The Purification and Biochemical Characterization of a Weissella cibaria F1 Derived β-Mannanase for Its Use in the Preparation of Konjac Oligo-Glucomannan with Immunomodulatory Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Cultures and Cell Line
2.2. Material and Reagents
2.3. Mannanase Activity and Protein Detection
2.4. Purification and Determination of Molecular Mass
2.5. Enzyme Identification by Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS)
2.6. Biochemical Characterization
2.7. Enzymatic Preparation KOGM
2.8. Immunomodulatory Activity Detection
2.8.1. Cell Culture
2.8.2. Cell Viability Assay
2.8.3. Phagocytosis Measurement
2.8.4. NO Assay
2.8.5. Cytokine Detection
2.9. Statistical Analysis
3. Results
3.1. Purification and Identification of W. cibaria F1 Mannanase
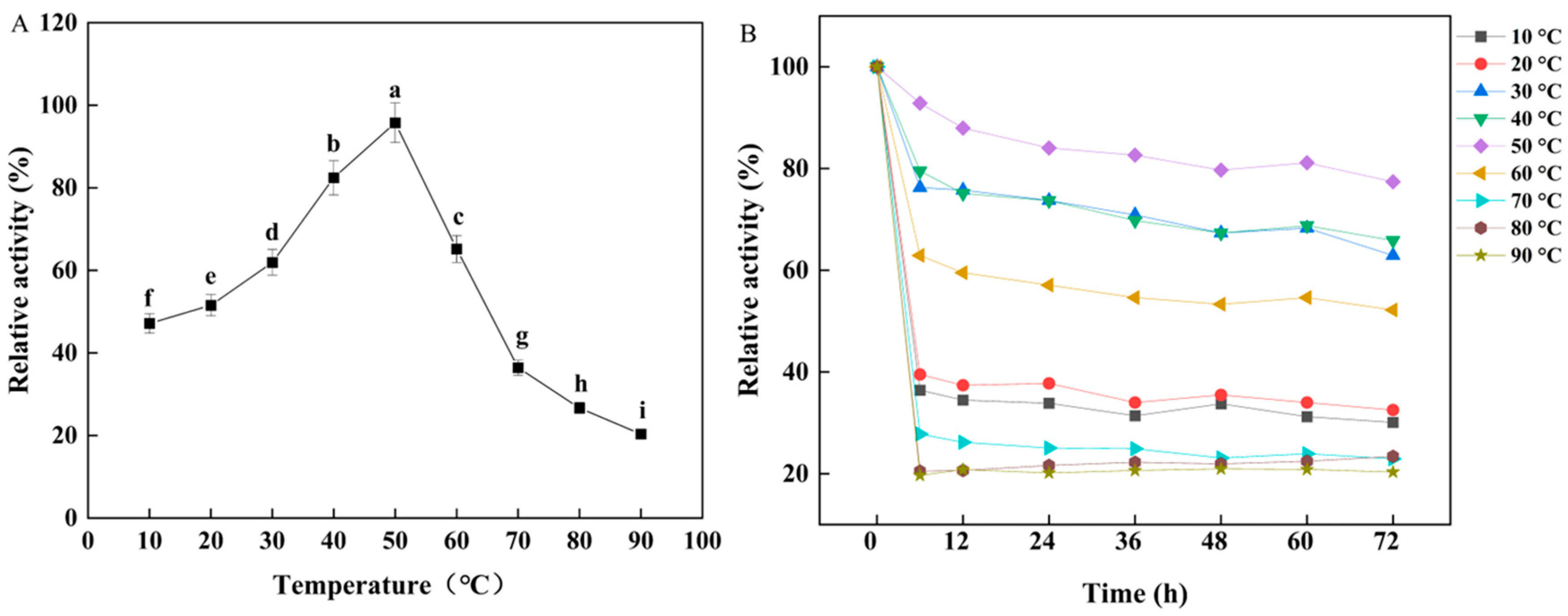
3.2. Effects of Temperature and pH on W. cibaria F1 Mannanase
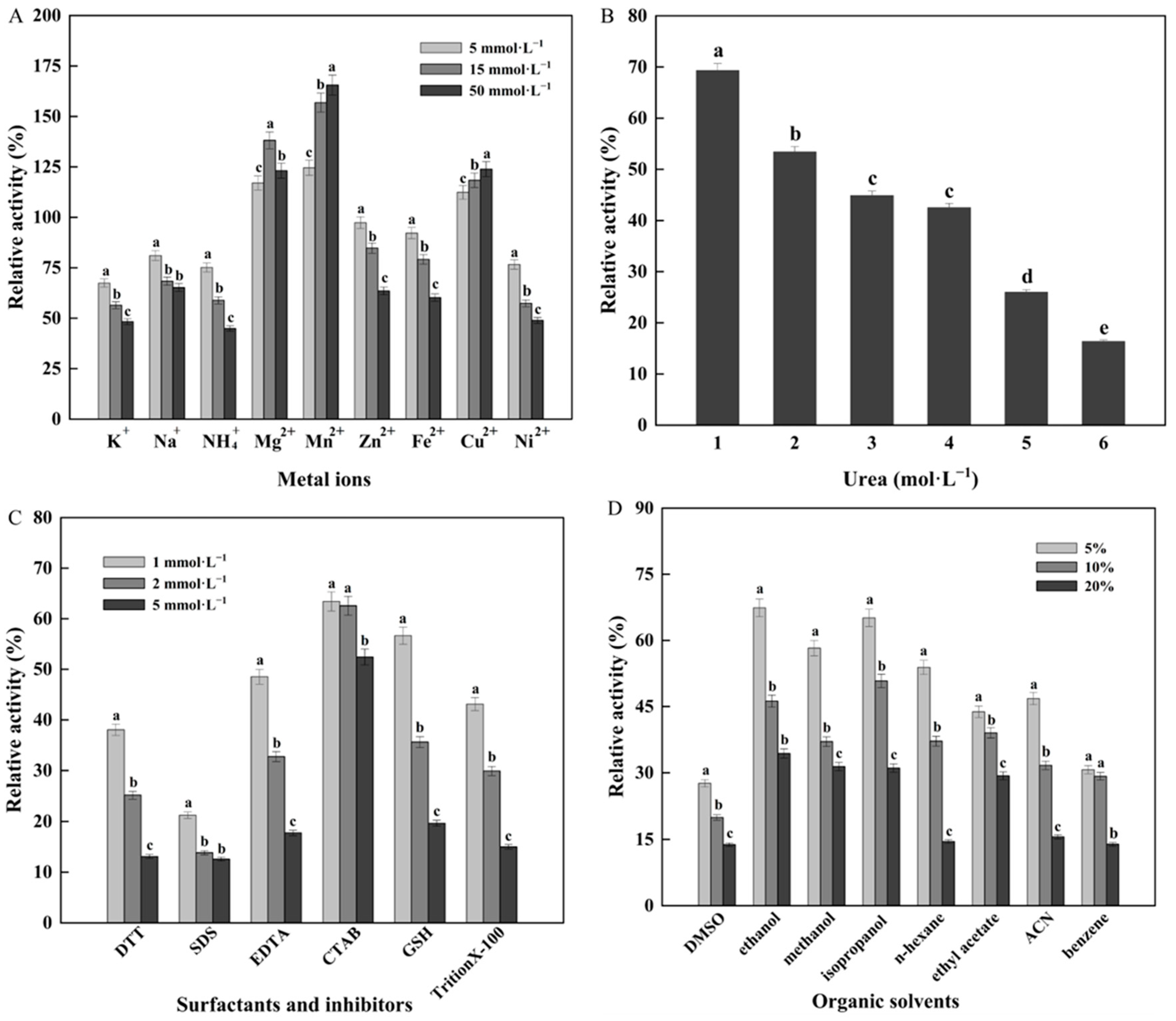
3.3. Effects of Metal Ions and Chemical Agents on W. cibaria F1 Mannanase
3.4. Kinetic Parameters of W. cibaria F1 Mannanase under Different Substrates
3.5. Immunomodulatory Activity of W. cibaria F1 Mannanase
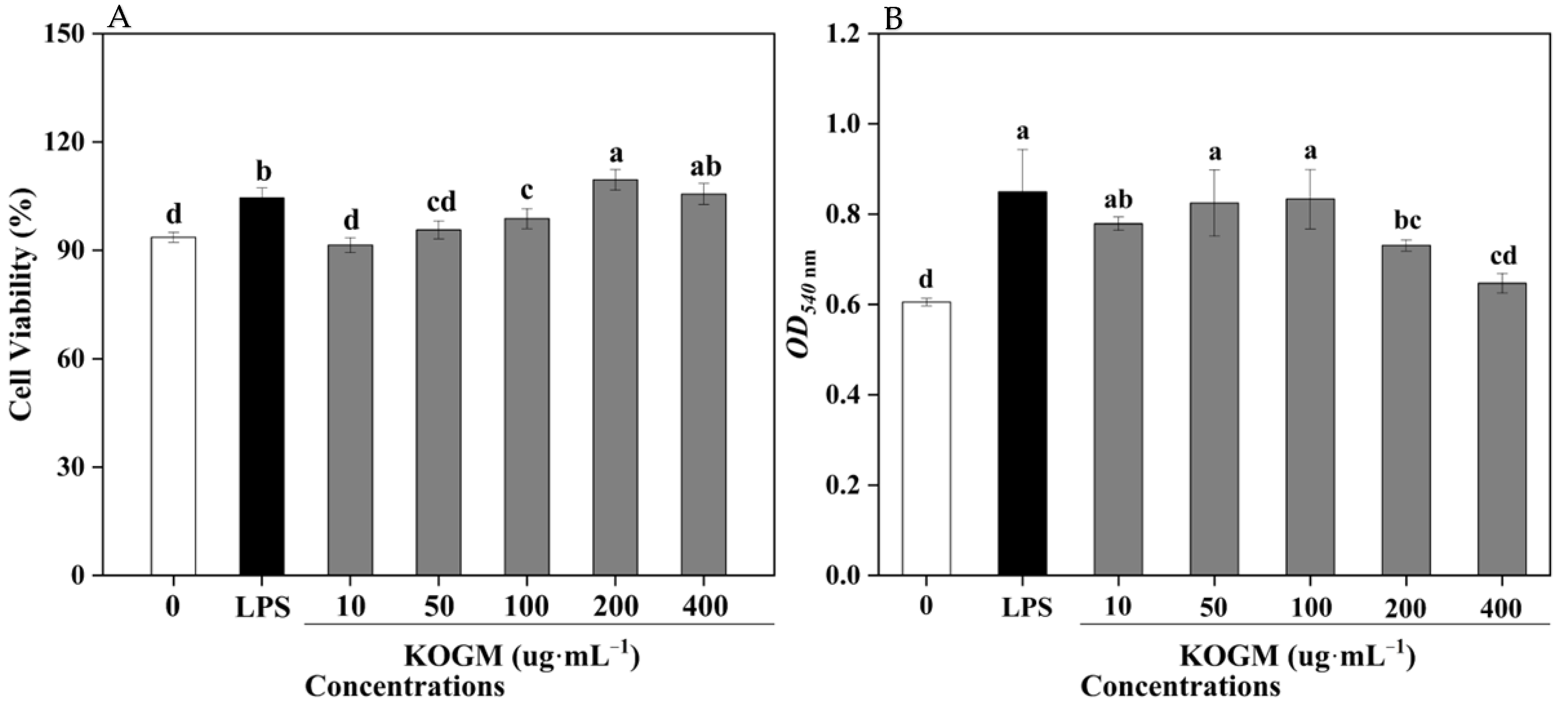
3.5.1. Effect of W. cibaria F1 Mannanase on Cell Viability
3.5.2. Effect of W. cibaria F1 Mannanase on Macrophages Phagocytosis
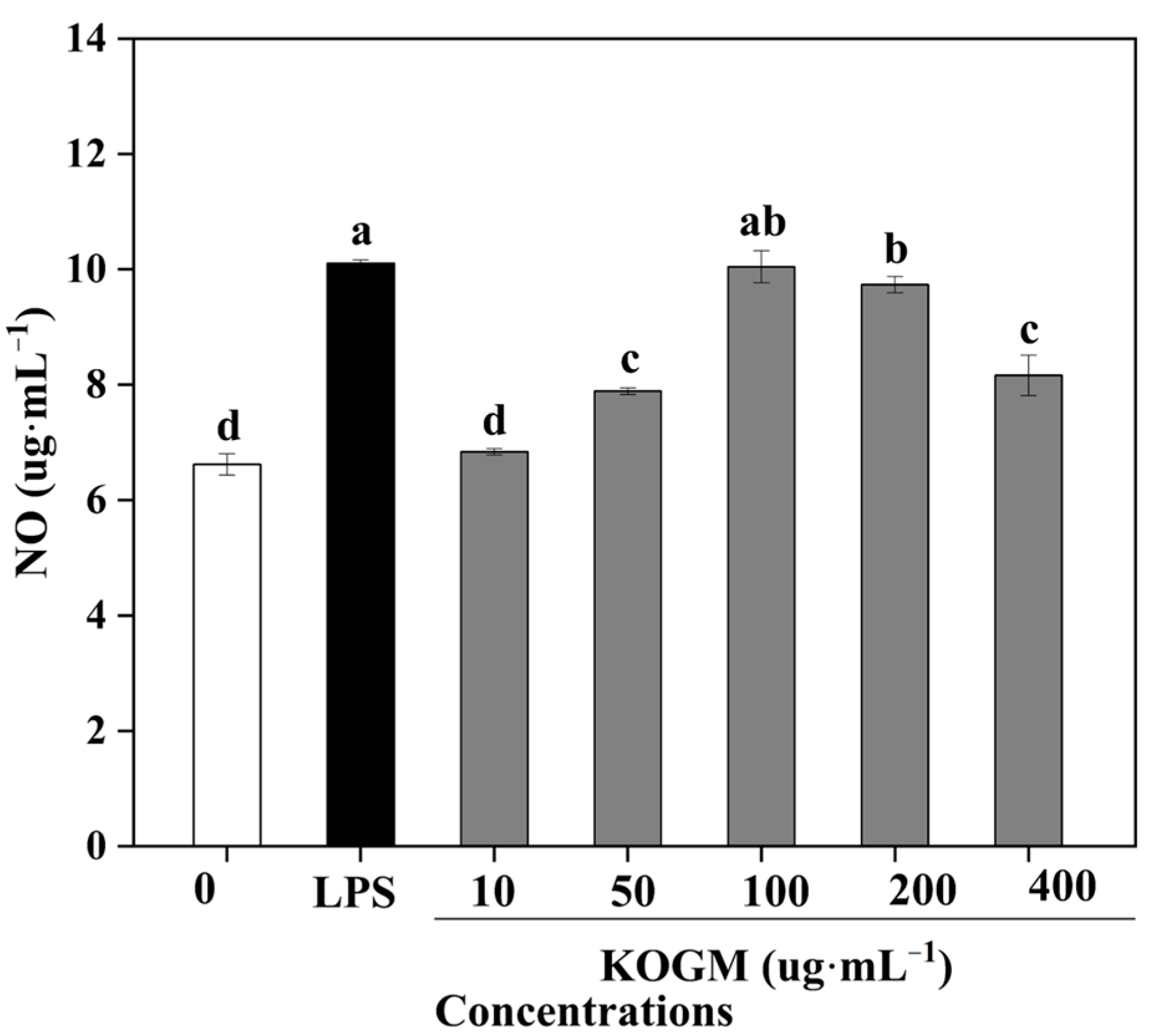
3.5.3. Effect of W. cibaria F1 Mannanase on Production of NO
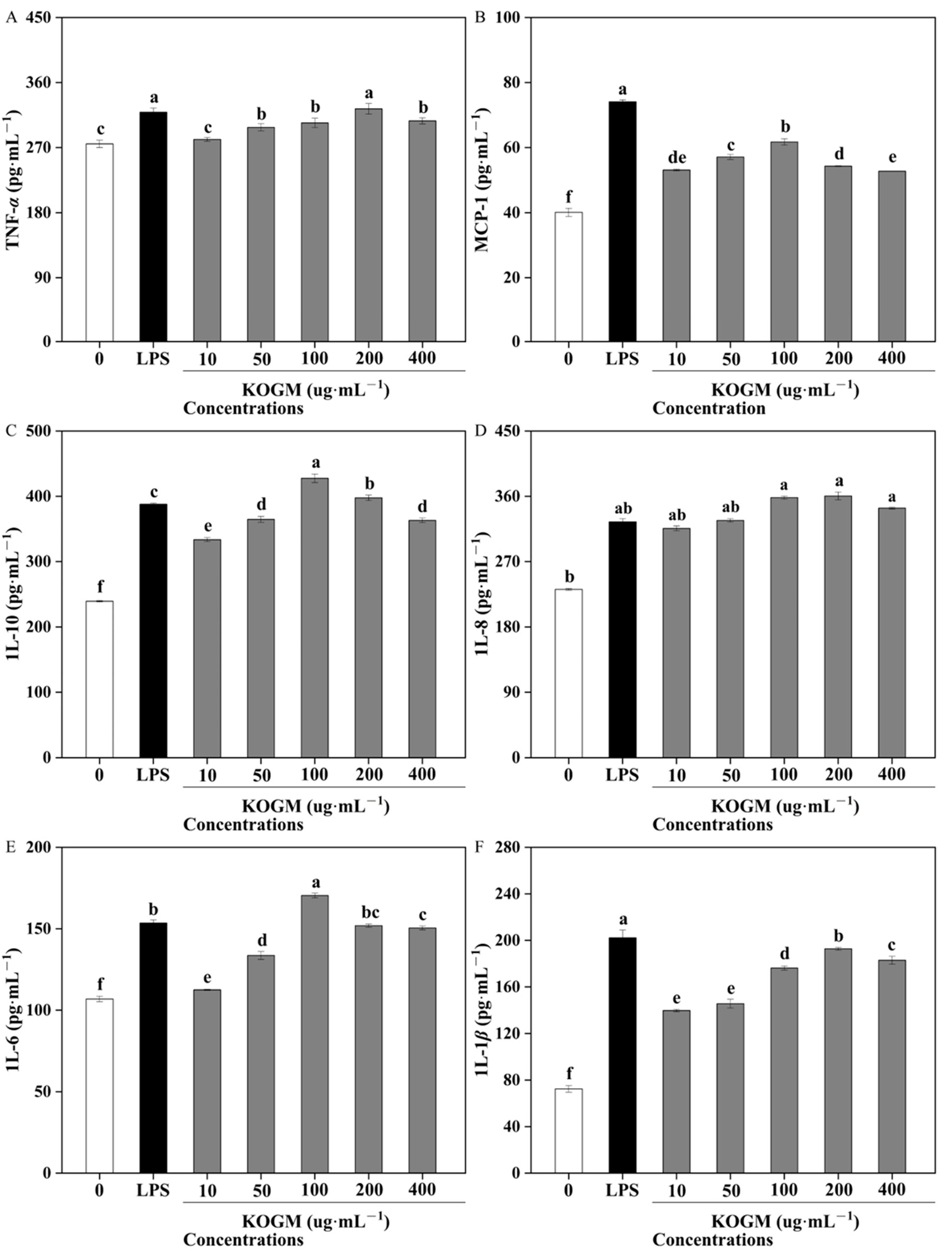
3.5.4. Effect of W. cibaria F1 Mannanase on Production of Cytokines
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Srivastava, P.K.; Kapoor, M. Production, properties, and applications of endo-β-mannanases. Biotechnol. Adv. 2016, 35, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Nopvichai, C.; Charoenwongpaiboon, T.; Luengluepunya, N.; Ito, K.; Pichyangkura, R. Production and purification of mannan oligosaccharide with epithelial tight junction enhancing activity. PeerJ 2019, 7, e7206. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, B.R. Characterization of β-mannanase extracted from a novel Streptomyces species Alg-S25 immobilized on chitosan nanoparticles. Biotechnol. Biotechnol. Equip. 2021, 35, 150–161. [Google Scholar] [CrossRef]
- Singh, S.; Singh, G.; Khatri, M.; Kaur, A.; Arya, S.K. Thermo and alkali stable β-mannanase: Characterization and application for removal of food (mannans based) stain. Int. J. Biol. Macromol. 2019, 134, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Gurler, H.N.; Yilmazer, C.; Erkan, S.B.; Ozcan, A.; Yatmaz, E.; Öziyci, H.R.; Karhan, M.; Turhan, I. Applicability of recombinant Aspergillus sojae crude mannanase enzyme in carrot juice production. J. Food Process. Preserv. 2020, 45, 1–9. [Google Scholar] [CrossRef]
- Dhawan, S.; Singh, R.; Kaur, R.; Kaur, J. A β-mannanase from Paenibacillus sp.: Optimization of production and its possible prebiotic potential. Biotechnol. Appl. Biochem. 2016, 63, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Ahirwar, S.; Soni, H.; Rawat, H.K.; Prajapati, B.P.; Kango, N. Experimental design of response surface methodology used for utilisation of palm kernel cake as solid substrate for optimised production of fungal mannanase. Mycology 2016, 7, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Peterbauer, C.; Maischberger, T.; Haltrich, D. Food-grade gene expression in lactic acid bacteria. Biotechnol. J. 2011, 6, 1147–1161. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, X.; Wang, Y.; Na, J.; Ge, J.P. Purification, biochemical and secondary structural characterisation of β-mannanase from Lactobacillus casei HDS-01 and juice clarification potential. Int. J. Biol. Macromol. 2020, 154, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Nadaroglu, H.; Adiguzel, G.; Adiguzel, A.; Sonmez, Z. A thermostable-endo-β-(1,4)-mannanase from Pediococcus acidilactici (M17): Purification, characterization and its application in fruit juice clarification. Eur. Food Res. Technol. 2017, 243, 193–201. [Google Scholar] [CrossRef]
- Nadaroglu, H.; Adiguzel, A.; Adiguzel, G. Purification and characterisation of β-mannanase from Lactobacillus plantarum (M24) and its applications in some fruit juices. Int. J. Food Sci. Technol. 2015, 50, 1158–1165. [Google Scholar] [CrossRef]
- Nadaroglu, H.; Dikbas, N. Purification and characterization of a β-mannanase from Lactobacillus plantarum (ATCC 14917TM). Int. J. Innov. Res. Rev. 2018, 2, 1–6. [Google Scholar]
- Zabidi, N.; Ling, F.H.; Chwen, L.T.; Rosfarizan, M.; Raha, A.R. Enhancement of versatile extracellular cellulolytic and hemicellulolytic enzyme productions by Lactobacillus plantarum RI 11 isolated from Malaysian food using renewable natural polymers. Molecules 2021, 25, 2607. [Google Scholar] [CrossRef]
- Adiguzel, G.; Sonmez, Z.; Adiguzel, A.; Nadaroglu, H. Purification and characterization of a thermostable endo-beta-1,4 mannanase from Weissella viridescens LB37 and its application in fruit juice clarification. Eur. Food Res. Technol. 2016, 242, 769–776. [Google Scholar] [CrossRef]
- Guo, L.; Yokoyama, W.; Chen, L.; Liu, F.; Zhong, F. Characterization and physicochemical properties analysis of konjac glucomannan: Implications for structure-properties relationships. Food Hydrocoll. 2021, 120, 106818. [Google Scholar] [CrossRef]
- Liu, J.H.; Xu, Q.H.; Zhang, J.J.; Zhou, X.X.; Lyu, F.; Zhao, P.C.; Ding, Y.T. Preparation, composition analysis and antioxidant activities of konjac oligo-glucomannan. Carbohydr. Polym. 2015, 130, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Yang, W. Preparation of konjac oligoglucomannans with different molecular weights and their in vitro and in vivo antioxidant activities. Open Life Sci. 2020, 15, 799–807. [Google Scholar] [CrossRef]
- Chen, Z.J.; Wang, S.S.; Shang, L.C.; Zhou, P.Y.; Li, J.; Li, B. An efficient and simple approach for the controlled preparation of partially degraded konjac glucomannan. Food Hydrocoll. 2020, 108, 106017. [Google Scholar] [CrossRef]
- Andrew, M.; Jayaraman, G. Structural features of microbial exopolysaccharides in relation to their antioxidant activity. Carbohydr. Res. 2020, 487, 107881. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.H.; Xu, Y.X.; Song, F.L.; Wang, Z.M.; Li, Y.L.; Zhao, M.Z.; He, F.; Tian, Z.Z.; Yang, Y. Protective effects of konjac glucomannan on gut microbiome with antibiotic perturbation in mice. Carbohydr. Polym. 2022, 290, 119476. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, B.; Yang, X.; Shang, W.; Ma, Q.; Strappe, P. Regulation of hyperglycemia in diabetic mice by autolysates from. J. Sci. Food Agric. 2019, 99, 6981–6988. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.R.; Cao, H.Y.; Zhao, L.; Na, R.Y.; Ping, W.X.; Ge, J.P.; Zhao, D. The response surface optimization of β-mannanase produced by Weissella cibaria F1 and its potential in juice clarification. Prep. Biochem. Biotechnol. 2022, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Toshiro, A.; Nobuyuki, N.; Koki, H. Characterization of three BETA-mannanases of an alkalophilic Bacillus sp. Agric. Biol. Chem. 1988, 52, 773–779. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Chem. 1976, 2, 248–253. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Q.Z.; Li, L.; Zhou, X.W. Immunostimulatory effects of the intracellular polysaccharides isolated from liquid culture of Ophiocordyceps sinensis (Ascomycetes) on RAW264.7 cells via the MAPK and PI3K/Akt signaling pathways. J. Ethnopharmacol. 2021, 275, 114130. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xie, J.H.; Jia, S.; Huang, L.X.; Wang, Z.J.; Li, C.; Xie, M.Y. Immunomodulatory effects of an acetylated Cyclocarya paliurus polysaccharide on murine macrophages RAW264.7. Int. J. Biol. Macromol. 2017, 98, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Kansoh, A.L.; Nagieb, Z.A. Xylanase and mannanase enzymes from Streptomyces galbus NR and their use in biobleaching of softwood kraft pulp. Antonie Van Leeuwenhoek 2004, 85, 103–114. [Google Scholar] [CrossRef]
- Lv, J.N.; Chen, Y.Q.; Pei, H.L.; Yang, W.H.; Li, Z.M.; Dong, B.; Cao, Y.H. Cloning, expression, and characterization of β-mannanase from Bacillus subtilis MAFIC-S11 in Pichia pastoris. Appl. Biochem. Biotechnol. 2013, 169, 2326–2340. [Google Scholar] [CrossRef]
- Lu, H.Q.; Luo, H.Y.; Shi, P.J.; Huang, H.Q.; Meng, K.; Yang, P.L.; Yao, B. A novel thermophilic endo-β-1,4-mannanase from Aspergillus nidulans XZ3: Functional roles of carbohydrate-binding module and Thr/Ser-rich linker region. Appl. Microbiol. Biotechnol. 2014, 98, 2155–2163. [Google Scholar] [CrossRef]
- Sudip, R.; Pradeep, G.C.; Yun, H.C.; Yoon, S.C.; Ji, E.; Seung, S.C.; Jin, C.Y. A multi-tolerant low molecular weight mannanase from Bacillus sp CSB39 and its compatibility as an industrial biocatalyst. Enzym. Microb. Technol. 2016, 92, 76–85. [Google Scholar] [CrossRef]
- Vu, T.T.; Quyen, D.T.; Dao, T.T.; Nguyen, S.T. Cloning, high-level expression, purification, and properties of a novel endo-beta-1,4-mannanase from Bacillus subtilis G1 in Pichia pastoris. J. Microbiol. Biotechnol. 2012, 22, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Leerawatthanakun, S.; Charoenwongpaiboon, T.; Klaewkla, M.; Chunsrivirot, S.; Sirirak, J.; Sriwitool, T.-e.; Wangpaiboon, K.; Pichyangkura, R. High surfactant-tolerant β-mannanase isolated from Dynastes hercules larvae excrement, and identification of its hotspot using site-directed mutagenesis and molecular dynamics simulation. Enzym. Microb. Technol. 2021, 154, 109956. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Kimoto, S.; Shinzawa, Y.; Minezawa, M.; Suzuki, K.; Jindou, S.; Kato, M.; Shimizu, M. Characterization of pH-tolerant and thermostable GH 134 β-1,4-mannanase SsGH134 possessing carbohydrate binding module 10 from Streptomyces sp. NRRL B-24484. J. Biosci. Bioeng. 2018, 125, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Syarifah, A.R.; Darah, I.; Ibrahim, C.O.; Ramli, H.; Tong, W.Y. Purification and physicochemical characterisation of Aspergillus niger USM F4 β-mannanase. Malays. J. Microbiol. 2020, 16, 396–406. [Google Scholar] [CrossRef]
- Dhawan, S. Purification of a thermostable β-mannanase from Paenibacillus Thiaminolyticus-characterization and its potential use as a detergent additive. J. Pure Appl. Microbiol. 2021, 15, 368–381. [Google Scholar] [CrossRef]
- Badejo, O.A.; Olaniyi, O.O.; Oluwadare, A.A.; Lawal, O.T. Biochemical properties of partially purified surfactant-tolerant alkalophilic endo beta-1,4 xylanase and acidophilic beta-mannanase from bacteria resident in ruminants’ guts. Biocatal. Agric. Biotechnol. 2021, 34, 101982. [Google Scholar] [CrossRef]
- Daniel, E.O. Protein unfolding in detergents: Effect of micelle structure, ionic strength, pH, and temperature. Biophys. J. 2002, 83, 2219–2230. [Google Scholar]
- Chauhan, P.S.; Sharma, P.; Puri, N.; Gupta, N. Purification and characterization of an alkali-thermostable β-mannanase from Bacillus nealsonii PN-11 and its application in mannooligosaccharides preparation having prebiotic potential. Eur. Food Res. Technol. 2014, 238, 927–936. [Google Scholar] [CrossRef]
- Cheng, L.F.; Duan, S.W.; Feng, X.Y.; Zheng, K.; Yang, Q.; Liu, Z.C. Purification and characterization of a thermostable -mannanase from Bacillus subtilis be-91: Potential application in inflammatory diseases. BioMed Res. Int. 2019, 2016, 6380147. [Google Scholar] [CrossRef]
- Karahalil, E.; Germe, M.; Karaoglan, M.; Yatmaz, E.; Turhan, I. Partial purification and characterization of a recombinant β-mannanase from Aspergillus fumigatus expressed in Aspergillus sojae grown on carob extract. Biomass Convers. Biorefinery 2020, 10, 1189–1205. [Google Scholar] [CrossRef]
- Uttam, K.J.; Rahul, K.S.; Bhanu, P.P.; Hemant, S.; Naveen, K. Production optimization and characterization of mannooligosaccharide generating β-mannanase from Aspergillus oryzae. Bioresour. Technol. 2018, 268, 308–314. [Google Scholar] [CrossRef]
- Jiang, Z.Q.; Yun, W.; Li, D.Y.; Li, L.; Chai, P.P.; Kusakabe, I. High-level production, purification and characterization of a thermostable β-mannanase from the newly isolated Bacillus subtilis WY34. Carbohydr. Polym. 2006, 66, 88–96. [Google Scholar] [CrossRef]
- Jian, W.; Chen, Y.H.; Wang, L.; Tu, L.; Xiong, H.; Sun, Y.M. Preparation and cellular protection against oxidation of Konjac oligosaccharides obtained by combination of γ -irradiation and enzymatic hydrolysis. Food Res. Int. 2018, 107, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fang, X.B.; Wu, T.; Fang, L.; Liu, C.L.; Min, W.H. In vitro immunomodulatory effects of acidic exopolysaccharide produced by Lactobacillus planetarium JLAU103 on RAW264.7 macrophages. Int. J. Biol. Macromol. 2020, 156, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zhang, J.G.; Ying, Z.; Yan, M.; Bo, Z.; Sun, Y.X. Prebiotic, immunomodulating, and antifatigue effects of Konjac Oligosaccharide. J. Food Sci. 2018, 12, 3110–3117. [Google Scholar] [CrossRef]
- Meng, X.X.; Yang, X.Y.; Lin, G.; Fang, Y.; Ruan, Z.L.; Liu, M.F.; Liu, G.X.; Li, M.Z.; Yang, D. Mannan oligosaccharide increases the growth performance, immunity and resistance capability against Vibro Parahemolyticus in juvenile abalone Haliotis discus hannai Ino. Fish Shellfish Immunol. 2019, 94, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sun, F.F.; Cheng, X.; Li, X.J.; Mu, H.B.; Wang, S.C.; Geng, H.L.; Duan, J.Y. Preparation and biological activities of an extracellular polysaccharide from Rhodopseudomonas palustris. Int. J. Biol. Macromol. 2019, 131, 933–940. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Li, Z.; Ma, K.; Zhang, C.L.; Chen, X.H.; Wang, G.X.; Yang, L.; Dong, M.S.; Rui, X.; Zhang, Q.Q.; et al. Structural characterization and immunomodulatory activity of an exopolysaccharide produced by Lactobacillus helveticus LZ-R-5. Carbohydr. Polym. 2020, 235, 115977. [Google Scholar] [CrossRef]
- Busse, M.; Campe, K.N.J.; Nowak, D.; Schumacher, A.; Zenclussen, A.C. IL-10 producing B cells rescue mouse fetuses from inflammation-driven fetal death and are able to modulate T cell immune responses. Sci. Rep. 2019, 9, 9335. [Google Scholar] [CrossRef]
- Shi, Y.; Ye, Y.F.; Zhang, B.W.; Liu, Y.; Wang, J.H. Purification, structural characterization and immunostimulatory activity of polysaccharides from Umbilicaria esculenta. Int. J. Biol. Macromol. 2021, 181, 743–751. [Google Scholar] [CrossRef]
- Akram, W.; Garud, N.; Joshi, R. Role of inulin as prebiotics on inflammatory bowel disease. Drug Discov. Ther. 2019, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Vulevic, J.; Juric, A.; Walton, G.E.; Claus, S.P.; Tzortzis, G.; Toward, R.E.; Gibson, G.R. Influence of galacto-oligosaccharide mixture (B-GOS) on gut microbiota, immune parameters and metabonomics in elderly persons. Br. J. Nutr. 2015, 114, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.N.; Wang, J.H.; Qi, Q.; Yang, L.; Sun, P.; Yuan, X.Y. Modulatory effect of fructooligosaccharide against triphenyltin-induced oxidative stress and immune suppression in goldfish (Carassius auratus). Ecotoxicol. Environ. Saf. 2021, 212, 111966. [Google Scholar] [CrossRef]
- Pang, J.M.; Zhou, X.J.; Ye, H.; Wu, Y.J.; Wang, Z.Y.; Lu, D.D.; Wang, J.J.; Han, D.D. The high level of xylooligosaccharides improves growth performance in weaned piglets by increasing antioxidant activity, enhancing immune function, and modulating gut microbiota. Front. Nutr. 2021, 8, 764556. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.P.; Jiao, K.P.; Luo, L.; Xiang, J.L.; Fan, J.L.; Zhang, X.Y.; Yi, J.P.; Zhu, W.X. Characterization and macrophage immunomodulatory activity of two polysaccharides from the flowers of Paeonia suffruticosa Andr. Int. J. Biol. Macromol. 2019, 124, 955–962. [Google Scholar] [CrossRef] [PubMed]
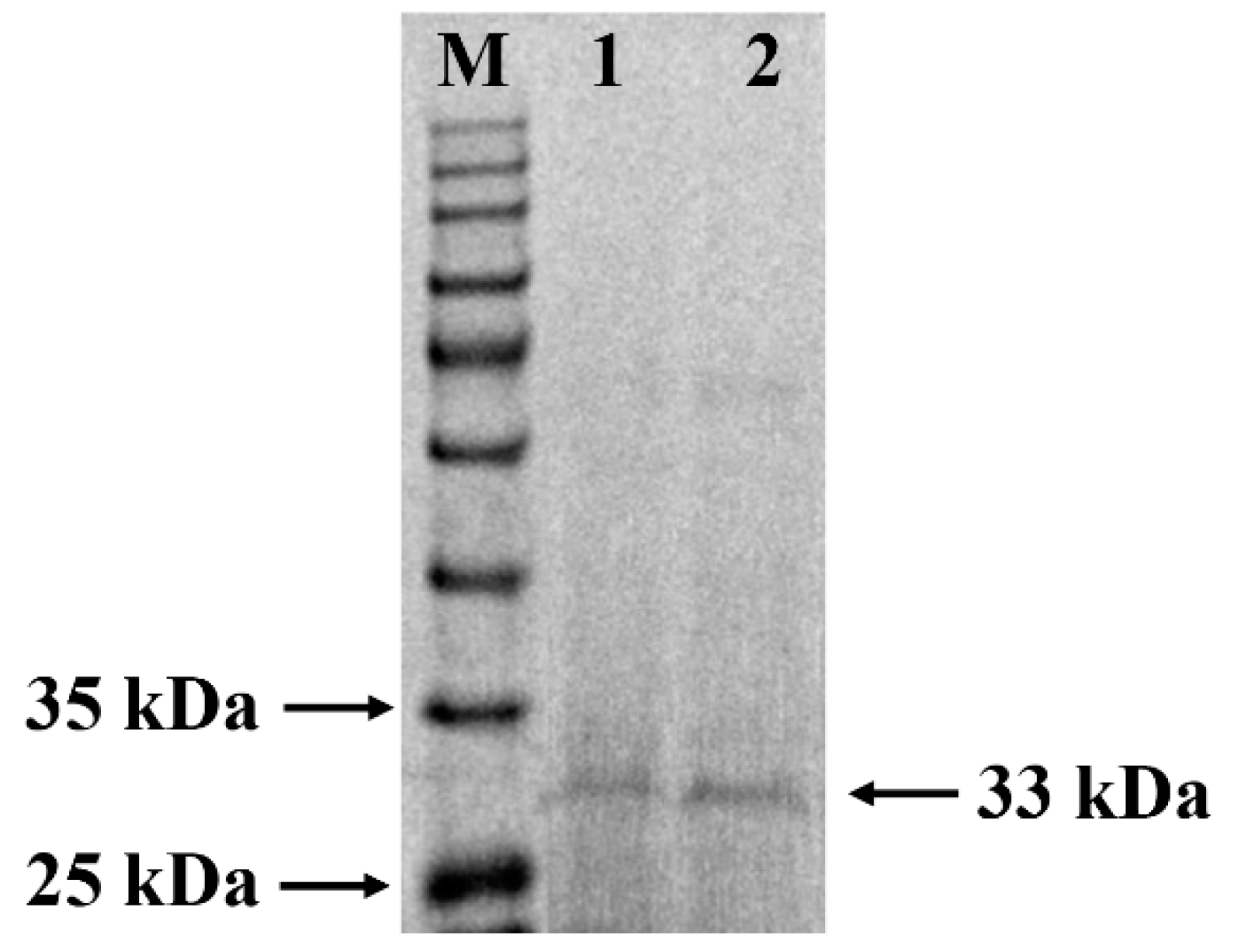


| Enzyme Fraction | Volume (mL) | Activity (U·mL−1) | Total Activity (U·mL−1) | Protein (mg·mL−1) | Recovery (%) | Specific Activity (U·mg−1) | Purification Fold |
|---|---|---|---|---|---|---|---|
| Crude extract | 1000 | 20.68 | 20680.0 | 107.00 | 100.00 | 193.27 | 1.00 |
| Acetone extraction | 100 | 65.77 | 6577.0 | 17.90 | 24.47 | 367.43 | 1.90 |
| DEAE-Sepharose FF | 10 | 127.28 | 1272.8 | 1.41 | 19.35 | 902.69 | 2.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Ji, H.; Du, R.; Ping, W.; Ge, J.; Zhao, D. The Purification and Biochemical Characterization of a Weissella cibaria F1 Derived β-Mannanase for Its Use in the Preparation of Konjac Oligo-Glucomannan with Immunomodulatory Properties. Fermentation 2022, 8, 468. https://doi.org/10.3390/fermentation8090468
Wang S, Ji H, Du R, Ping W, Ge J, Zhao D. The Purification and Biochemical Characterization of a Weissella cibaria F1 Derived β-Mannanase for Its Use in the Preparation of Konjac Oligo-Glucomannan with Immunomodulatory Properties. Fermentation. 2022; 8(9):468. https://doi.org/10.3390/fermentation8090468
Chicago/Turabian StyleWang, Shuo, Hairui Ji, Renpeng Du, Wenxiang Ping, Jingping Ge, and Dan Zhao. 2022. "The Purification and Biochemical Characterization of a Weissella cibaria F1 Derived β-Mannanase for Its Use in the Preparation of Konjac Oligo-Glucomannan with Immunomodulatory Properties" Fermentation 8, no. 9: 468. https://doi.org/10.3390/fermentation8090468
APA StyleWang, S., Ji, H., Du, R., Ping, W., Ge, J., & Zhao, D. (2022). The Purification and Biochemical Characterization of a Weissella cibaria F1 Derived β-Mannanase for Its Use in the Preparation of Konjac Oligo-Glucomannan with Immunomodulatory Properties. Fermentation, 8(9), 468. https://doi.org/10.3390/fermentation8090468






