Strategy Development for Microalgae Spirulina platensis Biomass Cultivation in a Bubble Photobioreactor to Promote High Carbohydrate Content
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalgae Strain, Maintenance, and Inoculum Preparation
2.2. Experimental Design for Nutrient Stress Optimization
2.3. Analytical Methods
2.3.1. Determination of the Growth Kinetics Parameters, pH, and Identification of Microalgae
2.3.2. Extraction of Chlorophyll-a and Chlorophyll-b, Total Carotenoids, and Phycocyanin
2.3.3. Total Carbohydrate Quantification
2.3.4. Quantification of Total Lipid in Dried Spirulina Platensis Biomass by Sulpho-Phospho-Vanillin (SPV) Method
2.3.5. Microalgae Protein Extraction from Dried Biomass
2.4. Statistical Analysis
3. Results and Discussion
3.1. Evaluating the Parameters That Influence Biomass and pH Determination
3.2. Morphological Behavior
3.3. Extraction of Pigments (Chlorophyll-a, Chlorophyll-b, Total Carotenoids, and Phycocyanin)
3.4. Carbohydrate Accumulation
3.5. Lipid Determination
3.6. Protein Quantification
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Velazquez-Lucio, J.; Rodríguez-Jasso, R.M.; Colla, L.M.; Sáenz-Galindo, A.; Cervantes-Cisneros, D.E.; Aguilar, C.N.; Fernandes, B.D.; Ruiz, H.A. Microalgal biomass pretreatment for bioethanol production: A review. Biofuel Res. J. 2018, 5, 780–791. [Google Scholar] [CrossRef]
- Aparicio, E.; Rodríguez-Jasso, R.M.; Pinales-Márquez, C.D.; Loredo-Treviño, A.; Robledo-Olivo, A.; Aguilar, C.N.; Kostas, E.T.; Ruiz, H.A. High-pressure technology for Sargassum spp. biomass pretreatment and fractionation in the third generation of bioethanol production. Bioresour. Technol. 2021, 329, 124935. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Conrad, M.; Sun, S.N.; Sanchez, A.; Rocha, G.J.; Romaní, A.; Castro, E.; Torres, A.; Rodríguez-Jasso, R.M.; Andrade, L.P.; et al. Engineering aspects of hydrothermal pretreatment: From batch to continuous operation, scale-up and pilot reactor under biorefinery concept. Bioresour. Technol. 2020, 299, 122685. [Google Scholar] [CrossRef]
- Rosero-Chasoy, G.; Rodríguez-Jasso, R.M.; Aguilar, C.N.; Buitrón, G.; Chairez, I.; Ruiz, H.A. Growth kinetics and quantification of carbohydrate, protein, lipids, and chlorophyll of Spirulina platensis under aqueous conditions using different carbon and nitrogen sources. Bioresour. Technol. 2022, 346, 126456. [Google Scholar] [CrossRef] [PubMed]
- Kostas, E.T.; Adams, J.M.M.; Ruiz, H.A.; Durán-Jiménez, G.; Lye, G.J. Macroalgal biorefinery concepts for the circular bioeconomy: A review on biotechnological developments and future perspectives. Renew. Sustain. Energy Rev. 2021, 151, 111553. [Google Scholar] [CrossRef]
- Saxena, R.; Rosero-Chasoy, G.; Aparicio, E.; Lara, A.; Loredo, A.; Robledo, A.; Kostas, E.T.; Rodríguez-Jasso, R.M.; Ruiz, H.A. Third generation biorefineries using micro-macro-alge. In Production of Biofuels and Chemicals from Sustainable Recycling of Organic Solid Waste; Fang, Z., Smith, R.L., Jr., Xu, L., Eds.; Springer: Singapore, 2022; pp. 373–411. [Google Scholar]
- Rosero-Chasoy, G.; Rodríguez-Jasso, R.M.; Aguilar, C.N.; Buitrón, G.; Chairez, I.; Ruiz, H.A. Microbial co-culturing strategies for the production high value compounds, a realiable framework towards sustainable biorefinery implementation—An overview. Bioresour. Technol. 2021, 321, 124458. [Google Scholar] [CrossRef]
- Rempel, A.; Machado, T.; Treichel, H.; Colla, E.; Margarites, A.C.; Colla, L.M. Saccharification of Spirulina Platensis biomass using free and immobilized amylolytic enzymes. Bioresour. Technol. 2018, 263, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, H.A.; Rodriguez, R.M.; Fernandes, B.D.; Vicente, A.; Teixeira, A. Hydrothermal processing, as an alternative for upgrading agriculture residues and marine biomass according to the biorefinery concept: A review. Renew. Sustain. Energy Rev. 2013, 21, 35–51. [Google Scholar] [CrossRef]
- Singh, P.; Guldhe, A.; Kumari, S.; Rawat, I.; Bux, F. Investigation of combined effect of nitrogen, phosphorus and iron on lipid productivity of microalgae Ankistrodesmus Falcatus KJ671624 using response surface methodology. Biochem. Eng. J. 2015, 94, 22–29. [Google Scholar] [CrossRef]
- Qin, L.; Alam, M.A.; Wang, Z. Open pond culture systems and photobioreactors for microalgal biofuel production. In Microalgae Biotechnology for Development of Biofuel and Wastewater Treatment; Springer: Singapore, 2019; pp. 45–74. [Google Scholar]
- Tamburic, B.; Evenhuis, C.R.; Crosswell, J.R.; Ralph, P.J. An empirical process model to predict microalgal carbon rates in photobioreactors. Algal Res. 2018, 31, 334–346. [Google Scholar] [CrossRef]
- Costa, J.A.V.; Colla, L.M.; Filho, P.D.; Kabke, K.; Weber, A. modelling of Spirulina Platensis growth in fresh water using response surface methodology. World J. Microbiol. Biotechnol. 2002, 18, 603–607. [Google Scholar] [CrossRef]
- Vieira Costa, J.A.; Colla, L.M.; Duarte Filho, P.F. Improving Spirulina Platensis biomass yield using a fed-batch process. Bioresour. Technol. 2004, 92, 237–241. [Google Scholar] [CrossRef]
- Ansari, F.A.; Singh, P.; Guldhe, A.; Bux, F. Microalgal cultivation using aquaculture wastewater: Integrated biomass generation and nutrient remediation. Algal Res. 2017, 21, 169–177. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Sarkar, S.; Manna, M.S.; Bhowmick, T.K.; Gayen, K. Extraction of chlorophylls and carotenoids from dry and wet biomass of isolated Chlorella Thermophila: Optimization of process parameters and modelling by artificial neural network. Process Biochem. 2020, 96, 58–72. [Google Scholar] [CrossRef]
- Mata, S.N.; de Souza Santos, T.; Cardoso, L.G.; Andrade, B.B.; Duarte, J.H.; Costa, J.A.V.; Oliveira de Souza, C.; Druzian, J.I. Spirulina sp. LEB 18 cultivation in a raceway-type bioreactor using wastewater from desalination process: Production of carbohydrate-rich biomass. Bioresour. Technol. 2020, 311, 123495. [Google Scholar] [CrossRef]
- Julianti, E.; Susanti; Singgih, M.; Neti Mulyani, L. Optimization of extraction method and characterization of phycocyanin pigment from Spirulina Platensis. J. Math. Fundam. Sci. 2019, 51, 168–176. [Google Scholar] [CrossRef]
- Bennett, A.; Bogobad, L. Complementary chromatic adaptation in a filamentous blue-green Aaga. J. Cell Biol. 1973, 58, 419–435. [Google Scholar] [CrossRef]
- Hernández, D.; Riaño, B.; Coca, M.; García-González, M.C. Saccharification of carbohydrates in microalgal biomass by physical, chemical and enzymatic pretreatments as a previous step for bioethanol production. Chem. Eng. J. 2015, 262, 939–945. [Google Scholar] [CrossRef]
- Waghmare, A.G.; Salve, M.K.; LeBlanc, J.G.; Arya, S.S. Concentration and characterization of microalgae proteins from Chlorella Pyrenoidosa. Bioresour. Bioprocess. 2016, 3, 16. [Google Scholar] [CrossRef]
- Dreywood, R. Qualitative test for carbohydrate material. Ind. Eng. Chem. Anal. Ed. 1946, 18, 499. [Google Scholar] [CrossRef]
- Loewus, F.A. Improvement in Anthrone method for determination of carbohydrates errors in volumetric analysis arising from adsorption. Anal. Chem. 1952, 24, 219. [Google Scholar] [CrossRef]
- Mishra, S.K.; Suh, W.I.; Farooq, W.; Moon, M.; Shrivastav, A.; Park, M.S.; Yang, J.W. Rapid quantification of microalgal lipids in aqueous medium by a simple colorimetric method. Bioresour. Technol. 2014, 155, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Anand, V.; Kashyap, M.; Sharma, M.P.; Bala, K. Impact of hydrogen peroxide on microalgae cultivated in varying salt-nitrate-phosphate conditions. J. Environ. Chem. Eng. 2021, 9, 105814. [Google Scholar] [CrossRef]
- Knight, J.A.; Anderson, S.; Rawle, J.M. Chemical basis of the sulfo-phospho-vanillin reaction for estimating total serum lipids. Clin. Chem. 1972, 18, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Eze, C.N.; Ogbonna, I.O.; Aoyagi, H.; Ogbonna, J.C. Comparison of growth, protein and carotenoid contents of some freshwater microalgae and the effects of urea and cultivation in a photobioreactor with reflective broth circulation guide on Desmodesmus Subspicatus LC172266. Braz. J. Chem. Eng. 2022, 39, 23–33. [Google Scholar] [CrossRef]
- Rausch, T. The estimation of micro-algal protein content and its meaning to the evaluation of algal biomass. Hydrobiologia 1981, 78, 237–251. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Slocombe, S.P.; Ross, M.; Thomas, N.; McNeill, S.; Stanley, M.S. A Rapid and general method for measurement of protein in micro-algal biomass. Bioresour. Technol. 2013, 129, 51–57. [Google Scholar] [CrossRef]
- Peter, A.P.; Chew, K.W.; Koyande, A.K.; Yuk-Heng, S.; Ting, H.Y.; Rajendran, S.; Munawaroh, H.S.H.; Yoo, C.K.; Show, P.L. Cultivation of Chlorella Vulgaris on dairy waste using vision imaging for biomass growth monitoring. Bioresour. Technol. 2021, 341, 125892. [Google Scholar] [CrossRef]
- Lu, W.; Liu, S.; Lin, Z.; Lin, M. Enhanced microalgae growth for biodiesel production and nutrients temoval in raw swine wastewater by carbon sources supplementation. Waste Biomass Valoriz. 2021, 12, 1991–1999. [Google Scholar] [CrossRef]
- Andrade, B.B.; Cardoso, L.G.; de Jesus Assis, D.; Costa, J.A.V.; Druzian, J.I.; da Cunha Lima, S.T. Production and characterization of Spirulina Sp. LEB 18 cultured in reused Zarrouk’s medium in a raceway-type Bioreactor. Bioresour. Technol. 2019, 284, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, S.; Nagarajan, P. Optimization of growth conditions for carotenoid production from Spirulina Platensis (Geitler). Int. J. Curr. Microbiol. Appl. Sci. 2013, 2, 325–328. [Google Scholar]
- Vonshak, A. Spirulina: Growth, physiology and biochemistry. In Spirulina Platensis Arthrospira; Vonshak, A., Ed.; CRC Press: Boca Raton, FL, USA, 1997; pp. 43–65. [Google Scholar] [CrossRef]
- Huang, Y.; Lou, C.; Luo, L.; Wang, X.C. Insight into nitrogen and phosphorus coupling effects on mixotrophic Chlorella Vulgaris growth under stably controlled nutrient conditions. Sci. Total Environ. 2021, 752, 141747. [Google Scholar] [CrossRef]
- Arbib, Z.; Ruiz, J.; Álvarez-Díaz, P.; Garrido-Pérez, C.; Barragan, J.; Perales, J.A. Photobiotreatment: Influence of nitrogen and phosphorus ratio in wastewater on growth kinetics of Scenedesmus Obliquus. Int. J. Phytoremediat. 2013, 15, 774–788. [Google Scholar] [CrossRef]
- Cardoso, L.G.; Lombardi, A.T.; de Jesus Silva, J.S.; Lemos, P.V.F.; Costa, J.A.V.; de Souza, C.O.; Druzian, J.I.; Chinalia, F.A. Scaling-up production of Spirulina Sp. LEB18 grown in aquaculture wastewater. Aquaculture 2021, 544, 737045. [Google Scholar] [CrossRef]
- Paes, C.R.P.S.; Faria, G.R.; Tinoco, N.A.B.; Castro, D.J.F.A.; Barbarino, E.; Lourenço, S.O. Growth, nutrient uptake and chemical composition of Chlorella Sp. and Nannochloropsis Oculata under nitrogen starvation. Lat. Am. J. Aquat. Res. 2016, 44, 275–292. [Google Scholar] [CrossRef]
- Cardoso, L.G.; Duarte, J.H.; Andrade, B.B.; Lemos, P.V.F.; Costa, J.A.V.; Druzian, J.I.; Chinalia, F.A. Spirulina sp. LEB 18 cultivation in outdoor pilot scale using aquaculture wastewater: High biomass, carotenoid, lipid and carbohydrate production. Aquaculture 2020, 525, 735272. [Google Scholar] [CrossRef]
- Ajayan, K.V.; Selvaraju, M.; Thirugnanamoorthy, K. Enrichment of chlorophyll and phycobiliproteins in Spirulina Platensis by the use of reflector light and nitrogen sources: An in-vitro Study. Biomass Bioenergy 2012, 47, 436–441. [Google Scholar] [CrossRef]
- García-López, D.A.; Olguín, E.J.; González-Portela, R.E.; Sánchez-Galván, G.; De Philippis, R.; Lovitt, R.W.; Llewellyn, C.A.; Fuentes-Grünewald, C.; Parra Saldívar, R. A novel two-phase bioprocess for the production of Arthrospira (Spirulina) Maxima LJGR1 at pilot plant scale during different seasons and for phycocyanin induction under controlled conditions. Bioresour. Technol. 2020, 298, 122548. [Google Scholar] [CrossRef]
- Werlang, E.B.; Julich, J.; Muller, M.V.G.; de Farias Neves, F.; Sierra-Ibarra, E.; Martinez, A.; Schneider, R. Bioethanol from hydrolyzed Spirulina (Arthrospira Platensis) biomass using ethanologenic bacteria. Bioresour. Bioprocess. 2020, 7, 27. [Google Scholar] [CrossRef]
- Liu, Q.; Yao, C.; Sun, Y.; Chen, W.; Tan, H.; Cao, X.; Xue, S.; Yin, H. Production and structural characterization of a new type of polysaccharide from nitrogen-limited Arthrospira Platensis cultivated in outdoor industrial-scale open raceway ponds. Biotechnol. Biofuels 2019, 12, 131. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.H.; Huang, S.W.; Chen, C.Y.; Hasunuma, T.; Kondo, A.; Chang, J.S. Characterization and optimization of carbohydrate production from an indigenous microalga Chlorella Vulgaris FSP-E. Bioresour. Technol. 2013, 135, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Rehman, Z.U.; Anal, A.K. Enhanced lipid and starch productivity of microalga (Chlorococcum Sp. TISTR 8583) with nitrogen limitation following effective pretreatments for biofuel production. Biotechnol. Rep. 2019, 21, e00298. [Google Scholar] [CrossRef] [PubMed]
- Campenni’, L.; Nobre, B.P.; Santos, C.A.; Oliveira, A.C.; Aires-Barros, M.R.; Palavra, A.M.F.; Gouveia, L. Carotenoid and lipid production by the autotrophic microalga Chlorella Protothecoides under nutritional, salinity, and luminosity stress conditions. Appl. Microbiol. Biotechnol. 2013, 97, 1383–1393. [Google Scholar] [CrossRef] [PubMed]
- Soni, R.A.; Sudhakar, K.; Rana, R.S. Spirulina—From growth to nutritional product: A review. Trends Food Sci. Technol. 2017, 69, 157–171. [Google Scholar] [CrossRef]

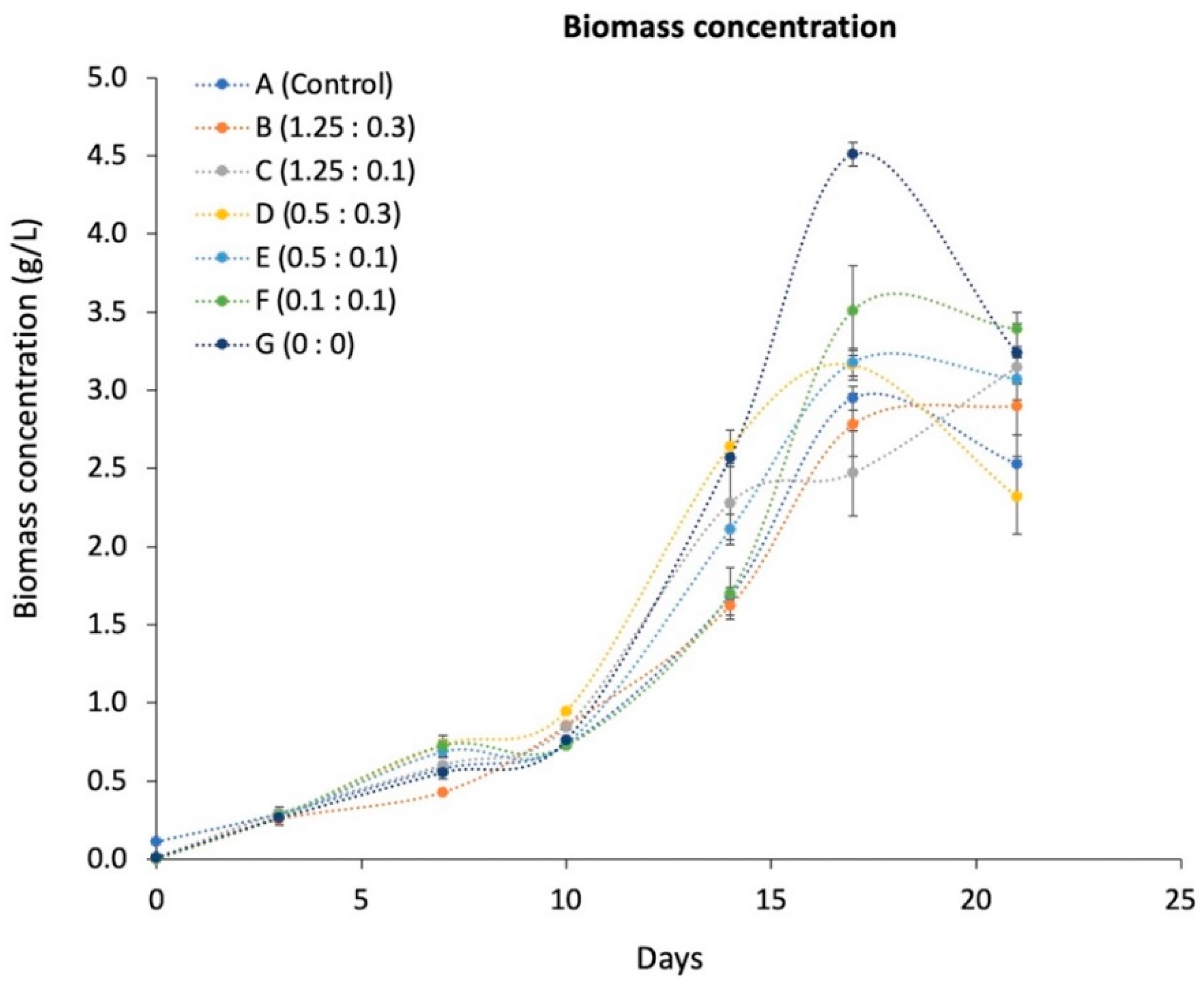

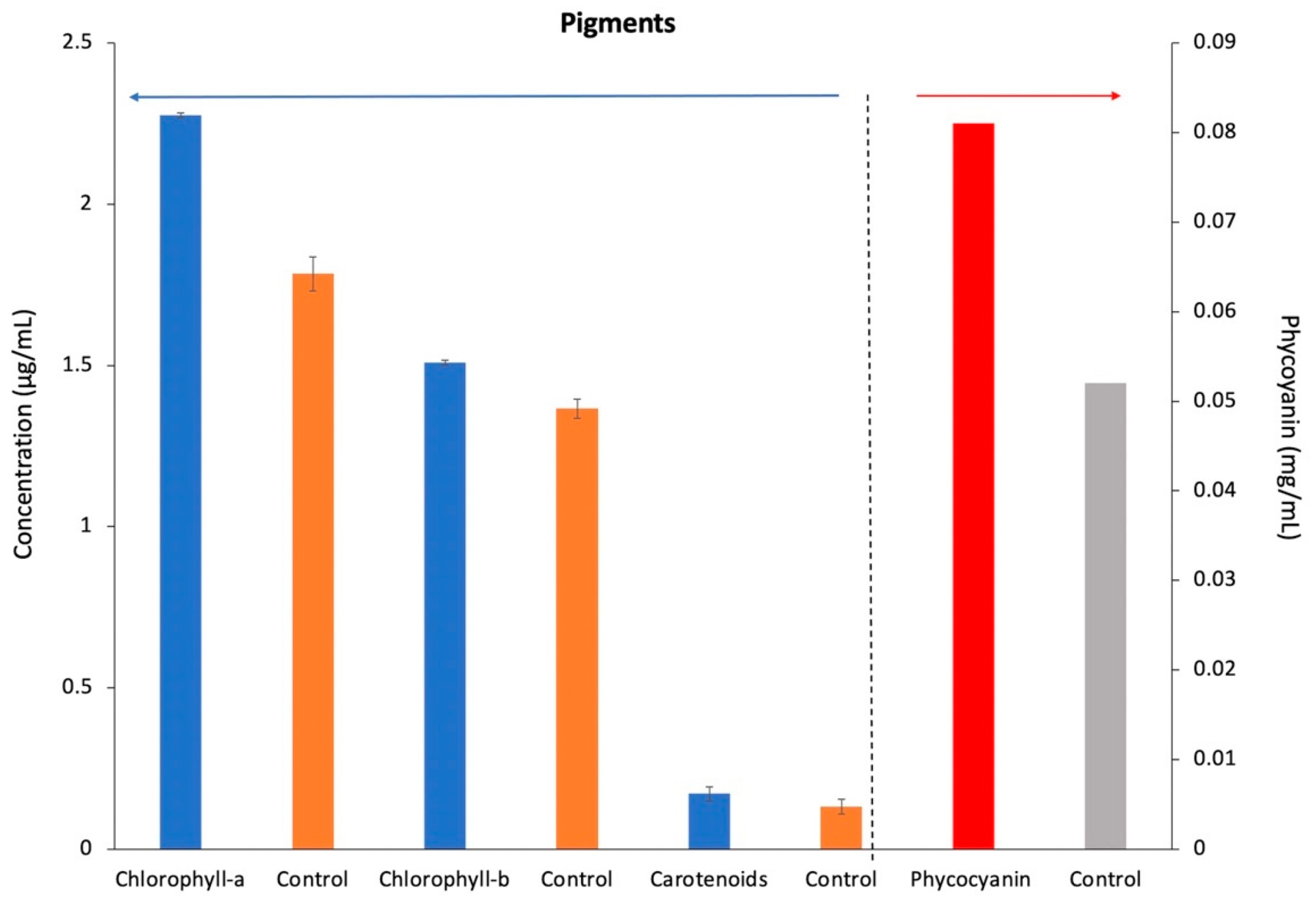
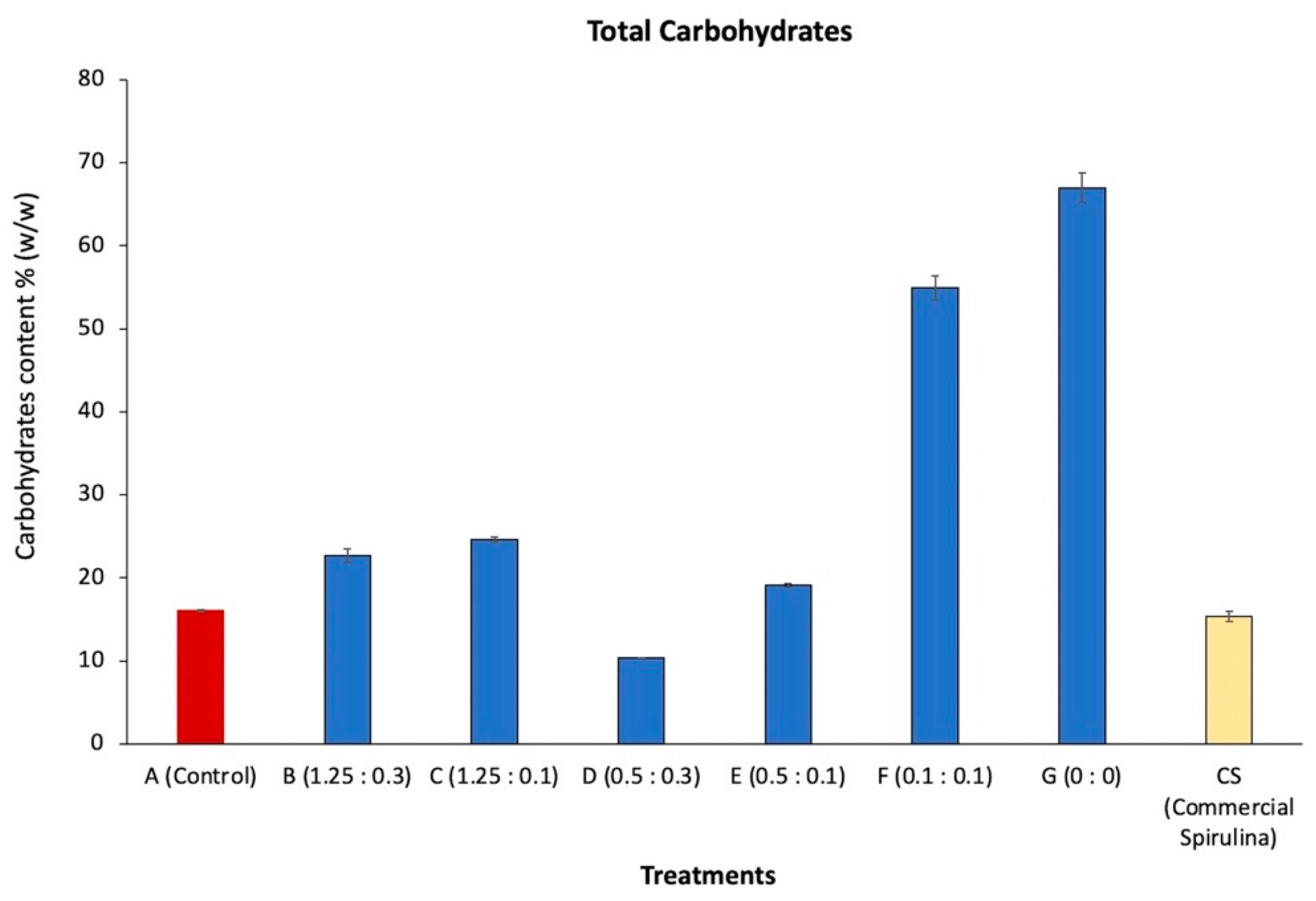
| Treatment | A | B | C | D | E | F | G |
|---|---|---|---|---|---|---|---|
| Chemical | g/L | ||||||
| NaHCO3 | 16.80 | 16.80 | 16.80 | 16.80 | 16.80 | 16.80 | 16.80 |
| NaNO3 | 2.50 | 1.25 | 1.25 | 0.50 | 0.50 | 0.10 | 0.00 |
| K2HPO4 | 0.50 | 0.30 | 0.10 | 0.30 | 0.10 | 0.10 | 0.00 |
| K2SO4 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| NaCl | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| MgSO4.7H2O | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| CaCl2.2H2O | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 |
| FeSO4.7H2O | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| EDTA | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 |
| Source of Variation | Degrees of Freedom | Sum Squares | Mean Squares | F-Test | p-Value |
|---|---|---|---|---|---|
| Treatment | 6 | 8312.47 | 1385.41 | 1674.52 | 0.00 |
| Error | 14 | 11.58 | 0.82 | ||
| Total | 20 | 8324.06 |
| Treatment | Xmax (g/L) | Pmax (g/L/d) | μ (1/d) | Dt |
|---|---|---|---|---|
| Control | 0.90 ± 0.00 | 0.89 ± 0.00 | 0.04 ± 0.00 | 15.93 ± 0.98 |
| G | 0.93 ± 0.00 | 0.92 ± 0.00 | 0.07 ± 0.00 | 9.32 ± 0.40 |
| Treatment | Carbohydrates Content % (w/w) | Lipid (μg/mg) | Protein (μg/mL) |
|---|---|---|---|
| Control | 16.07 ± 0.07 | 17.87 ± 0.33 | 40.35 ± 1.05 |
| G (0:0) | 74.43 ± 0.63 | 26.25 ± 0.46 | 16.84 ± 1.58 |
| Commercial Spirulina | 15.36 ± 0.62 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saxena, R.; Rodríguez-Jasso, R.M.; Chávez-Gonzalez, M.L.; Aguilar, C.N.; Quijano, G.; Ruiz, H.A. Strategy Development for Microalgae Spirulina platensis Biomass Cultivation in a Bubble Photobioreactor to Promote High Carbohydrate Content. Fermentation 2022, 8, 374. https://doi.org/10.3390/fermentation8080374
Saxena R, Rodríguez-Jasso RM, Chávez-Gonzalez ML, Aguilar CN, Quijano G, Ruiz HA. Strategy Development for Microalgae Spirulina platensis Biomass Cultivation in a Bubble Photobioreactor to Promote High Carbohydrate Content. Fermentation. 2022; 8(8):374. https://doi.org/10.3390/fermentation8080374
Chicago/Turabian StyleSaxena, Rohit, Rosa M. Rodríguez-Jasso, Mónica L. Chávez-Gonzalez, Cristóbal N. Aguilar, Guillermo Quijano, and Héctor A. Ruiz. 2022. "Strategy Development for Microalgae Spirulina platensis Biomass Cultivation in a Bubble Photobioreactor to Promote High Carbohydrate Content" Fermentation 8, no. 8: 374. https://doi.org/10.3390/fermentation8080374
APA StyleSaxena, R., Rodríguez-Jasso, R. M., Chávez-Gonzalez, M. L., Aguilar, C. N., Quijano, G., & Ruiz, H. A. (2022). Strategy Development for Microalgae Spirulina platensis Biomass Cultivation in a Bubble Photobioreactor to Promote High Carbohydrate Content. Fermentation, 8(8), 374. https://doi.org/10.3390/fermentation8080374








