Hydrogen and Methane Production from Anaerobic Co-Digestion of Sorghum and Cow Manure: Effect of pH and Hydraulic Retention Time
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrates
2.1.1. Types and Solutions of Sorghum
2.1.2. Liquid Cow Manure
2.2. Alkaline Pretreatment
2.3. Reactor Configuration
2.3.1. Effect of pH
2.3.2. Effect of HRT
2.4. Reactor Startup and Operation
2.4.1. Effect of pH
2.4.2. Effect of HRT
2.5. Analytical Methods
2.6. Bacterial Growth Model
3. Results and Discussion
3.1. Chemical Composition of Each Substrate
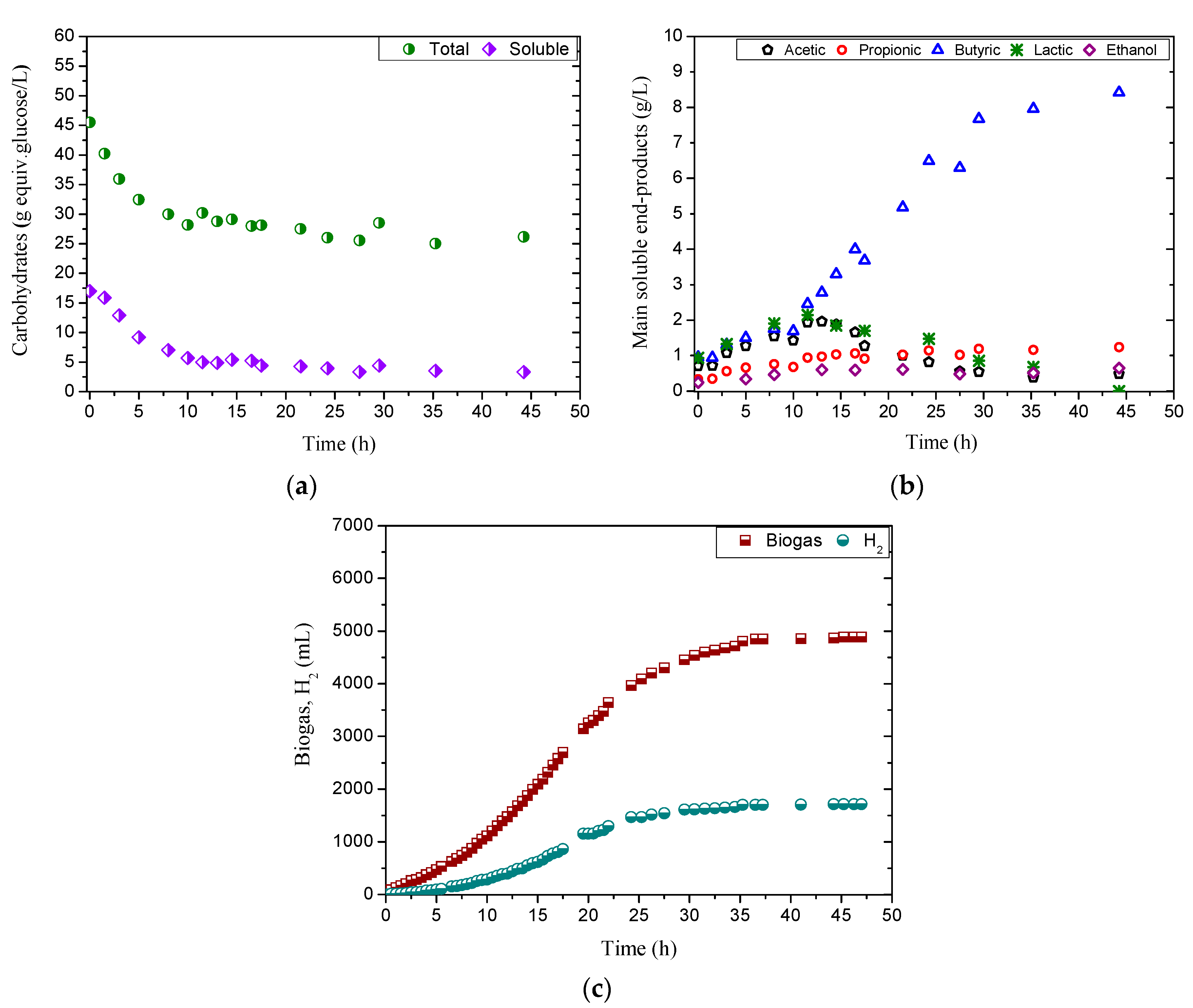
3.2. Effect of pH in Acidogenic Reactor
3.3. Kinetic Analysis
3.4. Effect of ES on Hydrogen Production
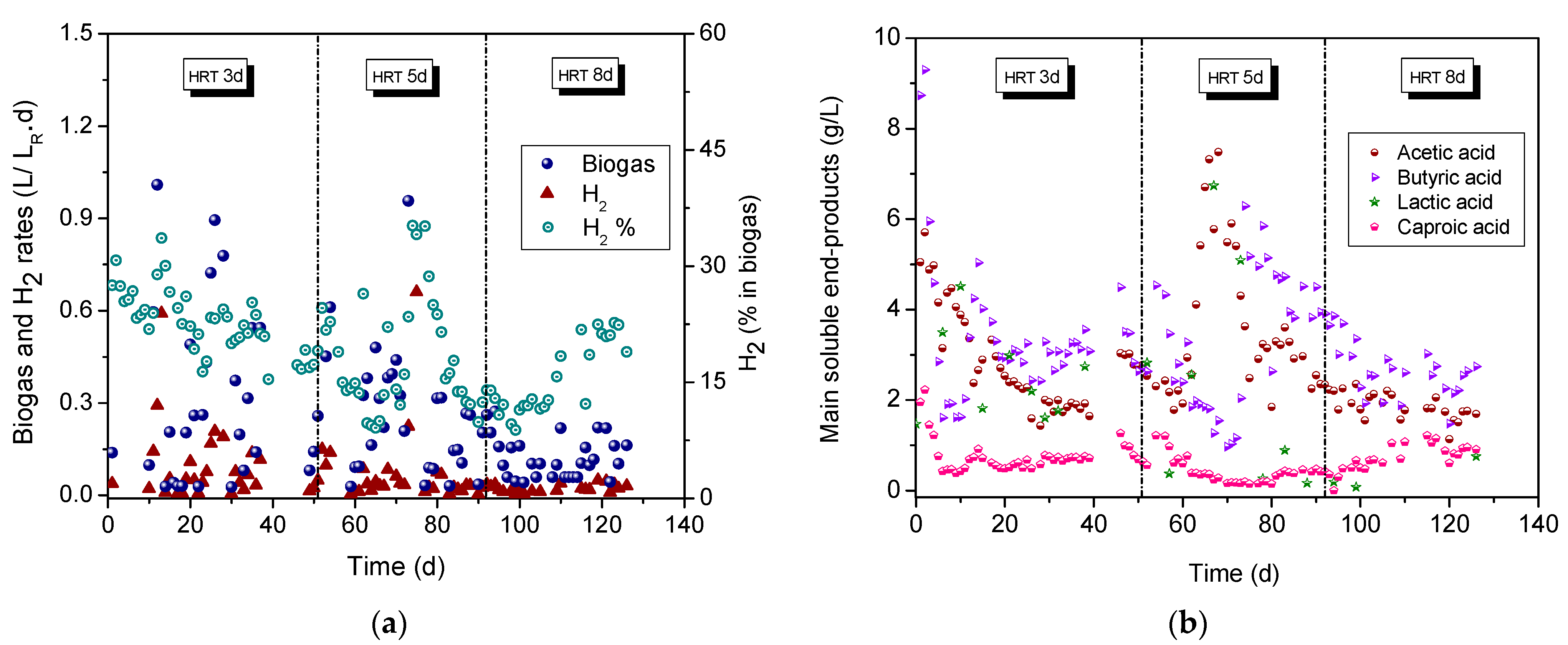
3.5. CSTR Acidogenic Reactor: Effect of HRT
3.6. CSTR Methanogenic Reactor: Effect of HRT
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roy, S.; Dikshit, P.K.; Sherpa, K.C.; Singh, A.; Jacob, S.; Rajak, R.C. Recent nanobiotechnological advancements in lignocellulosic biomass valorization: A review. J. Environ. Manag. 2021, 297, 113422. [Google Scholar] [CrossRef]
- Aravani, V.P.; Sun, H.; Yang, Z.; Liu, G.; Wang, W.; Anagnostopoulos, G.; Syriopoulos, G.; Charisiou, N.D.; Goula, M.A.; Kornaros, M.; et al. Agricultural and livestock sector’s residues in Greece & China: Comparative qualitative and quantitative characterization for assessing their potential for biogas production. Renew. Sustain. Energy Rev. 2022, 154, 111821. [Google Scholar] [CrossRef]
- Okolie, J.A.; Epelle, E.I.; Tabat, M.E.; Orivri, U.; Amenaghawon, A.N.; Okoye, P.U.; Gunes, B. Waste biomass valorization for the production of biofuels and value-added products: A comprehensive review of thermochemical, biological and integrated processes. Process Saf. Environ. Prot. 2022, 159, 323–344. [Google Scholar] [CrossRef]
- Aravani, V.P.; Tsigkou, K.; Papadakis, V.G.; Kornaros, M. Biochemical Μethane potential of most promising agricultural residues in Northern and Southern Greece. Chemosphere 2022, 296, 133985. [Google Scholar] [CrossRef]
- Piñas, J.A.V.; Venturini, O.J.; Lora, E.E.S.; del Olmo, O.A.; Roalcaba, O.D.C. An economic holistic feasibility assessment of centralized and decentralized biogas plants with mono-digestion and co-digestion systems. Renew. Energy 2019, 139, 40–51. [Google Scholar] [CrossRef]
- Schievano, A.; Tenca, A.; Lonati, S.; Manzini, E.; Adani, F. Can two-stage instead of one-stage anaerobic digestion really increase energy recovery from biomass? Appl. Energy 2014, 124, 335–342. [Google Scholar]
- Dareioti, M.A.; Vavouraki, A.I.; Tsigkou, K.; Kornaros, M. Assessment of Single- vs. Two-Stage Process for the Anaerobic Digestion of Liquid Cow Manure and Cheese Whey. Energies 2021, 14, 5423. [Google Scholar] [CrossRef]
- Tsigkou, K.; Tsafrakidou, P.; Kopsahelis, A.; Zagklis, D.; Zafiri, C.; Kornaros, M. Used disposable nappies and expired food products valorisation through one- & two-stage anaerobic co-digestion. Renew. Energy 2020, 147, 610–619. [Google Scholar] [CrossRef]
- Srisowmeya, G.; Chakravarthy, M.; Devi, G.N. Critical considerations in two-stage anaerobic digestion of food waste—A review. Renew. Sustain. Energy Rev. 2019, 119, 109587. [Google Scholar] [CrossRef]
- Cremonez, P.A.; Teleken, J.G.; Meier, T.R.W.; Alves, H.J. Two-Stage anaerobic digestion in agroindustrial waste treatment: A review. J. Environ. Manag. 2021, 281, 111854. [Google Scholar] [CrossRef]
- Hans, M.; Kumar, S. Biohythane production in two-stage anaerobic digestion system. Int. J. Hydrog. Energy 2019, 44, 17363–17380. [Google Scholar] [CrossRef]
- Tsigkou, K.; Sakarika, M.; Kornaros, M. Inoculum origin and waste solid content influence the biochemical methane potential of olive mill wastewater under mesophilic and thermophilic conditions. Biochem. Eng. J. 2019, 151, 107301. [Google Scholar] [CrossRef]
- Tsigkou, K.; Sivolapenko, N.; Kornaros, M. Thermophilic Dark Fermentation of Olive Mill Wastewater in Batch Reactors: Effect of pH and Organic Loading. Appl. Sci. 2022, 12, 2881. [Google Scholar] [CrossRef]
- Tsigkou, K.; Tsafrakidou, P.; Athanasopoulou, S.; Zafiri, C.; Kornaros, M. Effect of pH on the Anaerobic Fermentation of Fruit/Vegetables and Disposable Nappies Hydrolysate for Bio-hydrogen Production. Waste Biomass Valorization 2020, 11, 539–551. [Google Scholar] [CrossRef]
- Zakoura, M.; Kopsahelis, A.; Tsigkou, K.; Ntougias, S.; Ali, S.S.; Kornaros, M. Performance evaluation of three mesophilic upflow anaerobic sludge blanket bioreactors treating olive mill wastewater: Flocculent and granular inocula tests, organic loading rate effect and anaerobic consortia structure. Fuel 2022, 313, 122951. [Google Scholar] [CrossRef]
- Tsigkou, K.; Kornaros, M. Development of a high-rate anaerobic thermophilic upflow packed bed reactor for efficient bioconversion of diluted three-phase olive mill wastewater into methane. Fuel 2022, 310, 122263. [Google Scholar] [CrossRef]
- Tsigkou, K.; Zagklis, D.; Tsafrakidou, P.; Zapanti, P.; Manthos, G.; Karamitou, K.; Zafiri, C.; Kornaros, M. Expired food products and used disposable adult nappies mesophilic anaerobic co-digestion: Biochemical methane potential, feedstock pretreatment and two-stage system performance. Renew. Energy 2021, 168, 309–318. [Google Scholar] [CrossRef]
- Zagklis, D.; Papadionysiou, M.; Tsigkou, K.; Tsafrakidou, P.; Zafiri, C.; Kornaros, M. Effect of pH on the Economic Potential of Dark Fermentation Products from Used Disposable Nappies and Expired Food Products. Appl. Sci. 2021, 11, 4099. [Google Scholar] [CrossRef]
- Ghimire, A.; Frunzo, L.; Pirozzi, F.; Trably, E.; Escudie, R.; Lens, P.N.L.; Esposito, G. A review on dark fermentative biohydrogen production from organic biomass: Process parameters and use of by-products. Appl. Energy 2015, 144, 73–95. [Google Scholar] [CrossRef]
- Antonopoulou, G.; Lyberatos, G. Effect of Pretreatment of Sweet Sorghum Biomass on Methane Generation. Waste Biomass Valorization 2013, 4, 583–591. [Google Scholar] [CrossRef]
- Carrère, H.; Dumas, C.; Battimelli, A.; Batstone, D.J.; Delgenès, J.P.; Steyer, J.-P.; Ferrer, I. Pretreatment methods to improve sludge anaerobic degradability: A review. J. Hazard. Mater. 2010, 183, 1–15. [Google Scholar] [CrossRef]
- Giuliano, A.; Bolzonella, D.; Pavan, P.; Cavinato, C.; Cecchi, F. Co-digestion of livestock effluents, energy crops and agro-waste: Feeding and process optimization in mesophilic and thermophilic conditions. Bioresour. Technol. 2013, 128, 612–618. [Google Scholar] [CrossRef]
- Appels, L.; Lauwers, J.; Degrève, J.; Helsen, L.; Lievens, B.; Willems, K.; Van Impe, J.; Dewil, R. Anaerobic digestion in global bio-energy production: Potential and research challenges. Renew. Sustain. Energy Rev. 2011, 15, 4295–4301. [Google Scholar] [CrossRef]
- Atandi, E.; Rahman, S. Prospect of anaerobic co-digestion of dairy manure: A review. Environ. Technol. Rev. 2012, 1, 127–135. [Google Scholar] [CrossRef]
- Zhong, Y.; Chen, R.; Rojas-Sossa, J.P.; Isaguirre, C.; Mashburn, A.; Marsh, T.; Liu, Y.; Liao, W. Anaerobic co-digestion of energy crop and agricultural wastes to prepare uniform-format cellulosic feedstock for biorefining. Renew. Energy 2020, 147, 1358–1370. [Google Scholar] [CrossRef]
- Schmidt, A.; Lemaigre, S.; Delfosse, P.; von Francken-Welz, H.; Emmerling, C. Biochemical methane potential (BMP) of six perennial energy crops cultivated at three different locations in W-Germany. Biomass Convers. Biorefin. 2018, 8, 873–888. [Google Scholar] [CrossRef]
- Koçar, G.; Civaş, N. An overview of biofuels from energy crops: Current status and future prospects. Renew. Sustain. Energy Rev. 2013, 28, 900–916. [Google Scholar] [CrossRef]
- Dareioti, M.A.; Vavouraki, A.I.; Tsigkou, K.; Zafiri, C.; Kornaros, M. Dark Fermentation of Sweet Sorghum Stalks, Cheese Whey and Cow Manure Mixture: Effect of pH, Pretreatment and Organic Load. Processes 2021, 9, 1017. [Google Scholar] [CrossRef]
- Antonopoulou, G.; Gavala, H.N.; Skiadas, I.V.; Angelopoulos, K.; Lyberatos, G. Biofuels generation from sweet sorghum: Fermentative hydrogen production and anaerobic digestion of the remaining biomass. Bioresour. Technol. 2008, 99, 110–119. [Google Scholar] [CrossRef]
- Matsakas, L.; Rova, U.; Christakopoulos, P. Evaluation of Dried Sweet Sorghum Stalks as Raw Material for Methane Production. BioMed Res. Int. 2014, 2014, 1–7. [Google Scholar] [CrossRef][Green Version]
- Ma, H.; Hu, Y.; Kobayashi, T.; Xu, K.-Q. The role of rice husk biochar addition in anaerobic digestion for sweet sorghum under high loading condition. Biotechnol. Rep. 2020, 27, e00515. [Google Scholar] [CrossRef]
- Nozari, B.; Mirmohamadsadeghi, S.; Karimi, K. Bioenergy production from sweet sorghum stalks via a biorefinery perspective. Appl. Microbiol. Biotechnol. 2018, 102, 3425–3438. [Google Scholar] [CrossRef]
- Dareioti, M.A.; Kornaros, M. Anaerobic mesophilic co-digestion of ensiled sorghum, cheese whey and liquid cow manure in a two-stage CSTR system: Effect of hydraulic retention time. Bioresour. Technol. 2015, 175, 553–562. [Google Scholar] [CrossRef]
- Sarker, S.; Lamb, J.J.; Hjelme, D.R.; Lien, K.M. A Review of the Role of Critical Parameters in the Design and Operation of Biogas Production Plants. Appl. Sci. 2019, 9, 1915. [Google Scholar] [CrossRef]
- Mullet, J.; Morishige, D.; McCormick, R.; Truong, S.; Hilley, J.; Mckinley, B.; Anderson, R.; Olson, S.N.; Rooney, W. Energy Sorghum—A genetic model for the design of C4 grass bioenergy crops. J. Exp. Bot. 2014, 65, 3479–3489. [Google Scholar] [CrossRef]
- Padel, S.; Röcklinsberg, H.; Schmid, O. The implementation of organic principles and values in the European Regulation for organic food. Food Policy 2009, 34, 245–251. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Dareioti, M.A.; Kornaros, M. Effect of hydraulic retention time (HRT) on the anaerobic co-digestion of agro-industrial wastes in a two-stage CSTR system. Bioresour. Technol. 2014, 167, 407–415. [Google Scholar] [CrossRef]
- Eaton, A.D.; Clesceri, L.S.; Greenberg, A.E.; Franson, M.A.H. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; APHA: Washington, DC, USA, 2012. [Google Scholar]
- Dareioti, M.A.; Vavouraki, A.I.; Kornaros, M. Effect of pH on the anaerobic acidogenesis of agroindustrial wastewaters for maximization of bio-hydrogen production: A lab-scale evaluation using batch tests. Bioresour. Technol. 2014, 162, 218–227. [Google Scholar] [CrossRef]
- Yasin, N.H.M.; Rahman, N.A.; Man, H.C.; Yusoff, M.Z.M.; Hassan, M.A. Microbial characterization of hydrogen-producing bacteria in fermented food waste at different pH values. Int. J. Hydrogen Energy 2011, 36, 9571–9580. [Google Scholar] [CrossRef]
- Xu, Y.; Li, J.; Moore, C.; Xin, Z.; Wang, D. Physico-chemical characterization of pedigreed sorghum mutant stalks for biofuel production. Ind. Crop. Prod. 2018, 124, 806–811. [Google Scholar] [CrossRef]
- Usman, M.; Kavitha, S.; Kannah, Y.; Yogalakshmi, K.N.; Sivashanmugam, P.; Bhatnagar, A.; Kumar, G. A critical review on limitations and enhancement strategies associated with biohydrogen production. Int. J. Hydrogen Energy 2021, 46, 16565–16590. [Google Scholar]
- Khanal, S.K.; Chen, W.H.; Li, L.; Sung, S. Biological hydrogen production: Effects of pH and intermediate products. Int. J. Hydrogen Energy 2004, 29, 1123–1131. [Google Scholar] [CrossRef]
- Hwang, M.H.; Jang, N.J.; Hyun, S.H.; Kim, I.S. Anaerobic bio-hydrogen production from ethanol fermentation: The role of pH. J. Biotechnol. 2004, 111, 297–309. [Google Scholar] [CrossRef]
- Sivagurunathan, P.; Kumar, G.; Bakonyi, P.; Kim, S.-H.; Kobayashi, T.; Xu, K.Q.; Lakner, G.; Tóth, G.; Nemestothy, N.; Bélafi-Bakó, K. A critical review on issues and overcoming strategies for the enhancement of dark fermentative hydrogen production in continuous systems. Int. J. Hydrogen Energy 2016, 41, 3820–3836. [Google Scholar] [CrossRef]
- Pandey, A.K.; Pilli, S.; Bhunia, P.; Tyagi, R.; Surampalli, R.Y.; Zhang, T.C.; Kim, S.-H.; Pandey, A. Dark fermentation: Production and utilization of volatile fatty acid from different wastes—A review. Chemosphere 2022, 288, 132444. [Google Scholar] [CrossRef]
- Sekoai, P.T.; Ghimire, A.; Ezeokoli, O.T.; Rao, S.; Ngan, W.Y.; Habimana, O.; Yao, Y.; Yang, P.; Fung, A.H.Y.; Yoro, K.O.; et al. Valorization of volatile fatty acids from the dark fermentation waste Streams-A promising pathway for a biorefinery concept. Renew. Sustain. Energy Rev. 2021, 143, 110971. [Google Scholar] [CrossRef]
- Sambusiti, C.; Ficara, E.; Malpei, F.; Steyer, J.-P.; Carrere, H. Benefit of sodium hydroxide pretreatment of ensiled sorghum forage on the anaerobic reactor stability and methane production. Bioresour. Technol. 2013, 144, 149–155. [Google Scholar] [CrossRef]
- Seppälä, M.; Pyykkönen, V.; Väisänen, A.; Rintala, J. Biomethane production from maize and liquid cow manure–effect of share of maize, post-methanation potential and digestate characteristics. Fuel 2013, 107, 209–216. [Google Scholar]
- Molaey, R.; Yesil, H.; Calli, B.; Tugtas, A.E. Influence of volatile fatty acids in anaerobic bioleaching of potentially toxic metals. J. Environ. Manag. 2021, 285, 112118. [Google Scholar] [CrossRef]
- Náthia-Neves, G.; Berni, M.; Dragone, G.; Mussatto, S.I.; Forster-Carneiro, T. Anaerobic digestion process: Technological aspects and recent developments. Int. J. Environ. Sci. Technol. 2018, 15, 2033–2046. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, W.; Wang, Y.; Li, G.; Liu, Y.; Gong, X. Anaerobic cultivation of waste activated sludge to inoculate solid state anaerobic co-digestion of agricultural wastes: Effects of different cultivated periods. Bioresour. Technol. 2019, 294, 122078. [Google Scholar] [CrossRef]
- Karim, K.; Klasson, K.T.; Hoffmann, R.; Drescher, S.R.; DePaoli, D.W.; Al-Dahhan, M.H. Anaerobic digestion of animal waste: Effect of mixing. Bioresour. Technol. 2005, 96, 1607–1612. [Google Scholar] [CrossRef]




| Parameters | FS (%TS) | ES (%TS) | LCM (g/L) |
|---|---|---|---|
| pH 1 | 5.40 ± 0.01 | 4.10 ± 0.00 | 7.24 ± 0.18 |
| Moisture 2 | 74.00 ± 0.12 | 76.32 ± 0.10 | 96.69 ± 0.72 |
| TS 3 | 26.00 ± 0.03 | 23.73 ± 0.17 | 33.15 ± 1.98 |
| VS | 96.20 ± 0.51 | 94.08 ± 3.15 | 22.50 ± 0.98 |
| Ash 4 | 3.80 ± 0.09 | 5.93 ± 3.15 | 32.13 ± 1.50 |
| TOC | 57.00 ± 0.05 | 46.18 ± 0.00 | 16.72 ± 0.24 |
| Total Carbohydrates 5 | 58.00 ± 0.91 | 38.82 ± 1.29 | 6.99 ± 0.45 |
| Soluble Carbohydrates 5 | 28.04 ± 0.30 | 3.50 ± 0.85 | 0.45 ± 0.05 |
| Cellulose | 22.10 ± 1.31 | 37.60 ± 5.37 | N.A 6 |
| Hemicelluloses | 12.03 ± 1.55 | 25.51 ± 3.66 | N.A 6 |
| Lignin | 9.02 ± 2.01 | 17.28 ± 4.93 | N.A 6 |
| Alkalinity 7 | N.A 6 | N.A 6 | 12.38 ± 0.32 |
| Total Nitrogen (TKN) | 0.20 ± 0.01 | 0.96 ± 0.45 | 2.78 ± 0.00 |
| Proteins | 1.25 ± 0.06 | 6.00 ± 2.81 | 17.38 ± 0.00 |
| Lactic Acid | N.D8 | 4.28 ± 0.00 | N.D 8 |
| Operating Conditions | Acidogenic Reactor | ||
|---|---|---|---|
| HRT (d) | 3 | 5 | 8 |
| Flow rate (mL/d) | 167 | 100 | 62.5 |
| OLR (kg VS/m3·d) | 12.83 | 7.70 | 4.81 |
| OLR (kg COD/m3·d) | 23.03 | 13.82 | 8.64 |
| Methanogenic Reactor | |||
| HRT (d) | 20 | 25 | |
| Flow rate (mL/d) | 25 | 20 | |
| OLR (kg VS/m3·d) | 1.86 | 1.49 | |
| OLR (kg COD/m3·d) | 2.23 | 1.78 | |
| pH | P (mL) | Rm (mL/h) | λ (h) | r2 |
|---|---|---|---|---|
| 4.5 | 39.38 ± 0.62 | 1.71 ± 0.08 | - | 0.983 |
| 5.0 | 1759.29 ± 13.77 | 92.53 ± 2.24 | 7.49 ± 0.20 | 0.996 |
| 5.5 | 1701.10 ± 115.44 | 22.19 ± 0.46 | 15.89 ± 0.69 | 0.986 |
| 6.0 | 1365.80 ± 11.47 | 91.38 ± 2.34 | 0.48 ± 0.19 | 0.993 |
| Parameter | HRT (d) | ||
|---|---|---|---|
| 3 | 5 | 8 | |
| Biogas (L/LR·d) | 0.36 ± 0.10 | 0.46 ± 0.08 | 0.11 ± 0.02 |
| H2 (L/LR·d) | 0.09 ± 0.02 | 0.13 ± 0.02 | 0.02 ± 0.01 |
| H2 (%) | 22.0 ± 1.8 | 17.6 ± 2.2 | 20.9 ± 1.1 |
| Yield (mol H2/mol carbohydratesconsumed) | 0.63 ± 0.05 | 1.68 ± 0.08 | 0.48 ± 0.07 |
| Yield (mL H2/g carbohydratesconsumed) | 78.6 ± 7.7 | 209.2 ± 11.8 | 60.1 ± 5.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dareioti, M.A.; Tsigkou, K.; Vavouraki, A.I.; Kornaros, M. Hydrogen and Methane Production from Anaerobic Co-Digestion of Sorghum and Cow Manure: Effect of pH and Hydraulic Retention Time. Fermentation 2022, 8, 304. https://doi.org/10.3390/fermentation8070304
Dareioti MA, Tsigkou K, Vavouraki AI, Kornaros M. Hydrogen and Methane Production from Anaerobic Co-Digestion of Sorghum and Cow Manure: Effect of pH and Hydraulic Retention Time. Fermentation. 2022; 8(7):304. https://doi.org/10.3390/fermentation8070304
Chicago/Turabian StyleDareioti, Margarita A., Konstantina Tsigkou, Aikaterini I. Vavouraki, and Michael Kornaros. 2022. "Hydrogen and Methane Production from Anaerobic Co-Digestion of Sorghum and Cow Manure: Effect of pH and Hydraulic Retention Time" Fermentation 8, no. 7: 304. https://doi.org/10.3390/fermentation8070304
APA StyleDareioti, M. A., Tsigkou, K., Vavouraki, A. I., & Kornaros, M. (2022). Hydrogen and Methane Production from Anaerobic Co-Digestion of Sorghum and Cow Manure: Effect of pH and Hydraulic Retention Time. Fermentation, 8(7), 304. https://doi.org/10.3390/fermentation8070304









