Abstract
Biodiesel is seen as a successor to diesel of petrochemical origin, as it can be used in cycle and stationary engines and be obtained from renewable raw materials. Currently, the biodiesel production process on an industrial scale is mostly carried out through the transesterification reaction, also forming glycerol as a product. Pure glycerol is used in the pharmaceutical, cosmetic, cleaning, food, and other industries. Even presenting numerous applications, studies indicate that there is a saturation of glycerol in the market, which is directly related to the production of biodiesel. This increase causes a commercial devaluation of pure glycerol, making separation and purification processes unfeasible from an economic point of view. Despite the economic unfeasibility of the aforementioned processes, they continue to be carried out due to environmental issues. Faced with the problem presented, this work provides a bibliographical review of works that aimed to use glycerol as a raw material for the production of biofuels, with these processes being carried out mostly via fermentation.
1. Introduction
Currently, biodiesel stands out as a successor to diesel of petrochemical origin since there is worldwide concern about the depletion of fossil fuels as well as the emission of gases produced from their burning, gases that contribute to the aggravation of the greenhouse effect [1,2]. Biodiesel, like diesel, can be used in cycle and stationary engines [3]. The use of biodiesel has some advantages, such as being obtained from renewable sources and a lower rate of emission of gases that contribute to the worsening of the greenhouse effect [4,5].
Biodiesel on an industrial scale is mostly produced through the methyl transesterification reaction using basic homogeneous catalysts, such as sodium and potassium hydroxides [6,7,8], and can be obtained from different raw materials, e.g., vegetable oils and animal fats [9,10].
During the biodiesel production process via transesterification, there is also the formation of glycerol [4,11]. It is estimated that for every 10 kg of biodiesel produced, approximately 1 kg of crude glycerol is formed [12,13]. For the commercialization of glycerol produced from the biodiesel production process, crude glycerol is subjected to separation and purification processes, thus obtaining pure glycerol [14].
Pure glycerol is used in the food industry as a stabilizer, antioxidant, humectant, and emulsifier; in the pharmaceutical industry in cosmetics and medicines; and in the chemical industry in the composition of resins and polymers, among other uses [15,16]. Despite being used in several industrial sectors, the market is saturated with this product, as the demand for glycerol is less than the quantity produced, causing the commercial devaluation of glycerol [17]. According to Zhang et al. (2016), the energy expenditure during the separation and purification processes is unfeasible due to the low economic value attributed to purified glycerol, which is associated with the growing amount of glycerol in the market due to the production of biodiesel [18].
It is important to point out that although the separation and purification processes are impracticable, they are necessary since crude glycerol without treatment cannot be discarded in the environment and its use as fuel is unfeasible because its direct burning produces toxic gases [14].
As already mentioned, biodiesel is a potential successor to diesel, which is a reality in many countries. The biodiesel production process via the transesterification reaction is the most used, and during this process there is the production of glycerol, currently considered a by-product [19,20]. The considerable increase in the amount of glycerol in the market, according to studies, is directly associated with the production of biodiesel [21,22]. This increase in the amount of glycerol in the market has caused a devaluation of its commercial value, making separation and purification processes energetically and economically unfeasible [18]. Therefore, proposing the use of glycerol as a renewable raw material for the production of other biofuels is interesting from an economic, energy, and technological development point of view. It is an attempt to value this by-product that has saturated the market. In this way, considering the current scenario, including seeking the development of new forms of biofuel and the whole problem presented in relation to the biodiesel production process and glycerol, this review aims to present works that had the production of other biofuels as their objective, including methane, hydrogen, and ethanol, via fermentation processes using glycerol from biodiesel production as the raw material. It is important to highlight that this work aimed to carry out a review with a focus on fermentation because it is a more economically viable route, since it makes it possible to use crude glycerol as a source of raw material. We did not find a review in the consulted literature that performed this task, and it is thus our main contribution.
2. Glycerol
The official name of glycerol, according to IUPAC (International Union of Pure and Applied Chemistry), is propane-1,2,3-triol. The molecular formula of glycerol is C3H5(OH)3. Glycerol is a colorless liquid, has an oily appearance, is viscous, has a relatively high density, is soluble in water and alcohols, and is practically insoluble in hydrocarbons [23,24]. Table 1 presents the physicochemical properties of glycerol.

Table 1.
Physicochemical properties of glycerol.
As shown in Table 1, the decomposition point of glycerol is 290 °C. According to Gupta et al. (2012), it is at this temperature that the decomposition of glycerol into acrolein, a toxic and carcinogenic compound, occurs [14].
Glycerol can be obtained from sources considered renewable, including vegetable oils and animal fats, and also from sources considered non-renewable, such as petroleum. Glycerol can be obtained from fermentation processes, chemical processes such as the hydrogenation of carbohydrates of petrochemical origin, the saponification process, and the biodiesel production process. The physicochemical properties of glycerol may vary depending on the raw material used. The work carried out by Quispe et al. (2013) showed that when using different vegetable oils as the raw materials, the formed glycerol had different viscosities [25].
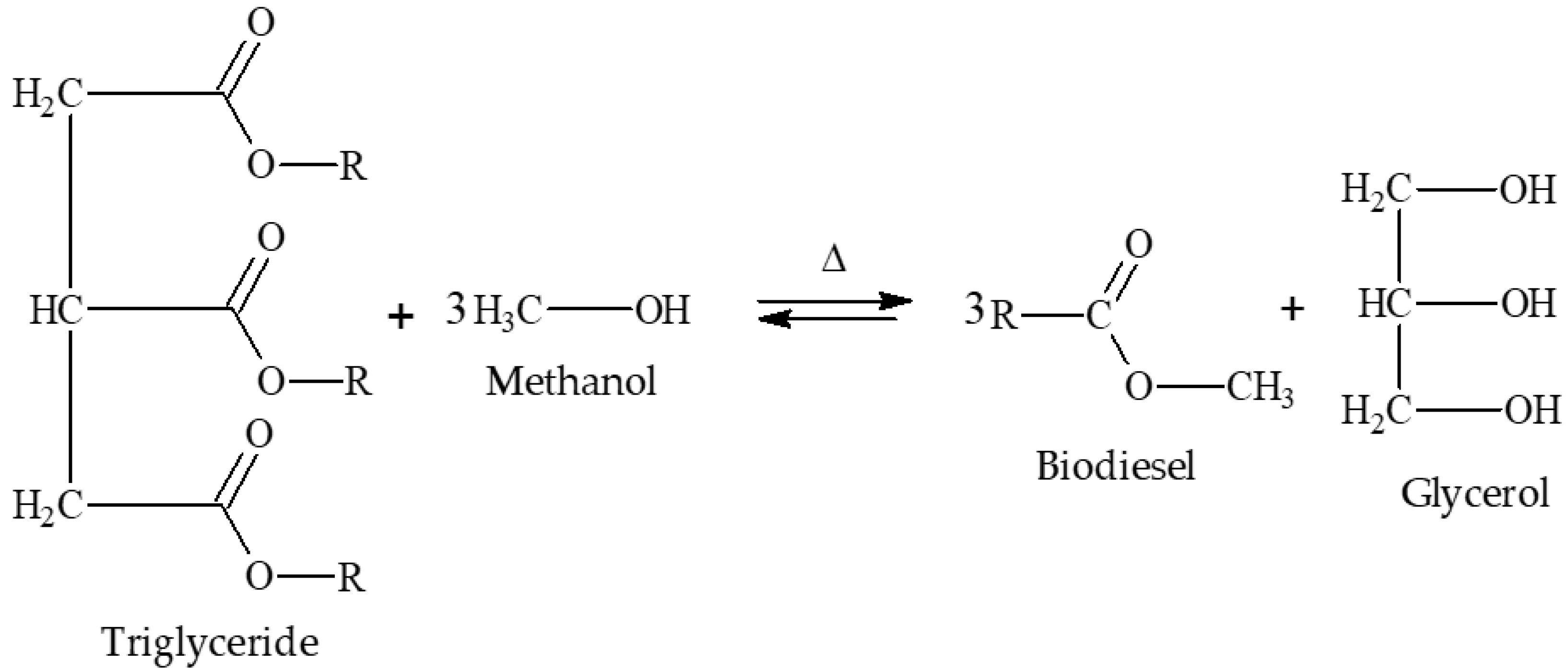
Glycerol from the biodiesel production process is mostly obtained through the transesterification reaction [26,27]. The transesterification reaction is represented in Figure 1.
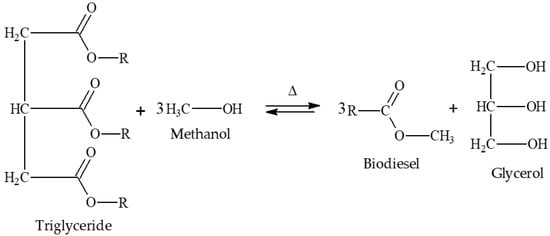
Figure 1.
Representation of the transesterification reaction adapted from the work by Almeida et al. (2018) [20].
The transesterification reaction is a reversible reaction, so it is necessary to add one of the reagents in excess; in this case, it is common to add alcohol in excess [28,29]. The alcohols frequently used are methanol and ethanol, with methanol being the most used since the use of ethanol in the production process requires a greater excess of product, presenting a greater propensity to form soaps and forming an emulsion that makes the processes of separation and purification difficult, in addition to the relatively higher commercial price when compared to methanol [30,31]. The catalysts frequently used on an industrial scale are homogeneous alkaline catalysts, especially sodium and potassium hydroxides [32]. The use of these catalysts has some advantages, such as lower economic cost, shorter reaction time, higher conversion rates, etc. However, these same catalysts have disadvantages, such as the need for a raw material with a low fatty acid content, since these catalysts in the presence of fatty acids and alcohol favor the formation of soaps [6,8,33,34,35,36]. It is important to highlight that there are works available in the literature that proposed the use of other catalysts, such as heterogeneous acid catalysts [20,37]. According to Alcañiz-Monge et al. (2013), heterogeneous acid catalysts have the advantage of obtaining biodiesel both through the esterification of fatty acids and transesterification of triglycerides, forming purer products and allowing possible reuse in a new process [38,39]. However, when compared to conventional catalysis, both heterogeneous and homogeneous acid catalysis have some disadvantages, such as requiring longer reaction time and higher operating costs [40,41].
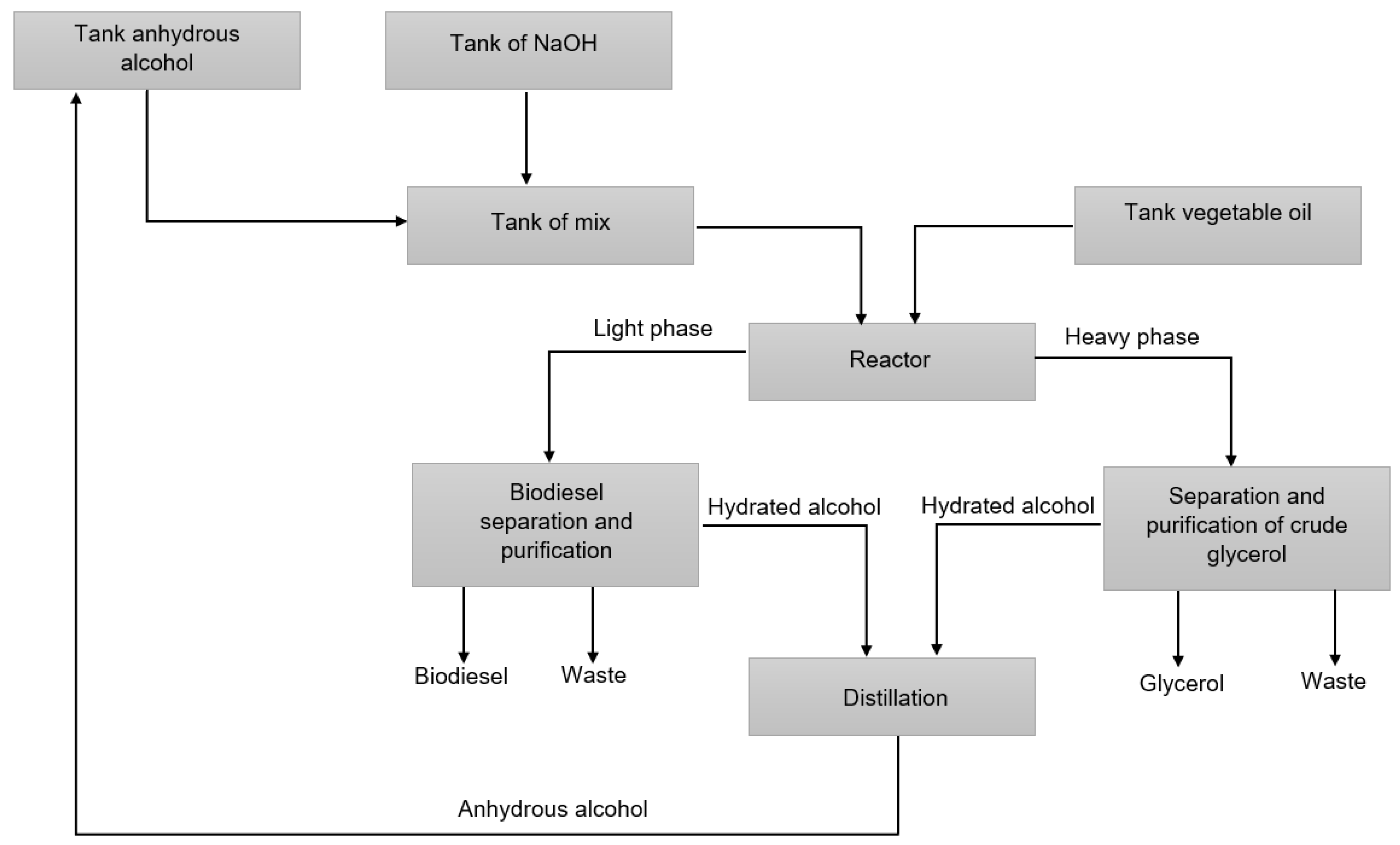
After the transesterification reaction, the reaction product goes through the separation and purification processes, where the catalyst is neutralized, the alcohol added in excess is recovered, and the purified products are obtained for commercialization [42,43,44]. Figure 2 presents a diagram of the biodiesel and glycerol production process.
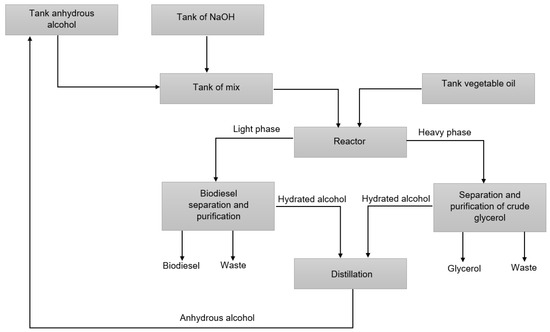
Figure 2.
Diagram of the biodiesel and glycerol production process adapted from Almeida et al. (2017) [45].
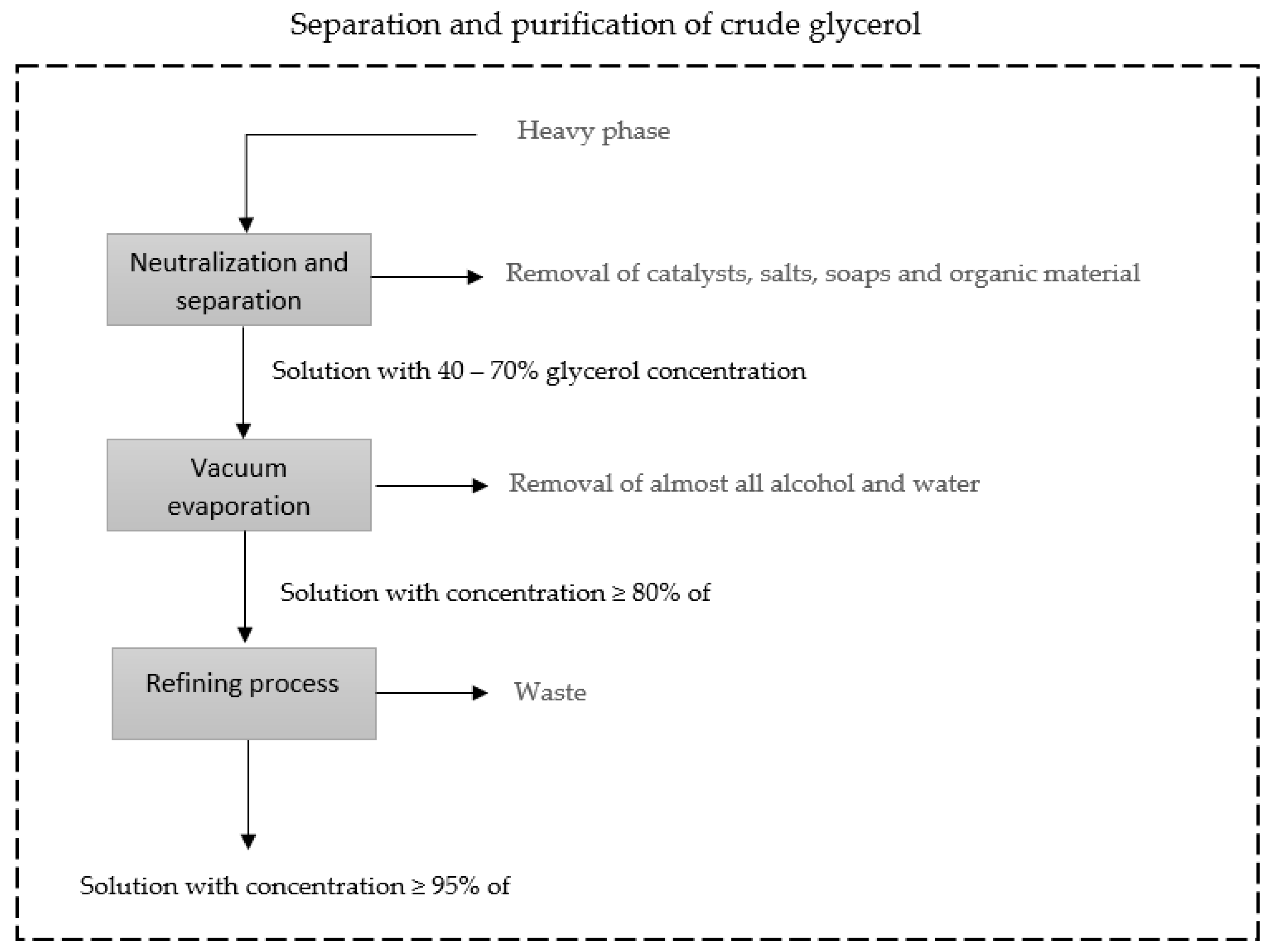
The purification process begins with the separation of the light phase, mostly made up of biodiesel, from the heavy phase, mostly made up of glycerol. The heavy phase, as mentioned, comprises mostly glycerol, alcohol, water, catalyst, impurities, soaps, and non-glycerol organic matter. The glycerol purification process begins with the process of neutralizing the catalyst present in the solution, followed by the process of separating the salts formed in the neutralization process, catalyst residues, soaps, and organic material. The resulting solution has a concentration of 40–70% glycerol, which undergoes a vacuum evaporation process in which almost all water and alcohol is removed, and the resulting solution has a concentration greater than or equal to 80% glycerol. The solution, in turn, undergoes a refining process until it reaches the purity level required for commercialization [46,47]. Vacuum distillation is the process often used to obtain purified glycerol [24,46]. Figure 3 presents a simplified diagram of the glycerol separation and purification processes.
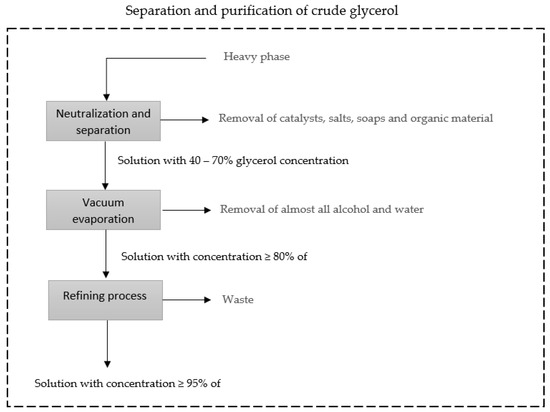
Figure 3.
Simplified diagram of the separation and purification processes of glycerol adapted from Luo et al. (2016) [46].
Biodiesel and glycerol production is a complex process that demands a high energy expenditure from the preparation of the raw material to obtaining the final products. This reinforces the need to make glycerol an energetically and economically attractive product, consequently increasing the viability of the biodiesel production process on an industrial scale [25].
3. Biofuel Production Processes Using Glycerol as Biomass
The use of glycerol as a carbon source for the production of biofuels has been gaining prominence due to its wide availability [18,48]. Another reason that corroborates the development of research in this area is the physicochemical characteristics of glycerol, such as high autoignition temperature and low heating value, causing glycerol to decompose into acrolein at 290 °C [49]. The following topics present works that aimed to use glycerol as a renewable raw material for the production of fuels.
3.1. Biogas
Recent studies present glycerol as a promising source of organic matter for the production of biogas [50,51,52,53]. In the biogas production process, glycerol can be used as a substrate or co-substrate [54]. Different bacteria are capable of metabolizing glycerol, including those of the genera Klebsiella, Clostridium, and Enterobacter [15,55,56,57].
The work carried out by Astals et al. (2012) aimed to produce biogas from the anaerobic digestion of swine effluents using crude glycerol as a co-substrate. They added 4% w/w, on a wet basis, of crude glycerol to the bioreactor and obtained a 400% increase in biogas production with respect to monodigestion. The authors concluded that the results were satisfactory, and the increase in biogas production may have been due to the increase in organic load, the carbon–nitrogen balance, and the decrease in ammonia concentration [58].
The work carried out by Siles et al. (2010) aimed to use glycerol in the co-digestion of wastewater formed during the biodiesel production process, with the purpose of treating the effluent as well as the production of methane. Some of the main factors evaluated by the authors were: biodegradability of the mixture formed by wastewater and glycerol, methane yield coefficient, and methane production kinetics. The inoculum used was the active methanogenic granular biomass used to treat wastewater from breweries. According to the authors, this biomass was selected due to its high methanogenic activity. Before forming the mixture, which was subsequently treated, the authors carried out the acidification and centrifugation of the glycerol, with the purpose of removing the catalysts used in the transesterification reaction and electrocoagulation process in the wastewater. The authors concluded that the results presented were satisfactory, as the mixture showed a high level of biodegradability, the kinetics of methane production remained constant, and the production of 310 mL of CH4/g was removed at 1 atm and 25 °C [59].
The work carried out by Beschkov et al. (2012) aimed to develop a mathematical model to describe the production of biogas and other products from the anaerobic digestion of crude glycerol using Klebsiella sp. bacteria. Experiments were carried out in a multistage bioreactor, and the results obtained were used to develop the proposed model. The choice of a multistage reactor was directly related to the intermediates formed during the glycerol digestion process, as these intermediates were acidic in character, lowering the pH of the medium and inhibiting the methanogenesis reaction. Thus, the chosen reactor separated the inhibition zone from the methanogenesis zone. The authors concluded that, although simple, the proposed model allowed estimating the number of compartments necessary for the total conversion of glycerol into biogas [60].
The work carried out by Fountoulakis et al. (2009) aimed to verify the influence of adding glycerol in continuous bioreactors that treated small fractions of urban organic waste and wastewater. The authors observed that the methane production rate increased with the addition of crude glycerol. The reactor was inoculated with anaerobic sludge from the municipal sewage treatment plant in the city of Iraklio, Greece. The authors observed the production of 1400 mL CH4/d without adding glycerol and 2094 mL CH4/d after adding glycerol. They concluded that the results were satisfactory [54].
The work carried out by Oliveira (2015) aimed to evaluate the optimal conditions for methane production using macroalgae Sargassum sp. co-digested with glycerol and residual frying oil. The authors noted that without the addition of glycerol and residual oil, the biochemical potential of Sargassum sp. was 181 ± 1 L CH4/L of COD and the methane rate increased 56% with the addition of glycerol and 46% with the addition of residual oil. The authors concluded that the addition of glycerol or residual oil was a promising alternative for methane production [61].
The work carried out by Sittijunda and Reungsang (2018) aimed to verify the methane production from the co-digestion of algae biomass with crude glycerol. The authors showed that under optimal conditions the maximum methane production was 58.88 mL CH4/L. The bacteria detected that were responsible for the digestion of the biomass were Methanosarcina sp., Methanoregula sp., Methanospirillum sp., and Methanoculleus sp. [62].
The work carried out by Baba et al. (2013) aimed to carry out an energy balance in a process plant in which crude glycerol was digested. The results presented, according to the authors, were satisfactory in terms of methane production. According to the analyses carried out, the digested sludge contained fertilizer components (N: 0.11%, P2O5: 0.036%, K2O: 0.19%). Thus, the authors concluded that crude glycerol was an attractive biomass for methane production and the residual sludge could be used as liquid fertilizer [50].
The work carried out by Chou and Su (2019) aimed to evaluate the feasibility of biogas production by anaerobic co-digestion of wastewater from dairy cattle and crude glycerol. The authors concluded that the conversion of residual crude glycerol into biogas showed satisfactory results and could be applied in the near future on an industrial scale to treat waste from slaughterhouses [63].
The work carried out by Sawasdee et al. (2019) aimed to evaluate the production of biogas from the co-digestion of glucose and glycerol in laboratory-scale batch reactors. The experiments involved varying glycerol/glucose ratios with fixed initial chemical oxygen demand for all conditions. The inoculum was obtained from cassava starch sludge. The highest yield of biogas production was obtained using the 5:5 ratio of glycerol/glucose, with a maximum production rate of 8 mL/h [64].
The work carried out by Alves et al. (2020) aimed to evaluate the effect of adding crude glycerol on the anaerobic digestion of primary sewage sludge. Crude glycerol was added in two fractions, 1% and 3% v/v. The methane yields were 223.8 mL CH4/g VS for 1% concentration and 368.8 mL CH4/g VS for 3% concentration, which represented increases in methane yield of 61% and 167% compared to the control test performed only with primary sludge digestion (138.2 mL CH4/g VS). For each percentage increase in applied SV load, the percentage increases in methane yield were 4.7% and 5.7% with 1% and 3% glycerol, respectively [65].
The work carried out by Prasertsan et al. (2021) aimed to evaluate the production of biogas using fluent raw material from a palm oil factory and evaluate which co-substrate had the highest yield, crude glycerol or ethanol. Concentrations of both substrates ranged from 1% to 5% v/v. The optimal concentrations of crude glycerol and ethanol were 1% and 5% v/v, respectively. The results presented by the authors showed that the removal of volatile solids using crude glycerol as a co-substrate presented better performance when compared to ethanol. However, regarding the biogas production rate, the results were the opposite, with a production rate of 553.46 mLCH4/g VS for glycerol and 582.12 mL CH4/g VS for ethanol. The dominant microorganisms were Methanosarcina sp. and Methanospirillum sp. [66].
The work carried out by Takeda et al. (2022) aimed to produce biogas using landfill leachate and crude glycerol as the raw materials. The authors analyzed the following parameters: removal of organic matter, time, glycerol content, and substrate/inoculum ratio. From the optimization of the mentioned parameters it was possible to maximize the efficiency of organic matter removal (90.15%) and specific production of biogas (403.15 mL/g SSV) under the conditions of 33.2 days, glycerol content of 1.71%, and substrate/inoculum ratio of 0.37 g COD/g VSS. The authors concluded that the average specific production of biogas was 20.3 times higher than that obtained by monodigestion of landfill leachate [67].
The work carried out by Bułkowska et al. (2022) aimed to evaluate the effect of glycerol on the anaerobic digestion of bovine manure under mesophilic conditions. The process was carried out in stirred tank semi-continuous reactor at a constant organic rate and hydraulic retention time of 30 days. It was found that the addition of glycerol to cattle manure produced 3.1 times more biogas than that by cattle manure alone (237.5 L vs. 76.4 L) [68].
The work carried out by Alves et al. (2022) evaluated the co-digestion of primary sewage treatment sludge, food waste, and crude glycerol. Crude glycerol concentrations were 1% and 3% v/v. The results presented by the authors were biogas yields of 432.4 and 692.6 mL/g VS for 1% and 3% crude glycerol, respectively, while the methane yields corresponded to 343.3 and 525.7 mL CH4/g VS for 1% and 3% crude glycerol, respectively. The latter represented increases of 45.4% and 122.7% in relation to those achieved with primary sludge and food residues in mixtures [69].
Table 2 summarizes the production of biogas using glycerol as the raw material.

Table 2.
Production of biogas using glycerol as the raw material.
3.2. Hydrogen
Currently, hydrogen is seen as a form of clean energy, since the only product of the combustion process is water [70]. Conventional forms of hydrogen production are catalytic reforming in petroleum processing and steam reforming of methane present in natural gas; however, these methods cannot be considered renewable since both use raw materials of fossil origin [71,72]. Another conventional method used for the production of hydrogen is the electrolysis of water; however, this method demands a high energy cost [70]. Due to the problems involved in conventional methods, the use of glycerol as biomass for the production of hydrogen is an interesting alternative method since it causes less environmental impact and requires less energy expenditure [73,74,75]. The process of producing hydrogen through the fermentation process is mainly carried out by anaerobic digestion, called dark fermentation [76,77]. According to Maru (2016), dark fermentation offers significant advantages when compared to other hydrogen production processes, as it requires less investment, the operating conditions are simpler, and it is environmentally more advantageous [78]. In this process, organic substrates, such as glycerol, are decomposed by hydrogen-producing microorganisms, and methanogenic microorganisms must be eliminated from the process so that they do not produce methane [79,80].
The work carried out by Maru et al. (2016) aimed to produce hydrogen through the fermentation process using pure and residual glycerol as the raw materials. The fermentation process was carried out and compared using three strains, Escherichia coli CECT432, Escherichia coli CECT434, and Enterobacter cloacae MCM2/1. Escherichia coli CECT432 was the strain that obtained the highest hydrogen production using pure glycerol, and a co-culture formed by Escherichia coli CECT432 and Enterobacter cloacae MCM2/1 showed the highest productivity. This same co-culture was used in the fermentation process using residual glycerol, and the results were satisfactory according to the authors. The authors concluded that the strains metabolized residual glycerol without any purification step, and the ability to produce H2 without prior purification of residual glycerol was attractive because it avoided extra costs in the process [78].
The work carried out by Sittijunda and Reungsang (2020) proposed the simultaneous production of hydrogen, ethanol, and 1,3-propanediol. The authors used pure and residual glycerol to convert to desirable products. The process was carried out via fermentation using a co-culture of Enterobacter sp., Klebsiella sp., and Klebsiella pneumoniae. The authors concluded that the use of crude glycerol as the raw material presented satisfactory results [81].
The work carried out by Prakash et al. (2018) aimed to use domestic wastewater and crude glycerol from the biodiesel production process to produce hydrogen via the fermentation process. The cultures used were Bacillus thuringiensis strain EGU4 and Bacillus amyloliquefaciens strain CD16. The results were satisfactory, but when comparing the performance of the two cultures, Bacillus amyloliquefaciens strain CD16 had the highest yield. The authors concluded that the results were satisfactory; however, they emphasized the need for biosafety when working with non-sterile sludge [82].
The work carried out by Chen et al. (2021) aimed to produce hydrogen via the fermentation process using raw glycerol as the raw material and compare the yield of fermentation with immobilized and suspended microorganisms. The inoculum used was collected at a wastewater treatment plant in Beijing, China, pre-treatment with ionizing radiation was performed in order to eliminate hydrogen-consuming bacteria, and the predominant bacterium in the culture was Clostridium sp. The results presented by the authors showed greater yield by fermentation using immobilized microorganisms as well as greater tolerance to the substrate [83].
The work carried out by Silva et al. (2020) aimed to use semi-continuous reactors for the production of hydrogen and volatile fatty acids via a fermentation process, using residual glycerol as a substrate. The microorganisms used were bacteria of the genera Enterobacter and Clostridium. The reactor that operated using bacteria of the genus Clostridium showed lower performance. The authors concluded that the yields found for the production of hydrogen using a semi-continuous reactor via a fermentation process with bacteria of the genus Enterobacter were satisfactory and consistent with the literature [84].
The work carried out by Mirzoyan et al. (2019) aimed to use a mixture of lactose and glycerol for the production of hydrogen via a fermentation process using the bacterium Escherichia coli at different pH values and concentrations. The authors concluded that the results were satisfactory and an alternative for the production of renewable hydrogen [85].
The work carried out by Toledo-Alarcon et al. (2020) aimed to produce hydrogen using glycerol as a substrate and verify the impact of sludge pre-treatment on the production process. Two types of inoculum were used, aerobic and anaerobic sludge, and two types of pre-treatment, aeration and thermal shock. Bacteria of the genus Clostridium were detected as dominant in all inocula. The best results were obtained using anaerobic sludge with thermal pre-treatment [86].
Table 3 summarizes the production of hydrogen using glycerol as the raw material.

Table 3.
Production of hydrogen using glycerol as the raw material.
3.3. Ethanol
Ethanol is mostly produced from renewable raw materials, especially from sugarcane and corn [87,88,89]. But, according to the study carried out by Yazdani and González (2007), the cost of producing ethanol from glycerol is almost 40% lower than that of ethanol produced from corn, taking into account the demand for raw materials and operational costs [90]. Currently, the ethanol production process using glycerol as a raw material is not considered economically viable when compared to other conventional processes [91,92]. However, the use of glycerol from the biodiesel production process is interesting because of some factors, such as making glycerol a source of biomass and producing ethanol from it, which is one of the alcohols that can be used in the biodiesel production process. This would encourage the development of integrated biorefineries (industrial symbiosis) [93,94] as well as the development of new methods and technologies that may be technically and economically viable in the future [95,96].
The work carried out by Sunarno et al. (2019) aimed to produce ethanol from crude glycerol using Enterobacter aerogenes TISTR 1468 supplemented with a cheaply available nutrient substrate, in this case, tuna condensate. The authors aimed to find the optimal conditions for the concentration of crude glycerol, tuna condensate, inorganic salts, and pH. The optimal conditions for ethanol production were 20 g/L of crude glycerol with the pH maintained at 7, yielding 12.33 g/L [97].
The work carried out by Oh et al. (2011) aimed to produce ethanol using glycerol as a carbon source. A mutant strain was used in the process, Klebsiella pneumoniae GEM167, which was obtained by irradiation. According to the authors, when comparing production with the control strain, an increase in ethanol production was expressly noticeable when using the mutant strain. The maximum production level was 21.5 g/L, with a productivity of 0.93 g/L/h [98].
The work carried out by Stepanov and Efremenko (2017) aimed to develop and use a biocatalyst in the form of a cryogel immobilizing cells of the yeast Pachysolen tannophilus to convert glycerol into ethanol in both batch and continuous modes. According to the authors, the conversion of glycerol into ethanol using this biocatalyst resulted in a yield of 90% in relation to the theoretical limit [99].
The work carried out by Sunarno et al. (2020) aimed to produce ethanol from crude glycerol using Enterobacter aerogenes TISTR 1468. The authors evaluated the conversion of glycerol into ethanol under aerobic and anaerobic conditions. The evaluation was carried out in a reactor with aeration in continuous and batch processes, and the conversion process without aeration was also evaluated. The aeration rate was controlled using the redox potential (OPR). The results presented by the authors showed that in the fermentation process without aeration, the ethanol yield was 18.78 g/L; with aeration, it was 30.31 g/L in the continuous process and 12.33 g/L in the batch process [100].
The work carried out by Suzuki et al. (2015) aimed to produce ethanol using glycerol from the development of a highly ethanol-tolerant Klebsiella variicola mutant, as well as improving the mutant’s ethanol production by optimizing the culture conditions. The mutant obtained from Klebsiella variicola TB-83, called TB-83D, was modified by means of ribosome engineering, and it was more resistant to streptomycin and tolerant to ethanol. The results indicated that the mutant strain showed higher ethanol production [101].
The work carried out by Vikromvarasiri et al. (2016) used an anaerobic sludge blanket reactor for wastewater treatment to test the possibility of producing ethanol in the fermentation process using glycerol as a co-substrate at various glycerol concentrations. The ethanol concentration and yield were highly dependent on the initial glycerol concentration. The highest ethanol concentration was 11.1 g/L obtained after 72 h of fermentation at an initial concentration of 45 g/L of glycerol. The main ethanol producers were identified as Enterobacter and Klebsiella strains [102].
The work carried out by Lee et al. (2017) aimed to convert glycerol into ethanol with Enterobacter aerogenes ATCC 29007 immobilized using alginate. The fermentation process was carried out in a continuous stirred tank reactor (CSTR) designed for continuous production with immobilized cells. The experiments were performed using pure and crude glycerol. Under optimal conditions, the ethanol production and yield were approximately 5.38 g/L and 0.96 mol ethanol/mol glycerol with pure glycerol, respectively, while the ethanol production and yield were approximately 5.29 g/L and 0.91 mol ethanol/mol glycerol with crude glycerol, respectively [103].
Table 4 summarizes the ethanol production using glycerol as the raw material.

Table 4.
Ethanol production using glycerol as the raw material.
4. Considerations
Biodiesel production is a complex process that demands high energy and economic expenditure [104,105]. Therefore, it is important for the industrial viability of biodiesel production to add economic value to glycerol, making glycerol a useful product and not an undesirable by-product [25,106,107].
The production of methane from glycerol is a sustainable alternative to methane extracted from natural gas [108]. Methane production is directly related to biogas production, and it is important to detect which other gases are present in this biogas in order to propose a treatment for its purification as well as the degree of purity of methane [108].
Hydrogen is a promising alternative to fossil fuels because it has a high energy yield (122 kJ/g) and its combustion product is water instead of gases that contribute to the worsening of the greenhouse effect [109]. Hydrogen produced through catalytic cracking of petroleum or through steam reforming of methane present in natural gas cannot be considered a renewable alternative since it is obtained from fossil fuels [110]. The production of hydrogen through water electrolysis demands a high energy cost, making the process unfeasible from an economic point of view. Thus, the fermentation pathway using glycerol as biomass can be attractive both from an environmental and economic point of view for the production of hydrogen [111,112,113].
Currently, ethanol is produced predominantly from sugarcane and corn [114,115,116,117]. However, producing ethanol from glycerol, which is currently seen as a by-product of the biodiesel production process, is a way of adding economic value to glycerol [35,118]. Another perspective is the use of ethanol produced from glycerol returns it to the biodiesel production process as one of the reagents [119].
Another alcohol that can be returned to the process is methanol, which in turn can be obtained from the biogas produced from glycerol, requiring biogas purification processes [120]. The work carried out by Magalhães et al. (2004) proposed the purification of biogas using a packing column and water as the solvent at pressures between 6 and 12 bar [121]. After purifying the biogas, methane underwent an oxidation process in order to obtain synthesis gas, and catalytic reform was employed to obtain methanol [122,123]. The proposal to use biogas to produce methanol is a way to obtain alcohol from a renewable source, since the methanol produced in the current context is predominantly from natural gas of fossil origin [124,125]. And, the methanol produced by this route can be returned to the biodiesel production process plant and for use as one of the reagents [62,126].
Finally, it is important to highlight that production is mostly carried out through biochemical processes; therefore, it is important that the residues from these processes receive adequate microbiological treatment for biosafety reasons [127]. The biofuels reported in these works can be applied in different industrial sectors, and according to the authors, they are attractive alternatives for the use of glycerol as a raw material.
5. Conclusions
The biodiesel production process is complex, demanding a high energy expenditure and consequently having a high economic cost, thereby reinforcing the need to make glycerol an attractive product energetically and economically. Thus, we can conclude that the works presented are alternatives to make the biodiesel production process viable since they sought to use glycerol as a raw material for the production of other biofuels.
Author Contributions
Conceptualization, E.L.A. and C.M.G.A.; formal analysis, E.L.A., J.E.O. and C.M.G.A.; writing—preparation of the original draft, E.L.A.; writing—review and editing, E.L.A. and C.M.G.A.; supervision, J.E.O. and C.M.G.A.; project administration, E.L.A., J.E.O. and C.M.G.A.; acquisition of financing, J.E.O. and C.M.G.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
The authors wish to thank the Coordination for the Improvement of Higher Education Personnel (CAPES) and National Council for Scientific and Technological Development (CNPq).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ghosh, S.K. Biomass & Bio-Waste Supply Chain Sustainability for Bio-Energy and Bio-Fuel Production. Procedia Environ. Sci. 2016, 31, 31–39. [Google Scholar] [CrossRef]
- Gaurav, N.; Sivasankari, S.; Kiran, G.S.; Ninawe, A.; Selvin, J. Utilization of Bioresources for Sustainable Biofuels: A Review. Renew. Sustain. Energy Rev. 2017, 73, 205–214. [Google Scholar] [CrossRef]
- Luque, R.; Lovett, J.C.; Datta, B.; Clancy, J.; Campelo, J.M.; Romero, A.A. Biodiesel as Feasible Petrol Fuel Replacement: A Multidisciplinary Overview. Energy Environ. Sci. 2010, 3, 1706–1721. [Google Scholar] [CrossRef]
- Chuah, L.F.; Klemeš, J.J.; Yusup, S.; Bokhari, A.; Akbar, M.M. A Review of Cleaner Intensification Technologies in Biodiesel Production. J. Clean. Prod. 2017, 146, 181–193. [Google Scholar] [CrossRef]
- Mamtani, K.; Shahbaz, K.; Farid, M.M. Deep Eutectic Solvents—Versatile Chemicals in Biodiesel Production. Fuel 2021, 295. [Google Scholar] [CrossRef]
- Demirbas, A. Competitive Liquid Biofuels from Biomass. Appl. Energy 2011, 88, 17–28. [Google Scholar] [CrossRef]
- Srilatha, K.; Prabhavathi Devi, B.L.A.; Lingaiah, N.; Prasad, R.B.N.; Sai Prasad, P.S. Biodiesel Production from Used Cooking Oil by Two-Step Heterogeneous Catalyzed Process. Bioresour. Technol. 2012, 119, 306–311. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Wu, X.; Leung, M.K.H. A Review on Biodiesel Production Using Catalyzed Transesterification. Appl. Energy 2010, 87, 1083–1095. [Google Scholar] [CrossRef]
- Abbaszaadeh, A.; Ghobadian, B.; Omidkhah, M.R.; Najafi, G. Current Biodiesel Production Technologies: A Comparative Review. Energy Convers. Manag. 2012, 63, 138–148. [Google Scholar] [CrossRef]
- Faruque, M.O.; Razzak, S.A.; Hossain, M.M. Application of Heterogeneous Catalysts for Biodiesel Production from Microalgal Oil—A Review. Catalysts 2020, 10, 1025. [Google Scholar] [CrossRef]
- Tachibana, Y.; Shi, X.; Graiver, D.; Narayan, R. The Use of Glycerol Carbonate in the Preparation of Highly Branched Siloxy Polymers. Silicon 2015, 7, 5–13. [Google Scholar] [CrossRef]
- Badia-Fabregat, M.; Rago, L.; Baeza, J.A.; Guisasola, A. Hydrogen Production from Crude Glycerol in an Alkaline Microbial Electrolysis Cell. Int. J. Hydrog. Energy 2019, 44, 17204–17213. [Google Scholar] [CrossRef]
- Zhou, J.J.; Shen, J.T.; Wang, X.L.; Sun, Y.Q.; Xiu, Z.L. Stability and Oscillatory Behavior of Microbial Consortium in Continuous Conversion of Crude Glycerol to 1,3-Propanediol. Appl. Microbiol. Biotechnol. 2018, 102, 8291–8305. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Kumar, N. Scope and Opportunities of Using Glycerol as an Energy Source. Renew. Sustain. Energy Rev. 2012, 16, 4551–4556. [Google Scholar] [CrossRef]
- da Silva, G.P.; Mack, M.; Contiero, J. Glycerol: A Promising and Abundant Carbon Source for Industrial Microbiology. Biotechnol. Adv. 2009, 27, 30–39. [Google Scholar] [CrossRef]
- Faccendini, P.L.; Ribone, M.É.; Lagier, C.M. Selective Application of Two Rapid, Low-Cost Electrochemical Methods to Quantify Glycerol According to the Sample Nature. Sens. Actuators B Chem. 2014, 193, 142–148. [Google Scholar] [CrossRef]
- Anitha, M.; Kamarudin, S.K.; Kofli, N.T. The Potential of Glycerol as a Value-Added Commodity. Chem. Eng. J. 2016, 295, 119–130. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y.; Valéro, J.R. Energy Balance of Biofuel Production from Biological Conversion of Crude Glycerol. J. Environ. Manag. 2016, 170, 169–176. [Google Scholar] [CrossRef]
- Elgharbawy, A.S.; Sadik, W.A.; Sadek, O.M.; Kasaby, M.A. Maximizing Biodiesel Production from High Free Fatty Acids Feedstocks through Glycerolysis Treatment. Biomass Bioenergy 2021, 146, 105997. [Google Scholar] [CrossRef]
- Almeida, E.L.; Andrade, C.M.G.; Andreo Dos Santos, O. Production of Biodiesel via Catalytic Processes: A Brief Review. Int. J. Chem. React. Eng. 2018, 16, 20170130. [Google Scholar] [CrossRef]
- Bournay, L.; Casanave, D.; Delfort, B.; Hillion, G.; Chodorge, J.A. New Heterogeneous Process for Biodiesel Production: A Way to Improve the Quality and the Value of the Crude Glycerin Produced by Biodiesel Plants. Catal. Today 2005, 106, 190–192. [Google Scholar] [CrossRef]
- Ramadhas, A.S.; Jayaraj, S.; Muraleedharan, C. Biodiesel Production from High FFA Rubber Seed Oil. Fuel 2005, 84, 335–340. [Google Scholar] [CrossRef]
- Ladero, M.; de Gracia, M.; Tamayo, J.J.; de Ahumada, I.L.; Trujillo, F.; Garcia-Ochoa, F. Kinetic Modelling of the Esterification of Rosin and Glycerol: Application to Industrial Operation. Chem. Eng. J. 2011, 169, 319–328. [Google Scholar] [CrossRef]
- Mendes, D.B.; Serra, J.C.V. Glicerina: Uma abordagem sobre a produção e o tratamento. Rev. Liberato. 2012, 13, 59–68. [Google Scholar] [CrossRef]
- Quispe, C.A.G.; Coronado, C.J.R.; Carvalho, J.A. Glycerol: Production, Consumption, Prices, Characterization and New Trends in Combustion. Renew. Sustain. Energy Rev. 2013, 27, 475–493. [Google Scholar] [CrossRef]
- Haigh, K.F.; Vladisavljević, G.T.; Reynolds, J.C.; Nagy, Z.; Saha, B. Kinetics of the Pre-Treatment of Used Cooking Oil Using Novozyme 435 for Biodiesel Production. Chem. Eng. Res. Des. 2014, 92, 713–719. [Google Scholar] [CrossRef]
- Perdomo, F.A.; Perdomo, L.; Millán, B.M.; Aragón, J.L. Design and Improvement of Biodiesel Fuels Blends by Optimization of Their Molecular Structures and Compositions. Chem. Eng. Res. Des. 2014, 92, 1482–1494. [Google Scholar] [CrossRef]
- Dasari, S.R.; Borugadda, V.B.; Goud, V.V. Reactive Extraction of Castor Seeds and Storage Stability Characteristics of Produced Biodiesel. Process Saf. Environ. Prot. 2016, 100, 252–263. [Google Scholar] [CrossRef]
- Dasari, S.R.; Goud, V.V. Simultaneous Extraction and Transesterification of Castor Seeds for Biodiesel Production: Assessment of Biodegradability. Process Saf. Environ. Prot. 2017, 107, 373–387. [Google Scholar] [CrossRef]
- Verma, P.; Sharma, M.P.; Dwivedi, G. Impact of Alcohol on Biodiesel Production and Properties. Renew. Sustain. Energy Rev. 2016, 56, 319–333. [Google Scholar] [CrossRef]
- Jafari, D.; Esfandyari, M. Optimization of Temperature and Molar Flow Ratios of Triglyceride/Alcohol in Biodiesel Production in a Batch Reactor. Biofuels 2020, 11, 261–267. [Google Scholar] [CrossRef]
- Moser, B.R. Biodiesel Production, Properties, and Feedstocks. In Vitr. Cell. Dev. Biol. Plant 2009, 45, 229–266. [Google Scholar] [CrossRef]
- Tan, K.T.; Lee, K.T. A Review on Supercritical Fluids (SCF) Technology in Sustainable Biodiesel Production: Potential and Challenges. Renew. Sustain. Energy Rev. 2011, 15, 2452–2456. [Google Scholar] [CrossRef]
- Atadashi, I.M.; Aroua, M.K.; Abdul Aziz, A.R.; Sulaiman, N.M.N. Production of Biodiesel Using High Free Fatty Acid Feedstocks. Renew. Sustain. Energy Rev. 2012, 16, 3275–3285. [Google Scholar] [CrossRef]
- Bateni, H.; Karimi, K. Biodiesel Production from Castor Plant Integrating Ethanol Production via a Biorefinery Approach. Chem. Eng. Res. Des. 2016, 107, 4–12. [Google Scholar] [CrossRef]
- Cristina Santos de Mello, M.; Gomes D’Amato Villardi, H.; Ferreira Young, A.; Luiz Pellegrini Pessoa, F.; Medeiros Salgado, A. Life Cycle Assessment of Biodiesel Produced by the Methylic-Alkaline and Ethylic-Enzymatic Routes. Fuel 2017, 208, 329–336. [Google Scholar] [CrossRef]
- Wong, W.Y.; Lim, S.; Pang, Y.L.; Shuit, S.H.; Chen, W.H.; Lee, K.T. Synthesis of Renewable Heterogeneous Acid Catalyst from Oil Palm Empty Fruit Bunch for Glycerol-Free Biodiesel Production. Sci. Total Environ. 2020, 727, 138534. [Google Scholar] [CrossRef]
- Alaba, P.A.; Sani, Y.M.; Ashri Wan Daud, W.M. Efficient Biodiesel Production: via Solid Superacid Catalysis: A Critical Review on Recent Breakthrough. RSC Adv. 2016, 6, 78351–78368. [Google Scholar] [CrossRef]
- Alcañiz-Monge, J.; Trautwein, G.; Marco-Lozar, J.P. Biodiesel Production by Acid Catalysis with Heteropolyacids Supported on Activated Carbon Fibers. Appl. Catal. A Gen. 2013, 468, 432–441. [Google Scholar] [CrossRef]
- Lee, A.F.; Wilson, K. Recent Developments in Heterogeneous Catalysis for the Sustainable Production of Biodiesel. Catal. Today 2015, 242, 3–18. [Google Scholar] [CrossRef]
- Vieira, S.S.; Magriotis, Z.M.; Santos, N.A.V.; Saczk, A.A.; Hori, C.E.; Arroyo, P.A. Biodiesel Production by Free Fatty Acid Esterification Using Lanthanum (La3+) and HZSM-5 Based Catalysts. Bioresour. Technol. 2013, 133, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, B.; Teixeira, J.C.; Dragone, G.; Teixeira, J.A. Oleaginous Yeasts for Sustainable Lipid Production—From Biodiesel to Surf Boards, a Wide Range of “Green” Applications. Appl. Microbiol. Biotechnol. 2019, 103, 3651–3667. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, M.; Karmegam, N.; Gundupalli, M.P.; Bizuneh Gebeyehu, K.; Tessema Asfaw, B.; Chang, S.W.; Ravindran, B.; Awasthi, M.K. Heterogeneous Base Catalysts: Synthesis and Application for Biodiesel Production—A Review. Bioresour. Technol. 2021, 331, 125054. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Pandey, A.; Larroche, C.; Madamwar, D. Algal Green Energy—R&D and Technological Perspectives for Biodiesel Production. Renew. Sustain. Energy Rev. 2018, 82, 2946–2969. [Google Scholar]
- Almeida, E.L.; Gomes, S.I.; Andrade, C.M.G.; dos Santos, O.A.A. Biodiesel Production Process Versus Bioethanol Production Process. Preliminary Analysis; Faculty of Law, University of Maribor: Maribor, Slovenija, 2017; pp. 333–342. [Google Scholar]
- Luo, X.; Ge, X.; Cui, S.; Li, Y. Value-Added Processing of Crude Glycerol into Chemicals and Polymers. Bioresour. Technol. 2016, 215, 144–154. [Google Scholar] [CrossRef]
- Ardi, M.S.; Aroua, M.K.; Hashim, N.A. Progress, Prospect and Challenges in Glycerol Purification Process: A Review. Renew. Sustain. Energy Rev. 2015, 42, 1164–1173. [Google Scholar] [CrossRef]
- Kondaveeti, S.; Kim, I.W.; Otari, S.; Patel, S.K.S.; Pagolu, R.; Losetty, V.; Kalia, V.C.; Lee, J.K. Co-Generation of Hydrogen and Electricity from Biodiesel Process Effluents. Int. J. Hydrog. Energy 2019, 44, 27285–27296. [Google Scholar] [CrossRef]
- Steinmetz, S.A.; Herrington, J.S.; Winterrowd, C.K.; Roberts, W.L.; Wendt, J.O.L.; Linak, W.P. Crude Glycerol Combustion: Particulate, Acrolein, and Other Volatile Organic Emissions. Proc. Combust. Inst. 2013, 34, 2749–2757. [Google Scholar] [CrossRef]
- Baba, Y.; Tada, C.; Watanabe, R.; Fukuda, Y.; Chida, N.; Nakai, Y. Bioresource Technology Anaerobic Digestion of Crude Glycerol from Biodiesel Manufacturing Using a Large-Scale Pilot Plant: Methane Production and Application of Digested Sludge as Fertilizer. Bioresour. Technol. 2013, 140, 342–348. [Google Scholar] [CrossRef]
- Fontes, G.C.; Ramos, N.M.; Amaral, P.F.F.; Nele, M.; Coelho, M.A.Z. Renewable Resources for Biosurfactant Production by Yarrowia Lipolytica. Braz. J. Chem. Eng. 2012, 29, 483–493. [Google Scholar] [CrossRef]
- Paulista, L.O.; Boaventura, R.A.R.; Vilar, V.J.P.; Pinheiro, A.L.N.; Martins, R.J.E. Enhancing Methane Yield from Crude Glycerol Anaerobic Digestion by Coupling with Ultrasound or A. Niger/E. Coli Biodegradation. Environ. Sci. Pollut. Res. 2020, 27, 1461–1474. [Google Scholar] [CrossRef] [PubMed]
- Maragkaki, A.E.; Fountoulakis, M.; Gypakis, A.; Kyriakou, A.; Lasaridi, K.; Manios, T. Pilot-Scale Anaerobic Co-Digestion of Sewage Sludge with Agro-Industrial by-Products for Increased Biogas Production of Existing Digesters at Wastewater Treatment Plants. Waste Manag. 2017, 59, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Fountoulakis, M.S.; Manios, T. Bioresource Technology Enhanced Methane and Hydrogen Production from Municipal Solid Waste and Agro-Industrial by-Products Co-Digested with Crude Glycerol. Bioresour. Technol. 2009, 100, 3043–3047. [Google Scholar] [CrossRef] [PubMed]
- Konstantinović, S.; Danilović, B.R.; Ćirić, J.T.; Ilić, S.B.; Savić, D.S.; Veljković, V.B. Valorization of Crude Glycerol from Biodiesel Production. Chem. Ind. Chem. Eng. Q. 2016, 22, 461–489. [Google Scholar] [CrossRef]
- Chiodo, V.; Zafarana, G.; Maisano, S.; Freni, S.; Galvagno, A.; Urbani, F. Molten Carbonate Fuel Cell System Fed with Biofuels for Electricity Production. Int. J. Hydrog. Energy 2016, 41, 18815–18821. [Google Scholar] [CrossRef]
- Ferreira, J.S.; Volschan, I.; Cammarota, M.C. Enhanced Biogas Production in Pilot Digesters Treating a Mixture of Sewage Sludge, Glycerol, and Food Waste. Energy Fuels 2018, 32, 6839–6846. [Google Scholar] [CrossRef]
- Astals, S.; Nolla-Ardèvol, V.; Mata-Alvarez, J. Anaerobic Co-Digestion of Pig Manure and Crude Glycerol at Mesophilic Conditions: Biogas and Digestate. Bioresour. Technol. 2012, 110, 63–70. [Google Scholar] [CrossRef]
- Siles, J.A.; Martín, M.A.; Chica, A.F.; Martín, A. Anaerobic Co-Digestion of Glycerol and Wastewater Derived from Biodiesel Manufacturing. Bioresour. Technol. 2010, 101, 6315–6321. [Google Scholar] [CrossRef]
- Beschkov, V.; Sapundzhiev, T.; Angelov, I. Modelling of Biogas Production from Glycerol by Anaerobic Process in a Baffled Multi-Stage Digestor. Biotechnol. Biotechnol. Equip. 2012, 26, 3244–3248. [Google Scholar] [CrossRef]
- Oliveira, J.V.; Alves, M.M.; Costa, J.C. Optimization of Biogas Production from Sargassum sp. Using a Design of Experiments to Assess the Co-Digestion with Glycerol and Waste Frying Oil. Bioresour. Technol. 2015, 175, 480–485. [Google Scholar] [CrossRef]
- Sittijunda, S.; Reungsang, A. Methane Production from the Co-Digestion of Algal Biomass with Crude Glycerol by Anaerobic Mixed Cultures. Waste Biomass Valorization 2018, 11, 1873–1881. [Google Scholar] [CrossRef]
- Chou, Y.C.; Su, J.J. Biogas Production by Anaerobic Co-Digestion of Dairy Wastewater with the Crude Glycerol from Slaughterhouse Sludge Cake Transesterification. Animals 2019, 9, 618. [Google Scholar] [CrossRef] [PubMed]
- Sawasdee, V.; Haosagul, S.; Pisutpaisal, N. Co-Digestion of Waste Glycerol and Glucose to Enhance Biogas Production. Int. J. Hydrog. Energy 2019, 44, 29575–29582. [Google Scholar] [CrossRef]
- Alves, I.R.F.S.; Mahler, C.F.; Oliveira, L.B.; Reis, M.M.; Bassin, J.P. Assessing the Use of Crude Glycerol from Biodiesel Production as an Alternative to Boost Methane Generation by Anaerobic Co-Digestion of Sewage Sludge. Biomass Bioenergy 2020, 143, 105831. [Google Scholar] [CrossRef]
- Prasertsan, P.; Leamdum, C.; Chantong, S.; Mamimin, C.; Kongjan, P.; O-Thong, S. Enhanced Biogas Production by Co-Digestion of Crude Glycerol and Ethanol with Palm Oil Mill Effluent and Microbial Community Analysis. Biomass Bioenergy 2021, 148, 106037. [Google Scholar] [CrossRef]
- Takeda, P.Y.; Gotardo, J.T.; Gomes, S.D. Anaerobic Co-Digestion of Leachate and Glycerol for Renewable Energy Generation. Environ. Technol. 2022, 43, 1118–1128. [Google Scholar] [CrossRef]
- Bułkowska, K.; Mikucka, W.; Pokój, T. Enhancement of Biogas Production from Cattle Manure Using Glycerine Phase as a Co-Substrate in Anaerobic Digestion. Fuel 2022, 317, 123456. [Google Scholar] [CrossRef]
- Alves, I.R.F.S.; Mahler, C.F.; Oliveira, L.B.; Reis, M.M.; Bassin, J.P. Investigating the Effect of Crude Glycerol from Biodiesel Industry on the Anaerobic Co-Digestion of Sewage Sludge and Food Waste in Ternary Mixtures. Energy 2022, 241, 122818. [Google Scholar] [CrossRef]
- Wang, R.; Liu, S.; Liu, S.; Li, X.; Zhang, Y.; Xie, C.; Zhou, S.; Qiu, Y.; Luo, S.; Jing, F.; et al. Glycerol Steam Reforming for Hydrogen Production over Bimetallic MNi/CNTs (M[Dbnd]Co, Cu and Fe) Catalysts. Catal. Today 2020, 355, 128–138. [Google Scholar] [CrossRef]
- Cortright, R.D.; Davda, R.R.; Dumesic, J.A. Hydrogen from Catalytic Reforming of Biomass-Derived Hydrocarbons in Liquid Water. Nature 2002, 418, 964–967. [Google Scholar] [CrossRef]
- Kondratenko, E.V.; Mul, G.; Baltrusaitis, J.; Larrazábal, G.O.; Pérez-Ramírez, J. Status and Perspectives of CO2 Conversion into Fuels and Chemicals by Catalytic, Photocatalytic and Electrocatalytic Processes. Energy Environ. Sci 2013, 6, 3112–3135. [Google Scholar] [CrossRef]
- Sarma, S.; Ortega, D.; Minton, N.P.; Dubey, V.K.; Moholkar, V.S. Homologous Overexpression of Hydrogenase and Glycerol Dehydrogenase in Clostridium Pasteurianum to Enhance Hydrogen Production from Crude Glycerol. Bioresour. Technol. 2019, 284, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Varella Rodrigues, C.; Oliveira Santana, K.; Nespeca, M.G.; Varella Rodrigues, A.; Oliveira Pires, L.; Maintinguer, S.I. Energy Valorization of Crude Glycerol and Sanitary Sewage in Hydrogen Generation by Biological Processes. Int. J. Hydrog. Energy 2020, 45, 11943–11953. [Google Scholar] [CrossRef]
- Pott, R.W.M.; Howe, C.J.; Dennis, J.S. The Purification of Crude Glycerol Derived from Biodiesel Manufacture and Its Use as a Substrate by Rhodopseudomonas Palustris to Produce Hydrogen. Bioresour. Technol. 2014, 152, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.M.; Wu, T.Y.; Juan, J.C. A Review of Sustainable Hydrogen Production Using Seed Sludge via Dark Fermentation. Renew. Sustain. Energy Rev. 2014, 34, 471–482. [Google Scholar] [CrossRef]
- De Gioannis, G.; Muntoni, A.; Polettini, A.; Pomi, R. A Review of Dark Fermentative Hydrogen Production from Biodegradable Municipal Waste Fractions. Waste Manag. 2013, 33, 1345–1361. [Google Scholar] [CrossRef]
- Maru, B.T.; López, F.; Kengen, S.W.M.; Constantí, M.; Medina, F. Dark Fermentative Hydrogen and Ethanol Production from Biodiesel Waste Glycerol Using a Co-Culture of Escherichia Coli and Enterobacter sp. Fuel 2016, 186, 375–384. [Google Scholar] [CrossRef]
- Chookaew, T.; Prasertsan, P.; Ren, Z.J. Two-Stage Conversion of Crude Glycerol to Energy Using Dark Fermentation Linked with Microbial Fuel Cell or Microbial Electrolysis Cell. New Biotechnol. 2014, 31, 179–184. [Google Scholar] [CrossRef]
- Ghimire, A.; Frunzo, L.; Pirozzi, F.; Trably, E.; Escudie, R.; Lens, P.N.L.; Esposito, G. A Review on Dark Fermentative Biohydrogen Production from Organic Biomass: Process Parameters and Use of by-Products. Appl. Energy 2015, 144, 73–95. [Google Scholar] [CrossRef]
- Sittijunda, S.; Reungsang, A. Valorization of Crude Glycerol into Hydrogen, 1,3-Propanediol, and Ethanol in an up-Flow Anaerobic Sludge Blanket (UASB) Reactor under Thermophilic Conditions. Renew. Energy 2020, 161, 361–372. [Google Scholar] [CrossRef]
- Prakash, J.; Sharma, R.; Patel, S.K.S.; Kim, I.W.; Kalia, V.C. Bio-Hydrogen Production by Co-Digestion of Domestic Wastewater and Biodiesel Industry Effluent. PLoS ONE 2018, 13, e0199059. [Google Scholar] [CrossRef]
- Chen, Y.; Yin, Y.; Wang, J. Comparison of Fermentative Hydrogen Production from Glycerol Using Immobilized and Suspended Mixed Cultures. Int. J. Hydrog. Energy 2021, 46, 8986–8994. [Google Scholar] [CrossRef]
- Cristina, M.; Silva, A.; Monteggia, L.O. Hydrogen Production Potential Comparison of Sucrose and Crude Glycerol Using Different Inoculums Sources; Inderscience Enterprises Ltd.: Cointrin-Geneva, Switzerland, 2020; Volume 25. [Google Scholar]
- Mirzoyan, S.; Trchounian, A.; Trchounian, K. Hydrogen Production by Escherichia Coli during Anaerobic Utilization of Mixture of Lactose and Glycerol: Enhanced Rate and Yield, Prolonged Production. Int. J. Hydrog. Energy 2019, 44, 9272–9281. [Google Scholar] [CrossRef]
- Toledo-Alarcón, J.; Cabrol, L.; Jeison, D.; Trably, E.; Steyer, J.P.; Tapia-Venegas, E. Impact of the Microbial Inoculum Source on Pre-Treatment Efficiency for Fermentative H2 Production from Glycerol. Int. J. Hydrog. Energy 2020, 45, 1597–1607. [Google Scholar] [CrossRef]
- Cardona, C.A.; Sánchez, Ó.J. Fuel Ethanol Production: Process Design Trends and Integration Opportunities. Bioresour. Technol. 2007, 98, 2415–2457. [Google Scholar] [CrossRef]
- Ganguly, A.; Chatterjee, P.K.; Dey, A. Studies on Ethanol Production from Water Hyacinth—A Review. Renew. Sustain. Energy Rev. 2012, 16, 966–972. [Google Scholar] [CrossRef]
- Acorsi, R.L.; De Giovanni, M.Y.G.; Andrade, C.M.G.; Olivo, J.E. Modeling and Simulation of Batch Sugarcane Alcoholic Fermentation Using the Metabolic Model. Fermentation 2022, 8, 82. [Google Scholar] [CrossRef]
- Yazdani, S.S.; Gonzalez, R. Anaerobic Fermentation of Glycerol: A Path to Economic Viability for the Biofuels Industry. Curr. Opin. Biotechnol. 2007, 18, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Posada, J.A.; Cardona, C.A. Design and Analysis of Fuel Ethanol Production from Raw Glycerol. Energy 2010, 35, 5286–5293. [Google Scholar] [CrossRef]
- Sunarno, J.N.; Prasertsan, P.; Duangsuwan, W.; Kongjan, P.; Cheirsilp, B. Mathematical Modeling of Ethanol Production from Glycerol by Enterobacter Aerogenes Concerning the Influence of Impurities, Substrate, and Product Concentration. Biochem. Eng. J. 2020, 155, 107471. [Google Scholar] [CrossRef]
- Chozhavendhan, S.; Karthiga Devi, G.; Bharathiraja, B.; Praveen Kumar, R.; Elavazhagan, S. Assessment of Crude Glycerol Utilization for Sustainable Development of Biorefineries. In Refining Biomass Residues for Sustainable Energy and Bioproducts: Technology, Advances, Life Cycle Assessment, and Economics; Elsevier: Amsterdam, The Netherlands, 2019; pp. 195–212. ISBN 9780128189962. [Google Scholar]
- Gonela, V.; Zhang, J. Design of the Optimal Industrial Symbiosis System to Improve Bioethanol Production. J. Clean. Prod. 2014, 64, 513–534. [Google Scholar] [CrossRef]
- Yu, K.O.; Kim, S.W.; Han, S.O. Engineering of Glycerol Utilization Pathway for Ethanol Production by Saccharomyces Cerevisiae. Bioresour. Technol. 2010, 101, 4157–4161. [Google Scholar] [CrossRef]
- Lee, J.H.; Yoo, H.Y.; Lee, S.K.; Chun, Y.; Kim, H.R.; Bankeeree, W.; Lotrakul, P.; Punnapayak, H.; Prasongsuk, S.; Kim, S.W. Significant Impact of Casein Hydrolysate to Overcome the Low Consumption of Glycerol by Klebsiella Aerogenes ATCC 29007 and Its Application to Bioethanol Production. Energy Convers. Manag. 2020, 221, 113181. [Google Scholar] [CrossRef]
- Sunarno, J.N.; Prasertsan, P.; Duangsuwan, W.; Cheirsilp, B.; Sangkharak, K. Biodiesel Derived Crude Glycerol and Tuna Condensate as an Alternative Low-Cost Fermentation Medium for Ethanol Production by Enterobacter Aerogenes. Ind. Crops Prod. 2019, 138, 111451. [Google Scholar] [CrossRef]
- Oh, Y.K.; Hwang, K.R.; Kim, C.; Kim, J.R.; Lee, J.S. Recent Developments and Key Barriers to Advanced Biofuels: A Short Review. Bioresour. Technol. 2018, 257, 320–333. [Google Scholar] [CrossRef]
- Stepanov, N.; Efremenko, E. Immobilised Cells of Pachysolen Tannophilus Yeast for Ethanol Production from Crude Glycerol. New Biotechnol. 2017, 34, 54–58. [Google Scholar] [CrossRef]
- Sunarno, J.N.; Prasertsan, P.; Duangsuwan, W.; Cheirsilp, B.; Sangkharak, K. Improve Biotransformation of Crude Glycerol to Ethanol of Enterobacter Aerogenes by Two-Stage Redox Potential Fed-Batch Process under Microaerobic Environment. Biomass Bioenergy 2020, 134, 105503. [Google Scholar] [CrossRef]
- Suzuki, T.; Seta, K.; Nishikawa, C.; Hara, E.; Shigeno, T.; Nakajima-Kambe, T. Improved Ethanol Tolerance and Ethanol Production from Glycerol in a Streptomycin-Resistant Klebsiella Variicola Mutant Obtained by Ribosome Engineering. Bioresour. Technol. 2015, 176, 156–162. [Google Scholar] [CrossRef]
- Vikromvarasiri, N.; Haosagul, S.; Boonyawanich, S.; Pisutpaisal, N. Microbial Dynamics in Ethanol Fermentation from Glycerol. Int. J. Hydrog. Energy 2016, 41, 15667–15673. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, J.H.; Yang, X.; Yoo, H.Y.; Han, S.O.; Park, C.; Kim, S.W. Re-Utilization of Waste Glycerol for Continuous Production of Bioethanol by Immobilized Enterobacter Aerogenes. J. Clean. Prod. 2017, 161, 757–764. [Google Scholar] [CrossRef]
- Pereyra, D.D.L.A.D.; Rueger, I.B.; Barbosa, P.A.M.D.A.; Peiter, F.S.; da Silva Freitas, D.M.; de Amorim, E.L.C. Co-Fermentation of Glycerol and Molasses for Obtaining Biofuels and Value-Added Products. Braz. J. Chem. Eng. 2020, 37, 653–660. [Google Scholar] [CrossRef]
- Ren, J.; Manzardo, A.; Mazzi, A.; Fedele, A.; Scipioni, A. Emergy Analysis and Sustainability Efficiency Analysis of Different Crop-Based Biodiesel in Life Cycle Perspective. Sci. World J. 2013, 2013, 918514. [Google Scholar] [CrossRef] [PubMed]
- Viana, M.B.; Freitas, A.V.; Leitão, R.C.; Pinto, G.A.S.; Santaella, S.T. Anaerobic Digestion of Crude Glycerol: A Review. Environ. Technol. Rev. 2012, 1, 81–92. [Google Scholar] [CrossRef]
- Bułkowska, K.; Białobrzewski, I.; Klimiuk, E.; Pokój, T. Kinetic Parameters of Volatile Fatty Acids Uptake in the ADM1 as Key Factors for Modeling Co-Digestion of Silages with Pig Manure, Thin Stillage and Glycerine Phase. Renew. Energy 2018, 126, 163–176. [Google Scholar] [CrossRef]
- Kapoor, R.; Ghosh, P.; Tyagi, B.; Vijay, V.K.; Vijay, V.; Thakur, I.S.; Kamyab, H.; Nguyen, D.D.; Kumar, A. Advances in Biogas Valorization and Utilization Systems: A Comprehensive Review. J. Clean. Prod 2020, 273, 123052. [Google Scholar] [CrossRef]
- Kurahashi, K.; Kimura, C.; Fujimoto, Y.; Tokumoto, H. Value-Adding Conversion and Volume Reduction of Sewage Sludge by Anaerobic Co-Digestion with Crude Glycerol. Bioresour. Technol. 2017, 232, 119–125. [Google Scholar] [CrossRef]
- Valvassore, M.S.; de Freitas, H.F.S.; Andrade, C.M.G.; Costa, C.B.B. Improving Feeding Profile Strategy for Hydrogen Production by Cyanothece sp. ATCC 51142 Using Meta-Heuristic Methods. Chem. Eng. Commun. 2023, 210, 1–15. [Google Scholar] [CrossRef]
- Vivek, N.; Pandey, A.; Binod, P. Biological Valorization of Pure and Crude Glycerol into 1,3-Propanediol Using a Novel Isolate Lactobacillus Brevis N1E9.3.3. Bioresour. Technol. 2016, 213, 222–230. [Google Scholar] [CrossRef]
- Johnson, E.E.; Rehmann, L. The Role of 1,3-Propanediol Production in Fermentation of Glycerol by Clostridium Pasteurianum. Bioresour. Technol. 2016, 209, 1–7. [Google Scholar] [CrossRef]
- Poladyan, A.; Baghdasaryan, L.; Trchounian, A. Escherichia Coli Wild Type and Hydrogenase Mutant Cells Growth and Hydrogen Production upon Xylose and Glycerol Co-Fermentation in Media with Different Buffer Capacities. Int. J. Hydrog. Energy 2018, 43, 15870–15879. [Google Scholar] [CrossRef]
- Jansen, M.L.A.; Bracher, J.M.; Papapetridis, I.; Verhoeven, M.D.; De Bruijn, H.; De Waal, P.P.; Van Maris, A.J.A.; Klaassen, P.; Pronk, J.T. Saccharomyces Cerevisiae Strains for Second-Generation Ethanol Production: From Academic Exploration to Industrial Implementation. FEMS Yeast Res. 2017, 17, fox044. [Google Scholar] [CrossRef]
- Mohsenzadeh, A.; Zamani, A.; Taherzadeh, M.J. Bioethylene Production from Ethanol: A Review and Techno-Economical Evaluation. ChemBioEng Rev. 2017, 4, 75–91. [Google Scholar] [CrossRef]
- De Freitas, H.F.S.; Olivo, J.E.; Andrade, C.M.G. Optimization of Bioethanol in Silico Production Process in a Fed-Batch Bioreactor Using Non-Linear Model Predictive Control and Evolutionary Computation Techniques. Energies 2017, 10, 1763. [Google Scholar] [CrossRef]
- Rodrigues, R.; Sperandio, L.C.C.; Andrade, C.M.G. Investigation of Color and Turbidity in the Clarification of Sugarcane Juice by Ozone. J. Food Process Eng. 2018, 41, e12661. [Google Scholar] [CrossRef]
- Yu, K.O.; Kim, S.W.; Han, S.O. Reduction of Glycerol Production to Improve Ethanol Yield in an Engineered Saccharomyces Cerevisiae Using Glycerol as a Substrate. J. Biotechnol. 2010, 150, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Farobie, O.; Sasanami, K.; Matsumura, Y. A Novel Spiral Reactor for Biodiesel Production in Supercritical Ethanol. Appl. Energy 2015, 147, 20–29. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Mardina, P.; Kim, D.; Kim, S.Y.; Kalia, V.C.; Kim, I.W.; Lee, J.K. Improvement in Methanol Production by Regulating the Composition of Synthetic Gas Mixture and Raw Biogas. Bioresour. Technol. 2016, 218, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, A.; De Souza, S.N.M.; De Lima Afonso, A.D.; Ricieri, R.P. Confecção e Avaliação de Um Sistema de Remoção Do CO2 Contido No Biogás. Acta Sci. Technol. 2004, 26, 11–19. [Google Scholar]
- Bozzano, G.; Manenti, F. Efficient Methanol Synthesis: Perspectives, Technologies and Optimization Strategies. Prog. Energy Combust. Sci. 2016, 56, 71–105. [Google Scholar] [CrossRef]
- Su, Z.; Ge, X.; Zhang, W.; Wang, L.; Yu, Z.; Li, Y. Methanol Production from Biogas with a Thermotolerant Methanotrophic Consortium Isolated from an Anaerobic Digestion System. Energy Fuels 2017, 31, 2970–2975. [Google Scholar] [CrossRef]
- Keshavarz, A.; Mirvakili, A.; Chahibakhsh, S.; Shariati, A.; Rahimpour, M.R. Simultaneous Methanol Production and Separation in the Methanol Synthesis Reactor to Increase Methanol Production. Chem. Eng. Process. Process Intensif. 2020, 158, 108176. [Google Scholar] [CrossRef]
- Riaz, A.; Zahedi, G.; Klemeš, J.J. A Review of Cleaner Production Methods for the Manufacture of Methanol. J. Clean. Prod. 2013, 57, 19–37. [Google Scholar] [CrossRef]
- Hogendoorn, C.; Pol, A.; Nuijten, G.H.L.; Op den Camp, H.J.M. Methanol Production by “Methylacidiphilum Fumariolicum” Solv under Different Growth Conditions. Appl. Environ. Microbiol. 2020, 86, e01188-20. [Google Scholar] [CrossRef] [PubMed]
- Bannantine, J.P.; Register, K.B.; White, D.M. Application of the Biosafety RAM and EProtocol Software Programs to Streamline Institutional Biosafety Committee Processes at the USDA-National Animal Disease Center. Appl. Biosaf. 2018, 23, 100–105. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).