Syngas Fermentation: Cleaning of Syngas as a Critical Stage in Fermentation Performance
Abstract
:1. Introduction
2. Gasification/Pyrolysis Technologies
3. Syngas Fermentation
3.1. Fundamentals of Syngas Fermentation
3.2. Experimental Work and Integration with Biomass Gasification
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Munasinghe, P.C.; Khanal, S.K. Biomass-derived syngas fermentation into biofuels: Opportunities and challenges. Bioresour. Technol. 2010, 101, 5013–5022. [Google Scholar] [CrossRef] [PubMed]
- European Commission: Circular Economy. The EU Aims to Transition to a Circular Economy to Make Europe Cleaner and More Competitive. Available online: https://environment.ec.europa.eu/topics/circular-economy_en (accessed on 8 August 2023).
- Geissdoerfer, M.; Savaget, P.; Bocken, N.M.P.; Hultink, E.J. The Circular Economy—A new sustainability paradigm? J. Clean. Prod. 2017, 143, 757–768. [Google Scholar] [CrossRef]
- Hazen, B.T.; Russo, I.; Confente, I.; Pellathy, D. Supply chain management for circular economy: Conceptual framework and research agenda. Int. J. Logist. Manag. 2021, 32, 510–537. [Google Scholar] [CrossRef]
- Koval, V.; Arsawan, I.W.; Suryantini, N.P.; Kovbasenko, S.; Fisunenko, N.; Aloshyna, T. Circular economy and sustainability-oriented innovation: Conceptual framework and energy future avenue. Energies 2022, 16, 243. [Google Scholar] [CrossRef]
- Ellacuriaga, M.; García-Cascallana, J.; Gómez, X. Biogas Production from Organic Wastes: Integrating Concepts of Circular Economy. Fuels 2021, 2, 144–167. [Google Scholar] [CrossRef]
- Steinmann, Z.J.N.; Huijbregts, M.A.J.; Reijnders, L. How to define the quality of materials in a circular economy? Resour. Conserv. Recycl. 2019, 141, 362–363. [Google Scholar] [CrossRef]
- Sevillano, C.A.; Pesantes, A.A.; Peña Carpio, E.; Martínez, E.J.; Gómez, X. Anaerobic Digestion for Producing Renewable Energy—The Evolution of This Technology in a New Uncertain Scenario. Entropy 2021, 23, 145. [Google Scholar] [CrossRef]
- Herwig, H. How to Teach Heat Transfer More Systematically by Involving Entropy. Entropy 2018, 20, 791. [Google Scholar] [CrossRef]
- Akhtar, A.; Krepl, V.; Ivanova, T. A Combined Overview of Combustion, Pyrolysis, and Gasification of Biomass. Energy Fuels 2018, 32, 7294–7318. [Google Scholar] [CrossRef]
- Elorf, A.; Kandasamy, J.; Belandria, V.; Bostyn, S.; Sarh, B.; Gökalp, I. Heating rate effects on pyrolysis, gasification and combustion of olive waste. Biofuels 2021, 12, 1157–1164. [Google Scholar] [CrossRef]
- Panepinto, D.; Tedesco, V.; Brizio, E.; Genon, G. Environmental Performances and Energy Efficiency for MSW Gasification Treatment. Waste Biomass Valoriz. 2015, 6, 123–135. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefin. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Soroush, S.; Ronsse, F.; Park, J.; Heynderickx, P.M. Comparison Study on the Water-to-Biomass Ratio in Hydrothermal Carbonization of Fresh Seaweed. Processes 2023, 11, 1123. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C. Hydrothermal Carbonization (HTC) of Lignocellulosic Biomass. Energy Fuels 2011, 25, 1802–1810. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Zielinska, B.; Felix, L. Hydrothermal carbonization (HTC) of selected woody and herbaceous biomass feedstocks. Biomass Convers. Biorefin. 2013, 3, 113–126. [Google Scholar] [CrossRef]
- Demirbas, A.; Arin, G. An Overview of Biomass Pyrolysis. Energy Sources 2002, 24, 471–482. [Google Scholar] [CrossRef]
- Zadeh, Z.E.; Abdulkhani, A.; Aboelazayem, O.; Saha, B. Recent Insights into Lignocellulosic Biomass Pyrolysis: A Critical Review on Pretreatment, Characterization, and Products Upgrading. Processes 2020, 8, 799. [Google Scholar] [CrossRef]
- Rasaq, W.A.; Golonka, M.; Scholz, M.; Białowiec, A. Opportunities and Challenges of High-Pressure Fast Pyrolysis of Biomass: A Review. Energies 2021, 14, 5426. [Google Scholar] [CrossRef]
- Chun, Y.; Lee, S.K.; Yoo, H.Y.; Kim, S.W. Recent advancements in biochar production according to feedstock classification, pyrolysis conditions, and applications: A review. BioResources 2021, 16, 6512–6547. [Google Scholar] [CrossRef]
- Piersa, P.; Unyay, H.; Szufa, S.; Lewandowska, W.; Modrzewski, R.; Ślężak, R.; Ledakowicz, S. An Extensive Review and Comparison of Modern Biomass Torrefaction Reactors vs. Biomass Pyrolysis—Part 1. Energies 2022, 15, 2227. [Google Scholar] [CrossRef]
- Sikarwar, V.S.; Zhao, M.; Clough, P.; Yao, J.; Zhong, X.; Memon, M.Z.; Shah, N.; Anthony, E.J.; Fennell, P.S. An overview of advances in biomass gasification. Energy Environ. Sci. 2016, 9, 2939–2977. [Google Scholar] [CrossRef]
- Sánchez, M.E.; Martínez, O.; Gómez, X.; Morán, A. Pyrolysis of mixtures of sewage sludge and manure: A comparison of the results obtained in the laboratory (semi-pilot) and in a pilot plant. Waste Manag. 2007, 27, 1328–1334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Che, Y.; Wang, Z.; Qiao, Y.; Tian, Y. Coupling Process of Heavy Oil Millisecond Pyrolysis and Coke Gasification: A Fundamental Study. Energy Fuels 2016, 30, 6698–6708. [Google Scholar] [CrossRef]
- Akyürek, Z. Synergetic Effects during Co-Pyrolysis of Sheep Manure and Recycled Polyethylene Terephthalate. Polymers 2021, 13, 2363. [Google Scholar] [CrossRef] [PubMed]
- Madanikashani, S.; Vandewalle, L.A.; De Meester, S.; De Wilde, J.; Van Geem, K.M. Multi-Scale Modeling of Plastic Waste Gasification: Opportunities and Challenges. Materials 2022, 15, 4215. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.; Li, Y.; Zhang, C.; Fang, Y.; Zhao, J. A review on resource utilization of oil sludge based on pyrolysis and gasification. J. Environ. Chem. Eng. 2023, 11, 109692. [Google Scholar] [CrossRef]
- Obileke, K.; Makaka, G.; Nwokolo, N. Recent Advancements in Anaerobic Digestion and Gasification Technology. Appl. Sci. 2023, 13, 5597. [Google Scholar] [CrossRef]
- Schmidt, P.; Batteiger, V.; Roth, A.; Weindorf, W.; Raksha, T. Power-to-Liquids as Renewable Fuel Option for Aviation: A Review. Chem. Ing. Tech. 2018, 90, 127–140. [Google Scholar] [CrossRef]
- Dieterich, V.; Buttler, A.; Hanel, A.; Spliethoff, H.; Fendt, S. Power-to-liquid via synthesis of methanol, DME or Fischer–Tropsch-fuels: A review. Energy Environ. Sci. 2020, 13, 3207–3252. [Google Scholar] [CrossRef]
- Teimouri, Z.; Abatzoglou, N.; Dalai, A.K. Kinetics and Selectivity Study of Fischer–Tropsch Synthesis to C5+ Hydrocarbons: A Review. Catalysts 2021, 11, 330. [Google Scholar] [CrossRef]
- Leckner, B. Process aspects in combustion and gasification Waste-to-Energy (WtE) units. Waste Manag. 2015, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.H.; Tao, L. Economic Perspectives of Biogas Production via Anaerobic Digestion. Bioengineering 2020, 7, 74. [Google Scholar] [CrossRef]
- Martínez, E.J.; González, R.; Ellacuriaga, M.; Gómez, X. Valorization of Fourth-Range Wastes: Evaluating Pyrolytic Behavior of Fresh and Digested Wastes. Fermentation 2022, 8, 744. [Google Scholar] [CrossRef]
- Xu, D.; Tree, D.R.; Lewis, R.S. The effects of syngas impurities on syngas fermentation to liquid fuels. Biomass Bioenergy 2011, 35, 2690–2696. [Google Scholar] [CrossRef]
- Benevenuti, C.; Amaral, P.; Ferreira, T.; Seidl, P. Impacts of Syngas Composition on Anaerobic Fermentation. Reactions 2021, 2, 391–407. [Google Scholar] [CrossRef]
- Ahuja, V.; Bhatt, A.K.; Ravindran, B.; Yang, Y.; Bhatia, S.K. A Mini-Review on Syngas Fermentation to Bio-Alcohols: Current Status and Challenges. Sustainability 2023, 15, 3765. [Google Scholar] [CrossRef]
- Sansaniwal, S.K.; Pal, K.; Rosen, M.A.; Tyagi, S.K. Recent advances in the development of biomass gasification technology: A comprehensive review. Renew. Sustain. Energy Rev. 2017, 72, 363–384. [Google Scholar] [CrossRef]
- Sun, X.; Atiyeh, H.K.; Huhnke, R.L.; Tanner, R.S. Syngas fermentation process development for production of biofuels and chemicals: A review. Bioresour. Technol. Rep. 2019, 7, 100279. [Google Scholar] [CrossRef]
- Hu, J.; Yu, F.; Lu, Y. Application of Fischer–Tropsch Synthesis in Biomass to Liquid Conversion. Catalysts 2012, 2, 303–326. [Google Scholar] [CrossRef]
- Böhme, C. BASF Develops Process for Climate-Friendly Methanol. Available online: https://www.basf.com/global/en/media/news-releases/2019/05/p-19-218.html (accessed on 12 August 2023).
- Vogt, C.; Monai, M.; Kramer, G.J.; Weckhuysen, B.M. The renaissance of the Sabatier reaction and its applications on Earth and in space. Nat. Catal. 2019, 2, 188–197. [Google Scholar] [CrossRef]
- Shadle, L.J.; Berry, D.A.; Syamlal, M. Coal Conversion Processes, Gasification. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley Online Library: Hoboken, NJ, USA, 2002. [Google Scholar] [CrossRef]
- IGCC PROJECT EXAMPLES. Available online: https://www.netl.doe.gov/research/coal/energy-systems/gasification/gasifipedia/project-examples (accessed on 12 August 2023).
- Wabash River Generating Station. Available online: https://www.gem.wiki/Wabash_River_Generating_Station#Unit_Retirements (accessed on 12 August 2023).
- Puertollano IGCC Power Station. Available online: https://www.gem.wiki/Puertollano_IGCC_power_station (accessed on 12 August 2023).
- Ward, C.; Goldstein, H.; Maurer, R.; Thimsen, D.; Sheets, B.J.; Hobbs, R.; Isgrigg, F.; Steiger, R.; Revay Madden, D.; Porcu, A.; et al. Making coal relevant for small scale applications: Modular gasification for syngas/engine CHP applications in challenging environments. Fuel 2020, 267, 117303. [Google Scholar] [CrossRef]
- Oliva, J.M.; Negro, M.J.; Manzanares, P.; Ballesteros, I.; Chamorro, M.Á.; Sáez, F.; Ballesteros, M.; Moreno, A.D. A Sequential Steam Explosion and Reactive Extrusion Pretreatment for Lignocellulosic Biomass Conversion within a Fermentation-Based Biorefinery Perspective. Fermentation 2017, 3, 15. [Google Scholar] [CrossRef]
- Hijosa-Valsero, M.; Garita-Cambronero, J.; Paniagua-García, A.I.; Díez-Antolínez, R. A global approach to obtain biobutanol from corn stover. Renew. Energy 2020, 148, 223–233. [Google Scholar] [CrossRef]
- Liu, S.; Yu, Y.; Xu, Z.; Chen, S.; Shen, G.; Yuan, X.; Deng, Q.; Shen, W.; Yang, S.; Zhang, C.; et al. Efficient Corncob Biorefinery for Ethanol Initiated by a Novel Pretreatment of Densifying Lignocellulosic Biomass with Sulfuric Acid. Fermentation 2022, 8, 661. [Google Scholar] [CrossRef]
- Kucharska, K.; Rybarczyk, P.; Hołowacz, I.; Łukajtis, R.; Glinka, M.; Kamiński, M. Pretreatment of Lignocellulosic Materials as Substrates for Fermentation Processes. Molecules 2018, 23, 2937. [Google Scholar] [CrossRef]
- Manandhar, A.; Shah, A. Techno-Economic Analysis of the Production of Lactic Acid from Lignocellulosic Biomass. Fermentation 2023, 9, 641. [Google Scholar] [CrossRef]
- Yogalakshmi, K.N.; Mohamed Usman, T.M.; Kavitha, S.; Sachdeva, S.; Thakur, S.; Adish Kumar, S.; Rajesh Banu, J. Lignocellulosic Biorefinery Technologies: A Perception into Recent Advances in Biomass Fractionation, Biorefineries, Economic Hurdles and Market Outlook. Fermentation 2023, 9, 238. [Google Scholar] [CrossRef]
- Schmatz, A.A.; Candido, J.P.; de Angelis, D.d.F.; Brienzo, M. Semi-Simultaneous Saccharification and Fermentation Improved by Lignin and Extractives Removal from Sugarcane Bagasse. Fermentation 2023, 9, 405. [Google Scholar] [CrossRef]
- Malhotra, M.; Suman, S.K. Laccase-mediated delignification and detoxification of lignocellulosic biomass: Removing obstacles in energy generation. Environ. Sci. Pollut. Res. 2021, 28, 58929–58944. [Google Scholar] [CrossRef]
- Petersen, I.; Werther, J. Experimental investigation and modeling of gasification of sewage sludge in the circulating fluidized bed. Chem. Eng. Process. Process Intensif. 2005, 44, 717–736. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, D.; Song, X.; Zhao, L. Study on the combined sewage sludge pyrolysis and gasification process: Mass and energy balance. Environ. Technol. 2012, 33, 2481–2488. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, K.; Gökalp, I. Pyrolysis, combustion and gasification characteristics of miscanthus and sewage sludge. Energy Convers. Manag. 2015, 89, 83–91. [Google Scholar] [CrossRef]
- Szwaja, S.; Poskart, A.; Zajemska, M.; Szwaja, M. Theoretical and Experimental Analysis on Co-Gasification of Sewage Sludge with Energetic Crops. Energies 2019, 12, 1750. [Google Scholar] [CrossRef]
- Schmid, M.; Hafner, S.; Scheffknecht, G. Experimental Parameter Study on Synthesis Gas Production by Steam-Oxygen Fluidized Bed Gasification of Sewage Sludge. Appl. Sci. 2021, 11, 579. [Google Scholar] [CrossRef]
- Buss, W. Pyrolysis Solves the Issue of Organic Contaminants in Sewage Sludge while Retaining Carbon—Making the Case for Sewage Sludge Treatment via Pyrolysis. ACS Sustain. Chem. Eng. 2021, 9, 10048–10053. [Google Scholar] [CrossRef]
- He, Y.D.; Zhai, Y.B.; Li, C.T.; Yang, F.; Chen, L.; Fan, X.P.; Peng, W.F.; Fu, Z.M. The fate of Cu, Zn, Pb and Cd during the pyrolysis of sewage sludge at different temperatures. Environ. Technol. 2010, 31, 567–574. [Google Scholar] [CrossRef]
- Li, B.; Ding, S.; Fan, H.; Ren, Y. Experimental Investigation into the Effect of Pyrolysis on Chemical Forms of Heavy Metals in Sewage Sludge Biochar (SSB), with Brief Ecological Risk Assessment. Materials 2021, 14, 447. [Google Scholar] [CrossRef]
- Nipattummakul, N.; Ahmed, I.I.; Kerdsuwan, S.; Gupta, A.K. Hydrogen and syngas production from sewage sludge via steam gasification. Int. J. Hydrogen Energy 2010, 35, 11738–11745. [Google Scholar] [CrossRef]
- Moško, J.; Pohořelý, M.; Skoblia, S.; Beňo, Z.; Jeremiáš, M. Detailed Analysis of Sewage Sludge Pyrolysis Gas: Effect of Pyrolysis Temperature. Energies 2020, 13, 4087. [Google Scholar] [CrossRef]
- Werle, S.; Dudziak, M. Analysis of Organic and Inorganic Contaminants in Dried Sewage Sludge and By-Products of Dried Sewage Sludge Gasification. Energies 2014, 7, 462–476. [Google Scholar] [CrossRef]
- Lu, T.; Yuan, H.; Wang, Y.; Huang, H.; Chen, Y. Characteristic of heavy metals in biochar derived from sewage sludge. J. Mater. Cycles Waste Manag. 2016, 18, 725–733. [Google Scholar] [CrossRef]
- Freda, C.; Cornacchia, G.; Romanelli, A.; Valerio, V.; Grieco, M. Sewage sludge gasification in a bench scale rotary kiln. Fuel 2018, 212, 88–94. [Google Scholar] [CrossRef]
- Chen, H.; Zhai, Y.; Xu, B.; Xiang, B.; Zhu, L.; Qiu, L.; Liu, X.; Li, C.; Zeng, G. Characterization of bio-oil and biochar from high-temperature pyrolysis of sewage sludge. Environ. Technol. 2015, 36, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Werle, S. Impact of feedstock properties and operating conditions on sewage sludge gasification in a fixed bed gasifier. Waste Manag. Res. 2014, 32, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Arjharn, W.; Hinsui, T.; Liplap, P.; Raghavan, G.V. Evaluation of an energy production system from sewage sludge using a pilot-scale downdraft gasifier. Energy Fuels 2013, 27, 229–236. [Google Scholar] [CrossRef]
- Abdelrahim, A.; Brachi, P.; Ruoppolo, G.; Fraia, S.D.; Vanoli, L. Experimental and numerical investigation of biosolid gasification: Equilibrium-based modeling with emphasis on the effects of different pretreatment methods. Ind. Eng. Chem. Res. 2019, 59, 299–307. [Google Scholar] [CrossRef]
- Gao, N.; Li, J.; Qi, B.; Li, A.; Duan, Y.; Wang, Z. Thermal analysis and products distribution of dried sewage sludge pyrolysis. J. Anal. Appl. Pyrolysis. 2014, 105, 43–48. [Google Scholar] [CrossRef]
- Wu, H.; Hanna, M.A.; Jones, D.D. Fluidized-bed gasification of dairy manure by Box–Behnken design. Waste Manag. Res. 2011, 30, 506–511. [Google Scholar] [CrossRef]
- Brassard, P.; Godbout, S.; Raghavan, V.; Palacios, J.H.; Grenier, M.; Zegan, D. The Production of Engineered Biochars in a Vertical Auger Pyrolysis Reactor for Carbon Sequestration. Energies 2017, 10, 288. [Google Scholar] [CrossRef]
- Nam, H.; Maglinao, A.L.; Capareda, S.C.; Rodriguez-Alejandro, D.A. Enriched-air fluidized bed gasification using bench and pilot scale reactors of dairy manure with sand bedding based on response surface methods. Energy 2016, 95, 187–199. [Google Scholar] [CrossRef]
- Pandey, D.S.; Katsaros, G.; Lindfors, C.; Leahy, J.J.; Tassou, S.A. Fast Pyrolysis of Poultry Litter in a Bubbling Fluidised Bed Reactor: Energy and Nutrient Recovery. Sustainability 2019, 11, 2533. [Google Scholar] [CrossRef]
- Ashraf, M.; Ramzan, N.; Azam, M.; Anwar, A.; Khan, R.U.; Durrani, A.K.; Rashid, M.U. Cattle dung conversion to syngas: Solar photovoltaic integrated gasification system. Biomass Convers. Biorefin. 2022, 1–12. [Google Scholar] [CrossRef]
- Bergfeldt, B.; Tomasi Morgano, M.; Leibold, H.; Richter, F.; Stapf, D. Recovery of Phosphorus and other Nutrients during Pyrolysis of Chicken Manure. Agriculture 2018, 8, 187. [Google Scholar] [CrossRef]
- Madadian, E.; Orsat, V.; Lefsrud, M. Comparative study of temperature impact on air gasification of various types of biomass in a research-scale down-draft reactor. Energy Fuels 2017, 31, 4045–4053. [Google Scholar] [CrossRef]
- Santana, K.V.R.; Apolônio, F.C.S.O.; Wisniewski, A. Valorization of cattle manure by thermoconversion process in a rotary kiln reactor to produce environmentally friendly products. BioEnergy Res. 2020, 13, 605–617. [Google Scholar] [CrossRef]
- Bläsingä, M.; Oga, I.O.; Müller, M. Co-Gasification of Turkey Manure and Meat Bone with Hard Coal and Lignite: Release of Condensable and Noncondensable Species. Ind. Eng. Chem. Res. 2017, 56, 14396–14400. [Google Scholar] [CrossRef]
- Tian, R.; Li, C.; Xie, S.; You, F.; Cao, Z.; Xu, Z.; Yu, G.; Wang, Y. Preparation of biochar via pyrolysis at laboratory and pilot scales to remove antibiotics and immobilize heavy metals in livestock feces. J. Soils Sediments 2019, 19, 2891–2902. [Google Scholar] [CrossRef]
- Schweitzer, D.; Gredinger, A.; Schmid, M.; Waizmann, G.; Beirow, M.; Spörl, R.; Scheffknecht, G. Steam gasification of wood pellets, sewage sludge and manure: Gasification performance and concentration of impurities. Biomass Bioenerg. 2018, 111, 308–319. [Google Scholar] [CrossRef]
- Vamvuka, D.; Raftogianni, A. Evaluation of Pig Manure for Environmental or Agricultural Applications through Gasification and Soil Leaching Experiments. Appl. Sci. 2021, 11, 12011. [Google Scholar] [CrossRef]
- Chojnacki, J.; Najser, J.; Rokosz, K.; Peer, V.; Kielar, J.; Berner, B. Syngas Composition: Gasification of Wood Pellet with Water Steam through a Reactor with Continuous Biomass Feed System. Energies 2020, 13, 4376. [Google Scholar] [CrossRef]
- Loha, C.; Chatterjee, P.K.; Chattopadhyay, H. Performance of fluidized bed steam gasification of biomass—Modeling and experiment. Energy Convers. Manag. 2011, 52, 1583–1588. [Google Scholar] [CrossRef]
- Meng, F.; Ma, Q.; Wang, H.; Liu, Y.; Wang, D. Effect of gasifying agents on sawdust gasification in a novel pilot scale bubbling fluidized bed system. Fuel 2019, 249, 112–118. [Google Scholar] [CrossRef]
- Ratnasari, D.K.; Bijl, A.; Yang, W.; Jönsson, P.G. Effect of H-ZSM-5 and Al-MCM-41 Proportions in Catalyst Mixtures on the Composition of Bio-Oil in Ex-Situ Catalytic Pyrolysis of Lignocellulose Biomass. Catalysts 2020, 10, 868. [Google Scholar] [CrossRef]
- González-Vázquez, M.D.P.; García, R.; Gil, M.V.; Pevida, C.; Rubiera, F. Comparison of the gasification performance of multiple biomass types in a bubbling fluidized bed. Energy Conver. Manag. 2018, 176, 309–323. [Google Scholar] [CrossRef]
- Osorio, J.; Chejne, F. Bio-Oil Production in Fluidized Bed Reactor at Pilot Plant from Sugarcane Bagasse by Catalytic Fast Pyrolysis. Waste Biomass Valoriz. 2019, 10, 187–195. [Google Scholar] [CrossRef]
- Dahmen, N.; Abeln, J.; Eberhard, M.; Kolb, T.; Leibold, H.; Sauer, J.; Stapf, D.; Zimmerlin, B. The bioliq process for producing synthetic transportation fuels. WIREs Energy Environ. 2017, 6, e236. [Google Scholar] [CrossRef]
- Michler, T.; Wippermann, N.; Toedter, O.; Niethammer, B.; Otto, T.; Arnold, U.; Pitter, S.; Koch, T.; Sauer, J. Gasoline from the bioliq® process: Production, characterization and performance. Fuel Process. Technol. 2020, 206, 106476. [Google Scholar] [CrossRef]
- Michler, T.; Niethammer, B.; Fuchs, C.; Toedter, O.; Arnold, U.; Koch, T.; Sauer, J. Further Development of Gasoline from the bioliq® Process with Focus on Particulate and Hydrocarbon Emissions. Fuels 2023, 4, 205–220. [Google Scholar] [CrossRef]
- Shell: Gasification-Technology. Available online: https://www.shell.com/business-customers/catalysts-technologies/licensed-technologies/refinery-technology/gasification-technology.html (accessed on 15 August 2023).
- Valmet Gasification. Available online: https://www.valmet.com/energyproduction/gasification/ (accessed on 15 August 2023).
- Eqtec Gasification Process. Available online: https://eqtec.com/gasification-projects/ (accessed on 15 August 2023).
- Ducom, G.; Tagutchou, J.-P.; Gautier, M.; Gaignaire, C.; Méhu, J.; Gourdon, R. Olive mill solid waste gasification in a pilot-scale downdraft gasifier with three-stage air supply: Performance, mass-energy balance and fate of inorganic elements. Fuel 2023, 340, 127469. [Google Scholar] [CrossRef]
- Thyssenkrupp-Uhde: Gasification. Available online: https://www.thyssenkrupp-uhde.com/en/products-and-technologies/hydrogen-and-gas-technologies/gasification (accessed on 15 August 2023).
- Sofia, D.; Coca Llano, P.; Giuliano, A.; Iborra Hernández, M.; García Peña, F.; Barletta, D. Co-gasification of coal–petcoke and biomass in the Puertollano IGCC power plant. Chem. Eng. Res. Des. 2014, 92, 1428–1440. [Google Scholar] [CrossRef]
- MHI Gasification Process. Available online: https://www.mhi.com/products/environment/pyrolysis_gasification_melting.html (accessed on 15 August 2023).
- MHI Technology. Available online: http://www.mhi.co.jp/technology/review/pdf/e483/e483037.pdf (accessed on 15 August 2023).
- Enerkem: Carbon Recycling. Available online: https://enerkem.com/process-technology/carbon-recycling/ (accessed on 15 August 2023).
- Bioliq. Available online: https://www.bioliq.de/english/55.php (accessed on 15 August 2023).
- Greentransitionholding: Gasification Technology. Available online: https://www.greentransitionholding.com/group-companies#aitos-gasification-technology (accessed on 15 August 2023).
- Urbas: Wood Gas CHP the Innovation. Available online: https://www.urbas.at/wp-content/uploads/2020/09/p_URBAS_DE_KWK_7.8.web_WF.PDF-en.pdf (accessed on 15 August 2023).
- Syncraft Climate Positive Solutions. Available online: https://en.syncraft.at/ (accessed on 16 August 2023).
- Burkhardt-Gruppe: Heat-and-Power-from-Wood. Available online: https://burkhardt-gruppe.de/en/power-engineering/heat-and-power-from-wood/wood-gas-generator/wood-gasifier-v-3-90/ (accessed on 16 August 2023).
- Repotec: Biomass Steam Gasification. Available online: http://www.repotec.at/index.php/technology.html (accessed on 16 August 2023).
- Pyrox. Available online: http://www.woodgas.eu/pyrox-tecnology (accessed on 16 August 2023).
- Eco20x Energia Rinnovabile. Available online: https://eco20cmd.com/en/eco20x/how-does-eco20x-work/ (accessed on 16 August 2023).
- Espegroup: CGiP50 Biomass Cogenerators. Available online: https://www.espegroup.com//app/uploads/2021/02/Datasheet_CHiP50_ENG.pdf (accessed on 16 August 2023).
- Allesina, G.; Pedrazzi, S. Barriers to Success: A Technical Review on the Limits and Possible Future Roles of Small Scale Gasifiers. Energies 2021, 14, 6711. [Google Scholar] [CrossRef]
- Patuzzi, F.; Basso, D.; Vakalis, S.; Antolini, D.; Piazzi, S.; Benedetti, V.; Cordioli, E.; Baratieri, M. State-of-the-art of small-scale biomass gasification systems: An extensive and unique monitoring review. Energy 2021, 223, 120039. [Google Scholar] [CrossRef]
- Van De Velde, K.; Kiekens, P. Thermal degradation of flax: The determination of kinetic parameters with thermogravimetric analysis. J. Appl. Polym. Sci. 2002, 83, 2634–2643. [Google Scholar] [CrossRef]
- Abdelouahed, L.; Leveneur, S.; Vernieres-Hassimi, L.; Balland, L.; Taouk, B. Comparative investigation for the determination of kinetic parameters for biomass pyrolysis by thermogravimetric analysis. J. Therm. Anal. Calorim. 2017, 129, 1201–1213. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Narayanan, S.K. Determination of kinetic parameters of biomass samples using thermogravimetric analysis. Environ. Prog. Sustain. Energy 2014, 33, 256–266. [Google Scholar] [CrossRef]
- Wang, W.; Lemaire, R.; Bensakhria, A.; Luart, D. Thermogravimetric Analysis and Kinetic Modeling of the AAEM-Catalyzed Pyrolysis of Woody Biomass. Molecules 2022, 27, 7662. [Google Scholar] [CrossRef]
- González-Vázquez, M.P.; García, R.; Gil, M.V.; Pevida, C.; Rubiera, F. Unconventional biomass fuels for steam gasification: Kinetic analysis and effect of ash composition on reactivity. Energy 2018, 155, 426–437. [Google Scholar] [CrossRef]
- Fernandez-Lopez, M.; López-González, D.; Valverde, J.L.; Sanchez-Silva, L. Kinetic study of the CO2 gasification of manure samples. J. Therm. Anal. Calorim. 2017, 127, 2499–2509. [Google Scholar] [CrossRef]
- Gil, M.V.; González-Vázquez, M.P.; García, R.; Rubiera, F.; Pevida, C. Assessing the influence of biomass properties on the gasification process using multivariate data analysis. Energy Conver. Manag. 2019, 184, 649–660. [Google Scholar] [CrossRef]
- Rabou, L.P.L.M.; Zwart, R.W.R.; Vreugdenhil, B.J.; Bos, L. Tar in Biomass Producer Gas, the Energy research Centre of The Netherlands (ECN) Experience: An Enduring Challenge. Energy Fuels 2009, 23, 6189–6198. [Google Scholar] [CrossRef]
- Kim, M.; Sohn, G.; Ye, I.; Ryu, C.; Kim, B.; Lee, J. Numerical analysis on the performance of a 300 MW IGCC coal gasifier under various operating conditions. Fuel 2019, 257, 116063. [Google Scholar] [CrossRef]
- Li, G.; Wang, L.; Wang, C.; Wang, C.; Wu, P.; Che, D. Experimental Study on Coal Gasification in a Full-Scale Two-Stage Entrained-Flow Gasifier. Energies 2020, 13, 4937. [Google Scholar] [CrossRef]
- Ataei, A.; Azimi, A.; Kalhori, S.B.; Abari, M.F.; Radnezhad, H. Performance analysis of a co-gasifier for organic waste in agriculture. Int. J. Recycl. Org. Waste Agric. 2012, 1, 6. [Google Scholar] [CrossRef]
- Fermoso, J.; Arias, B.; Gil, M.V.; Plaza, M.G.; Pevida, C.; Pis, J.J.; Rubiera, F. Co-gasification of different rank coals with biomass and petroleum coke in a high-pressure reactor for H2-rich gas production. Bioresour. Technol. 2010, 101, 3230–3235. [Google Scholar] [CrossRef]
- Martínez González, A.; Silva Lora, E.E.; Escobar Palacio, J.C. Syngas production from oil sludge gasification and its potential use in power generation systems: An energy and exergy analysis. Energy 2019, 169, 1175–1190. [Google Scholar] [CrossRef]
- Ba, Z.; Zhao, J.; Li, C.; Huang, J.; Fang, Y.; Zhang, L.; Kong, L.; Wang, Q. Developing efficient gasification technology for high-sulfur petroleum coke to hydrogen-rich syngas production. Fuel 2020, 267, 117170. [Google Scholar] [CrossRef]
- Chu, Z.; Gong, Z.; Wang, Z.; Zhang, H.; Liu, L.; Wu, J.; Wang, J. Experimental study on gasification of oil sludge with steam and its char characteristic. J. Hazard. Mater. 2021, 416, 125713. [Google Scholar] [CrossRef]
- Ongen, A.; Ozcan, H.K.; Ozbaş, E.E.; Aydin, S.; Yesildag, I. Co-gasification of oily sludge and chicken manure in a laboratory-scale updraft fixed bed gasifier. Clean Technol. Environ. Policy 2022, 24, 2229–2239. [Google Scholar] [CrossRef]
- Pattanayak, S.; Hauchhum, L.; Loha, C.; Sailo, L. Feasibility study of biomass gasification for power generation in Northeast India. Biomass Convers. Biorefin. 2023, 13, 999–1011. [Google Scholar] [CrossRef]
- Porcu, A.; Sollai, S.; Marotto, D.; Mureddu, M.; Ferrara, F.; Pettinau, A. Techno-Economic Analysis of a Small-Scale Biomass-to-Energy BFB Gasification-Based System. Energies 2019, 12, 494. [Google Scholar] [CrossRef]
- Heiskanen, H.; Virkajärvi, I.; Viikari, L. The effect of syngas composition on the growth and product formation of Butyribacterium methylotrophicum. Enzym. Microb. Technol. 2007, 41, 362–367. [Google Scholar] [CrossRef]
- Thi, H.N.; Park, S.; Li, H.; Kim, Y.-K. Medium Compositions for the Improvement of Productivity in Syngas Fermentation with Clostridium autoethanogenum. Biotechnol. Bioprocess Eng. 2020, 25, 493–501. [Google Scholar] [CrossRef]
- Johannes, B.; Volker, M. CO Metabolism in the Acetogen Acetobacterium woodii. Appl. Environ. Microbiol. 2015, 81, 5949–5956. [Google Scholar] [CrossRef]
- Richter, H.; Molitor, B.; Wei, H.; Chen, W.; Aristilde, L.; Angenent, L.T. Ethanol production in syngas-fermenting Clostridium ljungdahlii is controlled by thermodynamics rather than by enzyme expression. Energy Environ. Sci. 2016, 9, 2392–2399. [Google Scholar] [CrossRef]
- Lanzillo, F.; Ruggiero, G.; Raganati, F.; Russo, M.E.; Marzocchella, A. Batch Syngas Fermentation by Clostridium carboxidivorans for Production of Acids and Alcohols. Processes 2020, 8, 1075. [Google Scholar] [CrossRef]
- Voegele, E. Ineos Bio to Sell Ethanol Business, Including Vero Beach Plant. Available online: https://biomassmagazine.com/articles/13662/ineos-bio-to-sell-ethanol-business-including-vero-beach-plant (accessed on 18 August 2023).
- Dapile, L. Investigation: INEOS Failed Despite $129 Million in Taxpayer Subsidies. Available online: https://eu.tcpalm.com/story/news/2017/01/17/ineos-closes-vero-beach-biofuel-plant/96412616/ (accessed on 18 August 2023).
- Writer, N.E.S. Westinghouse Plasma Operations Delivers Clean Syngas to Coskata Ethanol Facility. Available online: https://www.nsenergybusiness.com/news/newswestinghouse_plasma_operations_delivers_clean_syngas_to_coskata_ethanol_facility_100106/# (accessed on 22 August 2023).
- Ce.eco: Datasheet Plasma. Available online: https://www.ce.eco/it/products/plasma/datasheet-plasma (accessed on 22 August 2023).
- Sassari, N.R.; Marcheti, M. Presentation of Plasma Waste Gasification Technology. Available online: http://www.ecogv.it/wp-content/uploads/2019/12/Presentazione-Serbia-CNR-1.pdf (accessed on 22 August 2023).
- Synata Bio. Available online: https://synatabio.com/technology/ (accessed on 22 August 2023).
- True North Eyes Ethanol Investment, Acquires Synata Bio in India. Available online: https://advancedbiofuelsusa.info/true-north-eyes-ethanol-investment-acquires-synata-bio-in-india/ (accessed on 22 August 2023).
- Steelanol, Fueling a Sustainable Future. Available online: http://www.steelanol.eu/en (accessed on 22 August 2023).
- Holmgren, J.; Zarraga, J.; Summers, Z.; Köpke, M.; Trukenbrod, G.; El-Sarkawy, O.; Patel, N.; Lanzatech. Transforming Carbon. Making Products. Available online: https://ir.lanzatech.com/static-files/21eb4d1f-3e47-4224-9957-03bfb658aeab (accessed on 22 August 2023).
- Shougang LanzaTech Obtains Industry’s First Product Carbon Label Certification. Available online: https://www.shougang.com.cn/en/ehtml/ShougangNews/20230313/1749.html (accessed on 22 August 2023).
- Stoll, I.K.; Boukis, N.; Sauer, J. Syngas Fermentation to Alcohols: Reactor Technology and Application Perspective. Chem. Ing. Tech. 2020, 92, 125–136. [Google Scholar] [CrossRef]
- Drake, H.L.; Gößner, A.S.; Daniel, S.L. Old Acetogens, New Light. Ann. N. Y. Acad. Sci. 2008, 1125, 100–128. [Google Scholar] [CrossRef] [PubMed]
- Schiel-Bengelsdorf, B.; Dürre, P. Pathway engineering and synthetic biology using acetogens. FEBS Lett. 2012, 586, 2191–2198. [Google Scholar] [CrossRef]
- Shanmugasundaram, T.; Wood, H.G. Interaction of ferredoxin with carbon monoxide dehydrogenase from Clostridium thermoaceticum. J. Biochem. Chem. 1992, 267, 897–900. [Google Scholar] [CrossRef]
- Henstra, A.M.; Sipma, J.; Rinzema, A.; Stams, A.J.M. Microbiology of synthesis gas fermentation for biofuel production. Curr. Opin. Biotechnol. 2007, 18, 200–206. [Google Scholar] [CrossRef]
- Daniell, J.; Köpke, M.; Simpson, S.D. Commercial Biomass Syngas Fermentation. Energies 2012, 5, 5372–5417. [Google Scholar] [CrossRef]
- Phillips, J.R.; Huhnke, R.L.; Atiyeh, H.K. Syngas Fermentation: A Microbial Conversion Process of Gaseous Substrates to Various Products. Fermentation 2017, 3, 28. [Google Scholar] [CrossRef]
- Bengelsdorf, F.R.; Beck, M.H.; Erz, C.; Hoffmeister, S.; Karl, M.M.; Riegler, P.; Wirth, S.; Poehlein, A.; Weuster-Botz, D.; Dürre, P. Bacterial anaerobic synthesis gas (syngas) and CO2+ H2 fermentation. In Advances in Applied Microbiology; Sarialani, S., Gadd, G., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 103, pp. 143–221. [Google Scholar] [CrossRef]
- Sahoo, J.; Patil, P.; Verma, A.; Lodh, A.; Khanna, N.; Prasad, R.; Pandit, S.; Fosso-Kankeu, E. Biochemical Aspects of Syngas Fermentation. In Recent Developments in Microbial Technologies; Prasad, R., Kumar, V., Singh, J., Upadhyaya, C.P., Eds.; Environmental and Microbial Biotechnology; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Gencic, S.; Grahame, D.A. Diverse energy-conserving pathways in Clostridium difficile: Growth in the absence of amino acid Stickland acceptors and the role of the Wood-Ljungdahl pathway. J. Bacteriol. 2020, 202, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Hurst, K.M.; Lewis, R.S. Carbon monoxide partial pressure effects on the metabolic process of syngas fermentation. Biochem. Eng. J. 2010, 48, 159–165. [Google Scholar] [CrossRef]
- Kennes, D.; Abubackar, H.N.; Diaz, M.; Veiga, M.C.; Kennes, C. Bioethanol production from biomass: Carbohydrate vs syngas fermentation. J. Chem. Technol. Biotechnol. 2016, 91, 304–317. [Google Scholar] [CrossRef]
- Martin, M.E.; Richter, H.; Saha, S.; Angenent, L.T. Traits of selected Clostridium strains for syngas fermentation to ethanol. Biotechnol. Bioeng. 2016, 113, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Klasson, K.T.; Ackerson, M.D.; Clausen, E.C.; Gaddy, J.L. Biological conversion of coal and coal-derived synthesis gas. Fuel 1993, 72, 1673–1678. [Google Scholar] [CrossRef]
- Mohammadi, M.; Younesi, H.; Najafpour, G.; Mohamed, A.R. Sustainable ethanol fermentation from synthesis gas by Clostridium ljungdahlii in a continuous stirred tank bioreactor. J. Chem. Technol. Biotechnol. 2012, 87, 837–843. [Google Scholar] [CrossRef]
- Liu, K.; Atiyeh, H.K.; Stevenson, B.S.; Tanner, R.S.; Wilkins, M.R.; Huhnke, R.L. Continuous syngas fermentation for the production of ethanol, n-propanol and n-butanol. Bioresour. Technol. 2014, 151, 69–77. [Google Scholar] [CrossRef]
- Elisiário, M.P.; Van Hecke, W.; De Wever, H.; Noorman, H.; Straathof, A.J.J. Acetic acid, growth rate, and mass transfer govern shifts in CO metabolism of Clostridium autoethanogenum. Appl. Microbiol. Biotechnol. 2023, 107, 5329–5340. [Google Scholar] [CrossRef]
- Calvo, D.C.; Luna, H.J.; Arango, J.A.; Torres, C.I.; Rittmann, B.E. Determining global trends in syngas fermentation research through a bibliometric analysis. J. Environ. Manag. 2022, 307, 114522. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Han, W.; Shao, L.; Lü, F. One-step production of C6–C8 carboxylates by mixed culture solely grown on CO. Biotechnol. Biofuels Bioprod. 2018, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Kucek, L.A.; Spirito, C.M.; Angenent, L.T. High n-caprylate productivities and specificities from dilute ethanol and acetate: Chain elongation with microbiomes to upgrade products from syngas fermentation. Energy Environ. Sci. 2016, 9, 3482–3494. [Google Scholar] [CrossRef]
- Gavala, H.N.; Grimalt-Alemany, A.; Asimakopoulos, K.; Skiadas, I.V. Gas Biological Conversions: The Potential of Syngas and Carbon Dioxide as Production Platforms. Waste Biomass Valoriz. 2021, 12, 5303–5328. [Google Scholar] [CrossRef]
- Phillips, J.R.; Atiyeh, H.K.; Tanner, R.S.; Torres, J.R.; Saxena, J.; Wilkins, M.R.; Huhnke, R.L. Butanol and hexanol production in Clostridium carboxidivorans syngas fermentation: Medium development and culture techniques. Bioresour. Technol. 2015, 190, 114–121. [Google Scholar] [CrossRef]
- Fernández-Naveira, Á.; Abubackar, H.N.; Veiga, M.C.; Kennes, C. Efficient butanol-ethanol (B-E) production from carbon monoxide fermentation by Clostridium carboxidivorans. Appl. Microbiol. Biotechnol. 2016, 100, 3361–3370. [Google Scholar] [CrossRef]
- Ramió-Pujol, S.; Ganigué, R.; Bañeras, L.; Colprim, J. Incubation at 25 °C prevents acid crash and enhances alcohol production in Clostridium carboxidivorans P7. Bioresour. Technol. 2015, 192, 296–303. [Google Scholar] [CrossRef]
- Tareen, A.K.; Punsuvon, V.; Sultan, I.N.; Khan, M.W.; Parakulsuksatid, P. Cellulase Addition and Pre-hydrolysis Effect of High Solid Fed-Batch Simultaneous Saccharification and Ethanol Fermentation from a Combined Pretreated Oil Palm Trunk. ACS Omega 2021, 6, 26119–26129. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, W.; Zahoor; Tan, X.; Zhuang, X.; Miao, C.; Guo, Y.; Chen, X.; Yu, Q.; Yuan, Z. Recycling of Black Liquor for Treating Sugarcane Bagasse at Low Temperature to Attain High Ethanol Production without Washing Step. ACS Sustain. Chem. Eng. 2020, 8, 17016–17021. [Google Scholar] [CrossRef]
- Production of Sustainable, Advanced Bio-ethANOL through an Innovative Gas-Fermentation Process Using Exhaust Gases Emitted in the STEEL Industry. Available online: https://cordis.europa.eu/project/id/656437 (accessed on 18 August 2023).
- Liew, F.; Henstra, A.M.; Köpke, M.; Winzer, K.; Simpson, S.D.; Minton, N.P. Metabolic engineering of Clostridium autoethanogenum for selective alcohol production. Metab. Eng. 2017, 40, 104–114. [Google Scholar] [CrossRef]
- Im, H.; An, T.; Kwon, R.; Park, S.; Kim, Y.-K. Effect of Organic Nitrogen Supplements on Syngas Fermentation Using Clostridium autoethanogenum. Biotechnol. Bioprocess Eng. 2021, 26, 476–482. [Google Scholar] [CrossRef]
- Xu, H.; Liang, C.; Yuan, Z.; Xu, J.; Hua, Q.; Guo, Y. A study of CO/syngas bioconversion by Clostridium autoethanogenum with a flexible gas-cultivation system. Enzym. Microb. Technol. 2017, 101, 24–29. [Google Scholar] [CrossRef]
- Cotter, J.L.; Chinn, M.S.; Grunden, A.M. Ethanol and acetate production by Clostridium ljungdahlii and Clostridium autoethanogenum using resting cells. Bioprocess Biosyst. Eng. 2009, 32, 369–380. [Google Scholar] [CrossRef]
- Mann, M.; Munch, G.; Regestein, L.; Rehmann, L. Cultivation Strategies of Clostridium autoethanogenum on Xylose and Carbon Monoxide Combination. ACS Sustain. Chem. Eng. 2020, 8, 2632–2639. [Google Scholar] [CrossRef]
- Dykstra, J.C.; van Oort, J.; Yazdi, A.T.; Vossen, E.; Patinios, C.; van der Oost, J.; Sousa, D.Z.; Kengen, S.W.M. Metabolic engineering of Clostridium autoethanogenum for ethyl acetate production from CO. Microb. Cell Fact. 2022, 21, 243. [Google Scholar] [CrossRef] [PubMed]
- Diender, M.; Stams, A.J.M.; Sousa, D.Z. Production of medium-chain fatty acids and higher alcohols by a synthetic co-culture grown on carbon monoxide or syngas. Biotechnol. Biofuels Bioprod. 2016, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Park, S.E.; Lee, H.; Yun, J.Y. Enhancement of bioethanol production in syngas fermentation with Clostridium ljungdahlii using nanoparticles. Bioresour. Technol. 2014, 159, 446–450. [Google Scholar] [CrossRef]
- Infantes, A.; Kugel, M.; Neumann, A. Evaluation of Media Components and Process Parameters in a Sensitive and Robust Fed-Batch Syngas Fermentation System with Clostridium ljungdahlii. Fermentation 2020, 6, 61. [Google Scholar] [CrossRef]
- Anggraini, I.D.; Keryanti; Kresnowati, M.T.A.P.; Purwadi, R.; Noda, R.; Watanabe, T.; Setiadi, T. Bioethanol Production via Syngas Fermentation of Clostridium Ljungdahlii in a Hollow Fiber Membrane Supported Bioreactor. Int. J. Technol. 2019, 10, 291–319. [Google Scholar] [CrossRef]
- Jack, J.; Lo, J.; Maness, P.-C.; Ren, Z.J. Directing Clostridium ljungdahlii fermentation products via hydrogen to carbon monoxide ratio in syngas. Biomass Bioenergy 2019, 124, 95–101. [Google Scholar] [CrossRef]
- Acharya, B.; Dutta, A.; Basu, P. Ethanol production by syngas fermentation in a continuous stirred tank bioreactor using Clostridium ljungdahlii. Biofuels 2019, 10, 221–237. [Google Scholar] [CrossRef]
- Sertkaya, S.; Azbar, N.; Abubackar, H.N.; Gundogdu, T.K. Design of Low-Cost Ethanol Production Medium from Syngas: An Optimization of Trace Metals for Clostridium ljungdahlii. Energies 2021, 14, 6981. [Google Scholar] [CrossRef]
- Han, Y.-F.; Xie, B.-T.; Wu, G.; Guo, Y.-Q.; Li, D.-M.; Huang, Z.-Y. Combination of Trace Metal to Improve Solventogenesis of Clostridium carboxidivorans P7 in Syngas Fermentation. Front. Microbiol. 2020, 11, 577266. [Google Scholar] [CrossRef] [PubMed]
- Rückel, A.; Hannemann, J.; Maierhofer, C.; Fuchs, A.; Weuster-Botz, D. Studies on Syngas Fermentation with Clostridium carboxidivorans in Stirred-Tank Reactors with Defined Gas Impurities. Front. Microbiol. 2021, 12, 655390. [Google Scholar] [CrossRef]
- Shen, S.; Wang, G.; Zhang, M.; Tang, Y.; Gu, Y.; Jiang, W.; Wang, Y.; Zhuang, Y. Effect of temperature and surfactant on biomass growth and higher-alcohol production during syngas fermentation by Clostridium carboxidivorans P7. Bioresour. Bioprocess 2020, 7, 56. [Google Scholar] [CrossRef]
- Shen, Y.; Brown, R.; Wen, Z. Syngas fermentation of Clostridium carboxidivoran P7 in a hollow fiber membrane biofilm reactor: Evaluating the mass transfer coefficient and ethanol production performance. Biochem. Eng. J. 2014, 85, 21–29. [Google Scholar] [CrossRef]
- Oh, H.J.; Ko, J.K.; Gong, G.; Lee, S.-M.; Um, Y. Production of Hexanol as the Main Product Through Syngas Fermentation by Clostridium carboxidivorans P7. Front. Bioeng. Biotechnol. 2022, 10, 850370. [Google Scholar] [CrossRef]
- Rückel, A.; Oppelt, A.; Leuter, P.; Johne, P.; Fendt, S. Conversion of Syngas from Entrained Flow Gasification of Biogenic Residues with Clostridium carboxidivorans and Clostridium autoethanogenum. Fermentation 2022, 8, 465. [Google Scholar] [CrossRef]
- Benevenuti, C.; Botelho, A.; Ribeiro, R.; Branco, M.; Pereira, A.; Vieira, A.C.; Ferreira, T.; Amaral, P. Experimental Design to Improve Cell Growth and Ethanol Production in Syngas Fermentation by Clostridium carboxidivorans. Catalysts 2020, 10, 59. [Google Scholar] [CrossRef]
- Kim, J.; Kim, K.-Y.; Ko, J.K.; Lee, S.-M.; Gong, G.; Kim, K.H.; Um, Y. Characterization of a Novel Acetogen Clostridium sp. JS66 for Production of Acids and Alcohols: Focusing on Hexanoic Acid Production from Syngas. Biotechnol. Bioprocess Eng. 2022, 27, 89–98. [Google Scholar] [CrossRef]
- Sun, X.; Thunuguntla, R.; Zhang, H.; Atiyeh, H. Biochar amended microbial conversion of C1 gases to ethanol and butanol: Effects of biochar feedstock type and processing temperature. Bioresour. Technol. 2022, 360, 127573. [Google Scholar] [CrossRef] [PubMed]
- Kundiyana, D.K.; Huhnke, R.L.; Wilkins, M.R. Effect of nutrient limitation and two-stage continuous fermentor design on productivities during “Clostridium ragsdalei” syngas fermentation. Bioresour. Technol. 2011, 102, 6058–6064. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Atiyeh, H.K.; Zhang, H.; Tanner, R.S.; Huhnke, R.L. Enhanced ethanol production from syngas by Clostridium ragsdalei in continuous stirred tank reactor using medium with poultry litter biochar. Appl. Energy 2019, 236, 1269–1279. [Google Scholar] [CrossRef]
- Patankar, S.; Dudhane, A.; Paradh, A.D.; Patil, S. Improved bioethanol production using genome-shuffled Clostridium ragsdalei (DSM 15248) strains through syngas fermentation. Biofuels 2021, 12, 81–89. [Google Scholar] [CrossRef]
- Gao, J.; Atiyeh, H.K.; Phillips, J.R.; Wilkins, M.R.; Huhnke, R.L. Development of low cost medium for ethanol production from syngas by Clostridium ragsdalei. Bioresour. Technol. 2013, 147, 508–515. [Google Scholar] [CrossRef]
- Kundiyana, D.K.; Huhnke, R.L.; Wilkins, M.R. Syngas fermentation in a 100-L pilot scale fermentor: Design and process considerations. J. Biosci. Bioeng. 2010, 109, 492–498. [Google Scholar] [CrossRef]
- Ramachandriya, K.D.; Kundiyana, D.K.; Sharma, A.M.; Kumar, A.; Atiyeh, H.K.; Huhnke, R.L.; Wilkins, M.R. Critical Factors Affecting the Integration of Biomass Gasification and Syngas Fermentation Technology. Available online: https://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1719&context=biosysengfacpub (accessed on 18 August 2023).
- Liakakou, E.T.; Infantes, A.; Neumann, A.; Vreugdenhil, B.J. Connecting gasification with syngas fermentation: Comparison of the performance of lignin and beech wood. Fuel 2021, 290, 120054. [Google Scholar] [CrossRef]
- Sharma, S.D.; Dolan, M.; Park, D.; Morpeth, L.; Ilyushechkin, A.; McLennan, K.; Harris, D.J.; Thambimuthu, K.V. A critical review of syngas cleaning technologies—fundamental limitations and practical problems. Powder Technol. 2008, 180, 115–121. [Google Scholar] [CrossRef]
- Woolcock, P.J.; Brown, R.C. A review of cleaning technologies for biomass-derived syngas. Biomass Bioenergy 2013, 52, 54–84. [Google Scholar] [CrossRef]
- Zwart, R.W.R.; Van der Drift, A.; Bos, A.; Visser, H.J.M.; Cieplik, M.K.; Könemann, H.W.J. Oil-based gas washing—Flexible tar removal for high-efficient production of clean heat and power as well as sustainable fuels and chemicals. Environ. Prog. Sustain. Energy 2009, 28, 324–335. [Google Scholar] [CrossRef]
- Thunman, H.; Seemann, M.; Berdugo Vilches, T.; Maric, J.; Pallares, D.; Ström, H.; Berndes, G.; Knutsson, P.; Larsson, A.; Breitholtz, C.; et al. Advanced biofuel production via gasification—Lessons learned from 200 man-years of research activity with Chalmers’ research gasifier and the GoBiGas demonstration plant. Energy Sci. Eng. 2018, 6, 6–34. [Google Scholar] [CrossRef]
- Frilund, C.; Tuomi, S.; Kurkela, E.; Simell, P. Small- to medium-scale deep syngas purification: Biomass-to-liquids multi-contaminant removal demonstration. Biomass Bioenergy 2021, 148, 106031. [Google Scholar] [CrossRef]
- Calì, G.; Deiana, P.; Bassano, C.; Meloni, S.; Maggio, E.; Mascia, M.; Pettinau, A. Syngas Production, Clean-Up and Wastewater Management in a Demo-Scale Fixed-Bed Updraft Biomass Gasification Unit. Energies 2020, 13, 2594. [Google Scholar] [CrossRef]
- Hai, I.U.; Sher, F.; Zarren, G.; Liu, H. Experimental investigation of tar arresting techniques and their evaluation for product syngas cleaning from bubbling fluidized bed gasifier. J. Clean. Prod. 2019, 240, 118239. [Google Scholar] [CrossRef]
- Oliveira, L.; Röhrenbach, S.; Holzmüller, V.; Weuster-Botz, D. Continuous sulfide supply enhanced autotrophic production of alcohols with Clostridium ragsdalei. Bioresour. Bioprocess 2022, 9, 15. [Google Scholar] [CrossRef]
- Oliveira, L.; Rückel, A.; Nordgauer, L.; Schlumprecht, P.; Hutter, E.; Weuster-Botz, D. Comparison of Syngas-Fermenting Clostridia in Stirred-Tank Bioreactors and the Effects of Varying Syngas Impurities. Microorganisms 2022, 10, 681. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Lewis, R.S. Syngas fermentation to biofuels: Effects of ammonia impurity in raw syngas on hydrogenase activity. Biomass Bioenergy 2012, 45, 303–310. [Google Scholar] [CrossRef]
- Oswald, F.; Zwick, M.; Omar, O.; Hotz, E.N.; Neumann, A. Growth and Product Formation of Clostridium ljungdahlii in Presence of Cyanide. Front. Microbiol. 2018, 9, 368587. [Google Scholar] [CrossRef]
- Infantes, A.; Kugel, M.; Raffelt, K.; Neumann, A. Side-by-Side Comparison of Clean and Biomass-Derived, Impurity-Containing Syngas as Substrate for Acetogenic Fermentation with Clostridium ljungdahlii. Fermentation 2020, 6, 84. [Google Scholar] [CrossRef]
- Monir, M.U.; Aziz, A.A.; Khatun, F.; Yousuf, A. Bioethanol production through syngas fermentation in a tar free bioreactor using Clostridium butyricum. Renew. Energy 2020, 157, 1116–1123. [Google Scholar] [CrossRef]
- Pacheco, M.; Pinto, F.; Brunsvik, A.; André, R.; Marques, P.; Mata, R.; Ortigueira, J.; Gírio, F.; Moura, P. Effects of Lignin Gasification Impurities on the Growth and Product Distribution of Butyribacterium methylotrophicum during Syngas Fermentation. Energies 2023, 16, 1722. [Google Scholar] [CrossRef]
- Monir, M.U.; Abd Aziz, A.; Yousuf, A.; Alam, M.Z. Hydrogen-rich syngas fermentation for bioethanol production using Sacharomyces cerevisiea. Int. J. Hydrogen Energy 2020, 45, 18241–18249. [Google Scholar] [CrossRef]
- Stoll, I.K.; Boukis, N.; Sauer, J. Syngas Fermentation at Elevated Pressure—Experimental Results. Available online: https://www.researchgate.net/profile/I-Stoll/publication/334697178_SYNGAS_FERMENTATION_AT_ELEVATED_PRESSURE_-EXPERIMENTAL_RESULTS/links/5d3ac241299bf1995b4b5088/SYNGAS-FERMENTATION-AT-ELEVATED-PRESSURE-EXPERIMENTAL-RESULTS.pdf (accessed on 18 August 2023).
- Sieblist, C.; Hägeholz, O.; Aehle, M.; Jenzsch, M.; Pohlscheidt, M.; Lübbert, A. Insights into large-scale cell-culture reactors: II. Gas-phase mixing and CO2 stripping. Biotechnol. J. 2011, 6, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Jia, Y.; Wang, L.; Cao, Y. Drag coefficient fluctuation prediction of a single bubble rising in water. Chem. Eng. J. 2017, 316, 553–562. [Google Scholar] [CrossRef]
- González, R.; Cabeza, I.O.; Casallas-Ojeda, M.; Gómez, X. Biological Hydrogen Methanation with Carbon Dioxide Utilization: Methanation Acting as Mediator in the Hydrogen Economy. Environments 2023, 10, 82. [Google Scholar] [CrossRef]
- Munasinghe, P.C.; Khanal, S.K. Syngas fermentation to biofuel: Evaluation of carbon monoxide mass transfer coefficient (kLa) in different reactor configurations. Biotechnol. Prog. 2010, 26, 1616–1621. [Google Scholar] [CrossRef]
- Ungerman, A.J.; Heindel, T.J. Carbon Monoxide Mass Transfer for Syngas Fermentation in a Stirred Tank Reactor with Dual Impeller Configurations. Biotechnol. Prog. 2007, 23, 613–620. [Google Scholar] [CrossRef]
- De Tissera, S.; Köpke, M.; Simpson, S.D.; Humphreys, C.; Minton, N.P.; Dürre, P. Syngas Biorefinery and Syngas Utilization. In Biorefineries; Advances in Biochemical Engineering/Biotechnology; Wagemann, K., Tippkötter, N., Eds.; Springer: Cham, Switzerland, 2017; Volume 166, pp. 247–280. [Google Scholar] [CrossRef]
- Orgill, J.J.; Atiyeh, H.K.; Devarapalli, M.; Phillips, J.R.; Lewis, R.S.; Huhnke, R.L. A comparison of mass transfer coefficients between trickle-bed, hollow fiber membrane and stirred tank reactors. Bioresour. Technol. 2013, 133, 340–346. [Google Scholar] [CrossRef]
- Devarapalli, M.; Lewis, R.S.; Atiyeh, H.K. Continuous Ethanol Production from Synthesis Gas by Clostridium ragsdalei in a Trickle-Bed Reactor. Fermentation 2017, 3, 23. [Google Scholar] [CrossRef]
- Kantarci, N.; Borak, F.; Ulgen, K.O. Bubble column reactors. Process Biochem. 2005, 40, 2263–2283. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Duduković, M.P. 18 Gas holdup and liquid recirculation in gas-lift reactors. Chem. Eng. Sci. 1980, 35, 135–141. [Google Scholar] [CrossRef]
- Ham, P.; Bun, S.; Painmanakul, P.; Wongwailikhit, K. Effective Analysis of Different Gas Diffusers on Bubble Hydrodynamics in Bubble Column and Airlift Reactors towards Mass Transfer Enhancement. Processes 2021, 9, 1765. [Google Scholar] [CrossRef]
- Elisiário, M.P.; De Wever, H.; Van Hecke, W.; Noorman, H.; Straathof, A.J.J. Membrane bioreactors for syngas permeation and fermentation. Crit. Rev. Biotechnol. 2022, 42, 856–872. [Google Scholar] [CrossRef]
- Sauer, M. Industrial production of acetone and butanol by fermentation—100 years later. FEMS Microbiol. Lett. 2016, 363, fnw134. [Google Scholar] [CrossRef] [PubMed]
- Amiri, H. Recent innovations for reviving the ABE fermentation for production of butanol as a drop-in liquid biofuel. Biofuel Res. J. 2020, 7, 1256–1266. [Google Scholar] [CrossRef]
- Schüler, M.A.; Stegmann, B.A.; Poehlein, A.; Daniel, R.; Dürre, P. Genome sequence analysis of the temperate bacteriophage TBP2 of the solvent producer Clostridium saccharoperbutylacetonicum N1-4 (HMT, ATCC 27021). FEMS Microbiol. Lett. 2020, 367, fnaa103. [Google Scholar] [CrossRef] [PubMed]
- Liberato, V.; Benevenuti, C.; Coelho, F.; Botelho, A.; Amaral, P.; Pereira, N.; Ferreira, T. Clostridium sp. As Bio-Catalyst for Fuels and Chemicals Production in a Biorefinery Context. Catalysts 2019, 9, 962. [Google Scholar] [CrossRef]
- Gungormusler, M.; Azbar, N.; Keskin, T. Bioethanol production from C1 gases using alternative media by syngas fermentation. Int. J. Glob. Warm. 2022, 28, 42–59. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.W.; Chae, C.G.; Kwon, S.J.; Kim, Y.J.; Lee, J.-H.; Lee, H.S. Domestication of the novel alcohologenic acetogen Clostridium sp. AWRP: From isolation to characterization for syngas fermentation. Biotechnol. Biofuels Bioprod. 2019, 12, 228. [Google Scholar] [CrossRef]
- You, S.; Ok, Y.S.; Tsang, D.C.W.; Kwon, E.E.; Wang, C.-H. Towards practical application of gasification: A critical review from syngas and biochar perspectives. Crit. Rev. Environ. Sci. Technol. 2018, 48, 1165–1213. [Google Scholar] [CrossRef]
- Straczewski, G.; Mai, R.; Gerhards, U.; Garbev, K.; Leibold, H. Catalytic Tar Conversion in Two Different Hot Syngas Cleaning Systems. Catalysts 2021, 11, 1231. [Google Scholar] [CrossRef]
- Hartwell, A.R.; Wilhelm, C.A.; Welles, T.S.; Milcarek, R.J.; Ahn, J. Effects of Synthesis Gas Concentration, Composition, and Operational Time on Tubular Solid Oxide Fuel Cell Performance. Sustainability 2022, 14, 7983. [Google Scholar] [CrossRef]
- Costa, P.; Pinto, F.; André, R.N.; Marques, P. Integration of Gasification and Solid Oxide Fuel Cells (SOFCs) for Combined Heat and Power (CHP). Processes 2021, 9, 254. [Google Scholar] [CrossRef]
- Ajvad, M.; Shih, H.Y. Combustion Analysis of Syngas Fuels Applied in a Micro Gas Turbine Combustor with a Rotating Casing. In Turbo Expo: Power for Land, Sea, and Air, Proceedings of the Turbomachinery Technical Conference and Exposition, Phoenix, AZ, USA, 17–21 June 2019; American Society of Mechanical Engineers: New York, NY, USA, 2019; Volume 58615, p. V04AT04A047. [Google Scholar] [CrossRef]
- Colantoni, S.; Della Gatta, S.; De Prosperis, R.; Russo, A.; Fantozzi, F.; Desideri, U. Gas Turbines Fired with Biomass Pyrolysis Syngas: Analysis of the Overheating of Hot Gas Path Components. J. Eng. Gas Turbines Power. 2010, 132. [Google Scholar] [CrossRef]
- Grimalt-Alemany, A.; Skiadas, I.V.; Gavala, H.N. Syngas biomethanation: State-of-the-art review and perspectives. Biofuels Bioprod. Biorefin. 2018, 12, 139–158. [Google Scholar] [CrossRef]
- Paniagua, S.; Lebrero, R.; Muñoz, R. Syngas biomethanation: Current state and future perspectives. Bioresour. Technol. 2022, 358, 127436. [Google Scholar] [CrossRef]
- Bellini, R.; Bassani, I.; Vizzarro, A.; Azim, A.A.; Vasile, N.S.; Pirri, C.F.; Verga, F.; Menin, B. Biological Aspects, Advancements and Techno-Economical Evaluation of Biological Methanation for the Recycling and Valorization of CO2. Energies 2022, 15, 4064. [Google Scholar] [CrossRef]
- Westman, S.Y.; Chandolias, K.; Taherzadeh, M.J. Syngas Biomethanation in a Semi-Continuous Reverse Membrane Bioreactor (RMBR). Fermentation 2016, 2, 8. [Google Scholar] [CrossRef]
- Mauerhofer, L.-M.; Zwirtmayr, S.; Pappenreiter, P.; Bernacchi, S.; Seifert, A.H.; Reischl, B.; Schmider, T.; Taubner, R.-S.; Paulik, C.; Rittmann, S.K.-M.R. Hyperthermophilic methanogenic archaea act as high-pressure CH4 cell factories. Commun. Biol. 2021, 4, 289. [Google Scholar] [CrossRef]
- Feickert Fenske, C.; Md, Y.; Strübing, D.; Koch, K. Preliminary gas flow experiments identify improved gas flow conditions in a pilot-scale trickle bed reactor for H2 and CO2 biological methanation. Bioresour. Technol. 2023, 371, 128648. [Google Scholar] [CrossRef]
- Janković, T.; Straathof, A.J.J.; Kiss, A.A. Advanced downstream processing of bioethanol from syngas fermentation. Sep. Purif. Technol. 2023, 322, 124320. [Google Scholar] [CrossRef]
- Yao, P.; Xiao, Z.; Chen, C.; Li, W.; Deng, Q. Cell growth behaviors of Clostridium acetobutylicum in a pervaporation membrane bioreactor for butanol fermentation. Biotechnol. Appl. Biochem. 2016, 63, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Sarchami, T.; Munch, G.; Johnson, E.; Kießlich, S.; Rehmann, L. A Review of Process-Design Challenges for Industrial Fermentation of Butanol from Crude Glycerol by Non-Biphasic Clostridium pasteurianum. Fermentation 2016, 2, 13. [Google Scholar] [CrossRef]
- Arregoitia-Sarabia, C.; González-Revuelta, D.; Fallanza, M.; Ortiz, A.; Gorri, D. PEBA/PDMS Composite Multilayer Hollow Fiber Membranes for the Selective Separation of Butanol by Pervaporation. Membranes 2022, 12, 1007. [Google Scholar] [CrossRef] [PubMed]
- Michailos, S.; Parker, D.; Webb, C. Design, sustainability analysis and multiobjective optimisation of ethanol production via syngas fermentation. Waste Biomass Valoriz. 2019, 10, 865–876. [Google Scholar] [CrossRef]
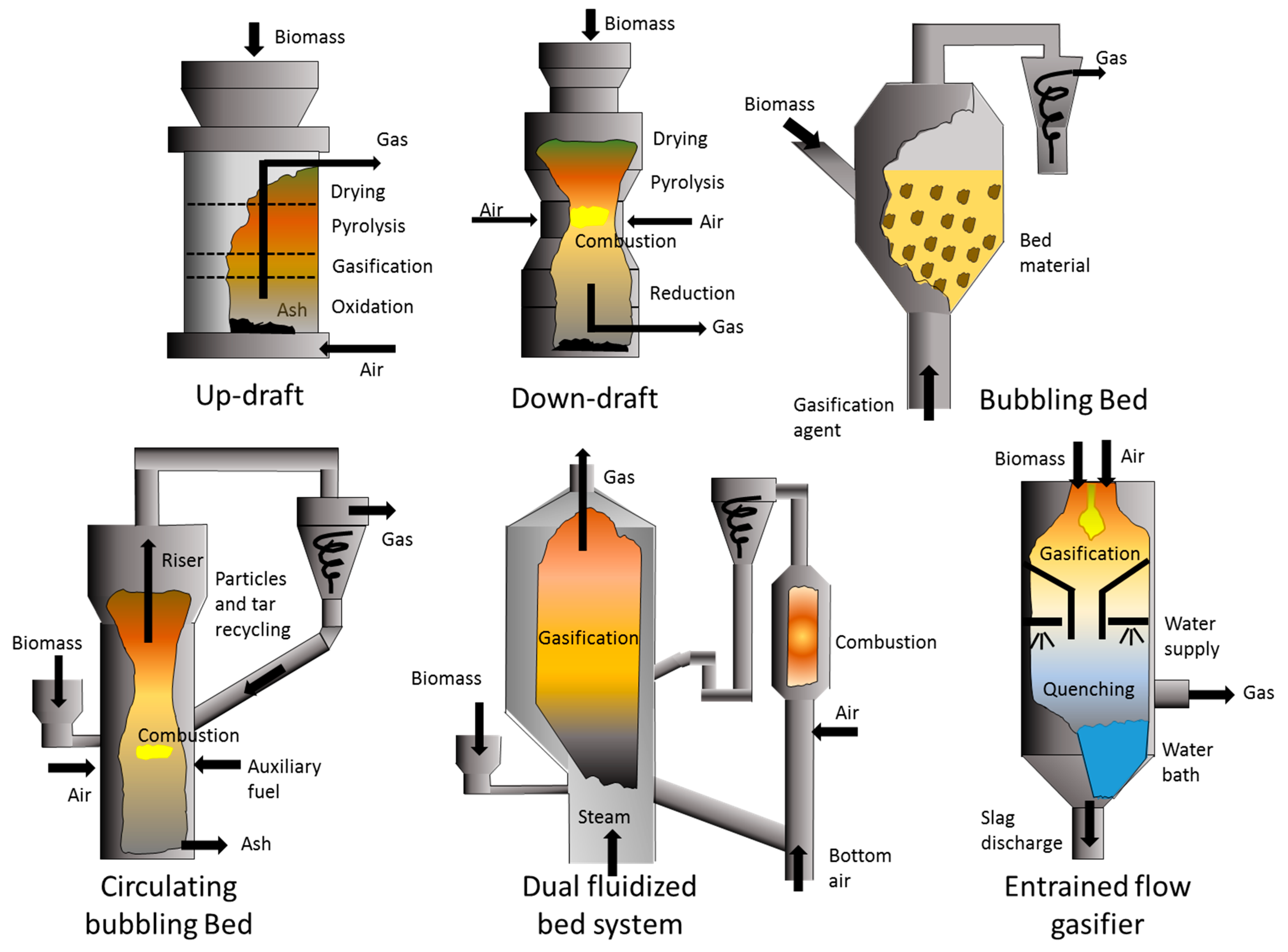
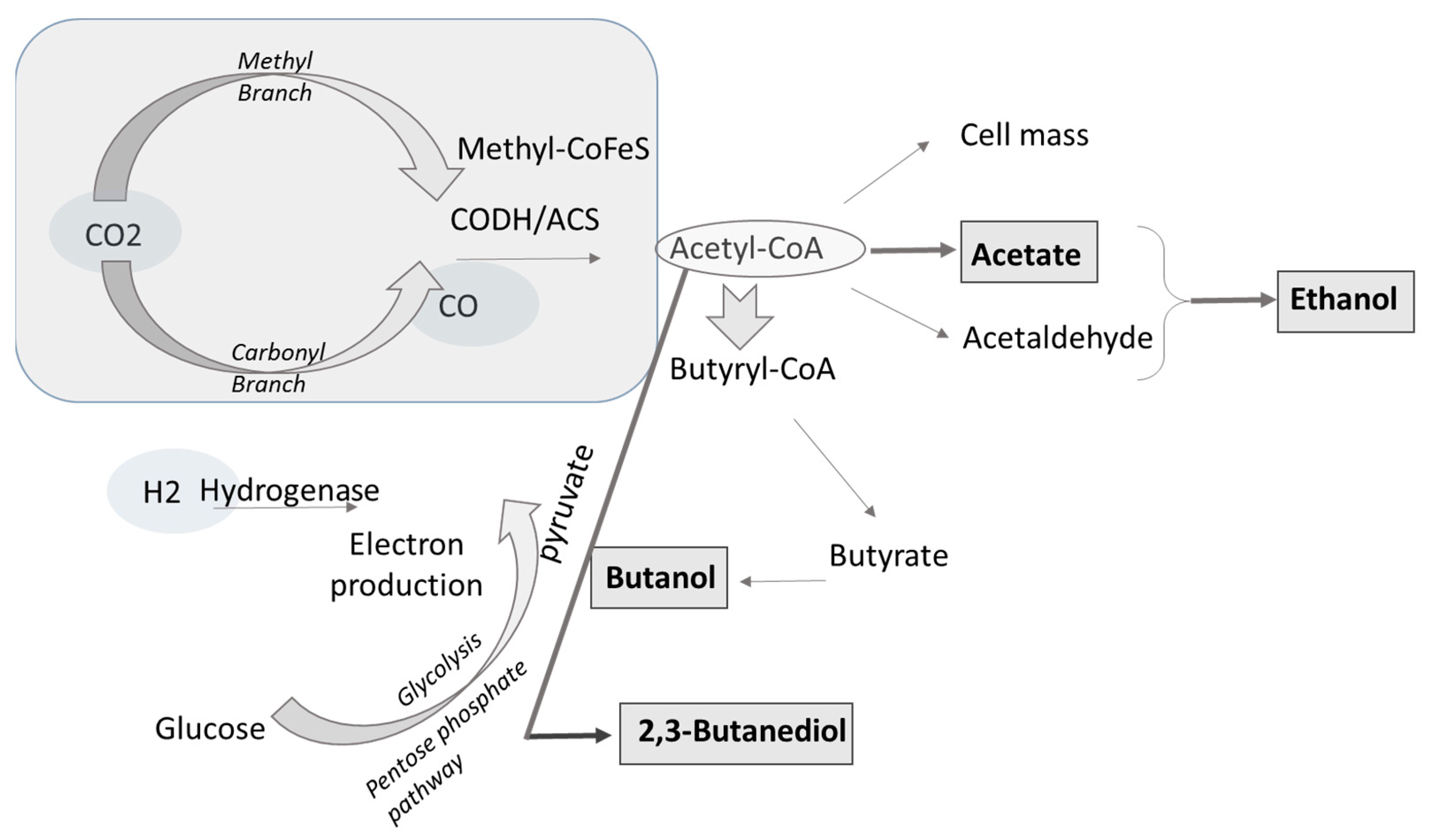
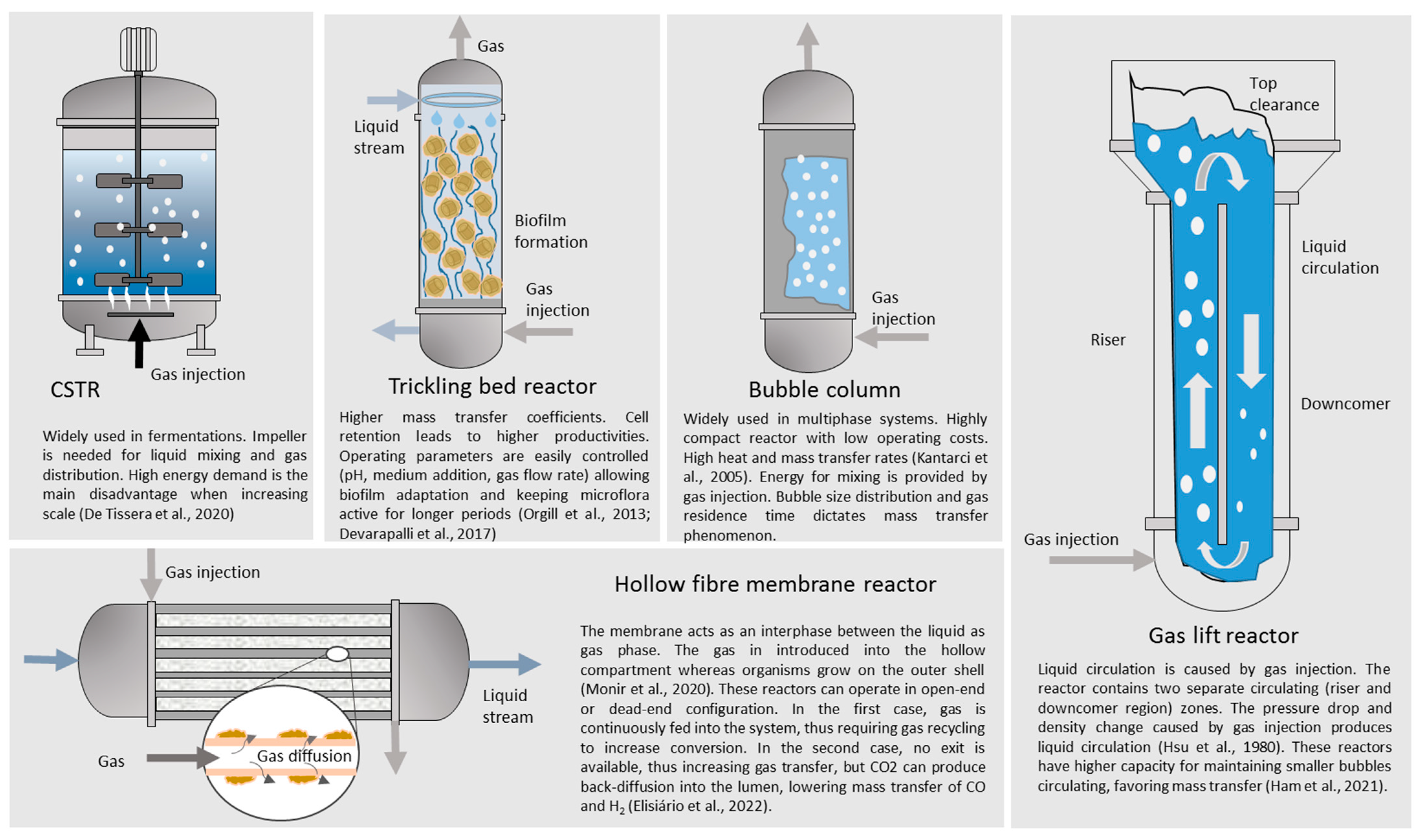
| Material | Gasification | Pyrolysis |
|---|---|---|
| Sewage sludge | Steam gasification, laboratory scale reactor. Total of 35 g of sample. Increasing the temperature from 700 to 1000 °C increases hydrogen content in syngas [64]. | Fixed bed reactor. Slow pyrolysis. Temperature: 400–800 °C. Pyrolysis gas was strongly temperature dependent [65]. |
| Air as the gasification agent. Analysis of tar and heavy metal content in tar. Concerns regarding final ash disposal after gasification [66]. | Quartz tube reactor. Temperature: 300–700 °C. The presence of heavy metals in biochar was studied, reporting an accumulation in char [67]. | |
| Use of rotary kiln reactor with long residence time (30–50 min). Temperature: 700–850 °C. Gas yields of 1 m3/kg sludge with HHV 1 of 6–9 MJ/m3 [68]. | Horizontal tube reactor. Temperature: 550–850 °C. Increase in temperature reduces bio-oil and char yield [69]. | |
| Fixed bed gasifier with varied ER 2 (0.12–0.27). Applying air preheating improved H2 and CO yields [70]. | Horizontal auger reactor. Pilot-plant. Electrically heated, temperature between 500 and 600 °C. Mixture of sewage sludge and manure. Feeding rate: Charges of 12 kg every 2 min [23]. | |
| Pilot-Scale Downdraft Gasifier. The effect of ER was evaluated. An increase in this parameter increased gasification temperature, thus affecting syngas composition and tar/dust formation [71]. | Sequential pyrolysis-gasification: Pyrolysis (400–550 °C) to produce char. Gasification of char. LHV 3 syngas: 5.31–5.65 MJ/m3 [57]. | |
| Atmospheric fluidized bed reactor. Better gas quality was obtained when a torrefaction pre-treatment was implemented. However, carbon conversion efficiency slightly decreases due to the lower volatile content of the torrefied material [72]. | Kinetic evaluation by use of thermal analysis. Use of tubular pyrolyzer. Fast pyrolysis maximized oil yields (550 °C) [73]. | |
| Manure | Dairy cattle manure. Fluidized bed gasifier. Steam gasification. Evaluated the effect of temperature. Syngas LHV 3: 2–4.7 MJ/m3 [74]. | Solid fraction of pig manure. Vertical auger pyrolysis reactor. Temperature: 500–650 °C. Flow rate: 0.4–0.8 kg/h [75]. |
| Dairy cattle manure Fluidized bed gasifier. Air–oxygen used as the gasification agent. Feeding rate: 8 kg/min Temperature: 600–800 °C. Syngas LHV: 1.78–8 MJ/m3 [76]. | Poultry litter. Laboratory-scale bubbling fluidized bed reactor using AL2O3 as bed material. Temperature: 460 and 530 °C. Residence time: 0.9 and 0.7 s. Feeding rates: 0.96 and 0.88 kg/h [77]. | |
| Cattle manure. Tubular furnace chamber. ER: 0.21–0.3. Syngas HHV: 4.89 MJ/m3. Use of electrical furnace. Electricity obtained from photovoltaic panels [78]. | Chicken manure. Pilot-scale screw pyrolysis reactor STYX. Char was produced and heavy metal toxicity was evaluated when applying the material to soils [79]. | |
| Switchgrass, hardwood, softwood, fiber, cardboard, and chicken manure. Pilot-scale 10 kWth down-draft gasification facility. The performance of materials and mixtures was evaluated. Significant differences were found between switchgrass and chicken manure mixed with wood chips, with the first one showing better results [80]. | Cattle manure Rotary kiln reactor Temperatures: 400, 500, and 600 °C. Best oil yield obtained at 500 °C [81]. | |
| Turkey manure and meat bone. Entrained flow gasification. Temperature: 1400 °C Biomass was tested as single input and in mixtures with lignite and hard coal. Significant condensable and noncondensable inorganics were measured although the high temperature applied [82]. | Pig and chicken manure. Temperature: 600 °C. Capacity: 6 t/d. Retention time: 45 min. Gas and oil phase was recycled into the chamber and burned to supply energy to the process [83]. | |
| Wood pellets, sewage sludge, and manure. Dual fluidized bed reactor. No bed agglomeration was observed at temperatures below 820 °C for the gasification stage and 950 °C for the combustion zone. Limestone addition allowed reducing impurities (tar, NH3, H2S) [84]. | Pig manure. Sequential pyrolysis-gasification. Pyrolysis: 700–800 °C. Gasification agent: Steam/CO2. Lower char yields were obtained in gasification with steam. Increase in the process temperature reduces char production [85]. | |
| Biomass | Wood pellet. Gasification agent: steam. Temperature: 750–850 °C. Pilot plant. Syngas composition was analyzed, attaining 43.3% of H2 when optimizing temperature and steam injection. The energy content in syngas was higher for tests where the methane concentration was higher [86]. | Wood pellets (Picea mariana and Pinus banksiana mixture). Vertical auger pyrolysis reactor. Temperature: 500–650 °C. Flow rate: 0.61–1.08 kg/h [74]. |
| Fluidized bed gasifier. Gasification agent: steam. Steam biomass ratio (0.75/2). Temperature: 650–850 °C. The increase in temperature increases the H2 and CO content in syngas. The increase in steam ratio increases H2 and methane content as fuel interest gases [87]. | Switchgrass (Panicum Virgatum L.) Vertical auger pyrolysis reactor. Temperature: 450–600 °C. Flow rate: 0.57 kg/h [75]. | |
| Sawdust. Bubbling fluidized bed gasifier. Gasification agent: air, oxygen enriched air, steam. H2 production was higher when using steam. LHV syngas: 5.95–7.16 MJ/m3 with air, 6.68–11.4 with oxygen enriched air and pure oxygen [88]. | Beech wood (Lignocel HBS 150–500 by J. Rettenmaier & Söhne GmbH + Co KG, Rosenberg, Germany) Bench-scale fixed bed reactor. Use of H-ZSM-5 and Al-MCM-41 pellet catalyst to reduce oxygen content in bio-oil [89]. | |
| Different biomass types. Bubbling fluidized bed reactor. Best gasification conditions were obtained at 900 °C with a steam to air ratio of 70:30. Torrified biomass showed higher efficiency. LHV syngas: 10.6–12 MJ/m3 [90]. | Bagasse. Fluidized bed reactor. Pilot plant flow rate: 3 kg/h. Retention time: 1 h. Temperature: 500 °C. Catalyst: CaO. Oxygen content of bio-oil is reduced but a lower oil yield is obtained [91]. |
| Process | Company | Technology Description | References |
|---|---|---|---|
| Shell gasification technology | Shell global | Large scale commercial technology. The technology is capable of upgrading the bottom-of-the-barrel fraction and low-value streams into synthesis gas. | [95] |
| Valmet gasifier: Circulating fluidized bed gasification | Valmet | Large scale plant with several units operating in Finland. Partial combustion of biomass/waste at high temperatures with controlled air addition. | [96] |
| Bubbling fluidized bed gasification | EQTEC | Demonstration plant at Movialsa (Ciudad Real, Spain) fueled with olive mill solid waste. Several projects under construction. | [97] |
| Co-current fixed bed downdraft gasification (GASCLEAN®) | Co-designed by PROVADEMSE, Villeurbanne, France (technological platform) and Cogebio (Loyettes, France) is the manufacturer. | TRL7, demonstration plant. The reactor contains different zones: drying, pyrolysis, oxidation, and reduction. | [98] |
| Uhde® entrained-flow gasification | Thyssenkrupp (Dortmund, Germany) | Demonstration plant in Puerto Llano Spain. Now shut down. Maximization of feedstock flexibility. Co-gasification of biomass and coal. | [99,100] |
| Mitsubishi Municipal Solid Waste (MSW) Gasification & Ash Melting System | Mitsubishi Heavy Industries Environmental & Chemical Engineering Co., Ltd. (Yokohama, Japan) | Commercial plant. Gasification plant with ash melting process to reduce the volume of fly ash produced. Metals can be recovered from the process. | [101] |
| Biomass gasification and chemicals from syngas | Mitsubishi Heavy Industries, Chubu Electric Power Co., Inc., (Nagoya, Japan), National Institute of Advanced Industrial Science and Technology | Commercial plant. Entrained-flow gasifier developed by MHI. The system gasifies pulverized biomass. No bed material is necessary; thus the temperature can be as high as 1000 °C. | [102] |
| Bubbling fluidized bed gasifier and methanol production | Enerkem (Montreal, Canada) | Gasification of wastes using a specialized product purification to obtain ultraclean syngas for catalytic conversion into methanol/ethanol. | [103] |
| Bioliq® process | KIT, Karlshruhe Institute of Technology (Karlsruhe, Germany) | Decentralized fast pyrolysis of biomass to produce bioSyncrude. Transport of pyrolysis-oil to a centralized unit for gasification and synthesis of fuels. | [104] |
| Aitos Gasification Technology | GTH, Green transition holding (Oslo, Norway) | Large scale plant. Waste gasification. Modular process. Low NOx emission process. | [105] |
| Wood gas CHP | URBAS energietechnik, (Völkermarkt, Austria) | Downdraft fixed bed gasifier. Feasible system for electricity production of less than 1000 kWel. | [106] |
| Syncraft®: Floating fixed bed gasification process | Syncraft Engineering GmbH, (Schwaz Austria) | The floating bed concept allows keeping char loose inside the reactor, preventing compaction and obtaining a permeable material. | [107] |
| Wood gasifier with CHP | Burkhardt Energy and Building Technology (Mühlhausen, Germany) | Ascending downdraft gasification. Fuel and air are fed to the gas reactor from the bottom, allowing pellets to swirl in a certain zone but avoiding being carried out. Wood pellets should be high quality and uniform to attain bed stabilization. | [108] |
| Biomass steam gasification | Repotec (Vienna, Austria) | Medium-sized power plants. The technology is based on steam-blown fluidized-bed gasifier. A nitrogen-free atmosphere is created, producing a low-tar content gas with high calorific value. Syngas is suited for power and heat generation and catalytic transformations. | [109] |
| Wood gasifier with CHP | Pyrox (Güstrow, Germany) | Medium scale. Combined updraft–downdraft fixed bed gasifier system. Four stages: drying–pyrolysis–oxidation–reduction. Oxidation takes place at high temperature (1000 °C). No wastewater is produced. Ash production is about 1% of the wood chip input. | [110] |
| ECO20x CHP System | ECO20x Energia rinnovabile (Caserta, Italy) | Downdraft gasifier capable of using high quality biomass and low-grade biomass such as crop waste with water content up to 20%, olive mill waste and textiles. | [111] |
| CHiP50 | ESPE energy expertise (Grantorto, Italy) | Downdraft gasifier treating high quality wood chips maximum 10% humidity content. Low ash production. | [112] |
| Microorganism | Product Concentration (g/L) | Main Characteristic of the Study | Reference |
|---|---|---|---|
| C. autoethanogenum | Ethanol: 2.24–3.76 | Addition of malt and vegetable extracts as replacement of yeast extract | [134] |
| Ethanol | Inactivation of adhE results in 180% increase in ethanol production | [175] | |
| Ethanol: 4.23–4.57 | Tryptone and peptone supplementation | [176] | |
| Ethanol: 3.45 | Yeast extract optimization Acetate supplementation increased ethanol yield | [177] | |
| Ethanol < 1.5 mM (0.069) | Unable to achieve cell resting conditions when a nitrogen limited environment was established | [178] | |
| Ethanol | Simultaneous feeding of xylose and CO | [179] | |
| Ethyl acetate | Metabolic engineering: expression of the Sce Atf1 resulted in ethyl acetate formation | [180] | |
| Values reported as production rate (mmol/L day) Butyrate: 8.5 Caproate: 0.63 Butanol: 3.5 Hexanol: 2.0 | Co-culture with Clostridium kluyveri Acetate and ethanol initially produced were converted into elongated products. Different pH conditions are necessary for the two species | [181] | |
| C. ljungdahlii | Ethanol: 0.3 | Nano particle addition to enhance syngas solubility | [182] |
| Ethanol | Nutrient availability and pH can be manipulated to achieve solventogenesis | [136] | |
| Ethanol: 2.0 | pH and gas flow regulation | [183] | |
| Ethanol: 1.09 | Improving bioreactor productivity by the use of hollow fiber system | [184] | |
| Ethanol: 0.34 2,3-Butanediol: 0.47 | Optimizing H2/CO ratio. Acetate production was favored by higher concentrations of hydrogen in the headspace. Alcohol production was favored by greater carbon monoxide concentrations in the headspace | [185] | |
| Ethanol: 0.85–3.75 | Evaluating gas flow rate, media and effluent flow rate, pH level, and stirrer speed. Pure CO and syngas mimic streams were fermented. Better results were obtained with synthetic syngas. | [186] | |
| Ethanol: 0.24–0.53 | Trace element medium optimization | [187] | |
| C. carboxidivorans | Ethanol: 2.0 Butanol: 1.0 | Trace element medium optimization | [188] |
| Ethanol: 1.7–3.23 Butanol: 0.09–1.02 | Effect of NH3, H2S and NOx | [189] | |
| Ethanol: 3.64 Butanol: 1.35 Hexanol: 0.66 | Temperature optimization (two-steps: 37–25 °C) | [190] | |
| Ethanol: 23.93 | Improving bioreactor productivity by the use of hollow fiber system | [191] | |
| Ethanol: 1.20 Butanol: 1.20 Hexanol: 1.90 | Hexanol production was enhanced to 2.34 g/L when ethanol was supplemented | [192] | |
| Ethanol: 1.40–1.50 Butanol: 0.40–0.50 Hexanol: 0.10–0.20 1 | Evaluating the effect of oxygen presence in syngas | [193] | |
| Ethanol: 2.28 Butanol: 0.74 | Optimizing medium and reducing costs | [194] | |
| Ethanol concentration not reported | Evaluating gas–liquid volume (VL/VG) ratio. Optimum growth rate takes place at CO partial pressure of 1.1 atm (equivalent to 25 mg/L) | [137] | |
| C6 acids/alcohols Clostridium sp. JS66 | Characterization of a new acetogen for producing hexanoic acid. Increase in gas feeding pressure reduces product yield | [195] | |
| Total alcohols (ethanol + butanol): 2.43–4.58 | Addition of char increased product yield | [196] | |
| C. ragsdalei | Ethanol: 2.01 | Key nutrients modulation: calcium pantothenate, vitamin B12, cobalt chloride (CoCl2) | [197] |
| Ethanol: 11–13.2 | Addition of poultry litter biochar as substitute of costly buffer components | [198] | |
| Ethanol: 14.92 | Random mutagenesis was performed using ethyl-methyl-sulfonate and UV followed by protoplast fusion | [199] | |
| Ethanol: 2.86–5.14 | Addition of char increased product yield | [196] | |
| Ethanol: 0.5–1.5 | Optimization of fermenting media and reducing medium costs | [200] |
| Contaminant | Culture | Effect | Reference |
|---|---|---|---|
| Ammonia | C. ragsdalei | Ammonia transforms into NH4+ ion, accumulating in the system and thus inhibiting hydrogenase activity. | [213] |
| Hydrogen cyanide | C. ljungdahlii | Product distribution was affected by the presence of cyanide, showing changes in ethanol-acetic acid ratio. Growth on fructose showed better tolerance, but growth on syngas was highly affected at a 0.1 mM concentration of cyanide in syngas. | [214] |
| Oxygen | C. carboxidivorans | The presence of oxygen led to drastically reduced growth and product formation without alcohol production. | [193] |
| Mixture of impurities. Testing commercially cleaned biomass syngas | C. ljungdahlii | Commercial syngas gas resulted in lower productivity when compared to clean syngas. Biomass derived syngas adversely affected carbon fixation. | [215] |
| Mixture of impurities. Testing tar free syngas obtained from absorbers and particle removal filters | C. butyricum | Impurities caused complete inhibition. Clean syngas produced 29.24 mmol of butanol from 1 L of syngas. | [216] |
| Condensables | Butyribacterium methylotrophicum | Complex mixtures of syngas impurities hinder the growth and alter the profile carboxylic acid production. | [217] |
| Tar | Saccharomyces cerevisiae | Total inhibition of cell growth. | [218] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ellacuriaga, M.; Gil, M.V.; Gómez, X. Syngas Fermentation: Cleaning of Syngas as a Critical Stage in Fermentation Performance. Fermentation 2023, 9, 898. https://doi.org/10.3390/fermentation9100898
Ellacuriaga M, Gil MV, Gómez X. Syngas Fermentation: Cleaning of Syngas as a Critical Stage in Fermentation Performance. Fermentation. 2023; 9(10):898. https://doi.org/10.3390/fermentation9100898
Chicago/Turabian StyleEllacuriaga, Marcos, María Victoria Gil, and Xiomar Gómez. 2023. "Syngas Fermentation: Cleaning of Syngas as a Critical Stage in Fermentation Performance" Fermentation 9, no. 10: 898. https://doi.org/10.3390/fermentation9100898
APA StyleEllacuriaga, M., Gil, M. V., & Gómez, X. (2023). Syngas Fermentation: Cleaning of Syngas as a Critical Stage in Fermentation Performance. Fermentation, 9(10), 898. https://doi.org/10.3390/fermentation9100898






