Abstract
Chrysosporium, a genus of ascomycete fungi in the family Onygenaceae, has the ability to produce abundant new bioactive natural products, providing a structural foundation in drug development. This review includes the sources, distribution, biological activities and structural characteristics of the compounds isolated from Chrysosporium from 1984 to 2021. The results show that 66% of the compounds isolated from Chrysosporium are new natural products. More than half of the Chrysosporium-isolated compounds are from marine-derived Chrysosporium. The chemical structures of Chrysosporium-derived compounds have different skeletons, which are concentrated in alkaloids, polyketides, and lactones. Eighty percent of the natural products isolated from Chrysosporium have been found to have various biological activities, including cytotoxic, antibacterial, antifungal and enzyme-inhibitory activities. These results demonstrate the potential of Chrysosporium for producing new bioactive secondary metabolites, which can be used as the structural basis for developing new drugs.
1. Introduction
The fungus Chrysosporium is classified into the Onygenaceae family, Onygenales order, Euascomycetes class and Ascomycota phylum, and was first established by Corda in 1833, identified as the model species C. corii [1]. The colonies of Chrysosporium are basically flat, white to beige to tan in color, often with a powdery or granular surface texture. The mycelial mesh of Chrysosporium is almost transparent, with a smooth wall and more or less irregular vertical branches [2]. In 1901, Saccardo took the genus Chrysosporium as a synonym of Sporotrichum Link ex Fr. [3], and there has been little research since then. In 1958, Hughes reintroduced the genus Chrysosporium [4]. Since then, Oorschot has greatly revised the classification and identification of Chrysosporium. The difference between the genus Chrysosporium and its related genera was redefined, a large number of strains were reconfirmed and identified, and 22 species of Chrysosporium were identified [5].
Chrysosporium is widely distributed and can be separated from many materials and environments, such as different soils from the equator to the polar regions, oceans, air, plants, river bed sludge, livestock manure, sewage, the surfaces or bodies of animals and birds, low-temperature caves, salt ponds, high-humidity bird nests, deserts, nuclear radiation areas, baked food, etc. [6,7,8,9,10,11]. The reason that Chrysosporium has such strong environmental adaptability is its capacity to produce different and unique enzymes and secondary metabolites, including alkaloids, polyketides and lactones [2].
Natural products have always been the structural inspiration of drug development because of their unique, complex structures and huge range of biological activities. Almost 50% of the approved drugs are natural products and their analogues, or are derived from natural products and inspired by them [12]. In 1984, chryscandin was found as a new antibiotic in the culture broth of the fungi C. pannorum No. 4629 by Michio Yamashita et al. [13,14], which is the first natural product discovered from the genus Chrysosporium. Although the first research on secondary metabolites of the genus Chrysosporium in 1984 occurred more than one century after the first identification of the genus Chrysosporium in 1833, the discovery of this antibiotic opened the door to the study of natural products from Chrysosporium.
The secondary metabolites isolated from Chrysosporium have various bioactive activities, including cytotoxic activity [15,16], antibacterial activity [13,14,17], antifungal activity [17,18], enzyme inhibition [17,19] and other biological activities [13,14,19]. They also show a diversity of structural frameworks, including polyketides [20,21], alkaloids [16], peptides [15,22], lactones [17], terpenoids [23] and steroids [18]. The activity and structural diversity of natural products isolated from Chrysosporium reflect its great potential to reveal new bioactive natural products. Until now, no review has summarized the secondary metabolites of the genus Chrysosporium. In view of this situation, the sources, distribution, biological activities and structural characteristics of the compounds isolated from Chrysosporium from 1984 to 2021 are reviewed in this paper.
2. Cytotoxic Activity
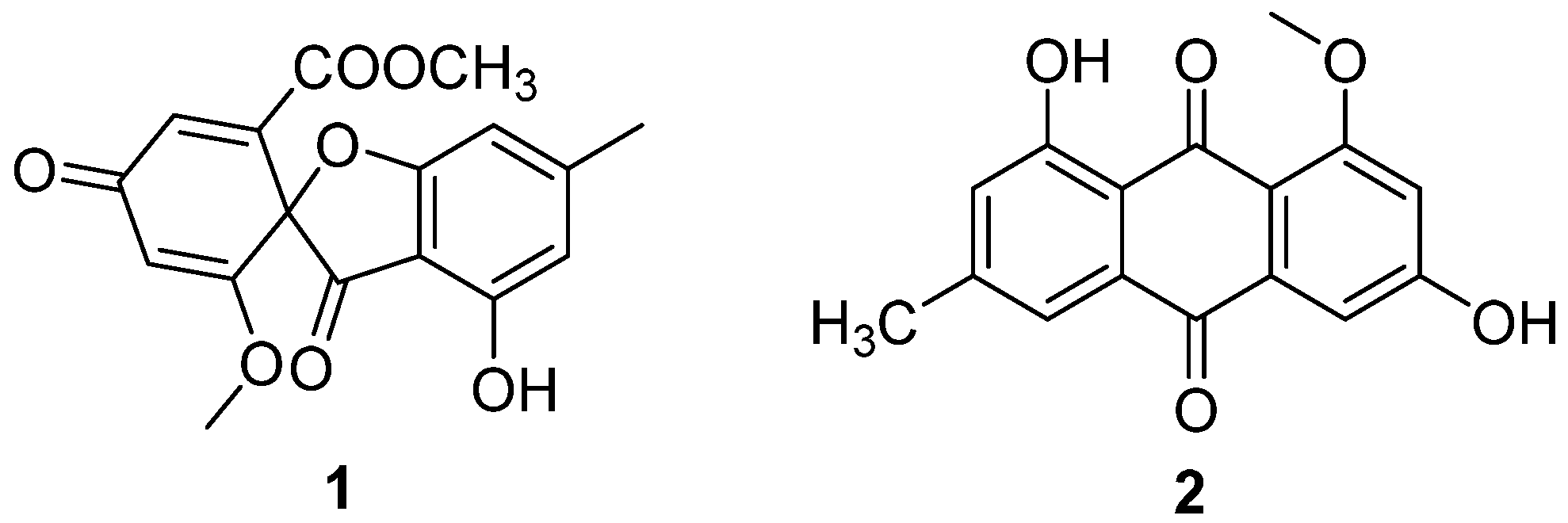
Two antibiotic components, C3368-A (1) and C3368-B (2) (Figure 1), were isolated from the fermentation broth of C. verrucosum Tubaki, which was obtained from the soil of King George Island, Antarctica [20]. Their structures were determined to be the same as those of R-(–)-bisdechlorogeodin and questin, respectively [21]. They had a strong inhibitory effect on the nucleoside transport of tumor cells and nucleoside uptake by spleen lymphocytes of mice. The IC50 values of the inhibition of nucleoside transport in Ehrlich ascites tumor cells of 1 and 2 were 4 μM and 6 μM (Table 1). Compounds 1 and 2 showed inhibition of nucleoside uptake by splenic lymphocytes in mice, with IC50 values of 5.8 μM and 2.0 μM (Table 1). This was the first Antarctic soil fungal-isolated antibiotic with the above-mentioned effects.
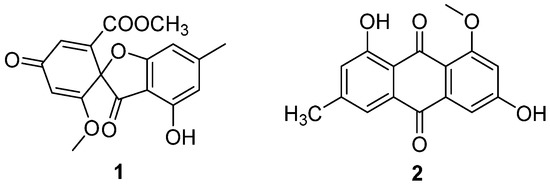
Figure 1.
Structure of polyketides 1 and 2 [20].
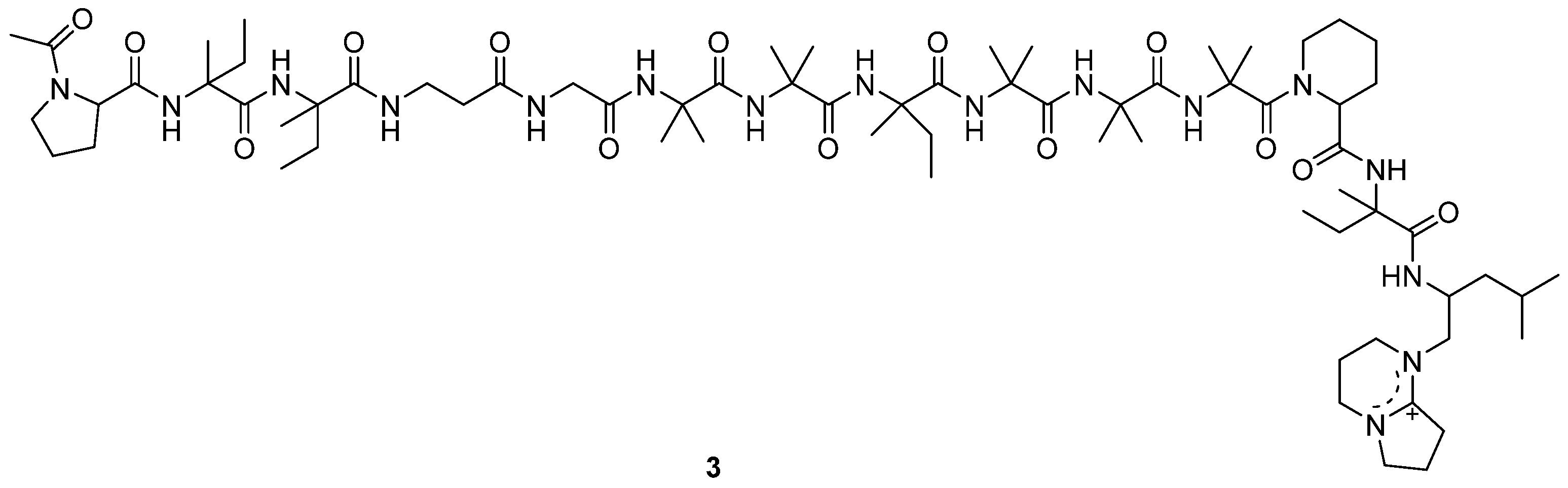
A new antitumor antibiotic, adenopeptin (3) (Figure 2), was isolated from the culture medium of Chrysosporium sp. PF1201 [15]. By using transformed rat glial cells and rat 3Y1 fibroblasts to study the antitumor activity of adenopeptin [24,25,26], the IC50 values of adenopeptin on normal and transformed cells were obtained (Table 1) [15]. Compound 3 showed significant antitumor activity against glial cells of RG-E1A-7 (oncogene E1A) and RG-E1-4 (oncogene E1A, E1B), with IC50 values of 4.5 nM and 34 pM, respectively (Table 1). Compound 3 also displayed strong antitumor activity against 3Y1 fibroblast Adl2-3Y1 (oncogene E1A, E1B), with the IC50 value of 11 pM (Table 1). It was found that adenopeptin (3) induced apoptotic cell death in the cells transformed by adenovirus type 12 oncogene (including E1A). However, 3 showed weak cytotoxicity against normal rat glial cells and rat 3Y1 fibroblasts (Table 1), indicating the potential of 3 to be developed into a selective anticancer drug.
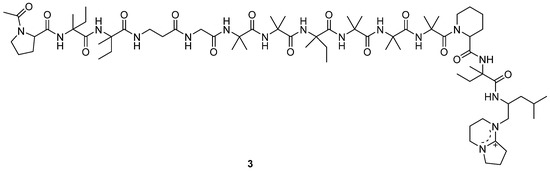
Figure 2.
Structure of peptide 3 [15].

Table 1.
Compounds with cytotoxic activity.
Table 1.
Compounds with cytotoxic activity.
| Compound | Cell | IC50 Value | Ability | Pro | Con | Prospect |
|---|---|---|---|---|---|---|
| 1/2 (μM) [20] (1992) | Inhibition of nucleoside transport in Ehrlich ascites tumor cells | 4/6 | Strong | Strong inhibitory effect on nucleoside transport of tumor cells and nucleoside uptake by spleen lymphocytes of mice | Structural foundation for antitumor drug development | |
| Inhibition of nucleoside uptake by splenic lymphocytes in mice | 5.8/2.0 | |||||
| 3 [15] (1998) | Glia | 9.1 μM | Weak | Selective cytotoxicity to transformed cell lines and normal cell lines | Potential to be developed into antitumor agent | |
| RG-E1A-7 | 4.5 nM | Strong | ||||
| RG-E1-4 | 34 pM | Strong | ||||
| 3Y1 | 11 μM | Weak | ||||
| Adl2-3Y1 | 11 pM | Strong | ||||
| SR-3Y1 | 590 nM | Strong | ||||
| HR-3Y1 | 3.2 μM | Moderate | ||||
| SV-3Y1 | 6.0 μM | Moderate | ||||
| 4/5/6 (μM) [27] (2001) | HL-60 | 0.43/-/0.10 | Strong | Strong cytotoxicity against a series of cell lines | Compound 4 was unstable | |
| WiDr | 1.87/-/0.47 | |||||
| HeLaS3 | 4.92/-/0.23 | |||||
| HCT-116 | 6.79/6.0/0.77 | |||||
| B16 | 6.56/7.6/1.87 | |||||
| P388D1 | 6.56/6.8/0.94 | |||||
| SK-BR3 | 7.96/-/1.17 | |||||
| 7/8 (μM) [28] (2013) | A549 | 2.10/13.91 | Weak | Broad-spectrum cytotoxic activity | Weak activity | To provide structural inspiration for new antitumor drugs |
| MDA MB-231 | 9.34/1.3 | |||||
| MCF-7 | 11.19/21.89 | |||||
| HeLa | 21.01/25.64 | |||||
| COLO 205 | 7.9/- | |||||
| 9/10/11 (μM) [29] (2013) | A549 | 147.3/63.2/34.5 | Weak | Weak activity | ||
| K562 | 164.0/63.0/25.4 |
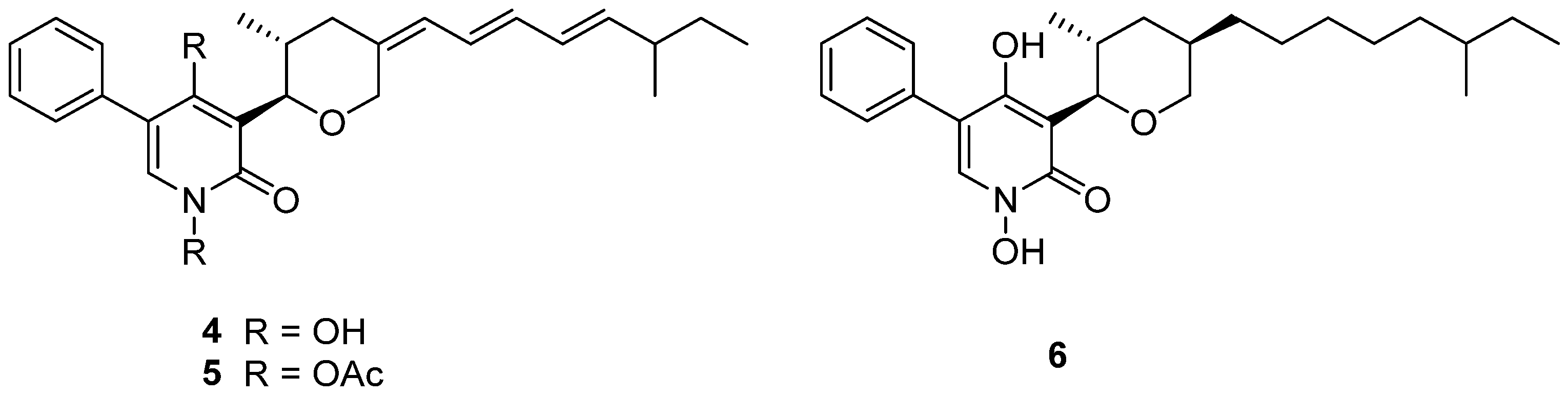
TMC-69 (4) (Figure 3) is a new antitumor antibiotic that was isolated from the fermentation broth of Chrysosporium sp. TC 1068 [16]. However, compound 4 was unstable, so more stable derivatives, diacetyl TMC-69 (5) and hexahydro TMC-69 (6), were prepared to research their structures and biological activities. TMC-69 (4) and its derivatives, 5 and 6, showed strong cytotoxicity to many tumor cell lines (Table 1). The survival time of mice-transplanted B16 melanoma and P388 leukemia was significantly prolonged by hexahydro TMC-69 (6). TMC-69 (4) had cytotoxicity on mouse tumor cells (P388D1 and B16) and human tumor cells such as HCT-116, HeLa S3, SK-BR3, WiDr and HL-60 cell lines, and showed the strongest cytotoxicity to HL-60 human promyelocytic leukemia cells, with an IC50 value of 0.43 μΜ (Table 1). Diacetyl TMC-69 (5) exhibited strong cytotoxicity against tumor cell lines HCT116, B16 and P388D1, with IC50 values ranging from 6.0 to 7.6 μΜ (Table 1) [16]. Hexahydro TMC-69 (6) also showed significant cytotoxicity to all tested cells, with IC50 values ranging from 0.1 to 1.87 μΜ (Table 1) [27]. The free N-hydroxyl group and the triene moiety are not necessary for cytotoxicity by analyzing the IC50 values of 4–6 (Table 1).
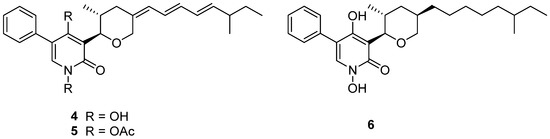
Figure 3.
Structure of alkaloids 4–6 [16].
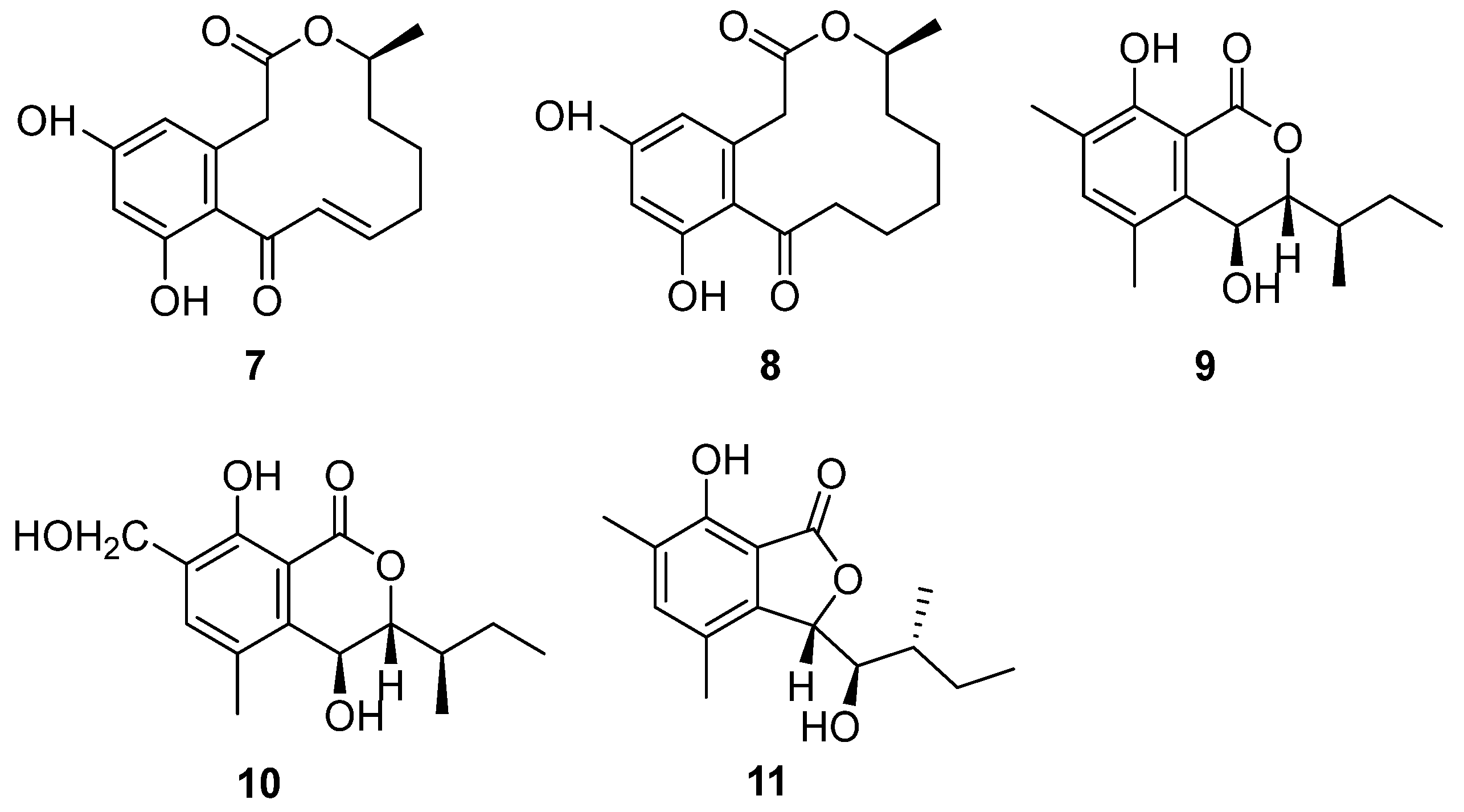
Strain BK-3 was identified as C. lobatum, which was isolated from forest soil samples collected in Kaziranga National Park, Assam, India [17]. Two bioactive compounds were isolated through biological activity-oriented purification and identified as α,β-dehydrocurvularin (7) and curvularin (8) (Figure 4), respectively [28]. Cytotoxicity tests were carried out on them and found that 7 and 8 showed similar antitumor activity towards HeLa, A549, MCF-7 and MDA MB-231. Furthermore, compound 7 was active towards COLO 205, with an IC50 value of 7.9 μM, while compound 8 was inactive (Table 1). The cytotoxic activities of compound 7 were better than those of compound 8, except for the MDA-MB-231 cell line, caused by the oxidation of 8 to format the unsaturation double bond in 7 (Figure 4), which was the only difference between these two compounds.
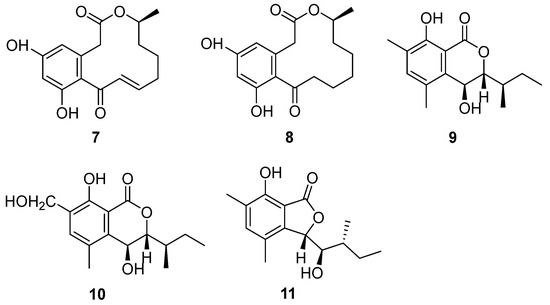
Figure 4.
Structure of lactones 7–11 [17,29].
The fungal strain C. articulatum, a marine-derived fungus, was isolated from an unidentified marine sponge collected in Gagu-do, Korea in October 2008. The strain was numbered F085 and stored in the Laboratory of Natural Product and Structure Determination, College of Pharmacy, Seoul National University [29]. Elin et al. isolated three new benzolactone metabolites, chrysoarticulins A–C (9–11) (Figure 4). Chrysoarticulins A–C were, respectively, identified as a new isocoumarin metabolite with a sec-butyl side chain, a benzyl oxidant of A and a new benzolactone metabolite. The new compounds showed weak cytotoxicity towards the A549 and K562 cell lines, and the activity of compound 11 was higher than that of other compounds, with IC50 values of 34.5 μM and 25.4 μM (Table 1). However, its cytotoxicity was far weaker than that of doxorubicin.
There are nine natural products (1–4 and 7–11) isolated from the genus Chrysosporium, along with two chemical derivatives (5 and 6), that exhibit cytotoxic activities. The cytotoxic isolated compounds include one peptide (3), three polyketides (1, 2 and 4) and five lactones (7–11). Among them, compound 1 displays significant antitumor activity against transformed cell lines and weak cytotoxicity against normal cell lines (Table 1), indicating the potential of 1 to be developed into an antitumor agent.
3. Antibacterial Activity
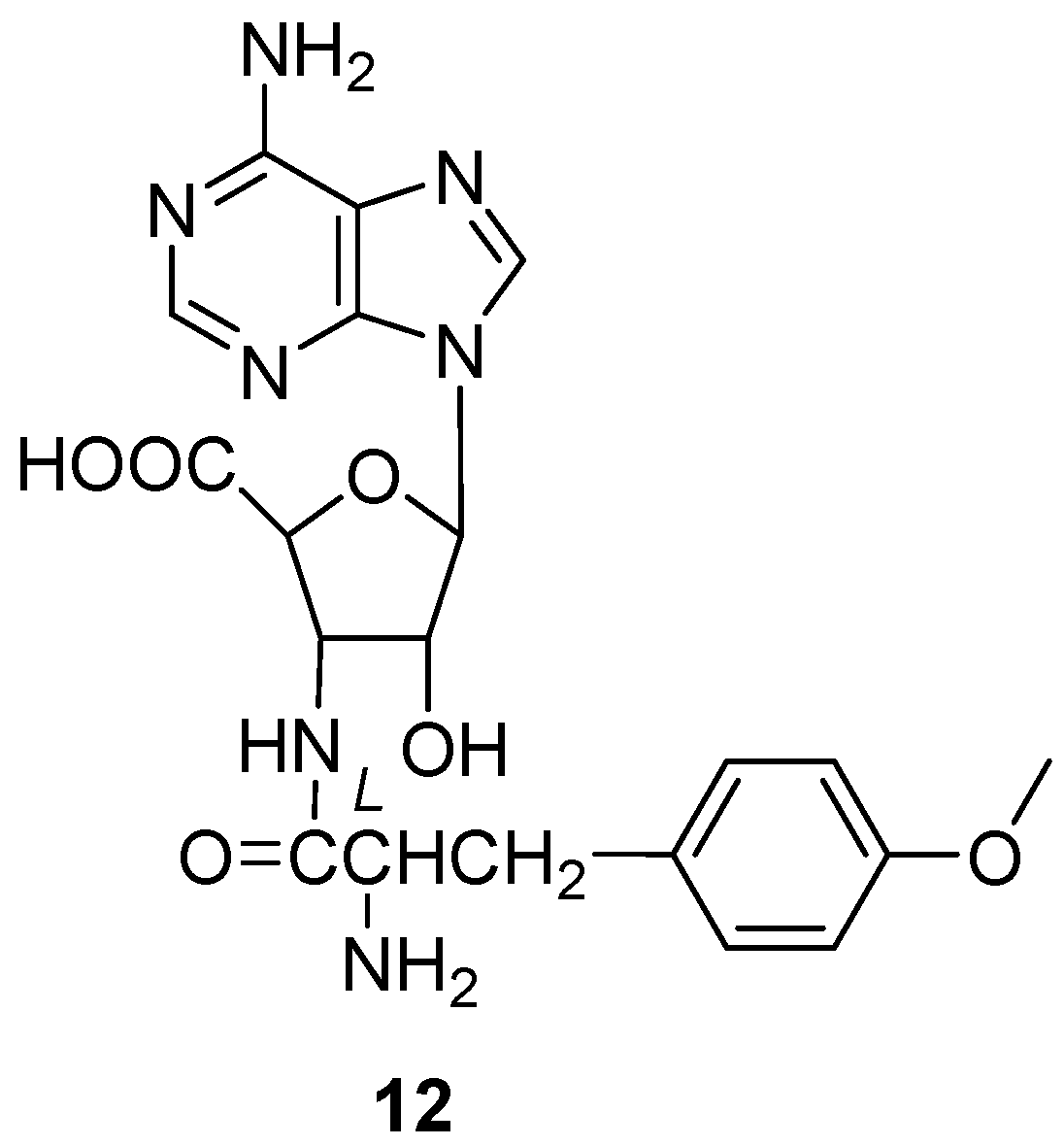
Yamashita et al. found a new antibiotic, chryscandin (12) (Figure 5) [14], from the fungus Chrysosporium pannorum in 1984. This was the first discovery of a secondary metabolite from the genus Chrysosporium. Compound 12 was found in the culture broth of the strain C. pannorum and had a unique structure, with an adenine nucleus and a 3-aminoribofuranuronic acid. The antimicrobial activity of 12 was tested and it showed activity against Gram-positive bacteria. The MIC value towards Staphylococcus aureus 209P JC-1 was 12.5 μg/mL (Table 2).
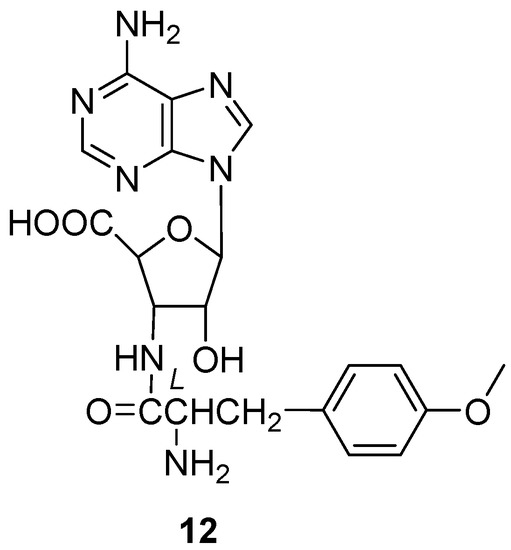
Figure 5.
Structure of alkaloid 12 [14].
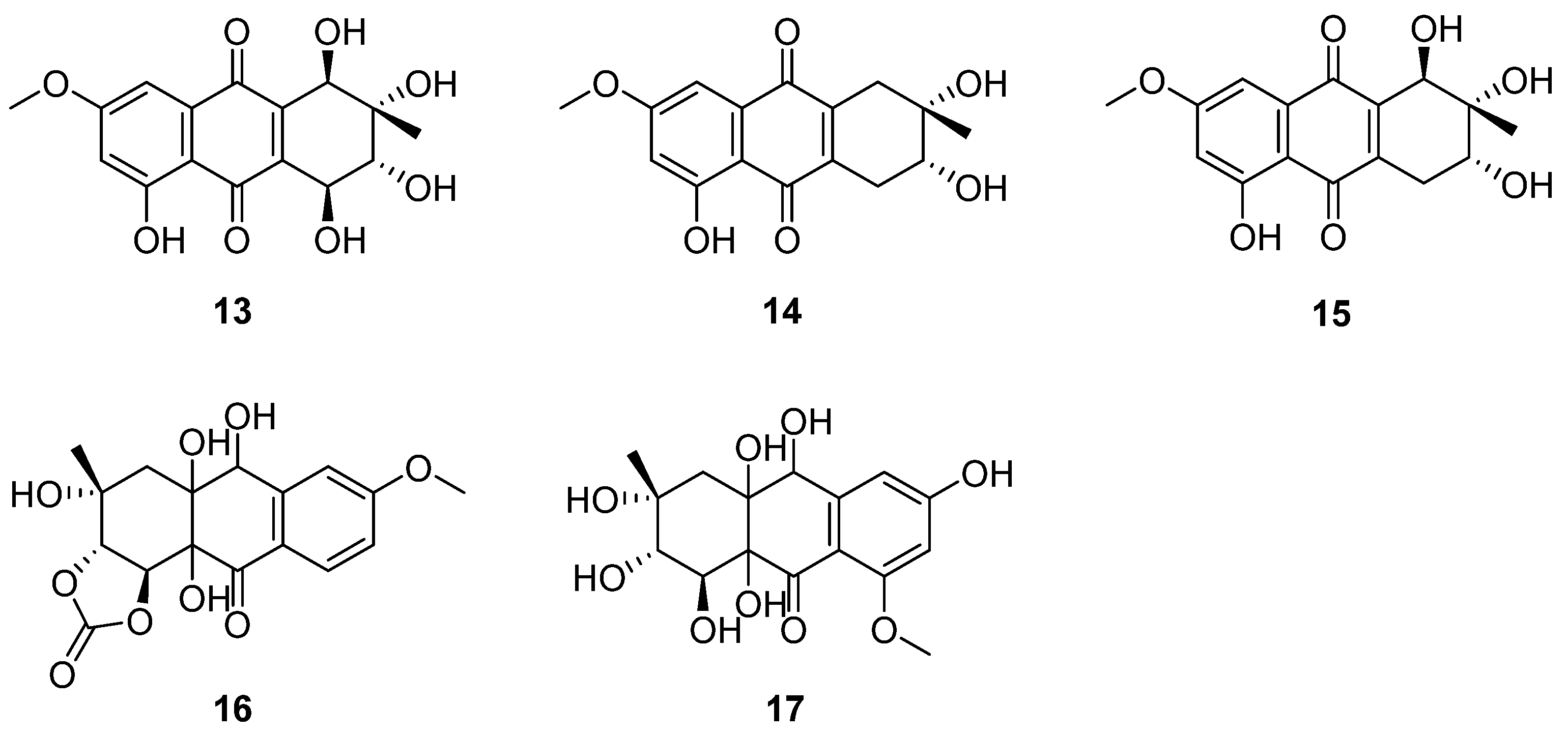
An antibacterial naphthoquinone antibiotic complex was isolated from the culture medium of C. queenslandicum IFM 51121, which was kept in the Microbial Strain Collection Center of the Research Center of Pathogenic Fungi and Microbial Toxicology, Chiba University, Japan [30]. The compounds of the complex were identified as altersolanols A–C (13–15) (Figure 6) [31,32]. They were active against all the tested Gram-positive bacteria and Pseudomonas aeruginosa IFO 3080, including Staphylococcus aureus IFO I2732, Micrococcus luteus IFO 3333 (13 MIC = 12.5, 14 MIC = 25, 15 MIC = 25) and Baccillus subtilis IFO 3007 (13 MIC = 50, 14 MIC = 25, 15 MIC = 25) [33] (Table 2). The MIC values of 13–15 against P. aeruginosa IFO 3080 and S. aureus IFO I2732 were all 12.5 μg/mL (Table 2) [33]. P. aeruginosa IFO 3080 was used to study the antimicrobial mechanism of 13. The incorporation of radioactive precursors into macromolecules (DNA, RNA and protein) in whole P. aeruginosa IFO 3080 cells was nonspecifically suppressed by compound 13. Moreover, the respiration of P. aeruginosa cells was enhanced; however, the proton conduction was unchanged through 13. In addition, compound 13 increased the oxidation of NADH in the membrane fraction isolated from P. aeruginosa. In addition, it stimulated the oxidation of NADH by the enzyme preparation of cytochrome c reductase in the absence of cytochrome c. In summary, altersolanol A (13) seemed to inhibit bacterial growth by interfering with the respiratory chain in the bacterial membrane as an electron acceptor [34]. In addition to the above metabolites, C. queenslandicum IFM 51121 co-produced new antibacterial compounds related to the members of the dihydro-naphthoquinone group [35,36,37], named chrysoqueen (16) and chrysolandol (17) [30]. They showed activity against Gram-positive bacteria such as Micrococcus luteus IFM 2066 and Bacillus subtilis PCI 219, which had the same MIC value of 33 μg/mL.
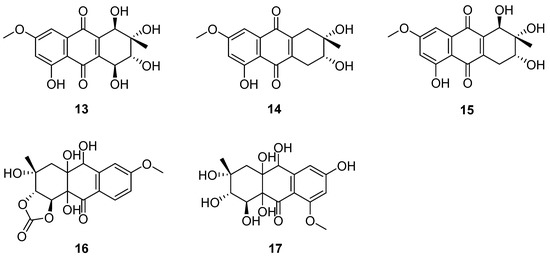
Figure 6.
Structure of polyketides 13–17 [30,31,32].
α,β-Dehydrocurvularin (7) and curvularin (8) (Figure 4) were isolated from the strain C. lobatum BK-3. Compound 7 exhibited weak antibacterial activity against Gram-positive and Gram-negative bacteria, such as B. subtilis, S. aureus and Escherichia coli, which had the same MIC value of 40 μg/mL [38] (Table 2). The possible antibacterial mechanism of 7 was its efflux pump inhibitory potential in ethidium bromide (EtBr) fluorescence. Compound 7 suppressed the efflux pump by 0.84-fold, decreasing in ethidium bromide (EtBr) fluorescence with the concentration of 100 μg/mL [39]. Curvularin (8) (Figure 4) could completely inhibit the growth of B. subtilis on the seeds of Phaseolus mungo at a concentration of 100 ppm [40].
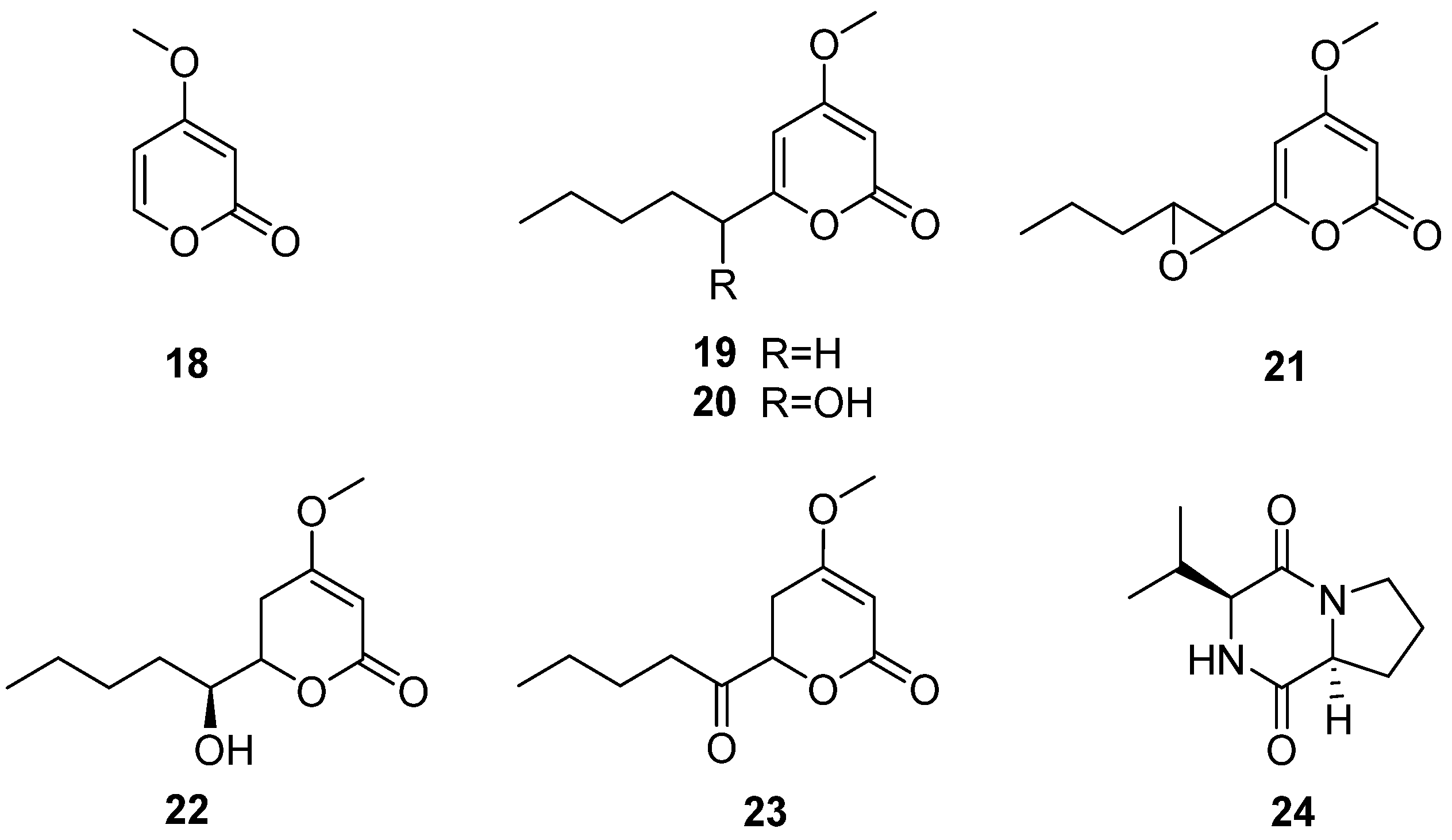
The fungus C. multifidum was isolated from the gut of Hermetia illucens BSF larvae [22]. The BSF larvae were taken from the breeding colony established by the Universidad Peruana Cayetano Heredia (Lima, Peru). The samples were fed with fresh and unsterilized chicken manure for 11 days. Chrysosporium was also isolated from chicken feces [41], which may be the Chrysosporium obtained from the diet of BSF larvae. Seven compounds were separated from the fungus C. multifidum, six α-pyranone derivatives (18–23) and a diketopiperazine (24) (Figure 7), and compound 24 was first reported in the Chrysosporium genus. The extract from the culture supernatant of C. multifidum showed moderate activity against methicillin-resistant Staphylococcus aureus (MRSA), with an MIC value of 62.5 μg/mL. Among these compounds, compound 21 had the highest activity towards MRSA (MIC = 62.5 μg/mL) (Table 2). These data suggest that the fungus C. multifidum isolated from the H. illucens gut may be a useful source to produce antimicrobial compounds that are active against other drug-resistant pathogens.
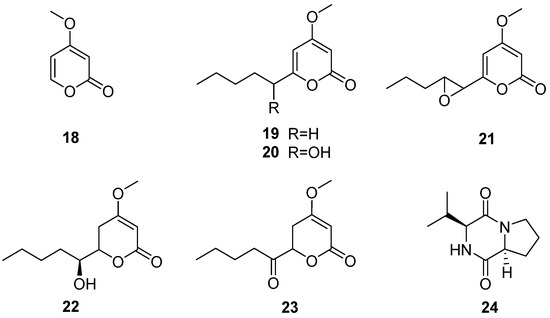
Figure 7.
Structure of lactones 18–23 and cyclic dipeptide 24 [22].

Table 2.
Compounds with antimicrobial activity.
Table 2.
Compounds with antimicrobial activity.
| Compound | Strain | MIC Value (μg/mL) | Ability | Pro | Con | Prospect |
|---|---|---|---|---|---|---|
| 12 [14] (1984) | Staphylococcus aureus 209P JC-1 | 12.5 | Weak | Broad-spectrum antimicrobial activity | Weak activity | Structural foundation for new antibiotics |
| Candida albicans FP-614 | 0.8 | |||||
| C. albicans FP-616 | 0.2 | |||||
| C. albicans FP-618 | 0.8 | |||||
| C. albicans FP-620 | 0.2 | |||||
| C. albicans FP-622 | 3.1 | |||||
| C. albicans FP-633 | 1.6 | |||||
| 13/14/15 [33] (2002) | S. aureus IFO I2732 | 12.5/12.5/12.5 | Weak | Broad-spectrum antibacterial activity and the antibacterial mechanism of 13 was studied. | Weak activity | To provide structural inspiration for new antibiotics |
| Micrococcus luteus IFO 3333 | 12.5/25/25 | |||||
| Baccillus subtilis IFO 3007 | 50/25/25 | |||||
| Pseudomonas aeruginosa IFO 3080 | 12.5/12.5/12.5 | |||||
| C. albicans IFO 1061 | >100 | |||||
| Aspergillus niger IFO 6275 | >100 | |||||
| Saccharomyces cereoisiae IFO 0203 | >100 | |||||
| 16/17 [30] (2002) | Bacillus subtilis PCI 219 | 33/33 | Weak | Weak activity | Structural foundation for developing new antibiotics | |
| Micrococcus luteus IFM 2066 | 33/33 | |||||
| 25 (Zone of inhibition) [42] (2002) | Aspergillus nidulans IFM 5369 | 52.1 mm | Strong | Significant antifungal activity against A. nidulans IFM 5369 and broad-spectrum antifungal activity | Potential to be developed into new antifungal drugs | |
| Penicillium chrysogenum IFM 40614 Paecilomyces variotii IFM 40913 A. terreus IFM 40851 A. fumigatus IFM 41088 Alternaria alternata IFM 41348 A. niger IFM 5368 A. niger IFM 41934 | Moderate | |||||
| 26 [18] (2003) | Candida albicans (FLZ-S) | 1 | Weak | Broad-spectrum antifungal activity | Weak activity | Structural foundation for developing new antibiotics |
| C. albicans (FLZ-R) | 0.5 | Weak | ||||
| C. dubliniensis (FLZ-R) | 8 | Weak | ||||
| C. krusei | 2 | Weak | ||||
| C. glabrate (FLZ-S) | 0.1 | Moderate | ||||
| C. glabrate (FLZ-R) | 0.5 | Weak | ||||
| Saccharomyces cerevisiae | <0.06 | Moderate | ||||
| Cryptococcus neoformans | 16 | Weak | ||||
| 27 [23] (2009) | S. cerevisiae (PM 503) | 60 | Weak | Weak activity | ||
| 7 [38] (2013) | B. subtilis | 40 | Weak | The antibacterial mechanism was studied | Weak activity | |
| S. aureus | 40 | |||||
| Escherichia coli | 40 | |||||
| Cladosporium herbarum | - | |||||
| 8 [40] (2013) | Chaetomium indicum Corda Phoma hibernicaa Grimes et al. Aspergillus flavus Link ex Fr. Drechslera tetramera McKiuney Fusarium oxysporum Schl. | Completely suppressed in 50 ppm | Strong | Completely suppressed the growth of a series of fungi on the seeds of Phaseolus mungo Roxb | Potential to be developed into agricultural antimicrobial drugs | |
| B. subtilis | Completely suppressed in 100 ppm | |||||
| Curvularia lunata (Walker) Boedijn Papulaspora sp. | Completely suppressed in 200 ppm | |||||
| Alternaria alternata Nees. | Reduced considerably in 200 ppm | |||||
| 21 | methicillin-resistant Staphylococcus aureus (MRSA) | 62.5 | Moderate | With antibacterial activity against drug-resistant strain | The low activity | Potential to be developed into antimicrobial resistance drugs |
| 18–20, 22–24 [41] (2019) | Weak |
4. Antifungal Activity
Besides antibacterial activity, chryscandin (12) (Figure 5) showed antifungal activity against C. albicans. The inhibition rates against C. albicans FP-614, FP-616, FP-618, FP-620, FP-622 and FP-633 were tested, and all the MIC values were less than 4 μg/mL [14] (Table 2). Such inhibition was accompanied by swelling of the Candida cells [43]. The acute toxicity of compound 12 was very low in mice, indicating the good drug safety performance of 12. The anti-infective experiment in vivo due to C. albicans of 12 was examined with the ED50 value of 10 mg/kg by the subcutaneous route and 25 mg/kg by the oral route [14], which was almost the same as the positive control, 5-fluorocytosine.
Altersolanols A–C (13–15) (Figure 6) and queenslandon (25) (Figure 8), isolated from C. queenslandicum IFM 51121, were found to have antifungal activity [31,32,42,44]. The MIC values of 13–15 towards C. albicans IFO 1061, Saccharomyces cereoisiae IFO 0203 and Aspergillus niger IFO 6275 were all over 100 μg/mL (Table 2). Queenslandon (25) belonged to the zearalenone family of mycotoxins [44]. Compound 25 exhibited significant antifungal activity, with a 52.1 mm maximum inhibition diameter against A. nidulans IFM 5369 by the 8 mm paper disc containing 50 μg of 25 [42], and its antifungal activity was even stronger than that of positive control amphotercin B. It also showed moderate antifungal activity against Penicillium chrysogenum IFM 40614, Paecilomyces variotii IFM 40913, A. terreus IFM 40851, A. fumigatus IFM 41088, Alternaria alternata IFM 41348, A. niger IFM 5368 and A. niger IFM 41934 [42] (Table 2). The inhibition diameters of the reference drug amphotercin B against A. niger IFM 5368 and A. nidulans IFM 5369 were 13 and 32 mm, respectively.
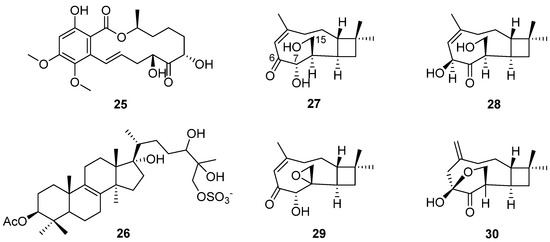
Figure 8.
Structure of lactone 25, steroid 26 and terpenoids 27–30 [18,23,42].
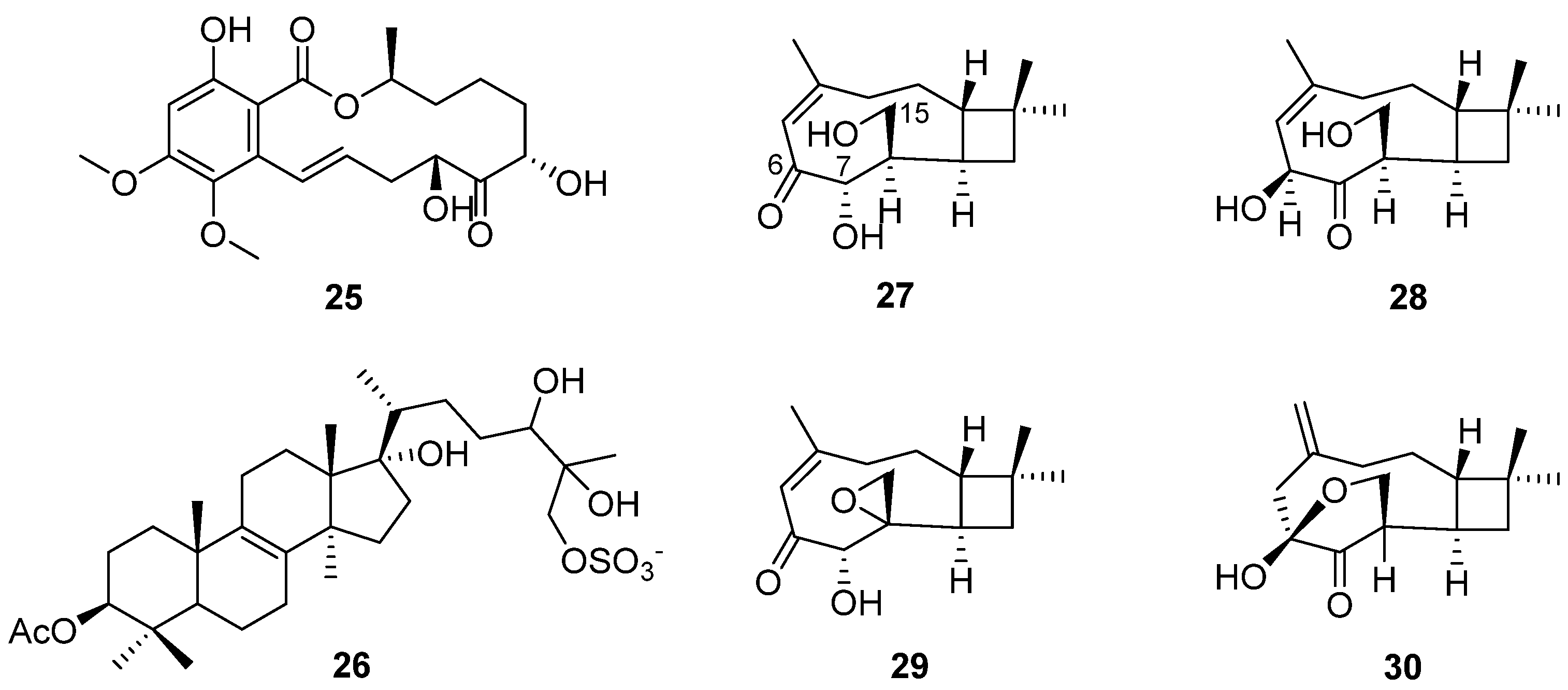
In order to search for antifungal drugs, a new antifungal agent, sch 601324 (26) (Figure 8), was separated from the fungal culture Chrysosporium pilosum [18], which belongs to sterol sulfates and has antifungal activity against various candidal strains, such as Candida albicans, C. dubliniensis, C. krusei, C. glabrate and Saccharomyces cerevisiae. The MIC values were under 2 μg/mL (Table 2). The collected microorganisms were purchased from commercial sources and named SPRI-IL15503. Four new caryophyllene sesquiterpenes (27–30) (Figure 8) were found by further study of the secondary metabolites of the microorganism [23]. Compound 27 showed antifungal activity, and its MIC value towards S. cerevisiae (PM 503) was 60 µg/mL (Table 2). Compounds 27–30 possess three oxygen atoms on C-6, C-7 and C-15, but their different oxidation states or cyclization positions may be related to the strength of their antifungal activity. The results show that C. pilosum has the ability to produce antimicrobial secondary metabolites, which provide a chemical structural foundation for drug development.
Two bioactive compounds, α,β-dehydrocurvularin (7) and curvularin (8) (Figure 4), isolated from the strain C. lobatum BK-3, showed antifungal activities. α,β-Dehydrocurvularin (7) exhibited antifungal activity against Cladosporium herbarum [45]. An antifungal activity evaluation indicated that curvularin (8) could completely suppress the growth of a series of fungi on the seeds of Phaseolus mungo Roxb with a concentration of 50 ppm (Table 2) [40].
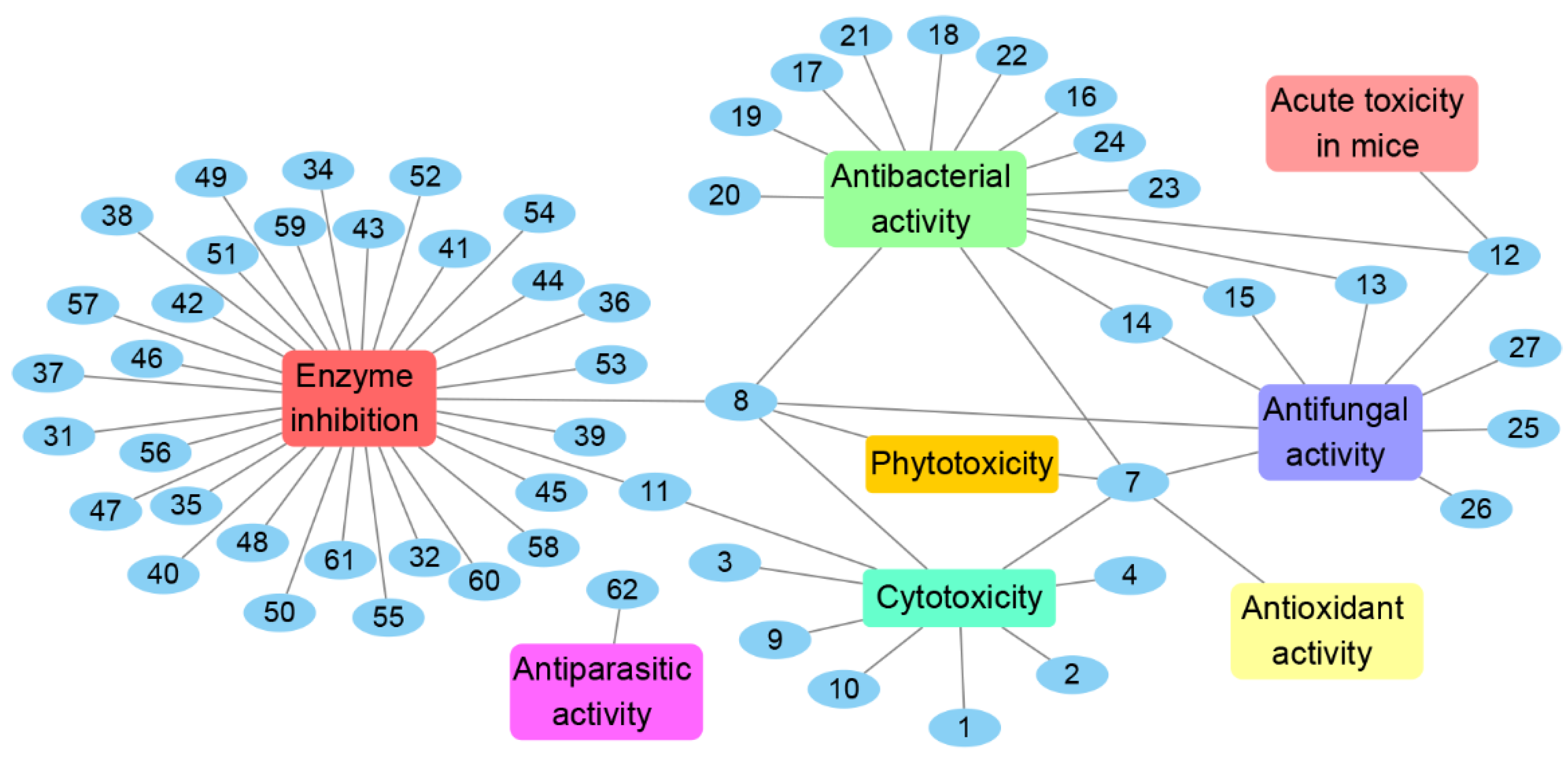
Eighteen Chrysosporium-isolated compounds exhibit antimicrobial activities. Fifteen of these compounds show antibacterial activities (7, 8, 12–24), nine of the compounds display antifungal activities (7, 8, 12–15, 25, 26, 27), and six compounds reveal both antibacterial and antifungal activities (7, 8, 12–15). Nine of the antimicrobial compounds belong to lactones (7, 8, 18–23, 25), five of the compounds belong to the family of polyketides (13–17), and others include an alkaloid (12), cyclic peptide (24), terpenoid (27) and steroid (26). Among them, compound 8 can completely suppress the growth of a series of fungi and bacterium B. subtilis on the seeds of Phaseolus mungo Roxb, meaning that it has the potential to be developed into agricultural antimicrobial drugs. Compound 25 exhibits stronger antifungal activity against A. nidulans IFM 5369 than positive control amphotercin B, which could be developed into antifungal drugs.
5. Enzyme Inhibition
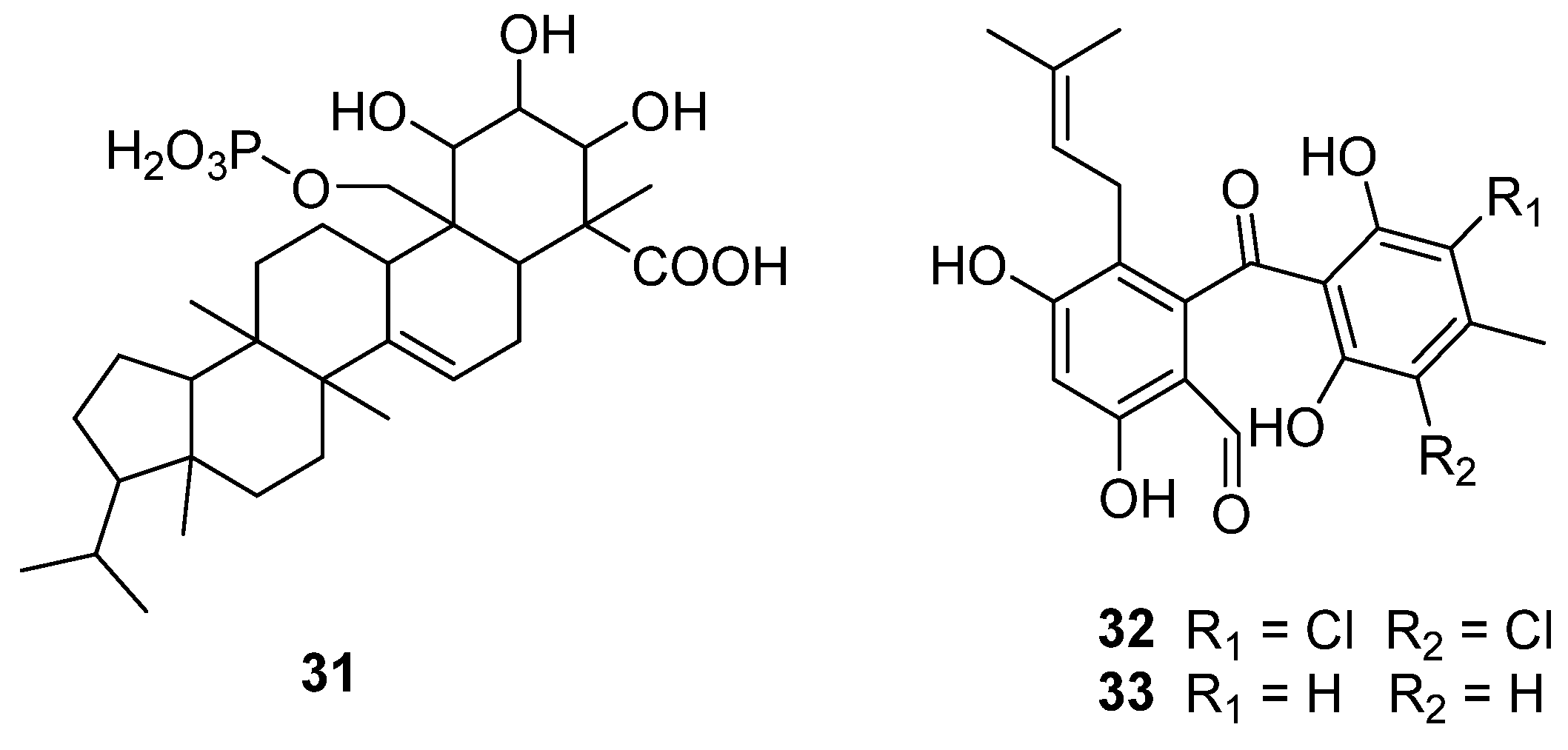
Inhibition of p21ras protein’s farnesylation is considered a potential strategy for antitumor chemotherapy research [46]. A new compound, R113228 (31) (Figure 9), was isolated from a fungus, C. lobatum, which was a new type of farnesyl protein transferase (FPTase) inhibitor [47]. This strain was numbered CBS12395 and is conserved in the Centraalbureau voor Schimmel Culturen, Baarn, Holland. Compound 31 showed selective inhibition towards FPTase. Because its IC50 towards human geranyl-geranyl protein transferase was 59 μM (Table 3), it showed no inhibitory effect on rat liver squalene synthase. This secondary metabolite represents a new and effective inhibitor of Ras post-translational processing and thus can be used as an anticancer agent.
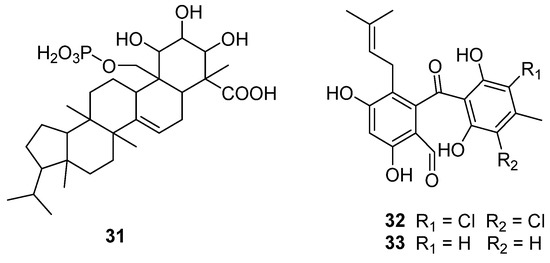
Figure 9.
Structure of triterpene 31 and polyketides 32 and 33 [47,48].
SB87-Cl (32) and SB87-H (33) (Figure 9) [48] were isolated from Chrysosporium sp. Compound 32 could inhibit reductase (Table 3), but had different structural characteristics from other inhibitors with chlorination. It also could be safely used as an active ingredient in hair growth stimulators [49].
In the search for HIV-1 protease inhibitors in microbial secondary metabolites, inhibitors from the fungus C. merdarium P-5656 were identified [19]. P-5656 came from the soil samples collected from a coconut forest near Tenacaita, Mexico, from which the known bisalkylated 2,5-dihydroxybenzoquinones didemethylasterriquinone D (34) and isocochliodinol (35), as well as the new metabolites semicochliodinol A (36) and B (37) (Figure 10), were separated. Compounds 34–36 exhibited strong enzyme inhibition activity against HIV-1 protease (HIV-1 prt), with IC50 values from 0.18 μM to 0.37 μM (Table 3). Compound 37 was a weak HIV-1 protease inhibitor. The IC50 values of compounds 34–37 for human cathepsin D (CD) were from 2.5 μM to 4.9 μM (Table 3). Nakikiqinones C and D, with a similar central hydroxybenzoquinone ring, have been described as c-ErbB-2 kinase inhibitors [50]. Compounds 34–37 inhibited epidermal growth factor receptor protein tyrosine kinase (EGF-R PTK) by approximately 20 μM (Table 3). The overlap of 34 with known EGF-R PTK [51] inhibitors or DAHP 1 [52] suggests that new derivatives of 34 with meta substitution on the benzoquinone ring can be more effective as inhibitors of this enzyme. Downregulation of PTK activity in tumor cells is associated with malignant transformation. EGF-R PTK inhibitors may have therapeutic potential as anticancer agents. Studies have shown that 34 and its derivatives are attractive lead compounds for HIV-1 protease and EGF-R PTK inhibitors.
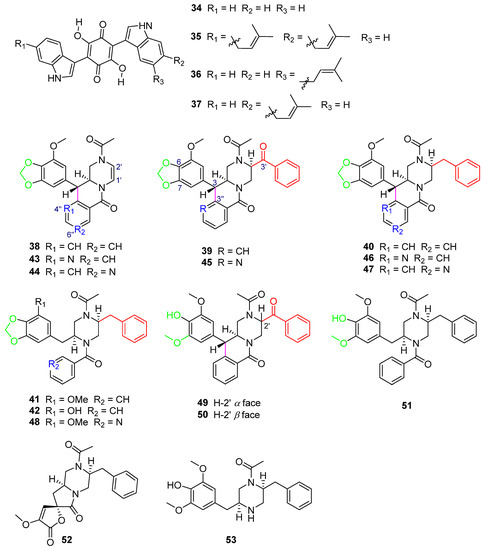
Figure 10.
Structure of alkaloids 34–53 [19,53,54]. The different color highlighted the different structural characteristics of 38–51.

Table 3.
Compounds with enzyme inhibition.
Table 3.
Compounds with enzyme inhibition.
| Compound | Enzyme | Inhibition Value | Ability | Pro | Con | Prospect |
|---|---|---|---|---|---|---|
| 31 (IC50/μM) [47] (1995) | FPTase | 59 | Moderate | Potential to be developed into new antitumor drugs | ||
| 32 [49] (1995) | Reductase | - | - | |||
| 34/35/36/37 (IC50/μM) [19] (1997) | HIV-1 prt | 0.24/0.18/0.37/>0.5 | Strong/weak (37) | Strong inhibition against HIV-1 prt | Weak inhibition against CD and EGF-R PTK | Attractive lead compounds for HIV-1 protease |
| CD | 4.2/4.1/2.5/4.9 | Weak | ||||
| EGF-R PTK | 15/20/20/60 | Weak | ||||
| 6 (IC50/μM) [27] (2001) | Cdc25A | 3.1 | Strong | Strong inhibition against Cdc25A and Cdc25B | Potential to be developed into new antitumor drugs | |
| Cdc25B | 4.4 | |||||
| 8 (IC50/μM) [28] (2013) | AChE | 1.36 | Strong | Strong inhibition | Potential to be developed into AChE inhibitory drugs to treat Alzheimer’s disease | |
| 11 (IC50/μM) [29] (2013) | Sortase A | 95.1 | Moderate | Potential to be developed into new antimicrobial drugs | ||
| ICL | 236.4 | Weak | Weak inhibition | Structural foundation for developing new anti-tuberculosis drugs | ||
| 38–42 (GS) [53] (2019) | P-gp | 3.6/7.2/6.3/1.6/1.8 | Weak | Compounds 43, 45, 46, 47 and 54 display significant enzyme inhibitory activity against P-gp and could reverse doxorubicin resistance in human colon cancer cells | Potential to be researched as assisted antitumor drugs to reduce the occurrence rate of drug resistance | |
| 43/44 (GS) | P-gp | 9.5/6.8 | Moderate | |||
| 45/46/47 (GS) | 20.5/21.3/19.8 | Strong | ||||
| 48–53 (GS) [54] (2021) | 1.62/0.81/1.11/1.13/1.39/1.27 | Weak | ||||
| 54 (GS) | P-gp | 14 | Strong | |||
| 55/58/59 (GS) | P-gp | 5.6/4.5/6.0 | Moderate | |||
| 56/57/60/61 [55] (2020) | P-gp | - | - |
TMC-69-6H (6) (Figure 3), a hexahydro derivative of 4, caused a dose-dependent inhibition of phosphatase Cdc25A (Cdc25 phosphatases are classified as dual-specificity protein phosphatases) activity, with an IC50 value of 3.1 μM, and also inhibited Cdc25B, with an IC50 of 4.4 μM (Table 3) [27].
The initial crude extract of the strain C. lobatum BK-3 showed 60% inhibition of acetylcholinesterase (AChE) [28]. Chemical investigation of the fungus led to the isolation of compound curvularin (8) (Figure 4), which showed strong AChE inhibitory activity, with 80% inhibition, and the IC50 value was 1.36 μmol/mL (Table 3), compared to the positive control galanthamine, with 75% inhibition and an IC50 value of 2.625 μmol/mL.
Chrysoarticulin C (11) (Figure 4), isolated from the fungus C. articulatum, had moderate activity on sortase A, the key enzyme related to the adhesion and invasion of Gram-positive bacteria, with the IC50 value of 95.1 μM. Compound 11 displayed weak inhibition against isocitrate lyase (ICL, a key enzyme in microbial biosynthesis), with an IC50 value of 236.4 μM (Table 3) [29].
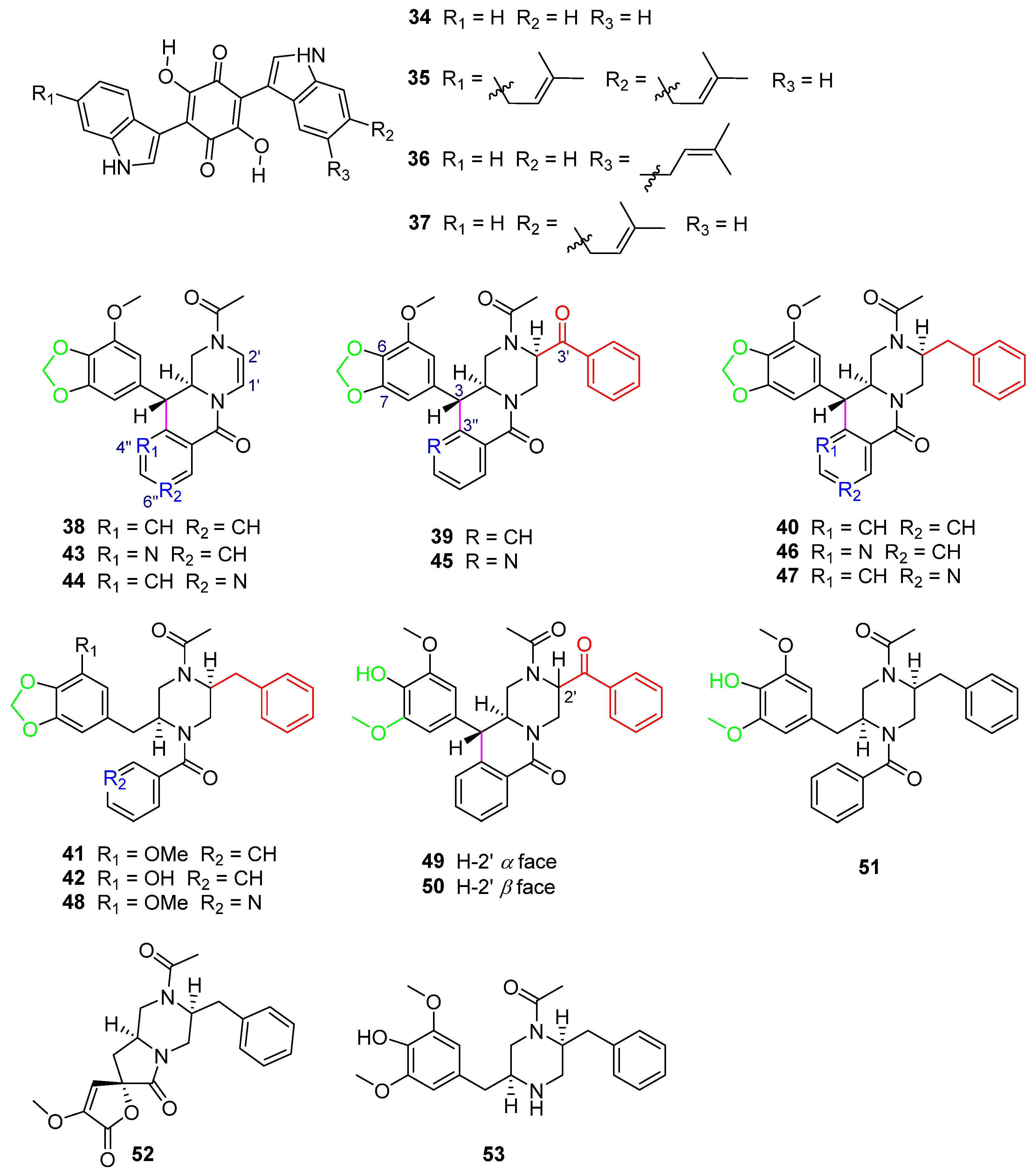
Five new phenylpropyl piperazines, chrysosporazines A–E (38–42) (Figure 10), featuring an unprecedented hexahydro-6H-pyrazino[1,2-b]isoquinolin-6-one scaffold [53], were obtained from the fungus Chrysosporium sp. CMB-F214 in the gastrointestinal tract of a Mugil mullet purchased from an Australian market. Chrysosporazines showed P-glycoprotein (P-gp) inhibition and could reverse doxorubicin resistance in human colon cancer cells (SW620 Ad300, P-gp overexpressing cell lines) with a 2.5 μM co-treatment, significantly improving the sensitivity of 38–40 to doxorubicin, with the GS (the ratio of doxorubicin IC50 without and with the addition of either 38–40 or verapamil) values of 3.6, 7.2 and 6.3, respectively, and the GS of verapamil as the positive control was 8.3 (Table 3).
Further chemical investigation of the fungus CMB-F214 led to the isolation of azachrysposorazines A1, A2, B1, C1, C2 and D1 (43–48), chrysosporazines N–P (49–51), spirochrysosporazine A (52) and known chrysosporazines A–D (38–41) (Figure 10), with the method of precursor-directed biosynthesis-mediated amplification [54]. The fungus CMB-F214 was cultured in an M1 agar plate medium containing sodium nicotinate. The new natural product chrysosporazine Q (53) was produced by the fungus CMB-F214 in an M2 medium without sodium nicotinate. Compounds 43–53 reversed the drug resistance of SW620 Ad300 cancer cells to doxorubicin. The doxorubicin sensitivity (GS) induced by C-2′-substituted analogues 45–47 was more than 2.5-times higher than that of the positive control, verapamil (Table 3).
Further investigation of the structure–activity relationship of the chrysosporazines and their derivatives found that the influences of the structures on reversing the doxorubicin resistance bioactivity of the compounds are mainly caused by the following aspects: C-2′ substitution by benzaldehyde or benzyl groups, C-3/C-3″ cyclization, whether there is a methylenedioxy ring and whether there is a replacement of CH by N in the location of 4″ or 6″.
The reduction of the double bond between C-1′ and C-2′ and the substitute in the location of C-2′ by a benzaldehyde group in 39 and 45 (Figure 10) or a benzyl group in 40 and 46 (Figure 10) can lead to significantly higher GS values (38 GS = 3.6, 39 GS = 7.2, 40 GS = 6.3, 43 GS = 9.5, 45 GS = 20.5, 46 GS = 21.3) (Table 3), when comparing the structures of 38/39/40, 43/45/46 (Figure 10), respectively.
The cyclization of C-3/C-3″ leads to higher activity when comparing the structures of 40 and 41 (Figure 10) (40 GS = 6.3, 41 GS = 1.6), and 46 and 48 (Figure 10) (46 GS = 21.3, 48 GS = 1.62), respectively (Table 3). The breakage of the C–C bond in C-3/C-3″ in 41, 42, 48 and 51 (Figure 10) leads to a lower ability to reverse doxorubicin resistance (41 GS = 1.6, 42 GS = 1.8, 48 GS = 1.62, 51 GS = 1.13) (Table 3), which further explains the importance of C-3/C-3″ cyclization to the activity of compounds.
The only difference in the structures of 39 and 49/50 (Figure 10) is whether the left sides of C-6 and C-7 contain a methylenedioxy ring, which causes their different GS values (39 GS = 7.2, 49 GS = 0.81, 50 GS = 1.11) (Table 3). It is found that the GS values of the compounds containing a methylenedioxy ring are higher than those of the compounds not containing it.
When comparing the structures of 38 and 43/44 (Figure 10) (38 GS = 3.6, 43 GS = 9.5, 44 GS = 6.8) (Table 3), substitution of CH with N at 4″ or 6″ significantly increases the activity of the compounds. The structure analysis of 39 and 45 (Figure 10) (39 GS = 7.2, 45 GS = 20.5) and 40 and 46/47 (Figure 10) (40 GS = 6.3, 46 GS = 21.3, 47 GS = 19.8) (Table 3) also reaches the same conclusion. The alternative CH by N at 4″ or 6″ has a positive impact on the activity of the compounds.
Compounds 45–47 (Figure 10), which have structures with all of the above characteristics, including C-2′ substitution, C-3/C-3″ cyclization, the methylenedioxy ring and alternative CH by N at 4″ or 6″, exhibited the best activity (45 GS = 20.5, 46 GS = 21.3, 47 GS = 19.8) (Table 3), with GS values > 2.5-fold higher than that of the positive control, verapamil (GS = 8.1), which further illustrated that these four structural features are the key determinants to improve the P-gp inhibition of chrysosporazines and their derivatives.
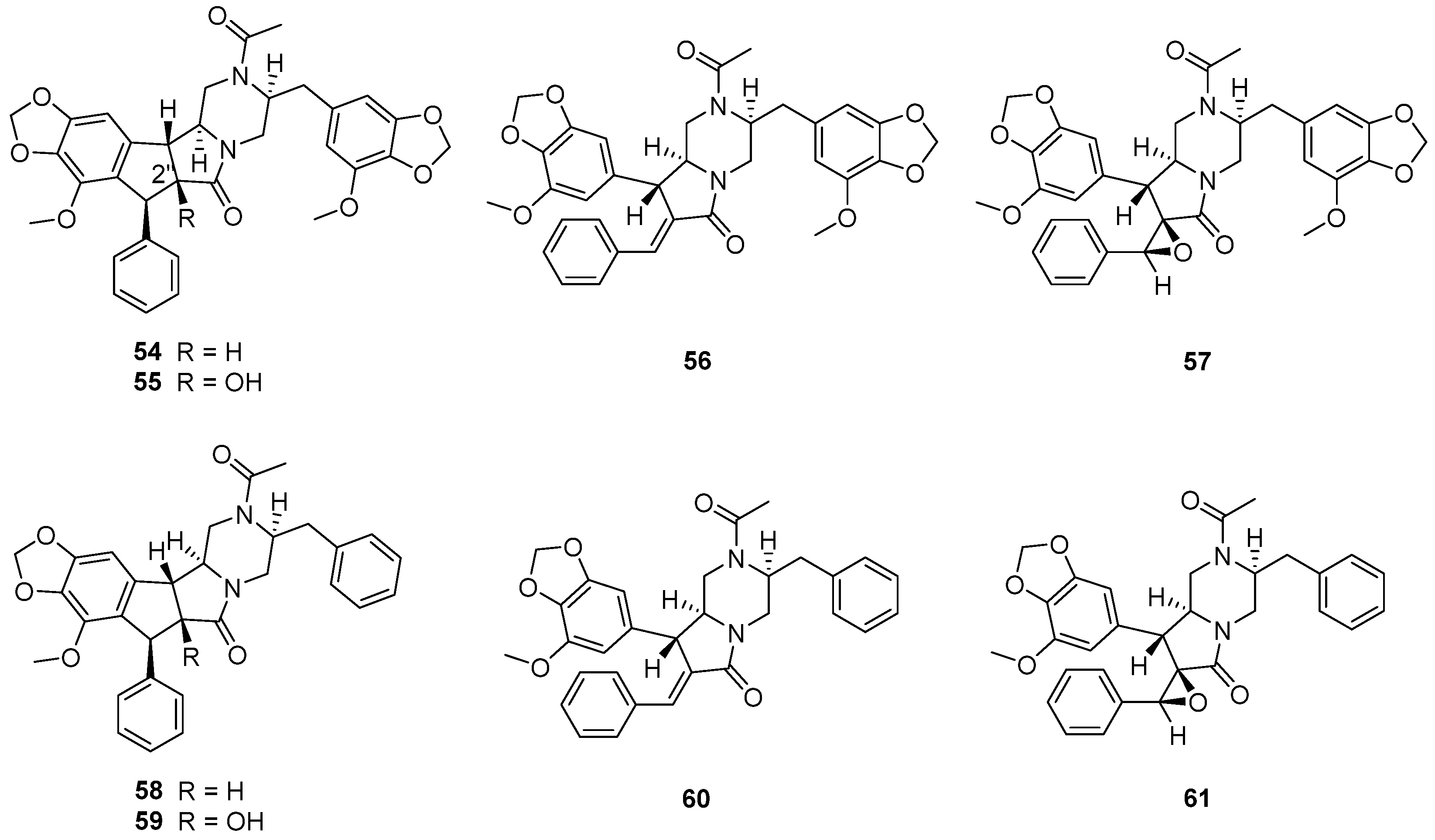
The fungus Chrysosporium sp. CMB-F294, derived from the Mugil mullet gastrointestinal-derived fungal isolate library, showed a potential ability to produce P-pg inhibitory compounds [55]. Chemical investigation of the fungus CMB-F294 led to the isolation of eight new alkaloids, chrysosporazines F–M (54–61) (Figure 11), belonging to phenylpropanoid piperazines. The cyclized analogues chrysosporazines F, G, J and K (54, 55, 58 and 59) showed significant inhibition towards the enzyme P-gp. Chrysosporazine F (54), with a 2.5 μM co-treatment, also induced an increase in doxorubicin sensitivity (GS = 14) (Table 3), which was over two times than that of positive control verapamil (GS = 6.0).
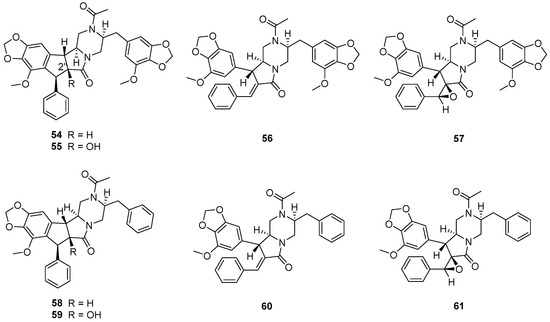
Figure 11.
Structure of alkaloids 54–61 [55].
Chrysosporazines and their derivatives (38–61), belonging to the rare class of phenylpropanoid piperazines, are the characteristic natural products of the genus Chrysosporium. Compounds 38–51 feature an unprecedented hexahydro-6H-pyrazino[1,2-b]isoquinolin-6-one scaffold, and 54–61 feature unprecedented carbocyclic and heterocyclic scaffolds.
Thirty-two compounds isolated from Chrysosporium sp. show enzyme inhibition. Most of the enzyme inhibitory compounds are alkaloids (34–61); others are lactones (8 and 11), a terpenoid (31) and a polyketide (32). Among them, compound 8 exhibits stronger enzyme inhibitory activity against AChE than that of the positive control galanthamine, meaning that 8 could be developed into AChE inhibitory drugs to treat Alzheimer’s disease. Moreover, compounds 43, 45, 46, 47 and 54 display significant enzyme inhibitory activity against P-gp and could reverse doxorubicin resistance in human colon cancer cells, which shows that they could be researched as assisted antitumor drugs to reduce the occurrence rate of drug resistance.
6. Other Biological Activities
A new biphenyl dicarboxylic acid, sporandol (62) (Figure 12), was isolated from C. meridarium under the guidance of antiparasitic activity [56]. This compound is much less toxic to mammals than other members of the binaphthyl group. Compound 62 is active against many parasites. At a dose of 190 mg/kg, it could inhibit the growth of the endoparasite (fluke) Fasciola hepatica and the ectoparasite Dipetalogaster maximus in a mouse [56]. When 620 mg/kg was administered daily for six consecutive days, the mouse did not suffer from serious poisoning. Therefore, Sporandol (62) has the potential to become an antiparasitic agent.
α,β-Dhydrohydrohcurvularin (7) (Figure 4) showed significant antioxidant properties. Its superoxide anion scavenging activity was much better than the positive controls (ascorbic acid, EC50 = 21.01 μg/mL, luteolin, EC50 = 31.01 μg/mL), with an EC50 value of 16.71 μg/mL. Moreover, compound 7 also showed DPPH scavenging activity, lipid peroxidation inhibition and erythrocyte hemolytic inhibition. The EC50 values were 50.75 μg/mL, 159.09 μg/mL and 141.79 μg/mL, respectively [28]. Among them, Curvularin (8) (Figure 4) was a potential nematicide against the root lesion nematode Pratylenchus penetrans [57]. Compounds 7 and 8 were found to have phytotoxicity. When the concentrations of 7 and 8 were as low as 0.1 and 0.33 mg/mL, respectively, it caused stem collapse and vascular necrosis in zinnia (Zinnia elegans Jacq. (Compositae)) seedlings within 24 h [45]. These two compounds led to the necrosis of cuttings of Canada thistle (Cirsium arvense (L.) Scop. (Compositae)) at a concentration of 0.33 mg/mL for 16 h. The activity of 7 and 8 toward oats (Avena byzantina cv. Coast Black) was low. Both of the compounds caused chlorosis at the lowest concentration tested (0.1 mg/mL) by 64 h of incubation [45]. α,β-Dhydrohydrohcurvularin (7) caused extensive necrosis of the leaves of various weeds at 688 μM, while corn and soybeans were insensitive to it. However, the investigation of the host range showed that the toxin was non-specific. Compound 7 significantly inhibited the mitosis of root tip cells, which supports the potential of 7 to be used as a natural biological herbicide [58].
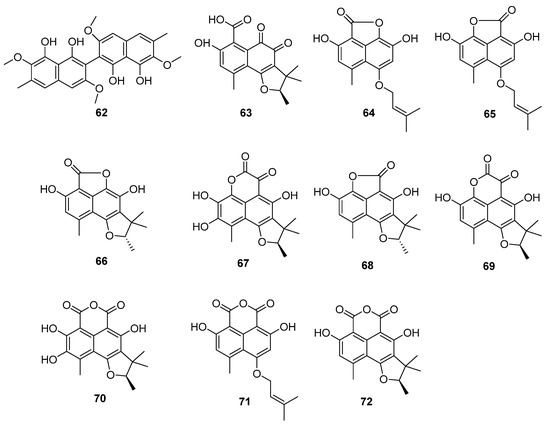
Figure 12.
Structure of polyketides 62–72 [56,59].
Figure 12.
Structure of polyketides 62–72 [56,59].
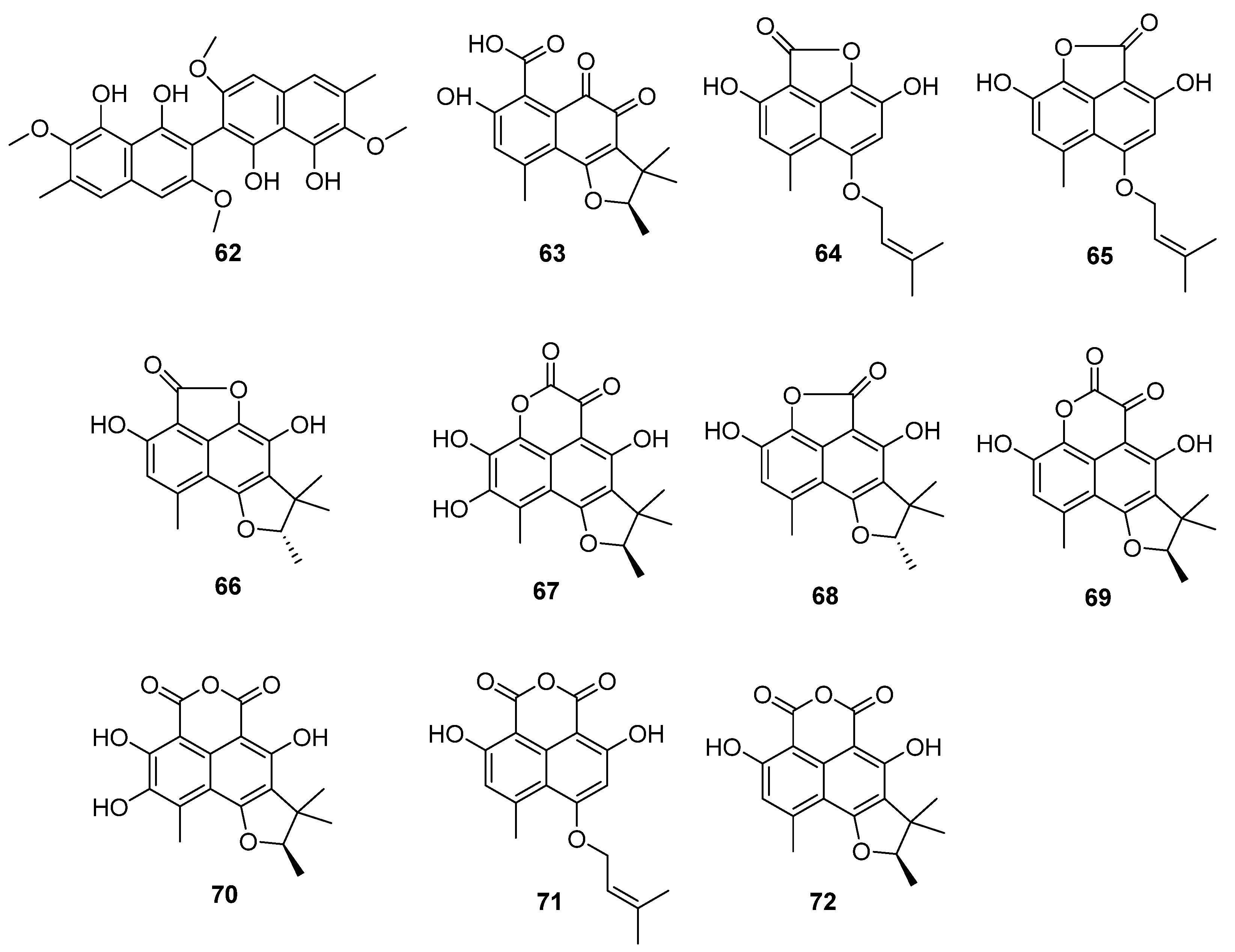
The fungus C. lobatum TM-237-S5 was isolated from the sponge Acanthella cavernosa, which was collected from the coral ecosystem of the Red Sea [59]. Ten compounds (63–72) (Figure 12) related to phenylacetones were isolated and identified: peniciphenalenin D (63), isoconiolactone (64), coniolactone (65), (−)-peniciphenalenin F (66), (+)-8-hydroxyscleroderolide (67), (−)-7,8-dihydro-3,6-dihydroxy-1,7,7,8-tetramethyl-5H-furo-[2′,3′:5,6] naphtho[1,8-bc]furan-5-one (68), (+)-scleroderolide (69), (+)-8-hydroxyslerodin (70), coniosclerodin (71) and (+)-sclerodin (72). Among them, 64, 66, 67 and 70 were new compounds.
7. Purification Techniques for the Compounds Isolated from Chrysosporium
The natural products isolated from the genus Chrysosporium were mainly purified through different chromatographic techniques, including silica gel column chromatography (CC) [22,28,30,42,56], medium-pressure liquid chromatography (MPLC) [22], Sephadex LH-20 CC [16,22,30,55], CM-Sephadex C-25 [14], Sepabeads SP20SS CC [15], MCI-gel CHP 20P CC [30], Diaion HP 20 CC [14,15,28], ODS CC [49], C18 reversed-phase vacuum flash chromatography [19,29], semi-preparative high-performance liquid chromatography (HPLC) [16,18,19,23,29,42,54,55,59] and preparative thin-layer chromatography (TLC) [30,42], combined with extraction, recrystallization [14,19,30,42], UPLC-DAD and UPLC-QTOF analyses [54], and bioactivity-guided isolation [30,42]. In the process of separation, most of the compounds could be disclosed on the TLC plates in UV254 light [28,30,42] and TLC-direct bioautography (DB) tests [22], conveniently.
The liquid culture medium of the genus Chrysosporium, concentrating most of the secondary metabolites, was first filtrated to discard the mycelium and obtain the supernatant fluid. The filtrate could be eluted through polymeric resin Diaion HP-20 [14,15,28] or MCI-gel CHP 20P CC [30] to remove the sugars and minerals and acquire the crude extract, or directly extracted by ethyl acetate (EA) to obtain the crude extract. Most of the fermentation broth was extracted by EA; however, the crude extracts of the fungi C. pilosum and Chrysosporium sp. were extracted with acetonitrile and then purified to obtain terpenoids 27–30 [23] and steroid 26 [18], respectively. Almost all of the compounds were in the organic phase, except polypeptide 3 [15]. The acetone–0.2 M HCl (1:1) eluate of the Diaion HP-20 column was partitioned between EA and water. The aqueous layer was extracted with chloroform, and the extract was subjected to Sepabeads SP20SS CC to obtain 3 [15]. Some of the fungi Chrysosporium were cultured in agar plates containing PDA medium [59], M1 agar media [54], M2 agar [54] or Yeast Extract Sucrose (YES) agar [55], and then extracted with EA, filtered and concentrated in a vacuum, to obtain the crude extract. The crude extract could be purified by different chromatographic techniques according to the different properties of the isolated compounds.
8. Conclusions
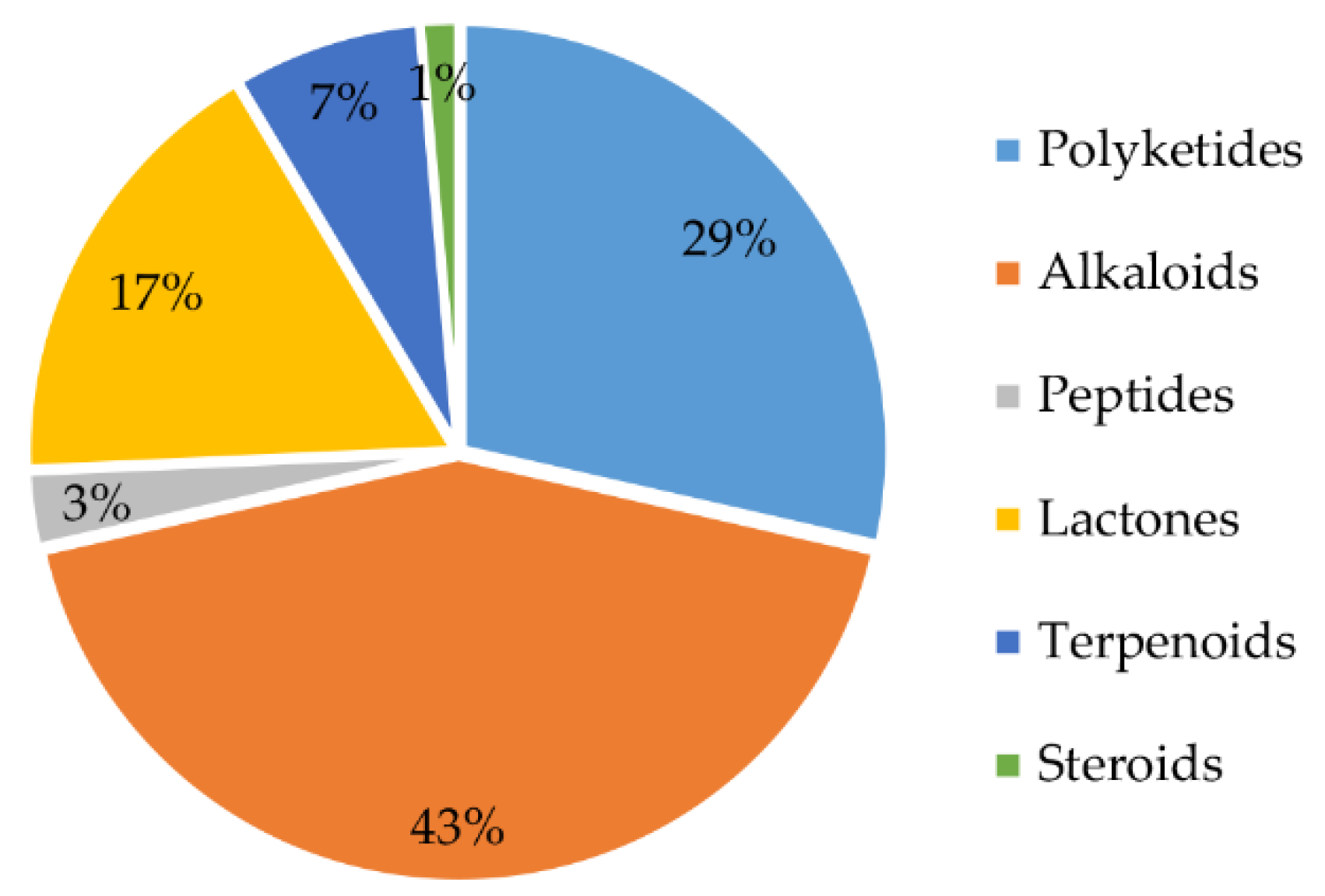
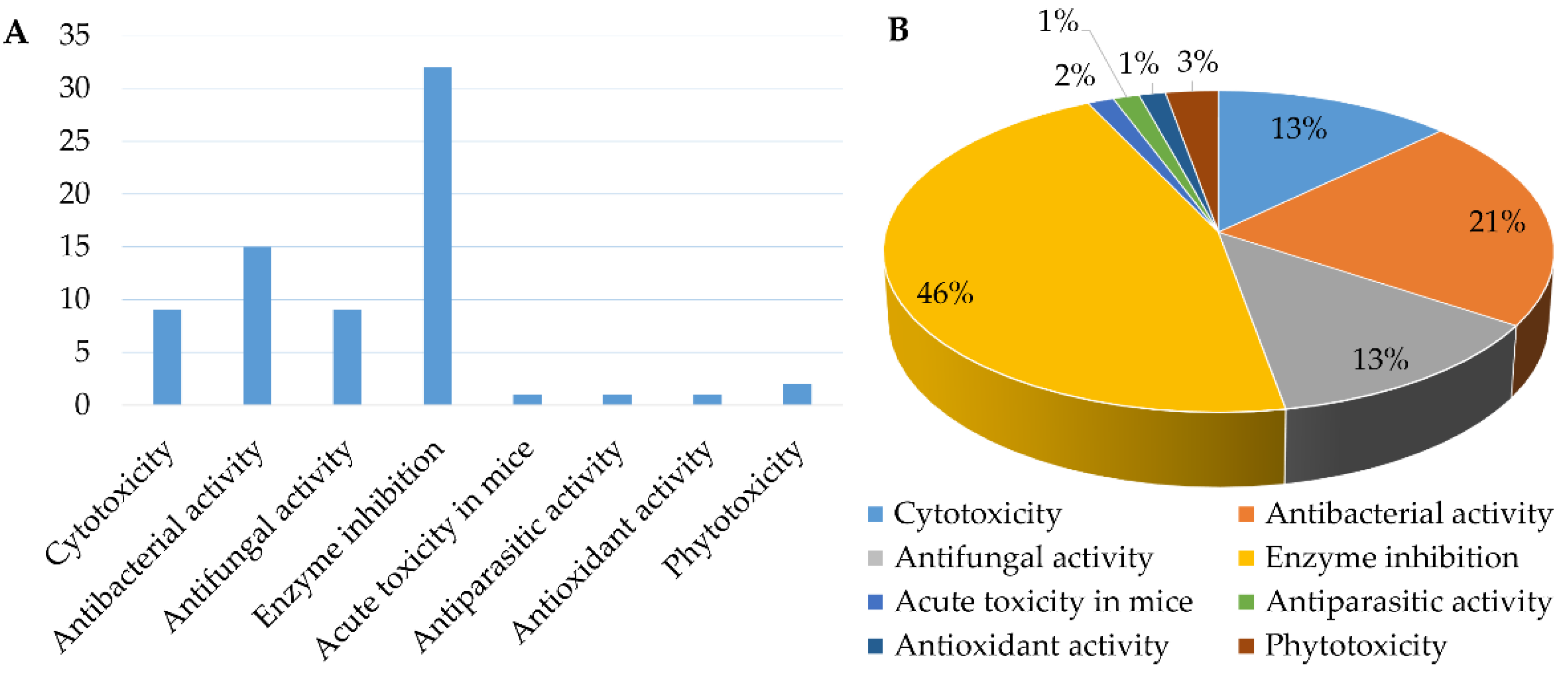
From 1984 to 2021, a total of 70 natural products were isolated from the genus Chrysosporium (Table 4), with 66% of these compounds being discovered for the first time. These findings demonstrate that the genus Chrysosporium has great potential to produce compounds with novel structures. The structures of the isolated compounds, with diverse skeletons, are mainly concentrated in the classes of alkaloids, polyketides and lactones (Figure 13). Forty-three percent of the compounds isolated from the genus Chrysosporium belong to the family of alkaloids (Figure 13); 29% are polyketides, and 17% are lactones. Terpenoids and peptides are both less than 10%. In addition, only 1% of the compounds belong to steroids (Figure 13).

Table 4.
Compounds isolated from Chrysosporium in 1984–2021.
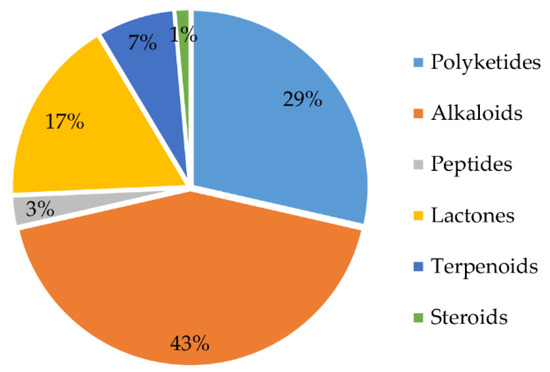
Figure 13.
Structural types of compounds isolated from Chrysosporium from 1984 to 2021.
Chrysosporazines, as well as their derivatives, belonging to the rare family of phenylpropanoid piperazine alkaloids, are the characteristic natural products of the genus Chrysosporium. Moreover, they are also the main secondary metabolites of the genus Chrysosporium, accounting for over one third of all the compounds isolated from Chrysosporium.
The sources of Chrysosporium are distributed in different ecosystems, including the Antarctic, forests and oceans. Half of the isolated natural products were separated from marine organism-derived Chrysosporium, including sponge, seaweed and mullet. The first marine-derived natural product was isolated from an unidentified sponge-derived Chrysosporium in 2013 [29]. Since then, more than 80% of the compounds have been isolated from the marine-derived genus Chrysosporium, indicating that marine fungi have great potential to produce new compounds.
The genus Chrysosporium has the potential to produce a variety of secondary metabolites with diverse bioactivities, including cytotoxicity, antimicrobial, enzyme inhibition, antiparasitic, antioxidant and phytotoxicity (Figure 14 and Figure 15). The activities of the Chrysosporium isolated compounds mainly focus on the categories of enzyme inhibition (46%), antimicrobial (34%) and cytotoxic (13%) activities (Figure 15). Around 80% of the compounds have biological activity, of which nine (3, 7, 8, 25, 43, 45, 46, 47, 54) showed stronger activities than those of the positive controls. Compound 3 shows intense cytotoxicity against transformed oncogene tumor cell lines, while it exhibits weak cytotoxicity to normal cell lines, indicating that 3 has the potential to be developed into a selective antitumor agent. Compound 7 shows strong antioxidant activity, which could be exploited for its anti-oxygen activity. Compound 8 exhibits strong enzyme inhibitory activity against AChE. Meanwhile, compounds 43, 45, 46, 47 and 54 display significant enzyme inhibitory activity against P-gp and could reverse doxorubicin resistance in human colon cancer cells, which shows that they could be developed into antitumor drugs to reduce the occurrence of drug resistance. Compound 25 exhibits significant antifungal activity and could be used as an antibiotic. Compounds 7 and 8 display a variety of different activities, including antimicrobial, antioxidant, cytotoxic, phytotoxic and enzyme inhibitory activities (Figure 14), which could be used in the medical, agricultural and industrial fields. All these results indicate that the genus Chrysosporium is a potential source of rich bioactive compounds.
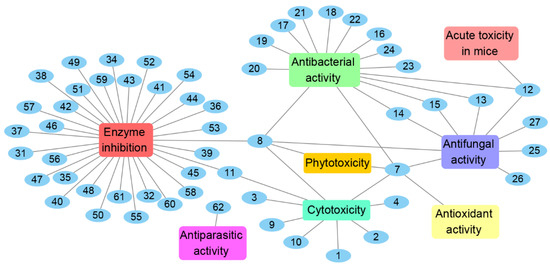
Figure 14.
Bioactivities of natural products isolated from Chrysosporium from 1984 to 2021.
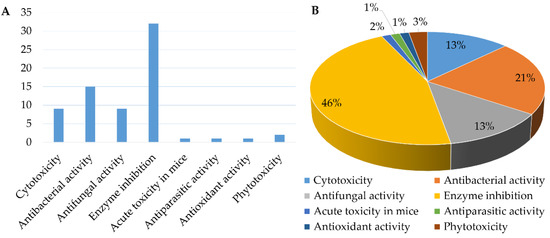
Figure 15.
The number (A) and percentage (B) of Chrysosporium isolated compounds with different bioactivities from 1984 to 2021.
In this review, we have summarized the chemical structure type, bioactivity, source and distribution of the secondary metabolites isolated from Chrysosporium from 1984 to 2021. The results of the literature survey show that Chrysosporium has great potential to produce a variety of new bioactive natural products. However, there are few studies about the bioactive mechanisms, which limits the practical application of the isolated compounds from Chrysosporium. The results demonstrate that the genus Chrysosporium is a promising germplasm resource for the discovery of novel compounds with pharmacological and biological activities, which have the potential to be developed into new drugs.
Author Contributions
Conceptualization, Y.W. and T.S.; Methodology, Y.W. and X.Y.; Software, Y.W. and T.S.; Validation, Y.W.; Formal Analysis, Y.W. and Y.L.; Investigation, Y.W. and X.Y.; Resources, Y.W. and Y.L.; Data Curation, Y.W.; Writing—Original Draft Preparation, Y.W.; Writing—Review and Editing, Y.W. and T.S.; Visualization, Y.W. and B.W.; Supervision, T.S. and B.W.; Project Administration, T.S.; Funding Acquisition, T.S. and B.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 82104029); the Shandong Provincial Natural Science Foundation (No. ZR2020QD111); the National Natural Science Foundation of China (No. 21868011); and the Talent Support Program of Shandong University of Science and Technology in 2019–2023.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sturm, J. Deutschlands Flora 3; Nabu Press: Nurnberg, Germany, 1833. [Google Scholar]
- Han, Y.-F.; Shen, X.; Liang, J.-D.; Liang, Z.-Q. Taxonomic advance and characteristics of the genus Chrysosporium. J. Mt. Agric. Biol. 2017, 36, 001–005. [Google Scholar]
- Saccardo, P.A. Sylloge Fungorum 15; Wentworth Press: Parisii, French, 1901. [Google Scholar]
- Hughes, S.J. Revisiones hyphomycetum aliquot cum appendice de nominibus rejiciendis. Can. J. Bot. 1958, 36, 727–836. [Google Scholar] [CrossRef]
- Van Oorschot, C.A. A revision of Chrysosporium and allied genera. Stud. Mycol. 1980, 73, 1013–1014. [Google Scholar]
- Da Silva, M.; Umbuzeiro, G.A.; Pfenning, L.H.; Canhos, V.P.; Esposito, E. Filamentous fungi isolated from estuarine sediments contaminated with industrial discharges. Soil Sediment Contam. 2003, 12, 345–356. [Google Scholar] [CrossRef]
- Deshmukh, S. Isolation of dermatophytes and other keratinophilic fungi from the vicinity of salt pan soils of Mumbai, India. Mycopathologia 2004, 157, 265–267. [Google Scholar] [CrossRef] [PubMed]
- Gock, M.A.; Hocking, A.D.; Pitt, J.I.; Poulos, P.G. Influence of temperature, water activity and pH on growth of some xerophilic fungi. Int. J. Food Microbiol. 2003, 81, 11–19. [Google Scholar] [CrossRef]
- Khizhnyak, S.; Tausheva, I.; Berezikova, A.; Nesterenko, E.; Rogozin, D.Y. Psychrophilic and psychrotolerant heterotrophic microorganisms of middle Siberian karst cavities. Russ. J. Ecol. 2003, 34, 231–235. [Google Scholar] [CrossRef]
- Kushwaha, R.K.S. The genus Chrysosporium, its physiology and biotechnological potential. Rev. Iberoam. Micol. 2000, 17, 66–76. [Google Scholar]
- Moore, R. Hot fungi from Chernobyl. Mycologist 2001, 2, 63–64. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Yamashita, M.; Kawai, Y.; Uchida, I.; Komori, T.; Kohsaka, M.; Imanaka, H.; Sakane, K.; Setoi, H.; Teraji, T. Chryscandin, a novel peptidyl nucleoside antibiotic II. Structure determination and synthesis. J. Antibiot. 1984, 37, 1284–1293. [Google Scholar] [CrossRef]
- Yamashita, M.; Tsurumi, Y.; Hosoda, J.; Komori, T.; Kohsaka, M.; Imanaka, H. Chryscandin, a novel peptidyl nucleoside antibiotic I. Taxonomy, fermentation, isolation and characterization. J. Antibiot. 1984, 37, 1279–1283. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, Y.; Adachi, H.; Kim, J.W.; Shin-ya, K.; Seto, H. Adenopeptin, a new apoptosis inducer in transformed cells from Chrysosporium sp. Tetrahedron 1998, 54, 15871–15878. [Google Scholar] [CrossRef]
- Kohno, J.; Hirano, N.; Sugawara, K.; Nishio, M.; Hashiyama, T.; Nakanishi, N.; Komatsubara, S. Structure of TMC-69, a new antitumor antibiotic from Chrysosporium sp. TC 1068. Tetrahedron 2001, 57, 1731–1735. [Google Scholar] [CrossRef]
- Kumar, C.G.; Mongolla, P.; Joseph, J.; Nageswar, Y.; Kamal, A. Antimicrobial activity from the extracts of fungal isolates of soil and dung samples from Kaziranga National Park, Assam, India. J. Mycol. Méd. 2010, 20, 283–289. [Google Scholar] [CrossRef]
- Yang, S.-W.; Buevich, A.; Chan, T.-M.; Terracciano, J.; Chen, G.; Loebenberg, D.; Patel, M.; Boehm, E.; Gullo, V.; Pramanik, B. A new antifungal sterol sulfate, sch 601324, from Chrysosporium sp. J. Antibiot. 2003, 56, 419–422. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fredenhagen, A.; Petersen, F.; Tintelnot-Blomley, M.; RÖSEL, J.; Mett, H.; Hug, P. Semicochliodinol A and B: Inhibitors of HIV-1 protease and EGF-R protein tyrosine kinase related to asterriquinones produced by the fungus Chrysosporium merdarium. J. Antibiot. 1997, 50, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y. Antibiotics produced by soil fungus C3368 from antarctica I. Taxonomy of strain C3368, fermentation and biological activity of antibiotics. Zhongguo Kangshengsu Zazhi 1992, 17, 401–403. [Google Scholar]
- Cheng, Y. Studies on antibiotics from Chrysosporium sp. strain C3368 one of the psychrophiles from antarctica II. Isolation and structure determination of antibiotics C3368. Zhongguo Kangshengsu Zazhi 1992, 17, 404–410. [Google Scholar]
- Correa, Y.; Cabanillas, B.; Jullian, V.; Álvarez, D.; Castillo, D.; Dufloer, C.; Bustamante, B.; Roncal, E.; Neyra, E.; Sheen, P. Identification and characterization of compounds from Chrysosporium multifidum, a fungus with moderate antimicrobial activity isolated from Hermetia illucens gut microbiota. PLoS ONE 2019, 14, e0218837. [Google Scholar] [CrossRef]
- Yang, S.-W.; Chan, T.-M.; Terracciano, J.; Boehm, E.; Patel, R.; Chen, G.; Loebenberg, D.; Patel, M.; Gullo, V.; Pramanik, B. Caryophyllenes from a fungal culture of Chrysosporium pilosum. J. Nat. Prod. 2009, 72, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Kimura, G.; Itagaki, A.; Summers, J. Rat cell line 3Y1 and its virogenic polyoma-and SV40-transformed derivatives. Int. J. Cancer 1975, 15, 694–706. [Google Scholar] [CrossRef] [PubMed]
- Shimura, H.; Mitsudomi, T.; Matsuzaki, A.; Kabemura, M.; Okuda, A.; Kimura, G. Transformation by vH-ras does not restore proliferation of a set of temperature-sensitive cell-cycle mutants of rat 3Y1 fibroblasts. Cell Struct. Funct. 1990, 15, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Zaitsu, H.; Tanaka, H.; Mitsudomi, T.; Matsuzaki, A.; Ohtsu, M.; Kimura, G. Differences in proliferation properties among sublines of rat 3Y1 fibroblasts transformed by various agents in vitro. Biomed. Res. 1988, 9, 181–197. [Google Scholar] [CrossRef]
- Hirano, N.; Kohno, J.; Tsunoda, S.; Nishio, M.; Kishi, N.; Okuda, T.; Kawano, K.; Komatsubara, S.; Nakanishi, N. TMC-69, a new antitumor antibiotic with Cdc25A inhibitory activity, produced by Chrysosporium sp. TCI068 taxonomy, fermentation and biological activities. J. Antibiot. 2001, 54, 421–427. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kumar, C.G.; Mongolla, P.; Sujitha, P.; Joseph, J.; Babu, K.S.; Suresh, G.; Ramakrishna, K.V.S.; Purushotham, U.; Sastry, G.N.; Kamal, A. Metabolite profiling and biological activities of bioactive compounds produced by Chrysosporium lobatum strain BK-3 isolated from Kaziranga National Park, Assam, India. SpringerPlus 2013, 2, 122. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.-e.; Julianti, E.; Oh, H.; Park, W.; Oh, D.-C.; Oh, K.-B.; Shin, J. Stereochemistry of hydroxy-bearing benzolactones: Isolation and structural determination of chrysoarticulins A–C from a marine-derived fungus Chrysosporium articulatum. Tetrahedron Lett. 2013, 54, 3111–3115. [Google Scholar] [CrossRef]
- Ivanova, V.B.; Hoshino, Y.; Yazawa, K.; Ando, A.; Mikami, Y.; Zaki, S.M.; Graefe, U. Isolation and structure elucidation of two new antibacterial compounds produced by Chrysosporium queenslandicum. J. Antibiot. 2002, 55, 914–918. [Google Scholar] [CrossRef]
- Wheeler, M.M. Anthraquinone pigments from the phytopathogen Phomopsis juniperovora Hahn. Phytochemistry 1975, 14, 288–289. [Google Scholar] [CrossRef]
- Becker, A.M.; Rickards, R.W.; Schmalzl, K.J.; Yick, H.C. Metabolites of Dactylaria lutea the structures of dactylariol and the antiprotozoal antibiotic dactylarin. J. Antibiot. 1978, 31, 324–329. [Google Scholar] [CrossRef]
- Yagi, A.; Okamura, N.; Haraguchi, H.; Abot, T.; Hashimoto, K. Antimicrobial tetrahydroanthraquinones from a strain of Alternaria solani. Phytochemistry 1993, 33, 87–91. [Google Scholar] [CrossRef]
- Haraguchi, H.; Abo, T.; Hashimoto, K.; Yagi, A. Action-mode of antimicrobial altersolanol A in Pseudomonas aeruginosa. Biosci. Biotechnol. Biochem. 1992, 56, 1221–1224. [Google Scholar] [CrossRef]
- Gerber, N.N.; Ammar, M.S. New antibiotic pigments related to fusarubin from Fusarium solani (Mart.) Sacc. II. Structure elucidations. J. Antibiot. 1979, 32, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Kurobane, I.; Vining, L.C.; Mcinnes, A.G.; Gerber, N.N. Metabolites of Fusarium solani related to dihydrofusarubin. J. Antibiot. 1980, 33, 1376–1379. [Google Scholar] [CrossRef]
- Barbier, M.; Devys, M.; Parisot, D. A new dihydrofusarubin O-ethyl ether produced by the fungus Nectria haematococca (Berk, and Br.) Wr. Can. J.Chem. 1988, 66, 2803–2804. [Google Scholar] [CrossRef]
- Caputo, O.; Viola, F. Isolation of α,β-dehydrocurvularin from Aspergillus aureofulgens. Planta Med. 1977, 31, 31–32. [Google Scholar] [CrossRef]
- Singh, S.; Verma, S.; Yadav, D.K.; Kumar, A.; Tyagi, R.; Gupta, P.; Bawankule, D.U.; Darokar, M.P.; Srivastava, S.K.; Kalra, A. The bioactive potential of culturable fungal endophytes isolated from the leaf of Catharanthus roseus (L.) G. Don. Curr. Top. Med. Chem. 2021, 21, 895–907. [Google Scholar] [CrossRef]
- Tandon, R.; Gupta, R.; Shukla, A.; Arora, D. Antagonistic interactions among seedborne microflora and dynamics of microbial incidence. Arch. Microbiol. 1979, 120, 77–80. [Google Scholar] [CrossRef]
- Allimuthu, V. Implication of Fungalgrowth in Poultry Management and Biogas Production. 2015. Available online: https://shodhganga.inflibnet.ac.in:8443/jspui/handle/10603/151954 (accessed on 17 April 2015).
- Hoshino, Y.; Ivanova, V.B.; Yazawa, K.; Ando, A.; Mikami, Y.; Zaki, S.M.; Karam, A.-Z.A.; Youssef, Y.A.; Graefe, U. Queenslandon, a new antifungal compound produced by Chrysosporium queenslandicum: Production, isolation and structure elucidation. J. Antibiot. 2002, 55, 516–519. [Google Scholar] [CrossRef]
- Isono, K. Nucleoside antibiotics: Structure, biological activity, and biosynthesis. J. Antibiot. 1988, 41, 1711–1739. [Google Scholar] [CrossRef]
- Plasencia, J.; Mirocha, C.J. Isolation and characterization of zearalenone sulfate produced by Fusarium spp. Appl. Environ. Microb. 1991, 57, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Robeson, D.J.; Strobel, G.A. αβ-Dehydrocurvularin and curvularin from Alternaria cinerariae. Z Naturforsch. C 1981, 36, 1081–1083. [Google Scholar] [CrossRef]
- Kato, K.; Cox, A.D.; Hisaka, M.M.; Graham, S.M.; Buss, J.E.; Der, C.J. Isoprenoid addition to Ras protein is the critical modification for its membrane association and transforming activity. Proc. Natl. Acad. Sci. USA 1992, 89, 6403–6407. [Google Scholar] [CrossRef]
- Van Der Pyl, D.; Cans, P.; Debernard, J.J.; Herman, F.; Lelievre, Y.; Tahraoui, L.; Vuilhorgne, M.; Leboul, J. RPR113228, a novel farnesyl-protein transferase inhibitor produced by Chrysosporium lobatum. J. Antibiot. 1995, 48, 736–737. [Google Scholar]
- Wachi, Y.; Yamashita, T.; Komatsu, K.; Yoshida, S. JP Patent JKXXAF. JP 07061950 A2, 7 March 1995. [Google Scholar]
- Iijima, D.; Tanaka, D.; Hamada, M.; Ogamino, T.; Ishikawa, Y.; Nishiyama, S. The first total synthesis of SB87-Cl and pestalone, novel bioactive benzophenone natural products. Tetrahedron Lett. 2004, 45, 5469–5471. [Google Scholar] [CrossRef]
- Kobayashi, J.i.; Madono, T.; Shigemori, H. Nakijiquinones C and D, new sesquiterpenoid quinones with a hydroxy amino acid residue from a marine sponge inhibiting c-erbB-2 kinase. Tetrahedron 1995, 51, 10867–10874. [Google Scholar] [CrossRef]
- Meyer, T.; Regenass, U.; Fabbro, D.; Alteri, E.; Röusel, J.; Möller, M.; Caravatti, G.; Matter, A. A derivative of staurosporine (CGP 41 251) shows selectivity for protein kinase C inhibition and in vitro anti-proliferative as well as in vivo anti-tumor activity. Int. J. Cancer 1989, 43, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Trinks, U.; Buchdunger, E.; Furet, P.; Kump, W.; Mett, H.; Meyer, T.; Mueller, M.; Regenass, U.; Rihs, G. Dianilinophthalimides: Potent and selective, ATP-competitive inhibitors of the EGF-receptor protein tyrosine kinase. J. Med. Chem. 1994, 37, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
- Elbanna, A.H.; Khalil, Z.G.; Bernhardt, P.V.; Capon, R.J. Chrysosporazines A–E: P-Glycoprotein inhibitory piperazines from an Australian marine fish gastrointestinal tract-derived fungus, Chrysosporium sp. CMB-F214. Org. Lett. 2019, 21, 8097–8100. [Google Scholar] [CrossRef]
- Elbanna, A.H.; Agampodi Dewa, A.; Khalil, Z.G.; Capon, R.J. Precursor-directed biosynthesis mediated amplification of minor aza phenylpropanoid piperazines in an Australian marine fish-gut-derived fungus, Chrysosporium sp. CMB-F214. Mar. Drugs 2021, 19, 478. [Google Scholar] [CrossRef]
- Mohamed, O.G.; Salim, A.A.; Khalil, Z.G.; Elbanna, A.H.; Bernhardt, P.V.; Capon, R.J. Chrysosporazines F–M: P-Glycoprotein inhibitory phenylpropanoid piperazines from an Australian marine fish derived fungus, Chrysosporium sp. CMB-F294. J. Nat. Prod. 2020, 83, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Tsipouras, A.; Goetz, M.A.; Hensens, O.D.; Liesch, J.M.; Ostlind, D.A.; Williamson, J.M.; Dombrowski, A.W.; Ball, R.G.; Singh, S.B. Sporandol: A novel antiparasitic binaphthalene from Chrysosporium meridarium. Bioorg. Med. Chem. Lett. 1997, 7, 1279–1282. [Google Scholar] [CrossRef]
- Kusano, M.; Nakagami, K.; Fujioka, S.; Kawano, T.; Shimada, A.; Kimura, Y. βγ-Dehydrocurvularin and related compounds as nematicides of pratylenchus penetrans from the fungus Aspergillus sp. Biosci. Biotech. Bioch. 2003, 67, 1413–1416. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.J.; Qiang, S.; Zhu, Y.Z.; Dong, Y.F. Isolation and phytotoxicity of a metabolite from Curvularia eragrostidis and characterisation of its modes of action. Ann. Appl. Biol. 2008, 152, 103–111. [Google Scholar] [CrossRef]
- Le Goff, G.; Lopes, P.; Arcile, G.; Vlachou, P.; Van Elslande, E.; Retailleau, P.; Gallard, J.-F.; Weis, M.; Benayahu, Y.; Fokialakis, N. Impact of the cultivation technique on the production of secondary metabolites by Chrysosporium lobatum TM-237-S5, isolated from the sponge Acanthella cavernosa. Mar. Drugs 2019, 17, 678. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).