Abstract
The objective of this research was to investigate the feasibility of integrating oat (1 → 3, 1 → 4)-β-D-glucan (β-glucan) dried by two different techniques (freeze drying and spray drying) into a synbiotic formulation with Akkermansia muciniphila. The study evaluated the impact of this synbiotic formulation on the growth of A. muciniphila and its effect on the fermentation process. The extracted oat β-glucans underwent freeze-drying (FD) and spray-drying (SD) processes before being introduced as supplementary carbon sources (1%) to brain heart infusion (BHI) medium containing A. muciniphila MSCL 1582. The BHI medium containing inulin, D-glucose, and BHI without added substrates served as the control. Bacterial growth and short-chain fatty acid (SCFA) production were measured before and after 72 h of fermentation. A light microscope and KOVA slides were used for the A. muciniphila count, and SCFA levels were measured via gas chromatography. Our findings revealed that oat β-glucans could effectively function as prebiotic substrates in complementary synbiotic composition with A. muciniphila, without inhibiting growth and causing metabolic impairment. Both FD and SD techniques demonstrated equivalent and favorable impacts on the fermentative capacity of A. muciniphila, rendering them suitable choices for the drying of β-glucans. Incorporating oat β-glucan into synbiotic formulations offers potential benefits, contributing to A. muciniphila growth and the fermentation process.
1. Introduction
The potential to positively impact the composition of gut microbiota in chronic conditions like type 2 diabetes (T2D), obesity, and inflammatory bowel diseases (IBDs) [1,2,3,4,5] is often attributed to probiotics, which are live microorganisms that, when administered in adequate amounts, confer a health benefit on the host [6]. Additionally, this potential is associated with prebiotics, which is a category of nutrients that are metabolized by the gut microbiota [7], and synbiotics, which are a combination of live microorganisms and specific substrate(s) selectively utilized by host microorganisms and confer a health benefit on the host [8]. Synbiotics are classified as either complementary or synergistic formulations [9,10]. Complementary synbiotics combine probiotics and prebiotics, with each component acting independently. On the other hand, synergistic synbiotics enhance the establishment and/or survival of companion microbes, resulting in better clinical outcomes compared to using probiotics or prebiotics alone [11].
This study focuses on oat (1 → 3, 1 → 4)-β-D-glucan (β-glucan), which is a prebiotic polysaccharide [12], and the feasibility of its use in a synbiotic composition with the probiotic bacterium Akkermansia muciniphila [13].
A. muciniphila, known as a new-generation probiotic, is a representative of the Verrucomicrobiota phylum [13] and resides within the intestinal mucus layer [14]. A decrease in A. muciniphila abundance in the gastrointestinal tract has been associated with conditions like type 2 diabetes, obesity, and inflammatory bowel diseases [5,15,16].
A recent study revealed that supplementation of β-glucans from oat bran led to a notable increase in the thickness of colonic mucus in mice, while this effect was not observed with β-glucans from mushrooms and curdlan supplements [17]. Furthermore, the modulation of the intestinal microbiota [18,19,20] and a rise in the prevalence of A. muciniphila [21,22] through the utilization of β-glucan have gained considerable attention in both animal models and clinical investigations.
Β-glucan cannot be absorbed in the small intestine due to the lack of enzymes that break it down. Consequently, it enters the large intestine, where intestinal bacteria facilitate its degradation through enzymatic processes and short-chain fatty acid (SCFA) production [23]. This β-glucan characteristic renders it suitable for application as a prebiotic [12,24] and promising option for synbiotic applications.
Considering the positive effects that both β-glucans and A. muciniphila have on their host, their interactions within a synbiotic composition still pose questions. Structural differences in oat β-glucan may have an impact on microbiota modulation [25]. Furthermore, the influence of diverse processing techniques, such as drying, on the prebiotic characteristics of β-glucans has also not been comprehensively elucidated. The choice of drying method for extracted β-glucans, whether spray drying (SD) or freeze drying (FD), could affect their accessibility to microbial enzymes and, as a result, impact their ability to undergo fermentation. SD and FD are prevalent methods employed for drying β-glucans [26].
An earlier investigation into the drying techniques for yeast β-glucan revealed that FD caused significant microstructure changes and the agglomeration of β-glucan; in contrast, SD β-glucan particles showed minimal reaggregation when resuspended in water [27]. Cereal powders comprising 33%, 18%, and 4% (w/w) (1 → 3, 1 → 4)-β-glucan also had distinct differences in their surface morphology depending both on the drying process (SD or FD) and the dietary fiber level [28]. FD resulted in powders characterized by a flake-like matrix structure, without any particle formation. This matrix morphology remained consistent regardless of the (1 → 3, 1 → 4)-β-glucan content. In contrast, SD produced powders with morphologies that varied depending on the (1 → 3, 1 → 4)-β-glucan content, featuring aggregated particles and microspheres [28].
The wet concentration of β-glucan assumes employing technological processes to remove excess aqueous liquid, typically by evaporating it through SD or FD. Although, it has been reported that the majority of processing procedures lead to certain levels of impairment to the structure of cereal β-glucans [29]. For instance, it has bene suggested that high temperatures can degrade β-glucan to lower molecular weight fragments, change its water holding capacity, or cause capacity displacement [30], subsequently changing its rheological behavior and assumingly reducing beneficial effects [31]. Thus, the apparent advantages, such as low-temperature regimes and the excellent preservation of components, when utilizing FD might not be sufficient enough due to significantly higher production costs compared to ubiquitously employed SD [28]. As a result, the drying method might be considered as a factor altering β-properties, influencing its subsequent utilization characteristics by microorganisms, in particular by A. muciniphila.
The current study discloses the production method of high-purity oat β-glucan and investigates the influence of the drying method of β-glucan on the A. muciniphila growth and fermentation process. Furthermore, the study discusses the feasibility of incorporating oat β-glucan and A. muciniphila into a synbiotic composition. The results contribute valuable insights into enhancing drying techniques for oat β-glucans, opening avenues for innovative applications in functional foods and health enhancement.
2. Materials and Methods
2.1. Extraction and Drying of Oat ß-Glucan
In this study, commercial oat flakes were used with the following composition in dry matter: protein 17.6 g 100 g−1, fats 5.7 g 100 g−1, fiber 2.13 g 100 g−1, carbohydrates 54.1 g 100 g−1, and β-glucan 4.4 g 100 g−1. The oat flakes were dried at a temperature of 65 ± 2 °C in a B5745-5-M incubator (AEG, Germany) for 24 h. Subsequently, the sample of 400 g dried flakes was mixed with 1600 g of 96.4° ethanol and reduced in size by stirring the mixture with the hand mixer Promix (Phillips, Hungary) for 3 min. Ethanol was added to the mixture to a final ratio of oat flakes to ethanol of 1 to 8 by wt. and stirring was maintained (15 s for each 10 min) by the hand mixer for 60 min at the temperature of 30 ± 2 °C. The resulting mixture was filtered through a 0.2 mm sieve and the retained material as coarse brans was collected and air dried in an incubator at 65 ± 2 °C for 24 h, cooled to room temperature, and then packed in polypropylene zip-lock silver bags and stored at room temperature. The aforementioned procedure was repeated until a sufficient amount of 320 g of dried coarse brans was produced, and then, the coarse brans were slurried in the aqueous solution of sodium carbonate at a pH of 9.5 at a ratio of 1 to 10 and continuously stirred for 16 h at 60 ± 2 °C. The slurry was cooled down to room temperature and adjusted to pH 4.5 by the addition of 2 M HCl while the slurry was continuously stirred. The resulting mixture was centrifuged at 1800× g for 15 min by a Hereus Multifuge X3 (Thermo Fisher Scientific, Langenselbold, Germany). The supernatant was adjusted to pH 7.0 by 2.0 M NaOH, and the enzyme α-amylase (SQzyme HSAL, Suntaq International, China) was added at the range of 0.01% by volume (prior to being introduced, the enzyme underwent heat treatment for 10 min at a temperature of 90 ± 2 °C to deactivate side enzymatic activities). Starch hydrolysis was performed at 85 ± 2 °C until an insufficient iodine test result was achieved. The resulting mixture was cooled to a temperature of 65 ± 2 °C and then filtered under vacuum through a Celpure P65 filter bed (Sartorius Lab instruments, Goettingen, DE). The filtered solution was subsequently concentrated four times using the rotary evaporator RE-2000A (Toption, Xi’an, China) at a temperature of 70 ± 1 °C. After concentration, the mixture was cooled down to 5 ± 2 °C and precipitated with ethanol. The ethanol was added at a temperature of −18 ± 2 °C to achieve a final volume corresponding to 25% (w/w), all while continuously stirring. The precipitated material, primarily composed of β-glucan, was carefully decanted after centrifugation at 2000× g for 10 min and re-suspended in deionized water at a temperature of 40 ± 2 °C to form a mixture containing 0.2% dry solids. The mixture was heated to 80 ± 2 °C to allow for sufficient dissolution of the precipitated material and then cooled to 40 ± 2 °C. Subsequently, the mixture was passed through a 20 kDa flat sheet polyamide ultrafiltration module based on cassette elements (PA-20, IPOC NAS, Minsk, Belarus). The four-times concentrated solution was diluted to a 4:1 volume ratio with 40 ± 2 °C deionized water, stirred, and concentrated to a 1/5 volume by the ultrafiltration module. Subsequently, the concentrated solution was subjected to drying using two methods, freeze and spray drying. The concentrate was freeze-dried using a FreeZone freeze-dry system (Labconco, Kansas City, MO, USA) at −51 ± 1 °C under a vacuum of 0.065–0.070 mbar for 72 h. The sample was coded as FD. Alternative drying involved the spray dryer YC-015 (Shanghai Pilotech Instrument Equipment Co., Shanghai, China) controlling the temperature of the exhausting gases at 90 ± 1 °C. The sample was coded as SD. The dried samples were packed in polypropylene zip-lock silver bags and stored at a temperature of −18 ± 1 °C.
The samples for β-glucans content were analyzed according to the McClear and Glennie-Holmes [32] method using a Megazyme assay kit (K-BGLU) for mixed-linkage beta-glucan AOAC 995.16.
The extracted and dried oat β-glucan samples with a concentration of 90% were further used in the fermentation experiment part of the study.
2.2. Microorganism Growth and Fermentation Medium
A. muciniphila MSCL 1582 was stored long term in a liquid nitrogen container (−196 °C) and preincubated in brain heart infusion (Neogen, Lab M. UK) agar [33] under anaerobic conditions (GasPak Anaerobic Pouch, Becton & Dickinson, Sparks, MD, USA) at 37 ± 1 °C for 72 h before the experiment. To each 50 mL bottle, 0.5 mL of A. muciniphila aqueous suspension was added, yielding approximately 5.5 × 106 CFU mL−1.
The fermentation medium in the 50 mL Simax bottles (Kavalierglass, a.s., Sázava Czech Republic) contained 37 g L−1 brain heart infusion broth (BHI, Neogen, Lab M. UK) dissolved in deionized water [33,34]. The prepared liquid fermentation medium was supplemented with either oat β-glucan 10 g L−1, whose purification and drying methods are described above, or the controls, inulin (Bio Planet, Poland) 10 g L−1 and D-glucose 10 g L−1. All samples of the fermentation medium were autoclaved at 121 °C for 15 min. Sterile filtered L-cysteine (0.5 g L−1) was added into all samples after sample autoclaving. The samples were prepared in triplicate.
A. muciniphila was inoculated at 5.5 × 106 CFU mL−1 into each sample of fermentation medium containing oat β-glucans, inulin, glucose, or broth without adding any additional carbon sources. The liquid growth media containing the A. muciniphila strain were incubated in a thermostat at 37 ± 1 °C. In vitro fermentation of all samples was carried out for 72 h under anaerobic conditions. The cultures were sampled at 0 and 72 h for metabolite analysis. After the 72 h incubation period, the triplicates of each sample were transferred to Eppendorf centrifuge tubes and centrifuged at 1100× g for 10 min (Sigma 1-14, Sigma Laborzentrifugen GmbH, Osterode am Harz, Germany). Approximately 0.5 mL of the supernatant was taken, and the triplicates of each sample were stored at −18 °C until the chromatography analysis.
2.3. Determination of A. muciniphila Count Using a Light Microscope and KOVA Slide
Since the viscosity of β-glucans does not allow one to determine the optical density of bacterial growth in the medium, microscopy on KOVA Glasstic slides (KOVA® Glasstic® Slide 10 with grids, Kova International, Inc., Garden Grove, CA, USA) was used to quantify it. This method is quite widely used because it greatly facilitates cell counting [35,36,37]. Bacterial counts were performed before and after 72 h of incubation.
To determine the number of cells in the bacterial suspension, 6.6 μL of the sample was injected into the separate wells of the KOVA slide and analyzed with a microscope (Leica BM E; Leica Microsystems CMS GmbH, Wetzlar,, Germany) at 600x magnification. The number of cells per μL was determined according to the manufacturer’s instructions. The experiments were repeated three times, with each measurement performed in triplicate.
2.4. Quantitative Determination of Short-Chain Fatty Acids
Acetic, butyric, and propionic acid concentrations were determined by gas chromatography (Agilent 7820A) with the flame ionization detection (GC-FID) method [38,39].
The samples for analysis were centrifuged at 1750× g for 4 min. (Sigma 1-14, Germany), filtered through a 0.22 μm Nylon pore-size filter (Millipore, Merck KGaA, Darmstadt, Germany), and diluted with purified water. The short-chain fatty acid (SCFA) concentrations were calculated by substituting the experimentally obtained peak areas into the calibration curve equation.
The external SCFA standards were acetic acid (CH3COOH), CAS: 64-19-7 (ASC reagent, >99.5%), propionic acid (CH3CH2COOH), CAS: 79-09-4 (ASC reagent, >99.5%), butyric acid (C3H7COOH), and CAS: 107-92-6 (ASC reagent, >99.5%), (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany).
All solvents were of analytical grade. Deionized water (18.2 MΩ) was prepared by a Milli-Q water purification system from Millipore (Merck KGaA, Darmstadt, Germany).
According to the reflected data, the regression equation of the trend line was calculated. Standard solutions were injected in triplicate, and the corresponding peak areas were recorded. The relative standard deviation between all solutions was determined to be less than 1.5%.
The calibration curves obtained showed linearity of the determination coefficient (R2 > 0.99) in the used concentration range (0.01–0.1 mg mL−1).
2.5. Statistical Analysis
The Friedman rank sum test was applied by analyzing the median differences among the samples and by analyzing the production of SCFAs and using one-way ANOVA for the microbial population with prior use of the Shapiro–Wilk normality test. Statistical analysis for bacterial growth and the SCFA-produced amount was conducted in R [40]. The figures and data were processed using different packages [41,42,43,44]. RStudio [45] was used for the integrated development environment for R. A coefficient of determination quantifying SCFA (R2) was calculated using Microsoft Excel 2018, p < 0.001. Analyses of SCFA measurements were carried out in triplicate and are expressed as the mean ± SD.
3. Results and Discussion
3.1. Calculation of A. muciniphila Bacterial Populations
Figure 1 illustrates the growth of A. muciniphila in the different samples under examination. The results collectively imply that both FD and SD β-glucans do not exert an antagonistic influence on A. muciniphila. Graphical representation of the statistics and detailed processed data is plotted within Figure 1.
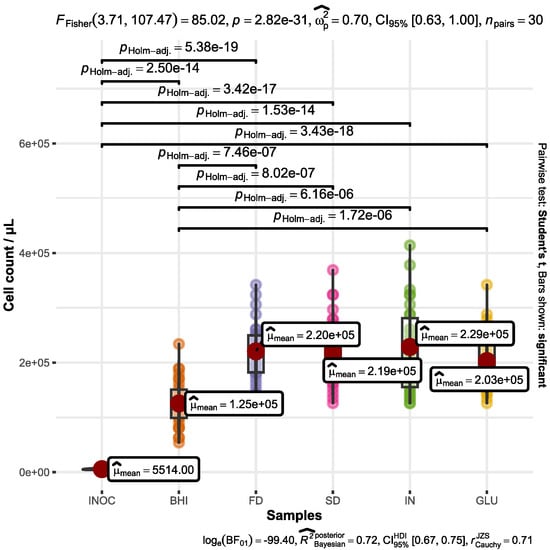
Figure 1.
Quantification of A. muciniphila cells in BHI medium after a 72 h fermentation period. The culture medium was enriched with supplemental substrates, including FD β-glucan (FD), SD β-glucan (SD), inulin (IN), D-glucose (GLU), and a control with no added substrates (BHI). The initial A. muciniphila quantity before fermentation is represented by INOC.
Bacterial growth within the control sample (BHI) exhibited a significantly lower magnitude (p < 0.001) when compared to the samples enriched with FD and SD β-glucans. Moreover, this growth discrepancy was similarly pronounced when compared with the control samples containing inulin (p < 0.001) and D-glucose (p < 0.001). In fact, the results show that the inclusion of an additional carbon source appears to exert a beneficial impact on A. muciniphila growth. This is in line with earlier studies which also indicated that A. muciniphila can grow on limited sugars and synthetic glucose-based media [40]. The highest cell number was determined for microorganisms fermenting media containing inulin which averaged at (2.29 ± 0.78) × 105 bacteria mL−1, while the lowest number was detected at the range (1.24 ± 0.43) × 105 bacteria mL−1 for the control samples. The bacterial growth in the samples containing β-glucan dried by spray and freeze dryers was counted at the rate of (2.18 ± 0.60) × 105 and (2.20 ± 0.51) × 105 bacteria mL−1, respectively.
The literature has shown that static cultivation could lead to metabolite accumulation, inhibiting A. muciniphila growth at concentrations above 80 mMol L−1 [46]. However, our chromatographic findings suggested that fatty acid production in the chosen substrates did not reach such levels.
A previous study demonstrated A. muciniphila’s log growth phase at 8–12 h in BHI, followed by a stable phase at 48 h [47]. In our research, bacterial growth and metabolite measurements were assessed after a 72 h fermentation period, mirroring a similar approach taken in another study where A. muciniphila was cultivated in BHI broth for a duration of 72 h [48].
3.2. Quantitative Determination of Propionic Acid
The initial concentration of propionic acid was 2 mg L−1 in the medium of all of the samples analyzed before commencing the fermentation process. Following a fermentation period of 72 h, there was a statistically significant difference in the amount of propionic acid produced across all samples (p < 0.05). The processed statistical data are plotted in Figure 2.
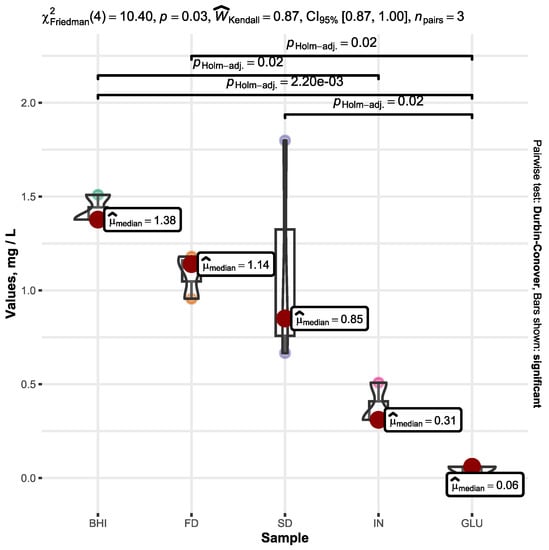
Figure 2.
Increase in the propionic acid concentration within the BHI medium containing A. muciniphila after a 72 h fermentation duration. The cultivation media were enriched with supplemental substrates, including FD β-glucan (FD), SD β-glucan (SD), inulin (IN), and D-glucose (GLU) alongside unaltered broth (BHI). The graph shows only the increase in propionic acid (mg L−1) throughout the fermentation process, omitting the initial quantity of propionic acid present in the BHI medium prior to fermentation. Only significant values are represented by the bars.
The rise in propionic acid levels within the medium, which was enriched with D-glucose (GLU), exhibited the smallest increment compared to all of the samples. This increase was notably and significantly less than that observed in the control BHI sample, whose median was determined to be 1.38 mg L−1, while the mean averaged at 1.42 ± 0.08 mg L−1. The increase in propionic acid in the FD and SD samples averaged at 1.09 ± 0.12 mg L−1 and 1.11 ± 0.61 mg L−1, respectively.
Although the propionic acid growth in the BHI sample exhibited the most elevated values, surpassing those in the control group containing the prebiotic inulin (IN) with statistical significance of p < 0.05, it is noteworthy that both FD and SD demonstrated the subsequent highest propionic acid levels following BHI. No statistically significant distinctions were observed in the quantities of propionic acid among the BHI, FD, and SD samples.
Notably, our study employed static fermentation conditions, which might have resulted in lower acid production. Research simulating intestinal motility showed increased acid production by A. muciniphila during motility [47], implying that optimizing in vitro culture conditions for A. muciniphila under dynamic digestion models could be a promising avenue.
Our investigation revealed that during in vitro fermentation carried out in BHI broth enriched with β-glucans, inulin, and D-glucose, the presence of both FD and SD β-glucans did not have an adverse impact on the production of propionic acid by the companion microorganism A. muciniphila. Furthermore, our studied samples contained SD and FD β-glucans and exhibited a statistically significant higher increase in propionic acid compared to the sample containing D-glucose. While, at that time, another prebiotic, inulin, did not show such a positive result.
As summarized above, the chromatographic data elucidate that the presence of A. muciniphila in the BHI fermentation medium, supplemented by either SD or FD oat β-glucan, showed positive bacterial metabolic activity, producing propionic acid.
The beneficial effects of propionic acid have been extensively studied. The recent study showed that propionic acid exhibits anti-inflammatory effects on human subcutaneous adipose tissue, which is accompanied by the improved expression of parameters associated with lipogenesis and glucose uptake [49]. Another clinical trial involving individuals with metabolic syndrome demonstrated that cereal β-glucan functions as a prebiotic, resulting in the increased presence of beneficial Bifidobacterium spp. and A. muciniphila as well as higher levels of propionic acid in feces [21,22]. This effect was observed after a four-week intervention study where the participants consumed 6 g of barley β-glucan within bread.
3.3. Quantitative Determination of Acetic Acid
Figure 3 illustrates the increase in acetic acid in the different samples under examination.
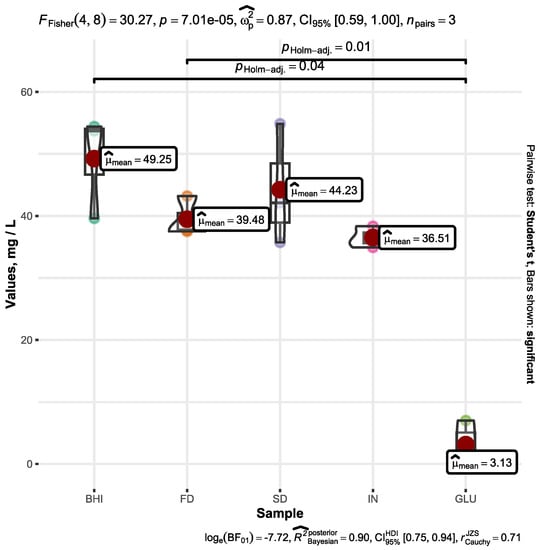
Figure 3.
Increase in the acetic acid concentration within the BHI medium containing A. muciniphila after a 72 h fermentation duration. The cultivation media were enriched with supplemental substrates, including FDβ-glucan (FD), SDβ-glucan (SD), inulin (IN), and D-glucose (GLU) alongside unaltered broth (BHI). The graph shows only the increase in acetic acid (mg L−1) throughout the fermentation process, omitting the initial quantity of acetic acid present in the growth medium prior to fermentation. Only significant values are represented by the bars.
The acetic acid’s initial concentration in the original BHI medium before commencing the fermentation process was determined to be 159 mg L−1. In the case of FD, the concentration was 152 mg L−1, in IN, it was 158 mg L−1, for GLU, it was 208 mg L−1, and for SD, it was 154 mg L−1.
Following a fermentation period of 72 h, the increased acetic acid amount was determined to be in the range of 49.25 ± 8.35 mg L−1 for BHI, 39.48 ± 3.22 mg L−1 for FD, 44.23 ± 9.74 mg L−1 for SD, 36.51 ± 1.71 mg L−1 for IN, and 3.13 ± 3.87 mg L−1 for GLU. Graphical representation of the statistics and detailed processed data is plotted in Figure 3.
The conducted Fisher’s one-way ANOVA showed that there were statistically significant differences across the tested samples (p < 0.001). The acetic acid concentration within the control sample (BHI) exhibited a significantly higher magnitude (p < 0.05) when compared to the sample enriched with D-glucose. Similarly, the heightened acetic acid concentration following 72 h of fermentation exhibited a noteworthy increase in the FD sample compared to GLU (p < 0.05). Statistically insignificant variations were observed in the acetic acid increase among the remaining samples.
The collective results indicate that the fermentation process of A. muciniphila is unaltered by both FD and SD β-glucans. Our findings demonstrated that the addition of D-glucose to the BHI medium with A. muciniphila led to a reduced increase in the acetic acid concentration compared to both the D-glucose-absent BHI medium and the BHI medium supplemented with FD β-glucans. It is worth noting the fact that the introduced monosaccharides suppressed the production of acetic acid by A. muciniphila. While the utilization of β-glucan by A. muciniphila in the fermentation process remains uncertain, we could speculate that the higher polymerization level of polysaccharides could beneficially affect the production of acetic acid by A. muciniphila. In addition, an earlier study indicated that the drying process, in particular FD, did not significantly affect the initial molecular weight nor solubility of β-glucan, which was derived from native oat bran or oatmeal [50]. However, in a recent investigation, a significant structural difference between oat β-glucan powders microencapsulated by the different drying methods (freeze and spay drying) was revealed. The authors highlighted that the SD method had a more favorable impact than FD due to the increased yield of β-glucan powder and improved the functional properties related to bioactive compound content [26]. Such an observation might facilitate the selection of a proper microencapsulation method for β-glucan as the difference between the investigated samples in terms of producing acetic acid was insignificant. However, the production of sole SCFA should not be considered as a determining factor. Previous studies have indicated that A. muciniphila possesses the capacity to synthesize acetic and propionic acids simultaneously, which were identified as substrates for both the microbiota and the host organism [49,50]. These compounds take on a crucial regulatory role by regulating the modulation of transcription factors, the control of cell cycle dynamics, the facilitation of lipolytic processes, and the complex regulation of satiety mechanisms [51,52]. In addition, in a recent study conducted on rats, it was observed that A. muciniphila played a contributory role in ameliorating age-associated ailments, facilitated by both A. muciniphila and its derived acetic acid [53].
3.4. Quantitative Determination of Butyric Acid
The butyric acid’s starting concentration was 8 mg L−1 in both the original BHI medium and all of the examined samples before starting the fermentation procedure.
After a fermentation duration of 72 h, a reduction in butyric acid in all of the samples was observed, with no statistically significant variation among the samples. Despite the relatively high dispersion, the lowest and highest amounts of the produced butyric acid were determined in the FD and BHI samples, which averaged at −1.17 ± 0.68 mg L−1 and −0.70 ± 0.54 mg L−1, respectively. Graphical representation of the statistics and detailed processed data is plotted in Figure 4.
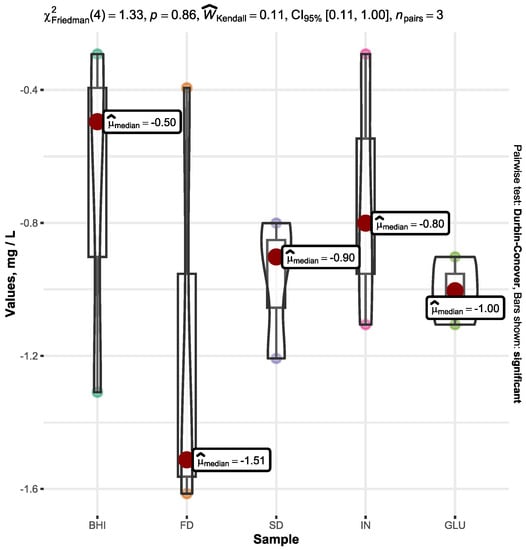
Figure 4.
Change in butyric acid content after 72 h of fermentation in BHI media with A. muciniphila depending on the added substrate: FD β-glucan (FD), SD β-glucan (SD), inulin (IN), D-glucose (GLU), and broth without any added substrate (BHI). The graph shows a decrease in butyric acid (mg L−1) compared to the original butyric acid amount in the medium before starting fermentation. Only significant values are represented by the bars.
The decline in butyric acid content within the samples suggests that the semi-starved condition of A. muciniphila is capable of utilizing butyric acid as a source of energy.
A previous study showed that the concentrations of butyric acid were significantly lower under in vitro static culture compared to dynamic culture (p < 0.05) [46]. Since our study used static culture, it is possible that there was no increase in butyric acid.
Further investigations are needed to determine whether A. muciniphila utilizes butyric acid in semi-starvation conditions. However, it is important to note that the process of fermentatively breaking down SCFAs like butyric acid presents a significant challenge. The conversion of these compounds into acetate, CO2, formate, and hydrogen requires a substantial amount of energy under normal conditions [54]. The degradation pathways for butyrate involve oxidation steps with comparatively high redox potentials [54].
4. Conclusions
In conclusion, our study’s findings indicate the feasibility of employing A. muciniphila in combination with β-glucan within a complementary synergistic composition. Notably, FD and SD β-glucans exhibit neither antagonistic effect on bacterial growth nor an inhibitory influence on the production of propionic and acetic acids.
Our study’s results suggests that FD and SD can potentially serve as protective agents for A. muciniphila growth and as additional substrates in a complementary synbiotic composition.
Subsequent investigations should incorporate dynamic culture conditions to assess bacterial metabolic activity, including the efficacy of butyric acid production.
We assume that the incorporation of oat FD and SD β-glucan into complementary synbiotic formulations alongside A. muciniphila holds promise for several advantageous outcomes. Primarily, oat β-glucan serves as a fermentable substrate for beneficial gut bacteria and potentially acts as a protective agent for A. muciniphila. Additionally, the immunomodulatory properties of oat β-glucan align with the anti-inflammatory properties of A. muciniphila, suggesting a potential synergy. Nevertheless, the successful integration of this approach necessitates addressing challenges encompassing formulation stability, compatibility, and the requisite exploration of optimal dosages and delivery mechanisms.
Author Contributions
Conceptualization, V.S.; methodology, V.S., D.S., A.P. and V.N.; software, D.S.; validation, A.P. and V.N.; formal analysis, V.S.; investigation, V.S.; resources, V.S., I.J. and V.N.; data curation, V.S. and V.N.; writing—original draft preparation, V.S.; writing—review and editing, V.N.; visualization, V.S. and D.S.; supervision, I.J. and V.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
We extend our appreciation to Jelena Dzjubenko and Viktorija Kapura, two students who provided valuable assistance in carrying out the laboratory experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ouyang, J.; Lin, J.; Isnard, S.; Fombuena, B.; Peng, X.; Marette, A.; Routy, B.; Messaoudene, M.; Chen, Y.; Routy, J.-P. The Bacterium Akkermansia muciniphila: A Sentinel for Gut Permeability and Its Relevance to HIV-Related Inflammation. Front. Immunol. 2020, 11, 645. [Google Scholar] [CrossRef] [PubMed]
- Yassour, M.; Lim, M.Y.; Yun, H.S.; Tickle, T.L.; Sung, J.; Song, Y.-M.; Lee, K.; Franzosa, E.A.; Morgan, X.C.; Gevers, D.; et al. Sub-Clinical Detection of Gut Microbial Biomarkers of Obesity and Type 2 Diabetes. Genome Med. 2016, 8, 17. [Google Scholar] [CrossRef]
- Van Immerseel, F.; Ducatelle, R.; De Vos, M.; Boon, N.; Van De Wiele, T.; Verbeke, K.; Rutgeerts, P.; Sas, B.; Louis, P.; Flint, H.J. Butyric Acid-Producing Anaerobic Bacteria as a Novel Probiotic Treatment Approach for Inflammatory Bowel Disease. J. Med. Microbiol. 2010, 59, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Liu, Z.; Zhu, C.; Mou, H.; Kong, Q. Nondigestible Carbohydrates, Butyrate, and Butyrate-Producing Bacteria. Crit. Rev. Food Sci. Nutr. 2019, 59, S130–S152. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Siles, M.; Enrich-Capó, N.; Aldeguer, X.; Sabat-Mir, M.; Duncan, S.H.; Garcia-Gil, L.J.; Martinez-Medina, M. Alterations in the Abundance and Co-Occurrence of Akkermansia muciniphila and Faecalibacterium Prausnitzii in the Colonic Mucosa of Inflammatory Bowel Disease Subjects. Front. Cell. Infect. Microbiol. 2018, 8, 281. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Megur, A.; Daliri, E.B.-M.; Baltriukienė, D.; Burokas, A. Prebiotics as a Tool for the Prevention and Treatment of Obesity and Diabetes: Classification and Ability to Modulate the Gut Microbiota. Int. J. Mol. Sci. 2022, 23, 6097. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Kolida, S.; Gibson, G.R. Synbiotics in Health and Disease. Annu. Rev. Food Sci. Technol. 2011, 2, 373–393. [Google Scholar] [CrossRef]
- Li, Y.; Qin, C.; Dong, L.; Zhang, X.; Wu, Z.; Liu, L.; Yang, J.; Liu, L. Whole Grain Benefit: Synergistic Effect of Oat Phenolic Compounds and β-Glucan on Hyperlipidemia via Gut Microbiota in High-Fat-Diet Mice. Food Funct. 2022, 13, 12686–12696. [Google Scholar] [CrossRef]
- Quintero, D.F.G.; Kok, C.R.; Hutkins, R. The Future of Synbiotics: Rational Formulation and Design. Front. Microbiol. 2022, 13, 919725. [Google Scholar] [CrossRef] [PubMed]
- Sivieri, K.; Oliveira, S.M.; Souza Marquez, A.; Pérez-Jiménez, J.; Diniz, S.N. Insights on β-Glucan as a Prebiotic Coadjuvant in the Treatment of Diabetes Mellitus: A Review. Food Hydrocoll. Health 2022, 2, 100056. [Google Scholar] [CrossRef]
- Jian, H.; Liu, Y.; Wang, X.; Dong, X.; Zou, X. Akkermansia muciniphila as a Next-Generation Probiotic in Modulating Human Metabolic Homeostasis and Disease Progression: A Role Mediated by Gut–Liver–Brain Axes? Int. J. Mol. Sci 2023, 24, 3900. [Google Scholar] [CrossRef]
- Liu, M.-J.; Yang, J.-Y.; Yan, Z.-H.; Hu, S.; Li, J.-Q.; Xu, Z.-X.; Jian, Y.-P. Recent Findings in Akkermansia muciniphila-Regulated Metabolism and Its Role in Intestinal Diseases. Clin. Nutr. 2022, 41, 2333–2344. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Q.; Cheng, L.; Buch, H.; Zhang, F. Akkermansia muciniphila Is a Promising Probiotic. Microb. Biotechnol. 2019, 12, 1109–1125. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-Talk between Akkermansia muciniphila and Intestinal Epithelium Controls Diet-Induced Obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, P.; Wang, R.; Zhou, M.; Pang, N.; Cui, X.; Ge, X.; Liu, X.; Huang, X.-F.; Yu, Y. Three Different Types of β-Glucans Enhance Cognition: The Role of the Gut-Brain Axis. Front. Nutr. 2022, 9, 848930. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Huang, X.; Wang, H.; Geng, F.; Nie, S. Effect of β-Glucan on Metabolic Diseases: A Review from the Gut Microbiota Perspective. Curr. Opin. Food Sci. 2022, 100907, 2214–7993. [Google Scholar] [CrossRef]
- Singh, R.P.; Bhardwaj, A. β-glucans: A potential source for maintaining gut microbiota and the immune system. Front. Nutr. 2023, 10, 1143682. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.M.; London, L.E.E.; Bjorndahl, T.C. Microbiome and Metabo-Lome Modifying Effects of Several Cardiovascular Disease Interventions in Apo-E−/− Mice. Microbiome 2017, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Liu, Y.; Wang, J.; Wang, X.; Tang, B.; Liu, M.; Liu, X. β-Glucan-Triggered Ak-Kermansia Muciniphila Expansion Facilitates the Expulsion of Intestinal Helminth via TLR2 in Mice. Carbohydr. Polym. 2021, 275, 118719. [Google Scholar] [CrossRef] [PubMed]
- Velikonja, A.; Lipoglavšek, L.; Zorec, M.; Orel, R.; Avguštin, G. Alterations in Gut Microbiota Composition and Metabolic Parameters after Dietary Intervention with Barley Beta Glucans in Patients with High Risk for Metabolic Syndrome Development. Anaerobe 2019, 55, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Aoe, S.; Mio, K.; Yamanaka, C.; Kuge, T. Low Molecular Weight Barley β-Glucan Affects Glucose and Lipid Metabolism by Prebiotic Effects. Nutrients 2020, 13, 130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, L.; Liu, L.; Wu, Z.; Pan, D.; Liu, L. Recent Advances of Stimuli-Responsive Polysaccharide Hydrogels in Delivery Systems: A Review. J. Agric. Food Chem. 2022, 70, 6300–6316. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Xia, Q.; Liu, L.; Wu, Z.; Pan, D. Recent Advances of Cereal β-Glucan on Immunity with Gut Microbiota Regulation Functions and Its Intelligent Gelling Application. Crit. Rev. Food Sci. Nutr. 2023, 63, 3895–3911. [Google Scholar] [CrossRef] [PubMed]
- Valková, V.; Ďúranová, H.; Falcimaigne-Cordin, A.; Rossi, C.; Nadaud, F.; Nesterenko, A.; Moncada, M.; Orel, M.; Ivanišová, E.; Chlebová, Z. Impact of Freeze- and Spray-Drying Microencapsulation Techniques on β-Glucan Powder Biological Activity: A Comparative Study. Foods 2022, 11, 2267. [Google Scholar] [CrossRef] [PubMed]
- Avramia, I.; Amariei, S. Spent Brewer’s Yeast as a Source of Insoluble β-Glucans. Int. J. Mol. Sci. 2021, 22, 825. [Google Scholar] [CrossRef]
- Chronakis, I.S.; Öste Triantafyllou, A.; Öste, R. Solid-State Characteristics and Redispersible Properties of Powders Formed by Spray-Drying and Freeze-Drying Cereal Dispersions of Varying (1→3,1→4)-β-Glucan Content. J. Cereal Sci. 2004, 40, 183–193. [Google Scholar] [CrossRef]
- Henrion, M.; Francey, C.; Lê, K.A.; Lamothe, L. Cereal B-Glucans: The Impact of Processing and How It Affects Physiological Responses. Nutrients 2019, 11, 1729. [Google Scholar] [CrossRef]
- Oliveira, L.D.C.; Oliveira, M.; Meneghetti, V.L.; Mazzutti, S.; Colla, L.M.; Elias, M.C.; Gutkoski, L.C. Effect of Drying Temperature on Quality of β-Glucan in White Oat Grains. Food Sci. Technol. 2012, 32, 775–783. [Google Scholar] [CrossRef]
- Butt, M.S.; Tahir-Nadeem, M.; Khan, M.K.I.; Shabir, R.; Butt, M.S. Oat: Unique among the Cereals. Eur. J. Nutr. 2008, 47, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Mcclear, B.V.; Glennie-Holmes, M. Enzymic Quantification of (1→3)(1→4)-β-D-Glucan in Barley and Malt. J. Inst. Brew. 1985, 91, 285–295. [Google Scholar] [CrossRef]
- Derrien, M.; Vaughan, E.E.; Plugge, C.M.; Vos, W.M. Akkermansia muciniphila Gen. Nov., Sp. Nov., a Human Intestinal Mucin-Degrading Bacterium. Int. J. Syst. Evol. Microbiol. 2004, 54, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Ouwerkerk, J.P.; Ark, K.C.H.; Davids, M.; Claassens, N.J.; Finestra, T.R.; Vos, W.M.; Belzer, C. Adaptation of Akkermansia muciniphila to the Oxic-Anoxic Inter-Face of the Mucus Layer. Appl. Environ. Microbiol. 2016, 82, 6983–6993. [Google Scholar] [CrossRef]
- Fowler, R.S. Quantification of normal vaginal constituents using a new wet preparation technique. J. Low. Genit. Tract Dis. 2012, 16, 437–441. [Google Scholar] [CrossRef]
- Emerson, J.F.; Emerson, S.S. Evaluation of a Standardized Procedure for Counting Microscopic Cells in Body Fluids. J. Clin. Lab. Anal. 2005, 19, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Piccinini, F.; Tesei, A.; Arienti, C.; Bevilacqua, A. Cell Counting and Viability Assessment of 2D and 3D Cell Cultures: Expected Reliability of the Trypan Blue Assay. Biol. Proced. Online 2017, 19, 8. [Google Scholar] [CrossRef]
- Zhang, C.; Fan, L.; Zhao, H. Rapid Detection of Short-Chain Fatty Acids in Biological Samples. Chromatographia 2019, 83, 305–310. [Google Scholar] [CrossRef]
- Kim, H.; Kwon, J.; Choi, S.Y. Method Development for the Quantitative Determination of Short Chain Fatty Acids in Microbial Samples by Solid Phase Extraction and Gas Chromatography with Flame Ionization Detection. J. Anal. Sci. Technol. 2019, 10, 28. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Graves, S.; Piepho, H.-P.; Selzer, L.; Dorai-Raj, S. multcompView: Visualizations of Paired Comparisons. 2023. Available online: https://cran.r-project.org/web/packages/multcompView/index.html (accessed on 10 August 2023).
- Kassambara, A. ggpubr: “ggplot2” Based Publication Ready Plots. 2023. Available online: https://rpkgs.datanovia.com/ggpubr/ (accessed on 10 August 2023).
- Patil, I. Visualizations with Statistical Details: The “Ggstatsplot” Approach. J. Open Source Softw. 2021, 6, 3167. [Google Scholar] [CrossRef]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Team, R. RStudio: Integrated Development Environment for R; RStudio; PBC: Boston, MA, USA, 2022. [Google Scholar]
- Li, Z.; Hu, G.; Zhu, L. Study of Growth, Metabolism, and Morphology of Akkermansia muciniphila with an in Vitro Advanced Bionic Intestinal Reactor. BMC Microbiol. 2021, 21, 61. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhu, L.; Hu, G.; Sun, Z.; Zhan, X.; Gao, M. Akkermansia muciniphila Fermentation Culture Based on a Novel Bionic Large Intestine Dynamic Digestion Model. Food Biosci. 2021, 43, 101260. [Google Scholar] [CrossRef]
- Daniel, N.; Gewirtz, A.T.; Chassaing, B. Akkermansia Muciniphila Counteracts the Deleterious Effects of Dietary Emulsifiers on Microbiota and Host Metabolism. Gut 2023, 72, 906–917. [Google Scholar] [CrossRef]
- Al-Lahham, S.; Rezaee, F. Propionic Acid Counteracts the Inflammation of Human Subcutaneous Adipose Tissue: A New Avenue for Drug Development. DARU J. Pharm. Sci. 2019, 27, 645–652. [Google Scholar] [CrossRef]
- Gamel, T.H.; Badali, K.; Tosh, S.M. Changes of β-Glucan Physico-Chemical Characteristics in Frozen and Freeze Dried Oat Bran Bread and Porridge. J. Cereal Sci. 2013, 58, 104–109. [Google Scholar] [CrossRef]
- Lukovac, S.; Belzer, C.; Pellis, L.; Keijser, B.J.; Vos, W.M.; Montijn, R.C.; Roeselers, G. Differential Modulation by Akkermansia muciniphila and Faecalibacterium prausnitzii of Host Peripheral Lipid Metabolism and Histone Acetylation in Mouse Gut Organoids. mBio 2014, 5, e01438-14. [Google Scholar] [CrossRef]
- Rodrigues, V.F.; Elias-Oliveira, J.; Pereira, Í.S.; Pereira, J.A.; Barbosa, S.C.; Machado, M.S.G.; Carlos, D. Akkermansia muciniphila and Gut Immune System: A Good Friendship That Attenuates Inflammatory Bowel Disease, Obesity, and Diabetes. Front. Immunol. 2022, 13, 934695. [Google Scholar] [CrossRef]
- Ma, J.; Liu, Z.; Gao, X.; Bao, Y.; Hong, Y.; He, X.; Zhu, W.; Li, Y.; Huang, W.; Zhen, N. Gut Microbiota Remodeling Improves Natural Aging-Related Disorders through Akkermansia muciniphila and Its Derived Acetic Acid. Pharmacol. Res. 2023, 189, 106687. [Google Scholar] [CrossRef]
- Müller, N.; Worm, P.; Schink, B.; Stams, A.J.; Plugge, C.M. Syntrophic Butyrate and Propionate Oxidation Processes: From Genomes to Reaction Mechanisms. Environ. Microbiol. Rep. 2010, 2, 489–499. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).