1. Introduction
The scarcity of animal feed, particularly protein-rich feed, has become increasingly apparent in recent years, severely impeding the growth of many agricultural sectors [
1]. Soybean meal (SBM) is an important source of plant protein in livestock and poultry nutrition. It has a crude protein content of 440–530 g/kg DMB and has an optimal amino acid profile with high digestibility [
2]. SBM is low in crude fiber (31–72 g/kg) and high in phosphorus (6.3–6.4 g/kg), mostly in the form of phytic acid [
2]. However, the presence of allergenic proteins and anti-nutritional factors limits the use of soybean meal, which can affect digestion and nutrient absorption in humans and animals [
3]. β-conglycinin (7S) and glycinin (11S) are known to induce various hypersensitivity reactions affecting the skin, intestines, and respiratory tract [
4]. The majority of the phosphorus in SBM is present as phytate, which is poorly absorbed in the digestive tract of livestock animals because many of them lack endogenous phytase [
5].
Fermentation was proved to improve the nutritional value of soybeans by increasing nutrient bioavailability and reducing antinutritional factors [
6]. Natural fermentation can significantly increase the content of water-soluble protein and the activity of digestive enzymes [
7]. Fermentation also contributes to a significant decrease in urease activity and trypsin inhibition activity, which in turn increases the digestibility of fermented soybean meal (FSBM) [
8].
The content of crude and soluble proteins, total phenol, and flavonoids increased significantly after solid phase fermentation of SBM with
Bacillus subtilis, while the content of trypsin inhibitor decreased. The result of a high-performance analysis of capillary electrophoresis showed an increase in the content of soluble components in FSBM compared to unfermented SBM. Antioxidant activity analysis showed that the reducing capacity, scavenging of 2,2-diphenyl-1-picrylhydrazyl and 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) radicals of FSBM was stronger than that of unfermented SBM. Antihypertensive activity was also found in SBM after fermentation [
9].
There are cases of SBM fermentation using microscopic fungi. Fungal fermentation with
Aspergillus ficuum and
Aspergillus niger caused the enrichment of soybean meal in protein and organic acids with a reduced content of dietary fiber, tannins, phytic acid, and urease, while the content of trypsin inhibitors was increased [
10].
Yang et al. [
11] evaluated the effect of solid-phase SBM fermentation using a mixed culture of
Lacticaseibacillus casei, yeast, and
Bacillus subtilis and found that fermented SBM had a lower in vitro IgE binding capacity than unfermented SBM, which reduced allergic reactions when FSBM is introduced into the diet.
Fermentation carried out in several stages is promising. Two-stage fermentation using
B. subtilis followed by
E. faecium is known to be an effective approach to improving the quality of feed from soybean meal. As a result, the content of lactic acid (a natural preservative), crude protein, and total phosphorus increased; but the amount of soybean antigenic proteins (β-conglycinin and glycinin), hemicellulose, and phytate decreased [
12].
2. Materials and Methods
2.1. Soybean Meal
Samples of SBM were provided by the Sodruzhestvo Group of Companies (Svetly, Kaliningrad region, Russia). This product is produced in-house by direct extraction of soybean seeds with a preliminary separation of the soy shell. SBM safety indicators comply with the Unified Veterinary (Veterinary and Sanitary) Requirements for Goods Subject to Veterinary Control (Surveillance) No. 317 of 18 June 2010. The content of crude protein in the provided samples was 48.58%, and urease activity was 0.04 pH unit.
2.2. Microorganisms
Microorganisms strains Lactiplantibacillus plantarum B5466, Saccharomyces cerevisiae Y4653, Aspergillus niger F1270, and Bacillus subtilis B7046 from the collection of the State Research Institute of Genetics and Breeding of Industrial Microorganisms of the Lactiplantibacillus Kurchatov Institute (Moscow, Russia) were used for SBM fermentation.
The Bacillus subtilis strain was cultivated at 37 °C for three days on meat-peptone agar (g/L): peptone—5, beef infusion—3, bacteriological agar—15. The Aspergillus niger strain was cultivated at 26 °C for three days on Czapek medium (g/L): sucrose—30, sodium nitrate—2, potassium hydrogen phosphate—1, magnesium sulfate—0.5, potassium chloride—0.5, iron sulfate—0.01, agar bacteriological—15.0. The Saccharomyces cerevisiae strain was cultivated at 28 °C for three days on YEPD agar (g/L): dextrose—20, peptone—20, yeast extract—10, bacteriological agar—15. The Lactiplantibacillus plantarum strain was cultivated at 28 °C for three days on a medium for lactic acid bacteria (g/L): casein hydrolyzate—20, yeast extract—5, gelatin—2.5, glucose—5, lactose—5, sucrose—5, sodium chloride—4, acetate sodium—1.5, ascorbic acid—0.5, bacteriological agar—15.
2.3. Soybean Meal Fermentation
Two hydromodules were used 30:110 and 30:130 (SBM:water), respectively. These SBM:water ratios were chosen because they provide optimal mixing of the biomass during deep fermentation, and also do not require a lot of energy to further dry the wet SBM. Before fermentation, a 30 g sample of SBM was placed in a 250 mL glass flask and 110 mL of distilled water was added for hydromodule 30:110 (SBM:water). Similarly, for the hydromodule 30:130 (SBM:water), a 30 g sample of SBM was placed in a 250 mL glass flask, followed by 130 mL of distilled water. SBM was autoclaved at 121 °C for 15 min. To prevent the development of foreign microflora, SBM samples were pre-sterilized at a temperature of 121 °C, a pressure of 0.1 MPa, and a duration of 20 min, as described by Huang et al. [
13]. After sterilization, the sample was cooled, and under sterile conditions, 1 ml of culture liquid of a mixed culture of microorganisms containing strains of
Bacillus subtilis,
Aspergillus niger,
Saccharomyces cerevisiae, and
Lactiplantibacillus plantarum was added. A mixed culture of microorganisms was prepared as follows: the cells of each culture were washed twice with a sterile 0.85% NaCl solution and inoculated to give a final inoculation of 1 × 10
7 CFU/mL. Then the resulting inoculums were mixed in a ratio of 1:1:1:1 in equal volumes. Fermentation was carried out at 37 °C and constant stirring (180 RPM) for five days. The parameters of the SBM microbial fermentation process were chosen according to the method described by the authors [
9]. Fermented soybean meal (FSBM) was subsequently freeze-dried in a FreeZone 2.5 L Triad freeze dryer, −84 °C (Labconco, Kansas City, MO, USA) and ground to powder.
2.4. Protein Content Analysis
The content of soluble protein in SBM was measured by the Kjeldahl method (GOST 10846–91. Grain and products of its processing. Method for determination of protein). The Kjeldahl method is based on the ashing of organic matter with concentrated sulfuric acid with copper sulfate to form ammonium sulfate. Then, an alkali solution is added to the cooled mineralizate, as a result of which ammonia is formed, which is distilled off and titrated. The result of the determination is the calculation of the mass fraction of nitrogen in the sample and the conversion to the mass crude fraction of protein using the coefficient (6.25).
The dye-binding method (the Bradford method) was used to determine the amount of protein after fermentation [
14]. The method is based on the binding of the Coomassie brilliant blue G-250 dye to amino acid residues of the protein, primarily to arginine, as well as to tryptophan, tyrosine, histidine, and phenylalanine of the SBM. It was carried out by adding 5 mL of Bradford reagent to 0.1 mL of the SBM solution in a test tube, mixing it, and letting it sit at room temperature for 30 minutes. Optical density was measured on a PE-5400UF spectrophotometer (Vikon-service, Astrakhan, Russia) at a wavelength of 595 nm in a cuvette with a layer thickness of 10 mm. A mixture of the same reagents without SBM was used as a reference solution. A calibration curve was plotted in the concentration range from 0.01 to 0.10 mg of a standard protein sample by measuring the optical density of solutions at 595 nm. The amount of dye initially added and still present in the solution was proportional to the amount of protein that was already present.
2.5. Urease Activity Analysis
Urease activity was evaluated according to GOST 13979.9–69 (Oilcakes and oilmeals. Method for measuring urease activity). The method is based on changing the pH of the phosphate buffer solution, which is formed as a result of the action of urease on the urea contained in the solution. The determination of urease activity as the main anti-nutritional factor was used, since this method is a reliable indicator of the effectiveness of hydrolysis in conditions of humidity, and also the values of this indicator in most cases correlate with another important index—trypsin inhibitor [
15].
2.6. Assessment of the Hydrolysis Degree
The method is based on the neutralization of carboxyl groups of monoaminodicarboxylic acids (aspartic, glutamic) of proteins with a solution of sodium hydroxide, the amount of which spent on neutralization is proportional to the mass fraction of the protein in the sample. The Sørensen formol titration is based on the interaction of amine groups with formaldehyde (as well as other aldehydes) to form methylene derivatives. At the same time, the amino groups lose their basic properties, and the carboxyl group is titrated with caustic alkali.
2.7. Macro- and Microelement Composition
The content of macroelements was determined by capillary electrophoresis using a KAPEL-105M system of capillary electrophoresis (Lumex, St. Petersburg, Russia). The measurement method is based on acid treatment of samples, followed by separation and quantitative determination of cations by capillary electrophoresis. The components are detected by indirect absorption at a wavelength of 267 nm.
The content of trace elements in SBM was determined by X-ray fluorescence spectroscopy using the MAKS Spectroscan (Spektron, St. Petersburg, Russia) according to the adapted method FR.1.31.2014.17343 “Determination of Mg, Al, Si, Zn, P, S, K, Ca, Ba, Ti, Cr, Mn, Fe, Ni, Br, Rb, Sr in plant materials and foodstuffs”.
2.8. Ash Content Determination
The ash content of the SBM samples was determined according to GOST 10847–2019. The essence of this method is the combustion of a ground grain sample, followed by a quantitative determination of the fireproof residue.
2.9. Statistical Analysis
Each experiment was repeated at least three times and data were presented as mean ± standard deviation. Data was processed out using standard methods of mathematical statistics. The software Statistica 10.0 (StatSoft Inc., Tulsa, OK, USA) was used to provide one way analysis of variance (ANOVA). Levene’s test was used to evaluate the equality of sample variances. Significant differences between the samples were detected using the Duncan test. The significance of differences between the mean values was taken at p < 0.05.
4. Discussion
In the present study, microbial fermentation was carried out using a mixed culture of microorganisms Bacillus subtilis, Aspergillus niger, Saccharomyces cerevisiae, and Lactiplantibacillus plantarum. We compared the degree of SBM hydrolysis by a consortium of probiotic microorganisms (Bacillus subtilis, Aspergillus niger, Saccharomyces cerevisiae, Lactiplantibacillus plantarum) for five days at different hydromodules (SBM: water) 30:110 and 30:130. It was found that the maximum accumulation of ammonium nitrogen was observed on the third day of hydrolysis for both modules (2.99% for the hydromodulus 30:110 and 2.45% for the hydromodule 30:130). With further exposure of the consortium of microorganisms to SBM, the content of ammonium nitrogen decreased. This is due to the fact that ammonium nitrogen, including ammonium salts, is the most active, mobile, and reactive form of nitrogenous compounds. It is also well absorbed by a consortium of probiotic microorganisms (Bacillus subtilis, Aspergillus niger, Saccharomyces cerevisiae, and Lactiplantibacillus plantarum), which are involved in chemical reactions. The most significant involvement of nitrogenous compounds in cellular metabolism and enzymatic reactions, which depends on the activity of enzyme systems like proteases and peptidases, occurs on the third day of the enzymatic hydrolysis process.
In addition to ammonium nitrogen, the effect of microbial fermentation on the content of crude protein in samples of soybean meal was evaluated. As a result of enzymatic hydrolysis on the third day of fermentation, there was an increase in crude protein by 6.94% at a hydromodulus of 30:110 (by the Kjeldahl method) and by 8.46% at a hydromodulus of 30:130 (by the Kjeldahl method). According to the Bradford method, the protein content increased by 7.13% at a water ratio of 30:110 and by 9.06% at a water ratio of 30:130 (by the Bradford method). The highest values of the protein content were recorded on the fifth day of hydrolysis. The values of the protein content determined by the Kjeldahl method and by the Bradford method were compared. The values differed in the direction of increased protein according to the Bradford method in the range from 2.5% to 5.4% for the 30:110 hydromodule and from 3.8% to 5.3% for the 30:130 hydromodule. The increase in the protein content determined by the Bradford method is explained by the fact that this method determines and takes into account, in addition of protein, and non-protein nitrogen. Cells of probiotic microorganisms (Bacillus subtilis, Aspergillus niger, Saccharomyces cerevisiae, Lactiplantibacillus plantarum) use nitrogenous substances to build tissues and cell structure, maintain vital activity, as well as high respiratory and enzymatic activity. The most important precursor of consumed organic nitrogen is ammonia or ammonium nitrogen, which these microorganisms assimilate in the first place, and only then consume amine nitrogen.
The urease activity of probiotic microorganisms was determined by changing the pH of the phosphate buffer solution, which is formed as a result of the action of urease on the urea contained in the solution. As a result of the studies, it was found that on the second day the value of urease activity slightly exceeds the initial value, which may be associated with the adaptation processes of microorganisms. The urease reaction can be thought of as a process of ecological mineralization of organic nitrogenous slag; as a result of this process, the water-soluble non-volatile organic substrate, urea, is transformed into volatile products, ammonia, and carbon dioxide, as a result of this enzymatic reaction. However, most of the products formed do not evaporate, but interact with each other in the SBM:water medium to form predominantly ammonium bicarbonate. The latter is absorbed by microorganisms and utilized as a source of nitrogen for the biosynthesis of proteins, nucleic acids, and other important nitrogenous bioorganic components of enzymatic hydrolysis.
However, no clear relationship between urease activity and duration of SBM hydrolysis was found in our studies. This can be explained by the fact that the urease activity of probiotic microorganisms is detected, but is at a relatively low level, in our opinion, for three reasons: due to the low concentration of urease-positive microorganisms of the consortium used for the enzymatic hydrolysis of SBM; due to low background concentration of urea in SBM; due to the possible inhibition of microbial urease by metal ions, the maximum content of which increased on days two–four of the process of enzymatic hydrolysis of SBM by a consortium of probiotic microorganisms (Bacillus subtilis, Aspergillus niger, Saccharomyces cerevisiae, Lactiplantibacillus plantarum).
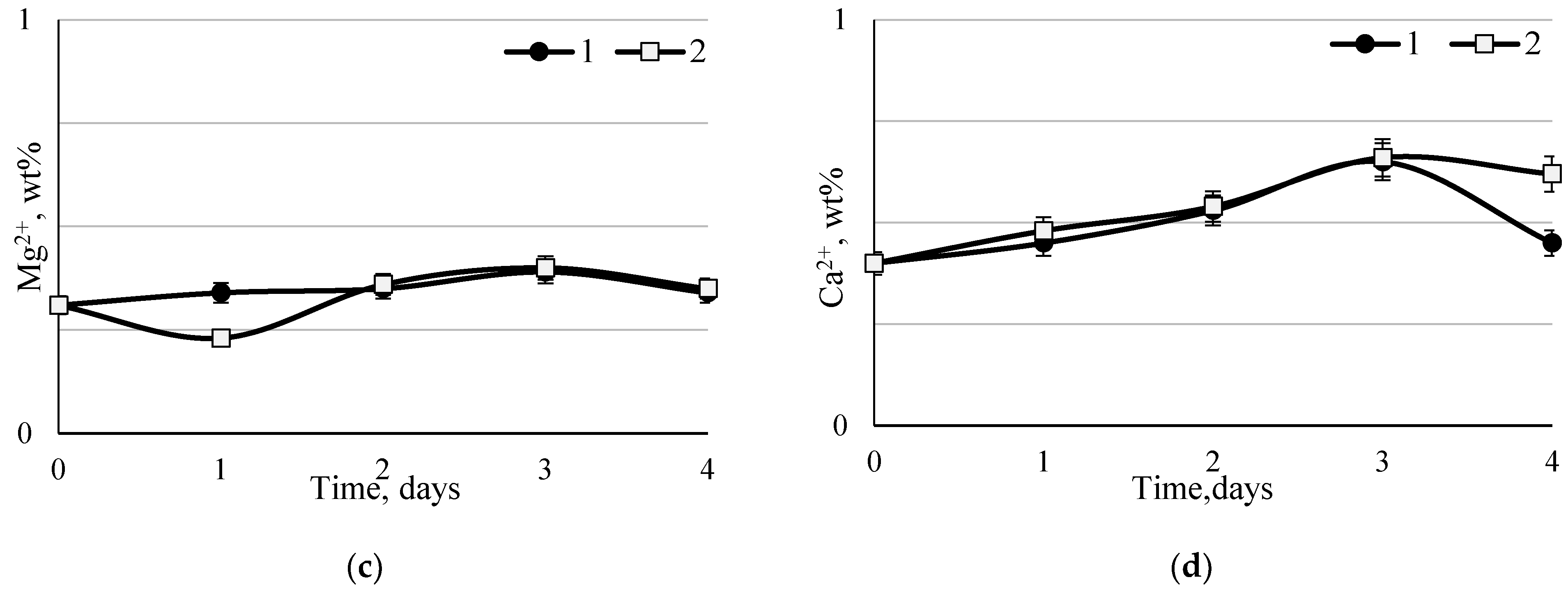
When using the hydromodulus 30:110, the ash content increased throughout the entire fermentation period and reached its maximum on the fifth day of fermentation—7.9%, which was 2.0% higher than the initial ash content. The increase in ash content as a result of enzymatic hydrolysis of SBM by a consortium of probiotic microorganisms (
Bacillus subtilis, Aspergillus niger, Saccharomyces cerevisiae, Lactiplantibacillus plantarum) is probably associated with the accumulation of macro- and microelements as a result of hydrolysis and their low solubility in the medium. Our studies are in good agreement with the studies of other authors [
16,
17,
18,
19,
20,
21,
22,
23,
24].
The basis of this consortium is based on similar work by researchers involved in the microbial conversion of SBM. For example, in the work of Jazi et al., a consortium containing
Lactiplantibacillus plantarum,
Bacillus subtilis и
Aspergillus oryzae was used to ferment SBM [
16]. However, the microorganism
Aspergillus niger was used in the present study, as studies are known where this fermented vegetable protein sources, while reducing the level of anti-nutrients and fiber in the finished product [
17,
18]. Obviously, in the consortium,
Aspergillus niger acts as a biocatalyst due to the destruction of the cellulose shell under the action of the synthesized enzyme β-glucosidase, which is also reported in the literature [
19]. In the present study, the microbial consortium was also supplemented with the microorganism
Saccharomyces cerevisiae. It was assumed that this microorganism contributes to an increase in protein content due to the rapid increase in its own biomass. This assumption was confirmed by the results obtained, indicating an increase in protein content by 9.42–10.45%. For example, in a study by Sharawy et al., using only
Saccharomyces cerevisiae, a 6% increase in crude protein content was achieved within 48 h of cultivation [
20]. These obtained results also give grounds to be convinced of the greater efficiency of fermentation by a consortium of a microorganism than by isolated strains.
The efficiency of microbial fermentation in this work was evaluated on the basis of several parameters, which included such a value as the percentage of amine nitrogen, which reflects the degree of SBM hydrolysis. That is, this value describes the ratio of the nitrogen of α-amino groups to the total nitrogen. The degree of hydrolysis of defatted soy flour by the microorganism
Aspergillus oryzae was evaluated by the formol titration method in the present study, as well as in research by Zhao et al. So, in the work of the authors, the degree of hydrolysis increased by 17.46% (at 24 h), then a decrease of 2.05% was observed (at 44 h). However, these data do not correlate with the results of the present study, since an increase in the degree of hydrolysis was observed only by 1.33%. However, this can be explained by the initially low content of crude protein in soy flour (8.45%) and its subsequent intensive increase to 58.31% [
21].
Urease activity is one of the main anti-nutritional factors evaluated during microbial fermentation of soybean meal. As already mentioned, urease activity reflects the efficiency of heat treatment of soybean meal. Urease, which is found in most legumes, can hydrolyze urea to carbon dioxide and ammonia [
22]. Li et al. showed a significant decrease in urease activity during the 72-hour fermentation. At the end of fermentation, complete inactivation of urease activity was found in fermented samples [
23]. The present study shows a two-fold decrease. I would also like to note that this index in most cases correlates with another important indicator of anti-nutritional factors—trypsin inhibitor [
15]. In this connection, it is promising and necessary to evaluate the dynamics of the trypsin inhibitor content in further studies of microbial fermentation of soybean meal.
The practical novelty of this study is a detailed analysis of the macro- and microelement composition of SBM and FSBM. In one of the few studies on the topic of assessing the microelement composition of FSBM, Dan et al. [
24] achieved an increase in the content of iron by 6.2%, zinc by 12.9%, and calcium by 46.1% using microbial fermentation of SBM. This study found that the content of macro- and microelements in FSBM increased to varying degrees, however, in general, it can be concluded that microbial hydrolysis has a positive effect on the FSBM composition. Based on the presented results, studies on the effect of various technological regimes and microbial communities on the macro- and microelement composition of soybean meal also seem promising.