Antibacterial Mechanism of Dellaglioa algida against Pseudomonas fluorescens and Pseudomonas fragi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell-Free Supernatant and Target Strains
2.2. Basic Antibacterial Experiments of D. algida
2.3. Antibacterial Effects under Various Culture Conditions
2.4. Antibacterial Effects on Biofilm of P. fluorescens and P. fragi
2.5. Antibacterial Effects on Swarming and Swimming of P. fluorescens and P. fragi
2.6. Statistical Analysis
3. Results and Discussions
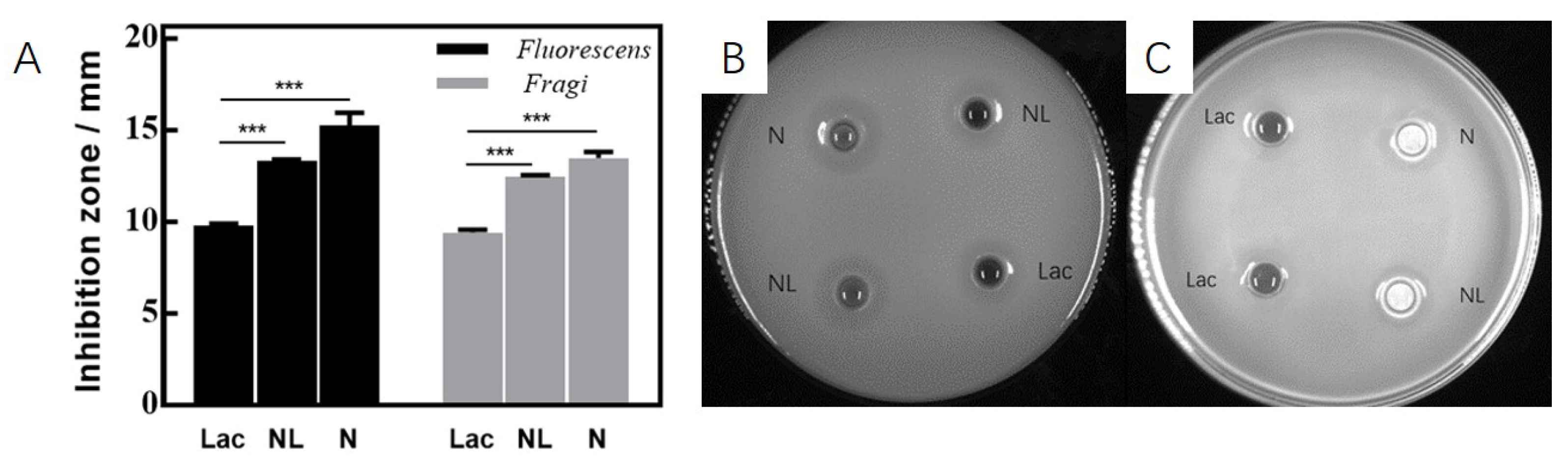
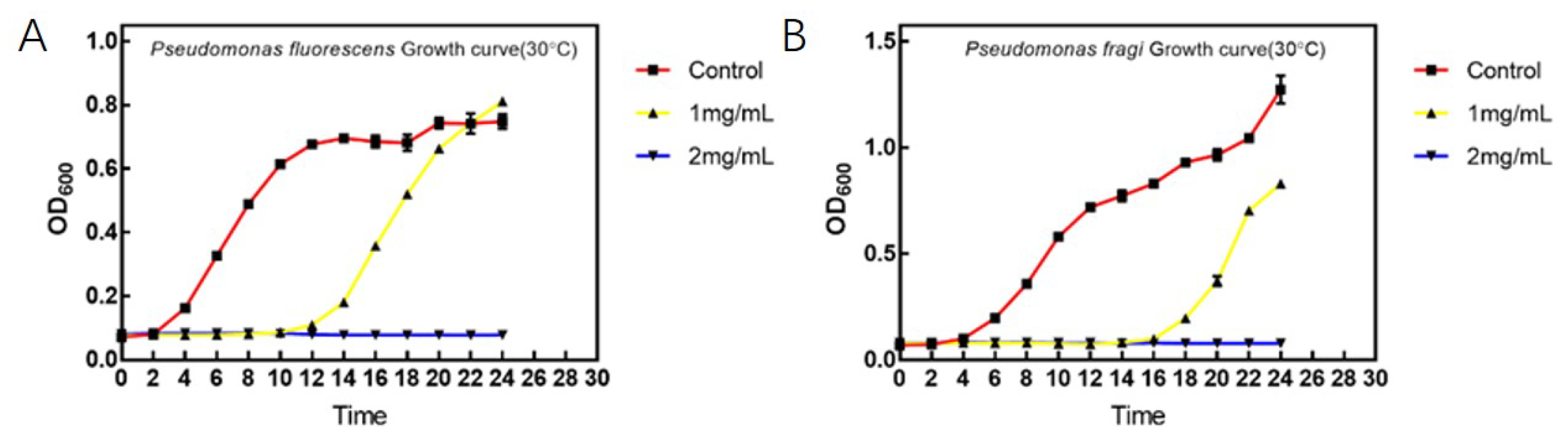
3.1. Basic Antibacterial Experiments of D. algida
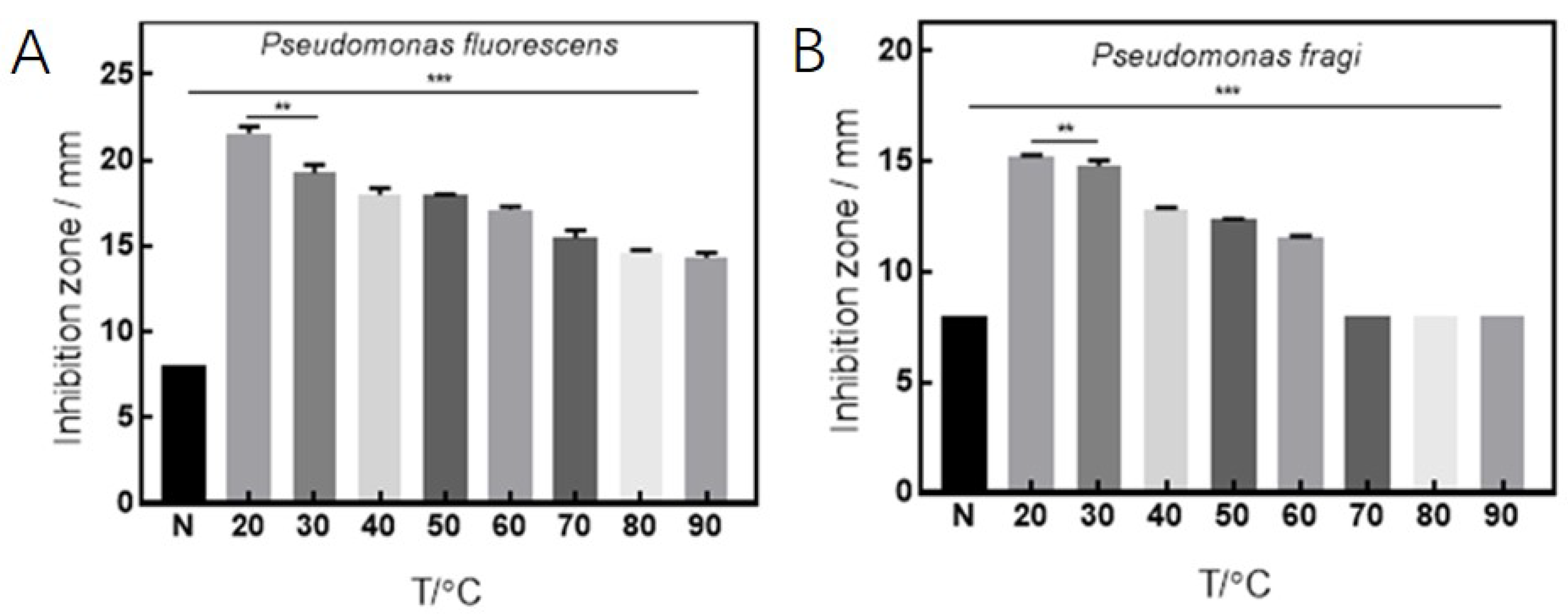
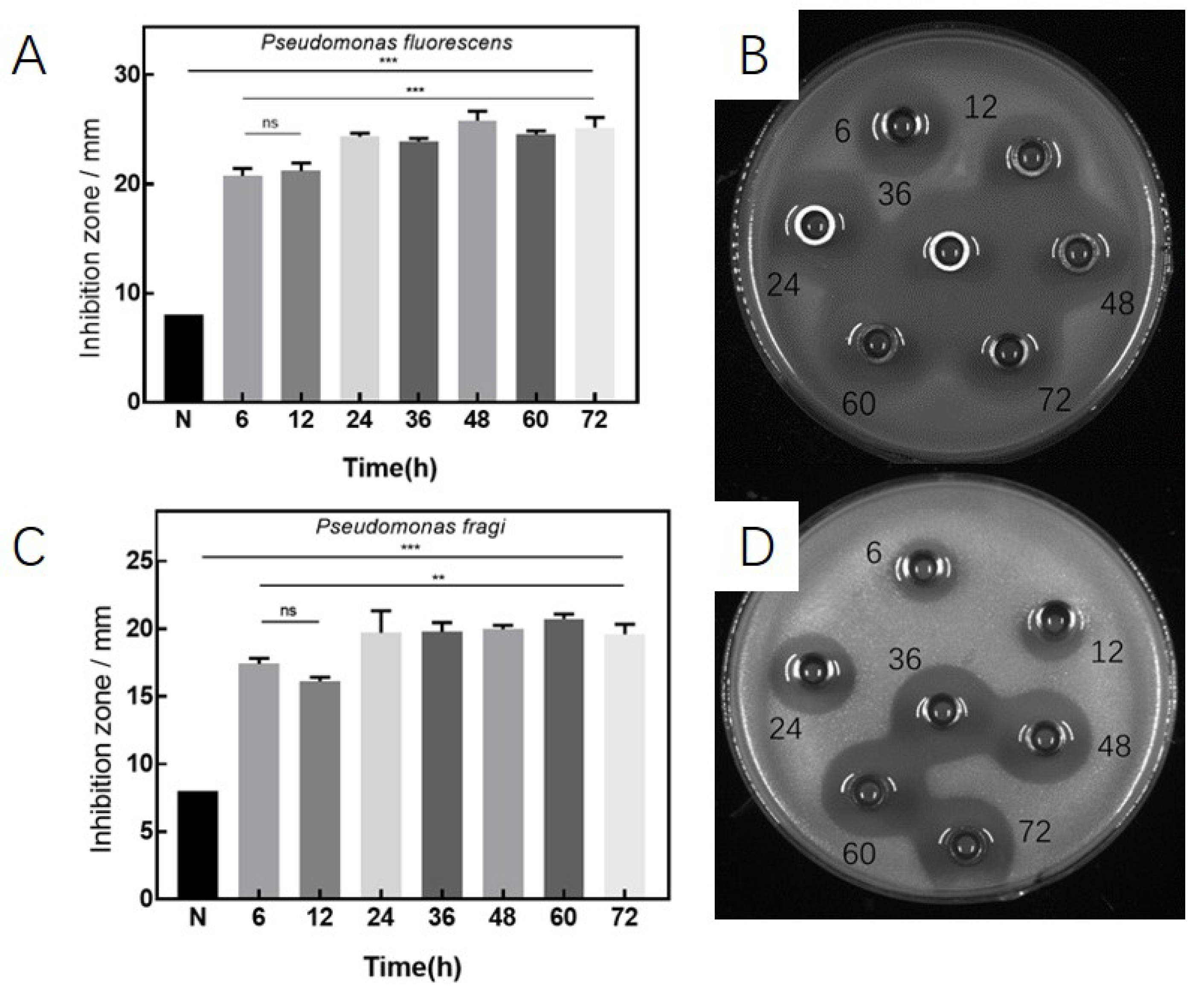
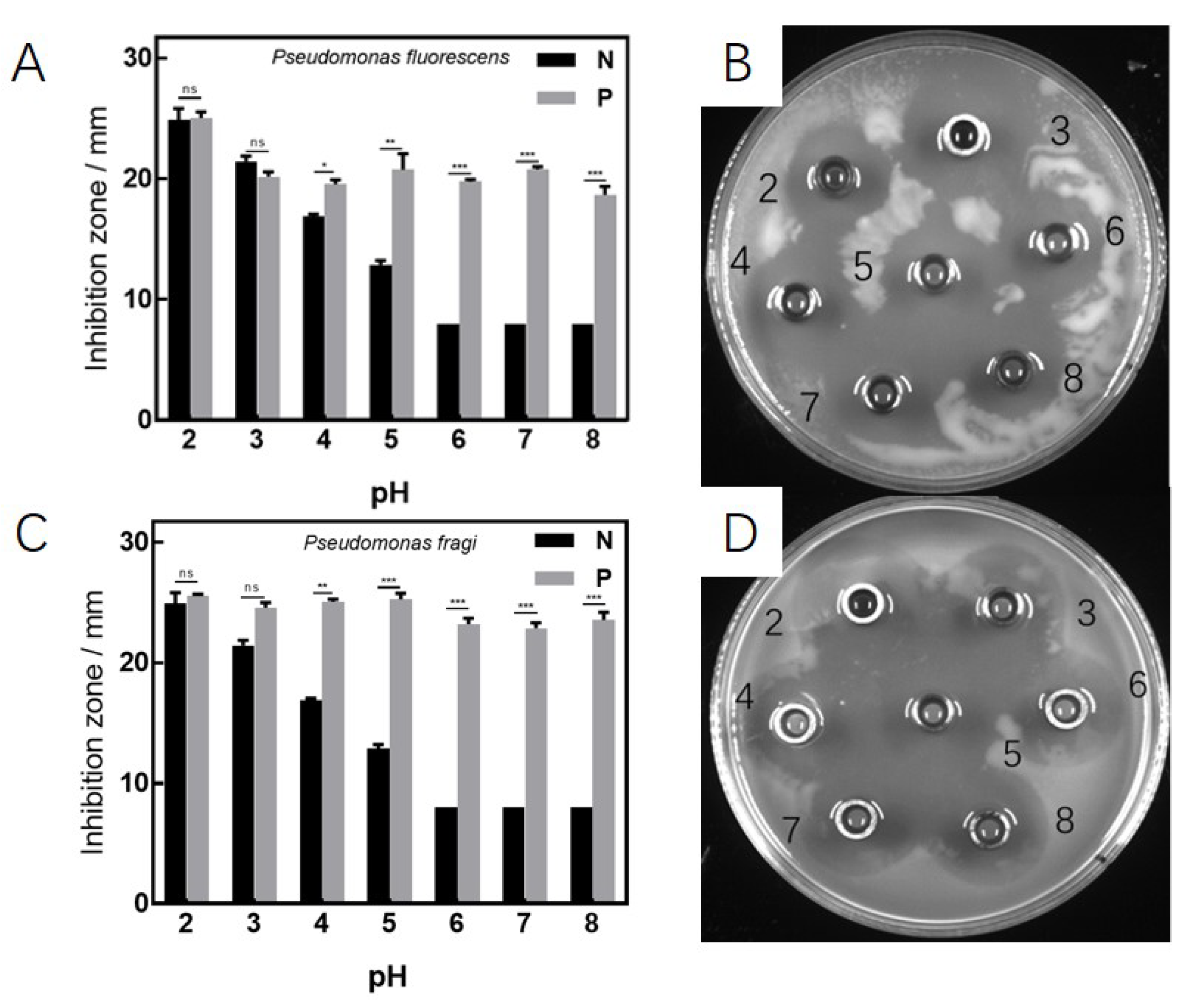
3.2. Antibacterial Effects under Various Culture Conditions
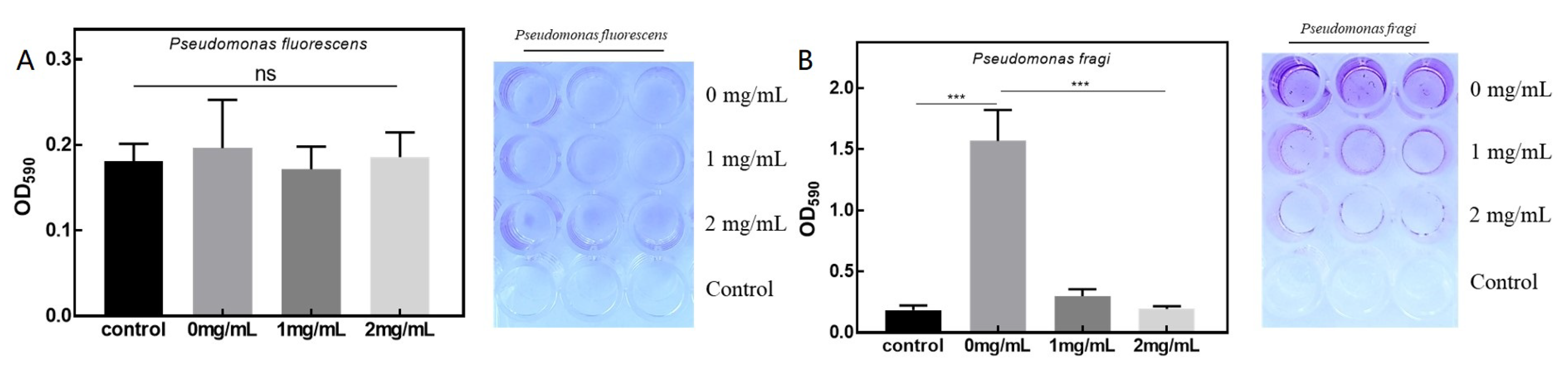
3.3. Antibacterial Effects on Biofilm of P. fluorescens and P. fragi
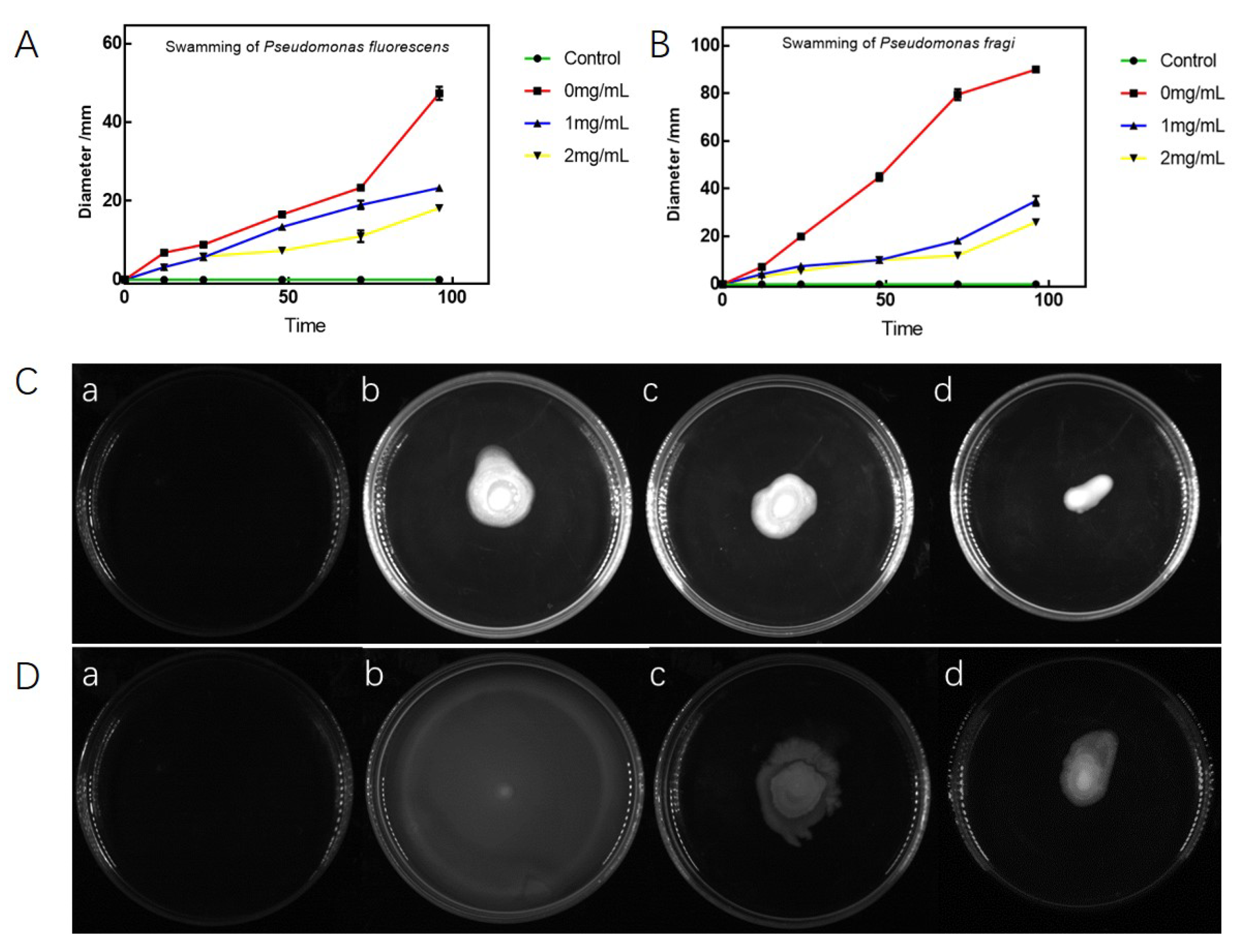
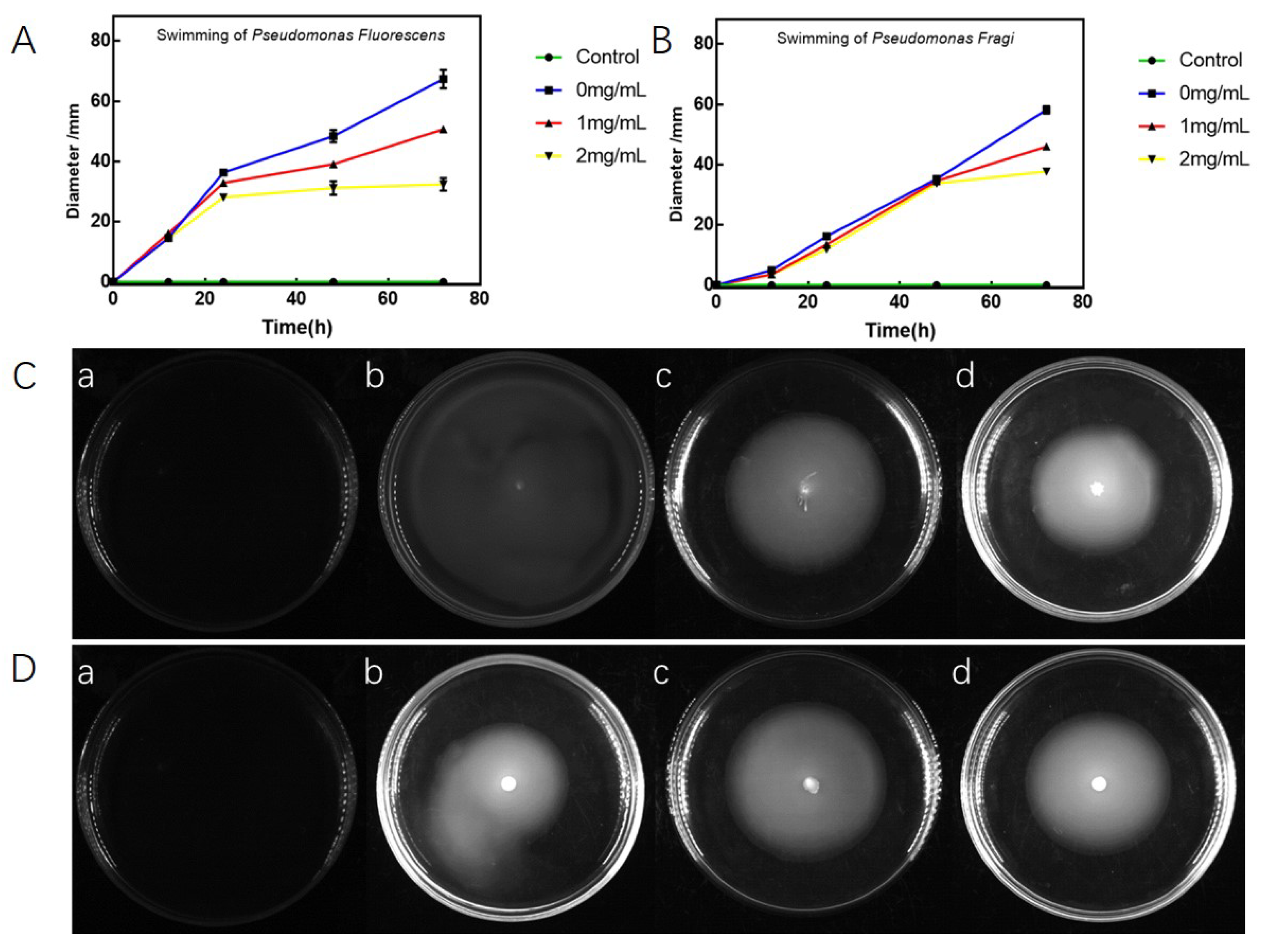
3.4. Antibacterial Effects on Swarming and Swimming of P. fluorescens and P. fragi
3.5. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramos, A.N.; Cabral, M.E.; Noseda, D.; Bosch, A.; Yantorno, O.M.; Valdez, J.C. Antipathogenic properties of Lactobacillus plantarum on Pseudomonas aeruginosa: The potential use of its supernatants in the treatment of infected chronic wounds. Wound Repair Regen. 2012, 20, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Jeyanathan, A.; Ramalhete, R.; Blunn, G.; Gibbs, H.; Pumilia, C.A.; Meckmongkol, T.; Lovejoy, J.; Coathup, M.J. Lactobacillus cell-free supernatant as a novel bioagent and biosurfactant against Pseudomonas aeruginosa in the prevention and treatment of orthopedic implant infection. J. Biomed. Mater. Res. Part B 2021, 109, 1634–1643. [Google Scholar] [CrossRef] [PubMed]
- Dambrauskienė, A.; Adukauskienė, D.; Jeroch, J.; Vitkauskienė, A. Pseudomonas aeruginosa bacteremia: Associations with a source of infection and antibiotic resistance. Med. Lith. 2009, 45, 1. [Google Scholar] [CrossRef] [Green Version]
- Lenzmeier, T.D.; Mudaliar, N.S.; Stanbro, J.A.; Watters, C.; Ahmad, A.; Simons, M.P.; Ventolini, G.; Zak, J.C.; Colmer, H.J.A.; Hamood, A.N. Application of Lactobacillus gasseri 63 AM supernatant to Pseudomonas aeruginosa-infected wounds prevents sepsis in murine models of thermal injury and dorsal excision. J. Med. Microbiol. 2019, 68, 1560–1572. [Google Scholar] [CrossRef]
- Ahmed, N.; Khalid, H.; Mushtaq, M.; Basha, S.; Rabaan, A.A.; Garout, M.; Halwani, M.A.; Al, M.A.; Alhumaid, S.; Al, A.Z.; et al. The molecular characterization of virulence determinants and antibiotic resistance patterns in human bacterial uropathogens. Antibiotics 2022, 11, 516. [Google Scholar] [CrossRef]
- Park, Y.; Koo, S.H. Epidemiology, molecular characteristics, and virulence factors of carbapenem-resistant Pseudomonas aeruginosa isolated from patients with urinary tract infections. Infect. Drug Resist. 2022, 15, 141–151. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Wu, R.Y.; Gui, M.; Jiang, Z.J.; Li, P.L. Identification of the specific spoilage organism in farmed sturgeon (Acipenser baerii) fillets and its associated quality and flavour change during ice storage. Foods 2021, 10, 2021. [Google Scholar] [CrossRef]
- Bellassi, P.; Rocchetti, G.; Morelli, L.; Senizza, B.; Lucini, L.; Cappa, F. A milk foodomics investigation into the effect of Pseudomonas fluorescens growth under cold chain conditions. Foods 2021, 10, 1173. [Google Scholar] [CrossRef]
- Dunstall, G.; Rowe, M.T.; Wisdom, G.B.; Kilpatrick, D. Effect of quorum sensing agents on the growth kinetics of Pseudomonas spp. of raw milk origin. J. Dairy Res. 2005, 72, 276–280. [Google Scholar] [CrossRef]
- Chierici, M.; Picozzi, C.; LaSpina, M.G.; Orsi, C.; Vigentini, I.; Zambrini, V.; Foschino, R. Strain diversity of Pseudomonas fluorescens group with Potential blue pigment phenotype isolated from dairy products. J. Food Prot. 2016, 79, 1430–1435. [Google Scholar] [CrossRef]
- Wang, G.Y.; Wang, H.H.; Han, Y.W.; Xing, T.; Ye, K.P.; Xu, X.L.; Zhou, G.H. Evaluation of the spoilage potential of bacteria isolated from chilled chicken in vitro and in situ. Food Microbiol. 2017, 63, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Almeida, K.M.D.; Bruzaroski, S.R.; Zanol, D.; Melo, M.D.; Santos, J.S.D.; Alegro, L.C.A.; Botaro, B.G.; Santana, E.H.W.D. Pseudomonas spp. and P. fluorescens: Population in refrigerated raw milk. Cienc. Rural 2017, 47, 2017. [Google Scholar] [CrossRef] [Green Version]
- Khailova, L.; Baird, C.H.; Rush, A.A.; McNamee, E.N.; Wischmeyer, P.E. Lactobacillus rhamnosus GG improves outcome in experimental pseudomonas aeruginosa pneumonia: Potential role of regulatory T cells. Shock 2013, 40, 496–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, X.X.; Chen, S.Y.; Duan, S.; Lan, C.M.; Yang, Z.N.; Cao, Y.; Miao, J.Y. Antibiotic activities of the natural antimicrobial substance produced by Lactobacillus paracasei FX-6 against Pseudomonas putida. LWT-Food Sci. Technol. 2020, 123, 109096. [Google Scholar] [CrossRef]
- Duan, X.X.; Duan, S.; Wang, Q.E.; Ji, R.; Cao, Y.; Miao, J.Y. Effects of the natural antimicrobial substance from Lactobacillus paracasei FX-6 on shelf life and microbial composition in chicken breast during refrigerated storage. Food Control 2020, 109, 106906. [Google Scholar] [CrossRef]
- Forestier, C.; Guelon, D.; Cluytens, V.; Gillart, T.; Sirot, J.; DeChamps, C. Oral probiotic and prevention of Pseudomonas aeruginosa infections: A randomized, double-blind, placebo-controlled pilot study in intensive care unit patients. Crit. Care 2008, 12, R69. [Google Scholar] [CrossRef] [Green Version]
- Alexandre, Y.; LeBerre, R.; Barbier, G.; LeBlay, G. Screening of Lactobacillus spp. for the prevention of Pseudomonas aeruginosa pulmonary infections. BMC Microbiol. 2014, 14, 107. [Google Scholar] [CrossRef] [Green Version]
- Valdez, J.C.; Peral, M.C.; Rachid, M.; Santana, M.; Perdigon, G. Interference of Lactobacillus plantarum with Pseudomonas aeruginosa in vitro and in infected burns: The potential use of probiotics in wound treatment. Clin. Microbiol. Infect. 2005, 11, 472–479. [Google Scholar] [CrossRef] [Green Version]
- Scillato, M.; Spitale, A.; Mongelli, G.; Privitera, G.F.; Mangano, K.; Cianci, A.; Stefani, S.; Santagati, M. Antimicrobial properties of Lactobacillus cell-free supernatants against multidrug-resistant urogenital pathogens. MicrobiologyOpen 2021, 10, e1173. [Google Scholar] [CrossRef]
- Wasfi, R.; AbdElRahman, O.A.; Zafer, M.M.; Ashour, H.M. Probiotic Lactobacillus sp. inhibit growth, biofilm formation and gene expression of caries-inducing Streptococcus mutans. J. Cell. Mol. Med. 2018, 22, 1972–1983. [Google Scholar] [CrossRef] [Green Version]
- Kato, Y.; Sakala, R.M.; Hayashidani, H.; Kiuchi, A.; Kaneuchi, C.; Ogawa, M. Lactobacillus algidus sp. nov., a psychrophilic lactic acid bacterium isolated from vacuum-packaged refrigerated beef. Int. J. Syst. Evol. Microbiol. 2000, 50, 1143–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J.S.; Wittouck, S.; Salvetti, E.; Franz, C.M.; Harris, H.; Mattarelli, P.; OToole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.H.; Harris, H.; McCann, A.; Guo, C.Y.; Argimón, S.; Zhang, W.Y.; Yang, X.W.; Jeffery, I.B.; Cooney, J.C.; Kagawa, T.F.; et al. Expanding the biotechnology potential of lactobacilli through comparative genomics of 213 strains and associated genera. Nat. Commun. 2015, 6, 8322. [Google Scholar] [CrossRef] [PubMed]
- Poirier, S.; Coeuret, G.; Champomier, V.M.C.; Chaillou, S. Draft genome sequences of nine strains of Brochothrix thermosphacta, Carnobacterium divergens, Lactobacillus algidus, Lactobacillus fuchuensis, Lactococcus piscium, Leuconostoc gelidum subsp. gasicomitatum, Pseudomonas lundensis, and Weissella viridescens, a collection of Psychrotrophic species involved in meat and seafood spoilage. Genome Announc. 2018, 6, e00479-18. [Google Scholar]
- Säde, E.; Johansson, P.; Heinonen, T.; Hultman, J.; Björkroth, J. Growth and metabolic characteristics of fastidious meat-derived Lactobacillus algidus strains. Int. J. Food Microbiol. 2020, 313, 108379. [Google Scholar] [CrossRef]
- Okaro, U.; Green, R.; Mohapatra, S.; Anderson, B. The trimeric autotransporter adhesin BadA is required for in vitro biofilm formation by Bartonella henselae. NPJ Biofilms Microbiomes. 2019, 5, 10. [Google Scholar] [CrossRef] [Green Version]
- Matsuo, S.; Wada, K.; Sadahira, T.; Mitsuhata, R.; Nasu, Y. Impact of Lactobacillus probiotics on biofilm formed by Pseudomonas aeruginosa. Eur. Urol. Suppl. 2019, 18, e548. [Google Scholar] [CrossRef]
- Wickramasinghe, N.N.; Hlaing, M.M.; Ravensdale, J.T.; Coorey, R.; Chandry, P.S.; Dykes, G.A. Characterization of the biofilm matrix composition of psychrotrophic, meat spoilage pseudomonads. Sci. Rep. 2020, 10, 16457. [Google Scholar] [CrossRef]
- Hossain, M.I.; Mizan, M.F.R.; Roy, P.K.; Nahar, S.; Toushik, S.H.; Ashrafudoulla, M.; Jahid, I.K.; Lee, J.; Ha, S.D. Listeria monocytogenes biofilm inhibition on food contact surfaces by application of postbiotics from Lactobacillus curvatus B.67 and Lactobacillus plantarum M.2. Food Res. Int. 2021, 148, 110595. [Google Scholar] [CrossRef]
- Wickramasinghe, N.N.; Ravensdale, J.; Coorey, R.; Dykes, G.A.; Chandry, P.S. Transcriptional profiling of biofilms formed on chilled beef by psychrotrophic meat spoilage bacterium, Pseudomonas fragi 1793. Biofilm 2021, 3, 100045. [Google Scholar] [CrossRef]
- Cui, T.Q.; Bai, F.L.; Sun, M.T.; Lv, X.R.; Li, X.P.; Zhang, D.F.; Du, H. Lactobacillus crustorum ZHG 2-1 as novel quorum-quenching bacteria reducing virulence factors and biofilms formation of Pseudomonas aeruginosa. LWT-Food Sci. Technol. 2020, 117, 108696. [Google Scholar] [CrossRef]
- Layus, B.I.; Gerez, C.L.; Rodriguez, A.V. Antibacterial activity of Lactobacillus plantarum CRL 759 against methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa. Arab. J. Sci. Eng. 2020, 45, 4503–4510. [Google Scholar] [CrossRef]
- deAlcântara, A.L.D.A.; Bruzaroski, S.R.; Luiz, L.L.; de Souza, C.H.B.; Poli-Frederico, R.C.; Fagnani, R.; deSantana, E.H.W. Antimicrobial activity of Lactobacillus rhamnosus against Pseudomonas fluorescens and Pseudomonas putida from raw milk. J. Food Process. Preserv. 2019, 43, e14082. [Google Scholar]
- Dai, M.; Li, Y.; Xu, L.; Wu, D.; Zhou, Q.; Li, P.; Gu, Q. A novel bacteriocin from Lactobacillus pentosus ZFM94 and its antibacterial mode of action. Front. Nutr. 2021, 8, 710862. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Ma, H.; Sun, M.; Lin, Y.; Bai, F.; Li, J.; Zhang, B. A novel bacteriocin DY4-2 produced by Lactobacillus plantarum from cutlassfish and its application as bio-preservative for the control of Pseudomonas fluorescens in fresh turbot (Scophthalmus maximus) fillets. Food Control 2018, 89, 22–31. [Google Scholar] [CrossRef]
- Aman, M.; Aneeqha, N.; Bristi, K.; Deeksha, J.; Afza, N.; Sindhuja, V.; Shastry, R.P. Lactic acid bacteria inhibits quorum sensing and biofilm formation of Pseudomonas aeruginosa strain JUPG01 isolated from rancid butter. Biocatal. Agric. Biotechnol. 2021, 36, 102115. [Google Scholar] [CrossRef]
- El-Mokhtar, M.A.; Hassanein, K.M.; Ahmed, A.S.; Gad, G.F.M.; Amin, M.M.; Hassanein, O.F.E. Antagonistic activities of cell-free supernatants of lactobacilli against extended-spectrum β-lactamase producing Klebsiella pneumoniae and Pseudomonas aeruginosa. Infect. Drug Resist. 2020, 13, 543. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.M.; Kim, J.S.; Kim, H.S.; Kim, Y.Y.; Oh, J.K.; Jung, H.W.; Park, D.S.; Bae, K.H. Lactobacillus reuteri AN417 cell-free culture supernatant as a novel antibacterial agent targeting oral pathogenic bacteria. Sci. Rep. 2021, 11, 1631. [Google Scholar] [CrossRef]
- Liu, Y.; Bu, Y.; Li, J.; Liu, Y.; Liu, A.; Gong, P.; Liu, T.; Zhang, L.; Wang, S.; Yi, H. Inhibition activity of plantaricin Q7 produced by Lactobacillus plantarum Q7 against Listeria monocytogenes and its biofilm. Fermentation. 2022, 8, 75. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Zhou, Q.Q.; Li, P.; Gu, Q. Purification, characterization, and mode of action of Paracin 54, a novel bacteriocin against Staphylococci. Appl. Microbiol. Biotechnol. 2021, 105, 6735–6748. [Google Scholar] [CrossRef]
- Marques, V.D.; Franzolin, M.R.; Sanabani, S.S.; Vigerelli, H.; Piazza, R.M.F.; Pimenta, D.C.; Venâncio, T.; Neves, I.V.; deSousa, S.H.G.; dosSantos, C.D.; et al. A new class of antimicrobial molecules derived from kefir, effective againstPseudomonas aeruginosaand methicillin resistantStaphylococcus aureus (MRSA) strains. Sci. Rep. 2020, 10, 17434. [Google Scholar] [CrossRef] [PubMed]
- Chappell, T.C.; Nair, N.U. Engineered lactobacilli display anti-biofilm and growth suppressing activities against Pseudomonas aeruginosa. npj Biofilms Microbomes. 2020, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Vuotto, C.; Longo, F.; Donelli, G. Probiotics to counteract biofilm-associated infections: Promising and conflicting data. Int. J. Oral Sci. 2014, 6, 189–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhubail, M.; O’Neill, C.A.; Mcbain, A.; Cruickshank, S. The spent culture fluid of Bifidobacterium longum, Lactobacillus rhamnosus GG and Escherichia coli Nissle 1917 inhibits the growth and biofilm formation of Pseudomonas aeruginosa. J. Investig. Dermatol. 2019, 139, S329. [Google Scholar] [CrossRef] [Green Version]




| Composition | Content | Composition | Content |
|---|---|---|---|
| Peptone | 10.00 g | Triammonium citrate | 2.00 g |
| Beef paste | 10.00 g | Dipotassium hydrogen phosphate | 2.00 g |
| Yeast powder | 4.00 g | Manganese sulfate | 0.04 g |
| Glucose | 20.00 g | Tween-80 | 1.00 g |
| Magnesium sulfate | 0.20 g | Water | 1.00 L |
| Sodium acetate | 5.00 g | pH | 5.70 ± 0.20 |
| Group | P. fluorescens | P. fragi | ||
|---|---|---|---|---|
| Control | Treatment | Control | Treatment | |
| Proteinase K | 22.625 ± 0.072 * | 17.703 ± 0.395 | 22.355 ± 0.757 * | 10.625 ± 0.022 |
| Trypsin | 22.694 ± 1.620 | 21.045 ± 0.072 | 23.697 ± 0.985 * | 17.634 ± 0.548 |
| Papain | 21.965 ±0.721 | 20.926 ± 0.371 | 25.021 ± 0.003 * | 16.583 ± 0.021 |
| -Amylase | 21.683 ± 0.076 | 21.633 ± 0.016 | 25.470 ± 0.368 | 25.750 ± 0.013 |
| Lipase | 21.866 ± 0.061 | 21.454 ± 0.045 | 24.863 ± 0.697 | 24.637 ± 0.036 |
| Catalase | 22.701 ± 0.003 | 22.407 ± 0.004 | 26.131 ± 0.051 | 25.189 ± 0.095 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Liu, S.; Zhan, Z.; Wei, T.; Ma, T.; Sun, J.; Song, J. Antibacterial Mechanism of Dellaglioa algida against Pseudomonas fluorescens and Pseudomonas fragi. Fermentation 2022, 8, 298. https://doi.org/10.3390/fermentation8070298
Sun Y, Liu S, Zhan Z, Wei T, Ma T, Sun J, Song J. Antibacterial Mechanism of Dellaglioa algida against Pseudomonas fluorescens and Pseudomonas fragi. Fermentation. 2022; 8(7):298. https://doi.org/10.3390/fermentation8070298
Chicago/Turabian StyleSun, Yao, Shiyu Liu, Zhe Zhan, Tianhui Wei, Tongqing Ma, Jie Sun, and Jinzhu Song. 2022. "Antibacterial Mechanism of Dellaglioa algida against Pseudomonas fluorescens and Pseudomonas fragi" Fermentation 8, no. 7: 298. https://doi.org/10.3390/fermentation8070298
APA StyleSun, Y., Liu, S., Zhan, Z., Wei, T., Ma, T., Sun, J., & Song, J. (2022). Antibacterial Mechanism of Dellaglioa algida against Pseudomonas fluorescens and Pseudomonas fragi. Fermentation, 8(7), 298. https://doi.org/10.3390/fermentation8070298






