Pigment Production by Paracoccus spp. Strains through Submerged Fermentation of Valorized Lignocellulosic Wastes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microorganisms, Culture Media and Growth Conditions
2.3. Pretreatment of Lignocellulosic Feedstocks
2.4. Saccharification of Pretreated Lignocellulosic Feedstocks
2.5. Fermentation Conditions for Pigment Production
2.6. Pigment Extraction and Assessment
3. Results and Discussion
3.1. Morphological Characteristics of Paracoccus Strains
3.2. Pretreatment of Lignocellulosic Feedstocks
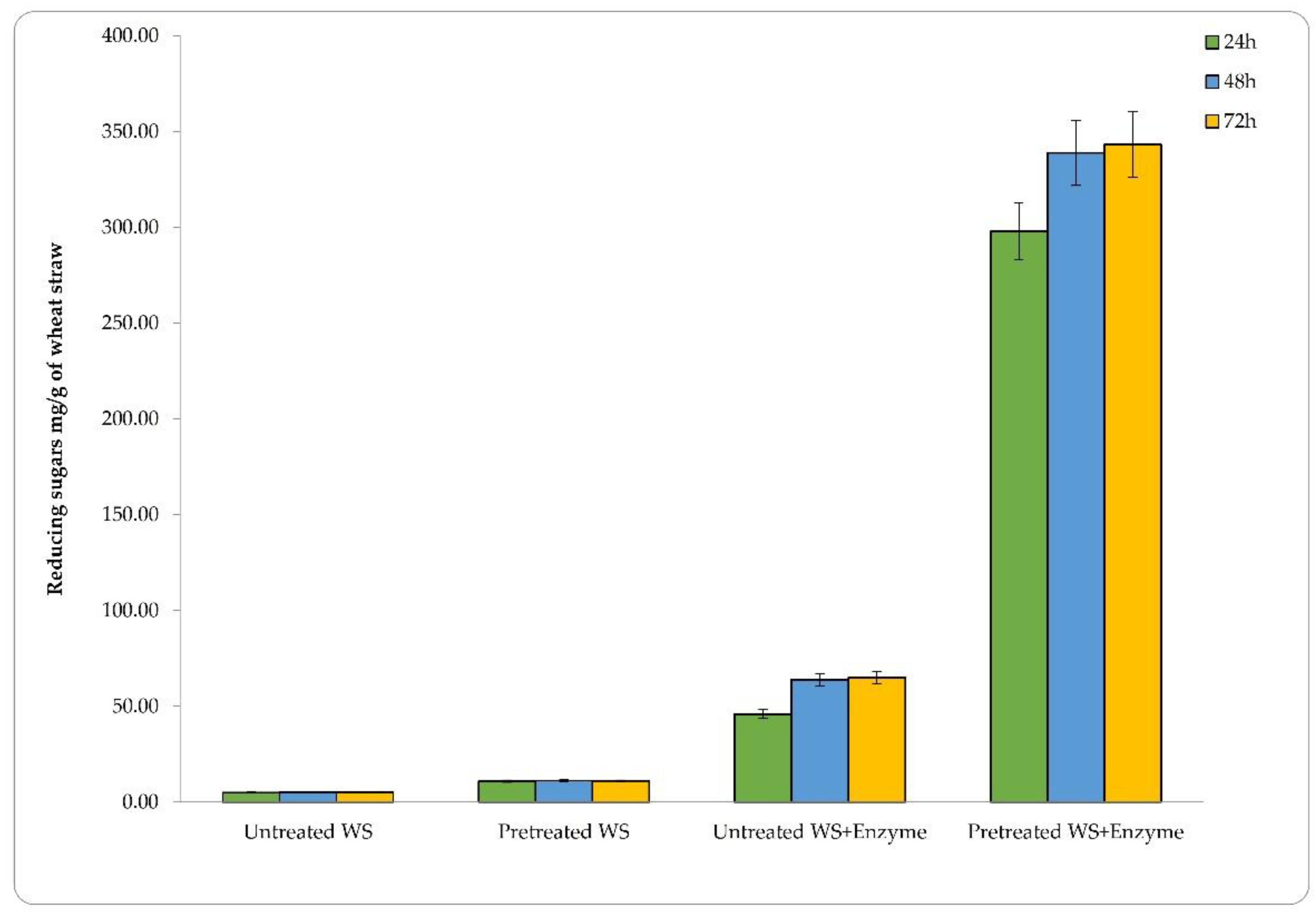
3.3. Saccharification of Pretreated Lignocellulosic Feedstocks
3.4. Carotenoid Production by Paracoccus Strains via the Fermentation of Lignocellulosic Hydrolysates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Downham, A.; Collins, P. Colouring Our Foods in the Last and next Millennium. Int. J. food Sci. Technol. 2000, 35, 5–22. [Google Scholar]
- Grewal, J.; Woła̧cewicz, M.; Pyter, W.; Joshi, N.; Drewniak, L.; Pranaw, K. Colorful Treasure from Agro-Industrial Wastes: A Sustainable Chassis for Microbial Pigment Production. Front. Microbiol. 2022, 13, 832918. [Google Scholar] [PubMed]
- BEUC—The European Consumer Organization—The Chemicals Strategy for Sustainability. 2020. Available online: https://www.beuc.eu/sites/default/files/publications/beuc-x-2020-105_beuc_welcomes_the_chemicals_strategy_for_sustainability_letter_to_commissioner_sinkevicius.pdf (accessed on 17 August 2022).
- Mohammadi, M.A.; Ahangari, H.; Mousazadeh, S.; Hosseini, S.M.; Dufossé, L. Microbial Pigments as an Alternative to Synthetic Dyes and Food Additives: A Brief Review of Recent Studies. Bioprocess Biosyst. Eng. 2021, 45, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mussagy, C.U.; Khan, S.; Kot, A.M. Current Developments on the Application of Microbial Carotenoids as an Alternative to Synthetic Pigments. Crit. Rev. Food Sci. Nutr. 2021, 62, 6932–6946. [Google Scholar] [CrossRef] [PubMed]
- Sigurdson, G.T.; Tang, P.; Giusti, M.M. Natural Colorants: Food Colorants from Natural Sources. Annu. Rev. Food Sci. Technol. 2017, 8, 261–280. [Google Scholar] [CrossRef]
- Venil, C.K.; Dufossé, L.; Devi, P.R. Bacterial Pigments: Sustainable Compounds with Market Potential for Pharma and Food Industry. Front. Sustain. Food Syst. 2020, 4, 100. [Google Scholar]
- Pailliè-Jiménez, M.E.; Stincone, P.; Brandelli, A. Natural Pigments of Microbial Origin. Front. Sustain. Food Syst. 2020, 4, 590439. [Google Scholar]
- Chatragadda, R.; Dufossé, L. Ecological and Biotechnological Aspects of Pigmented Microbes: A Way Forward in Development of Food and Pharmaceutical Grade Pigments. Microorganisms 2021, 9, 637. [Google Scholar] [CrossRef]
- Ram, S.; Mitra, M.; Shah, F.; Tirkey, S.R.; Mishra, S. Bacteria as an Alternate Biofactory for Carotenoid Production: A Review of Its Applications, Opportunities and Challenges. J. Funct. Foods 2020, 67, 103867. [Google Scholar] [CrossRef]
- Maj, A.; Dziewit, L.; Drewniak, L.; Garstka, M.; Krucon, T.; Piatkowska, K.; Gieczewska, K.; Czarnecki, J.; Furmanczyk, E.; Lasek, R. In Vivo Creation of Plasmid PCRT01 and Its Use for the Construction of Carotenoid-Producing Paracoccus spp. Strains That Grow Efficiently on Industrial Wastes. Microb. Cell Fact. 2020, 19, 141. [Google Scholar] [CrossRef]
- Ashokkumar, V.; Venkatkarthick, R.; Jayashree, S.; Chuetor, S.; Dharmaraj, S.; Kumar, G.; Chen, W.-H.; Ngamcharussrivichai, C. Recent Advances in Lignocellulosic Biomass for Biofuels and Value-Added Bioproducts-A Critical Review. Bioresour. Technol. 2022, 344, 126195. [Google Scholar] [PubMed]
- Yadav, N.; Pranaw, K.; Khare, S.K. Screening of Lactic Acid Bacteria Stable in Ionic Liquids and Lignocellulosic By-Products for Bio-Based Lactic Acid Production. Bioresour. Technol. Reports 2020, 11, 100423. [Google Scholar]
- Liu, Y.; Nie, Y.; Lu, X.; Zhang, X.; He, H.; Pan, F.; Zhou, L.; Liu, X.; Ji, X.; Zhang, S. Cascade Utilization of Lignocellulosic Biomass to High-Value Products. Green Chem. 2019, 21, 3499–3535. [Google Scholar]
- Kobayashi, T.; Nakajima, L. Sustainable Development Goals for Advanced Materials Provided by Industrial Wastes and Biomass Sources. Curr. Opin. Green Sustain. Chem. 2021, 28, 100439. [Google Scholar]
- FAO. Food and Agriculture Organization of the United Nations. Database (FAOSTAT). 2021. Available online: https://www.fao.org/faostat/en/#data (accessed on 24 June 2021).
- Koryś, K.A.; Latawiec, A.E.; Grotkiewicz, K.; Kuboń, M. The Review of Biomass Potential for Agricultural Biogas Production in Poland. Sustainability 2019, 11, 6515. [Google Scholar]
- De Medeiros, T.D.M.; Dufossé, L.; Bicas, J.L. Lignocellulosic Substrates as Starting Materials for the Production of Bioactive Biopigments. Food Chem. X 2022, 13, 100223. [Google Scholar] [PubMed]
- Lopes, F.C.; Ligabue-Braun, R. Agro-Industrial Residues: Eco-Friendly and Inexpensive Substrates for Microbial Pigments Production. Front. Sustain. Food Syst. 2021, 5, 589414. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass. Lab. Anal. Proced. 2008, 1617, 1–16. [Google Scholar]
- Sharma, A.; Nain, V.; Tiwari, R.; Singh, S.; Adak, A.; Nain, P.K.S.; Nain, L. Simultaneous Saccharification and Fermentation of Alkali-Pretreated Corncob under Optimized Conditions Using Cold-Tolerant Indigenous Holocellulase. Korean J. Chem. Eng. 2017, 34, 773–780. [Google Scholar]
- Jin, S.; Zhang, G.; Zhang, P.; Li, F.; Wang, S.; Fan, S.; Zhou, S. Microwave Assisted Alkaline Pretreatment to Enhance Enzymatic Saccharification of Catalpa Sawdust. Bioresour. Technol. 2016, 221, 26–30. [Google Scholar]
- Miller, G.L.; Blum, R.; Glennon, W.E.; Burton, A.L. Measurement of Carboxymethylcellulase Activity. Anal. Biochem. 1960, 1, 127–132. [Google Scholar] [CrossRef]
- Liaaen-Jensen, S.; Jensen, A. Quantitative determination of carotenoids in photosynthetic tissues. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1971; Volume 23, pp. 586–602. ISBN 0076-6879. [Google Scholar]
- Ali, N.; Zhang, Q.; Liu, Z.-Y.; Li, F.-L.; Lu, M.; Fang, X.-C. Emerging Technologies for the Pretreatment of Lignocellulosic Materials for Bio-Based Products. Appl. Microbiol. Biotechnol. 2020, 104, 455–473. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K.; Jagtap, S.S.; Bedekar, A.A.; Bhatia, R.K.; Patel, A.K.; Pant, D.; Banu, J.R.; Rao, C.V.; Kim, Y.-G.; Yang, Y.-H. Recent Developments in Pretreatment Technologies on Lignocellulosic Biomass: Effect of Key Parameters, Technological Improvements, and Challenges. Bioresour. Technol. 2020, 300, 122724. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, J.; Kontogianni, N.; Barampouti, E.M.; Mai, S.; Moustakas, K.; Malamis, D.; Loizidou, M. Towards Upscaling the Valorization of Wheat Straw Residues: Alkaline Pretreatment Using Sodium Hydroxide, Enzymatic Hydrolysis and Biogas Production. Environ. Sci. Pollut. Res. 2021, 28, 24486–24498. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wang, M.; Zhao, Z.; Hu, J.; Zhang, J.; Liu, P. Effect of Different Pretreatment of Birch Sawdust on the Production of Active Polysaccharides by Inonotus Obliquus under Submerged Fermentation and Its Structural Mechanism. Appl. Biochem. Biotechnol. 2021, 193, 1545–1557. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.K.; Da Silva, S.S.; Singh, O.V. Detoxification of Lignocellulose Hydrolysates: Biochemical and Metabolic Engineering toward White Biotechnology. BioEnergy Res. 2013, 6, 388–401. [Google Scholar] [CrossRef]
- Rusanen, A.; Lappalainen, K.; Kärkkäinen, J.; Tuuttila, T.; Mikola, M.; Lassi, U. Selective Hemicellulose Hydrolysis of Scots Pine Sawdust. Biomass Convers. Biorefinery 2019, 9, 283–291. [Google Scholar] [CrossRef]
- Kumar, P.; Jun, H.-B.; Kim, B.S. Co-Production of Polyhydroxyalkanoates and Carotenoids through Bioconversion of Glycerol by Paracoccus Sp. Strain LL1. Int. J. Biol. Macromol. 2018, 107, 2552–2558. [Google Scholar] [CrossRef]
- Muhammad, M.; Aloui, H.; Khomlaem, C.; Hou, C.T.; Kim, B.S. Production of Polyhydroxyalkanoates and Carotenoids through Cultivation of Different Bacterial Strains Using Brown Algae Hydrolysate as a Carbon Source. Biocatal. Agric. Biotechnol. 2020, 30, 101852. [Google Scholar] [CrossRef]
- Choi, S.S.; Seo, Y.B.; Nam, S.-W.; Kim, G.-D. Enhanced Production of Astaxanthin by Co-Culture of Paracoccus Haeundaensis and Lactic Acid Bacteria. Front. Mar. Sci. 2021, 7, 597553. [Google Scholar]
- Lee, J.H.; Kim, Y.T. Cloning and Characterization of the Astaxanthin Biosynthesis Gene Cluster from the Marine Bacterium Paracoccus Haeundaensis. Gene 2006, 370, 86–95. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyter, W.; Grewal, J.; Bartosik, D.; Drewniak, L.; Pranaw, K. Pigment Production by Paracoccus spp. Strains through Submerged Fermentation of Valorized Lignocellulosic Wastes. Fermentation 2022, 8, 440. https://doi.org/10.3390/fermentation8090440
Pyter W, Grewal J, Bartosik D, Drewniak L, Pranaw K. Pigment Production by Paracoccus spp. Strains through Submerged Fermentation of Valorized Lignocellulosic Wastes. Fermentation. 2022; 8(9):440. https://doi.org/10.3390/fermentation8090440
Chicago/Turabian StylePyter, Weronika, Jasneet Grewal, Dariusz Bartosik, Lukasz Drewniak, and Kumar Pranaw. 2022. "Pigment Production by Paracoccus spp. Strains through Submerged Fermentation of Valorized Lignocellulosic Wastes" Fermentation 8, no. 9: 440. https://doi.org/10.3390/fermentation8090440
APA StylePyter, W., Grewal, J., Bartosik, D., Drewniak, L., & Pranaw, K. (2022). Pigment Production by Paracoccus spp. Strains through Submerged Fermentation of Valorized Lignocellulosic Wastes. Fermentation, 8(9), 440. https://doi.org/10.3390/fermentation8090440








