Deciphering Microbial Diversity and Functional Codes of Traditional Fermented Whole Grain Tianpei from Typical Regions of China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collections of Tianpei Samples
2.2. Tianpei Genomic Extraction, Metagenomic Library Construction, and Sequencing
2.3. Sequence Processing and Megenomic Assembly
2.4. Microbial Gene Prediction, Taxonomy, Functional Annotations, and Analysis
2.5. Tianpei Properties and Tianpei-Making in Laboratory
3. Results
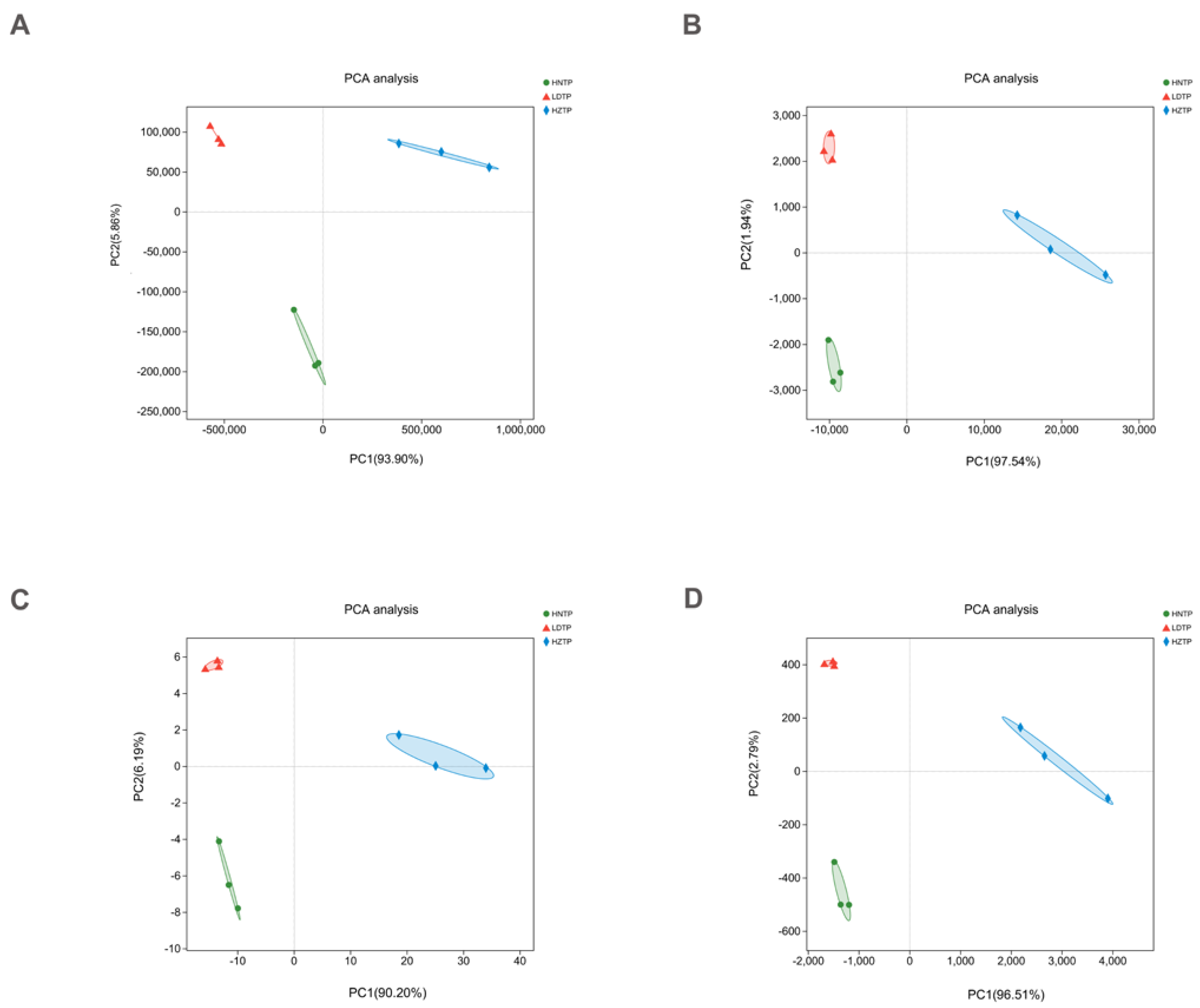
3.1. Sequencing Data Overview of Tianpei Microbiome
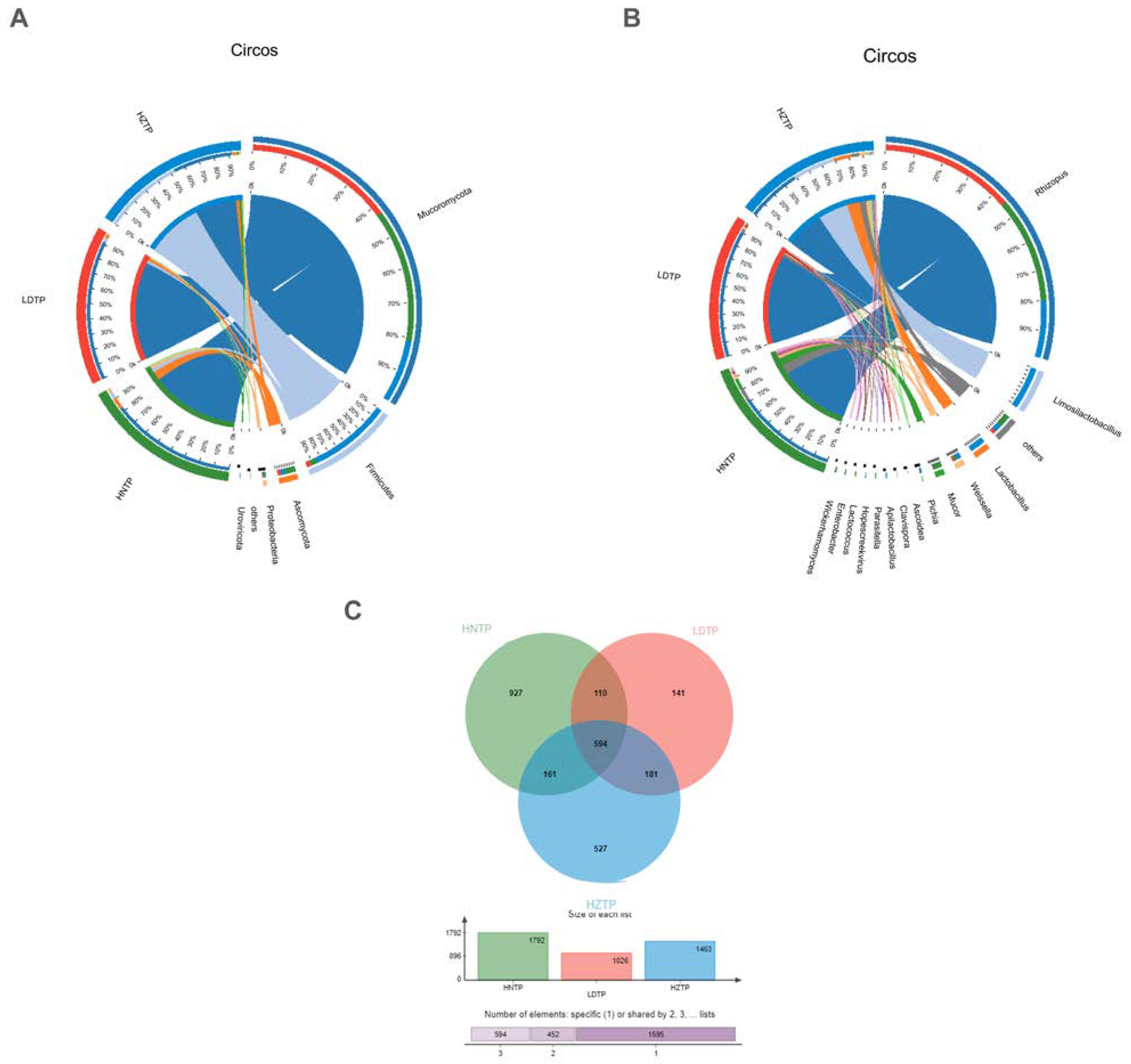
3.2. Microbial Community Composition among Tianpei Samples
3.3. Microbial Functional Annotation and Compositions of Tianpei Samples
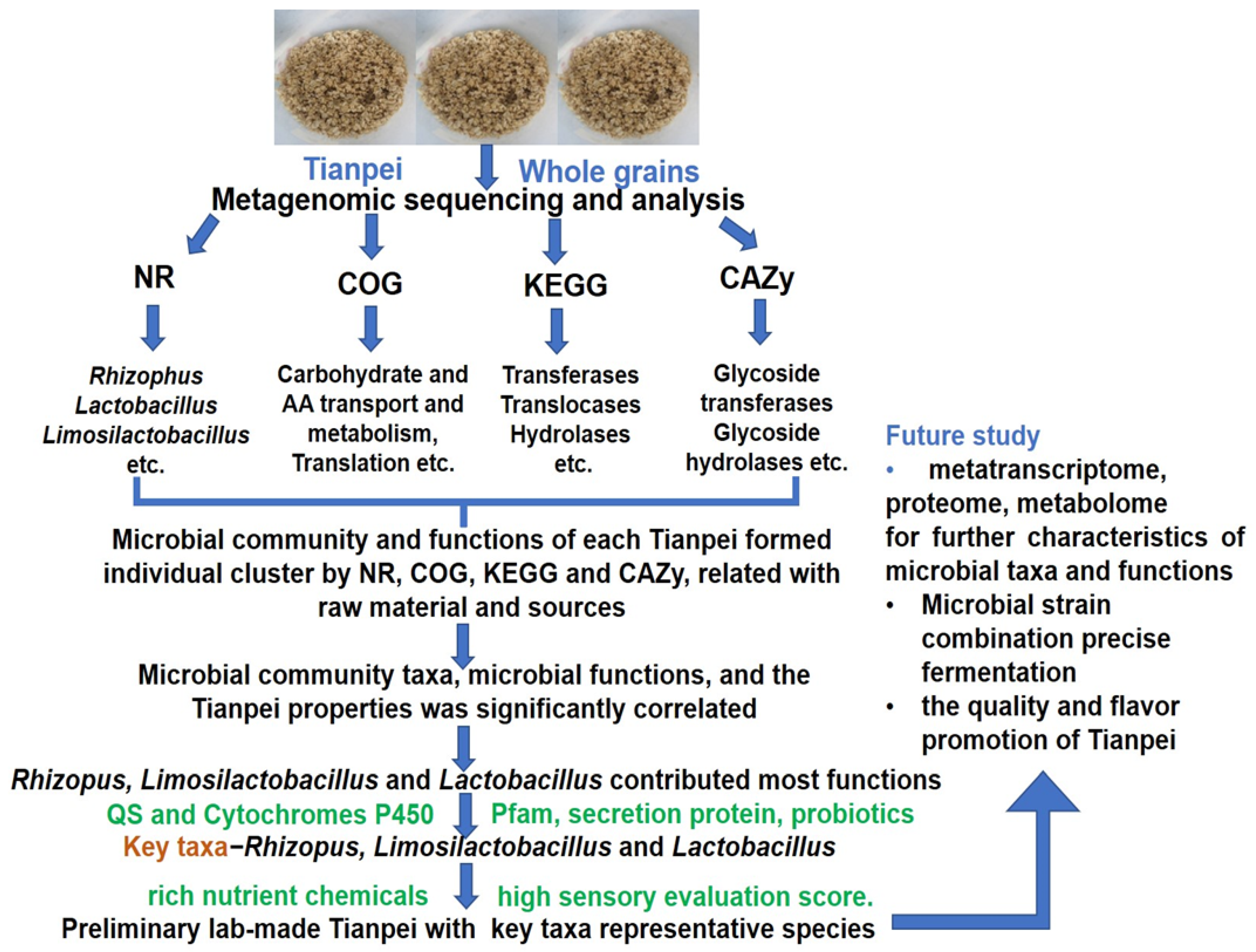
3.4. Comparative Analysis of Microbial Taxa and Functions and Correlation among Microbial Taxa, Functions, and Tianpei Properties
3.5. Analysis of Taxa and Functional Differences, Taxa and Function Contribution Analysis of Tianpei Samples
3.6. Analysis of Quorum Sensing, Pfam, Secretion Protein, Probio, and Cytochromes P450
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nugent, A.P.; Thielecke, F. Wholegrains and health: Many benefits but do contaminants pose any risk? Nutr. Bull. 2019, 44, 107–115. [Google Scholar] [CrossRef]
- Alastair, B.R.; van der Jan-Willem, K.; Robert, K.; Seal, C.J. Perspective: A definition for whole-grain food products -recom mendations from the healthgrain forum. Adv. Nutr. 2017, 8, 525–531. [Google Scholar]
- Tan, B. Whole Grains Nutrition, Health Benefits and Processing; Science Press: Beijing, China, 2021; pp. 1–275. [Google Scholar]
- Bouchard, J.; Malalgoda, M.; Storsley, J.; Malunga, L.; Netticadan, T.; Thandapilly, S.J. Health benefits of cereal grain-and pulse-derived proteins. Molecules 2022, 27, 3746. [Google Scholar] [CrossRef] [PubMed]
- Kissock, K.R.; Neale, E.P.; Beck, E.J. Whole grain food definition effects on determining associations of whole grain intake and body weight changes: A systematic review. Adv. Nutr. 2021, 12, 693–707. [Google Scholar] [CrossRef]
- Marklund, M.; Biskup, I.; Kamal-Eldin, A.; Landberg, R. Alkylresorcinols and their metabolites as biomarkers for whole grain wheat and rye. In Whole Grains and Health, 2nd ed.; Landberg, R., Scheers, N., Eds.; John Wiley & Sons, Ltd: West Suss, UK, 2021; pp. 99–136. [Google Scholar]
- Hajihashemi, P.; Azadbakht, L.; Hashemipour, M.; Kelishadi, R.; Saneei, P.; Esmaillzadeh, A. Whole grain intake favorably affects blood glucose and serum triacylglycerols in overweight and obese children: A randomized controlled crossover clinical trial. Nutrition 2021, 87, 111200. [Google Scholar] [CrossRef]
- Allai, F.M.; Azad, Z.; Gul, K.; Dar, B.N. Wholegrains: A review on the amino acid profile, mineral content, physicochemical, bioactive composition and health benefits. Int. J. Food Sci. 2022, 57, 1849–1865. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, P.; Warner, R.D.; Shen, S.; Fang, Z. Cereal grain-based functional beverages: From cereal grain bioactive phytochemicals to beverage processing technologies, health benefits and product features. Crit. Rev. Food Sci. Nutr. 2022, 62, 2404–2431. [Google Scholar] [CrossRef]
- Stern, A.L.; Blackstone, N.T.; Economos, C.D.; Griffin, T.S. Less animal protein and more whole grain in US school lunches could greatly reduce environmental impacts. Commun. Earth Environ. 2022, 3, 138. [Google Scholar] [CrossRef]
- van der Kamp, J.W.; Jones, J.M.; Miller, K.B.; Ross, A.B.; Seal, C.J.; Tan, B.; Beck, E.J. Consensus, global definitions of whole grain as a food ingredient and of whole-grain foods presented on behalf of the whole grain initiative. Nutrients 2021, 14, 138. [Google Scholar] [CrossRef]
- Tan, B.; Qiao, C.C. Dilemma, opportunity and thoughts on development of China’s whole green food industry. Biotechnol. Bus 2019, 6, 64–74. [Google Scholar]
- Zhao, H.M.; Guo, X.N.; Zhu, K.X. Impact of solid state fermentation on nutritional, physical and flavor properties of wheat bran. Food Chem. 2017, 217, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.X. Studies on the Fermentation Technology and Nutritional Functionality of Whole Grain Yogurt. Master Thesis, South China University of Technology, Guangzhou, China, 2019. [Google Scholar]
- Adebo, O.A.; Gabriela, M.M.I. Impact of fermentation on the phenolic compounds and antioxidant activity of whole cereal grains: A mini review. Molecules 2020, 25, 927. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ye, Y.; Wang, L.; Tan, B. Nutrition and sensory vvaluation of solid-state fermented brown rice based on cluster and principal component analysis. Foods 2022, 11, 1560. [Google Scholar] [CrossRef] [PubMed]
- Masiá, C.; Geppel, A.; Jensen, P.E.; Buldo, P. Effect of Lactobacillus rhamnosus on physicochemical properties of fermented plant-based raw materials. Foods 2021, 10, 573. [Google Scholar] [CrossRef]
- Kewuyemi, Y.O.; Njobeh, P.B.; Kayitesi, E.; Adebiyi, J.A.; Oyedeji, A.B.; Adefisoye, M.A.; Adebo, O.A. Metabolite profile of whole grain ting (a Southern African fermented product) obtained using two strains of Lactobacillus fermentum. J. Cereal Sci. 2020, 95, 103042. [Google Scholar] [CrossRef]
- Wronkowska, M.; Rostek, D.; Lenkiewicz, M.; Kurantowicz, E.; Yaneva, T.G.; Starowicz, M. Oat flour fermented by Lactobacillus strains–Kinetics of volatile compound formation and antioxidant capacity. J. Cereal Sci. 2021, 103, 103392. [Google Scholar] [CrossRef]
- Angelov, A.; Yaneva-Marinova, T.; Gotcheva, V. Oats as a matrix of choice for developing fermented functional beverages. J. Food Sci. Technol. 2018, 55, 2351–2360. [Google Scholar] [CrossRef]
- Zhang, W.; Yuan, L. The quality influence of compound distiller’s yeast to oats fermented sweet grains. Food Ind. 2019, 40, 48–50. [Google Scholar]
- Shi, X.; Chen, J.; Liang, H.; Cheng, C.; Zhao, Z.; Qi, Y. Analysis of microorganism, physicochemical parameters and active components during fermentation of oats. Food Ferment. Ind. 2018, 44, 49–52. [Google Scholar]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Liu, C.M.; Luo, R.; Sadakane, K.; Lam, T.W. MEGAHIT: An ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyatt, D.; Chen, G.L.; LoCascio, P.F.; Land, M.L.; Larimer, F.W.; Hauser, L.J. Prodigal: Prokaryotic gene recognition and translation initiation site identification. BMC Bioinform. 2010, 11, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noguchi, H.; Park, J.; Takagi, T. MetaGene: Prokaryotic gene finding from environmental genome shotgun sequences. Nucleic Acids Res. 2006, 34, 5623–5630. [Google Scholar] [CrossRef]
- Fu, L.; Niu, B.; Zhu, Z.; Wu, S.; Li, W. CD-HIT: Accelerated for clustering the next-generation sequencing data. Bioinformatics 2012, 28, 3150–3152. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Kristiansen, K.; Wang, J. SOAP: Short oligonucleotide alignment program. Bioinformatics 2008, 24, 713–714. [Google Scholar] [CrossRef] [Green Version]
- Kang, C.H.; So, J.S. Antibiotic and heavy metal resistance in Shewanella putrefaciens strains isolated from shellfishes collected from West Sea, Korea. Mar. Pollut. Bull. 2015, 112, 111–116. [Google Scholar] [CrossRef] [PubMed]
- HMMER. Available online: http://hmmer.org/ (accessed on 11 September 2022).
- CAZY. Available online: http://www.cazy.org/ (accessed on 15 September 2022).
- Pfam. Available online: http://pfam.xfam.org (accessed on 18 September 2022).
- DTU Health Tech. Available online: https://services.healthtech.dtu.dk/service.php?SignalP-5.0 (accessed on 20 September 2022).
- Probio. Available online: http://bidd.group/probio/homepage.htm (accessed on 21 September 2022).
- Wang, Z.; Xu, Z.; Sun, L.; Dong, L.; Wang, Z.; Du, M. Dynamics of microbial communities, texture and flavor in Suan zuo yu during fermentation. Food Chem. 2020, 332, 127364. [Google Scholar] [CrossRef]
- Ling, H.Z.; Shi, H.L.; Chen, X.C.; Cheng, K.K. Detection of the microbial diversity and flavour components of northeastern Chinese soybean paste during storage. Food Chem. 2021, 374, 131686. [Google Scholar] [CrossRef]
- Ren, F.; Dong, W.; Yan, D.H. Organs, cultivars, soil, and fruit properties affect structure of endophytic mycobiota of pinggu peach trees. Microorganisms 2019, 7, 322. [Google Scholar] [CrossRef] [Green Version]
- Delgado-Baquerizo, M.; Oliverio, A.M.; Brewer, T.E.; Benavent-González, A.; Eldridge, D.J.; Bardgett, R.D.; Fernando, T.; Brajesh, K.; Fierer, N. A global atlas of the dominant bacteria found in soil. Science 2018, 359, 320–325. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.Y.; Guo, S.Y.; Huang, M.R.; Thorsten, L.H.; Wei, J.C. Ascomycota has a faster evolutionary rate and higher species diversity than Basidiomycota. Sci. China Life Sci. 2010, 53, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.J. Study on the Process of Immobilized Fermentation of Fumaric Acid from Rhizopus arrhizus. Master Thesis, Beijing University of Chemical Technology, Beijing, China, 2019. [Google Scholar]
- Wu, H.; Liu, H.N.; Ma, A.M.; Zhou, J.Z.; Xia, X.D. Synergetic effects of Lactobacillus plantarum and Rhizopus oryzae on physicochemical, nutritional and antioxidant properties of whole-grain oats (Avena sativa L.) during solid-state fermentation. LWT 2022, 154, 112687. [Google Scholar] [CrossRef]
- Wang, C.; Song, X.; Li, C.; He, L.; Wang, X.; Zeng, X. Mixed fermentation with Lactobacillus plantarum, Bifidobacteriµm animalis subsp. lactis and Candida utilis improves the fermentation quality of Hong Suan Tang. Food Chem. 2023, 402, 134488. [Google Scholar] [PubMed]
- Ojetti, V.; Saviano, A.; Brigida, M.; Petruzziello, C.; Caronna, M.; Gayani, G.; Franceschi, F. Randomized control trial on the efficacy of Limosilactobacillus reuteri ATCC PTA 4659 in reducing inflammatory markers in acute uncomplicated diverticulitis. Eur. J. Gastroen. Hepat. 2022, 34, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Zoumpopoulou, G.; Ioannou, M.; Anastasiou, R.; Antoniou, A.; Alexandraki, V.; Papadimitriou, K.; Moschopoulou, E.; Tsakalidou, E. Kaimaki ice cream as a vehicle for Limosilactobacillus fermentum ACA-DC 179 to exert potential probiotic effects: Overview of strain stability and final product quality. Int. Dairy J. 2021, 123, 105177. [Google Scholar] [CrossRef]
- Zannini, E.; Mauch, A.; Galle, S.; Gänzle, M.; Coffey, A.; Arendt, E.K.; Taylor, J.P.; Waters, D.M. Barley malt wort fermentation by exopolysaccharide-forming Weissella cibaria MG 1 for the production of a novel beverage. J. Appl. Microbiol. 2013, 115, 1379–1387. [Google Scholar] [CrossRef]
- Molaverdi, M.; Mirmohamadsadeghi, S.; Karimi, K.; Aghbashlo, M.; Tabatabaei, M. Efficient ethanol production from rice straw through cellulose restructuring and high solids loading fermentation by Mucor indicus. J. Clean Prod. 2022, 339, 130702. [Google Scholar] [CrossRef]
- Tan, Y.N.; Lee, P.P.; Chen, W.N. Dual extraction of crustacean and fungal chitosan from a single Mucor circinelloides fermentation. Fermentation 2020, 6, 40. [Google Scholar] [CrossRef] [Green Version]
- Bi, L.; Qiu, X.H.; Zeng, S.L. Screening of yeast with biosurfactant production from pickled Vegetables. Microbiol. China 2022, 49, 153–162. [Google Scholar]
- Li, N.; Wang, L.; Yin, J.; Ma, N.; Tao, Y. Adjustment of impact odorants in Hutai-8 rose wine by co-fermentation of Pichia fermentans and Saccharomyces cerevisiae. Food Res. Int. 2022, 153, 110959. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.J.; Julien, P.; Kuhn, M.; von Mering, C.; Muller, J.; Doerks, T.; Bork, P. eggNOG: Automated construction and annotation of orthologous groups of genes. Nucleic Acids Res. 2007, 36, D250–D254. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Lombard, V.; Golaconda, R.H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, R.N. Glutathione S-transferases: Structure and mechanism of an archetypical detoxication enzyme. Adv. Enzymol. Relat. Areas Mol. Biol. 1994, 69, 1–44. [Google Scholar]
- Sami, A.A.; Arabia, S.; Sarker, R.H.; Islam, T. Deciphering the role of helicases and translocases: A multifunctional gene family safeguarding plants from diverse environmental adversities. Curr. Plant Biol. 2021, 26, 100204. [Google Scholar] [CrossRef]
- Bourne, Y.; Henrissat, B. Glycoside hydrolases and glycosyltransferases: Families and functional modules. Curr. Opin. Struc. Biol. 2001, 11, 593–600. [Google Scholar] [CrossRef]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The Carbohydrate-Active EnZymes database (CAZy): An expert resource for glycogenomics. Nucleic Acids Res. 2009, 37, D233–D238. [Google Scholar] [CrossRef]
- Sionov, R.V.; Steinberg, D. Targeting the holy triangle of quorum sensing, biofilm formation, and antibiotic resistance in pathogenic nacteria. Microorganisms 2022, 10, 1239. [Google Scholar] [CrossRef]
- Wu, L.; Luo, Y. Bacterial quorum-sensing systems and their role in intestinal bacteria-host crosstalk. Front. Microbiol. 2021, 12, 611413. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, G.; Zhou, J.; Zeng, Y.; Cheng, K.; Cai, Z. Dynamic patterns of quorum sensing signals in phycospheric microbes during a marine algal bloom. Environ. Res. 2022, 212, 113443. [Google Scholar] [CrossRef] [PubMed]
- Venema, K.; Chikindas, M.L.; Seegers, J.F.M.; Haandrikman, A.J.; Leenhouts, K.J.; Venema, G.; Kok, J. Rapid and efficient purification method for small, hydrophobic, cationic bacteriocins: Purification of lactococcin B and pediocin PA-1. Appl. Environ. Microbiol. 1997, 63, 305–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holo, H.; Nilssen, Ø.; Nes, I. Lactococcin A, a new bacteriocin from Lactococcus lactis subsp. cremoris: Isolation and characterization of the protein and its gene. J. Bacteriol. 1991, 173, 3879–3887. [Google Scholar]
- Nebert, D.W.; Russell, D.W. Clinical importance of the cytochromes P450. Lancet 2002, 360, 1155–1162. [Google Scholar] [CrossRef]
- Dangi, B.; Davydova, N.Y.; Maldonado, M.A.; Ahire, D.; Prasad, B.; Davydov, D.R. Probing functional interactions between cytochromes P450 with principal component analysis of substrate saturation profiles and targeted proteomics. Arch. Biochem. Biophys. 2021, 708, 108937. [Google Scholar] [CrossRef]
- Fuchs, R.; Reed, J.; Backes, W. Identification of a disordered region-targeting motif in cytochromes P450. FASEB J. 2021, 35, 02991. [Google Scholar] [CrossRef]


| Samples | Mean Clean Reads | Mean Clean Bases (bp) | Percent in Raw Reads (%) | Percent in Raw Bases (%) |
|---|---|---|---|---|
| HNTP | 43,266,315.33 | 6,519,779,808 | 98.57 | 98.37 |
| HZTP | 47,782,730.67 | 7,197,564,757 | 98.63 | 98.39 |
| LDTP | 42,708,644.00 | 6,431,474,771 | 98.42 | 98.15 |
| Removing the host genome | ||||
| Mean Optimized reads | Mean optimized bases (bp) | Percent in raw reads (%) | Percent in raw bases (%) | |
| HNTP | 26,046,762 | 3,929,623,528 | 59.36% | 59.31% |
| HZTP | 24,257,476 | 3,659,932,373 | 50.05% | 50.01% |
| LDTP | 22,573,880 | 3,404,879,219 | 52.01% | 51.95% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, F.; Liu, M.; Liu, Y.; Tian, X.; Jiang, P.; Tan, B. Deciphering Microbial Diversity and Functional Codes of Traditional Fermented Whole Grain Tianpei from Typical Regions of China. Fermentation 2023, 9, 53. https://doi.org/10.3390/fermentation9010053
Ren F, Liu M, Liu Y, Tian X, Jiang P, Tan B. Deciphering Microbial Diversity and Functional Codes of Traditional Fermented Whole Grain Tianpei from Typical Regions of China. Fermentation. 2023; 9(1):53. https://doi.org/10.3390/fermentation9010053
Chicago/Turabian StyleRen, Fei, Ming Liu, Yanxiang Liu, Xiaohong Tian, Ping Jiang, and Bin Tan. 2023. "Deciphering Microbial Diversity and Functional Codes of Traditional Fermented Whole Grain Tianpei from Typical Regions of China" Fermentation 9, no. 1: 53. https://doi.org/10.3390/fermentation9010053
APA StyleRen, F., Liu, M., Liu, Y., Tian, X., Jiang, P., & Tan, B. (2023). Deciphering Microbial Diversity and Functional Codes of Traditional Fermented Whole Grain Tianpei from Typical Regions of China. Fermentation, 9(1), 53. https://doi.org/10.3390/fermentation9010053




