Abstract
This current work revealed a single-step fabrication of tungsten oxide nanoflakes (WO3 NFs) with the help of Terminalia arjuna bark extract. Bioactive phytoconstituents of T. arjuna bark extract were involved in the nucleation process and promoted the material crystalline growth in a particular direction. The as-prepared sample thermal decomposition was analyzed by TG/DTG. The as-prepared sample was annealed at 300 °C for 2 h, and the annealed sample was characterized by UV-Vis-DRS, FTIR, Raman, XRD, SEM, EDX, and TEM. Synthesized WO3 samples showed a monoclinic phase of the flake-like structure with lengths of 25~230 nm and diameters of 25~120 nm. The WO3 NFs were evaluated against S. aureus and E. coli. Over 3 mg concentrations of WO3 NFs outperform the positive control in antibacterial activity. The pseudo-first-order kinetics of the WO3 NFs enhanced the photocatalytic performance of methylene blue (MB). These results prove that WO3 NFs have sustainable performance in antibacterial and MB degradation applications.
1. Introduction
Bacterial infections have recently been identified as a major global health threat [1]. For the most part, organic antibiotics have been utilized to treat human bacterial diseases, resulting in bacterial pathogens becoming resistant to antibiotics. However, novel classes of antibiotics slow their further development [2]. On the other hand, the prime source of all living organisms for sustainable development needs is water. The discharges of industrial effluents, especially from the textile industry, such as azo dye, phenyl matter, and toxic organic substances resistant to degradation and decomposition by biological treatment, cause severe water pollution. Chemical markers and biological staining procedures employ methylene blue (MB), a cationic dye. Due to the high chromatic nature and poor biodegradability, it has been complicated to process degradability. More importantly, it causes various harmful effects on human health care, such as diarrhea, vomiting, severe headache, and methemoglobinemia-like syndrome. In addition, it significantly affects the waterbody phytoplankton photosynthesis and microorganism metabolic activity in the water environment [3,4,5,6]. Photocatalyst is one of the novel methods to prevent water pollution. Two-dimensional (2D) nanomaterials are efficient catalysts for solving bacterial pathogens, mosquito vectors, and environmental remediation problems. Nowadays, researchers have placed their attention on nanomaterials because they have been used for catalysts’ action to produce bio-alcohols due to their reusability. Hitherto, metal and metal oxide Co, Mn, Fe2O3, CuO, ZnO, SiO2, and composites of CoFe2O4@SiO2, SrO/SiO2, CaO-Au, and ZnO–Ag have been used in bioethanol and biobutanol fermentation [7]. Because of their high surface-to-volume ratio, 2D nanostructured materials have recently gained interest for their potential for separation and catalysis. Mainly tungsten oxide (WO3)-based nanomaterials have been utilized for photocatalysis, sensing, electrochromic devices, paints, dye-sensitized solar cells, CO2 reduction, batteries, air purification, cancer therapy, and antimicrobial agents [8,9]. They have become more prevalent in recent years because of their inexpensive production costs, configurable bandgap, and great solubility in aqueous solutions under acidic conditions [10]. Typically, WO3 nanoparticles (NPs) are synthesized by physical and chemical methods such as solvothermal [11], sol-gel [12], reactive magnetron sputtering [10], thermal decomposition [13], acid precipitation [14], hydrothermal synthesis [15], and ion-exchange processes [16]. Supothina et al. [14] reported WO3 NPs synthesized by the acid precipitation method. The 5.3, 10, and 16 mM concentrations of ammonium tungstate were investigated using 1.5, 3.0, 4.5, and 6.0 pH. In addition, >3 pH concentrations showed a better yield of WO3. The average size was 30 nm, and the nanoplate dimension was 190 ± 15 nm wide and 50 ± 5 nm thick. The acid precipitation method leads to a 2D nanostructure. However, these physical and chemical methods are expensive; reducing agents and organic solvents are harmful to the environment, high energy, and time-consuming. Therefore, researchers have paid attention to the single-step green synthesis method as an alternative for toxic reducing agents, which is less time-consuming and has large-scale production and ease of handling. Plant-based extracts such as leaves, stems, bark, and roots are rich sources of bioactive reducing compounds and capping agents and can be used for metal and metal oxide NPs or nanomaterial synthesis [17]. The size and shape of nanomaterials from plant extracts vary. According to the literature survey, we chose Terminalia arjuna bark extract to prepare WO3 nanomaterial. It contained phytoconstituents of apigenin, arjun glucoside, arjunic acid, arjunetin, flavonoids, tannins, and triterpenoids [18]. Previously, metal and metal oxide NPs have successfully been synthesized by this plant bark extract [19,20]. We expect that plant bark extract phytoconstituents are helpful in improving the nucleation process leading to different orientations of WO3 material growth.
Our research focuses on the single-step synthesis of WO3 nanoflakes (NFs) utilizing T. arjuna bark extract. The optical, functional, structural, and topological features of synthesized WO3 NFs were investigated. Then, they can be used for antibacterial and photocatalytic applications. The synthesized WO3 NFs had a considerable effect on all activities. To our knowledge, this is the first report on the single-step fabrication of 2D-WO3 NFs utilizing T. arjuna bark extract.
2. Materials and Methods
2.1. Chemical
Himedia, India, provided the nutrient agar and positive control growth media, sterile disk used in this study, whereas Sigma Aldrich supplied the tungstic acid 99%. The experiment was conducted using water that was twice as far apart as normal.
2.2. Plant Material and Microorganism Collection
The T. arjuna bark utilized in this research was obtained from Alagappa University Botanical Garden in Karaikudi, Tamil Nadu. The Department Herbarium completed the taxonomical identification of plants with the help of the Flora of the Madras Presidency, India. The Ramakrishna Mission Vivekananda College’s Postgraduate and Research Department of Plant Biology and Plant Biotechnology graciously contributed to the bacterium strains (Staphylococcus aureus and Escherichia coli).
2.3. Plant Bark Extraction Method
Boiling 10 g of T. arjuna bark for 10 min in 100 mL of double-distilled water and allowing it cool to 35 °C produced the extract, and it was filtered with Lab world test filter paper 100 dia with 12.5 cm. The obtained solution was used for further experimental uses.
2.4. Synthesis of WO3 NFs
WO3 NFs were produced in a typical experiment through a one-step green synthesis process. For this experiment, we used 475 mL of double-distilled water to dissolve 2.5 g of tungstic acid and kept the mixture constantly stirred using a magnetic rod. We added 25 mL of bark extract and stirred it with a magnetic stirrer for 12 h at 80 °C. Subsequently, this yellowish mixture turned into a greenish-yellow color. The continuous stirring pH of the solution was revealed to decrease from 3.5 to 2.5. Parallelly, the non-plant extract method synthesized WO3 as-prepared sample (reduced the pH 2.5 by diluted HCl). Further, the greenish-yellow precipitate was obtained at two-fourths of the precursor, and this sample was annealed at 300 °C for 2 h in an air atmosphere. Finally, collected WO3 as-prepared nano-powder was gently grounded by the mortar and pistil. Lastly, we obtained one-half of the sample. Obtained WO3 nano-powder was stored in a desiccator chamber to avoid the atmosphere’s moisture content.
2.5. Characterization of WO3 NFs
The STA 6000 thermogravimetric analyzer (Perkin Elmer Co., Ltd., Shelton, CT, USA) was used to assess the thermal behavior of the as-prepared sample in a flow of (20 mL/min) N2 atmosphere; the temperature increased at 5 °C/min from 30 to 725 °C. Samples weighed a total of 21.26 mg. UV-Vis spectroscopy examination was performed on synthetic WO3 NFs, and a wavelength range of 100 to 1500 nm was recorded using the Shimadzu UV-1600, Tokyo, Japan. Samples of WO3-NFs were mixed with KBr pellets, and FT–IR spectrophotometers (Bruker Vector 26 spectrometers) were used to identify the functional groups in the sample. RigakuMiniflex X-ray diffractometer (Cu K, 1.5406 A°) was used to analyze the material’s crystalline structure from 10° to 90°. The vibrational frequency of the sample was characterized with Bruker-Tensor 27 series spectrophotometer (Bruker Optik GmbH, Ettlingen, Germany) via Raman microscopy (Renishaw, Wotton-under-Edge, UK). We used a Zeiss Field emission scanning electron microscope (FE–SEM) coupled with energy dispersive spectroscopy (EDS) to examine the surface morphology and elemental compositional features. The image was taken at 20 kV voltage and 10 mm working distance. The particle in the sample was dispersed using ultrasonic separation. The WO3 NFs were studied using a high-resolution transmission electron microscope (HT-TEM), Tecnai G2 20 S-TWIN TEM, with an accelerating voltage of 200 kV. A minute sample was added to 1 mL ethanol for HR-TEM sample preparation and sonicated for 60 min. Then, the solution was drop cast on a carbon-coated Cu-grid.
2.6. Antibacterial Activity
The antibacterial activity of WO3 NFs was assessed using the agar-disk diffusion technique on S. aureus and E. coli. Each bacterial strain was cultivated in an aseptic nutrient broth medium for 24 h at 37 °C. It was then carefully poured onto Petri plates to cool. Then, each bacterial suspension was dispersed uniformly across nutrient agar plates. The sterile disk containing 1, 3, 5, and 10 mg WO3 NFs was then put over the medium using sterile forceps. The positive control was amoxicillin (30 mg). After 24 h of incubation at 37 °C, the inhibition zone diameter produced around the disk was measured. The antibacterial activity was performed as n = 3 independent experiments. All results were expressed as means ± standard error (means ± SE).
2.7. Photocatalytic Activity of Methylene Blue
Photodegrading methylene blue (MB) was evaluated for its photocatalytic activity under 300 W Xe lamp illumination. Before, 40 mL L−1 MB suspension and 40 mg WO3 NFs were used. MB absorption–desorption equilibrium was established on the photocatalyst by stirring this solution for 30 min before light illumination. Irradiated samples were taken out every 10 min for extraction and centrifugation for 10 min. A UV-Vis spectrometer measured the MB dye absorption wavelength at 663 nm to determine the dye concentration [20].
3. Results and Discussion
3.1. TG/DTG Measurements of WO3 NFs
TG is a technique that determines the mass of a sample over time as the temperature varies in a controlled environment. DTG is used to determine a sample’s phase transition and thermal behavior. This measurement offers information regarding thermal breakdown absorption, desorption, and kinetic composition analysis. TG/DTG curves of as-synthesized WO3 as-prepared nano-powder exhibited three thermal decomposition steps. Whereas, over 600 °C, no weight loss was observed in TG, as shown in Figure 1. The first weight loss < 100 °C (2.5%) was assigned to water dehydration for the DTG curve. The second weight loss from 200 to 300 °C (7.5%) was attributed to the decomposition and combustion process of the bio-organic matrix. From 500 to 600 °C (7%), the third step was the related crystallization of tungsten oxide.
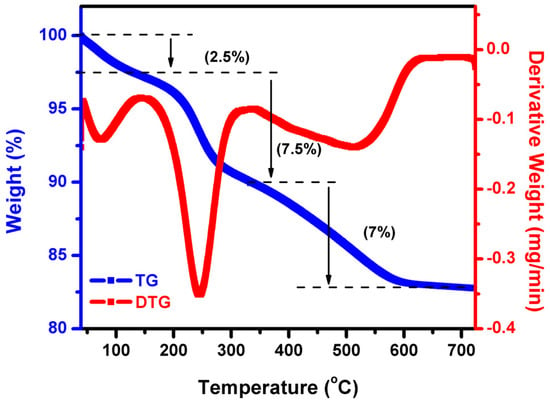
Figure 1.
TG/DTG analysis of WO3 NFs.
3.2. Optical Properties of WO3 NFs
3.2.1. UV-Vis-DRS Analysis
Figure 2 shows the UV-Vis-diffuse reflectance spectrum of WO3 NFs. Its n-type W=O bond shifted its absorption to the π-type at 330 nm. For this reason, the Kubelka–Munk equation uses the function equation below [21].
where R = reflectance, F(R) = absorbance. After calculation, the bandgap value was determined to be 2.66 eV. Similarly, Huang et al. reported WO3 nanorods and revealed a bandgap at 2.48 eV. WO3 nanorods have a 450 nm absorption peak [22]. Simultaneously, WO3 nanosheets displayed a red-shifted band edge with 2.75 and 2.38 eV band gaps. The bulk WO3 bandgap was typically 2.6–3.0 eV. The photogenerated electron-hole pairs on the WO3 surface lower the bandgap when electrons move from valance to the conduction band [23].
Kubelka–Munk equation: K = (1 − R)2/2R
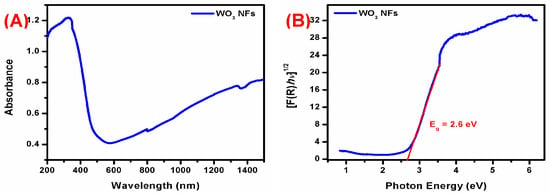
Figure 2.
(A) UV-Vis-DRS spectrum and (B) bandgap analysis of WO3 NFs.
3.2.2. FT-IR Analysis
Figure 3 shows the FT-IR spectrum of synthesized WO3 NFs. The band at 3006 cm−1 was due to tungsten oxide -OH stretching vibrations [22]. It relates to O-H bending vibrations. Strong bands at 809 and 733 cm−1 correspond to W-O-W stretching vibration [24,25]. The wide absorption peak at 1000–500 cm−1 was previously thought to be due to stretching vibrations in the WO3 crystal lattice [26,27]. Earlier studies showed a similar tendency [28]. Non-plant extract synthesized WO3 spectrum did not show OH stretching and bending vibration. At the same time, the FTIR spectrum of plant bark extracts with various functional group peaks related to the bioactive compounds of T. arjuna [18,19]. However, this plant bark-extract functional groups did not appear in the 300 °C 2 h annealing sample. It confirmed the formation of WO3 material.
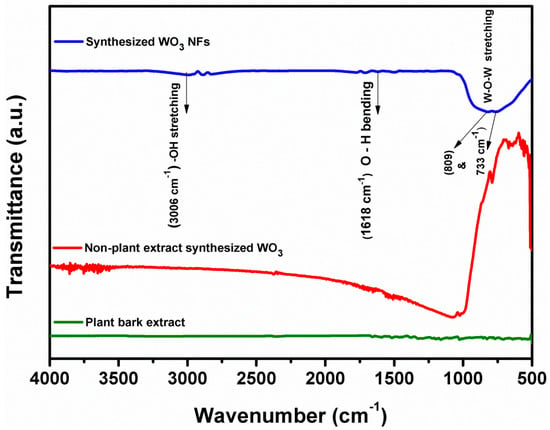
Figure 3.
FT-IR analysis of plant extract synthesized WO3 NFs, non-plant extract synthesized WO3, and plant bark extract.
3.2.3. Raman Analysis
Figure 4 depicts the intensive Raman bands observed at 71 and 135 cm−1 in the low-frequency region, owing to the lattice vibration modes. Similar vibration modes were obtained below at 200 cm−1 in the WO3, which agrees with the previous results [29]. In the Raman spectrum of WO3 NFs, there were four distinct peaks at 273, 326, 712, and 807 cm−1. The W-O-W bending vibration produced two smaller peaks at 273 and 326 cm−1. Subsequently, the two high-intensity peaks centered at 712 and 807 cm−1 are related to W−O−W stretching vibration [30,31]. Recently, WO3 NPs produced by Fusarium solani revealed two lower-intensity peaks at 267 and 324 cm−1 with a flake-like appearance [32].
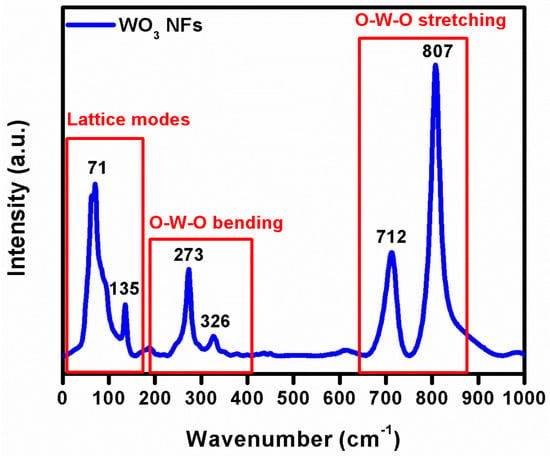
Figure 4.
Raman analysis of WO3 NFs.
3.3. XRD Analysis
The XRD analysis provides phase identification, sample purity, and crystallite size information. Depicted in Figure 5, the XRD patterns of the WO3 NFs show four main peaks at 2ɵ = 23.11, 23.58, 24.38, and 34.15° ascribed to indexed with the planes (0 0 2), (0 2 0), (2 0 0), and (2 0 2), respectively. The XRD pattern categorized the monoclinic phase of WO3 with unit-cell values of a = 7.29 Å, b = 7.53 Å, and c = 7.68 Å, according to JCPDS card no.1314358. The relative four intensities planes (0 0 2), (0 2 0), (2 0 0), and (2 0 2) indicated the highly crystalline nature of the WO3 phase. Parthibavarman et al. reported that WO3 nanorods and nanosheets showed (0 0 2), (0 2 0), and (2 0 0) planes leading to a high crystalline nature [33]. However, the intensity of the (0 0 2) plane was increased for WO3 nanorods compared to WO3 nanosheets. Our result was consistent with the aforementioned WO3 nanosheet’s crystalline nature [33].
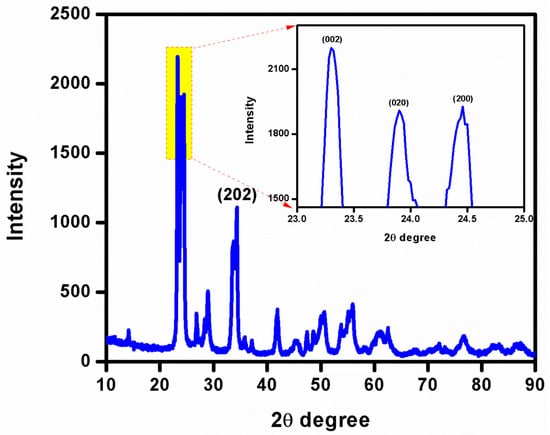
Figure 5.
XRD analysis of WO3 NFs.
3.4. FE-SEM with EDX Analysis
FE-SEM analysis provides a surface topology that leads to two-dimensional images with high resolution. As shown in Figure 6, this synthesized method obtained the flake-like morphology of WO3 NFs. Furthermore, the elemental composition, weight, and atomic percentage are classified by the EDX spectral analysis. The EDX profile of WO3 NFs showed W and O elements and did not find any more impurity peaks. The atomic weight percentage of W and O was 25 and 75%, respectively, which confirmed the formation of WO3 NFs of purity.
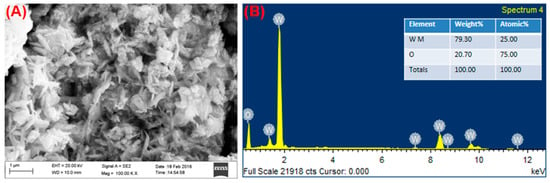
Figure 6.
(A) SEM and (B) EDX analysis of WO3 NFs.
3.5. HR-TEM Analysis of WO3 NFs
The accurate particle size, shape, and crystalline behavior of WO3 NFs were confirmed by HR-TEM images and SAED. Figure 7 shows the flake-like structure with lengths of 25~230 nm and diameters of 25~120 nm. The interplanar spacing was 0.373 nm, related to the (020) planes of monoclinic WO3 NFs. An HR-TEM result of synthesized WO3 NFs clearly showed that the growth direction is along the (020) plane. On the HR-TEM picture of WO3 NFs SAED patterns highlighted the NF’s crystalline structure.

Figure 7.
(A–C) TEM images, (D) SAED patterns of WO3 NFs.
3.6. Antibacterial Activity of WO3 NFs
The size, shape, aspect ratio, surface charge, stability, and surface defect of nanoparticles were all shown to be important factors in their antibacterial activity [34]. The current study shows the antibacterial activity of WO3 NFs, as shown in Figure 8 and Figure 9. The E. coli bacterial strain showed a maximum zone of inhibition compared to the S. aureus at 10 mg concentration, related to the bacterial cell wall structure. The antibacterial activity results exhibited are dependent on increasing the content of WO3 NFs. The WO3 NFs contact the bacterial cell wall by electrostatic attraction. This is followed by ROS produced by the bacterial cell wall: superoxide-anions (O2•–), hydroxyl radicals (•OH), hydrogen peroxides (H2O2), and singlet oxygen (1O2). This ROS generation leads to inhibits bacterial survivability. It caused the malfunction of intracellular organelles, interference of cell signaling, collapses in DNA replication, protein and ATP synthesis, imbalance in the electron transfer system, and enzyme kinetics. These factors inhibited bacterial growth and caused the loss of cytoplasmic electrolyte fluids, which led to cell death, as shown in Figure 10. The WO3 NFs induced ROS generation as follows.
WO3 + hν → e– + h+
h++ H2O → •OH + H–
e– + O2 → •O2–
•O2– + H+ → HO2•
HO2• + H+ + e– → H2O2
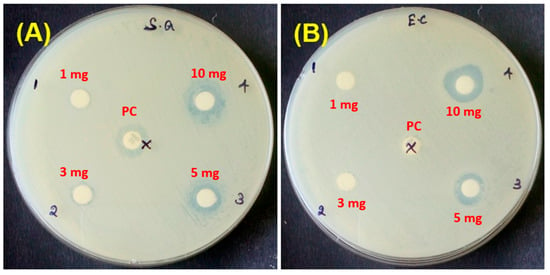
Figure 8.
Antibacterial activity of WO3 NFs; (A) S. aureus and (B) E. coli.
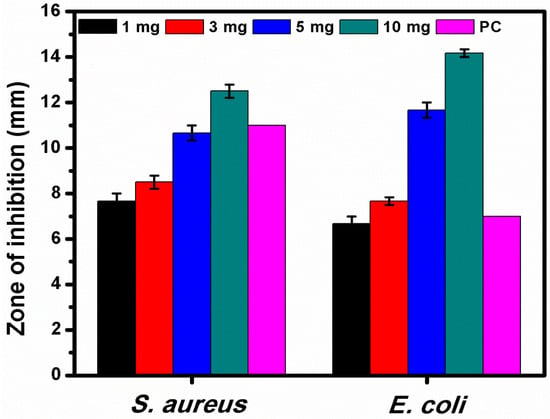
Figure 9.
Different concentrations of WO3 NFs tested against S. aureus and E. coli. PC, positive control (Amoxicillin—30 mg).
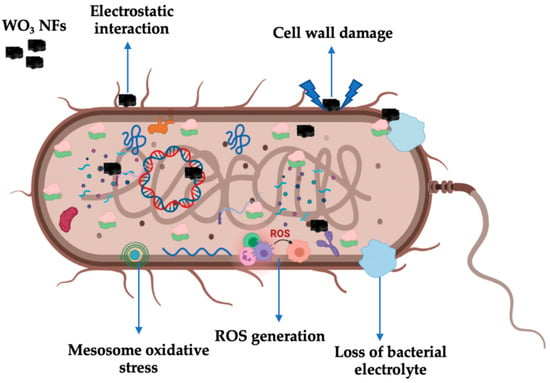
Figure 10.
Antibacterial mechanism of WO3 NFs.
Both E. coli and S. aureus may be killed by the WO3-X nanodots in a dose and time-dependent way to better rupture the bacterial membranes [35]. WO3 and rGO-WO3 (100 g/mL) composite sheets in 2D rectangular shapes inhibited the development of bacteria and severely damaged the cell wall of B. subtilis, according to Ahmed et al. [36]. This study suggested that WO3 NFs influence the control of bacterial growth, especially against Gram-negative bacteria.
3.7. Methylene Blue Photocatalytic Degradation
Under visible light irradiation, the photocatalytic performance of WO3 NFs towards the decolorization of MB dye was investigated. Figure 11A shows the variation of MB concentration (C/C0) at time intervals. The WO3 NFs exhibit higher photocatalytic performance compared to the plant extract treatment of MB (Figure 11C). Almost 85.7% of MB was degraded within 40 min using WO3 NFs, as shown in Figure 11B,D. Due to the increased charge separation between photogenerated electron and hole pairs, significant improvements in photodegradation were seen. Photodegradation of MB dyes using pseudo-first-order kinetic model ln (C0/C) = Kt value is for WO3 NFs.
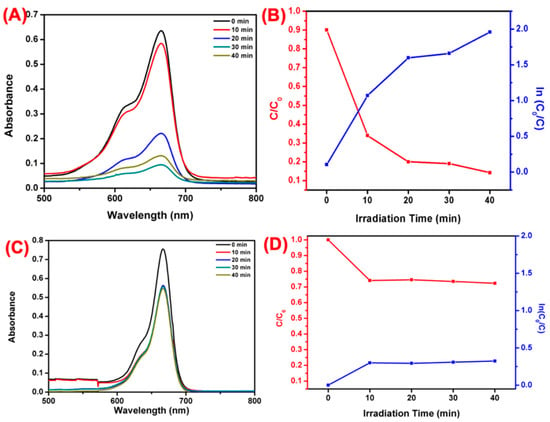
Figure 11.
Visible-light-induced photodegradation of MB. (A) Different time irradiation WO3 NFs, (B) plotted against; (C/C0) and ln(C0/C). (C) Different time irradiation plant bark extract, (D) plotted against (C/C0) and ln(C0/C).
4. Conclusions
We successfully synthesized WO3 NFs using T. arjuna bark extract by a one-step method and evaluated the antibacterial activity and photocatalytic degradation with MB dye. Optical band gap values showed at 2.6 eV, and the FTIR functional groups revealed the formation of WO3 by forming two high-intensity peaks centered at 712 and 807 cm−1. The XRD results exhibit high crystalline nature with a monoclinic phase. The topological analysis of SEM and TEM analysis showed a flake-like structure. The antibacterial activity of WO3 NFs excellently inhibited E. coli at the 10 mg concentration compared to S. aureus due to the Gram-negative bacteria’s thin cell wall structural configuration. Overall, antibacterial activity illustrates a dose-dependent effect. At the same time, ROS generation of H2O2 causes DNA damage, protein denaturation, and cytoplasmic electrolyte leakage leading to bacterial lysis. In addition, MB photodegradation of WO3 NFs showed an efficiency of 85.7%. This process was easy, fast, inexpensive, sustainable, and scalable. Conveniently, the one-step green route alternates between physical and chemical methods. Synthesized WO3 NFs can be used for antibacterial and photocatalytic applications. As a result, this material may be used in various disciplines, including energy storage and biological applications.
Author Contributions
Conceptualization, S.T., G.K., M.G.; methodology, S.T., G.K.; software, M.G.; validation, S.T., G.K., M.G., A.A., S.K., K.A.A.-G.; formal analysis, A.A., S.T., G.K.; resources, M.G., K.A.A.-G.; visualization, M.N.R.; writing—original draft preparation, S.T., G.K.; writing—review and editing, M.G., M.N.R.; funding acquisition, K.A.A.-G. All authors have read and agreed to the published version of the manuscript.
Funding
Researchers Supporting Project Number (RSP2023R48) King Saud University, Riyadh, Saudi Arabia. This research was supported by the Post-Doctoral Fellowship Reinventing University program fund of Chiang Mai University, Chiang Mai, Contract number: 10/2022. This research work was also partially supported by Chiang Mai University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors express their sincere appreciation to the Researchers Supporting Project Number (RSP2023R48), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
These authors declare no competing financial interest.
References
- Zou, Z.; Sun, J.; Li, Q.; Pu, Y.; Liu, J.; Sun, R.; Wang, L.; Jiang, T. Vancomycin modified copper sulfide nanoparticles for photokilling of vancomycin-resistant enterococci bacteria. Colloids Surf. B Biointerfaces 2020, 189, 110875. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cheng, X.; Liao, J.; Lin, Z.; Chen, L.; Liu, D.; Zhang, T.; Li, L.; Lu, Y.; Xia, H. Synergistic photothermal and photodynamic therapy for effective implant-related bacterial infection elimination and biofilm disruption using Cu9S8 nanoparticles. ACS Biomater. Sci. Eng. 2019, 5, 6243–6253. [Google Scholar] [CrossRef] [PubMed]
- Rai, H. Removal of dyes from textile manufacturing effluent of textile and dyestuff manufacturing industry: A review of emerging techniques with reference to biological treatment. Crit. Rev. Environ. Sci. Technol. 2005, 35, 219–238. [Google Scholar] [CrossRef]
- Shokoohi, R.; Nematollahi, D.; Samarghandi, M.R.; Azarian, G.; Latifi, Z. Optimization of three-dimensional electrochemical process for degradation of methylene blue from aqueous environments using central composite design. Environ. Technol. Innov. 2020, 18, 100711. [Google Scholar] [CrossRef]
- Indana, M.K.; Gangapuram, B.R.; Dadigala, R.; Bandi, R.; Guttena, V. A novel green synthesis and characterization of silver nanoparticles using gum tragacanth and evaluation of their potential catalytic reduction activities with methylene blue and Congo red dyes. J. Anal. Sci. Technol. 2016, 7, 19. [Google Scholar] [CrossRef]
- Kuang, Y.; Zhang, X.; Zhou, S. Adsorption of methylene blue in water onto activated carbon by surfactant modification. Water 2020, 12, 587. [Google Scholar] [CrossRef]
- Kushwaha, D.; Upadhyay, S.; Mishra, P. Nanotechnology in bioethanol/biobutanol production. In Green Nanotechnology for Biofuel Production; Springer: Berlin/Heidelberg, Germany, 2018; pp. 115–127. [Google Scholar]
- Moudgil, A.; Dhyani, V.; Das, S. High speed efficient ultraviolet photodetector based on 500 nm width multiple WO3 nanowires. Appl. Phys. Lett. 2018, 113, 101101. [Google Scholar] [CrossRef]
- Mardare, C.C.; Hassel, A.W. Review on the versatility of tungsten oxide coatings. Phys. Status Solidi 2019, 216, 1900047. [Google Scholar] [CrossRef]
- Verma, M.; Singh, K.P.; Kumar, A. Reactive magnetron sputtering based synthesis of WO3 nanoparticles and their use for the photocatalytic degradation of dyes. Solid State Sci. 2020, 99, 105847. [Google Scholar] [CrossRef]
- Jeevitha, G.; Mangalaraj, D. Ammonia sensing at ambient temperature using tungsten oxide (WO3) nanoparticles. Mater. Today Proc. 2019, 18, 1602–1609. [Google Scholar] [CrossRef]
- Susanti, D.; Diputra, A.G.P.; Tananta, L.; Purwaningsih, H.; Kusuma, G.E.; Wang, C.; Shih, S.; Huang, Y. WO3 nanomaterials synthesized via a sol-gel method and calcination for use as a CO gas sensor. Front. Chem. Sci. Eng. 2014, 8, 179–187. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, X.; Zhou, S.; Xie, X.; Luo, Y.; Yu, Y. L232- Synthesis of WO3 nanoparticles for photocatalytic O2 evolution by thermal decomposition of ammonium tungstate loading on g-C3N4. J. Alloys Compd. 2011, 509, L235. [Google Scholar] [CrossRef]
- Supothina, S.; Seeharaj, P.; Yoriya, S.; Sriyudthsak, M. Synthesis of tungsten oxide nanoparticles by acid precipitation method. Ceram. Int. 2007, 33, 931–936. [Google Scholar] [CrossRef]
- Ahmadi, M.; Younesi, R.; Guinel, M.J.-F. Synthesis of tungsten oxide nanoparticles using a hydrothermal method at ambient pressure. J. Mater. Res. 2014, 29, 1424–1430. [Google Scholar] [CrossRef]
- Lu, Z.; Kanan, S.M.; Tripp, C.P. Synthesis of high surface area monoclinic WO3 particles using organic ligands and emulsion based methods. J. Mater. Chem. 2002, 12, 983–989. [Google Scholar] [CrossRef]
- Alharbi, N.S.; Govindarajan, M.; Kadaikunnan, S.; Khaled, J.M.; Almanaa, T.N.; Alyahya, S.A.; Al-Anbr, M.N.; Gopinath, K.; Sudha, A. Nanosilver crystals capped with Bauhinia acuminata phytochemicals as new antimicrobials and mosquito larvicides. J. Trace Elem. Med. Biol. 2018, 50, 146–153. [Google Scholar] [CrossRef]
- Gopinath, K.; Kumaraguru, S.; Bhakyaraj, K.; Thirumal, S.; Arumugam, A. Eco-friendly synthesis of TiO2, Au and Pt doped TiO2 nanoparticles for dye sensitized solar cell applications and evaluation of toxicity. Superlattices Microstruct. 2016, 92, 100–110. [Google Scholar] [CrossRef]
- Gopinath, K.; Sundaravadivelan, C.; Arumugam, A. Green synthesis, characterization of silver, gold and bimetallic nanoparticles using bark extract of Terminalia arjuna and their larvicidal activity against malaria vector, Anopheles stephensi. Int. J. Recent Sci. Res. 2013, 4, 904–910. [Google Scholar]
- Mahboob, S.; Nivetha, R.; Gopinath, K.; Balalakshmi, C.; Al-Ghanim, K.A.; Al-Misned, F.; Ahmed, Z.; Govindarajan, M. Facile synthesis of gold and platinum doped titanium oxide nanoparticles for antibacterial and photocatalytic activity: A photodynamic approach. Photodiagnosis Photodyn. Ther. 2021, 33, 102148. [Google Scholar] [CrossRef]
- Choudhury, B.; Dey, M.; Choudhury, A. Defect generation, dd transition, and band gap reduction in Cu-doped TiO2 nanoparticles. Int. Nano Lett. 2013, 3, 25. [Google Scholar] [CrossRef]
- Huang, D.; Zheng, C.; Huang, L.; Wu, X.; Chen, L. Linear and nonlinear optical properties of ultrafine WO3 nanorods. Optik 2018, 156, 994–998. [Google Scholar] [CrossRef]
- Huang, L.; Xu, H.; Li, Y.; Li, H.; Cheng, X.; Xia, J.; Xu, Y.; Cai, G. Visible-light-induced WO3/gC3N4 composites with enhanced photocatalytic activity. Dalton Trans. 2013, 42, 8606–8616. [Google Scholar] [CrossRef] [PubMed]
- Mane, A.; Navale, S.; Sen, S.; Aswal, D.; Gupta, S.; Patil, V. Nitrogen dioxide (NO2) sensing performance of p-polypyrrole/n-tungsten oxide hybrid nanocomposites at room temperature. Org. Electron. 2015, 16, 195–204. [Google Scholar] [CrossRef]
- Li, S.; Lin, P.; Zhao, L.; Wang, C.; Liu, D.; Liu, F.; Sun, P.; Liang, X.; Liu, F.; Yan, X. The room temperature gas sensor based on Polyaniline@flower-like WO3 nanocomposites and flexible PET substrate for NH3 detection. Sens. Actuators B Chem. 2018, 259, 505–513. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, K.; Wang, L.; Sun, J.; Luo, R.; Li, D.; Chen, A. Synthesis mechanism and gas-sensing application of nanosheet-assembled tungsten oxide microspheres. J. Mater. Chem. A 2014, 2, 7927–7934. [Google Scholar] [CrossRef]
- Liu, F.; Chen, X.; Xia, Q.; Tian, L.; Chen, X. Ultrathin tungsten oxide nanowires: Oleylamine assisted nonhydrolytic growth, oxygen vacancies and good photocatalytic properties. RSC Adv. 2015, 5, 77423–77428. [Google Scholar] [CrossRef]
- Badilescu, S.; Ashrit, P. Study of sol–gel prepared nanostructured WO3 thin films and composites for electrochromic applications. Solid State Ion. 2003, 158, 187–197. [Google Scholar] [CrossRef]
- De Wijs, G.; De Groot, R. Amorphous WO3: A first-principles approach. Electrochim. Acta 2001, 46, 1989–1993. [Google Scholar] [CrossRef]
- Xu, L.; Yin, M.-L. Agx@WO3 core-shell nanostructure for LSP enhanced chemical sensors. Sci. Rep. 2014, 4, 6745. [Google Scholar] [CrossRef]
- Boruah, P.J.; Khanikar, R.R.; Bailung, H. Synthesis and characterization of oxygen vacancy induced narrow bandgap tungsten oxide (WO3−x) nanoparticles by plasma discharge in liquid and its photocatalytic activity. Plasma Chem. Plasma Process. 2020, 40, 1019–1036. [Google Scholar] [CrossRef]
- Kavitha, N.; Venkatesh, K.; Palani, N.; Ilangovan, R. Fungus mediated biosynthesis of WO3 nanoparticles using Fusarium solani extract. Proc. AIP Conf. Proc. 2017, 1832, 050130. [Google Scholar]
- Parthibavarman, M.; Karthik, M.; Prabhakaran, S. Facile and one step synthesis of WO3 nanorods and nanosheets as an efficient photocatalyst and humidity sensing material. Vacuum 2018, 155, 224–232. [Google Scholar] [CrossRef]
- Kasi, G.; Viswanathan, K.; Sadeghi, K.; Seo, J. Optical, thermal, and structural properties of polyurethane in Mg-doped zinc oxide nanoparticles for antibacterial activity. Prog. Org. Coat. 2019, 133, 309–315. [Google Scholar] [CrossRef]
- Duan, G.; Chen, L.; Jing, Z.; De Luna, P.; Wen, L.; Zhang, L.; Zhao, L.; Xu, J.; Li, Z.; Yang, Z. Robust antibacterial activity of tungsten oxide (WO3−X) nanodots. Chem. Res. Toxicol. 2019, 32, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Ojha, A.K.; Singh, A.; Hirsch, F.; Fischer, I.; Patrice, D.; Materny, A. Well-controlled in-situ growth of 2D WO3 rectangular sheets on reduced graphene oxide with strong photocatalytic and antibacterial properties. J. Hazard. Mater. 2018, 347, 266–278. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).