Relationship between the Physiological Activity of Japanese Post-Fermented Teas and Lactic Acid Bacteria
Abstract
:1. Introduction
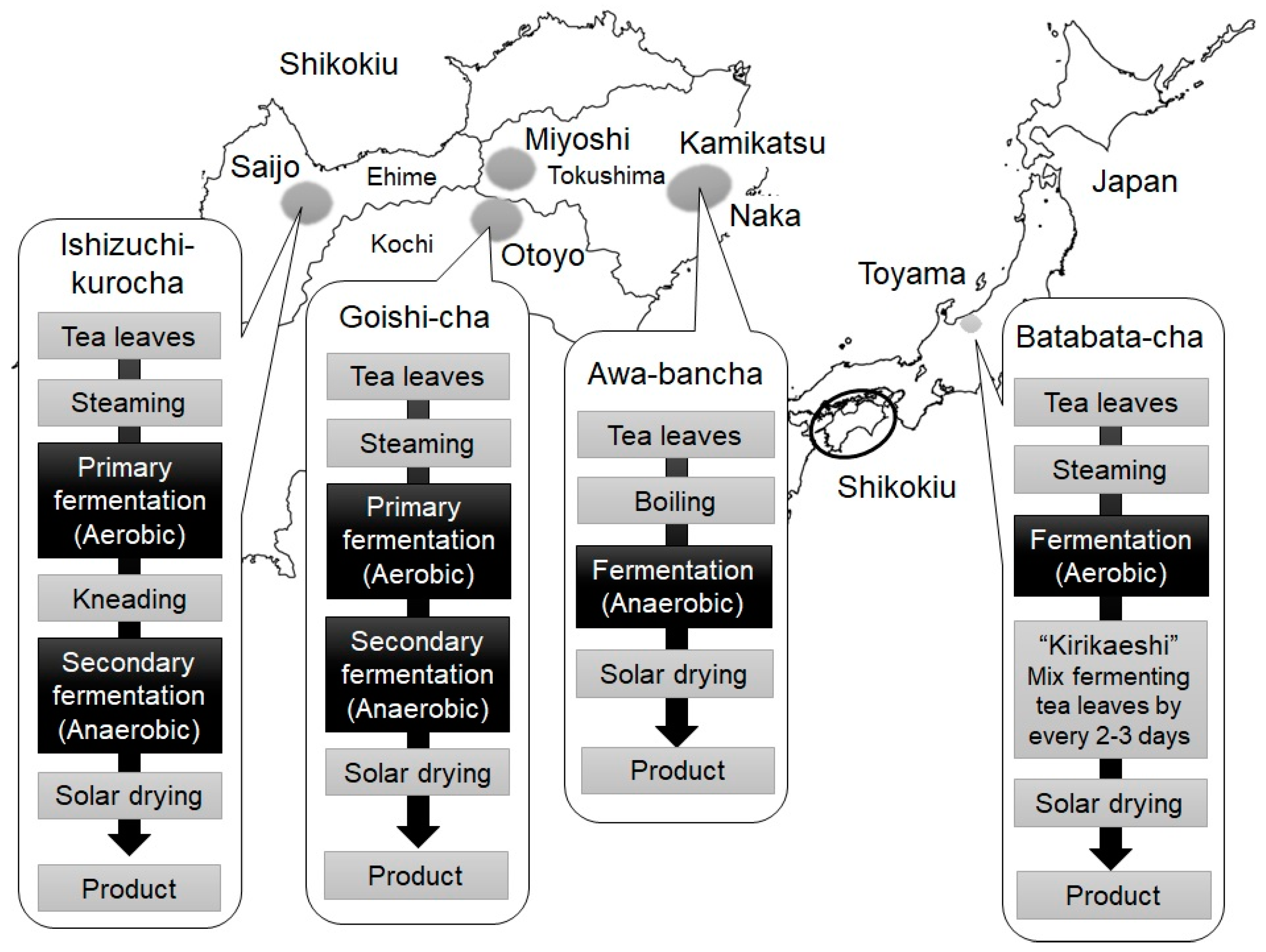
2. Production Method of Japanese Post-Fermented Teas
3. Chemical Composition of Post-Fermented Tea
| Production Year | Reported Year | Caffeine | EGC | EGCg | EC | ECg | C | Reference | |
|---|---|---|---|---|---|---|---|---|---|
| Green tea | Unknown | 2011 | 3025 | 3086 | 6241 | 869 | 1755 | 74 | [6] |
| 2276 | 4023 | 5842 | 1017 | 1496 | 123 | ||||
| 2014 | 2016 | 2913 | 1633 | 3060 | 633 | 460 | n.d. | [12] | |
| Unknown | 2019 | 2300 | 3100 | 6600 | 850 | 1200 | 69 | [4] | |
| Ishizuchi-kurocha | 1992 | 1995 | n.t. | 1617 | 234 | 1188 | n.d. | n.t. | [7] |
| 1998 | 1999 | n.t. | 160 | 10 | 50 | n.d. | n.t. | [13] | |
| n.t. | 80 | 40 | 40 | 50 | n.t. | ||||
| n.t. | 1130 | 140 | 320 | 60 | n.t. | ||||
| Unknown | 2011 | 1978 | 1234 | n.d. | 212 | n.d. | 372 | [6] | |
| 2014 | 2016 | 927 | 1067 | 133 | 127 | 6.7 | n.t. | [12] | |
| 1047 | 1300 | 66.7 | 320 | 6.7 | n.t. | ||||
| 2014 | 2019 | 1775 | 1265 | 504 | 428 | 20 | 299 | [4] | |
| 2015 | 500 | 700 | 100 | 0 | 0 | 1700 | |||
| 400 | 300 | 100 | 0 | 0 | 2100 | ||||
| 2016 | 1500 | 1100 | 220 | 330 | 0 | 280 | |||
| 2100 | 130 | 100 | 0 | 0 | 220 | ||||
| 2017 | 2200 | 590 | 360 | 260 | 0 | 420 | |||
| 2200 | 70 | 490 | 20 | 0 | 260 | ||||
| 2500 | 210 | 110 | 70 | 0 | 240 | ||||
| Goishi-cha | Unknown | 2015 | 1445 | 26.2 | 6.4 | 5.5 | n.d. | n.t. | [14] |
| 2008 | n.t. | 151 | 115 | 33 | n.d. | 224 | [15] | ||
| n.t. | 87 | 66 | 19 | n.d. | 232 | ||||
| 2011 | 1793 | 249 | n.d. | 48 | n.d. | 382 | [6] | ||
| 1519 | 986 | n.d. | 241 | n.d. | 221 | ||||
| 2016 | 773 | 433 | 26.7 | 13.3 | n.d. | n.t. | [12] | ||
| 2019 | 2272 | 1338 | 471 | 413 | 16 | 409 | [5] | ||
| Awa-bancha | 2006 | 2007 | 1430 | 710 | 2080 | 390 | 460 | 650 | [16] |
| Unknown | 2015 | 1733 | 2672 | 2644 | 500 | 764 | n.t. | [14] | |
| 2011 | 1636 | 1239 | 2430 | 132 | 822 | 31 | [6] | ||
| 1374 | 2789 | 2115 | 525 | 592 | 76 | ||||
| 1918 | 332 | 2962 | 36 | 908 | 57 | ||||
| 2016 | 407 | 467 | trace | 40 | 6.7 | n.t. | [12] | ||
| 253 | 520 | 13.3 | 73.3 | 33.3 | n.t. | ||||
| 2019 | 1025 | 3219 | 1833 | 1070 | 479 | 142 | [4] | ||
| 2018 | 2020 | 1587.5 | 772.4 | 539.6 | 164.6 | 94.4 | 267.7 | [5] | |
| 1533.7 | 1427.7 | 283.7 | trace | trace | 226.1 | ||||
| 1111.8 | 775.3 | 1103 | trace | 226.7 | 58.8 | ||||
| 915 | 388.3 | 2116.9 | 178.9 | 370.2 | trace | ||||
| 1334.9 | 4734.6 | 1621.2 | 1312.6 | 454.7 | 120.9 | ||||
| 1860.9 | 2420 | 1210.8 | trace | 458.4 | 25.6 | ||||
| Batabata-cha | Unknown | 2015 | 2106 | n.d. | 2.3 | 0.6 | 1.9 | n.t. | [11] |
| 2016 | 520 | 26.7 | n.d. | 6.7 | n.d. | n.t. | [12] | ||
| 2019 | 2221 | 0 | 0 | 0 | 0 | 0 | [4] |
4. Physiological Activity of Post-Fermented Tea
5. Lactic Acid Bacteria in Japanese Post-Fermented Tea
6. Contribution of Lactic Acid Bacteria to Bioactivity
7. Possibility of the Presence of Bacteriocin in Microbial-Fermented Tea
8. Brewed Tea from Artificial Post-Fermented Tea: Kamigare Lactic Acid Bacteria-Fermented Tea
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Horie, M.; Nishioka, H.; Tada, A.; Sugino, S.; Mizuno, T.; Toyotome, T.; Iwahashi, H. Microorganisms involved in fermentation of Batabata-cha. J. Jpn. Soc. Tast. Technol. 2019, 18, 62–70. [Google Scholar]
- Horie, M.; Ruengsomwong, S.; Wannissorn, B. Field research for production method of Miang: Post-fermented tea in Thailand. Jpn. J. Food Eng. 2020, 21, 125–137. [Google Scholar] [CrossRef]
- Mizuno, T.; Iwahashi, H.; Horie, M. Identification of microbial flora associated with fermentation of Ishizuchi-kurocha. J. Jpn. Soc. Tast. Technol. 2020, 19, 46–52. [Google Scholar]
- Horie, M.; Tada, A.; Kanamoto, N.; Tamai, T.; Fukuda, N.; Sugino, S.; Toyotome, T.; Tabei, Y. Evaluation of lactic acid bacteria and component change during fermentation of Ishizuchi-kurocha. J. Food Process. Preserv. 2019, 43, e14186. [Google Scholar] [CrossRef]
- Nishioka, H.; Mizuno, T.; Iwahashi, H.; Horie, M. Changes in lactic acid bacteria and components of Awa-bancha by anaerobic fermentation. Biosci. Biotechnol. Biochem. 2020, 84, 1921–1935. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Nagai, S.; Shii, T.; Matsuo, Y.; Kouno, I. Isolation of 1,3-diphenylpropan-2-ols, identical to tea catechin metabolites produced by intestinal bacteria, and pyrogallol from Japanese post-fermented tea. Jpn. J. Food Chem. Saf. 2011, 18, 6–11. [Google Scholar]
- Kato, M.; Tamura, A.; Saitou, H.; Omori, M.; Nanba, A.; Miyagawa, K. Changes of flavor during manufacturing process of Japanese fermented tea (Ishizuchi-kurocha) and its characteristic. J. Home Econ. Jpn. 1995, 46, 525–530. [Google Scholar]
- Horie, M.; Ohmiya, Y.; Ohmori, T. Analysis of D-amino acid in Japanese post-fermented tea, Ishizuchi-kurocha. Biosci Microbiota Food Health, 2023; in press. [Google Scholar] [CrossRef]
- Shleper, M.; Kartvelishvily, E.; Wolosker, H. D-serine is the dominant endogenous coagonist for NMDA receptor neurotoxicity in organotypic hippocampal slices. J. Neurosci. 2005, 25, 9413–9417. [Google Scholar] [CrossRef]
- Nakade, Y.; Iwata, Y.; Furuichi, K.; Mita, M.; Hamase, K.; Konno, R.; Miyake, T.; Sakai, N.; Kitajima, S.; Toyama, T.; et al. Gut microbiota-derived D-serine protects against acute kidney injury. JCI Insight 2018, 3, e97957. [Google Scholar] [CrossRef]
- Sato, T.; Umekawa, Y.; Shindo, S. Suppressive effects of D-amino acids on the li-pid accumulation in human hepatocyte. J. Brew. Soc. Jpn. 2022, 117, 131–138. [Google Scholar]
- Horie, M.; Nara, K.; Sugino, S.; Umeno, A.; Yoshida, Y. Comparison of antioxidant activities among four kinds of Japanese traditional fermented tea. Food Sci. Nutr. 2016, 5, 639–645. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Mon’ya, S.; Bessho, Y.; Shintani, T.; Morimoto, S. Development of component characteristics and storage method of Ishizuchi-kurocha (Ishizuchi-kurocha no seibun tokusei to chozouhou no kaitatsu). Norin Suisan Kako Riyou Kaihatsu Kaigi Gijyutukaihatsu Kenkyuseika Houkokusho Ehime Prefect. 1999, 11–29. [Google Scholar]
- Nakayama, H.; Nakazono, Y.; Yakashiro, I.; Matsuo, Y.; Tanaka, T.; Usui, A.; Ishimaru, K. HPLC and HPLC-TOFMS analyses of post-fermented teas in Japan. Jpn. J. Food Chem. Saf. 2015, 22, 94–99. [Google Scholar]
- Shimamura, T.; Matsuura, R.; Moriyama, H.; Takeda, N.; Ukeda, H. Changes in catechin content and superoxide anion scavenging activity of Goishi tea during manufacturing. Nippon. Shokuhin Kagaku Kogaku Kaishi 2008, 55, 640–644. [Google Scholar] [CrossRef]
- Miyazaki, E.; Nakanishi, K. Various component analysis during manufacturing process of Awa-bancha made in spring. Rep. Tokushima Prefect. Ind. Technol. Cent. 2007, 16, 37–40. [Google Scholar]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial Properties of Green Tea Catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef]
- Peluso, I.; Serafini, M. Antioxidants from black and green tea: From dietary modulation of oxidative stress to pharmacological mechanisms. Br. J. Pharmacol. 2017, 174, 1195–1208. [Google Scholar] [CrossRef]
- Miyamura, M.; Moriyama, H.; Murata, S.; Yokota, J.; Yoshioka, S.; Takuma, D.; Hamada, A.; Nishioka, Y. Inhibitory Effects of “Goishi-tea” as a Post-Fermented-tea on Dietary-Induced Hypercholesteremia and Atherosclerosis in Rabbits. Yakugaku Zasshi 2008, 128, 1037–1044. [Google Scholar] [CrossRef]
- Shimamura, T.; Kashiwagi, T.; Matsumoto, Y.; Yoshitsugu, K.; Hiraoka, A.; Yamazoe, C.; Moriyama, H.; Ohishi, M.; Miyamura, M.; Ukeda, H. Elucidation of antioxidant compounds in Goishi tea. Food Preserv. Sci. 2017, 43, 103–110. [Google Scholar] [CrossRef]
- Kondo, M.; Nishi, K.; Sugahara, T. Ishizuchi dark tea suppresses IgE-mediated degranulation of RBL-2H3 cells and nasal rubbing behavior of pollinosis in mice. J. Funct. Foods 2015, 14, 659–669. [Google Scholar] [CrossRef]
- Yokota, J.; Jobu, K.; Yoshioka, S.; Moriyama, H.; Murata, S.; Oishi, M.; Ukeda, H.; Miyamura, M. Effect of Goishi-tea on Adipocytokine Changes. Nippon. Shokuhin Kagaku Kogaku Kaishi 2011, 58, 398–402. [Google Scholar] [CrossRef]
- Nishioka, H.; Ohno, T.; Iwahashi, H.; Horie, M. Diversity of Lactic Acid Bacteria Involved in the Fermentation of Awa-bancha. Microbes Env. 2021, 36, ME21029. [Google Scholar] [CrossRef]
- Horie, M.; Sato, H.; Tada, A.; Nakamura, S.; Sugino, S.; Tabei, Y.; Katoh, M.; Toyotome, T. Regional characteristics of Lactobacillus plantarum group strains isolated from two kinds of Japanese post-fermented teas, Ishizuchi-kurocha and Awa-bancha. Biosci. Microbiota Food Health 2019, 38, 11–22. [Google Scholar] [CrossRef]
- López de Felipe, F.; Curiel, J.A.; Muñoz, R. Improvement of the fermentation performance of Lactobacillus plantarum by the flavanol catechin is uncoupled from its degradation. J. Appl. Microbiol. 2010, 109, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Renzetti, A.; Betts, J.W.; Fukumoto, K.; Rutherford, R.N. Antibacterial green tea catechins from a molecular perspective: Mechanisms of action and structure-activity relationships. Food Funct. 2020, 11, 9370–9396. [Google Scholar] [CrossRef]
- Tabasco, R.; Sánchez-Patán, F.; Monagas, M.; Bartolomé, B.; Victoria Moreno-Arribas, M.; Peláez, C.; Requena, T. Effect of grape polyphenols on lactic acid bacteria and bifidobacteria growth: Resistance and metabolism. Food Microbiol. 2011, 28, 1345–1352. [Google Scholar] [CrossRef]
- Shintani, T.; Matsumoto, Y.; Bessyo, Y. Analysis of microflora in Ishizuchi-kurocha by using ribosome RNA gene and BiOLOG system. Bull. Ind. Res. Cent. Ehime Prefect. 2000, 38, 73–79. [Google Scholar]
- Tamura, A.; Kato, M.; Omori, M.; Nanba, A.; Miyagawa, K. Characterization of microorganisms in post-heating fermented teas in Japan. J. Home Econ. Jpn. 1994, 45, 1095–1101. [Google Scholar]
- Okada, S.; Takahashi, N.; Ohara, N.; Uchimura, T.; Kozai, M. Microorganisms in Fermentation of Goishi-cha, Japanese Fermented Tea Leaves (Microorganisms Involving in the Fermentation of Japanese Fermented Tea Leaves Part II). Nippon. Shokuhin Kagaku Kogaku Kaishi 1996, 43, 1019–1027. [Google Scholar] [CrossRef]
- Okada, S.; Takahashi, N.; Ohara, N.; Uchimura, T.; Kozai, M. Microorganisms Involving in Fermentation of Awa-bancha, Japanese Fermented Tea Leaves (Microorganisms Involving in Fermentation of Japanese Fermented Tea Leaves Part I). Nippon. Shokuhin Kagaku Kogaku Kaishi 1996, 43, 12–20. [Google Scholar] [CrossRef]
- Yamamoto, M.; Horie, M.; Fukushima, M.; Toyotome, T. Culture-based analysis of fungi in leaves after the primary and secondary fermentation processes during Ishizuchi-kurocha production and lactate assimilation of P. kudriavzevii. Int. J. Food Microbiol. 2019, 306, 108263. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, H.; Iwahashi, H.; Horie, M. Characterization of lactic acid bacteria isolated from the Japanese post-fermented tea Awa-bamcha. J. Jpn. Soc. Tast. Technol. 2022, 21, 12–19. [Google Scholar]
- Alberto, M.R.; Gómez-Cordovés, C.; Manca de Nadra, M.C. Metabolism of gallic acid and catechin by Lactobacillus hilgardii from wine. J. Agric. Food Chem. 2004, 52, 6465–6469. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, R.; Zhang, Y.; Yang, Y.; Sun, X.; Zhang, Q.; Yang, N. Biotransformation of phenolics and metabolites and the change in antioxidant activity in kiwifruit induced by Lactobacillus plantarum fermentation. J. Sci. Food Agric. 2020, 100, 3283–3290. [Google Scholar] [CrossRef]
- Chen, H.; Hayek, S.; Rivera Guzman, J.; Gillitt, N.D.; Ibrahim, S.A.; Jobin, C.; Sang, S. The microbiota is essential for the generation of black tea theaflavins-derived metabolites. PLoS ONE 2012, 7, e51001. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Ohhata, E.; Masuda, T.; Okada, S.; Miyazaki, Y.; Yamasita, T.; Yasui, H. Oral administration of Lactobacillus plantarum FG4-4 ameliorates the development of dermatitis in atopic dermatitis model NC/Nga mice. Jpn. J. Lact. Acid Bact. 2010, 21, 214–220. [Google Scholar] [CrossRef]
- Gomaa, E.Z.; Abdelall, M.F.; El-Mahdy, O.M. Detoxification of Aflatoxin B1 by Antifungal Compounds from Lactobacillus brevis and Lactobacillus paracasei, Isolated from Dairy Products. Probiotics Antimicrob Proteins 2018, 10, 201–209. [Google Scholar] [CrossRef]
- Peng, Q.; Yang, J.; Wang, Q.; Suo, H.; Hamdy, A.M.; Song, J. Antifungal Effect of Metabolites from a New Strain Lactiplantibacillus Plantarum LPP703 Isolated from Naturally Fermented Yak Yogurt. Foods 2023, 12, 181. [Google Scholar] [CrossRef]
- Bukhari, S.A.; Salman, M.; Numan, M.; Javed, M.R.; Zubair, M.; Mustafa, G. Characterization of antifungal metabolites produced by Lactobacillus plantarum and Lactobacillus coryniformis isolated from rice rinsed water. Mol. Biol. Rep. 2020, 47, 1871–1881. [Google Scholar] [CrossRef]
- Marcone, G.L.; Rosini, E.; Crespi, E.; Pollegioni, L. D-amino acids in foods. Appl. Microbiol. Biotechnol. 2020, 104, 555–574. [Google Scholar] [CrossRef] [PubMed]
- Syaputri, Y.; Iwahashi, H. Characteristics of heterologous plantaricin from Lactobacillus plantarum and its future in food preservation. Rev. Agric. Sci. 2020, 8, 124–137. [Google Scholar] [CrossRef]
- Heeney, D.D.; Zhai, Z.; Bendiks, Z.; Barouei, J.; Martinic, A.; Slupsky, C.; Marco, M.L. Lactobacillus plantarum bacteriocin is associated with intestinal and systemic improvements in diet-induced obese mice and maintains epithelial barrier integrity in vitro. Gut Microbes 2019, 10, 382–397. [Google Scholar] [CrossRef]
- Todorov, S.D. Bacteriocins from Lactobacillus plantarum—Production, genetic organization and mode of action: Produção, organização genética e modo de ação. Braz. J. Microbiol. 2009, 40, 209–221. [Google Scholar] [CrossRef]
- Niwa, R.; Syaputri, Y.; Horie, M.; Iwahashi, H. Draft Genome Sequence of Lactobacillus plantarum IYO1511, Isolated from Ishizuchi-Kurocha. Microbiol. Resour. Announc. 2020, 9, e00143-e20. [Google Scholar] [CrossRef]
- Ito, F.; Niwa, R.; Syaputri, Y.; Ikagawa, Y.; Mizuno, T.; Horie, M.; Iwahashi, H. Draft Genome Sequence of Lactiplantibacillus pentosus AWA1501, Isolated from Awa-bancha. Microbiol. Resour. Announc. 2021, 10, e0051821. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.S.; Ray, R.C.; Zdolec, N. Lactobacillus plantarum with Functional Properties: An Approach to Increase Safety and Shelf-Life of Fermented Foods. Biomed. Res. Int. 2018, 2018, 9361614. [Google Scholar] [CrossRef] [PubMed]
- Ziliu, L.; Kubo, M.; Lei, J.; Zuoqian, L.; Syaputri, Y.; Ohno, T.; Nishioka, H.; Horie, M.; Naitou, K.; Iwahashi, H. Study on the establishment of Kamigare Lactobacillus brewed tea. J. Jpn. Soc. Tast. Technol. 2022, 21, 6–11. [Google Scholar]

| Species | Reference | |
|---|---|---|
| Ishiduchi-kurocha | Lactiplantibacillus plantarum | [3,4,8,23,28,29] |
| Lactiplantibacillus pentosus | [29] | |
| Levilactobacillus brevis | [3,4,8,23] | |
| Lacticaseibacillus pantheris | [4] | |
| Lactiplantibacillus plantarum | ||
| Paucilactobacillus vaccinostercus | ||
| Goishi-cha | Lactiplantibacillus plantarum | [29,30] |
| Lactiplantibacillus pentosus | [29] | |
| Awa-bancha | Lactiplantibacillus pentosus | [5,23,24,29] |
| Lactiplantibacillus plantarum | [23,24,29,31] | |
| Levilactobacillus brevis | [5,24] | |
| Lacticaseibacillus pantheris | ||
| Paucilactobacillus suebicus | [5] | |
| Secundilactobacillus collinoides | ||
| Lacticaseibacillus pantheris | ||
| Loigolactobacillus coryniformis | [24] | |
| Lactiplantibacillus paraplantarum | ||
| Secundilactobacillus collinoides | ||
| Lactiplantibacillus mudanjiangensi | ||
| Leuconostoc mesenteroides |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horie, M.; Iwahashi, H. Relationship between the Physiological Activity of Japanese Post-Fermented Teas and Lactic Acid Bacteria. Fermentation 2023, 9, 876. https://doi.org/10.3390/fermentation9100876
Horie M, Iwahashi H. Relationship between the Physiological Activity of Japanese Post-Fermented Teas and Lactic Acid Bacteria. Fermentation. 2023; 9(10):876. https://doi.org/10.3390/fermentation9100876
Chicago/Turabian StyleHorie, Masanori, and Hitoshi Iwahashi. 2023. "Relationship between the Physiological Activity of Japanese Post-Fermented Teas and Lactic Acid Bacteria" Fermentation 9, no. 10: 876. https://doi.org/10.3390/fermentation9100876
APA StyleHorie, M., & Iwahashi, H. (2023). Relationship between the Physiological Activity of Japanese Post-Fermented Teas and Lactic Acid Bacteria. Fermentation, 9(10), 876. https://doi.org/10.3390/fermentation9100876






