Abstract
The commercial importance of lactic acid (LA) is due to its versatility, especially in the food industry, and for being the precursor of poly-lactic acid, which demands a high-quality LA precursor. The overall LA production process still has some bottlenecks related to costs; thus, alternative substrates such as sugarcane juice may reduce the cost of the fermentation medium and provide a favorable environment for the Lactobacillus pentosus strain, which continues to be explored. In this context, this work presents the process of producing LA from sugarcane juice. The LA purification method is also described using different ion-exchange resins, both in packed columns and in a stirred tank. The fermentation kinetics showed the highest LA production of 113.74 g/L in 96 h, in which a productivity of 1.18 g LA/L∙h was reached. Among the purification techniques, the combined use of Amberlite IR120 and IRA-67 resins under agitation in a stirred tank was the best condition, and resulted in a final LA concentration of 189.11 g/L after 120 min, with 95% LA mass recovery. This result demonstrates a simplified way to use ion-exchange resins safely and in a controlled environment, and with process scale-up viability.
1. Introduction
Lactic acid (LA) is the popular name for 2-hydroxypropanoic acid (C3H6O3), whose molecule has a chiral carbon, which means that there are two optically active isomers: L(+) and D(-)lactic acid. It has wide industrial application, mainly in the food industry; more recently, its application in the polymerized form of poly-lactic acid (PLA) has become indispensable in the cosmetic, pharmaceutical and biomedical industries [1]. The production of those LA active forms depends on the production of the microbial strain, resulting in significantly different properties when polymerized. LA-producing bacteria (LAB) comprise a group of bacteria that produce LA as the main metabolic product, such as the strain Lactobacillus pentosus, which follows the homofermentative route [2]. Other LABs that are known for LA bioconversion with a high rate of production, namely between 99–222 g/L, are as follows: Sporolactobacillus inulinus, L. bulgaricus, L. plantarum, L. delbrueckii and L. rhamnosus [1,3,4]. Several wastes and/or subproducts can be used as substrates to produce LA, such as cheese whey, coffee mucilage, lignocellulosic hydrolysates, urban waste and food waste [1,5,6]. Sugarcane juice is obtained from sugarcane, which is employed in the Brazilian sugar production and alcohol industry. Brazil is the main sugarcane producer, and its 2023/2024 harvest is expected to reach 637.1 million tons [7]. Sugarcane juice is extracted directly from sugarcane through a mechanical grinding process. It comprises phenolic compounds and chlorophyll, a large amount of water (80%), sucrose (17%), glucose (0.4%) and fructose (0.2%) [8,9]. From its solid fraction, nitrogenous substances, fats, pectins, organic acids and ash can be obtained [10]. Sugarcane juice’s composition varies according to the cultivar, harvest time, soil and climate [11]. After submerged fermentation, calcium lactate is the main product created due to the use of sodium carbonate to control the strong inhibition that the microorganism undergoes when the pH decreases throughout the process. The conventional LA recovery process using fermentation broth requires several steps, depending on the employed substrate. The most widespread and industrially applied method consists of a precipitation method using sulfuric acid. Allied to the use of activated carbon, this technique promotes a higher yield (around 60–70%) than other recovery methods, but it still presents losses and leads to the production of undesirable components, such as calcium sulfate [12,13,14].
Ion-exchange resins consist of a flexible polymeric base (phenolic, acrylic or polystyrene divinylbenzene bases) with a loaded functional group. Such functional groups determine the type of resin, the main ones being sulfonic acid, carboxylic and phosphoric groups, which are strong and weak acid-exchange resins, respectively, and quaternary ammonium and amino groups, which are strong and weak acid-exchange resins, respectively [15,16]. When acid resins are used in a medium in which the pH is greater than their pKa value, they carry a negative charge and attract positive compounds, thus creating cationic resins. Following the same reasoning, basic resins are anionic. Strong acid and basic resins have approximate pKas of 1 and 14, respectively, while weak ones have approximate pKas of 4 and 10. Thus, strong resins are applied in most cases, since they are present an ionized form most of the time [17]. The main advantages of this type of resin are its versatility and adsorption capacity, in addition to its lower cost when compared to other types of resin. However, it is sensitive to extreme temperatures [18]. The resins used in this study have characteristics (Table 1) that meet different process requirements.

Table 1.
Characteristics of Amberlite and Purolite resins.
Considering its high availability during the year, and the low cost of acquiring sugarcane juice, it was used as a substrate by the bacteria L. pentosus to produce LA, with a subsequent recovery step involving the use of activated carbon and precipitation with sulfuric acid solution. In an alternative process, the use of ion-exchange resins for LA purification was also proposed, where the performances of resins manufactured by Amberlite and Purolite were compared. The purification systems under agitation, carried out in a tank with mechanical agitation, as well as the system using packed columns, were also compared. The objective of this work was to develop the LA purification process from the use of fermented and recovered broth, and present a feasible option to be transferred to an industrial scale.
2. Materials and Methods
2.1. Material
The sugarcane juice used as the substrate in the LA production process was obtained from a local market in Curitiba, Brazil, mechanically extracted from sugarcane, then subjected to conventional filtration in order to remove suspended solids.
Ion-exchange resins from two brands, Amberlite and Purolite, were used. Amberlite IR-120 and IRA-67 resins were purchased from the Merck group. Purolite C150SH and A847S resins were supplied by the company.
2.2. Microorganism
Lactobacillus pentosus was cultured in MRS broth (Man–Rogosa–Sharpe) at 35 °C for 24 h. After incubation, glycerol was added to the culture for storage at −20 °C, with the periodic renewal of the culture.
2.3. LA Production Using Sugarcane Juice as Substrate
2.3.1. Inoculum
The growth medium and conditions were previously optimized by Oliveira et al. [19]; these were composed of sugarcane juice that was diluted to a concentration of 20 g/L of total reducing sugars and 25 g/L of yeast extract. Inoculum propagation was carried out in a 125 mL Erlenmeyer flask with 75 mL of culture medium and 10% (v/v) of inoculum proportion, maintained at 30 °C under agitation at 120 rpm, for 24 h.
2.3.2. LA Production Kinetics
The medium composition was previously optimized by Oliveira et al. [19]: sugarcane juice at a concentration of 230 g/L of total reducing sugars, 15 g/L of yeast extract, 90 g/L of CaCO3, and inoculation proportion of 10% (v/v). The LA production kinetics were evaluated in 125 mL Erlenmeyer flasks containing 75 mL of culture medium, incubated at 30 °C, andagitated at 120 rpm for 168 h. Samples were withdrawn every 24 h to monitor the concentration of LA produced (g/L), the pH profile and sugar consumption.
2.4. Conventional LA Recovery
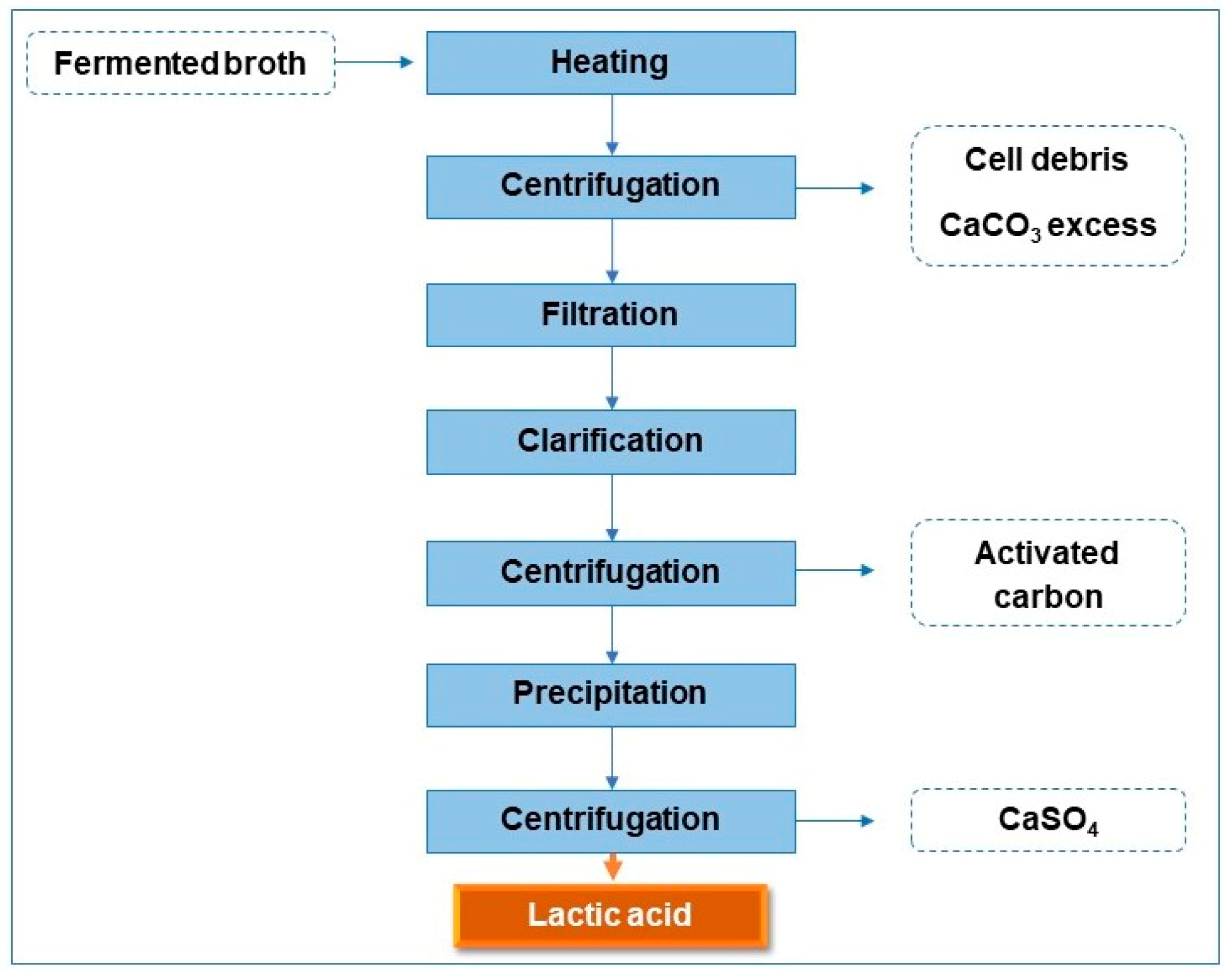
The LA recovery process using the fermented broth containing the form of calcium lactate comprises several steps, as shown in Figure 1.
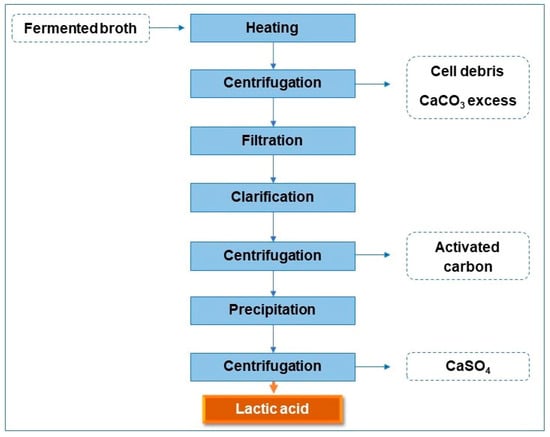
Figure 1.
Steps of LA recovery.
The fermented broth containing calcium lactate was heated in a water bath at a temperature of 50 °C, then centrifuged at 1800× g (times gravity) for 20 min (Centribio, model 80-2B). Then, the centrifuged broth was filtered using a Büchner funnel and Whatman No. 1 filter paper. The conditions described by Oliveira et al. [19] were used for clarification and precipitation processes. The activated carbon proportion was 15% (w/v) considering the fermented broth. Then, the solution was kept in a water bath at 50 °C under agitation at 100 rpm for 25 min. After this time, the activated carbon was removed via centrifugation (1800× g/20 min), and a clarified broth was obtained; this, in turn, passed through acid precipitation with a sulfuric acid solution (4 mol/L) under magnetic stirring until complete homogenization. The solution was then centrifuged again to separate the converted LA from the calcium sulfate.
2.5. LA Purification through Ion-Exchange Resins
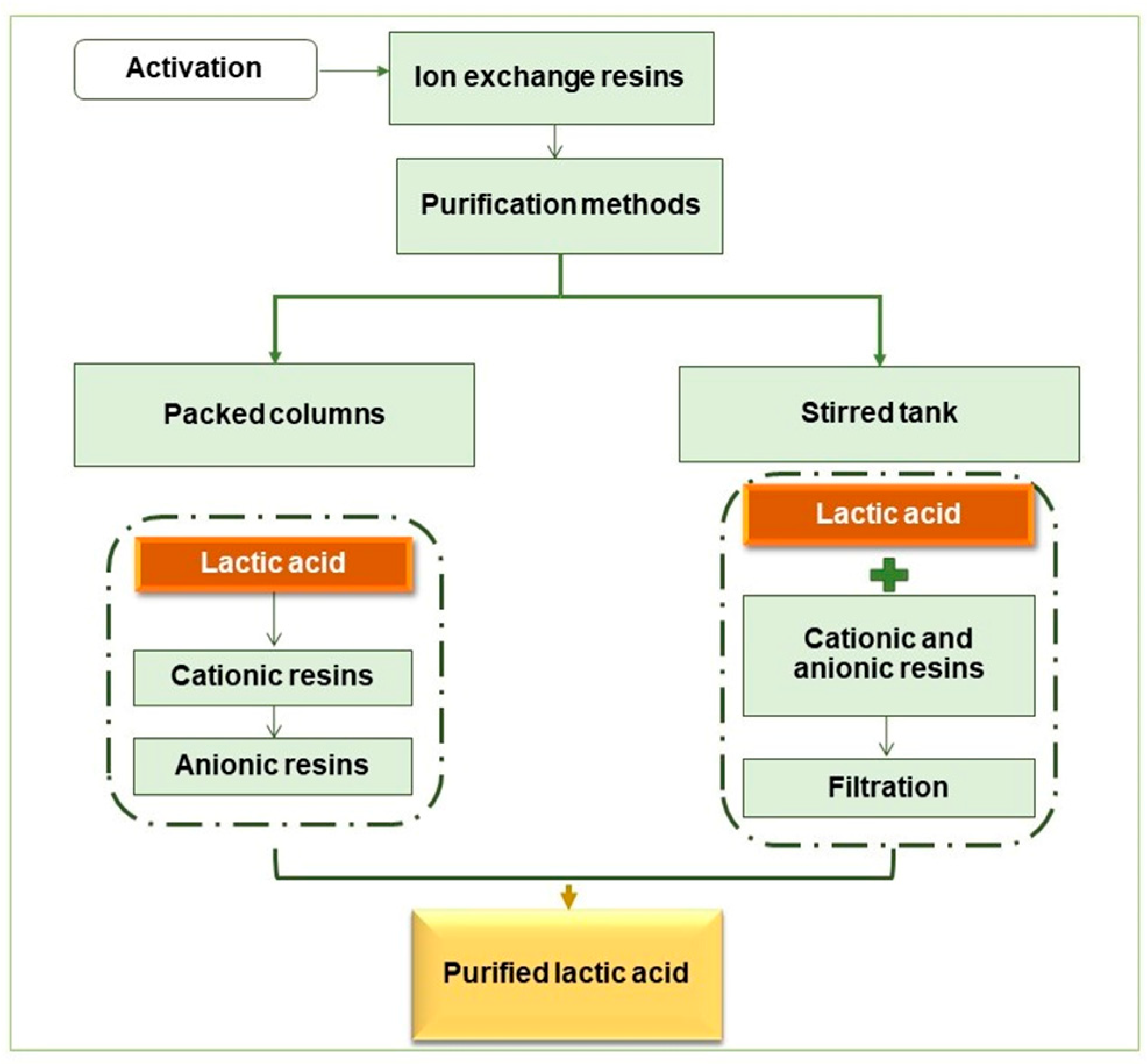
Two LA purification systems using ion-exchange resins were tested (Figure 2), and a protocol provided by the Purolite company was followed regarding the resins’ usage. When necessary, the resins initially underwent an activation procedure.
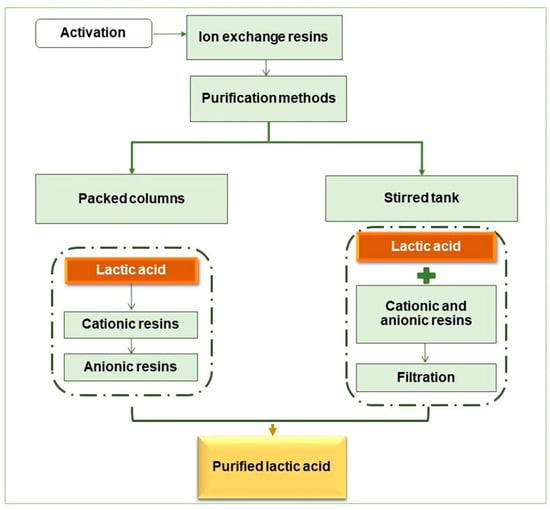
Figure 2.
Diagram of the LA purification steps.
2.5.1. Resin Activation
Amberlite resins: for anionic resins in Cl- form, sequential washes with 1N HCl–deionized water–1N NaOH–deionized water until pH 7. For cationic resins in H+ form, sequential washes with 1N HCl–deionized water until pH 7. Purolite resins: they are available in their active forms, needing successive washings with deionized water until the solution is clear.
2.5.2. LA Purification Using Cationic and Anionic resins (Glass Column)
The glass column (450 mm × 20 mm) contained a minimum resin bed of 30 cm, and each column was packed using deionized water, avoiding the formation of bubbles. The first column was prepared with cationic resins, and the second column was prepared with anionic resins with a flow rate of 0.2 BV/h for LA percolation. Aliquots were withdrawn before percolation and at the end of the procedure to evaluate the pH and LA concentration. After each sample percolation, distilled water was percolated to wash the resins. For this assay, the efficiency of the adsorption process (E) was calculated considering the values of the initial (C0) and final (Ce) concentration of adsorbate.
The adsorption capacity of the resin (q) was calculated considering the initial (C0) and final (Ce) concentration of adsorbate, the solution volume (V) and the resin mass (W).
2.5.3. LA Purification Using Cationic and Anionic Resins Simultaneously (Stirred Tank)
Assays were carried out in a stirred tank (Tecnal TE-054MAG, with Tecnal TE-139 mechanical agitator) equipped with a digital controlling system for temperature and mechanical agitation, a total volume capacity of 2 L, and a propeller-type impeller for axial flow. Samples were kept in contact with both resins, simultaneously, for 120 min, at a temperature of 30 °C and stirring at 100 rpm. After this time, conventional filtration was performed to separate the resins and analyze the LA concentration.
2.6. Analytical Methods
The pH measurement was performed using a potentiometer. The total reducing sugars were determined via the reaction with dinitrosalicylic acid–DNS [20], which was adapted for the determination of the total sugar content; this underwent acid hydrolysis using HCl. L. pentosus is a homofermentative LAB, which prioritizes the production of 2 mols of LA per mol of glucose. Therefore, the yield of the LA fermentation process was calculated from the conversion ratio of the substrate (total sugars) into product (LA), considering the maximum theoretical yield, in molecular weight, of the conversion of glucose into LA, equivalent to 1.0. The LA solution obtained after the precipitation step was eluted in a column (10 mL) of solid-phase extraction packed with 5 g of C18 resin, as a clean-up step prior to chromatography analysis. The procedure consisted of activating the column with methanol (8 mL), washing with MilliQ water (8 mL), sample elution (6 mL), and washing with MilliQ water (20 mL). The samples were analyzed using HPLC equipment (Agilent 1260 Infinity), a Hi-Plex column (300 × 7.7 mm) at 60 °C, an isocratic regime of mobile phase H2SO4 0.005 M, and a flow rate of 0.6 mL/min.
2.7. Statistics
All results are expressed as average ± standard deviation (in triplicate). Analysis of variance (ANOVA) was used to assess the data significance, where p ≤ 0.05 was considered statistically significant. The results were analyzed using the Software Statistica 5.0 (StatSoft, Tulsa, OK, USA).
3. Results and Discussion
3.1. LA Production Kinetics
The L. pentosus strain showed good adaptation to the medium containing sugarcane juice, liable to cell growth and product accumulation. The LA production kinetics were monitored for 168 h; however, from 120 h onwards, a solid calcium lactate was observed, making the recovery and analysis steps more difficult. Samples were withdrawn every 24 h, and in every sample, it was found that the LA concentration in the fermented broth was very low, where the predominant form was the precursor calcium lactate. The best production was reached in 96 h (1.18 g/L∙h), accumulating 113.74 g/L of LA (Table 2).

Table 2.
Data for LA production kinetics over 96 h.
LA concentrations produced from different substrates are found in the literature. Karp et al. [21] reached a productivity of 1.13 g/L∙h using L. agilis, and 138 g/L of LA was produced after optimization using vinasse as a substrate enriched with soy molasses. Bernardo et al. [22] used cheese whey and corn liquor as a fermentation medium for L. rhamnosus B103. At first, they obtained 57 g/L of LA and a productivity of 1.18 g/L∙h; then, LA production was increased to 106 g/L using the fed-batch operation. In optimized synthetic medium, Wang et al. [23] immobilized L. pentosus ATCC 8041 cells in an optimized hydrogel mix. The LA production through free cells was 90 g/L, with a productivity of 1.3 g/L∙h; through immobilized cells, LA production reached 106 g/L and a productivity of 2.2 g/L∙h was obtained. Paulova et al. [24] achieved an LA productivity of 4.0 g/L∙h using chicken feather hydrolysate and L. casei. In addition, de la Torre et al. [25] achieved a high LA productivity of 6.7 g/L∙h using orange peel hydrolysate and L. delbrueckii. It should be noted that in the present work, a high production of LA was achieved using sugarcane juice directly as a substrate, without prior treatment or cell manipulation.
pH control is essential in lactic fermentation, promoting better LA production, yield, and productivity. LAB have a certain sensitivity to changes in the pH of the fermentation medium. When LA is produced, the pH decreases as LA has the ability to dissociate and release protons [12]. According to the pH data obtained during kinetics, CaCO3 was effective in controlling pH variations, supporting the variations that occur in the period between cell maintenance and LA production, with the ideal range being between 6–6.5.
The relationship between a high sugar intake (Table 2) and low LA production in the first 24 h of fermentation indicates the consumption of the available sugars for the energy necessary for cell maintenance (sugar metabolism and pyruvate production). In turn, LAB convert pyruvate into LA, contributing to the decrease in pH, and they may slow down their growth, LA production and sugar consumption [12]. After 48 h, LAB may adapt to the medium conditions via enzymatic regulation mechanisms, allowing them to retake the metabolism and leading to the rapid formation of a solid block of calcium lactate. Li et al. [14] describe a rapid increase in LA production on the third day of fermentation using Lactobacillus strains. From the fourth day, the production begins to fall in a medium composed of food waste. Cirlini et al. [26] also observed log phase cycles during the initial 48 h of fermentation in a screening study of Lactobacillus strains using elderberry juice as the substrate. In this study, there was a clear formation of a solid block of calcium lactate before 120 h when the considerable acidification of the medium occurred, causing analytical difficulties. Therefore, kinetics ended at this time.
3.2. LA Recovery
At the end of fermentation, the calcium lactate present in the fermented broth precipitated; then, this broth was heated to 50 °C in a water bath to remove excess CaCO3 and bacterial cells through a centrifugation step. The supernatant was subjected to clarification using activated carbon in an environment with controlled temperature and agitation; this was in order to remove pigments and organic substances. After centrifugation, the clarified supernatant was subjected to precipitation using a 4 mol/L H2SO4 solution, thus obtaining the LA. The best results for LA production were found in 96 h (Table 2), with 49% of LA recovered; this percentage is compatible with the average found in the literature, from 50% [27]. These values related to the precipitation process demonstrate the need for a later step in LA purification, namely, obtaining a molecule with a high level of purity.
3.3. LA Purification through Ion-Exchange Resins
3.3.1. LA Purification Using Cationic and Anionic resins (Glass Column)
The initially treated sample contained 144.91 g/L of LA, which was converted using sulfuric acid at pH 1. The results of the purification procedures with cationic and anionic resins packed in columns are shown in Table 3.

Table 3.
Data for purified samples in packed columns with ion-exchange resins.
Amberlite’s resins showed better results than Purolite’s resins, with final samples of purified LA of 62.24 g/L and 36.35 g/L, respectively. It is possible that this fact is due to the Amberlite resins being supplied in an inactive form. In this way, there is a guarantee that the resins are pure without contamination, and there is greater control of their activation through sequential washes and pH monitoring. Purolite resins are supplied in their active form, with a pre-washing step being part of the protocol to remove chemical residues and dust. This is a positive point considering the facility and that it reduces the time required to prepare the experiments; however, the quality control is reduced.
Regarding efficiency (Table 4), considering that the sample was in subsequent contact with cationic and anionic resins, on average, the rates obtained were very close between the manufacturers, Amberlite (66.68%) and Purolite (68.85%). The average recovery in LA mass was also close between Purolite (25%) and Amberlite (27%), but these were very low rates. The proper use of resins requires studies of different parameters and different adaptation possibilities. It is known that ion-exchange resins have high selectivity, being able to reach a high LA yield and generate a minimum amount of waste. Cationic resins showed a good adsorption capacity for both manufacturers, Amberlite (165 mg/mL) and Purolite (136 mg/mL), respectively. For the anionic resins, the results were low due to the previous treatment in cationic resins.

Table 4.
The LA mass recovery rates, efficiency and adsorption capacity of resins, in packed columns.
Several types of ion exchangers have been used for LA recovery, and investigations on two-step separation techniques, including the use of anionic and cationic resins, are constant in the literature. Bishai et al. [28] observed that pH directly influences ion exchange. From pH 2–3, the LA did not bind with anionic resins. No binding with the resins was observed when the LA reached its pKa (3.85). The authors also observed that a decrease in purity can be attributed to the release of other contaminating anionic groups in the medium, which can be competitive for ion exchange. Msuya et al. [29] assembled an ion-exchange resin system similar to the system proposed in this work (Section 2.5.2), comprising a cation-exchange resin column connected to an anion-exchange resin column. The authors used the weak anionic resin Indion FFIP (Ion Exchange India Ltd., Mumbai, India) and the strong acid cationic resin Indion 225H (Ion Exchange India Ltd., Mumbai, India), also using water for washing. As a result, a higher LA concentration (108 g/L) was obtained, representing a concentration of 4.5 times the initial concentration (24 g/L) before the process. Arcanjo et al. [30] used an initial LA solution (120 g/L) for recovery in Amberlite IRA-67 and IRA-96 resin, where the total LA recovery was 25.8% (31 g/L). They also tested a binary solution containing LA (345 g/L) and glycerol (230 g/L). LA recovery from this solution was lower than the value obtained for the pure LA solution. Furthermore, the authors concluded that LA and glycerol molecules are similar, which may interfere in the selectivity of ion-exchange resins.
Zaini et al. [31] also started LA purification using a strong acid cationic resin (IRA 120) to convert the lactate salt into LA. Due to the functional groups of sulfonic acid, theoretically, the hydrogen ions (H+) that are linked to the functional group of the resin will bind to the sodium lactate obtained at the end of the fermentation, and convert it into LA. Subsequently, the non-dissociated form of LA obtained via the IRA 120 was kept in contact with the resin IRA-67 (Cl−) for the removal of anions. The pH of the effluent was around 5.5–6.0, indicating that the organic acids present in the fermentation broth (LA and acetic acid) were adsorbed by the resin. After washing the column with water, the LA was desorbed with 0.5M HCl. The first acid to be eluted was acetic acid, followed by LA; thus, competition between organic acids for binding to the functional groups of the resins was observed.
According to the literature data survey, weak base resins are more efficient than strong base resins for LA adsorption. This is mainly because weak base resins have greater resistance to oxidation and organic fouling. Furthermore, the selectivity of the resin for most organic acids is higher due to the elevated number of aromatic rings in styrene resins, making it hydrophobic. Anion-exchange resins generally have different levels of affinity towards the nutrients and compounds available in the medium, which could be the main reason for the inhibitory effects observed towards each different type of anion-exchange resin. The presence of this type of competitive adsorption can therefore reduce the adsorption capacity of the resin by blocking available sites for LA adsorption [15].
3.3.2. LA Purification Using Cationic and Anionic Resins Simultaneously (Stirred Tank)
In this study, the two resins (cationic and anionic) were kept in contact with the LA sample in a stirred tank, and the obtained data are listed in Table 5. After 120 min, conventional filtration was performed. The initial sample (450 mL) contained 144.91 g/L of LA, which was converted using sulfuric acid, with pH 1.

Table 5.
Data for purified samples in stirred tank with ion-exchange resins.
The stirred tank test showed an excellent result (189.11 g/L) when using the Amberlite IR 120 and IRA 67 resins simultaneously in a controlled environment; meanwhile, the Purolite resins showed a lower performance (42.38 g/L). Usually, ion-exchange resins present high rates of LA purification, due to the selectively active groups. Cationic and anionic resins packed in columns achieve between 91 and 98% purification [32]. Under the optimized conditions of batch experiments, anionic resins present between 85 and 90% purification [33,34]. The simultaneous use of cationic and anionic resins is not reported. The purification of samples by means of ionic resins in stirred tanks presents good prospects. The system is temperature-, agitation- and time-controlled, without other concerns regarding the flow rate oscillation; for example, packing glass columns are also prepared with each resin. Also, with agitation using a propeller-type impeller, the sample is not compacted, as the liquid is directed towards the base of the tank, leading to axial circulation, better homogenization and better mass transfer; this demonstrates the adequacy of the sensitive processes, and their ability to provide good heat distribution. In this way, the flow of washing residues is reduced, and the separation between the resins can be carried out using the difference in density for subsequent regeneration, in addition to the use of a smaller volume of solution. This is a much simpler procedure, aimed at removing the residual sulfuric acid and the residual cation for the LA purification, scaling up the process, and jointly using both resins (cationic and anionic). Table 6 briefly compares the costs of each method for LA recovery and purification, in terms of the products used at a lab scale. The total prices are close between the methods applied. Regarding the resins, Purolite has the lowest cost; however, the difference between Purolite resins and Amberlite resins is USD 82.88, which shows better results. At larger quantities, these products may be less expensive. Also, an economic analysis including the energy consumption could be considered for the next study.

Table 6.
Brief price description of products used in LA recovery and purification methods.
4. Conclusions
LA was efficiently produced using sugarcane juice, an alternative substrate, which was favorable to the cell multiplication of LAB L. pentosus. It was observed that the optimal production conditions were reached after four days of fermentation, with satisfactory productivity. The employed substrate does not need to undergo any pretreatment, which means that it can be used directly in submerged fermentation conditions with simple adjustments. In order to purify LA in a single step, both anionic and cationic exchange resins were used together in contact with the sample in a stirred tank. This method is simple and stable in terms of flow rates, time, and temperature. It does not require successive washings with chemical solvents, which reduces the generation of effluents. It allows the use of higher sample volumes in less time, and the resins’ recovery is facilitated through density difference using water as a solvent. This study also provides insights into scalability, and the results at the bench scale show great potential. The usage of combined ionic exchange resins in contact with larger volumes of samples in a stirred tank could be an option for industrial processes. This is feasible considering the operational volumes and controlling conditions. This method can be used for transitioning scales, and future studies concerning the adsorption and efficiency capacities of these resins when operating simultaneously are envisaged. The purified LA must be then carefully analyzed, showing good characteristics for polymerization.
Author Contributions
Conceptualization, P.Z.d.O. and L.P.d.S.V.; methodology, P.Z.d.O. and L.P.d.S.V.; formal analysis, P.Z.d.O. and L.P.d.S.V.; investigation, P.Z.d.O.; writing—original draft preparation, P.Z.d.O.; writing—review and editing; Review, L.P.d.S.V., P.Z.d.O. and L.P.d.S.V.; supervision, L.P.d.S.V.; project administration, L.P.d.S.V.; funding acquisition, L.P.d.S.V. and C.R.S. All authors have read and agreed to the published version of the manuscript.
Funding
National Council for Scientific and Technological Development: Process306336/2022-7; Coordenação de Aperfeicoamento de Pessoal de Nível Superior: CAPES-PROEX.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico do Brasil (CNPq) for the research funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Klotz, S.; Kuenza, A.; Prüßea, U. Nutritional requirements and the impact of yeast extract on the D-lactic acid production by Sporolactobacillus inulinus. Green Chem. 2017, 19, 4633–4641. [Google Scholar] [CrossRef]
- Martinez, F.A.C.; Balciunas, E.M.; Salgado, J.M.; González, J.M.D.; Converti, A.; Oliveira, R.P.S. Lactic acid properties, applications and production: A review. Trends Food Sci. Technol. 2013, 30, 70–83. [Google Scholar] [CrossRef]
- Awasthi, D.; Wang, L.; Rhee, M.S.; Wang, Q.; Chauliac, D.; Ingram, L.O.; Shanmugam, K.T. Metabolic engineering of Bacillus subtilis for production of D-lactic acid. Biotechnol. Bioeng. 2018, 115, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Alexandri, M.; Schneider, R.; Mehlmann, K.; Venus, J. Recent Advances in D-Lactic Acid Production from Renewable Resources: Case Studies on Agro-Industrial Waste Streams. Food Technol. Biotechnol. 2019, 57, 293–304. [Google Scholar] [CrossRef]
- Chandrapala, J.; Duke, M.C.; Gray, S.R.; Weeks, M.; Palmer, M.; Vasiljevic, T. Strategies for maximizing removal of lactic acid from acid whey—Addressing the un-processability issue. Sep. Purif. Technol. 2017, 172, 489–497. [Google Scholar] [CrossRef]
- Orrego, D.; Zapata-Zapata, A.D.; Kim, D. Optimization and Scale-Up of Coffee Mucilage Fermentation for Ethanol Production. Energies 2018, 11, 786. [Google Scholar] [CrossRef]
- CONAB (Companhia Nacional de Abastecimento). Histórico Mensal da Cana-de-Açúcar; CONAB: Brasília, Brazil, 2023. Available online: https://www.conab.gov.br/info-agro/analises-do-mercado-agropecuario-e-extrativista/analises-do-mercado/historico-mensal-de-cana-de-acucar (accessed on 17 August 2023).
- Marasinghege, C.; Broadfoot, R.; Bottle, S.; Bartley, J.; Doherty, W.O.S.; Rackemann, D.W. Investigation on the effect of the heating surface temperature of 1st evaporator on sucrose loss and the degradation of sugarcane juice constituents. J. Food Eng. 2022, 329, 111074. [Google Scholar] [CrossRef]
- Panigrahi, C.; Mishra, H.N.; De, S. Combined ultrafiltration and ozone processing of sugarcane juice: Quantitative assessment of polyphenols, investigation of storage effects by multivariate techniques and shelf-life prediction. Food Chem. Adv. 2023, 2, 100214. [Google Scholar] [CrossRef]
- Tarafdar, A.; Kumar, Y.; Kaur, B.P.; Badgujar, P.C. High-pressure microfluidization of sugarcane juice: Effect on total phenols, total flavonoids, antioxidant activity, and microbiological quality. J. Food Process. Preserv. 2021, 45, e15428. [Google Scholar] [CrossRef]
- Geremias-Andrade, I.M.; Rocheto, A.C.; Gallo, F.A.; Petrus, R.R. The shelf life of standardized sugarcane juice stored under refrigeration. Food Sci. Technol. 2020, 40, 95–101. [Google Scholar] [CrossRef]
- Papadimitriou, K.; Alegría, Á.; Bron, P.A.; de Angelis, M.; Gobbetti, M.; Kleerebezem, M.; Lemos, J.A.; Linares, D.M.; Ross, P.; Stanton, C.; et al. Stress Physiology of Lactic Acid Bacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 837–890. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, W.; Gu, X.; Guo, Z.; Song, J.; Zhu, D.; Liu, Y.; Liu, Y.; Xue, G.; Li, X.; et al. Stabilizing lactate production through repeated batch fermentation of food waste and waste activated sludge. Bioresour. Technol. 2020, 300, 122709. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sadiq, S.; Zhang, W.; Chen, Y.; Xu, X.; Abbas, A.; Chen, S.; Zhang, R.; Xue, G.; Sobotka, D.; et al. Salinity enhances high optically active L-lactate production from co-fermentation of food waste and waste activated sludge: Unveiling the response of microbial community shift and functional profiling. Bioresour. Technol. 2021, 319, 124124. [Google Scholar] [CrossRef]
- Othman, M.; Ariff, A.B.; Kapri, M.R.; Rios-Solis, L.; Halim, M. Growth Enhancement of Probiotic Pediococcus acidilactici by Extractive Fermentation of Lactic Acid Exploiting Anion-Exchange Resin. Front. Microbiol. 2018, 29, 2554. [Google Scholar] [CrossRef] [PubMed]
- Luongo, V.; Palma, A.; Rene, E.R.; Fontana, A.; Pirozzi, F.; Esposito, G.; Lens, P.N.L. Lactic acid recovery from a model of Thermotoga neapolitana fermentation broth using ion exchange resins in batch and fixed-bed reactors. Sep. Sci. Technol. 2019, 54, 1008–1025. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Poulopoulos, S.G. Adsorption and Ion Exchange. In Adsorption, Ion Exchange and Catalysis: Design of Operations and Environmental Applications, 1st ed.; Inglezakis, V.J., Poulopoulos, S.G., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2006; pp. 243–353. [Google Scholar]
- Harrison, R.G.; Todd, P.W.; Rudge, S.R.; Petrides, D.P. Bioseparations Science and Engineering, 2nd ed.; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Oliveira, J.; Vandenberghe, L.P.S.; Oliveira, P.Z.; Mello, A.F.M.; Rodrigues, C.; Nigam, P.S.; Faraco, V.; Soccol, C.R. Bioconversion of potato-processing wastes into an industrially-important chemical lactic acid. Bioresour. Technol. Rep. 2021, 15, 100698. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Karp, S.G.; Igashiyama, A.H.; Siqueira, P.F.; Carvalho, J.C.; Vandenberghe, L.P.S.; Thomaz-Soccol, V.; Coral, J.; Tholozan, J.L.; Pandey, A.; Soccol, C.R. Application of the biorefinery concept to produce L-lactic acid from the soybean vinasse at laboratory and pilot scale. Bioresour. Technol. 2011, 102, 1765–1772. [Google Scholar] [CrossRef]
- Bernardo, M.P.; Coelho, L.F.; Sass, D.C.; Contiero, J. L-(+)-Lactic acid production by Lactobacillus rhamnosus B103 from dairy industry waste. Braz. J. Microbiol. 2016, 47, 640–646. [Google Scholar] [CrossRef]
- Wang, J.; Huang, J.; Laffend, H.; Jiang, S.; Zhang, J.; Ning, Y.; Fang, M.; Liu, S. Optimization of immobilized Lactobacillus pentosus cell fermentation for lactic acid production. Bioresour. Bioprocess. 2020, 7, 15. [Google Scholar] [CrossRef]
- Paulova, L.; Chmelik, J.; Branska, B.; Patakova, P.; Drahokoupil, M.; Melzoch, K. Comparison of Lactic Acid Production by L. casei in Batch, Fed-batch and Continuous Cultivation, Testing the use of Feather Hydrolysate as a Complex Nitrogen Source. Braz. Arch. Biol. Technol. 2020, 63, e20190151. [Google Scholar] [CrossRef]
- de la Torre, I.; Acedos, M.G.; Ladero, M.; Santos, V.E. On the use of resting L. delbrueckii spp. delbrueckii cells for D-lactic acid production from orange peel wastes hydrolysates. Biochem. Eng. J. 2019, 145, 162–169. [Google Scholar] [CrossRef]
- Cirlini, M.; Ricci, A.; Galaverna, G.; Lazzi, C. Application of lactic acid fermentation to elderberry juice: Changes in acidic and glucidic fractions. LWT 2020, 118, 108779. [Google Scholar] [CrossRef]
- Vidra, A.; Németh, Á.; Salgó, A. Factors Affecting Precipitation of Calcium Lactate from Fermentation Broths and from Aqueous Solution. Period. Polytech. Chem. Eng. 2019, 63, 533–540. [Google Scholar] [CrossRef]
- Bishai, M.; De, S.; Adhikari, B.; Banerjee, R. A platform technology of recovery of lactic acid from a fermentation broth of novel substrate Zizyphus oenophlia. 3 Biotech 2015, 5, 455–463. [Google Scholar] [CrossRef]
- Msuya, N.; Minja, R.J.A.; Katima, J.H.Y.; Masanja, E.; Temu, A.K. Separation and Purification of Lactic Acid from Sisal Wastes. Am. J. Chem. 2018, 8, 13–18. [Google Scholar] [CrossRef]
- Arcanjo, M.R.A.; Fernandes, F.A.N.; Silva, I.J. Separation of Lactic Acid Produced by Hydrothermal Conversion of Glycerol Using Ion-Exchange Chromatography. Adsorpt. Sci. Technol. 2015, 33, 139–151. [Google Scholar] [CrossRef]
- Zaini, N.A.M.; Chatzifragkou, A.; Tverezovskiy, V.; Charalampopoulos, D. Purification and polymerisation of microbial D-lactic acid from DDGS hydrolysates fermentation. Biochem. Eng. J. 2019, 150, 107265. [Google Scholar] [CrossRef]
- Ahmad, A.; Othman, I.; Taher, H.; Banat, F. Lactic acid recovery from date pulp waste fermentation broth by ions exchange resins. Environ. Technol. Innov. 2021, 22, 101438. [Google Scholar] [CrossRef]
- Vecino, X.; Reig, M.; Valderrama, C.; Cortina, J.L. Ion-Exchange Technology for Lactic Acid Recovery in Downstream Processing: Equilibrium and Kinetic Parameters. Water 2021, 13, 1572. [Google Scholar] [CrossRef]
- Pleissner, D.; Schneider, R.; Venus, J.; Koch, T. Separation of lactic acid and recovery of salt-ions from fermentation broth. J. Chem. Technol. Biotechnol. 2017, 92, 504–511. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).