Physicochemical, Functional, and Technological Properties of Protein Hydrolysates Obtained by Microbial Fermentation of Broiler Chicken Gizzards
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Ingredients
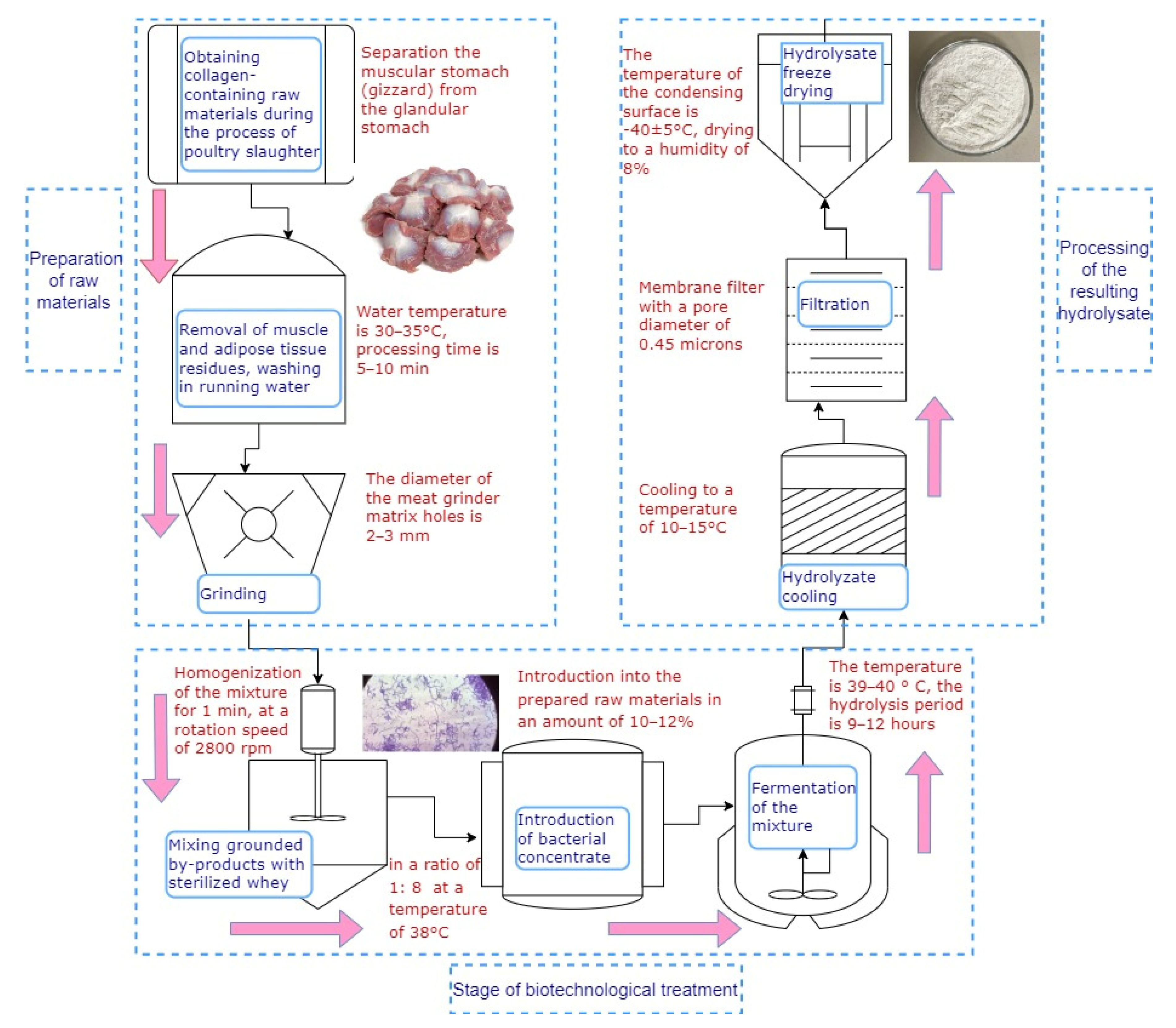
2.2. Preparation of Protein Hydrolysate
2.3. Determination of Proximate Composition
2.4. Determination of Functional and Technological Properties
2.5. Determination of Antioxidant Activity
2.6. Determination of Microbiological Indicators
2.7. Determination of Antimicrobial Activity
2.8. Differential-Scanning Calorimetry (DSC) and Thermal Gravimetric Analysis
2.9. Analysis of Average Particle Size
2.10. Statistical Analysis
3. Results and Discussions
3.1. Physicochemical and Technological Indicators of Protein Hydrolysates
3.2. Differential-Scanning Calorimetry (DSC) and Thermal Gravimetric Analysis
3.3. Antimicrobial Activity
3.4. Antioxidant Properties
3.5. Microbiological Indicators
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arihara, K.; Yokoyamaa, I.; Ohatab, M. Bioactivities generated from meat proteins by enzymatic hydrolysis and the Maillard reaction. Meat Sci. 2021, 180, 108561. [Google Scholar] [CrossRef]
- Thoresenb, P.; García Álvareza, R.; Risa Vakaa, M.; Rustadb, T.; Sonea, I.; Noriega Fernándeza, E. Potential of innovative pre-treatment technologies for the revalorisation of residual materials from the chicken industry through enzymatic hydrolysis. Innov. Food Sci. Emerg. Technol. 2020, 64, 102377. [Google Scholar] [CrossRef]
- Hong, H.; Fan, H.; Chalamaiah, M.; Wu, J. Preparation of low-molecular-weight, collagen hydrolysates (peptides): Current progress, challenges, and future perspectives. Food Chem. 2019, 301, 125222. [Google Scholar] [CrossRef] [PubMed]
- Toldra, F.; Reig, M.; Mora, L. Management of meat by- and co-products for an improved meat processing sustainability. Meat Sci. 2021, 181, 108608. [Google Scholar] [CrossRef]
- Rathina Raj, K.; Mahendrakar, N.S. Effect of ensiling and organic solvents treatment on proteolytic enzymes of layer chicken intestine. J. Food Sci. Technol. 2010, 47, 320–324. [Google Scholar] [CrossRef] [Green Version]
- Lafarga, T.; Hayes, M. Bioactive peptides from meat muscle and by-products: Generation, functionality and application as functional ingredients. Meat Sci. 2014, 98, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Santos, B.A.S.; Azambuja, S.P.H.; Ávila, P.F.; Pacheco, M.T.B.; Goldbeck, R. n-Butanol production by Saccharomyces cerevisiae from protein-rich agro-industrial by-products. Braz. J. Microbial. 2020, 51, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.; Rezende, P.C.; Corrêa, N.M.; Rocha, J.S.; Martins, M.A.; Andrade, T.C.; Fracalossi, D.M.; Vieira, F.N. Protein hydrolysates from poultry by-product and swine liver as an alternative dietary protein source for the Pacific white shrimp. Aquac. Rep. 2020, 17, 100344. [Google Scholar] [CrossRef]
- Lasekan, A.; Bakar, F.A.; Hashim, D. Potential of chicken by-products as sources of useful biological resources. Waste Manag. 2013, 33, 552–565. [Google Scholar] [CrossRef]
- Vikman, Y.M.; Siipola, V.; Kanerva, H.; Šližyte, R.; Wikberg, H. Poultry By-products as a Potential Source of Nutrients. Adv. Recycl. Waste Manag. 2017, 2, 142. [Google Scholar] [CrossRef]
- Tram, N.X.T.; Ishikawa, K.; Minh, T.H.; Benson, D.; Tsuru, K. Characterization of carbonate apatite derived from chicken bone and its in-vitro evaluation using MC3T3-E1 cells. Mater. Res. Express 2021, 8, 025401. [Google Scholar] [CrossRef]
- Mozuriene, E.; Bartkiene, E.; Krungleviciute, V.; Zadeike, D.; Juodeikiene, G.; Damasius, J.; Baltusnikiene, A. Effect of natural marinade based on lactic acid bacteria on pork meat quality parameters and biogenic amine contents. LWT Food Sci. Technol. 2016, 69, 319–326. [Google Scholar] [CrossRef]
- Heyman, M. Evaluation of the impact of food technology on the allergenicity of cow’s milk proteins. Proc. Nutr. Soc. 1999, 58, 587–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halavach, T.N.; Kurchenko, V.P. Milk protein hydrolysis with enzyme preparation and proteolytic systems of lactic acid bacteria. Tr. BGU 2012, 7, 106–126. (In Russian) [Google Scholar]
- Chakka, A.K.; Elias, M.; Jini, R.; Sakhare, P.Z.; Bhaskar, N. In-vitro antioxidant and antibacterial properties of fermentatively and enzymatically prepared chicken liver protein hydrolysates. J. Food Sci. Technol. 2015, 52, 8059–8067. [Google Scholar] [CrossRef] [Green Version]
- Vlahova-Vangelova, D.B.; Dragoev, S.G.; Balev, D.K.; Assenova, B.K.; Amirhanov, K.J. Quality, Microstructure, and Technological Properties of Sheep Meat Marinated in Three Different Ways. J. Food Qual. 2017, 2017, 5631532. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Wu, Z.; Dai, Z.; Wang, G.; Wu, G. Proteinhydrolysates in animal nutrition: Industrial production, bioactive peptides, and functional significance. J. Anim. Sci. Biotechnol. 2017, 8, 24. [Google Scholar] [CrossRef] [Green Version]
- Izzo, L.; Luz, C.; Ritieni, A.; Mañes, J.; Meca, G. Whey fermented by using Lactobacillus plantarum strains: A promising approach to increase the shelf life of pita bread. J. Dairy Sci. 2020, 103, 5906–5915. [Google Scholar] [CrossRef]
- Kęska, P.; Wójciak, K.M.; Stadnik, J. Bioactive peptides from beef products fermented by acid whey—In vitro and in silico study. Sci. Agric. 2019, 76, 311–320. [Google Scholar] [CrossRef]
- Wan, M.Y.; Dong, G.; Yang, B.Q.; Feng, H. Identification and characterization of a novel antioxidant peptide from feather keratin hydrolysate. Biotechnol. Lett. 2016, 38, 643–649. [Google Scholar] [CrossRef]
- Mundi, S.; Aluko, R.E. Inhibitory properties of kidney bean protein hydrolysate and its membrane fractions against renin, angiotensin converting enzyme, and free radicals. Austin J. Nutr. Food Sci. 2014, 2, 1008–1019. [Google Scholar]
- Tadesse, S.A.; Emire, S.A. Production and processing of antioxidant bioactive peptides: A driving force for the functional food market. Heliyon 2020, 6, e04765. [Google Scholar] [CrossRef] [PubMed]
- Unsal, M.; Aktas, N. Fractionation and characterization of edible sheep tail fat. Meat Sci. 2003, 63, 235. [Google Scholar] [CrossRef]
- Tkaczewska, J. Peptides and protein hydrolysates as food preservatives and bioactive components of edible films and coatings-A review. Trends Food Sci. Technol. 2020, 106, 298–311. [Google Scholar] [CrossRef]
- Di Bernardini, R.; Harnedy, P.; Bolton, D.; Kerry, J.; O’Neill, E.; Mullen, A.M.; Hayes, M. Antioxidant and antimicrobial peptidic hydrolysates from muscle protein sources and by-products. Food Chem. 2011, 124, 1296–1307. [Google Scholar] [CrossRef]
- Zinina, O.; Merenkova, S.; Galimov, D. Optimization of microbial hydrolysis parameters of poultry by-products using probiotic microorganisms to obtain protein hydrolysates. Fermentation 2021, 7, 122. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Assaad, H.; Zhou, L.; Carroll, R.J.; Wu, G. Rapid publication-ready MS-Word tables for one-way ANOVA. SpringerPlus 2014, 3, 474. [Google Scholar] [CrossRef] [Green Version]
- Karami, Z.; Akbariadergani, B. Bioactive food derived peptides: A review on correlation between structure of bioactive peptides and their functional properties. J. Food Sci. Technol. 2019, 56, 535–547. [Google Scholar] [CrossRef]
- Zinina, O.V.; Merenkova, S.P.; Knyazeva, A.A.; Marushkevich, M.A.; Gavrilova, K.S. Microbial fermentation of poultry by-products. Bull. South Ural. State Univ. Ser. Food Biotechnol. 2021, 9, 77–89. (In Russian) [Google Scholar] [CrossRef]
- Karpukhina, P.A.; Krasnoshtanova, A.A. Protein fractions poultry egg white hydrolysis and measurement of its functional and technological properties. Success Chem. Chem. Technol. 2021, 35, 76–78. (In Russian) [Google Scholar]
- Gbogouri, G.A.; Linder, M.; Fanni, J.; Parmentier, M. Influence of hydrolysis degree on the functional properties of salmon byproduct hydrolysates. J. Food Sci. 2004, 69, 615–622. [Google Scholar] [CrossRef]
- Klompong, V.; Benjakul, S.; Kantachote, D.; Shahidi, F. Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem. 2007, 102, 1317–1327. [Google Scholar] [CrossRef]
- Glotova, I.A.; Litovkin, A.N.; Artemov, E.S.; Ermolova, A.V.; Shakhov, S.V. Research of the dehydration processes of biopolymer systems in poultry products. Polythematic Online Sci. J. Kuban State Agrar. Univ. 2016, 121, 45. [Google Scholar] [CrossRef]
- Nilova, L.; Naumenko, N.; Kalinina, I. A study of the forms of bound water in bread and bakery products using differential thermal analysis. Agron. Res. 2017, 15, 1386–1398. [Google Scholar]
- Sultanova, S.A.; Safarov, J.E.; Ikromova, S. The study of moisture bond forms in edible vegetable raw materials by differential thermal analysis. Int. J. Adv. Sci Technol. 2020, 29, 5839–5845. [Google Scholar]
- Hickey, R.M.; Twomey, D.P.; Ross, R.P.; Hill, C. Production of enterolysin A by a raw milk enterococcal isolate exhibiting multiple virulence factors. Microbiology 2003, 149, 655–664. [Google Scholar] [CrossRef] [Green Version]
- Hayes, M.; Ross, R.P.; Fitzgerald, G.F.; Hill, C.; Stanton, C. Casein-derived antimicrobial peptides generated by Lactobacillus acidophilus DPC6026. Appl. Environ. Microbiol. 2006, 72, 2260–2264. [Google Scholar] [CrossRef] [Green Version]
- Alemán, A.; Giménez, B.; Pérez-Santin, E.; Gómez-Guillén, M.C.; Montero, P. Contribution of Leu and Hyp residues to antioxidant and ACE-inhibitory activities of peptide sequences isolated from squid gelatin hydrolysate. Food Chem. 2011, 125, 334–341. [Google Scholar] [CrossRef] [Green Version]
- Iwaniak, A.; Minkiewicz, P.; Darewicz, M.; Hrynkiewicz, M. Food protein originating peptides as tastants—Physiological, technological, sensory, and bioinformatic approaches. Food Res. Int. 2016, 89, 27–38. [Google Scholar] [CrossRef]
- O’Sullivan, S.M.; Lafarga, T.; Hayes, M.; O’Brien, N.M. Bioactivity of bovine lung hydrolysates prepared using papain, pepsin, and Alcalase. J. Food Biochem. 2017, 41, 12406. [Google Scholar] [CrossRef]
- Verma, A.K.; Chatli, M.K.; Mehta, N.; Kumar, P. Assessment of physicochemical, antioxidant and antimicrobial activity of porcine blood protein hydrolysate in pork emulsion stored under aerobic packaging condition at 4 ± 1 °C. LWT 2018, 88, 71–79. [Google Scholar] [CrossRef]
- Jin, S.-K.; Choi, J.-S.; Kim, G.-D. Effect of porcine plasma hydrolysate on physicochemical, antioxidant, and antimicrobial properties of emulsion-type pork sausage during cold storage. Meat Sci. 2021, 171, 108293. [Google Scholar] [CrossRef] [PubMed]
- Lima, K.O.; de Quadros, C.d.C.; da Rocha, M.; de Lacerda, J.T.J.G.; Juliano, M.A.; Dias, M.; Mendes, M.A.; Prentice, C. Bioactivity and bioaccessibility of protein hydrolyzates from industrial byproducts of Stripped weakfish (Cynoscion guatucupa). LWT 2019, 111, 408–413. [Google Scholar] [CrossRef]
- Mhina, C.F.; Jung, H.Y.; Kim, J.K. Recovery of antioxidant and antimicrobial peptides through the reutilization of Nile perch wastewater by biodegradation using two Bacillus species. Chemosphere 2020, 253, 126728. [Google Scholar] [CrossRef] [PubMed]
- Bougatef, A.; Nedjar-Arroume, N.; Manni, L.; Ravallec, R.; Barkia, A.; Guillochon, D.; Nasri, M. Purification and identification of novel antioxidant peptides from enzymatic hydrolysates of sardinelle (Sardinella aurita) by-products proteins. Food Chem. 2010, 118, 559–565. [Google Scholar] [CrossRef]
- Verma, A.K.; Chatli, M.K.; Kumar, P.A.V.A.N.; Mehta, N. Antioxidant and antimicrobial activity of protein hydrolysate extracted from porcine liver. Indian J. Anim. Sci. 2017, 87, 711–717. [Google Scholar]
- Yu, H.C.; Hsu, J.L.; Chang, C.I.; Tan, F.J. Antioxidant properties of porcine liver proteins hydrolyzed using Monascus purpureus. Food Sci. Biotechnol. 2017, 26, 1217–1225. [Google Scholar] [CrossRef]
- Jamdar, S.N.; Rajalakshmi, V.; Sharma, A. Antioxidant and ace inhibitory properties of poultry viscera protein hydrolysate and its peptide fractions. J. Food Biochem. 2012, 36, 494–501. [Google Scholar] [CrossRef]
- Qian, Z.-J.; Jung, W.-K.; Kim, S.-K. Free radical scavenging activity of a novel antioxidative peptide purified from hydrolysate of bullfrog skin, Rana catesbeiana Shaw. Bioresour. Technol. 2008, 99, 1690–1698. [Google Scholar] [CrossRef]
- Rajapakse, N.; Mendis, E.; Jung, W.-K.; Je, J.-Y.; Kim, S.-K. Purification of a radical scavenging peptide from fermented mussel sauce and its antioxidant properties. Food Res. Int. 2005, 38, 175–182. [Google Scholar] [CrossRef]
- Sheveleva, S.A.; Kuvaeva, I.B.; Efimochkina, N.R.; Minaeva, L.P. Microbiological safety of food: Development of normative and methodological base. Probl. Nutr. 2020, 89, 125–145. (In Russian) [Google Scholar] [CrossRef]





| Indicator | Control Hydrolysate | Hydrolysate Fermented by Propionix LCSC | Hydrolysate Fermented by BLC |
|---|---|---|---|
| Mass fraction of protein, % | 36.26 ± 0.015 b | 49.79 ± 0.040 a | 51.42 ± 0.128 a |
| Mass fraction of fat, % | 2.61 ± 0.006 a | 2.58 ± 0.007 a | 2.62 ± 0.004 a |
| Mass fraction of moisture, % | 7.91 ± 0.019 a | 7.54 ± 0.013 a | 7.34 ± 0.013 a |
| Mass fraction of ash, % | 6.03 ± 0.007 a | 6.29 ± 0.010 a | 6.18 ± 0.012 a |
| Indicator | Control Hydrolysate | Hydrolysate Fermented by Propionix LCSC | Hydrolysate Fermented by BLC |
|---|---|---|---|
| FHC, % g/g | 139.5 ± 2.1 c 4.05 ± 0.02 c | 220.5 ± 1.8 b 6.52 ± 0.01 b | 351.1 ± 3.2 a 10.35 ± 0.02 a |
| WHC, % g/g | 170.3 ± 2.2 c 2.43 ± 0.01 b | 315.0 ± 2.4 b 4.27 ± 0.01 a | 363.0 ± 2.6 a 4.92 ± 0.02 a |
| FEA, % | 47 ± 1.0 b | 53 ± 1.0 b | 61 ± 1.5 a |
| SE, % | 24 ± 1.0 b | 36 ± 1.5 a | 42 ± 1.0 a |
| FC, % | 240 ± 2.0 b | 275 ± 3.0 a | 310 ± 2.0 a |
| FR, % | 120 ± 1.0 b | 132 ± 1.0 b | 160 ± 2.0 a |
| S, % | 88.9 ± 1.1 a | 90.1 ± 1.5 a | 91.4 ± 0.8 a |
| Sample | Number of Interval | Temperature Interval | Mass Losses, % | |
|---|---|---|---|---|
| °C | K | |||
| Hydrolysate fermented by BLC | 0 | up to 30 | up to 303 | 28.4 |
| I | 30–115 | 303–388 | 15.5 | |
| II | 115–125 | 388–398 | 1.2 | |
| III | 125–170 | 398–443 | 9.5 | |
| IV | 170–250 | 443–523 | 6.1 | |
| Hydrolysate fermented by Propionix LCSC | 0 | up to 30 | up to 303 | 19.1 |
| I | 30–110 | 303–383 | 21.7 | |
| II | 110–120 | 383–393 | 3.9 | |
| III | 120–170 | 393–443 | 1.9 | |
| IV | 170–250 | 443–523 | 6.2 | |
| Whey protein hydrolysate | 0 | up to 30 | up to 303 | 32.8 |
| I | 30–100 | 303–373 | 10.7 | |
| II | 100–125 | 373–398 | 0.3 | |
| III | 125–155 | 398–428 | 1.5 | |
| IV | 155–240 | 428–513 | 7.1 | |
| Type of Hydrolysate | The Width of the Inhibition Zone for the Microorganism, mm | |
|---|---|---|
| Escherichia coli | Staphylococcus aureus | |
| Control hydrolysate | 2.0 ± 0.45 b | 1.0 ± 0.48 c |
| Hydrolysate fermented by BLC | 5.0 ± 0.52 a | 5.0 ± 0.54 a |
| Hydrolysate fermented by Propionix LCSC | 4.0 ± 0.46 a | 3.0 ± 0.52 b |
| Effect of hydrolysates on culture |  |  |
| Indicator, CFU/g | Control Hydrolysate | Hydrolysate Fermented by Propionix LCSC | Hydrolysate Fermented by BLC |
|---|---|---|---|
| Total viable counts (TVC) | ((1.5 ± 0.05) × 102) a | ((1.0 ± 0.03) × 102) b | ((1.5 ± 0.04) × 102) a |
| Total coliforms count (TCC) | not detected | not detected | not detected |
| Mold | not detected | not detected | not detected |
| Yeasts | not detected | not detected | not detected |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zinina, O.; Merenkova, S.; Rebezov, M.; Galimov, D.; Khayrullin, M.; Burkov, P. Physicochemical, Functional, and Technological Properties of Protein Hydrolysates Obtained by Microbial Fermentation of Broiler Chicken Gizzards. Fermentation 2022, 8, 317. https://doi.org/10.3390/fermentation8070317
Zinina O, Merenkova S, Rebezov M, Galimov D, Khayrullin M, Burkov P. Physicochemical, Functional, and Technological Properties of Protein Hydrolysates Obtained by Microbial Fermentation of Broiler Chicken Gizzards. Fermentation. 2022; 8(7):317. https://doi.org/10.3390/fermentation8070317
Chicago/Turabian StyleZinina, Oksana, Svetlana Merenkova, Maksim Rebezov, Damir Galimov, Mars Khayrullin, and Pavel Burkov. 2022. "Physicochemical, Functional, and Technological Properties of Protein Hydrolysates Obtained by Microbial Fermentation of Broiler Chicken Gizzards" Fermentation 8, no. 7: 317. https://doi.org/10.3390/fermentation8070317






