence of Lactococcus lactis IO-1, a lactic acid bacterium that utilizes xylose and produces high levels of L-lactic acid. J. Bacteriol. 2012, 194, 2102–2103. [Google Scholar] [CrossRef]
- Su, J.H. Kinetics study on fermentation of l-lactic acid by lactobacillus thermophilus(T-1). Mod. Food Sci. Technol. 2007, 23, 30–32. [Google Scholar]
- Zhang, L.; Li, X.; Yong, Q.; Yang, S.T.; Ouyang, J.; Yu, S. Impacts of lignocellulose-derived inhibitors on L-lactic acid fermentation by Rhizopus oryzae. Bioresour. Technol. 2016, 203, 173–180. [Google Scholar] [CrossRef]
- Takano, M.; Hoshino, K. Lactic acid production from paper sludge by SSF with thermotolerant Rhizopus sp. Bioresour. Bioproc. 2016, 3, 1–10. [Google Scholar] [CrossRef]
- Kwan, T.H.; Vlysidis, A.; Wu, Z.; Hu, Y.; Koutinas, A.; Lin, C. Lactic acid fermentation modelling of Streptococcus thermophilus YI-B1 and Lactobacillus casei Shirota using food waste derived media. Biochem. Eng. J. 2017, 6, 97–109. [Google Scholar] [CrossRef]
- Regiane, A.; Schneider, R.; Rossell, C.V.; Filho, R.M.V.J. Polymer grade L-lactic acid production from sugarcane bagasse hemicellulosic hydrolysate using Bacillus coagulans. Bioresour. Technol. Rep. 2019, 11, 26–31. [Google Scholar]
- Wang, L.; Cai, Y.; Zhu, L.; Guo, H.; Yu, B. Major role of NAD-dependent lactate dehydrogenases in the production of L-lactic acid with high optical purity by the thermophile Bacillus coagulans. Appl. Environ. Microbiol. 2014, 80, 7134–7141. [Google Scholar] [CrossRef] [PubMed]
- Thomasser, C.; Danner, H.; Neureiter, M.; Saidi, B.; Braun, R. Thermophilic lactic acid production on hemicellulose hydrolysate. Meded. Rijksuniv. Gent. Fak. Landbouwkd. Toegep. Biol. Wet. 2001, 66, 325–328. [Google Scholar] [PubMed]
- Juturu, V.; Wu, J.C. Production of high concentration of l-lactic acid from oil palm empty fruit bunch by thermophilic Bacillus coagulans JI12. Biotechnol. Appl. Biochem. 2018, 65, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Eldarov, M.A.; Kishkovskaia, S.A.; Tanaschuk, T.N.; Mardanov, a.v. Genomics and biochemistry of Saccharomyces cerevisiae wine yeast strains. Biochem. Biokhimiia 2016, 81, 1650–1668. [Google Scholar] [CrossRef] [PubMed]
- Colombie, S.; Sablayrolles, J.M. Nicotinic acid controls lactate production by K1-LDH: A Saccharomyces cerevisiae strain expressing a bacterial LDH gene. J. Ind. Microbiol. Biotechnol. 2004, 31, 209–215. [Google Scholar] [CrossRef]
- Novy, V.; Brunner, B.; Nidetzky, B. L-Lactic acid production from glucose and xylose with engineered strains of Saccharomyces cerevisiae: Aeration and carbon source influence yields and productivities. Microb. Cell Fact. 2018, 17, 59. [Google Scholar] [CrossRef]
- Zhang, L.H.; Chen, X.Z.; Shen, W.; Fan, Y. Progresses in the metabolic engineering of Escherichia coli for lactic acid production. Chin. J. Bioprocess Eng. 2017, 15, 48–56. [Google Scholar]
- Niu, D.; Tian, K.; Prior, B.A.; Wang, M.; Wang, Z.; Lu, F.; Singh, S. Highly efficient L-lactate production using engineered Escherichia coli with dissimilar temperature optima for L-lactate formation and cell growth. Microb. Cell Fact. 2014, 13, 78. [Google Scholar] [CrossRef]
- Le, X.J.; Wang, C.L.; Gu, X.B. Breeding of L-lactic Acid Producing Bacteria. China Food Addit. 2004, 5, 67–71. [Google Scholar]
- Gu, S.B.; Ge, C.M.; Wang, Q.H. Characteristics of L-lactic acid producing strain Rhizopus oryzae PW352 and high productive mutant by low energy ions implantation. Ind. Microbiol. 2004, 34, 12–16. [Google Scholar]
- Xian, M.; Zou, H.B.; Liu, W. The Invention Discloses Lactobacillus casei Producing High Photopurity L-lactic acid and a Fermentation Method. Patent CN/2013/10474231, 12 October 2013. Available online: https://d.wanfangdata.com.cn/patent/CN201310474231.1 (accessed on 23 May 2022).
- Jiang, A.L.; Hu, W.; Li, W.J.; Liu, L.; Tian, X.J.; Liu, J.; Wang, S.Y.; Lu, D.; Chen, J.H. Enhanced production of l-lactic acid by Lactobacillus thermophilus SRZ50 mutant generated by high-linear energy transfer heavy ion mutagenesis. Eng. Life Sci. 2018, 18, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Ishida, N.; Saitoh, S.; Tokuhiro, K.; Nagamori, E.; Matsuyama, T.; Kitamoto, K.; Takahashi, H. Efficient production of L-Lactic acid by metabolically engineered Saccharomyces cerevisiae with a genome-integrated L-lactate dehydrogenase gene. Appl. Environ. Microb. 2005, 71, 1964–1970. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Zhang, B.; Hua, Y.; Zhu, Y.; Li, W.; Wang, D.; Hong, J. Efficient L-lactic acid production from corncob residue using metabolically engineered thermo-tolerant yeast. Bioresour. Technol. 2019, 273, 220–230. [Google Scholar] [CrossRef]
- Redden, H.; Alper, H.S. The development and characterization of synthetic minimal yeast promoters. Nat. Commun. 2015, 6, 7810. [Google Scholar] [CrossRef]
- Flagfeldt, D.B.; Siewers, V.; Huang, L.; Nielsen, J. Characterization of chromosomal integration sites for heterologous gene expression in Saccharomyces cerevisiae. Yeast 2009, 26, 545–551. [Google Scholar] [CrossRef]
- Reider, A.A.; D’Espaux, L.; Wehrs, M.; Sachs, D.; Li, R.A.; Tong, G.J.; Garber, M.; Nnadi, O.; Zhuang, W.; Hillson, N.J.; et al. A Cas9-based toolkit to program gene expression in Saccharomyces cerevisiae. Nucleic Acids Res. 2017, 45, 496–508. [Google Scholar] [CrossRef]
- Nobuhiro, I.; Satoshi, S.; Toru, O.; Kenro, T.; Eiji, N.; Katsuhiko, K.; Haruo, T. The effect of pyruvate decarboxylase gene knockout in Saccharomyces cerevisiae on L-lactic acid production. Biosci. Biotechnol. Biochem. 2006, 70, 1148–1153. [Google Scholar]
- Kenro, T.; Nobuhiro, I.; Eiji, N.; Satoshi, S.; Toru, O.; Akihiko, K.; Haruo, T. Double mutation of the PDC1 and ADH1 genes improves lactate production in the yeast Saccharomyces cerevisiae expressing the bovine lactate dehydrogenase gene. Appl. Microbiol. Biotechnol. 2009, 82, 883–890. [Google Scholar]
- Mazumdar, S.; Blankschien, M.D.; Clomburg, J.M.; Gonzalez, R. Efficient synthesis of L-lactic acid from glycerol by metabolically engineered Escherichia coli. Microb. Cell Fact. 2013, 12, 7–12. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, Y.G.; Kim, S.J.; Jin, Y.S.; Seo, J.H. Deletion of glycerol-3-phosphate dehydrogenase genes improved 2,3-butanediol production by reducing glycerol production in pyruvate decarboxylase-deficient Saccharomyces cerevisiae. J. Biotechnol. 2019, 304, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Pontes, R.; Romani, A.; Michelin, M.; Domingues, L.; Teixeira, J.; Nunes, J. L-lactic acid production from multi-supply autohydrolyzed economically unexploited lignocellulosic biomass. Ind. Crop. Prod. 2021, 170, 113775. [Google Scholar] [CrossRef]
- Li, J.; Shi, S.; Wang, Y.; Jiang, Z. Integrated production of optically pure L-lactic acid from paper mill sludge by simultaneous saccharification and co-fermentation (SSCF). Waste Manag. 2021, 129, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.T.; Zhang, Q.; Guo, X.P. Effect of ptsG and mglB genes knock-out on the production of L-lactic acid by Escherichia coli from mixed sugars. China Brew. 2021, 40, 82–86. [Google Scholar]
- Lopez-Gomez, J.P.; Alexandri, M.; Schneider, R.; Latorre-Sanchez, M.; Coll Lozano, C.; Venus, J. Organic fraction of municipal solid waste for the production of L-lactic acid with high optical purity. J. Clean. Prod. 2020, 247, 119165. [Google Scholar] [CrossRef]
- Unban, K.; Khanongnuch, R.; Kanpiengjai, A.; Shetty, K.; Khanongnuch, C. Utilizing gelatinized starchy waste from rice noodle factory as substrate for L(+)-lactic acid production by amylolytic lactic acid bacterium Enterococcus faecium k-1. Appl. Biochem. Biotechnol. 2020, 192, 353–366. [Google Scholar] [CrossRef]
- Bae, J.; Kim, H.; Kim, M.; Sung, B.H.; Jeon, J.; Kim, H.; Jin, Y.; Kweon, D.; Sohn, J. Direct fermentation of Jerusalem artichoke tuber powder for production of L-lactic acid and d-lactic acid by metabolically engineered Kluyveromyces marxianus. J. Biotechnol. 2018, 266, 27–33. [Google Scholar] [CrossRef]
- Okano, K.; Uematsu, G.; Hama, S.; Tanaka, T.; Noda, H.; Kondo, A.; Honda, K. Metabolic engineering of lactobacillus plantarum for direct L-lactic acid production from raw corn starch. Biotechnol. J. 2018, 13, e1700517. [Google Scholar] [CrossRef]
- Lee, J.W.; In, J.H.; Park, J.B.; Shin, J.; Park, J.H.; Sung, B.H.; Kweon, D.H. Co-expression of two heterologous lactate dehydrogenases genes in Kluyveromyces marxianus for L-lactic acid production. J. Biotechnol. 2017, 241, 81–86. [Google Scholar] [CrossRef]
- Novy, V.; Brunner, B.; Muller, G.; Nidetzky, B. Toward “homolactic” fermentation of glucose and xylose by engineered Saccharomyces cerevisiae harboring a kinetically efficient L-lactate dehydrogenase within pdc1-pdc5 deletion background. Biotechnol. Bioeng. 2017, 114, 163–171. [Google Scholar] [CrossRef]
- De Lima, P.B.; Mulder, K.C.; Melo, N.T.; Carvalho, L.S.; Menino, G.S.; Mulinari, E.; de Castro, V.H.; Dos, R.T.; Goldman, G.H.; Magalhaes, B.S.; et al. Novel homologous lactate transporter improves L-lactic acid production from glycerol in recombinant strains of Pichia pastoris. Microb. Cell Fact. 2016, 15, 158. [Google Scholar] [CrossRef]
- Timothy, L.T.; Guo, C.Z.; Eun, J.O.; Vijay, S.; Andrew, A.; Vimal, S.; Christopher, D.S.; Ji, Y.J.; Byung, J.Y.; In, P.; et al. Lactic acid production from cellobiose and xylose by engineered Saccharomyces cerevisiae. Biotechnol. Bioeng. 2016, 113, 1075–1083. [Google Scholar]
- Garrett, B.G.; Srinivas, K.; Ahring, B.K. Performance and stability of Amberlite (TM) IRA-67 ion exchange resin for product extraction and pH control during homolactic fermentation of corn stover sugars. Biochem. Eng. J. 2015, 94, 1–8. [Google Scholar] [CrossRef]
- Xu, K.; Xu, P. Efficient production of L-lactic acid using co-feeding strategy based on cane molasses/glucose carbon sources. Bioresour. Technol. 2014, 153, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Y.; Zhang, G.Y. Construction of Commercial Saccharomyces cerevisiae with L-lactic acid Efficient Production Ability. Food Food Res. Dev. 2012, 33, 164–168. [Google Scholar]
- Gao, T.; Wong, Y.; Ng, C.; Ho, K. L-lactic acid production by Bacillus subtilis MUR1. Bioresour. Technol. 2012, 121, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, J.; Zhou, J.; Liu, L.; Du, G.; Chen, J. Modification of carbon flux in Sacchromyces cerevisiae to improve L-lactic acid production. Acta Microbiol. Sin. 2011, 51, 50–58. [Google Scholar]
- Zhang, Q.; Zhang, L.; Ding, Z.; Wang, Z.; Shi, G. Metabolic engineering of wild acid-resistant yeast for L-lactic acid production. Chin. J. Biotechnol. 2011, 27, 1024–1031. [Google Scholar]
- Ikushima, S.; Fujii, T.; Kobayashi, O.; Yoshida, S.; Yoshida, A. Genetic engineering of Candida utilis yeast for efficient production of L-lactic acid. Biosci. Biotechnol. Biochem. 2009, 73, 1818–1824. [Google Scholar] [CrossRef]
- Osawa, F.; Fujii, T.; Nishida, T.; Tada, N.; Ohnishi, T.; Kobayashi, O.; Komeda, T.; Yoshida, S. Efficient production of L-lactic acid by Crabtree-negative yeast Candida boidinii. Yeast 2009, 26, 485–496. [Google Scholar] [CrossRef]
- Saitoh, S.; Ishida, N.; Onishi, T.; Tokuhiro, K.; Nagamori, E.; Kitamoto, K.; Takahashi, H. Genetically engineered wine yeast produces a high concentration of L-lactic acid of extremely high optical purity. Appl. Environ. Microb. 2005, 71, 2789–2792. [Google Scholar] [CrossRef]
- Hou, J.; Suo, F.; Wang, C.; Li, X.; Shen, Y.; Bao, X. Fine-tuning of NADH oxidase decreases byproduct accumulation in respiration deficient xylose metabolic Saccharomyces cerevisiae. BMC Biotechnol. 2014, 14, 13. [Google Scholar] [CrossRef]
- Heux, S.; Cachon, R.; Dequin, S. Cofactor engineering in Saccharomyces cerevisiae: Expression of a H2O-forming NADH oxidase and impact on redox metabolism. Metab. Eng. 2006, 8, 303–314. [Google Scholar] [CrossRef]
- Bhatt, S.M.; Srivastava, S. Mannitol increases lactic acid production by shifting NADH/NAD+ ratio inhibiting ethanol production. J. Biotechnol. 2008, 136, 22–27. [Google Scholar] [CrossRef]
- Kozak, B.U.; van Rossum, H.M.; Benjamin, K.R.; Wu, L.; Daran, J.M.; Pronk, J.T.; van Maris, A.J. Replacement of the Saccharomyces cerevisiae acetyl-CoA synthetases by alternative pathways for cytosolic acetyl-CoA synthesis. Metab. Eng. 2014, 21, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Si, T.; Nair, N.U.; Zhao, H. Design and construction of acetyl-CoA overproducing Saccharomyces cerevisiae strains. Metab. Eng. 2014, 24, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, A.; Talaia, G.; Sa-Pessoa, J.; Bessa, D.; Goncalves, M.J.; Moreira, R.; Paiva, S.; Casal, M.; Queiros, O. Lactic acid production in Saccharomyces cerevisiae is modulated by expression of the monocarboxylate transporters Jen1 and Ady2. FEMS Yeast Res. 2012, 12, 375–381. [Google Scholar] [CrossRef]
- Paiva, S.; Vieira, N.; Nondier, I.; Haguenauer-Tsapis, R.; Casal, M.; Urban-Grimal, D. Glucose-induced ubiquitylation and endocytosis of the yeast Jen1 transporter: Role of lysine 63-linked ubiquitin chains. J. Biol. Chem. 2009, 284, 19228–19236. [Google Scholar] [CrossRef]
- Wakamatsu, M.; Tomitaka, M.; Tani, T.; Taguchi, H.; Kida, K.; Akamatsu, T. Improvement of ethanol production from D-lactic acid by constitutive expression of lactate transporter Jen1p in Saccharomyces cerevisiae. Biosci. Biotechnol. Biochem. 2013, 77, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.P.; Casal, M. Expression of the lactate permease gene JEN1 from the yeast Saccharomyces cerevisiae. Fungal Genet. Biol. 2001, 32, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Lisby, M.; Rothstein, R. Cell biology of mitotic recombination. Cold Spring Harb. Perspect. Biol. 2015, 7, a16535. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yang, D.; Zhang, Z.; Liu, T.; Hu, G.; He, M.; Zhao, S.; Peng, N. High-efficiency genome editing based on endogenous crispr-cas system enhances cell growth and lactic acid production in Pediococcus acidilactici. Appl. Environ. Microb. 2021, 87, e94821. [Google Scholar] [CrossRef] [PubMed]
- Tavares, T.G.; Malcata, F. Whey proteins as source of bioactive peptides against hypertension. Bioact. Food Pept. Health Dis. 2017, 4, 75–84. [Google Scholar]
- Juodeikiene, G.; Zadeike, D.; Bartkiene, E.; Klupsaite, D. Application of acid tolerant Pedioccocus strains for increasing the sustainability of lactic acid production from cheese whey. LWT-Food Sci. Technol. 2016, 72, 399–406. [Google Scholar] [CrossRef]
- Turner, T.L.; Kim, E.; Hwang, C.; Zhang, G.C.; Liu, J.J.; Jin, Y.S. Short communication: Conversion of lactose and whey into lactic acid by engineered yeast. J. Dairy Sci. 2017, 100, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Huimin, L.I.; Wang, X.; Wang, J.; Zhang, X.; Liu, Q. Efficient production of lactic acid and other organic acids from sugarcane molasses by Lactic acid bacteria. Food Ind. 2019, 1, 109–112. [Google Scholar]
- Liu, Y.T.; Wu, M.Y.; Jin, Y.L.; Shen, W.L.; Fang, Y.Z.H. Lactic acid fermentation by Lactobacillus rhamnosus from sweet potato residue. Sci. Agric. Sin. 2016, 9, 1767–1777. [Google Scholar]
- Nurkhamidah, S.; Altway, A.; Susianto; Rahmawati, Y.; Ramadhani, A. Utilization of molasses to produce lactic acid by using Lactobacillus delbrueckii and Lactobacillus plantarum. IOP Conf. Ser. Mater. Sci. Eng. 2019, 1, 5–12. [Google Scholar] [CrossRef]
- Trakarnpaiboon, S.; Srisuk, N.; Piyachomkwan, K.; Yang, S.; Kitpreechavanich, V. L-Lactic acid production from liquefied cassava starch by thermotolerant Rhizopus microsporus: Characterization and optimization. Process Biochem. 2017, 63, 26–34. [Google Scholar] [CrossRef]
- Panesar, P.S.; Kaur, S. Screenning of media components and process parameters for production of L(+) lactic acid from potato wast liquid using amylolytic Rhizopus oryzae. Acta Aliment. 2017, 46, 312–322. [Google Scholar] [CrossRef]
- Zacharof, M.P. Industrial Symbiosis: Beer Brewery Wastewater-Based Biorefinery. Circ. Econ. Sustain. 2021, 1, 593–609. [Google Scholar] [CrossRef]
- Gui, X.; Luo, Y.; Li, Z.; Nie, M.; Yang, Y.; Zhang, C.; Liu, J. Co-fermentation of kitchen waste and excess sludge for organic acid production: A review. Chin. J. Biotechnol. 2021, 37, 448–460. [Google Scholar] [CrossRef]
- Buenavista, R.; Siliveru, K.; Zheng, Y. Utilization of distiller’s dried grains with solubles: A review. J. Agric. Food Res. 2021, 1, 100195. [Google Scholar] [CrossRef]
- Alexandri, M.; Neu, A.K.; Schneider, R.; Lopez-Gomez, J.P.; Venus, J. Evaluation of various bacillus coagulans isolates for the production of high purity L-lactic acid using defatted rice bran hydrolysates. Int. J. Food Sci. Technol. 2018, 54, 1321–1329. [Google Scholar] [CrossRef]
- Nalawade, K.; Saikia, P.; Behera, S.; Konde, K.; Patil, S. Assessment of multiple pretreatment strategies for 2G L-lactic acid production from sugarcane bagasse. Biomass Convers. Biorefin. 2020, 7, 1–14. [Google Scholar] [CrossRef]
- Hu, J.L. High-Efficient and Titer Lactic acid Production from Corn Stover by Bacillus coagulans LA204 Using Simultaneous Saccharification and Fermentation. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2015. [Google Scholar]
- Li, X.; Chen, Y.G.; Zhao, S.; Chen, H.; Zheng, X.; Luo, J.Y.; Liu, Y.N. Efficient production of optically pure L-lactic acid from food waste at ambient temperature by regulating key enzyme activity. Water Res. 2015, 70, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Mendes, F.; Sieuwerts, S.; de Hulster, E.; Almering, M.J.H.; Luttik, M.A.H.; Pronk, J.T.; Smid, E.J.; Bron, P.A.; Daran-Lapujade, P. Transcriptome-based characterization of interactions between Saccharomyces cerevisiae and Lactobacillus delbrueckii subsp bulgaricus in lactose-grown chemostat cocultures. Appl. Environ. Microbiol. 2013, 79, 5949–5961. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Murata, Y.; Yamazumi, H.; Tau, Y.; Mori, M.; Moriguch, M.; Shira, R.Y. Selective proliferation of lactic acid bacteria and accumulation of lactic acid during open fermentation of kitchen refuse with intermittent pH adjustment. Food Sci. Technol. Res. 2000, 6, 140–145. [Google Scholar] [CrossRef]
- Tashiro, Y.; Inokuchi, S.; Poudel, P.; Okugawa, Y.; Miyamoto, H.; Miayamoto, H.; Sakai, K. Novel pH control strategy for efficient production of optically active L-lactic acid from kitchen refuse using a mixed culture system. Bioresour. Technol. 2016, 216, 52–59. [Google Scholar] [CrossRef]
- Policastro, G.; Carraturo, F.; Compagnone, M.; Giugliano, M.; Guida, M.; Luongo, V.; Napolitano, R.; Fabbricino, M. A preliminary study on a novel bioaugmentation technique enhancing lactic acid production by mixed cultures fermentation. Bioresour. Technol. 2021, 340, 125595. [Google Scholar] [CrossRef]
- Ma, X.Y.; Gao, M.; Li, C.L.; Wang, N.H.; Wang, Q.H.; Sun, X.H. Effects of different lignocellulosic wastes on alleviating acidification of L-lactic acid production from food waste fermentation. Bioresour. Technol. 2021, 342, 126043. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huo, K.; Gao, L.; Zhao, G.; Wang, B.; Liu, J. Open simultaneous saccharification and fermentation of l-lactic acid by complete utilization of sweet sorghum stalk: A water-saving process. RSC Adv. 2021, 11, 5284–5290. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Primary Study on Prodution of L-Lactic Acid by High Cell Density and Semi-Continuous Fermentation with Rhizopus oryzae. Master’s Thesis, Hefei University of Technology, Hefei, China, 2009. [Google Scholar]
- Zhao, L.; Jiang, X.W.; Bian, J.W. Process conditions for L-lactic acid production by corncob-immobilized Rhizopus oryzae. Food Sci. 2013, 34, 201–205. [Google Scholar]
- Luongo, V.; Policastro, G.; Ghimire, A.; Pirozzi, F.F.M. Repeated-batch fermentation of cheese whey for semi-continuous lactic acid production using mixed cultures at uncontrolled pH. Sustainability 2019, 11, 3330. [Google Scholar] [CrossRef]
- Radosavljevic, M.; Levic, S.; Belovic, M.; Pejin, J.; Djukic-Vukovic, A.; Mojovic, L.; Nedovic, V. Immobilization of Lactobacillus rhamnosus in polyvinyl alcohol/calcium alginate matrix for production of lactic acid. Bioproc. Biosyst. Eng. 2020, 43, 315–322. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, Y.; Zhang, J.; Pan, J. Using tobacco waste extract in pre-culture medium to improve xylose utilization for L-lactic acid production from cellulosic waste by Rhizopus oryzae. Bioresour. Technol. 2016, 218, 344–350. [Google Scholar] [CrossRef]
- Danner, H.; Madzingaidzo, L.; Thomasser, C.; Neureiter, M.; Braun, R. Thermophilic production of Lactic acid using integrated membrane bioreactor systems coupled with monopolar electrodialysis. Appl. Microbiol. Biotechnol. 2002, 59, 160–169. [Google Scholar] [CrossRef]
- Russo, F.; Luongo, V.; Mattei, M.R.; Frunzo, L. Mathematical modeling of metal recovery from E-waste using a dark-fermentation-leaching process. Sci. Rep. 2022, 12, 4274. [Google Scholar] [CrossRef]
- Gadhamshetty, V.; Arudchelvam, Y.; Nirmalakhandan, N.; Johnson, D.C. Modeling dark fermentation for biohydrogen production: ADM1-based model vs. Gompertz model. Int. J. Hydrogen Energy 2010, 35, 479–490. [Google Scholar] [CrossRef]
- Do Nascimento, T.R.; Cavalcante, W.A.; de Oliveira, G.H.D.; Zaiat, M.; Ribeiro, R. Modeling dark fermentation of cheese whey for H2 and n-butyrate production considering the chain elongation perspective. Bioresour. Technol. Rep. 2022, 17, 100940. [Google Scholar] [CrossRef]
- Kim, M.S.; Cho, K.H.; Park, K.H.; Jang, J.; Hahn, J.S. Activation of Haa1 and War1 transcription factors by differential binding of weak acid anions in Saccharomyces cerevisiae. Nucleic Acids Res. 2019, 47, 1211–1224. [Google Scholar] [CrossRef] [PubMed]
- Heredia, M.Y.; Ikeh, M.; Gunasekaran, D.; Conrad, K.A.; Filimonava, S.; Marotta, D.H.; Nobile, C.J.; Rauceo, J.M. An expanded cell wall damage signaling network is comprised of the transcription factors Rlm1 and Sko1 in Candida albicans. PLoS Genet. 2020, 16, e1008908. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).