Certain Fermented Foods and Their Possible Health Effects with a Focus on Bioactive Compounds and Microorganisms
Abstract
:1. Introduction
2. Fermented Dairy Products
2.1. Kefir
2.2. Yogurt
2.3. Cheese
3. Fermented Meats
| Fermented Foods | Certain Bioactive Compounds | Effects on Health | References | |
|---|---|---|---|---|
| Intestine | ||||
| Fermented mutton jerky | x3-2b Lactiplantibacillus plantarum and composite bacteria | Purine content of fermented mutton jerky by x3-2b Lactobacillus plantarum and composite bacteria ↓ In vitro digestion, decreasing purine content by 37x-3 Pediococcus pentosaceus ↑ | [170] | |
| Cured beef | - | Gastric protein carbonylation ↑ Colonic Ruminococcaceae ↑ Cecal propionate ↑ TBARs and diacetyl in feces ↑ Levels of cecal butyrate, fecal phenol, dimethyl disulfide ↓ Level of fecal carbon disulfide ↑ Colonic Ruminococcaceae ↑ | [171] | |
| Fermented sausage | Enterococcus faecium CRL 183 | Lactobacillus spp. in ascending colon, transverse colon, and descending colon ↓ Bacteroides spp. in descending colon ↓ Enterobacteriaceae in transverse colon and descending colon ↓ Colonic ammonium ions ↑ Butyric acid concentration in transverse colon, ascending colon, and descending colon ↑ Concentration of propionic acids in ascending colon and transverse colon ↑ Concentration of acetic acid in ascending colon, transverse colon, and descending colon ↓ | [172] | |
| Fermented sausage | - | Release of free iron in digestive system ↑ Concentration of gastric N-nitrosamine ↑ | [173] | |
| Fermented sausage | Enterococcus faecium S27 | Transfer of tetracycline resistance determinant (tet(M)) to E. faecium and Enterococcus faecalis ↑ Transfer of Enterococcus faecium’s streptomycin resistance ↑ | [174] | |
| Fermented sausage | Bologna sausage (a) Dry fermented sausage (b) | Calcium transporter in Caco-2 cells: in (a) ↑, in (b) ↓ | [175] | |
| Fermented salami | Plant extracts | Phenol and p-cresol in colon ↓ Acetate, propionate, butyrate in colon ↑ Enterobacteriaceae ↓ Bifidobacteriaceae ↑ | [176] | |
| Fermented fish | Staphylococcus sp. DBOCP6 | Non-hemolytic and non-pathogenic effects against broad and narrow spectrum antibiotics Ability to adhere to the intestinal wall | [177] | |
| Cardiovascular diseases and ACE-I inhibitory effects | ||||
| - | - | Cardiovascular disease risk, stroke risk ↑ Total mortality risk ↑ | [178] | |
| Salami Sausage | Cardiovascular disease risk ↑ | [179] | ||
| - | Cardiovascular disease risk ↑ | [180] | ||
| - | Total stroke incidence ↑ No association between ischemic stroke and coronary heart disease mortality | [181] | ||
| Bacon Sausage | - | Cardiovascular death risk ↑ Ischemic heart disease risk ↑ | [182] | |
| Dry-cured pork ham | - | Levels of total cholesterol, LDL, basal glucose ↓ | [183] | |
| Semi-dry fermented camel sausage | Lactiplantibacillus plantarum KX881772 | Inhibition of ACE ↑ Cytotoxicity activity towards Caco-2 cell line ↑ α-amylase inhibition ↑ α-glucosidase inhibition ↑ | [184] | |
| Fermented pork sausage | Staphylococcus simulans NJ201 Lactiplantibacillus plantarum CD101 | ACE inhibition ↑ Superoxide anion scavenging activities ↑ Ferric-reducing antioxidant activity ↑ | [185] | |
| Dry fermented camel sausage | Staphylococcus xylosus and Lactiplantibacillus plantarum Staphylococcus caarnosus and Latilactobacillus sakei Staphylococcus xylosus and Lactobacillus pentosus | Antioxidant capacity by <3 kDa peptides ↑ Maximum ACE inhibition by <3 kDa peptides Maximum ACE inhibition in sausages with S. xylosus and L. plantarum | [186] | |
| Dry-cured ham | - | ACE inhibition ↑ Radical scavenging activity ↑ PAF-AH inhibitory effect ↑ | [187] | |
| Fermented meat | - | Antioxidant activity against OH-radical by GlnTyr-Pro ↑ | [188] | |
| Dry-fermented sausage | Starter culture (P200S34) and protease (EPg222) | ACE inhibition ↑ Antioxidant activity ↑ | [189] | |
| - | Risk of cardiovascular mortality, stroke, myocardial infarction via reduction in processed meat ↓ | [190] | ||
| - | Risk of all-mortality cause and cardiometabolic disease via lower consumption ↓ | [191] | ||
| - | Risk of heart failure ↑ | [192] | ||
| Cancer | ||||
| - | Risk of colon cancer, rectal cancer, breast cancer, lung cancer, and colorectal cancer ↑ | [193] | ||
| Ham Sausage Bacon | - | Breast cancer risk ↑ | [194] | |
| - | Weak positive association with breast cancer | [195] | ||
| - | Breast cancer risk with diet rich in processed meat ↑ | [196] | ||
| Ham Sausage Bacon | - | Gastric cancer risk ↑ | [197] | |
| Ham Sausage Bacon | - | Colorectal cancer risk ↑ | [198] | |
| - | Colorectal cancer risk with lower consumption ↓ | [199] | ||
| - | Colorectal cancer risk with lower consumption ↓ | [200] | ||
| - | Colorectal cancer risk ↑ | [201] | ||
| Ham Sausage Bacon | - | Colorectal cancer risk ↑ | [202] | |
| Ham Sausage Bacon | - | Colorectal cancer risk ↑ | [203] | |
| - | Colorectal adenoma risk ↑ | [204] | ||
| Ham | - | Risk of renal cell carcinoma ↑ Risk of bladder cancer ↑ | [205] | |
| Ham Sausage Bacon | - | Bladder cancer risk ↑ | [206] | |
| Ham Sausage Bacon | - | Minimal connection to kidney cancer risk | [207] | |
| Ham Salami Sausage Bacon | - | No association with gliomas | [208] | |
| Risk of hepatocellular carcinoma ↑ | [209] | |||
| Other diseases | ||||
| - | Risk of type 2 diabetes ↑ | [210] | ||
| Bacon Salami Sausages | - | Risk of diabetes as well as stroke and coronary heart disease ↑ | [211] | |
| - | Risk of type 2 diabetes ↑ | [212] | ||
| - | Type 2 diabetes risk ↑ | [213] | ||
| - | Gestational diabetes mellitus risk ↑ | [214] | ||
| - | No change in Crohn’s disease flares | [215] | ||
| - | Risk of mortality via increase in consumption ↑ | [216] | ||
| - | Mortality risk of all causes (except cancer) and cardiovascular-caused mortality ↑ | [217] | ||
| - | Depression risk ↑ | [218] | ||
| N-Nitrosodimethylamine | No change in glioma | [219] | ||
| Diethylnitrosamine | Probability of hepatocarcinogenesis | [220] | ||
4. Fermented Vegetables and Fruits
4.1. Fermented Vegetables
4.2. Fermented Fruits
5. Fermented Legumes
6. Fermented Cereals
7. The Other Side of Fermented Foods
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ray, R.; Joshi, V. Fermented Foods: Past, Present and Future. In Microorganisms and Fermentation of Traditional Foods; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Marco, M.L.; Sanders, M.E.; Gänzle, M.; Arrieta, M.C.; Cotter, P.D.; De Vuyst, L.; Hill, C.; Holzapfel, W.; Lebeer, S.; Merenstein, D. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented foods. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 196–208. [Google Scholar] [PubMed]
- Annunziata, G.; Arnone, A.; Ciampaglia, R.; Tenore, G.C.; Novellino, E. Fermentation of foods and beverages as a tool for increasing availability of bioactive compounds. Focus on short-chain fatty acids. Foods 2020, 9, 999. [Google Scholar] [CrossRef]
- Melini, F.; Melini, V.; Luziatelli, F.; Ficca, A.G.; Ruzzi, M. Health-Promoting Components in Fermented Foods: An Up-to-Date Systematic Review. Nutrients 2019, 11, 1189. [Google Scholar] [CrossRef]
- Leeuwendaal, N.K.; Stanton, C.; O’Toole, P.W.; Beresford, T.P. Fermented Foods, Health and the Gut Microbiome. Nutrients 2022, 14, 1527. [Google Scholar] [CrossRef] [PubMed]
- Mathur, H.; Beresford, T.P.; Cotter, P.D. Health Benefits of Lactic Acid Bacteria (LAB) Fermentates. Nutrients 2020, 12, 1679. [Google Scholar] [CrossRef] [PubMed]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar]
- Terefe, N.S. Food Fermentation. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- FAO; WHO. Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria. pp. 1–29. Available online: https://www.fao.org/3/y6398e/y6398e.pdf (accessed on 11 September 2023).
- International Scientific Association for Probiotics and Prebiotics, ISAPP. Probiotics: Dispelling Myths; ISAPP: Sacramento, CA, USA, 2018. [Google Scholar]
- Ilango, S.; Antony, U. Probiotic microorganisms from non-dairy traditional fermented foods. Trends Food Sci. Technol. 2021, 118, 617–638. [Google Scholar] [CrossRef]
- Diez-Ozaeta, I.; Astiazaran, O.J. Fermented foods: An update on evidence-based health benefits and future perspectives. Food Res. Int. 2022, 156, 111133. [Google Scholar] [CrossRef]
- Jaiswal, S.; Pant, T.; Suryavanshi, M.; Antony, U. Microbiological diversity of fermented food Bhaati Jaanr and its antioxidant and anti-inflammatory properties: Effect against colon cancer. Food Biosci. 2023, 55, 102822. [Google Scholar] [CrossRef]
- Papadimitriou, C.G.; Vafopoulou-Mastrojiannaki, A.; Silva, S.V.; Gomes, A.-M.; Malcata, F.X.; Alichanidis, E. Identification of peptides in traditional and probiotic sheep milk yoghurt with angiotensin I-converting enzyme (ACE)-inhibitory activity. Food Chem. 2007, 105, 647–656. [Google Scholar] [CrossRef]
- Gu, Y.; Li, X.; Chen, H.; Sun, Y.; Yang, L.; Ma, Y.; Yong Chan, E.C. Antidiabetic effects of multi-species probiotic and its fermented milk in mice via restoring gut microbiota and intestinal barrier. Food Biosci. 2022, 47, 101619. [Google Scholar] [CrossRef]
- Khakhariya, R.; Sakure, A.A.; Maurya, R.; Bishnoi, M.; Kondepudi, K.K.; Padhi, S.; Rai, A.K.; Liu, Z.; Patil, G.B.; Mankad, M.; et al. A comparative study of fermented buffalo and camel milk with anti-inflammatory, ACE-inhibitory and anti-diabetic properties and release of bio active peptides with molecular interactions: In vitro, in silico and molecular study. Food Biosci. 2023, 52, 102373. [Google Scholar] [CrossRef]
- Tunick, M.H.; Van Hekken, D.L. Dairy Products and Health: Recent Insights. J. Agric. Food Chem. 2015, 63, 9381–9388. [Google Scholar] [CrossRef] [PubMed]
- Nongonierma, A.B.; FitzGerald, R.J. Bioactive properties of milk proteins in humans: A review. Peptides 2015, 73, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Shiby, V.K.; Mishra, H.N. Fermented milks and milk products as functional foods—A review. Crit. Rev. Food Sci. Nutr. 2013, 53, 482–496. [Google Scholar] [CrossRef]
- Fernández, M.; Hudson, J.A.; Korpela, R.; de los Reyes-Gavilán, C.G. Impact on human health of microorganisms present in fermented dairy products: An overview. Biomed. Res. Int. 2015, 2015, 412714. [Google Scholar] [CrossRef]
- Lin, M.Y.; Young, C.M. Folate levels in cultures of lactic acid bacteria. Int. Dairy J. 2000, 10, 409–413. [Google Scholar] [CrossRef]
- Van Wyk, J.; Witthuhn, R.C.; Britz, T.J. Optimisation of vitamin B12 and folate production by Propionibacterium freudenreichii strains in kefir. Int. Dairy J. 2011, 21, 69–74. [Google Scholar] [CrossRef]
- Hugenschmidt, S.; Schwenninger, S.M.; Lacroix, C. Concurrent high production of natural folate and vitamin B12 using a co-culture process with Lactobacillus plantarum SM39 and Propionibacterium freudenreichii DF13. Process Biochem. 2011, 46, 1063–1070. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Gyawali, R.; Awaisheh, S.S.; Ayivi, R.D.; Silva, R.C.; Subedi, K.; Aljaloud, S.O.; Anusha Siddiqui, S.; Krastanov, A. Fermented foods and probiotics: An approach to lactose intolerance. J. Dairy Res. 2021, 88, 357–365. [Google Scholar] [CrossRef]
- Prado, M.R.; Blandón, L.M.; Vandenberghe, L.P.; Rodrigues, C.; Castro, G.R.; Thomaz-Soccol, V.; Soccol, C.R. Milk kefir: Composition, microbial cultures, biological activities, and related products. Front. Microbiol. 2015, 6, 1177. [Google Scholar] [CrossRef]
- Azizi, N.F.; Kumar, M.R.; Yeap, S.K.; Abdullah, J.O.; Khalid, M.; Omar, A.R.; Osman, M.A.; Mortadza, S.A.S.; Alitheen, N.B. Kefir and Its Biological Activities. Foods 2021, 10, 1210. [Google Scholar] [CrossRef]
- Rosa, D.D.; Dias, M.M.S.; Grześkowiak, Ł.M.; Reis, S.A.; Conceição, L.L.; Peluzio, M. Milk kefir: Nutritional, microbiological and health benefits. Nutr. Res. Rev. 2017, 30, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; Ferrocino, I.; Reale, A.; Sabbatini, R.; Milanović, V.; Alkić-Subašić, M.; Boscaino, F.; Aquilanti, L.; Pasquini, M.; Trombetta, M.F.; et al. Study of kefir drinks produced by backslopping method using kefir grains from Bosnia and Herzegovina: Microbial dynamics and volatilome profile. Food Res. Int. 2020, 137, 109369. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Gu, F.; Abdella, N.H.; Ruan, H.; He, G. Investigation on culturable microflora in Tibetan kefir grains from different areas of China. J. Food Sci. 2012, 77, M425–M433. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, X.; Jiang, H.; Dong, M. Analysis of the microflora in Tibetan kefir grains using denaturing gradient gel electrophoresis. Food Microbiol. 2009, 26, 770–775. [Google Scholar] [CrossRef]
- Altay, F.; Karbancıoglu-Güler, F.; Daskaya-Dikmen, C.; Heperkan, D. A review on traditional Turkish fermented non-alcoholic beverages: Microbiota, fermentation process and quality characteristics. Int. J. Food Microbiol. 2013, 167, 44–56. [Google Scholar] [CrossRef]
- Bourrie, B.C.; Willing, B.P.; Cotter, P.D. The Microbiota and Health Promoting Characteristics of the Fermented Beverage Kefir. Front. Microbiol. 2016, 7, 647. [Google Scholar] [CrossRef]
- Yirmibesoglu, S.; Tefon Öztürk, B. Comparing microbiological profiles, bioactivities, and physicochemical and sensory properties of donkey milk kefir and cow milk kefir. Turk. J. Vet. Anim. Sci. 2020, 44, 774–781. [Google Scholar] [CrossRef]
- Aires, R.; Gobbi Amorim, F.; Côco, L.Z.; da Conceição, A.P.; Zanardo TÉ, C.; Taufner, G.H.; Nogueira, B.V.; Vasquez, E.C.; Melo Costa Pereira, T.; Campagnaro, B.P.; et al. Use of kefir peptide (Kef-1) as an emerging approach for the treatment of oxidative stress and inflammation in 2K1C mice. Food Funct. 2022, 13, 1965–1974. [Google Scholar] [CrossRef]
- Maalouf, K.; Baydoun, E.; Rizk, S. Kefir induces cell-cycle arrest and apoptosis in HTLV-1-negative malignant T-lymphocytes. Cancer Manag. Res. 2011, 3, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, F.S.; Ozarslan, S.; Guzel-Seydim, Z.B.; Kök Taş, T. The effect of kefir produced from natural kefir grains on the intestinal microbial populations and antioxidant capacities of Balb/c mice. Food Res. Int. 2019, 115, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Ton, A.M.M.; Campagnaro, B.P.; Alves, G.A.; Aires, R.; Côco, L.Z.; Arpini, C.M.; Guerra, E.O.T.; Campos-Toimil, M.; Meyrelles, S.S.; Pereira, T.M.C.; et al. Oxidative Stress and Dementia in Alzheimer’s Patients: Effects of Synbiotic Supplementation. Oxidative Med. Cell Longev. 2020, 2020, 2638703. [Google Scholar] [CrossRef]
- Bellikci-Koyu, E.; Sarer-Yurekli, B.P.; Akyon, Y.; Aydin-Kose, F.; Karagozlu, C.; Ozgen, A.G.; Brinkmann, A.; Nitsche, A.; Ergunay, K.; Yilmaz, E.; et al. Effects of Regular Kefir Consumption on Gut Microbiota in Patients with Metabolic Syndrome: A Parallel-Group, Randomized, Controlled Study. Nutrients 2019, 11, 2089. [Google Scholar] [CrossRef]
- Karaffová, V.; Mudroňová, D.; Mad’ar, M.; Hrčková, G.; Faixová, D.; Gancarčíková, S.; Ševčíková, Z.; Nemcová, R. Differences in Immune Response and Biochemical Parameters of Mice Fed by Kefir Milk and Lacticaseibacillus paracasei Isolated from the Kefir Grains. Microorganisms 2021, 9, 831. [Google Scholar] [CrossRef]
- Chen, H.L.; Tung, Y.T.; Chuang, C.H.; Tu, M.Y.; Tsai, T.C.; Chang, S.Y.; Chen, C.M. Kefir improves bone mass and microarchitecture in an ovariectomized rat model of postmenopausal osteoporosis. Osteoporos. Int. 2015, 26, 589–599. [Google Scholar] [CrossRef]
- Malta, S.M.; Batista, L.L.; Silva, H.C.G.; Franco, R.R.; Silva, M.H.; Rodrigues, T.S.; Correia, L.I.V.; Martins, M.M.; Venturini, G.; Espindola, F.S.; et al. Identification of bioactive peptides from a Brazilian kefir sample, and their anti-Alzheimer potential in Drosophila melanogaster. Sci. Rep. 2022, 12, 11065. [Google Scholar] [CrossRef]
- Hamet, M.F.; Medrano, M.; Pérez, P.F.; Abraham, A.G. Oral administration of kefiran exerts a bifidogenic effect on BALB/c mice intestinal microbiota. Benef. Microbes 2016, 7, 237–246. [Google Scholar] [CrossRef]
- Youn, H.Y.; Kim, D.H.; Kim, H.J.; Bae, D.; Song, K.Y.; Kim, H.; Seo, K.H. Survivability of Kluyveromyces marxianus Isolated from Korean Kefir in a Simulated Gastrointestinal Environment. Front. Microbiol. 2022, 13, 842097. [Google Scholar] [CrossRef]
- Maccaferri, S.; Klinder, A.; Brigidi, P.; Cavina, P.; Costabile, A. Potential probiotic Kluyveromyces marxianus B0399 modulates the immune response in Caco-2 cells and peripheral blood mononuclear cells and impacts the human gut microbiota in an in vitro colonic model system. Appl. Env. Microbiol. 2012, 78, 956–964. [Google Scholar] [CrossRef]
- Youn, H.Y.; Kim, H.J.; Kim, D.H.; Jang, Y.S.; Kim, H.; Seo, K.H. Gut microbiota modulation via short-term administration of potential probiotic kefir yeast Kluyveromyces marxianus A4 and A5 in BALB/c mice. Food Sci. Biotechnol. 2023, 32, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Youn, H.Y.; Kim, D.H.; Kim, H.J.; Jang, Y.S.; Song, K.Y.; Bae, D.; Kim, H.; Seo, K.H. A Combined In Vitro and In Vivo Assessment of the Safety of the Yeast Strains Kluyveromyces marxianus A4 and A5 Isolated from Korean Kefir. Probiotics Antimicrob. Proteins 2023, 15, 129–138. [Google Scholar] [CrossRef]
- Tang, W.; Li, C.; He, Z.; Pan, F.; Pan, S.; Wang, Y. Probiotic Properties and Cellular Antioxidant Activity of Lactobacillus plantarum MA2 Isolated from Tibetan Kefir Grains. Probiotics Antimicrob. Proteins 2018, 10, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Serafini, F.; Turroni, F.; Ruas-Madiedo, P.; Lugli, G.A.; Milani, C.; Duranti, S.; Zamboni, N.; Bottacini, F.; van Sinderen, D.; Margolles, A.; et al. Kefir fermented milk and kefiran promote growth of Bifidobacterium bifidum PRL2010 and modulate its gene expression. Int. J. Food Microbiol. 2014, 178, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Jenab, A.; Roghanian, R.; Ghorbani, N.; Ghaedi, K.; Emtiazi, G. The Efficacy of Electrospun PAN/Kefiran Nanofiber and Kefir in Mammalian Cell Culture: Promotion of PC12 Cell Growth, Anti-MCF7 Breast Cancer Cells Activities, and Cytokine Production of PBMC. Int. J. Nanomed. 2020, 15, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, E.; Farooq, M.; Dailin, D.; El-Enshasy, H.; Othman, N.; Malek, R.; Danial, E.N.; Wadaan, M. In vitro and in vivo biological screening of kefiran polysaccharide produced by Lactobacillus kefiranofaciens. Biomed. Res. 2017, 28, 594–600. [Google Scholar]
- Bahari, A.; Shahabi-Ghahfarrokhi, I.; Koolivand, D. Kefiran ameliorates malfunctions in primary and functional immune cells caused by lipopolysaccharides. Int. J. Biol. Macromol. 2020, 165, 619–624. [Google Scholar] [CrossRef]
- Vinderola, G.; Perdigón, G.; Duarte, J.; Farnworth, E.; Matar, C. Effects of the oral administration of the exopolysaccharide produced by Lactobacillus kefiranofaciens on the gut mucosal immunity. Cytokine 2006, 36, 254–260. [Google Scholar] [CrossRef]
- Radhouani, H.; Gonçalves, C.; Maia, F.R.; Oliveira, J.M.; Reis, R.L. Biological performance of a promising Kefiran-biopolymer with potential in regenerative medicine applications: A comparative study with hyaluronic acid. J. Mater. Sci. Mater. Med. 2018, 29, 124. [Google Scholar] [CrossRef]
- Hasheminya, S.-M.; Dehghannya, J. Novel ultrasound-assisted extraction of kefiran biomaterial, a prebiotic exopolysaccharide, and investigation of its physicochemical, antioxidant and antimicrobial properties. Mater. Chem. Phys. 2020, 243, 122645. [Google Scholar] [CrossRef]
- Uchida, M.; Ishii, I.; Inoue, C.; Akisato, Y.; Watanabe, K.; Hosoyama, S.; Toida, T.; Ariyoshi, N.; Kitada, M. Kefiran reduces atherosclerosis in rabbits fed a high cholesterol diet. J. Atheroscler. Thromb. 2010, 17, 980–988. [Google Scholar] [CrossRef]
- Riaz Rajoka, M.S.; Mehwish, H.M.; Fang, H.; Padhiar, A.A.; Zeng, X.; Khurshid, M.; He, Z.; Zhao, L. Characterization and anti-tumor activity of exopolysaccharide produced by Lactobacillus kefiri isolated from Chinese kefir grains. J. Funct. Foods 2019, 63, 103588. [Google Scholar] [CrossRef]
- Wang, X.; Tian, J.; Zhang, X.; Tang, N.; Rui, X.; Zhang, Q.; Dong, M.; Li, W. Characterization and Immunological Activity of Exopolysaccharide from Lacticaseibacillus paracasei GL1 Isolated from Tibetan Kefir Grains. Foods 2022, 11, 3330. [Google Scholar] [CrossRef]
- You, X.; Yang, L.; Zhao, X.; Ma, K.; Chen, X.; Zhang, C.; Wang, G.; Dong, M.; Rui, X.; Zhang, Q.; et al. Isolation, purification, characterization and immunostimulatory activity of an exopolysaccharide produced by Lactobacillus pentosus LZ-R-17 isolated from Tibetan kefir. Int. J. Biol. Macromol. 2020, 158, 408–419. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Li, Z.; Ma, K.; Zhang, C.; Chen, X.; Wang, G.; Yang, L.; Dong, M.; Rui, X.; Zhang, Q.; et al. Structural characterization and immunomodulatory activity of an exopolysaccharide produced by Lactobacillus helveticus LZ-R-5. Carbohydr. Polym. 2020, 235, 115977. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Xu, D.; Tang, N.; Rui, X.; Zhang, Q.; Chen, X.; Dong, M.; Li, W. Biosynthesis of exopolysaccharide and structural characterization by Lacticaseibacillus paracasei ZY-1 isolated from Tibetan kefir. Food Chem. Mol. Sci. 2021, 3, 100054. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, X.; Jiang, Y.; Zhao, W.; Guo, T.; Cao, Y.; Teng, J.; Hao, X.; Zhao, J.; Yang, Z. Antioxidant status and gut microbiota change in an aging mouse model as influenced by exopolysaccharide produced by Lactobacillus plantarum YW11 isolated from Tibetan kefir. J. Dairy Sci. 2017, 100, 6025–6041. [Google Scholar] [CrossRef] [PubMed]
- Bengoa, A.A.; Dardis, C.; Gagliarini, N.; Garrote, G.L.; Abraham, A.G. Exopolysaccharides from Lactobacillus paracasei Isolated from Kefir as Potential Bioactive Compounds for Microbiota Modulation. Front. Microbiol. 2020, 11, 583254. [Google Scholar] [CrossRef]
- Lim, J.; Kale, M.; Kim, D.H.; Kim, H.S.; Chon, J.W.; Seo, K.H.; Lee, H.G.; Yokoyama, W.; Kim, H. Antiobesity Effect of Exopolysaccharides Isolated from Kefir Grains. J. Agric. Food Chem. 2017, 65, 10011–10019. [Google Scholar] [CrossRef]
- Brasil, G.A.; Silva-Cutini, M.A.; Moraes, F.S.A.; Pereira, T.M.C.; Vasquez, E.C.; Lenz, D.; Bissoli, N.S.; Endringer, D.C.; de Lima, E.M.; Biancardi, V.C.; et al. The benefits of soluble non-bacterial fraction of kefir on blood pressure and cardiac hypertrophy in hypertensive rats are mediated by an increase in baroreflex sensitivity and decrease in angiotensin-converting enzyme activity. Nutrition 2018, 51–52, 66–72. [Google Scholar] [CrossRef]
- Khoury, N.; El-Hayek, S.; Tarras, O.; El-Sabban, M.; El-Sibai, M.; Rizk, S. Kefir exhibits anti-proliferative and pro-apoptotic effects on colon adenocarcinoma cells with no significant effects on cell migration and invasion. Int. J. Oncol. 2014, 45, 2117–2127. [Google Scholar] [CrossRef]
- Liu, J.R.; Wang, S.Y.; Lin, Y.Y.; Lin, C.W. Antitumor activity of milk kefir and soy milk kefir in tumor-bearing mice. Nutr. Cancer 2002, 44, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Jia, H.; Zhang, X.; Wang, X.; Wang, Z.; Gao, Z.; Yuan, Y.; Yue, T. Supplementation of kefir ameliorates azoxymethane/dextran sulfate sodium induced colorectal cancer by modulating the gut microbiota. Food Funct. 2021, 12, 11641–11655. [Google Scholar] [CrossRef]
- Ben Taheur, F.; Mansour, C.; Mechri, S.; Skhiri, S.S.; Jaouadi, B.; Mzoughi, R.; Chaieb, K.; Zouari, N. Does probiotic Kefir reduce dyslipidemia, hematological disorders and oxidative stress induced by zearalenone toxicity in wistar rats? Toxicon X 2022, 14, 100121. [Google Scholar] [CrossRef]
- Grishina, A.; Kulikova, I.; Alieva, L.; Dodson, A.; Rowland, I.; Jin, J. Antigenotoxic effect of kefir and ayran supernatants on fecal water-induced DNA damage in human colon cells. Nutr. Cancer 2011, 63, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, İ.; Dolar, M.E.; Özpınar, H. Effect of administering kefir on the changes in fecal microbiota and symptoms of inflammatory bowel disease: A randomized controlled trial. Turk. J. Gastroenterol. 2019, 30, 242–253. [Google Scholar] [CrossRef]
- Albuquerque Pereira, M.F.; Morais de Ávila, L.G.; Ávila Alpino, G.C.; Dos Santos Cruz, B.C.; Almeida, L.F.; Macedo Simões, J.; Ladeira Bernardes, A.; Xisto Campos, I.; de Oliveira Barros Ribon, A.; de Oliveira Mendes, T.A.; et al. Milk kefir alters fecal microbiota impacting gut and brain health in mice. Appl. Microbiol. Biotechnol. 2023, 107, 5161–5178. [Google Scholar] [CrossRef] [PubMed]
- Ostadrahimi, A.; Taghizadeh, A.; Mobasseri, M.; Farrin, N.; Payahoo, L.; Beyramalipoor Gheshlaghi, Z.; Vahedjabbari, M. Effect of probiotic fermented milk (kefir) on glycemic control and lipid profile in type 2 diabetic patients: A randomized double-blind placebo-controlled clinical trial. Iran. J. Public Health 2015, 44, 228–237. [Google Scholar]
- Kakisu, E.J.; Abraham, A.G.; Pérez, P.F.; De Antoni, G.L. Inhibition of Bacillus cereus in milk fermented with kefir grains. J. Food Prot. 2007, 70, 2613–2616. [Google Scholar] [CrossRef]
- Iraporda, C.; Abatemarco Júnior, M.; Neumann, E.; Nunes, Á.C.; Nicoli, J.R.; Abraham, A.G.; Garrote, G.L. Biological activity of the non-microbial fraction of kefir: Antagonism against intestinal pathogens. J. Dairy Res. 2017, 84, 339–345. [Google Scholar] [CrossRef]
- Amorim, F.G.; Coitinho, L.B.; Dias, A.T.; Friques, A.G.F.; Monteiro, B.L.; Rezende, L.C.D.; Pereira, T.M.C.; Campagnaro, B.P.; De Pauw, E.; Vasquez, E.C.; et al. Identification of new bioactive peptides from Kefir milk through proteopeptidomics: Bioprospection of antihypertensive molecules. Food Chem. 2019, 282, 109–119. [Google Scholar] [CrossRef]
- Ebner, J.; Arslan, A.A.; Fedorova, M.; Hoffmann, R.; Küçükçetin, A.; Pischetsrieder, M. Peptide profiling of bovine kefir reveals 236 unique peptides released from caseins during its production by starter culture or kefir grains. J. Proteom. 2015, 117, 41–57. [Google Scholar] [CrossRef]
- Quirós, A.; Hernández-Ledesma, B.; Ramos, M.; Amigo, L.; Recio, I. Angiotensin-Converting Enzyme Inhibitory Activity of Peptides Derived from Caprine Kefir. J. Dairy Sci. 2005, 88, 3480–3487. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chen, H.L.; Fan, H.C.; Tung, Y.T.; Kuo, C.W.; Tu, M.Y.; Chen, C.M. Anti-Inflammatory, Antioxidant, and Antifibrotic Effects of Kefir Peptides on Salt-Induced Renal Vascular Damage and Dysfunction in Aged Stroke-Prone Spontaneously Hypertensive Rats. Antioxidants 2020, 9, 790. [Google Scholar] [CrossRef]
- de Lima, M.; da Silva, R.A.; da Silva, M.F.; da Silva, P.A.B.; Costa, R.; Teixeira, J.A.C.; Porto, A.L.F.; Cavalcanti, M.T.H. Brazilian Kefir-Fermented Sheep’s Milk, a Source of Antimicrobial and Antioxidant Peptides. Probiotics Antimicrob. Proteins 2018, 10, 446–455. [Google Scholar] [CrossRef]
- Miao, J.; Liu, G.; Ke, C.; Fan, W.; Li, C.; Chen, Y.; Dixon, W.; Song, M.; Cao, Y.; Xiao, H. Inhibitory effects of a novel antimicrobial peptide from kefir against Escherichia coli. Food Control 2016, 65, 63–72. [Google Scholar] [CrossRef]
- Tu, M.Y.; Han, K.Y.; Chang, G.R.; Lai, G.D.; Chang, K.Y.; Chen, C.F.; Lai, J.C.; Lai, C.Y.; Chen, H.L.; Chen, C.M. Kefir Peptides Prevent Estrogen Deficiency-Induced Bone Loss and Modulate the Structure of the Gut Microbiota in Ovariectomized Mice. Nutrients 2020, 12, 3432. [Google Scholar] [CrossRef] [PubMed]
- Yanni, A.E.; Kartsioti, K.; Karathanos, V.T. The role of yoghurt consumption in the management of type II diabetes. Food Funct. 2020, 11, 10306–10316. [Google Scholar] [CrossRef]
- Qing, J.; Peng, C.; Chen, H.; Li, H.; Liu, X. Small molecule linoleic acid inhibiting whey syneresis via interact with milk proteins in the fermentation of set yogurt fortified with c9,t11-conjugated linoleic acid. Food Chem. 2023, 429, 136849. [Google Scholar] [CrossRef] [PubMed]
- Paszczyk, B.; Czarnowska-Kujawska, M.; Klepacka, J.; Tońska, E. Health-Promoting Ingredients in Goat’s Milk and Fermented Goat’s Milk Drinks. Animals 2023, 13, 907. [Google Scholar] [CrossRef]
- Serafeimidou, A.; Zlatanos, S.; Laskaridis, K.; Sagredos, A. Chemical characteristics, fatty acid composition and conjugated linoleic acid (CLA) content of traditional Greek yogurts. Food Chem. 2012, 134, 1839–1846. [Google Scholar] [CrossRef] [PubMed]
- Serafeimidou, A.; Zlatanos, S.; Kritikos, G.; Tourianis, A. Change of fatty acid profile, including conjugated linoleic acid (CLA) content, during refrigerated storage of yogurt made of cow and sheep milk. J. Food Compos. Anal. 2013, 31, 24–30. [Google Scholar] [CrossRef]
- Paszczyk, B.; Czarnowska-Kujawska, M. Fatty Acid Profile, Conjugated Linoleic Acid Content, and Lipid Quality Indices in Selected Yogurts Available on the Polish Market. Animals 2022, 12, 96. [Google Scholar] [CrossRef]
- Dilzer, A.; Park, Y. Implication of conjugated linoleic acid (CLA) in human health. Crit. Rev. Food Sci. Nutr. 2012, 52, 488–513. [Google Scholar] [CrossRef]
- Le Roy, C.I.; Kurilshikov, A.; Leeming, E.R.; Visconti, A.; Bowyer, R.C.E.; Menni, C.; Falchi, M.; Koutnikova, H.; Veiga, P.; Zhernakova, A.; et al. Yoghurt consumption is associated with changes in the composition of the human gut microbiome and metabolome. BMC Microbiol. 2022, 22, 39. [Google Scholar] [CrossRef]
- Sadrzadeh-Yeganeh, H.; Elmadfa, I.; Djazayery, A.; Jalali, M.; Heshmat, R.; Chamary, M. The effects of probiotic and conventional yoghurt on lipid profile in women. Br. J. Nutr. 2010, 103, 1778–1783. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, R.; Yang, X.; Dai, J.; Huang, M.; Ji, X.; Li, Y.; Okekunle, A.P.; Gao, G.; Onwuka, J.U.; et al. Yogurt improves insulin resistance and liver fat in obese women with nonalcoholic fatty liver disease and metabolic syndrome: A randomized controlled trial. Am. J. Clin. Nutr. 2019, 109, 1611–1619. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Pei, R.; Raghuvanshi, R.; Liu, Z.; Bolling, B.W. Yogurt Supplementation Attenuates Insulin Resistance in Obese Mice by Reducing Metabolic Endotoxemia and Inflammation. J. Nutr. 2023, 153, 703–712. [Google Scholar] [CrossRef]
- Douglas, S.M.; Ortinau, L.C.; Hoertel, H.A.; Leidy, H.J. Low, moderate, or high protein yogurt snacks on appetite control and subsequent eating in healthy women. Appetite 2013, 60, 117–122. [Google Scholar] [CrossRef]
- Rezazadeh, L.; Gargari, B.P.; Jafarabadi, M.A.; Alipour, B. Effects of probiotic yogurt on glycemic indexes and endothelial dysfunction markers in patients with metabolic syndrome. Nutrition 2019, 62, 162–168. [Google Scholar] [CrossRef]
- Wongrattanapipat, S.; Chiracharoenchitta, A.; Choowongwitthaya, B.; Komsathorn, P.; La-Ongkham, O.; Nitisinprasert, S.; Tunsagool, P.; Nakphaichit, M. Selection of potential probiotics with cholesterol-lowering properties for probiotic yoghurt production. Food Sci. Technol. Int. 2022, 28, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Asgharian, H.; Homayouni-Rad, A.; Mirghafourvand, M.; Mohammad-Alizadeh-Charandabi, S. Effect of probiotic yoghurt on plasma glucose in overweight and obese pregnant women: A randomized controlled clinical trial. Eur. J. Nutr. 2020, 59, 205–215. [Google Scholar] [CrossRef]
- Mazani, M.; Nemati, A.; Amani, M.; Haedari, K.; Mogadam, R.A.; Baghi, A.N. The effect of probiotic yoghurt consumption on oxidative stress and inflammatory factors in young females after exhaustive exercise. J. Pak. Med. Assoc. 2018, 68, 1748–1754. [Google Scholar]
- Mirjalili, M.; Salari Sharif, A.; Sangouni, A.A.; Emtiazi, H.; Mozaffari-Khosravi, H. Effect of probiotic yogurt consumption on glycemic control and lipid profile in patients with type 2 diabetes mellitus: A randomized controlled trial. Clin. Nutr. ESPEN 2023, 54, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Odamaki, T.; Kato, K.; Sugahara, H.; Xiao, J.Z.; Abe, F.; Benno, Y. Effect of probiotic yoghurt on animal-based diet-induced change in gut microbiota: An open, randomised, parallel-group study. Benef. Microbes 2016, 7, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Del Carmen, S.; de Moreno de LeBlanc, A.; LeBlanc, J.G. Development of a potential probiotic yoghurt using selected anti-inflammatory lactic acid bacteria for prevention of colitis and carcinogenesis in mice. J. Appl. Microbiol. 2016, 121, 821–830. [Google Scholar] [CrossRef]
- Negm El-Dein, A.; Ezzat, A.; Aly, H.F.; Awad, G.; Farid, M. Lactobacillus-fermented yogurt exerts hypoglycemic, hypocholesterolemic, and anti-inflammatory activities in STZ-induced diabetic Wistar rats. Nutr. Res. 2022, 108, 22–32. [Google Scholar] [CrossRef]
- Velasco, M.; Requena, T.; Delgado-Iribarren, A.; Peláez, C.; Guijarro, C. Probiotic Yogurt for the Prevention of Antibiotic-associated Diarrhea in Adults: A Randomized Double-blind Placebo-controlled Trial. J. Clin. Gastroenterol. 2019, 53, 717–723. [Google Scholar] [CrossRef]
- Fox, M.J.; Ahuja, K.D.; Robertson, I.K.; Ball, M.J.; Eri, R.D. Can probiotic yogurt prevent diarrhoea in children on antibiotics? A double-blind, randomised, placebo-controlled study. BMJ Open 2015, 5, e006474. [Google Scholar] [CrossRef]
- Barengolts, E.; Smith, E.D.; Reutrakul, S.; Tonucci, L.; Anothaisintawee, T. The Effect of Probiotic Yogurt on Glycemic Control in Type 2 Diabetes or Obesity: A Meta-Analysis of Nine Randomized Controlled Trials. Nutrients 2019, 11, 671. [Google Scholar] [CrossRef]
- Ivey, K.L.; Hodgson, J.M.; Kerr, D.A.; Thompson, P.L.; Stojceski, B.; Prince, R.L. The effect of yoghurt and its probiotics on blood pressure and serum lipid profile; a randomised controlled trial. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 46–51. [Google Scholar] [CrossRef]
- Bandiera, N.S.; Carneiro, I.; da Silva, A.S.; Honjoya, E.R.; de Santana, E.H.; Aragon-Alegro, L.C.; de Souza, C.H. Viability of probiotic Lactobacillus casei in yoghurt: Defining the best processing step to its addition. Arch. Latinoam. Nutr. 2013, 63, 58–63. [Google Scholar]
- Sah, B.N.P.; Vasiljevic, T.; McKechnie, S.; Donkor, O.N. Effect of refrigerated storage on probiotic viability and the production and stability of antimutagenic and antioxidant peptides in yogurt supplemented with pineapple peel. J. Dairy Sci. 2015, 98, 5905–5916. [Google Scholar] [CrossRef] [PubMed]
- Mani-López, E.; Palou, E.; López-Malo, A. Probiotic viability and storage stability of yogurts and fermented milks prepared with several mixtures of lactic acid bacteria. J. Dairy Sci. 2014, 97, 2578–2590. [Google Scholar] [CrossRef] [PubMed]
- Taha, S.; El Abd, M.; De Gobba, C.; Abdel-Hamid, M.; Khalil, E.; Hassan, D. Antioxidant and antibacterial activities of bioactive peptides in buffalo’s yoghurt fermented with different starter cultures. Food Sci. Biotechnol. 2017, 26, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Aloğlu, H.S.; Oner, Z. Determination of antioxidant activity of bioactive peptide fractions obtained from yogurt. J. Dairy Sci. 2011, 94, 5305–5314. [Google Scholar] [CrossRef]
- Jin, Y.; Yu, Y.; Qi, Y.; Wang, F.; Yan, J.; Zou, H. Peptide profiling and the bioactivity character of yogurt in the simulated gastrointestinal digestion. J. Proteom. 2016, 141, 24–46. [Google Scholar] [CrossRef]
- Giacometti Cavalheiro, F.; Parra Baptista, D.; Domingues Galli, B.; Negrão, F.; Nogueira Eberlin, M.; Lúcia Gigante, M. High protein yogurt with addition of Lactobacillus helveticus: Peptide profile and angiotensin-converting enzyme ACE-inhibitory activity. Food Chem. 2020, 333, 127482. [Google Scholar] [CrossRef]
- Plaisancié, P.; Claustre, J.; Estienne, M.; Henry, G.; Boutrou, R.; Paquet, A.; Léonil, J. A novel bioactive peptide from yoghurts modulates expression of the gel-forming MUC2 mucin as well as population of goblet cells and Paneth cells along the small intestine. J. Nutr. Biochem. 2013, 24, 213–221. [Google Scholar] [CrossRef]
- Abd El-Fattah, A.; Sakr, S.; El-Dieb, S.; Elkashef, H. Developing functional yogurt rich in bioactive peptides and gamma-aminobutyric acid related to cardiovascular health. LWT 2018, 98, 390–397. [Google Scholar] [CrossRef]
- Heydari, S.; Hosseini, S.E.; Mortazavian, A.M.; Taheri, S. Extraction of bioactive peptides produced in probiotic yoghurt and determination of their biological activities. Int. Dairy J. 2023, 139, 105544. [Google Scholar] [CrossRef]
- Bintsis, T. Yeasts in different types of cheese. AIMS Microbiol. 2021, 7, 447–470. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich-Wyder, M.T.; Arias-Roth, E.; Jakob, E. Cheese yeasts. Yeast 2019, 36, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Dong, X.; Huang, Z.; Li, X.; Zhao, Y.; Wang, Y.; Zhu, H.; Fang, A.; Giovannucci, E.L. Cheese consumption and multiple health outcomes: An umbrella review and updated meta-analysis of prospective studies. Adv. Nutr. 2023, 14, 1170–1186. [Google Scholar] [CrossRef]
- Hu, M.J.; Tan, J.S.; Gao, X.J.; Yang, J.G.; Yang, Y.J. Effect of Cheese Intake on Cardiovascular Diseases and Cardiovascular Biomarkers. Nutrients 2022, 14, 2936. [Google Scholar] [CrossRef]
- Hjerpsted, J.; Tholstrup, T. Cheese and Cardiovascular Disease Risk: A Review of the Evidence and Discussion of Possible Mechanisms. Crit. Rev. Food Sci. Nutr. 2016, 56, 1389–1403. [Google Scholar] [CrossRef]
- Kurbanova, M.; Voroshilin, R.; Kozlova, O.; Atuchin, V. Effect of Lactobacteria on Bioactive Peptides and Their Sequence Identification in Mature Cheese. Microorganisms 2022, 10, 2068. [Google Scholar] [CrossRef] [PubMed]
- Shafique, B.; Murtaza, M.A.; Hafiz, I.; Ameer, K.; Basharat, S.; Mohamed Ahmed, I.A. Proteolysis and therapeutic potential of bioactive peptides derived from Cheddar cheese. Food Sci. Nutr. 2023, 11, 4948–4963. [Google Scholar] [CrossRef]
- Helal, A.; Tagliazucchi, D. Peptidomics Profile, Bioactive Peptides Identification and Biological Activities of Six Different Cheese Varieties. Biology 2023, 12, 78. [Google Scholar] [CrossRef]
- Álvarez Ramos, L.; Arrieta Baez, D.; Dávila Ortiz, G.; Carlos Ruiz Ruiz, J.; Manuel Toledo López, V. Antioxidant and antihypertensive activity of Gouda cheese at different stages of ripening. Food Chem. X 2022, 14, 100284. [Google Scholar] [CrossRef]
- Martín-Del-Campo, S.T.; Martínez-Basilio, P.C.; Sepúlveda-Álvarez, J.C.; Gutiérrez-Melchor, S.E.; Galindo-Peña, K.D.; Lara-Domínguez, A.K.; Cardador-Martínez, A. Production of Antioxidant and ACEI Peptides from Cheese Whey Discarded from Mexican White Cheese Production. Antioxidants 2019, 8, 158. [Google Scholar] [CrossRef] [PubMed]
- Timón, M.L.; Andrés, A.I.; Otte, J.; Petrón, M.J. Antioxidant peptides (<3 kDa) identified on hard cow milk cheese with rennet from different origin. Food Res. Int. 2019, 120, 643–649. [Google Scholar] [CrossRef]
- Abedin, M.M.; Chourasia, R.; Chiring Phukon, L.; Singh, S.P.; Kumar Rai, A. Characterization of ACE inhibitory and antioxidant peptides in yak and cow milk hard chhurpi cheese of the Sikkim Himalayan region. Food Chem. X 2022, 13, 100231. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, Z.; Chorbadjiyska, E.; Gotova, I.; Pashova, K.; Ilieva, S. Selected adjunct cultures remarkably increase the content of bioactive peptides in Bulgarian white brined cheese. Biotechnol. Biotechnol. Equip. 2015, 29, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Helal, A.; Cattivelli, A.; Conte, A.; Tagliazucchi, D. Effect of Ripening and In Vitro Digestion on Bioactive Peptides Profile in Ras Cheese and Their Biological Activities. Biology 2023, 12, 948. [Google Scholar] [CrossRef]
- Munir, M.; Nadeem, M.; Mahmood Qureshi, T.; Gamlath, C.J.; Martin, G.J.O.; Hemar, Y.; Ashokkumar, M. Effect of sonication, microwaves and high-pressure processing on ACE-inhibitory activity and antioxidant potential of Cheddar cheese during ripening. Ultrason. Sonochem. 2020, 67, 105140. [Google Scholar] [CrossRef]
- Crippa, G.; Zabzuni, D.; Bravi, E.; Piva, G.; De Noni, I.; Bighi, E.; Rossi, F. Randomized, double blind placebo-controlled pilot study of the antihypertensive effects of Grana Padano D.O.P. cheese consumption in mild—Moderate hypertensive subjects. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7573–7581. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Rivas, I.K.; Gutiérrez-Méndez, N.; Rentería-Monterrubio, A.L.; Sánchez-Vega, R.; Tirado-Gallegos, J.M.; Santellano-Estrada, E.; Arevalos-Sánchez, M.M.; Chávez-Martínez, A. Effect of Packaging and Salt Content and Type on Antioxidant and ACE-Inhibitory Activities in Requeson Cheese. Foods 2022, 11, 1264. [Google Scholar] [CrossRef]
- Sánchez-Rivera, L.; Recio, I.; Ramos, M.; Gómez-Ruiz, J. Short communication: Peptide profiling in cheeses packed using different technologies. J. Dairy Sci. 2013, 96, 3551–3557. [Google Scholar] [CrossRef]
- Donmez, M.; Kemal Seckin, A.; Sagdic, O.; Simsek, B. Chemical characteristics, fatty acid compositions, conjugated linoleic acid contents and cholesterol levels of some traditional Turkish cheeses. Int. J. Food Sci. Nutr. 2005, 56, 157–163. [Google Scholar] [CrossRef]
- Laskaridis, K.; Serafeimidou, A.; Zlatanos, S.; Gylou, E.; Kontorepanidou, E.; Sagredos, A. Changes in fatty acid profile of feta cheese including conjugated linoleic acid. J. Sci. Food Agric. 2013, 93, 2130–2136. [Google Scholar] [CrossRef]
- Santurino, C.; López-Plaza, B.; Fontecha, J.; Calvo, M.V.; Bermejo, L.M.; Gómez-Andrés, D.; Gómez-Candela, C. Consumption of Goat Cheese Naturally Rich in Omega-3 and Conjugated Linoleic Acid Improves the Cardiovascular and Inflammatory Biomarkers of Overweight and Obese Subjects: A Randomized Controlled Trial. Nutrients 2020, 12. [Google Scholar] [CrossRef]
- Koba, K.; Yanagita, T. Health benefits of conjugated linoleic acid (CLA). Obes. Res. Clin. Pr. 2014, 8, e525–e532. [Google Scholar] [CrossRef]
- den Hartigh, L.J. Conjugated Linoleic Acid Effects on Cancer, Obesity, and Atherosclerosis: A Review of Pre-Clinical and Human Trials with Current Perspectives. Nutrients 2018, 11, 370. [Google Scholar] [CrossRef]
- Omer, A.K.; Mohammed, R.R.; Ameen, P.S.M.; Abas, Z.A.; Ekici, K. Presence of Biogenic Amines in Food and Their Public Health Implications: A Review. J. Food Prot. 2021, 84, 1539–1548. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Fresno, A.; Martínez, N.; Sánchez-Llana, E.; Díaz, M.; Fernández, M.; Martin, M.C.; Ladero, V.; Alvarez, M.A. Lactobacillus casei strains isolated from cheese reduce biogenic amine accumulation in an experimental model. Int. J. Food Microbiol. 2012, 157, 297–304. [Google Scholar] [CrossRef]
- Ołdak, A.; Zielińska, D.; Rzepkowska, A.; Kołożyn-Krajewska, D. Comparison of Antibacterial Activity of Lactobacillus plantarum Strains Isolated from Two Different Kinds of Regional Cheeses from Poland: Oscypek and Korycinski Cheese. Biomed. Res. Int. 2017, 2017, 6820369. [Google Scholar] [CrossRef]
- de Souza, B.M.S.; Borgonovi, T.F.; Casarotti, S.N.; Todorov, S.D.; Penna, A.L.B. Lactobacillus casei and Lactobacillus fermentum Strains Isolated from Mozzarella Cheese: Probiotic Potential, Safety, Acidifying Kinetic Parameters and Viability under Gastrointestinal Tract Conditions. Probiotics Antimicrob. Proteins 2019, 11, 382–396. [Google Scholar] [CrossRef]
- Meira, S.M.; Helfer, V.E.; Velho, R.V.; Lopes, F.C.; Brandelli, A. Probiotic potential of Lactobacillus spp. isolated from Brazilian regional ovine cheese. J. Dairy Res. 2012, 79, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Summer, A.; Formaggioni, P.; Franceschi, P.; Di Frangia, F.; Righi, F.; Malacarne, M. Cheese as Functional Food: The Example of Parmigiano Reggiano and Grana Padano. Food Technol. Biotechnol. 2017, 55, 277–289. [Google Scholar] [CrossRef]
- Smoke, T.; Smoking, I. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. In Red Meat and Processed Meat; International Agency for Research on Cancer: Lyon, France, 2018. [Google Scholar]
- Toldrá, F.; Reig, M. Innovations for healthier processed meats. Trends Food Sci. Technol. 2011, 22, 517–522. [Google Scholar] [CrossRef]
- Kołożyn-Krajewska, D.; Dolatowski, Z.J. Probiotics in fermented meat products. Acta Sci. Pol. Technol. Aliment. 2009, 8, 61–74. [Google Scholar]
- Lücke, F.-K. Fermented meat products. Food Res. Int. 1994, 27, 299–307. [Google Scholar] [CrossRef]
- Rodzi, N.A.R.M.; Lee, L.K. Traditional fermented foods as vehicle of non-dairy probiotics: Perspectives in South East Asia countries. Food Res. Int. 2021, 150, 110814. [Google Scholar] [CrossRef]
- Leroy, F.; Verluyten, J.; De Vuyst, L. Functional meat starter cultures for improved sausage fermentation. Int. J. Food Microbiol. 2006, 106, 270–285. [Google Scholar] [CrossRef]
- Yılmaz, I.; Velioğlu, H. Fermented meat products. In Quality of Meat and Meat Products; Yilmaz, I., Ed.; Transworld Research Network: Trivandrum, India, 2009; pp. 1–16. [Google Scholar]
- Lücke, F.-K. Utilization of microbes to process and preserve meat. Meat Sci. 2000, 56, 105–115. [Google Scholar] [CrossRef]
- Swetwiwathana, A.; Visessanguan, W. Potential of bacteriocin-producing lactic acid bacteria for safety improvements of traditional Thai fermented meat and human health. Meat Sci. 2015, 109, 101–105. [Google Scholar] [CrossRef]
- Belleggia, L.; Ferrocino, I.; Reale, A.; Corvaglia, M.R.; Milanović, V.; Cesaro, C.; Boscaino, F.; Di Renzo, T.; Garofalo, C.; Cardinali, F.; et al. Unfolding microbiota and volatile organic compounds of Portuguese Painho de Porco Preto fermented sausages. Food Res. Int. 2022, 155, 111063. [Google Scholar] [CrossRef]
- Settanni, L.; Barbaccia, P.; Bonanno, A.; Ponte, M.; Di Gerlando, R.; Franciosi, E.; Di Grigoli, A.; Gaglio, R. Evolution of indigenous starter microorganisms and physicochemical parameters in spontaneously fermented beef, horse, wild boar and pork salamis produced under controlled conditions. Food Microbiol. 2020, 87, 103385. [Google Scholar] [CrossRef]
- López-Pedrouso, M.; Zaky, A.A.; Lorenzo, J.M.; Camiña, M.; Franco, D. A review on bioactive peptides derived from meat and by-products: Extraction methods, biological activities, applications and limitations. Meat Sci. 2023, 204, 109278. [Google Scholar] [CrossRef] [PubMed]
- Papavergou, E.J.; Savvaidis, I.N.; Ambrosiadis, I.A. Levels of biogenic amines in retail market fermented meat products. Food Chem. 2012, 135, 2750–2755. [Google Scholar] [CrossRef]
- Kukleci, E.; Smulders, F.J.M.; Hamidi, A.; Bauer, S.; Paulsen, P. Prevalence of Foodborne Pathogenic Bacteria, Microbial Levels of Hygiene Indicator Bacteria, and Concentrations of Biogenic Amines in Ready-to-Eat Meat Products at Retail in the Republic of Kosovo. J. Food Prot. 2019, 82, 1135–1140. [Google Scholar] [CrossRef]
- Song, M.Y.; Van-Ba, H.; Park, W.S.; Yoo, J.Y.; Kang, H.B.; Kim, J.H.; Kang, S.M.; Kim, B.M.; Oh, M.H.; Ham, J.S. Quality Characteristics of Functional Fermented Sausages Added with Encapsulated Probiotic Bifidobacterium longum KACC 91563. Korean J. Food Sci. Anim. Resour. 2018, 38, 981–994. [Google Scholar] [CrossRef] [PubMed]
- Stadnik, J.; J. Dolatowski, Z. Biogenic amines in meat and fermented meat products. Acta Sci. Pol. Technol. Aliment. 2010, 9, 251–263. [Google Scholar]
- Ashaolu, T.J.; Khalifa, I.; Mesak, M.A.; Lorenzo, J.M.; Farag, M.A. A comprehensive review of the role of microorganisms on texture change, flavor and biogenic amines formation in fermented meat with their action mechanisms and safety. Crit. Rev. Food Sci. Nutr. 2023, 63, 3538–3555. [Google Scholar] [CrossRef]
- Özbay, S. Determination of volatile N-nitrosamines formed in salami cooked by different processes. J. Food Compos. Anal. 2022, 112, 104691. [Google Scholar] [CrossRef]
- Herrmann, S.S.; Duedahl-Olesen, L.; Granby, K. Occurrence of volatile and non-volatile N-nitrosamines in processed meat products and the role of heat treatment. Food Control 2015, 48, 163–169. [Google Scholar] [CrossRef]
- Jofré, A.; Aymerich, T.; Garriga, M. Probiotic Fermented Sausages: Myth or Reality? Procedia Food Sci. 2015, 5, 133–136. [Google Scholar] [CrossRef]
- Łepecka, A.; Szymański, P.; Okoń, A.; Zielińska, D. Antioxidant activity of environmental lactic acid bacteria strains isolated from organic raw fermented meat products. LWT 2023, 174, 114440. [Google Scholar] [CrossRef]
- Gallego, M.; Mora, L.; Escudero, E.; Toldrá, F. Bioactive peptides and free amino acids profiles in different types of European dry-fermented sausages. Int. J. Food Microbiol. 2018, 276, 71–78. [Google Scholar] [CrossRef]
- Xie, Y.; Geng, Y.; Yao, J.; Ji, J.; Chen, F.; Xiao, J.; Hu, X.; Ma, L. N-nitrosamines in processed meats: Exposure, formation and mitigation strategies. J. Agric. Food Res. 2023, 13, 100645. [Google Scholar] [CrossRef]
- Yang, D.; Li, C.; Li, L.; Yang, X.; Chen, S.; Wu, Y.; Feng, Y. Novel insight into the formation and inhibition mechanism of dipeptidyl peptidase-Ⅳ inhibitory peptides from fermented mandarin fish (Chouguiyu). Food Sci. Hum. Wellness 2023, 12, 2408–2416. [Google Scholar] [CrossRef]
- Gupta, S.; Mohanty, U.; Majumdar, R.K. Isolation and characterization of lactic acid bacteria from traditional fermented fish product Shidal of India with reference to their probiotic potential. LWT 2021, 146, 111641. [Google Scholar] [CrossRef]
- Liu, J.; Sun, X.; Zhang, Y.; Jin, Y.; Sun, L.; Chai, X.; Wang, D.; Su, L.; Zhao, L. The impact of different fermenting microbes on residual purine content in fermented lamb jerky following in vitro digestion. Food Chem. 2023, 405, 134997. [Google Scholar] [CrossRef] [PubMed]
- Van Hecke, T.; Vossen, E.; Goethals, S.; Boon, N.; De Vrieze, J.; De Smet, S. In vitro and in vivo digestion of red cured cooked meat: Oxidation, intestinal microbiota and fecal metabolites. Food Res. Int. 2021, 142, 110203. [Google Scholar] [CrossRef]
- Roselino, M.N.; Sakamoto, I.K.; Tallarico Adorno, M.A.; Márcia Canaan, J.M.; de Valdez, G.F.; Rossi, E.A.; Sivieri, K.; Umbelino Cavallini, D.C. Effect of fermented sausages with probiotic Enterococcus faecium CRL 183 on gut microbiota using dynamic colonic model. LWT 2020, 132, 109876. [Google Scholar] [CrossRef]
- Keuleyan, E.; Bonifacie, A.; Sayd, T.; Duval, A.; Aubry, L.; Bourillon, S.; Gatellier, P.; Promeyrat, A.; Nassy, G.; Scislowski, V.; et al. In vitro digestion of nitrite and nitrate preserved fermented sausages—New understandings of nitroso-compounds’ chemical reactivity in the digestive tract. Food Chem. X 2022, 16, 100474. [Google Scholar] [CrossRef]
- Jahan, M.; Zhanel, G.G.; Sparling, R.; Holley, R.A. Horizontal transfer of antibiotic resistance from Enterococcus faecium of fermented meat origin to clinical isolates of E. faecium and Enterococcus faecalis. Int. J. Food Microbiol. 2015, 199, 78–85. [Google Scholar] [CrossRef]
- Soto, A.M.; Morales, P.; Haza, A.I.; García, M.L.; Selgas, M.D. Bioavailability of calcium from enriched meat products using Caco-2 cells. Food Res. Int. 2014, 55, 263–270. [Google Scholar] [CrossRef]
- Nissen, L.; Casciano, F.; Di Nunzio, M.; Galaverna, G.; Bordoni, A.; Gianotti, A. Effects of the replacement of nitrates/nitrites in salami by plant extracts on colon microbiota. Food Biosci. 2023, 53, 102568. [Google Scholar] [CrossRef]
- Borah, D.; Gogoi, O.; Adhikari, C.; Kakoti, B.B. Isolation and characterization of the new indigenous Staphylococcus sp. DBOCP06 as a probiotic bacterium from traditionally fermented fish and meat products of Assam state. Egypt. J. Basic. Appl. Sci. 2016, 3, 232–240. [Google Scholar] [CrossRef]
- Iqbal, R.; Dehghan, M.; Mente, A.; Rangarajan, S.; Wielgosz, A.; Avezum, A.; Seron, P.; AlHabib, K.F.; Lopez-Jaramillo, P.; Swaminathan, S.; et al. Associations of unprocessed and processed meat intake with mortality and cardiovascular disease in 21 countries [Prospective Urban Rural Epidemiology (PURE) Study]: A prospective cohort study. Am. J. Clin. Nutr. 2021, 114, 1049–1058. [Google Scholar] [CrossRef]
- Bovalino, S.; Charleson, G.; Szoeke, C. The impact of red and processed meat consumption on cardiovascular disease risk in women. Nutrition 2016, 32, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Damigou, E.; Kosti, R.I.; Anastasiou, C.; Chrysohoou, C.; Barkas, F.; Adamidis, P.S.; Kravvariti, E.; Pitsavos, C.; Tsioufis, C.; Liberopoulos, E.; et al. Associations between meat type consumption pattern and incident cardiovascular disease: The ATTICA epidemiological cohort study (2002−2022). Meat Sci. 2023, 205, 109294. [Google Scholar] [CrossRef]
- de Medeiros, G.; Mesquita, G.X.B.; Lima, S.; Silva, D.F.O.; de Azevedo, K.P.M.; Pimenta, I.; de Oliveira, A.; Lyra, C.O.; Martínez, D.G.; Piuvezam, G. Associations of the consumption of unprocessed red meat and processed meat with the incidence of cardiovascular disease and mortality, and the dose-response relationship: A systematic review and meta-analysis of cohort studies. Crit. Rev. Food Sci. Nutr. 2022, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hayden, K.; Jackson, R.; Schutte, R. Association of red and processed meat consumption with cardiovascular morbidity and mortality in participants with and without obesity: A prospective cohort study. Clin. Nutr. 2021, 40, 3643–3649. [Google Scholar] [CrossRef] [PubMed]
- Montoro-García, S.; Zafrilla-Rentero, M.P.; Celdrán-de Haro, F.M.; Piñero-de Armas, J.J.; Toldrá, F.; Tejada-Portero, L.; Abellán-Alemán, J. Effects of dry-cured ham rich in bioactive peptides on cardiovascular health: A randomized controlled trial. J. Funct. Foods 2017, 38, 160–167. [Google Scholar] [CrossRef]
- Ayyash, M.; Liu, S.-Q.; Al Mheiri, A.; Aldhaheri, M.; Raeisi, B.; Al-Nabulsi, A.; Osaili, T.; Olaimat, A. In vitro investigation of health-promoting benefits of fermented camel sausage by novel probiotic Lactobacillus plantarum: A comparative study with beef sausages. LWT 2019, 99, 346–354. [Google Scholar] [CrossRef]
- Kong, Y.-w.; Feng, M.-q.; Sun, J. Effects of Lactobacillus plantarum CD101 and Staphylococcus simulans NJ201 on proteolytic changes and bioactivities (antioxidant and antihypertensive activities) in fermented pork sausage. LWT 2020, 133, 109985. [Google Scholar] [CrossRef]
- Mejri, L.; Vásquez-Villanueva, R.; Hassouna, M.; Marina, M.L.; García, M.C. Identification of peptides with antioxidant and antihypertensive capacities by RP-HPLC-Q-TOF-MS in dry fermented camel sausages inoculated with different starter cultures and ripening times. Food Res. Int. 2017, 100, 708–716. [Google Scholar] [CrossRef]
- Li, H.; Wu, J.; Wan, J.; Zhou, Y.; Zhu, Q. Extraction and identification of bioactive peptides from Panxian dry-cured ham with multifunctional activities. LWT 2022, 160, 113326. [Google Scholar] [CrossRef]
- Ohata, M.; Uchida, S.; Zhou, L.; Arihara, K. Antioxidant activity of fermented meat sauce and isolation of an associated antioxidant peptide. Food Chem. 2016, 194, 1034–1039. [Google Scholar] [CrossRef]
- Fernández, M.; Benito, M.J.; Martín, A.; Casquete, R.; Córdoba, J.J.; Córdoba, M.G. Influence of starter culture and a protease on the generation of ACE-inhibitory and antioxidant bioactive nitrogen compounds in Iberian dry-fermented sausage “salchichón”. Heliyon 2016, 2, e00093. [Google Scholar] [CrossRef]
- Zeraatkar, D.; Han, M.A.; Guyatt, G.H.; Vernooij, R.W.M.; El Dib, R.; Cheung, K.; Milio, K.; Zworth, M.; Bartoszko, J.J.; Valli, C.; et al. Red and Processed Meat Consumption and Risk for All-Cause Mortality and Cardiometabolic Outcomes: A Systematic Review and Meta-analysis of Cohort Studies. Ann. Intern. Med. 2019, 171, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Vernooij, R.W.M.; Zeraatkar, D.; Han, M.A.; El Dib, R.; Zworth, M.; Milio, K.; Sit, D.; Lee, Y.; Gomaa, H.; Valli, C.; et al. Patterns of Red and Processed Meat Consumption and Risk for Cardiometabolic and Cancer Outcomes: A Systematic Review and Meta-analysis of Cohort Studies. Ann. Intern. Med. 2019, 171, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.; Liu, Y.; Zhu, L.; Mei, X.; Jin, P.; Luo, Y. Association between intake of red and processed meat and the risk of heart failure: A meta-analysis. BMC Public Health 2019, 19, 354. [Google Scholar] [CrossRef]
- Farvid, M.S.; Sidahmed, E.; Spence, N.D.; Mante Angua, K.; Rosner, B.A.; Barnett, J.B. Consumption of red meat and processed meat and cancer incidence: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2021, 36, 937–951. [Google Scholar] [CrossRef]
- Anderson, J.J.; Darwis, N.D.M.; Mackay, D.F.; Celis-Morales, C.A.; Lyall, D.M.; Sattar, N.; Gill, J.M.R.; Pell, J.P. Red and processed meat consumption and breast cancer: UK Biobank cohort study and meta-analysis. Eur. J. Cancer 2018, 90, 73–82. [Google Scholar] [CrossRef]
- Alexander, D.D.; Morimoto, L.M.; Mink, P.J.; Cushing, C.A. A review and meta-analysis of red and processed meat consumption and breast cancer. Nutr. Res. Rev. 2010, 23, 349–365. [Google Scholar] [CrossRef]
- Dandamudi, A.; Tommie, J.; Nommsen-Rivers, L.; Couch, S. Dietary Patterns and Breast Cancer Risk: A Systematic Review. Anticancer. Res. 2018, 38, 3209–3222. [Google Scholar] [CrossRef]
- Kim, S.R.; Kim, K.; Lee, S.A.; Kwon, S.O.; Lee, J.K.; Keum, N.; Park, S.M. Effect of Red, Processed, and White Meat Consumption on the Risk of Gastric Cancer: An Overall and Dose–Response Meta-Analysis. Nutrients 2019, 11, 826. [Google Scholar] [CrossRef]
- Chan, D.S.; Lau, R.; Aune, D.; Vieira, R.; Greenwood, D.C.; Kampman, E.; Norat, T. Red and processed meat and colorectal cancer incidence: Meta-analysis of prospective studies. PLoS ONE 2011, 6, e20456. [Google Scholar] [CrossRef]
- Ubago-Guisado, E.; Rodríguez-Barranco, M.; Ching-López, A.; Petrova, D.; Molina-Montes, E.; Amiano, P.; Barricarte-Gurrea, A.; Chirlaque, M.D.; Agudo, A.; Sánchez, M.J. Evidence Update on the Relationship between Diet and the Most Common Cancers from the European Prospective Investigation into Cancer and Nutrition (EPIC) Study: A Systematic Review. Nutrients 2021, 13, 3582. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Schwedhelm, C.; Hoffmann, G.; Knüppel, S.; Laure Preterre, A.; Iqbal, K.; Bechthold, A.; De Henauw, S.; Michels, N.; Devleesschauwer, B.; et al. Food groups and risk of colorectal cancer. Int. J. Cancer 2018, 142, 1748–1758. [Google Scholar] [CrossRef]
- Vieira, A.R.; Abar, L.; Chan, D.S.M.; Vingeliene, S.; Polemiti, E.; Stevens, C.; Greenwood, D.; Norat, T. Foods and beverages and colorectal cancer risk: A systematic review and meta-analysis of cohort studies, an update of the evidence of the WCRF-AICR Continuous Update Project. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 1788–1802. [Google Scholar] [CrossRef]
- Händel, M.N.; Rohde, J.F.; Jacobsen, R.; Nielsen, S.M.; Christensen, R.; Alexander, D.D.; Frederiksen, P.; Heitmann, B.L. Processed meat intake and incidence of colorectal cancer: A systematic review and meta-analysis of prospective observational studies. Eur. J. Clin. Nutr. 2020, 74, 1132–1148. [Google Scholar] [CrossRef]
- Alexander, D.D.; Miller, A.J.; Cushing, C.A.; Lowe, K.A. Processed meat and colorectal cancer: A quantitative review of prospective epidemiologic studies. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. (ECP) 2010, 19, 328–341. [Google Scholar] [CrossRef]
- Aune, D.; Chan, D.S.M.; Vieira, A.R.; Navarro Rosenblatt, D.A.; Vieira, R.; Greenwood, D.C.; Kampman, E.; Norat, T. Red and processed meat intake and risk of colorectal adenomas: A systematic review and meta-analysis of epidemiological studies. Cancer Causes Control 2013, 24, 611–627. [Google Scholar] [CrossRef] [PubMed]
- Rosato, V.; Negri, E.; Serraino, D.; Montella, M.; Libra, M.; Lagiou, P.; Facchini, G.; Ferraroni, M.; Decarli, A.; La Vecchia, C. Processed Meat and Risk of Renal Cell and Bladder Cancers. Nutr. Cancer 2018, 70, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Crippa, A.; Larsson, S.C.; Discacciati, A.; Wolk, A.; Orsini, N. Red and processed meat consumption and risk of bladder cancer: A dose-response meta-analysis of epidemiological studies. Eur. J. Nutr. 2018, 57, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.D.; Cushing, C.A. Quantitative assessment of red meat or processed meat consumption and kidney cancer. Cancer Detect. Prev. 2009, 32, 340–351. [Google Scholar] [CrossRef]
- Saneei, P.; Willett, W.; Esmaillzadeh, A. Red and processed meat consumption and risk of glioma in adults: A systematic review and meta-analysis of observational studies. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2015, 20, 602–612. [Google Scholar] [CrossRef]
- Yu, J.; Liu, Z.; Liang, D.; Li, J.; Ma, S.; Wang, G.; Chen, W. Meat Intake and the Risk of Hepatocellular Carcinoma: A Meta-Analysis of Observational Studies. Nutr. Cancer 2022, 74, 3340–3350. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G.; Lampousi, A.M.; Knüppel, S.; Iqbal, K.; Schwedhelm, C.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food groups and risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Micha, R.; Wallace, S.K.; Mozaffarian, D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: A systematic review and meta-analysis. Circulation 2010, 121, 2271–2283. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Y.; Wang, C.; Mao, Z.; Zhou, W.; Zhang, L.; Fan, M.; Cui, S.; Li, L. Meat and fish intake and type 2 diabetes: Dose–response meta-analysis of prospective cohort studies. Diabetes Metab. 2020, 46, 345–352. [Google Scholar] [CrossRef]
- Zhang, R.; Fu, J.; Moore, J.B.; Stoner, L.; Li, R. Processed and Unprocessed Red Meat Consumption and Risk for Type 2 Diabetes Mellitus: An Updated Meta-Analysis of Cohort Studies. Int. J. Environ. Res. Public Health 2021, 18, 10788. [Google Scholar] [CrossRef]
- Mijatovic-Vukas, J.; Capling, L.; Cheng, S.; Stamatakis, E.; Louie, J.; Cheung, N.W.; Markovic, T.; Ross, G.; Senior, A.; Brand-Miller, J.C.; et al. Associations of Diet and Physical Activity with Risk for Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 698. [Google Scholar] [CrossRef]
- Albenberg, L.; Brensinger, C.M.; Wu, Q.; Gilroy, E.; Kappelman, M.D.; Sandler, R.S.; Lewis, J.D. A Diet Low in Red and Processed Meat Does Not Reduce Rate of Crohn’s Disease Flares. Gastroenterology 2019, 157, 128–136.e5. [Google Scholar] [CrossRef]
- Taneri, P.E.; Wehrli, F.; Roa-Díaz, Z.M.; Itodo, O.A.; Salvador, D.; Raeisi-Dehkordi, H.; Bally, L.; Minder, B.; Kiefte-de Jong, J.C.; Laine, J.E.; et al. Association Between Ultra-Processed Food Intake and All-Cause Mortality: A Systematic Review and Meta-Analysis. Am. J. Epidemiol. 2022, 191, 1323–1335. [Google Scholar] [CrossRef]
- Wang, X.; Lin, X.; Ouyang, Y.Y.; Liu, J.; Zhao, G.; Pan, A.; Hu, F.B. Red and processed meat consumption and mortality: Dose-response meta-analysis of prospective cohort studies. Public Health Nutr. 2016, 19, 893–905. [Google Scholar] [CrossRef]
- Nucci, D.; Fatigoni, C.; Amerio, A.; Odone, A.; Gianfredi, V. Red and Processed Meat Consumption and Risk of Depression: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 6686. [Google Scholar] [CrossRef] [PubMed]
- Michaud, D.S.; Holick, C.N.; Batchelor, T.T.; Giovannucci, E.; Hunter, D.J. Prospective study of meat intake and dietary nitrates, nitrites, and nitrosamines and risk of adult glioma12. Am. J. Clin. Nutr. 2009, 90, 570–577. [Google Scholar] [CrossRef]
- Travis, C.C.; McClain, T.W.; Birkner, P.D. Diethylnitrosamine-induced hepatocarcinogenesis in rats: A theoretical study. Toxicol. Appl. Pharmacol. 1991, 109, 289–304. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Remize, F.; Poucheret, P. Fruits and vegetables, as a source of nutritional compounds and phytochemicals: Changes in bioactive compounds during lactic fermentation. Food Res. Int. 2018, 104, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Sun, T.Y.; He, Y.; Gou, W.; Zuo, L.S.; Fu, Y.; Miao, Z.; Shuai, M.; Xu, F.; Xiao, C.; et al. Dietary fruit and vegetable intake, gut microbiota, and type 2 diabetes: Results from two large human cohort studies. BMC Med. 2020, 18, 371. [Google Scholar] [CrossRef]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef]
- Wang, D.D.; Li, Y.; Bhupathiraju, S.N.; Rosner, B.A.; Sun, Q.; Giovannucci, E.L.; Rimm, E.B.; Manson, J.E.; Willett, W.C.; Stampfer, M.J.; et al. Fruit and Vegetable Intake and Mortality: Results from 2 Prospective Cohort Studies of US Men and Women and a Meta-Analysis of 26 Cohort Studies. Circulation 2021, 143, 1642–1654. [Google Scholar] [CrossRef]
- Di Cagno, R.; Coda, R.; De Angelis, M.; Gobbetti, M. Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiol. 2013, 33, 1–10. [Google Scholar] [CrossRef]
- Sabater, C.; Ruiz, L.; Delgado, S.; Ruas-Madiedo, P.; Margolles, A. Valorization of Vegetable Food Waste and By-Products through Fermentation Processes. Front. Microbiol. 2020, 11, 581997. [Google Scholar] [CrossRef]
- Harris, J.; Tan, W.; Raneri, J.E.; Schreinemachers, P.; Herforth, A. Vegetables for Healthy Diets in Low- and Middle-Income Countries: A Scoping Review of the Food Systems Literature. Food Nutr. Bull. 2022, 43, 232–248. [Google Scholar] [CrossRef]
- Irakoze, M.L.; Wafula, E.N.; Owaga, E. Potential Role of African Fermented Indigenous Vegetables in Maternal and Child Nutrition in Sub-Saharan Africa. Int. J. Food Sci. 2021, 2021, 3400329. [Google Scholar] [CrossRef]
- Ashaolu, T.J.; Reale, A. A holistic review on Euro-Asian lactic acid bacteria fermented cereals and vegetables. Microorganisms 2020, 8, 1176. [Google Scholar] [PubMed]
- Lee, S.J.; Jeon, H.S.; Yoo, J.Y.; Kim, J.H. Some Important Metabolites Produced by Lactic Acid Bacteria Originated from Kimchi. Foods 2021, 10, 2148. [Google Scholar] [CrossRef]
- Park, K.Y.; Jeong, J.K.; Lee, Y.E.; Daily, J.W., 3rd. Health benefits of kimchi (Korean fermented vegetables) as a probiotic food. J. Med. Food 2014, 17, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Noh, J.S.; Song, Y.O. Beneficial Effects of Kimchi, a Korean Fermented Vegetable Food, on Pathophysiological Factors Related to Atherosclerosis. J. Med. Food 2018, 21, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.; Kim, M.J.; Song, Y.O. Bioactive Compounds in Kimchi Improve the Cognitive and Memory Functions Impaired by Amyloid Beta. Nutrients 2018, 10, 1554. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.S.; Chung, H.Y.; Song, S.H.; Suh, H.; Noh, J.S.; Song, Y.O. 3-(4′-hydroxyl-3′,5′-dimethoxyphenyl)propionic acid, an active principle of kimchi, inhibits development of atherosclerosis in rabbits. J. Agric. Food Chem. 2007, 55, 10486–10492. [Google Scholar] [CrossRef]
- Yun, Y.R.; Kim, H.J.; Song, Y.O. Kimchi methanol extract and the kimchi active compound, 3′-(4′-hydroxyl-3′,5′-dimethoxyphenyl)propionic acid, downregulate CD36 in THP-1 macrophages stimulated by oxLDL. J. Med. Food 2014, 17, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.S.; Kim, H.J.; Kwon, M.J.; Song, Y.O. Active principle of kimchi, 3-(4′-hydroxyl-3′,5′-dimethoxyphenyl)propionic acid, retards fatty streak formation at aortic sinus of apolipoprotein E knockout mice. J. Med. Food 2009, 12, 1206–1212. [Google Scholar] [CrossRef]
- Jeong, J.W.; Choi, I.W.; Jo, G.H.; Kim, G.Y.; Kim, J.; Suh, H.; Ryu, C.H.; Kim, W.J.; Park, K.Y.; Choi, Y.H. Anti-Inflammatory Effects of 3-(4′-Hydroxyl-3′,5′-Dimethoxyphenyl)Propionic Acid, an Active Component of Korean Cabbage Kimchi, in Lipopolysaccharide-Stimulated BV2 Microglia. J. Med. Food 2015, 18, 677–684. [Google Scholar] [CrossRef]
- Jeon, H.L.; Lee, N.K.; Yang, S.J.; Kim, W.S.; Paik, H.D. Probiotic characterization of Bacillus subtilis P223 isolated from kimchi. Food Sci. Biotechnol. 2017, 26, 1641–1648. [Google Scholar] [CrossRef]
- Yu, H.S.; Lee, N.K.; Choi, A.J.; Choe, J.S.; Bae, C.H.; Paik, H.D. Anti-Inflammatory Potential of Probiotic Strain Weissella cibaria JW15 Isolated from Kimchi through Regulation of NF-κB and MAPKs Pathways in LPS-Induced RAW 264.7 Cells. J. Microbiol. Biotechnol. 2019, 29, 1022–1032. [Google Scholar] [CrossRef]
- Sohn, H.; Chang, Y.H.; Yune, J.H.; Jeong, C.H.; Shin, D.M.; Kwon, H.C.; Kim, D.H.; Hong, S.W.; Hwang, H.; Jeong, J.Y.; et al. Probiotic Properties of Lactiplantibacillus plantarum LB5 Isolated from Kimchi Based on Nitrate Reducing Capability. Foods 2020, 9, 1777. [Google Scholar] [CrossRef]
- Yoon, S.; Cho, H.; Nam, Y.; Park, M.; Lim, A.; Kim, J.H.; Park, J.; Kim, W. Multifunctional Probiotic and Functional Properties of Lactiplantibacillus plantarum LRCC5314, Isolated from Kimchi. J. Microbiol. Biotechnol. 2022, 32, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Cheon, M.J.; Lee, N.K.; Paik, H.D. Neuroprotective Effects of Heat-Killed Lactobacillus plantarum 200655 Isolated from Kimchi against Oxidative Stress. Probiotics Antimicrob Proteins 2021, 13, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Moon, J.H.; Shin, C.M.; Jeong, D.; Kim, B. Effect of Lactobacillus sakei, a Probiotic Derived from Kimchi, on Body Fat in Koreans with Obesity: A Randomized Controlled Study. Endocrinol. Metab. 2020, 35, 425–434. [Google Scholar] [CrossRef]
- Jang, H.J.; Lee, N.K.; Paik, H.D. Probiotic characterization of Lactobacillus brevis KU15153 showing antimicrobial and antioxidant effect isolated from kimchi. Food Sci. Biotechnol. 2019, 28, 1521–1528. [Google Scholar] [CrossRef]
- Kim, K.T.; Yang, S.J.; Paik, H.D. Probiotic properties of novel probiotic Levilactobacillus brevis KU15147 isolated from radish kimchi and its antioxidant and immune-enhancing activities. Food Sci. Biotechnol. 2021, 30, 257–265. [Google Scholar] [CrossRef]
- Youn, H.S.; Kim, J.H.; Lee, J.S.; Yoon, Y.Y.; Choi, S.J.; Lee, J.Y.; Kim, W.; Hwang, K.W. Lactobacillus plantarum Reduces Low-Grade Inflammation and Glucose Levels in a Mouse Model of Chronic Stress and Diabetes. Infect. Immun. 2021, 89, e0061520. [Google Scholar] [CrossRef]
- An, J.M.; Kang, E.A.; Han, Y.M.; Oh, J.Y.; Lee, D.Y.; Choi, S.H.; Kim, D.H.; Hahm, K.B. Dietary intake of probiotic kimchi ameliorated IL-6-driven cancer cachexia. J. Clin. Biochem. Nutr. 2019, 65, 109–117. [Google Scholar] [CrossRef]
- Shankar, T.; Palpperumal, S.; Kathiresan, D.; Sankaralingam, S.; Balachandran, C.; Baskar, K.; Hashem, A.; Alqarawi, A.A.; Abd Allah, E.F. Biomedical and therapeutic potential of exopolysaccharides by Lactobacillus paracasei isolated from sauerkraut: Screening and characterization. Saudi J. Biol. Sci. 2021, 28, 2943–2950. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Qiao, Y.; Peng, Q.; Shi, B.; Dia, V.P. Antioxidant and Immunomodulatory Properties of Partially purified Exopolysaccharide from Lactobacillus Casei Isolated from Chinese Northeast Sauerkraut. Immunol. Investig. 2022, 51, 748–765. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Peng, Q.; Zhang, Y.; Tian, D.; Zhang, P.; Huang, Y.; Ma, L.; Dia, V.P.; Qiao, Y.; Shi, B. Antibacterial potential of a novel Lactobacillus casei strain isolated from Chinese northeast sauerkraut and the antibiofilm activity of its exopolysaccharides. Food Funct. 2020, 11, 4697–4706. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, X.; Li, S.; Li, C.; Li, D.; Yang, Z. Evaluation of probiotic properties of Lactobacillus plantarum strains isolated from Chinese sauerkraut. World J. Microbiol. Biotechnol. 2013, 29, 489–498. [Google Scholar] [CrossRef]
- Kim, N.; Lee, J.; Song, H.S.; Oh, Y.J.; Kwon, M.S.; Yun, M.; Lim, S.K.; Park, H.K.; Jang, Y.S.; Lee, S.; et al. Kimchi intake alleviates obesity-induced neuroinflammation by modulating the gut-brain axis. Food Res. Int. 2022, 158, 111533. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Park, E.S.; Choi, Y.S.; Park, S.J.; Kim, J.H.; Chang, H.K.; Park, K.Y. Kimchi improves irritable bowel syndrome: Results of a randomized, double-blind placebo-controlled study. Food Nutr. Res. 2022, 66, 1–12. [Google Scholar] [CrossRef]
- Park, J.M.; Lee, W.H.; Seo, H.; Oh, J.Y.; Lee, D.Y.; Kim, S.J.; Hahm, K.B. Fecal microbiota changes with fermented kimchi intake regulated either formation or advancement of colon adenoma. J. Clin. Biochem. Nutr. 2021, 68, 139–148. [Google Scholar] [CrossRef]
- Park, S.E.; Kwon, S.J.; Cho, K.M.; Seo, S.H.; Kim, E.J.; Unno, T.; Bok, S.H.; Park, D.H.; Son, H.S. Intervention with kimchi microbial community ameliorates obesity by regulating gut microbiota. J. Microbiol. 2020, 58, 859–867. [Google Scholar] [CrossRef]
- An, S.Y.; Lee, M.S.; Jeon, J.Y.; Ha, E.S.; Kim, T.H.; Yoon, J.Y.; Ok, C.O.; Lee, H.K.; Hwang, W.S.; Choe, S.J.; et al. Beneficial effects of fresh and fermented kimchi in prediabetic individuals. Ann. Nutr. Metab. 2013, 63, 111–119. [Google Scholar] [CrossRef]
- Islam, M.S.; Choi, H. Antidiabetic effect of Korean traditional Baechu (Chinese cabbage) kimchi in a type 2 diabetes model of rats. J. Med. Food 2009, 12, 292–297. [Google Scholar] [CrossRef]
- Palani, K.; Harbaum-Piayda, B.; Meske, D.; Keppler, J.K.; Bockelmann, W.; Heller, K.J.; Schwarz, K. Influence of fermentation on glucosinolates and glucobrassicin degradation products in sauerkraut. Food Chem. 2016, 190, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Tai, A.; Fukunaga, K.; Ohno, A.; Ito, H. Antioxidative properties of ascorbigen in using multiple antioxidant assays. Biosci. Biotechnol. Biochem. 2014, 78, 1723–1730. [Google Scholar] [CrossRef]
- Amarakoon, D.; Lee, W.J.; Tamia, G.; Lee, S.H. Indole-3-Carbinol: Occurrence, Health-Beneficial Properties, and Cellular/Molecular Mechanisms. Annu. Rev. Food Sci. Technol. 2023, 14, 347–366. [Google Scholar] [CrossRef] [PubMed]
- Pathak, D.R.; Stein, A.D.; He, J.P.; Noel, M.M.; Hembroff, L.; Nelson, D.A.; Vigneau, F.; Shen, T.; Scott, L.J.; Charzewska, J.; et al. Cabbage and Sauerkraut Consumption in Adolescence and Adulthood and Breast Cancer Risk among US-Resident Polish Migrant Women. Int. J. Environ. Res. Public Health 2021, 18, 10795. [Google Scholar] [CrossRef]
- Nielsen, E.S.; Garnås, E.; Jensen, K.J.; Hansen, L.H.; Olsen, P.S.; Ritz, C.; Krych, L.; Nielsen, D.S. Lacto-fermented sauerkraut improves symptoms in IBS patients independent of product pasteurisation—A pilot study. Food Funct. 2018, 9, 5323–5335. [Google Scholar] [CrossRef]
- Fideler, J.; Johanningsmeier, S.D.; Ekelöf, M.; Muddiman, D.C. Discovery and quantification of bioactive peptides in fermented cucumber by direct analysis IR-MALDESI mass spectrometry and LC-QQQ-MS. Food Chem. 2019, 271, 715–723. [Google Scholar] [CrossRef]
- Moore, J.F.; DuVivier, R.; Johanningsmeier, S.D. Changes in the free amino acid profile of pickling cucumber during lactic acid fermentation. J. Food Sci. 2022, 87, 599–611. [Google Scholar] [CrossRef]
- Petroski, W.; Minich, D.M. Is There Such a Thing as “Anti-Nutrients”? A Narrative Review of Perceived Problematic Plant Compounds. Nutrients 2020, 12, 2929. [Google Scholar] [CrossRef]
- Cuadrado, C.; Hajos, G.; Burbano, C.; Pedrosa, M.M.; Ayet, G.; Muzquiz, M.; Pusztai, A.; Gelencser, E. Effect of natural fermentation on the lectin of lentils measured by immunological methods. Food Agric. Immunol. 2002, 14, 41–49. [Google Scholar]
- Sangija, F.; Martin, H.; Matemu, A. Effect of lactic acid fermentation on the nutritional quality and consumer acceptability of African nightshade. Food Sci. Nutr. 2022, 10, 3128–3142. [Google Scholar] [CrossRef]
- Knez, E.; Kadac-Czapska, K.; Grembecka, M. Effect of Fermentation on the Nutritional Quality of the Selected Vegetables and Legumes and Their Health Effects. Life 2023, 13, 655. [Google Scholar] [CrossRef] [PubMed]
- Layla, A.; Syed, Q.A.; Zahoor, T.; Shahid, M. Investigating the role of Lactiplantibacillus plantarum vs. spontaneous fermentation in improving nutritional and consumer safety of the fermented white cabbage sprouts. Int. Microbiol. 2023, 1–12. [Google Scholar] [CrossRef]
- Dreher, M.L. Whole Fruits and Fruit Fiber Emerging Health Effects. Nutrients 2018, 10, 1833. [Google Scholar] [CrossRef]
- Leitão, M.; Ribeiro, T.; García, P.A.; Barreiros, L.; Correia, P. Benefits of Fermented Papaya in Human Health. Foods 2022, 11, 563. [Google Scholar] [CrossRef]
- Cousin, F.J.; Le Guellec, R.; Schlusselhuber, M.; Dalmasso, M.; Laplace, J.M.; Cretenet, M. Microorganisms in Fermented Apple Beverages: Current Knowledge and Future Directions. Microorganisms 2017, 5, 39. [Google Scholar] [CrossRef]
- Lee, B.H.; Hsu, W.H.; Hou, C.Y.; Chien, H.Y.; Wu, S.C. The Protection of Lactic Acid Bacteria Fermented-Mango Peel against Neuronal Damage Induced by Amyloid-Beta. Molecules 2021, 26, 3503. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Hsiao, S.Y.; Lin, Y.H.; Tsai, G.J. Effects of Fermented Citrus Peel on Ameliorating Obesity in Rats Fed with High-Fat Diet. Molecules 2022, 27, 8966. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Huang, Y.W.; Hou, C.Y.; Chen, Y.T.; Dong, C.D.; Chen, C.W.; Singhania, R.R.; Leang, J.Y.; Hsieh, S.L. Lemon fermented products prevent obesity in high-fat diet-fed rats by modulating lipid metabolism and gut microbiota. J. Food Sci. Technol. 2023, 60, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Sun, Y.; Gao, T.; Wu, Y.; Sun, H.; Zhu, Q.; Liu, C.; Zhou, C.; Han, Y.; Tao, Y. Fermentation and Storage Characteristics of “Fuji” Apple Juice Using Lactobacillus acidophilus, Lactobacillus casei and Lactobacillus plantarum: Microbial Growth, Metabolism of Bioactives and in vitro Bioactivities. Front. Nutr. 2022, 9, 833906. [Google Scholar] [CrossRef]
- Yassunaka Hata, N.N.; Surek, M.; Sartori, D.; Vassoler Serrato, R.; Aparecida Spinosa, W. Role of Acetic Acid Bacteria in Food and Beverages. Food Technol. Biotechnol. 2023, 61, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Perjéssy, J.; Hegyi, F.; Nagy-Gasztonyi, M.; Zalán, Z. Effect of the lactic acid fermentation by probiotic strains on the sour cherry juice and its bioactive compounds. Food Sci. Technol. Int. 2022, 28, 408–420. [Google Scholar] [CrossRef]
- Muhialdin, B.J.; Kadum, H.; Zarei, M.; Meor Hussin, A.S. Effects of metabolite changes during lacto-fermentation on the biological activity and consumer acceptability for dragon fruit juice. LWT 2020, 121, 108992. [Google Scholar] [CrossRef]
- Cirlini, M.; Ricci, A.; Galaverna, G.; Lazzi, C. Application of lactic acid fermentation to elderberry juice: Changes in acidic and glucidic fractions. LWT 2020, 118, 108779. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, Y.; Yang, N.; Jiang, T.; Xu, H.; Lei, H. Fermentation of kiwifruit juice from two cultivars by probiotic bacteria: Bioactive phenolics, antioxidant activities and flavor volatiles. Food Chem. 2022, 373, 131455. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, S.; Tao, Y.; Li, D.; Han, Y.; Show, P.L.; Wen, G.; Zhou, J. Fermentation of blueberry and blackberry juices using Lactobacillus plantarum, Streptococcus thermophilus and Bifidobacterium bifidum: Growth of probiotics, metabolism of phenolics, antioxidant capacity in vitro and sensory evaluation. Food Chem. 2021, 348, 129083. [Google Scholar] [CrossRef]
- Zhong, H.; Abdullah; Zhao, M.; Tang, J.; Deng, L.; Feng, F. Probiotics-fermented blueberry juices as potential antidiabetic product: Antioxidant, antimicrobial and antidiabetic potentials. J. Sci. Food Agric. 2021, 101, 4420–4427. [Google Scholar] [CrossRef]
- Ousaaid, D.; Mechchate, H.; Laaroussi, H.; Hano, C.; Bakour, M.; El Ghouizi, A.; Conte, R.; Lyoussi, B.; El Arabi, I. Fruits Vinegar: Quality Characteristics, Phytochemistry, and Functionality. Molecules 2021, 27, 222. [Google Scholar] [CrossRef]
- Bakir, S.; Toydemir, G.; Boyacioglu, D.; Beekwilder, J.; Capanoglu, E. Fruit Antioxidants during Vinegar Processing: Changes in Content and in Vitro Bio-Accessibility. Int. J. Mol. Sci. 2016, 17, 1658. [Google Scholar] [CrossRef]
- Budak, H.N.; Guzel-Seydim, Z.B. Antioxidant activity and phenolic content of wine vinegars produced by two different techniques. J. Sci. Food Agric. 2010, 90, 2021–2026. [Google Scholar] [CrossRef]
- Hadi, A.; Pourmasoumi, M.; Najafgholizadeh, A.; Clark, C.C.T.; Esmaillzadeh, A. The effect of apple cider vinegar on lipid profiles and glycemic parameters: A systematic review and meta-analysis of randomized clinical trials. BMC Complement. Med. Ther. 2021, 21, 179. [Google Scholar] [CrossRef]
- Gheflati, A.; Bashiri, R.; Ghadiri-Anari, A.; Reza, J.Z.; Kord, M.T.; Nadjarzadeh, A. The effect of apple vinegar consumption on glycemic indices, blood pressure, oxidative stress, and homocysteine in patients with type 2 diabetes and dyslipidemia: A randomized controlled clinical trial. Clin. Nutr. ESPEN 2019, 33, 132–138. [Google Scholar] [CrossRef]
- Ousaaid, D.; Laaroussi, H.; Bakour, M.; ElGhouizi, A.; Aboulghazi, A.; Lyoussi, B.; ElArabi, I. Beneficial Effects of Apple Vinegar on Hyperglycemia and Hyperlipidemia in Hypercaloric-Fed Rats. J. Diabetes Res. 2020, 2020, 9284987. [Google Scholar] [CrossRef]
- Halima, B.H.; Sonia, G.; Sarra, K.; Houda, B.J.; Fethi, B.S.; Abdallah, A. Apple Cider Vinegar Attenuates Oxidative Stress and Reduces the Risk of Obesity in High-Fat-Fed Male Wistar Rats. J. Med. Food 2018, 21, 70–80. [Google Scholar] [CrossRef]
- Yagnik, D.; Serafin, V.; Shah, A.J. Antimicrobial activity of apple cider vinegar against Escherichia coli, Staphylococcus aureus and Candida albicans; downregulating cytokine and microbial protein expression. Sci. Rep. 2018, 8, 1732. [Google Scholar] [CrossRef]
- Tripathi, S.; Kumari, U.; Mitra Mazumder, P. Ameliorative effects of apple cider vinegar on neurological complications via regulation of oxidative stress markers. J. Food Biochem. 2020, 44, e13504. [Google Scholar] [CrossRef]
- Shams, F.; Aghajani-Nasab, M.; Ramezanpour, M.; Fatideh, R.H.; Mohammadghasemi, F. Effect of apple vinegar on folliculogenesis and ovarian kisspeptin in a high-fat diet-induced nonalcoholic fatty liver disease in rat. BMC Endocr. Disord. 2022, 22, 330. [Google Scholar] [CrossRef]
- Hlebowicz, J.; Darwiche, G.; Björgell, O.; Almér, L.O. Effect of apple cider vinegar on delayed gastric emptying in patients with type 1 diabetes mellitus: A pilot study. BMC Gastroenterol. 2007, 7, 46. [Google Scholar] [CrossRef]
- Bounihi, A.; Bitam, A.; Bouazza, A.; Yargui, L.; Koceir, E.A. Fruit vinegars attenuate cardiac injury via anti-inflammatory and anti-adiposity actions in high-fat diet-induced obese rats. Pharm. Biol. 2017, 55, 43–52. [Google Scholar] [CrossRef] [PubMed]
- James, A.; Yao, T.; Ke, H.; Wang, Y. Microbiota for production of wine with enhanced functional components. Food Sci. Hum. Wellness 2023, 12, 1481–1492. [Google Scholar] [CrossRef]
- Garaguso, I.; Nardini, M. Polyphenols content, phenolics profile and antioxidant activity of organic red wines produced without sulfur dioxide/sulfites addition in comparison to conventional red wines. Food Chem. 2015, 179, 336–342. [Google Scholar] [CrossRef]
- Pintać, D.; Bekvalac, K.; Mimica-Dukić, N.; Rašeta, M.; Anđelić, N.; Lesjak, M.; Orčić, D. Comparison study between popular brands of coffee, tea and red wine regarding polyphenols content and antioxidant activity. Food Chem. Adv. 2022, 1, 100030. [Google Scholar] [CrossRef]
- Ju, Y.; Yang, L.; Yue, X.; Li, Y.; He, R.; Deng, S.; Yang, X.; Fang, Y. Anthocyanin profiles and color properties of red wines made from Vitis davidii and Vitis vinifera grapes. Food Sci. Hum. Wellness 2021, 10, 335–344. [Google Scholar] [CrossRef]
- Rodriguez-Naranjo, M.I.; Gil-Izquierdo, A.; Troncoso, A.M.; Cantos-Villar, E.; Garcia-Parrilla, M.C. Melatonin is synthesised by yeast during alcoholic fermentation in wines. Food Chem. 2011, 126, 1608–1613. [Google Scholar] [CrossRef]
- Viegas, O.; Esteves, C.; Rocha, J.; Melo, A.; Ferreira, I.M.P.L.V.O. Simultaneous determination of melatonin and trans-resveratrol in wine by dispersive liquid–liquid microextraction followed by HPLC-FLD. Food Chem. 2021, 339, 128091. [Google Scholar] [CrossRef]
- Álvarez-Fernández, M.A.; Fernández-Cruz, E.; Cantos-Villar, E.; Troncoso, A.M.; García-Parrilla, M.C. Determination of hydroxytyrosol produced by winemaking yeasts during alcoholic fermentation using a validated UHPLC–HRMS method. Food Chem. 2018, 242, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Micallef, M.; Lexis, L.; Lewandowski, P. Red wine consumption increases antioxidant status and decreases oxidative stress in the circulation of both young and old humans. Nutr. J. 2007, 6, 27. [Google Scholar] [CrossRef]
- Tedesco, I.; Spagnuolo, C.; Russo, G.L.; Russo, M.; Cervellera, C.; Moccia, S. The Pro-Oxidant Activity of Red Wine Polyphenols Induces an Adaptive Antioxidant Response in Human Erythrocytes. Antioxidants 2021, 10, 800. [Google Scholar] [CrossRef]
- Wang, P.; Gao, J.; Ke, W.; Wang, J.; Li, D.; Liu, R.; Jia, Y.; Wang, X.; Chen, X.; Chen, F.; et al. Resveratrol reduces obesity in high-fat diet-fed mice via modulating the composition and metabolic function of the gut microbiota. Free Radic. Biol. Med. 2020, 156, 83–98. [Google Scholar] [CrossRef]
- Di Lorenzo, A.; Bloise, N.; Meneghini, S.; Sureda, A.; Tenore, G.C.; Visai, L.; Arciola, C.R.; Daglia, M. Effect of Winemaking on the Composition of Red Wine as a Source of Polyphenols for Anti-Infective Biomaterials. Materials 2016, 9, 316. [Google Scholar] [CrossRef]
- Chalons, P.; Courtaut, F.; Limagne, E.; Chalmin, F.; Cantos-Villar, E.; Richard, T.; Auger, C.; Chabert, P.; Schini-Kerth, V.; Ghiringhelli, F.; et al. Red Wine Extract Disrupts Th17 Lymphocyte Differentiation in a Colorectal Cancer Context. Mol. Nutr. Food Res. 2020, 64, e1901286. [Google Scholar] [CrossRef]
- Mahjabeen, W.; Khan, D.A.; Mirza, S.A. Role of resveratrol supplementation in regulation of glucose hemostasis, inflammation and oxidative stress in patients with diabetes mellitus type 2: A randomized, placebo-controlled trial. Complement. Ther. Med. 2022, 66, 102819. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.M.; Zhang, Y.M.; Feng, Y.Z.; Zhang, K.X.; Zhang, J.Y.; Chen, J.; Luo, B.L.; Li, X.Y.; Chen, G.H. Resveratrol ameliorates maternal separation-induced anxiety- and depression-like behaviors and reduces Sirt1-NF-kB signaling-mediated neuroinflammation. Front. Behav. Neurosci. 2023, 17, 1172091. [Google Scholar] [CrossRef]
- Martínez-Flórez, S.; Gutiérrez-Fernández, B.; Sánchez-Campos, S.; González-Gallego, J.; Tuñón, M.J. Quercetin attenuates nuclear factor-kappaB activation and nitric oxide production in interleukin-1beta-activated rat hepatocytes. J. Nutr. 2005, 135, 1359–1365. [Google Scholar] [CrossRef]
- Ortega, M.G.; Saragusti, A.C.; Cabrera, J.L.; Chiabrando, G.A. Quercetin tetraacetyl derivative inhibits LPS-induced nitric oxide synthase (iNOS) expression in J774A.1 cells. Arch. Biochem. Biophys. 2010, 498, 105–110. [Google Scholar] [CrossRef]
- Wu, H.; Chen, L.; Zhu, F.; Han, X.; Sun, L.; Chen, K. The Cytotoxicity Effect of Resveratrol: Cell Cycle Arrest and Induced Apoptosis of Breast Cancer 4T1 Cells. Toxins 2019, 11, 731. [Google Scholar] [CrossRef]
- Li, J.; Fan, Y.; Zhang, Y.; Liu, Y.; Yu, Y.; Ma, M. Resveratrol Induces Autophagy and Apoptosis in Non-Small-Cell Lung Cancer Cells by Activating the NGFR-AMPK-mTOR Pathway. Nutrients 2022, 14, 2413. [Google Scholar] [CrossRef] [PubMed]
- Iban-Arias, R.; Sebastian-Valverde, M.; Wu, H.; Lyu, W.; Wu, Q.; Simon, J.; Pasinetti, G.M. Role of Polyphenol-Derived Phenolic Acid in Mitigation of Inflammasome-Mediated Anxiety and Depression. Biomedicines 2022, 10, 1264. [Google Scholar] [CrossRef]
- Ye, S.; Fang, L.; Xie, S.; Hu, Y.; Chen, S.; Amin, N.; Fang, M.; Hu, Z. Resveratrol alleviates postpartum depression-like behavior by activating autophagy via SIRT1 and inhibiting AKT/mTOR pathway. Behav. Brain Res. 2023, 438, 114208. [Google Scholar] [CrossRef]
- Wong, R.H.; Thaung Zaw, J.J.; Xian, C.J.; Howe, P.R. Regular Supplementation with Resveratrol Improves Bone Mineral Density in Postmenopausal Women: A Randomized, Placebo-Controlled Trial. J. Bone Min. Res. 2020, 35, 2121–2131. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Malcangi, G.; Inchingolo, A.M.; Piras, F.; Settanni, V.; Garofoli, G.; Palmieri, G.; Ceci, S.; Patano, A.; De Leonardis, N.; et al. Benefits and Implications of Resveratrol Supplementation on Microbiota Modulations: A Systematic Review of the Literature. Int. J. Mol. Sci. 2022, 23, 4027. [Google Scholar] [CrossRef]
- Avellone, G.; Di Garbo, V.; Campisi, D.; De Simone, R.; Raneli, G.; Scaglione, R.; Licata, G. Effects of moderate Sicilian red wine consumption on inflammatory biomarkers of atherosclerosis. Eur. J. Clin. Nutr. 2006, 60, 41–47. [Google Scholar] [CrossRef]
- Loke, W.M.; Hodgson, J.M.; Proudfoot, J.M.; McKinley, A.J.; Puddey, I.B.; Croft, K.D. Pure dietary flavonoids quercetin and (-)-epicatechin augment nitric oxide products and reduce endothelin-1 acutely in healthy men. Am. J. Clin. Nutr. 2008, 88, 1018–1025. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, X.; Chen, K.; Lang, H.; Zhang, Y.; Hou, P.; Ran, L.; Zhou, M.; Zheng, J.; Yi, L.; et al. Resveratrol prevents sarcopenic obesity by reversing mitochondrial dysfunction and oxidative stress via the PKA/LKB1/AMPK pathway. Aging 2019, 11, 2217–2240. [Google Scholar] [CrossRef]
- Su, L.; Zeng, Y.; Li, G.; Chen, J.; Chen, X. Quercetin improves high-fat diet-induced obesity by modulating gut microbiota and metabolites in C57BL/6J mice. Phytother. Res. 2022, 36, 4558–4572. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Indias, I.; Sánchez-Alcoholado, L.; Pérez-Martínez, P.; Andrés-Lacueva, C.; Cardona, F.; Tinahones, F.; Queipo-Ortuño, M.I. Red wine polyphenols modulate fecal microbiota and reduce markers of the metabolic syndrome in obese patients. Food Funct. 2016, 7, 1775–1787. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Zheng, J.; Xu, F.; Xi, Y.; Chen, J.; Xu, X. Resveratrol Alleviates Dextran Sulfate Sodium-Induced Acute Ulcerative Colitis in Mice by Mediating PI3K/Akt/VEGFA Pathway. Front. Pharmacol. 2021, 12, 693982. [Google Scholar] [CrossRef]
- Sabzevary-Ghahfarokhi, M.; Soltani, A.; Luzza, F.; Larussa, T.; Rahimian, G.; Shirzad, H.; Bagheri, N. The protective effects of resveratrol on ulcerative colitis via changing the profile of Nrf2 and IL-1β protein. Mol. Biol. Rep. 2020, 47, 6941–6947. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Wei, C.; Wang, J.Y.; Zhang, R.; Li, Y.X.; Wang, L.S. Effect of resveratrol on Treg/Th17 signaling and ulcerative colitis treatment in mice. World J. Gastroenterol. 2015, 21, 6572–6581. [Google Scholar] [CrossRef]
- Chitimus, D.M.; Popescu, M.R.; Voiculescu, S.E.; Panaitescu, A.M.; Pavel, B.; Zagrean, L.; Zagrean, A.M. Melatonin’s Impact on Antioxidative and Anti-Inflammatory Reprogramming in Homeostasis and Disease. Biomolecules 2020, 10, 1211. [Google Scholar] [CrossRef]
- Tu, Y.; Song, E.; Wang, Z.; Ji, N.; Zhu, L.; Wang, K.; Sun, H.; Zhang, Y.; Zhu, Q.; Liu, X.; et al. Melatonin attenuates oxidative stress and inflammation of Müller cells in diabetic retinopathy via activating the Sirt1 pathway. Biomed. Pharmacother. 2021, 137, 111274. [Google Scholar] [CrossRef]
- Rehman, S.U.; Ikram, M.; Ullah, N.; Alam, S.I.; Park, H.Y.; Badshah, H.; Choe, K.; Kim, M.O. Neurological Enhancement Effects of Melatonin against Brain Injury-Induced Oxidative Stress, Neuroinflammation, and Neurodegeneration via AMPK/CREB Signaling. Cells 2019, 8, 760. [Google Scholar] [CrossRef]
- Kang, J.Y.; Xu, M.M.; Sun, Y.; Ding, Z.X.; Wei, Y.Y.; Zhang, D.W.; Wang, Y.G.; Shen, J.L.; Wu, H.M.; Fei, G.H. Melatonin attenuates LPS-induced pyroptosis in acute lung injury by inhibiting NLRP3-GSDMD pathway via activating Nrf2/HO-1 signaling axis. Int. Immunopharmacol. 2022, 109, 108782. [Google Scholar] [CrossRef]
- de Pablos, R.M.; Espinosa-Oliva, A.M.; Hornedo-Ortega, R.; Cano, M.; Arguelles, S. Hydroxytyrosol protects from aging process via AMPK and autophagy; a review of its effects on cancer, metabolic syndrome, osteoporosis, immune-mediated and neurodegenerative diseases. Pharmacol. Res. 2019, 143, 58–72. [Google Scholar] [CrossRef]
- Karković Marković, A.; Torić, J.; Barbarić, M.; Jakobušić Brala, C. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef]
- D’Adamo, S.; Cetrullo, S.; Guidotti, S.; Borzì, R.M.; Flamigni, F. Hydroxytyrosol modulates the levels of microRNA-9 and its target sirtuin-1 thereby counteracting oxidative stress-induced chondrocyte death. Osteoarthr. Cartil. 2017, 25, 600–610. [Google Scholar] [CrossRef]
- Wang, W.; Jing, T.; Yang, X.; He, Y.; Wang, B.; Xiao, Y.; Shang, C.; Zhang, J.; Lin, R. Hydroxytyrosol regulates the autophagy of vascular adventitial fibroblasts through the SIRT1-mediated signaling pathway. Can. J. Physiol. Pharmacol. 2018, 96, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Bolarinwa, I.; Al-Ezzi, M.; Carew, I.; Muhammad, K. Nutritional Value of Legumes in Relation to Human Health: A Review. Adv. J. Food Sci. Technol. 2019, 17, 72–85. [Google Scholar] [CrossRef]
- Cakir, Ö.; Ucarli, C.; TARHAN, Ç.; Pekmez, M.; Turgut-Kara, N. Nutritional and health benefits of legumes and their distinctive genomic properties. Food Sci. Technol. 2019, 39, 1–12. [Google Scholar]
- Juárez-Chairez, M.F.; Cid-Gallegos, M.S.; Meza-Márquez, O.G.; Jiménez-Martínez, C. Biological functions of peptides from legumes in gastrointestinal health. A review legume peptides with gastrointestinal protection. J. Food Biochem. 2022, 46, e14308. [Google Scholar] [CrossRef]
- Schuster-Gajzágó, I. Nutritional aspects of legumes. In Cultivated Plants, Primarily as Food Sources; Encyclopedia of Food and Agricultural Sciences, Engineering and Technology Resources; Encyclopedia of Life Support System (EOLSS): Abu Dhabi, United Arab Emirates, 2004; Volume 1, pp. 1–7. [Google Scholar]
- Kubota, M.; Shimizu, H. Nutrition and bone health. Soybean and soy foods, and bone health. Clin. Calcium 2009, 19, 1514–1519. [Google Scholar] [PubMed]
- Garrido-Galand, S.; Asensio-Grau, A.; Calvo-Lerma, J.; Heredia, A.; Andrés, A. The potential of fermentation on nutritional and technological improvement of cereal and legume flours: A review. Food Res. Int. 2021, 145, 110398. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Zhang, K.; Zhang, Z.; Zhang, C.; Sun, Y.; Feng, Z. Fermented soybean foods: A review of their functional components, mechanism of action and factors influencing their health benefits. Food Res. Int. 2022, 158, 111575. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, X.; Hao, L.; Zhang, G.; Jin, Z.; Li, C.; Yang, Y.; Rao, J.; Chen, B. Traditional fermented soybean products: Processing, flavor formation, nutritional and biological activities. Crit. Rev. Food Sci. Nutr. 2022, 62, 1971–1989. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Hwang, C.W.; Yang, W.S.; Kim, C.H. Current Perspectives on the Physiological Activities of Fermented Soybean-Derived Cheonggukjang. Int. J. Mol. Sci. 2021, 22, 5746. [Google Scholar] [CrossRef]
- Kaufman, P.B.; Duke, J.A.; Brielmann, H.; Boik, J.; Hoyt, J.E. A comparative survey of leguminous plants as sources of the isoflavones, genistein and daidzein: Implications for human nutrition and health. J. Altern. Complement. Med. 1997, 3, 7–12. [Google Scholar] [CrossRef]
- Cichońska, P.; Ziarno, M. Legumes and Legume-Based Beverages Fermented with Lactic Acid Bacteria as a Potential Carrier of Probiotics and Prebiotics. Microorganisms 2021, 10, 91. [Google Scholar] [CrossRef]
- Takagi, A.; Kano, M.; Kaga, C. Possibility of breast cancer prevention: Use of soy isoflavones and fermented soy beverage produced using probiotics. Int. J. Mol. Sci. 2015, 16, 10907–10920. [Google Scholar] [CrossRef]
- Kimura, K.; Yokoyama, S. Trends in the application of Bacillus in fermented foods. Curr. Opin. Biotechnol. 2019, 56, 36–42. [Google Scholar] [CrossRef]
- Cao, Z.H.; Green-Johnson, J.M.; Buckley, N.D.; Lin, Q.Y. Bioactivity of soy-based fermented foods: A review. Biotechnol. Adv. 2019, 37, 223–238. [Google Scholar] [CrossRef]
- Allwood, J.G.; Wakeling, L.T.; Bean, D.C. Fermentation and the microbial community of Japanese koji and miso: A review. J. Food Sci. 2021, 86, 2194–2207. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.M.; Kim, H.J.; Jeon, M.S.; Yoo, S.J.; Moon, H.Y.; Jeon, E.J.; Jeon, C.O.; Eyun, S.I.; Kang, H.A. Genomic and functional features of yeast species in Korean traditional fermented alcoholic beverage and soybean products. FEMS Yeast Res. 2023, 23, foac066. [Google Scholar] [CrossRef]
- Owusu-Kwarteng, J.; Parkouda, C.; Adewumi, G.A.; Ouoba, L.I.I.; Jespersen, L. Technologically relevant Bacillus species and microbial safety of West African traditional alkaline fermented seed condiments. Crit. Rev. Food Sci. Nutr. 2022, 62, 871–888. [Google Scholar] [CrossRef] [PubMed]
- Ogueke, C.C.; Nwosu, J.N.; Owuamanam, C.I.; Iwouno, J.N. Ugba, the fermented African oilbean seeds; its production, chemical composition, preservation, safety and health benefits. Pak. J. Biol. Sci. PJBS 2010, 13, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Labba, I.M.; Andlid, T.; Lindgren, Å.; Sandberg, A.S.; Sjöberg, F. Isolation, identification, and selection of strains as candidate probiotics and starters for fermentation of Swedish legumes. Food Nutr. Res. 2020, 64, 1–13. [Google Scholar] [CrossRef]
- Jayachandran, M.; Xu, B. An insight into the health benefits of fermented soy products. Food Chem. 2019, 271, 362–371. [Google Scholar] [CrossRef]
- Nikmaram, N.; Dar, B.N.; Roohinejad, S.; Koubaa, M.; Barba, F.J.; Greiner, R.; Johnson, S.K. Recent advances in γ-aminobutyric acid (GABA) properties in pulses: An overview. J. Sci. Food Agric. 2017, 97, 2681–2689. [Google Scholar] [CrossRef]
- Das, G.; Paramithiotis, S.; Sundaram Sivamaruthi, B.; Wijaya, C.H.; Suharta, S.; Sanlier, N.; Shin, H.S.; Patra, J.K. Traditional fermented foods with anti-aging effect: A concentric review. Food Res. Int. 2020, 134, 109269. [Google Scholar] [CrossRef]
- Belobrajdic, D.P.; James-Martin, G.; Jones, D.; Tran, C.D. Soy and Gastrointestinal Health: A Review. Nutrients 2023, 15, 1959. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Sarkar, S.; Borsingh Wann, S.; Kalita, J.; Manna, P. Current perspectives on the anti-inflammatory potential of fermented soy foods. Food Res. Int. 2022, 152, 110922. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Huang, H.; Li, H.; Wei, Y.; Yao, C. Legume-Derived Bioactive Peptides in Type 2 Diabetes: Opportunities and Challenges. Nutrients 2023, 15, 1096. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Sarkar, S.; Bordoloi, J.; Wann, S.B.; Kalita, J.; Manna, P. Daidzein, its effects on impaired glucose and lipid metabolism and vascular inflammation associated with type 2 diabetes. BioFactors 2018, 44, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Monk, J.M.; Zhang, C.P.; Wu, W.; Zarepoor, L.; Lu, J.T.; Liu, R.; Pauls, K.P.; Wood, G.A.; Tsao, R.; Robinson, L.E.; et al. White and dark kidney beans reduce colonic mucosal damage and inflammation in response to dextran sodium sulfate. J. Nutr. Biochem. 2015, 26, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Pang, W.; Wang, D.; Zuo, Z.; Wang, Y.; Sun, W.; Zhang, N.; Zhang, D. Kidney Bean Fermented Broth Alleviates Hyperlipidemic by Regulating Serum Metabolites and Gut Microbiota Composition. Nutrients 2022, 14, 3202. [Google Scholar] [CrossRef]
- Georgetti, S.R.; Casagrande, R.; Vicentini, F.T.; Baracat, M.M.; Verri, W.A., Jr.; Fonseca, M.J. Protective effect of fermented soybean dried extracts against TPA-induced oxidative stress in hairless mice skin. BioMed Res. Int. 2013, 2013, 340626. [Google Scholar] [CrossRef]
- Yang, J.H.; Byeon, E.H.; Kang, D.; Hong, S.G.; Yang, J.; Kim, D.R.; Yun, S.P.; Park, S.W.; Kim, H.J.; Huh, J.W.; et al. Fermented Soybean Paste Attenuates Biogenic Amine-Induced Liver Damage in Obese Mice. Cells 2023, 12, 822. [Google Scholar] [CrossRef]
- Frias, J.; Song, Y.S.; Martínez-Villaluenga, C.; González de Mejia, E.; Vidal-Valverde, C. Immunoreactivity and amino acid content of fermented soybean products. J. Agric. Food Chem. 2008, 56, 99–105. [Google Scholar] [CrossRef]
- Ali, N.M.; Yeap, S.K.; Yusof, H.M.; Beh, B.K.; Ho, W.Y.; Koh, S.P.; Abdullah, M.P.; Alitheen, N.B.; Long, K. Comparison of free amino acids, antioxidants, soluble phenolic acids, cytotoxicity and immunomodulation of fermented mung bean and soybean. J. Sci. Food Agric. 2016, 96, 1648–1658. [Google Scholar] [CrossRef]
- Das, D.; Sarkar, S.; Dihingia, A.; Afzal, N.U.; Wann, S.B.; Kalita, J.; Dewanjee, S.; Manna, P. A popular fermented soybean food of Northeast India exerted promising antihyperglycemic potential via stimulating PI3K/AKT/AMPK/GLUT4 signaling pathways and regulating muscle glucose metabolism in type 2 diabetes. J. Food Biochem. 2022, 46, e14385. [Google Scholar] [CrossRef]
- Sapbamrer, R.; Visavarungroj, N.; Suttajit, M. Effects of dietary traditional fermented soybean on reproductive hormones, lipids, and glucose among postmenopausal women in northern Thailand. Asia Pac. J. Clin. Nutr. 2013, 22, 222–228. [Google Scholar] [CrossRef]
- Rim, H.K.; Kim, K.Y.; Ryu, J.G.; Song, Y.H.; Kim, H.H.; Han, J.H.; Jeong, H.J.; Kim, H.M. Alcohol-fermented soybean increases the expression of receptor-interacting protein 2 and IκB kinase β in mouse peritoneal macrophages. J. Med. Food 2011, 14, 1181–1189. [Google Scholar] [CrossRef]
- Chen, K.; Luo, H.; Li, Y.; Han, X.; Gao, C.; Wang, N.; Lu, F.; Wang, H. Lactobacillus paracasei TK1501 fermented soybeans alleviate dextran sulfate sodium-induced colitis by regulating intestinal cell function. J. Sci. Food Agric. 2023, 103, 5422–5431. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.C.; Pathak, S.; Kumar, V.; Panda, B.P. Attenuation of neurobehavioral and neurochemical abnormalities in animal model of cognitive deficits of Alzheimer’s disease by fermented soybean nanonutraceutical. Inflammopharmacology 2018, 26, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.C.; Kuo, L.H.; Chang, Y.Y.; Tung, Y.C.; Lo, Y.C.; Pan, M.H. Modulatory Effect of Fermented Black Soybean and Adlay on Gut Microbiota Contributes to Healthy Aging. Mol. Nutr. Food Res. 2023, 67, e2200700. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Wu, P.S.; Liang, D.W.; Kwan, C.C.; Chen, Y.S. Quality, antioxidative ability, and cell proliferation-enhancing activity of fermented black soybean broths with various supplemental culture medium. J. Food Sci. 2012, 77, C95–C101. [Google Scholar] [CrossRef]
- Malardé, L.; Vincent, S.; Lefeuvre-Orfila, L.; Efstathiou, T.; Groussard, C.; Gratas-Delamarche, A. A fermented soy permeate improves the skeletal muscle glucose level without restoring the glycogen content in streptozotocin-induced diabetic rats. J. Med. Food 2013, 16, 176–179. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.B.; Lee, H.S.; Kim, S.J.; Yoo, H.J.; Hwang, J.S.; Chen, G.; Youn, H.J. Ethanol extract of fermented soybean, Chungkookjang, inhibits the apoptosis of mouse spleen, and thymus cells. J. Microbiol. 2007, 45, 256–261. [Google Scholar]
- Lee, D.H.; Kim, M.J.; Ahn, J.; Lee, S.H.; Lee, H.; Kim, J.H.; Park, S.H.; Jang, Y.J.; Ha, T.Y.; Jung, C.H. Nutrikinetics of Isoflavone Metabolites After Fermented Soybean Product (Cheonggukjang) Ingestion in Ovariectomized Mice. Mol. Nutr. Food Res. 2017, 61, 1700322. [Google Scholar] [CrossRef]
- Cho, B.O.; Shin, J.Y.; Kim, J.S.; Che, D.N.; Kang, H.J.; Jeong, D.Y.; Jang, S.I. Soybean Fermented with Bacillus amyloliquefaciens (Cheonggukjang) Ameliorates Atopic Dermatitis-Like Skin Lesion in Mice by Suppressing Infiltration of Mast Cells and Production of IL-31 Cytokine. J. Microbiol. Biotechnol. 2019, 29, 827–837. [Google Scholar] [CrossRef]
- Choi, J.; Kwon, S.H.; Park, K.Y.; Yu, B.P.; Kim, N.D.; Jung, J.H.; Chung, H.Y. The anti-inflammatory action of fermented soybean products in kidney of high-fat-fed rats. J. Med. Food 2011, 14, 232–239. [Google Scholar] [CrossRef]
- Lee, J.H.; Paek, S.H.; Shin, H.W.; Lee, S.Y.; Moon, B.S.; Park, J.E.; Lim, G.D.; Kim, C.Y.; Heo, Y. Effect of fermented soybean products intake on the overall immune safety and function in mice. J. Vet. Sci. 2017, 18, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.E.; Kim, K.A.; Han, M.J.; Kim, D.H. Doenjang, a fermented Korean soybean paste, inhibits lipopolysaccharide production of gut microbiota in mice. J. Med. Food 2014, 17, 67–75. [Google Scholar] [CrossRef]
- Sumi, H.; Hamada, H.; Tsushima, H.; Mihara, H.; Muraki, H. A novel fibrinolytic enzyme (nattokinase) in the vegetable cheese Natto; a typical and popular soybean food in the Japanese diet. Experientia 1987, 43, 1110–1111. [Google Scholar] [CrossRef] [PubMed]
- Oba, M.; Rongduo, W.; Saito, A.; Okabayashi, T.; Yokota, T.; Yasuoka, J.; Sato, Y.; Nishifuji, K.; Wake, H.; Nibu, Y.; et al. Natto extract, a Japanese fermented soybean food, directly inhibits viral infections including SARS-CoV-2 in vitro. Biochem. Biophys. Res. Commun. 2021, 570, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Uenishi, K. Recommended soy and soy products intake to prevent bone fracture and osteoporosis. Clin. Calcium 2005, 15, 1393–1398. [Google Scholar]
- Katsuyama, H.; Ideguchi, S.; Fukunaga, M.; Saijoh, K.; Sunami, S. Usual dietary intake of fermented soybeans (Natto) is associated with bone mineral density in premenopausal women. J. Nutr. Sci. Vitaminol. 2002, 48, 207–215. [Google Scholar] [CrossRef]
- Murai, U.; Sawada, N.; Charvat, H.; Inoue, M.; Yasuda, N.; Yamagishi, K.; Tsugane, S. Soy product intake and risk of incident disabling dementia: The JPHC Disabling Dementia Study. Eur. J. Nutr. 2022, 61, 4045–4057. [Google Scholar] [CrossRef]
- Sasaki, H.; Pham Thi Ngoc, D.; Nishikawa, M.; Kanauchi, M. Lipopolysaccharide neutralizing protein in Miso, Japanese fermented soybean paste. J. Food Sci. 2020, 85, 2498–2505. [Google Scholar] [CrossRef]
- Matsuo, M. Chemical components, palatability, antioxidant activity and antimutagenicity of oncom miso using a mixture of fermented soybeans and okara with Neurospora intermedia. J. Nutr. Sci. Vitaminol. 2006, 52, 216–222. [Google Scholar] [CrossRef]
- Nagata, C.; Shimizu, H.; Takami, R.; Hayashi, M.; Takeda, N.; Yasuda, K. Hot flushes and other menopausal symptoms in relation to soy product intake in Japanese women. Climacteric 1999, 2, 6–12. [Google Scholar] [CrossRef]
- Uemura, H.; Katsuura-Kamano, S.; Nakamoto, M.; Yamaguchi, M.; Fujioka, M.; Iwasaki, Y.; Arisawa, K. Inverse association between soy food consumption, especially fermented soy products intake and soy isoflavone, and arterial stiffness in Japanese men. Sci. Rep. 2018, 8, 9667. [Google Scholar] [CrossRef]
- Nozue, M.; Shimazu, T.; Sasazuki, S.; Charvat, H.; Mori, N.; Mutoh, M.; Sawada, N.; Iwasaki, M.; Yamaji, T.; Inoue, M.; et al. Fermented Soy Product Intake Is Inversely Associated with the Development of High Blood Pressure: The Japan Public Health Center-Based Prospective Study. J. Nutr. 2017, 147, 1749–1756. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M. Low-salt O-miso produced from Koji fermentation of oncom improves redox state and cholesterolemia in rats more than low-salt soybean-miso. J. Nutr. Sci. Vitaminol. 2004, 50, 362–366. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.J.; Shin, H.W.; Jung, S.; Ha, J.H. Effect of Soybean and Soybean Koji on Obesity and Dyslipidemia in Rats Fed a High-Fat Diet: A Comparative Study. Int. J. Environ. Res. Public Health 2021, 18, 6032. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Kim, S.A.; Jo, Y.M.; Seo, H.; Kim, G.Y.; Cheon, S.W.; Yang, S.H.; Jeon, C.O.; Han, N.S. Probiotic potential of Tetragenococcus halophilus EFEL7002 isolated from Korean soy Meju. BMC Microbiol. 2022, 22, 149. [Google Scholar] [CrossRef]
- Kulprachakarn, K.; Chaipoot, S.; Phongphisutthinant, R.; Paradee, N.; Prommaban, A.; Ounjaijean, S.; Rerkasem, K.; Parklak, W.; Prakit, K.; Saengsitthisak, B.; et al. Antioxidant Potential and Cytotoxic Effect of Isoflavones Extract from Thai Fermented Soybean (Thua-Nao). Molecules 2021, 26, 7432. [Google Scholar] [CrossRef]
- Arumugam, S.; Dioletis, E.; Paiva, R.; Fields, M.R.; Weiss, T.R.; Secor, E.R.; Ali, A. Fermented Soy Beverage Q-CAN Plus Consumption Improves Serum Cholesterol and Cytokines. J. Med. Food 2020, 23, 560–563. [Google Scholar] [CrossRef]
- Lin, C.Y.; Tsai, Z.Y.; Cheng, I.C.; Lin, S.H. Effects of fermented soy milk on the liver lipids under oxidative stress. World J. Gastroenterol. 2005, 11, 7355–7358. [Google Scholar] [CrossRef] [PubMed]
- Biscola, V.; de Olmos, A.R.; Choiset, Y.; Rabesona, H.; Garro, M.S.; Mozzi, F.; Chobert, J.M.; Drouet, M.; Haertlé, T.; Franco, B. Soymilk fermentation by Enterococcus faecalis VB43 leads to reduction in the immunoreactivity of allergenic proteins β-conglycinin (7S) and glycinin (11S). Benef. Microbes 2017, 8, 635–643. [Google Scholar] [CrossRef]
- Hwang, J.H.; Wu, S.J.; Wu, P.L.; Shih, Y.Y.; Chan, Y.C. Neuroprotective effect of tempeh against lipopolysaccharide-induced damage in BV-2 microglial cells. Nutr. Neurosci. 2019, 22, 840–849. [Google Scholar] [CrossRef]
- McKevith, B. Nutritional aspects of cereals. Nutr. Bull. 2004, 29, 111–142. [Google Scholar] [CrossRef]
- Mishra, S.; Mithul Aravind, S.; Charpe, P.; Ajlouni, S.; Ranadheera, C.S.; Chakkaravarthi, S. Traditional rice-based fermented products: Insight into their probiotic diversity and probable health benefits. Food Biosci. 2022, 50, 102082. [Google Scholar] [CrossRef]
- Patra, M.; Bashir, O.; Amin, T.; Wani, A.W.; Shams, R.; Chaudhary, K.S.; Mirza, A.A.; Manzoor, S. A comprehensive review on functional beverages from cereal grains-characterization of nutraceutical potential, processing technologies and product types. Heliyon 2023, 9, e16804. [Google Scholar] [CrossRef]
- Tsafrakidou, P.; Michaelidou, A.M.; Biliaderis, C.G. Fermented Cereal-based Products: Nutritional Aspects, Possible Impact on Gut Microbiota and Health Implications. Foods 2020, 9, 734. [Google Scholar] [CrossRef]
- Goksen, G.; Sugra Altaf, Q.; Farooq, S.; Bashir, I.; Cappozzi, V.; Guruk, M.; Lucia Bavaro, S.; Kumar Sarangi, P. A Glimpse into Plant-based Fermented Products Alternative to Animal Based Products: Formulation, Processing, Health Benefits. Food Res. Int. 2023, 173, 113344. [Google Scholar] [CrossRef]
- Hlangwani, E.; Njobeh, P.B.; Chinma, C.E.; Oyedeji, A.B.; Fasogbon, B.M.; Oyeyinka, S.A.; Sobowale, S.S.; Dudu, O.E.; Molelekoa, T.B.J.; Kesa, H.; et al. Chapter 2—African cereal-based fermented products. In Indigenous Fermented Foods for the Tropics; Adebo, O.A., Chinma, C.E., Obadina, A.O., Soares, A.G., Panda, S.K., Gan, R.-Y., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 15–36. [Google Scholar]
- Pswarayi, F.; Gänzle, M. African cereal fermentations: A review on fermentation processes and microbial composition of non-alcoholic fermented cereal foods and beverages. Int. J. Food Microbiol. 2022, 378, 109815. [Google Scholar] [CrossRef]
- Zannou, O.; Agossou, D.J.; Miassi, Y.; Agani, O.B.; Darino Aisso, M.; Chabi, I.B.; Euloge Kpoclou, Y.; Azokpota, P.; Koca, I. Traditional fermented foods and beverages: Indigenous practices of food processing in Benin Republic. Int. J. Gastron. Food Sci. 2022, 27, 100450. [Google Scholar] [CrossRef]
- Kütt, M.-L.; Orgusaar, K.; Stulova, I.; Priidik, R.; Pismennõi, D.; Vaikma, H.; Kallastu, A.; Zhogoleva, A.; Morell, I.; Kriščiunaite, T. Starter culture growth dynamics and sensory properties of fermented oat drink. Heliyon 2023, 9, e15627. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.I.K.; Tuaño, A.P.P.; Juanico, C.B. Microbial quality, safety, sensory acceptability, and proximate composition of a fermented nixtamalized maize (Zea mays L.) beverage. J. Cereal Sci. 2022, 107, 103521. [Google Scholar] [CrossRef]
- Rebaza-Cardenas, T.; Montes-Villanueva, N.D.; Fernández, M.; Delgado, S.; Ruas-Madiedo, P. Microbiological and physical-chemical characteristics of the Peruvian fermented beverage “Chicha de siete semillas”: Towards the selection of strains with acidifying properties. Int. J. Food Microbiol. 2023, 406, 110353. [Google Scholar] [CrossRef]
- Oyeyinka, A.T.; Siwela, M.; Pillay, K. A mini review of the physicochemical properties of amahewu, a Southern African traditional fermented cereal grain beverage. LWT 2021, 151, 112159. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Sarkar, I.; Sen, G.; Ghosh, C.; Sen, A. Biochemical and Metagenomic sketching of microbial populations in the starter culture of ‘Chokot’, a rice-based fermented liquor of Rabha Tribe in North Bengal, India. Ecol. Genet. Genom. 2023, 29, 100193. [Google Scholar] [CrossRef]
- Osimani, A.; Garofalo, C.; Aquilanti, L.; Milanović, V.; Clementi, F. Unpasteurised commercial boza as a source of microbial diversity. Int. J. Food Microbiol. 2015, 194, 62–70. [Google Scholar] [CrossRef]
- Yeğin, S.; Üren, A. Biogenic amine content of boza: A traditional cereal-based, fermented Turkish beverage. Food Chem. 2008, 111, 983–987. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, M.; Zhao, Y.; Zhu, Y.; Bai, J.; Fan, S.; Zhu, L.; Song, C.; Xiao, X. Recent Developments in Fermented Cereals on Nutritional Constituents and Potential Health Benefits. Foods 2022, 11, 2243. [Google Scholar]
- Shangpliang, H.N.J.; Tamang, J.P. Metagenomics and metagenome-assembled genomes mining of health benefits in jalebi batter, a naturally fermented cereal-based food of India. Food Res. Int. 2023, 172, 113130. [Google Scholar] [CrossRef] [PubMed]
- Banwo, K.; Oyeyipo, A.; Mishra, L.; Sarkar, D.; Shetty, K. Improving phenolic bioactive-linked functional qualities of traditional cereal-based fermented food (Ogi) of Nigeria using compatible food synergies with underutilized edible plants. NFS J. 2022, 27, 1–12. [Google Scholar] [CrossRef]
- Gebre, T.S.; Emire, S.A.; Chelliah, R.; Aloo, S.O.; Oh, D.-H. Isolation, functional activity, and safety of probiotics from Ethiopian traditional cereal-based fermented beverage, “Borde”. LWT 2023, 184, 115076. [Google Scholar] [CrossRef]
- Ogunremi, O.R.; Freimüller Leischtfeld, S.; Mischler, S.; Miescher Schwenninger, S. Antifungal activity of lactic acid bacteria isolated from kunu-zaki, a cereal-based Nigerian fermented beverage. Food Biosci. 2022, 49, 101648. [Google Scholar] [CrossRef]
- Bayoï, J.R.; Ndegoue, S.V.; Etoa, F.-X. Traditional processing and quality attributes of “kounou”, a fermented indigenous cereal-based beverage from the northern zone of Cameroon. J. Agric. Food Res. 2021, 6, 100209. [Google Scholar] [CrossRef]
- Rasheed, H.A.; Tuoheti, T.; Zhang, Y.; Azi, F.; Tekliye, M.; Dong, M. Purification and partial characterization of a novel bacteriocin produced by bacteriocinogenic Lactobacillus fermentum BZ532 isolated from Chinese fermented cereal beverage (Bozai). LWT 2020, 124, 109113. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Wu, S.-j.; Fang, J.-Y.; Wang, Y.-P.; Shyu, Y.-T. Cardiovascular and intestinal protection of cereal pastes fermented with lactic acid bacteria in hyperlipidemic hamsters. Food Res. Int. 2012, 48, 428–434. [Google Scholar] [CrossRef]
- Oguntoyinbo, F.A.; Narbad, A. Multifunctional properties of Lactobacillus plantarum strains isolated from fermented cereal foods. J. Funct. Foods 2015, 17, 621–631. [Google Scholar] [CrossRef]
- Ayyash, M.; Johnson, S.K.; Liu, S.-Q.; Al-Mheiri, A.; Abushelaibi, A. Cytotoxicity, antihypertensive, antidiabetic and antioxidant activities of solid-state fermented lupin, quinoa and wheat by Bifidobacterium species: In-vitro investigations. LWT 2018, 95, 295–302. [Google Scholar] [CrossRef]
- Zhu, C.; Guan, Q.; Song, C.; Zhong, L.; Ding, X.; Zeng, H.; Nie, P.; Song, L. Regulatory effects of Lactobacillus fermented black barley on intestinal microbiota of NAFLD rats. Food Res. Int. 2021, 147, 110467. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-García, J.; Muñoz-Pina, S.; García-Hernández, J.; Heredia, A.; Andrés, A. Fermented quinoa flour: Implications of fungal solid-state bioprocessing and drying on nutritional and antioxidant properties. LWT 2023, 182, 114885. [Google Scholar] [CrossRef]
- Kingamkono, R.; Sjögren, E.; Svanberg, U. Enteropathogenic bacteria in faecal swabs of young children fed on lactic acid-fermented cereal gruels. Epidemiol. Infect. 1999, 122, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xue, K.; Iversen, K.N.; Qu, Z.; Dong, C.; Jin, T.; Hallmans, G.; Åman, P.; Johansson, A.; He, G.; et al. The effects of fermented rye products on gut microbiota and their association with metabolic factors in Chinese adults—An explorative study. Food Funct. 2021, 12, 9141–9150. [Google Scholar] [CrossRef]
- Ren, R.; Zeng, H.; Mei, Q.; Xu, Z.; Mazhar, M.; Qin, L. Effects of Monascus purpureus-fermented tartary buckwheat extract on the blood lipid profile, glucose tolerance and antioxidant enzyme activities in KM mice. J. Cereal Sci. 2022, 105, 103465. [Google Scholar] [CrossRef]
- Salar, R.K.; Purewal, S.S.; Sandhu, K.S. Fermented pearl millet (Pennisetum glaucum) with in vitro DNA damage protection activity, bioactive compounds and antioxidant potential. Food Res. Int. 2017, 100, 204–210. [Google Scholar] [CrossRef]
- Ofosu, F.K.; Elahi, F.; Daliri, E.B.-M.; Aloo, S.O.; Chelliah, R.; Han, S.-I.; Oh, D.-H. Fermented sorghum improves type 2 diabetes remission by modulating gut microbiota and their related metabolites in high fat diet-streptozotocin induced diabetic mice. J. Funct. Foods 2023, 107, 105666. [Google Scholar] [CrossRef]
- Li, L.; Wang, P.; Xu, X.; Zhou, G. Influence of various cooking methods on the concentrations of volatile N-nitrosamines and biogenic amines in dry-cured sausages. J. Food Sci. 2012, 77, C560–C565. [Google Scholar] [CrossRef]
- Drabik-Markiewicz, G.; Dejaegher, B.; De Mey, E.; Kowalska, T.; Paelinck, H.; Vander Heyden, Y. Influence of putrescine, cadaverine, spermidine or spermine on the formation of N-nitrosamine in heated cured pork meat. Food Chem. 2011, 126, 1539–1545. [Google Scholar] [CrossRef]
- Gushgari, A.J.; Halden, R.U. Critical review of major sources of human exposure to N-nitrosamines. Chemosphere 2018, 210, 1124–1136. [Google Scholar] [CrossRef] [PubMed]
- WHO. Iarc Monographs on the Identification of Carcinogenic Hazards to Humans; WHO: Geneva, Switzerland, 2023; pp. 1–33. [Google Scholar]
- Ahmad, W.; Mohammed, G.I.; Al-Eryani, D.A.; Saigl, Z.M.; Alyoubi, A.O.; Alwael, H.; Bashammakh, A.S.; O’Sullivan, C.K.; El-Shahawi, M.S. Biogenic Amines Formation Mechanism and Determination Strategies: Future Challenges and Limitations. Crit. Rev. Anal. Chem. 2020, 50, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Doeun, D.; Davaatseren, M.; Chung, M.S. Biogenic amines in foods. Food Sci. Biotechnol. 2017, 26, 1463–1474. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, W.; Łukasiewicz, M.; Puppel, K. Biogenic amines: Formation, action and toxicity—A review. J. Sci. Food Agric. 2021, 101, 2634–2640. [Google Scholar] [CrossRef]
- Jaguey-Hernández, Y.; Aguilar-Arteaga, K.; Ojeda-Ramirez, D.; Añorve-Morga, J.; González-Olivares, L.G.; Castañeda-Ovando, A. Biogenic amines levels in food processing: Efforts for their control in foodstuffs. Food Res. Int. 2021, 144, 110341. [Google Scholar] [CrossRef]
- Lee, Y.C.; Kung, H.F.; Huang, Y.L.; Wu, C.H.; Huang, Y.R.; Tsai, Y.H. Reduction of Biogenic Amines during Miso Fermentation by Lactobacillus plantarum as a Starter Culture. J. Food Prot. 2016, 79, 1556–1561. [Google Scholar] [CrossRef]
- Lee, Y.C.; Kung, H.F.; Huang, C.Y.; Huang, T.C.; Tsai, Y.H. Reduction of histamine and biogenic amines during salted fish fermentation by Bacillus polymyxa as a starter culture. J. Food Drug Anal. 2016, 24, 157–163. [Google Scholar] [CrossRef]
- Lin, X.; Tang, Y.; Hu, Y.; Lu, Y.; Sun, Q.; Lv, Y.; Zhang, Q.; Wu, C.; Zhu, M.; He, Q.; et al. Sodium Reduction in Traditional Fermented Foods: Challenges, Strategies, and Perspectives. J. Agric. Food Chem. 2021, 69, 8065–8080. [Google Scholar] [CrossRef]
- Bautista-Gallego, J.; Rantsiou, K.; Garrido-Fernández, A.; Cocolin, L.; Arroyo-López, F.N. Salt Reduction in Vegetable Fermentation: Reality or Desire? J. Food Sci. 2013, 78, R1095–R1100. [Google Scholar] [CrossRef] [PubMed]
- Laranjo, M.; Gomes, A.; Agulheiro-Santos, A.C.; Potes, M.E.; Cabrita, M.J.; Garcia, R.; Rocha, J.M.; Roseiro, L.C.; Fernandes, M.J.; Fraqueza, M.J.; et al. Impact of salt reduction on biogenic amines, fatty acids, microbiota, texture and sensory profile in traditional blood dry-cured sausages. Food Chem. 2017, 218, 129–136. [Google Scholar] [CrossRef]
- Dugat-Bony, E.; Sarthou, A.S.; Perello, M.C.; de Revel, G.; Bonnarme, P.; Helinck, S. The effect of reduced sodium chloride content on the microbiological and biochemical properties of a soft surface-ripened cheese. J. Dairy Sci. 2016, 99, 2502–2511. [Google Scholar] [CrossRef]
- Laranjo, M.; Gomes, A.; Agulheiro-Santos, A.C.; Potes, M.E.; Cabrita, M.J.; Garcia, R.; Rocha, J.M.; Roseiro, L.C.; Fernandes, M.J.; Fernandes, M.H.; et al. Characterisation of “Catalão” and “Salsichão” Portuguese traditional sausages with salt reduction. Meat Sci. 2016, 116, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zang, S.; Zhao, Z.; Li, X. Dynamic changes of bacterial communities and nitrite character during northeastern Chinese sauerkraut fermentation. Food Sci. Biotechnol. 2018, 27, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Zeng, X.; Kong, L.; Sun, X.; Shi, J.; Wu, Z.; Guo, Y.; Pan, D. Research Progress of Nitrite Metabolism in Fermented Meat Products. Foods 2023, 12, 1485. [Google Scholar]
- Li, F.; Zhuang, H.; Qiao, W.; Zhang, J.; Wang, Y. Effect of partial substitution of NaCl by KCl on physicochemical properties, biogenic amines and N-nitrosamines during ripening and storage of dry-cured bacon. J. Food Sci. Technol. 2016, 53, 3795–3805. [Google Scholar] [CrossRef]
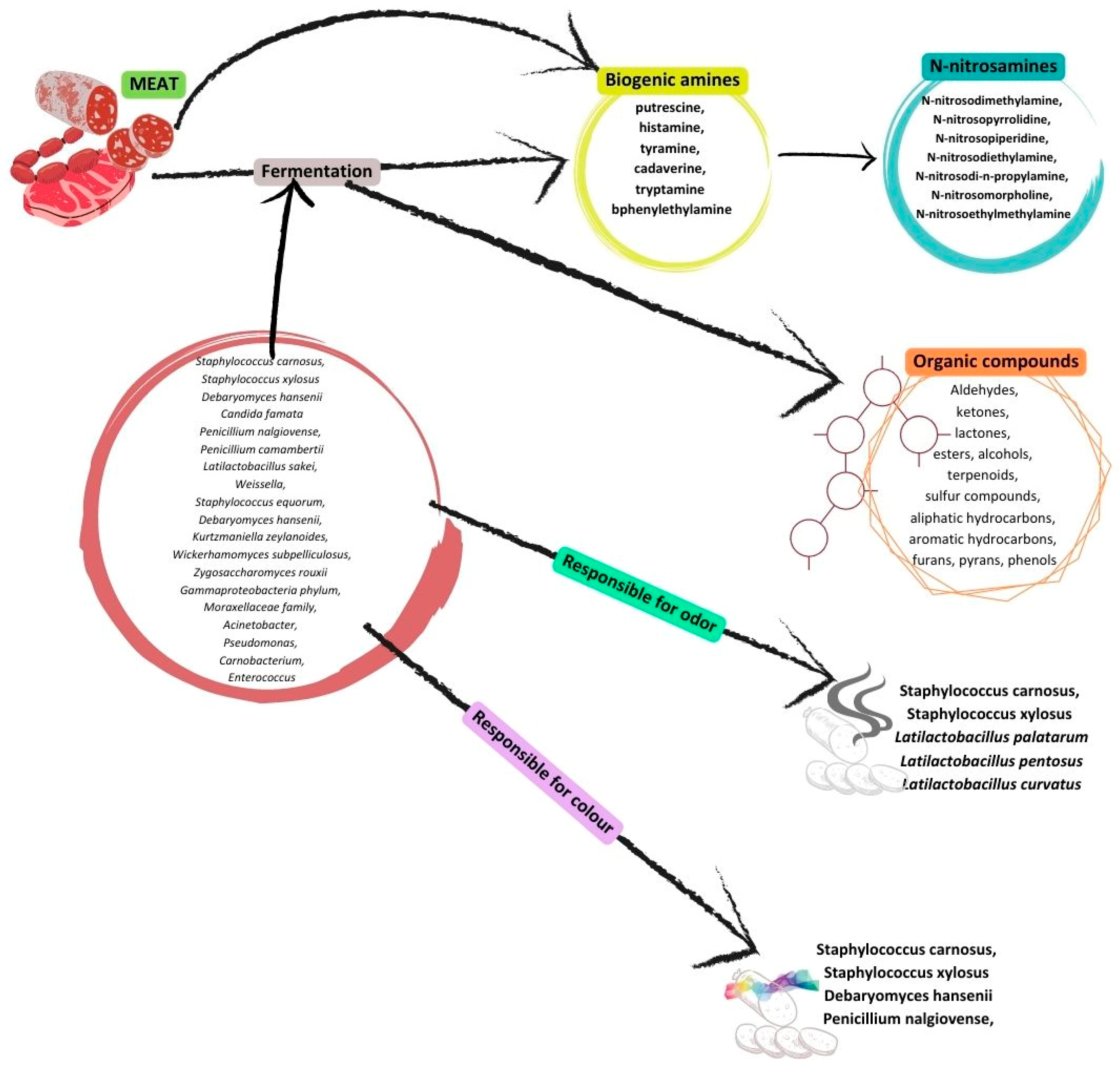
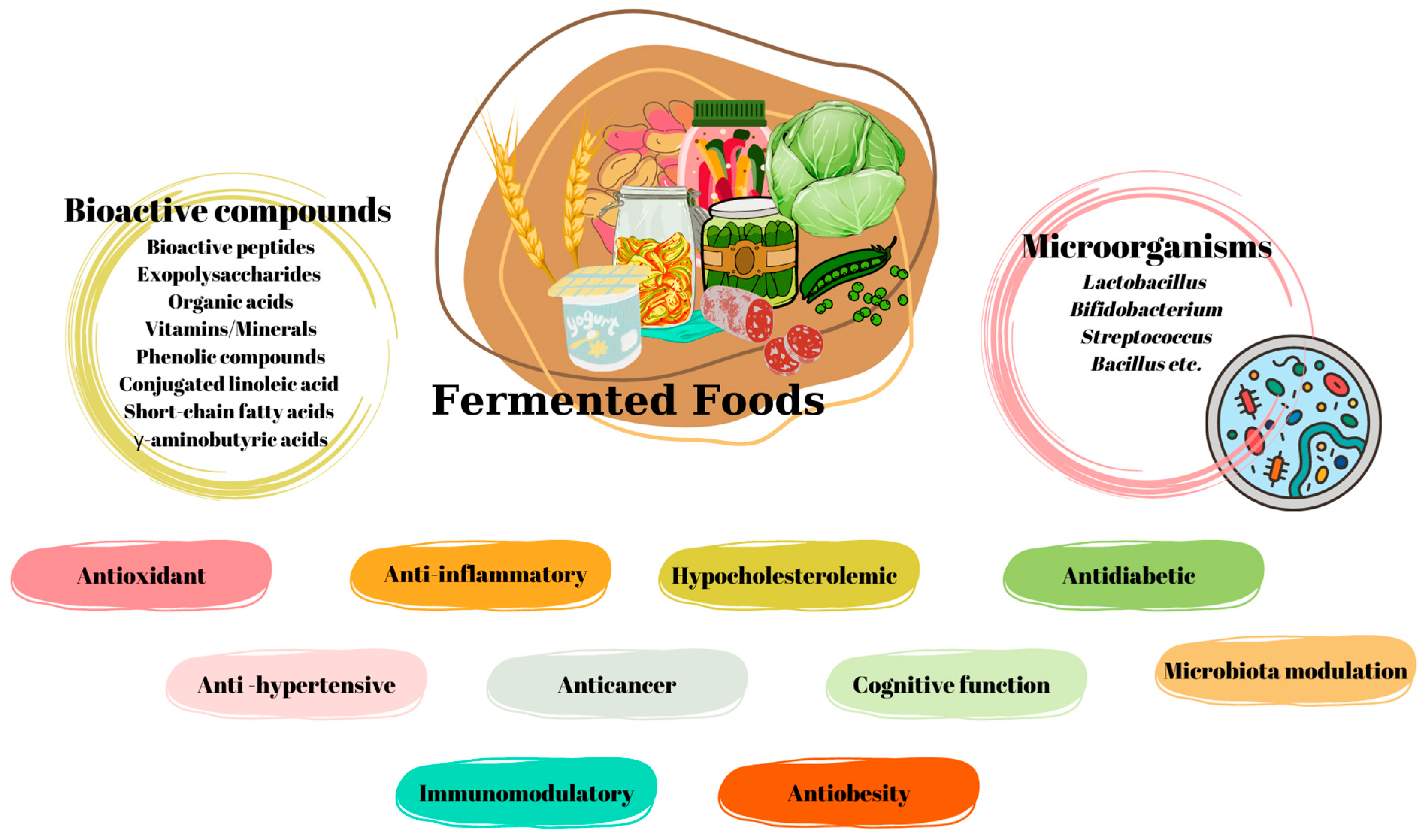
| Bioactive Components | Health Effects | Specific Effects | References |
|---|---|---|---|
| Kefir and kefir grains | Antihypertensive | ACE inhibitory activity Blood pressure ↓ | [34] |
| Mean arterial pressure ↓ Cardiac hypertrophy ↓ TNF-α/IL-10 ↓ ACE activity ↓ | [64] | ||
| Anticancer | TGF-α downregulation and TGF-β1 mRNA expression upregulation has antiproliferative effects Dose-dependent effects: Transcriptional levels of TGF-α ↓ Transcriptional levels of TGF-β1 ↑ Apoptotic cells ↑ | [35] | |
| Expression of TGF-α ↓ TGF-β1 ↓ p53-independent p21 expression ↑ Upregulation in Bax/Bcl-2 ratio Kefir may induce apoptosis and inhibit proliferation | [65] | ||
| Tumor growth 64.8% ↓ | [66] | ||
| The size and the amount of tumor ↓ In colon tissue: The mRNA expression levels of mRNA of TNF-α, IL-6, and IL-17a ↓ TNF-α, IL-6, and IL-17a ↓ Proliferating cell indicators (Ki67, NF-κB, β-Catenin) ↓ Claudin 1, ZO-1 mRNA, and protein levels ↑ Serum LPS ↓ In feces: Butyric acid, acetic acid, and propionic acid ↑ Ascomycota/Basidiomycota ratio and Firmicutes/Bacteroidetes ratio ↓ Lactobacillus and Bifidobacterium ↑ The relative abundance of probiotics ↑ The pathogenic bacteria (Aspergillus, Clostridium sensu stricto, and Talaromyces) ↓ | [67] | ||
| Antioxidant | TAS ↑ | [36] | |
| Serum levels of ·O2−, H2O2, and ONOO−/OH− ↓ NO levels ↑ Protein oxidation ↓ p53 expression↑ DNA fragmentation ↓ Apoptosis ↓ | [37] | ||
| MDA↓, CAT ↑, SOD ↑, GPx ↑ | [68] | ||
| DNA damage ↓ Antioxidant capacity of kefir according to milk ↑ | [69] | ||
| Anti-inflammatory | TNF-α, IL12p70, and IL-8 ↓ IL-8/IL-10 and IL-12/IL-10 ↓ | [37] | |
| TNF-α, IFN-γ ↓ | [38] | ||
| Microbiota modulation | Bifidobacterium bifidum PRL2010 ↑ | [48] | |
| Lactobacillus quantity of treatment group for Crohn’s disease ↑ Lactobacillus quantity of treatment group for ulcerative colitis ↑ | [70] | ||
| Relative abundance of Actinobacteria ↑ | [38] | ||
| Firmicutes/Bacteroidetes ratio, Ascomycota/Basidiomycota ratio ↓ Lactobacillus and Bifidobacterium ↑ Probiotics’ relative abundance ↑ The pathogenic bacterium (Clostridium sensu stricto, Aspergillus, and Talaromyces) ↓ Clostridium_sensu_stricto_1, Bacteroides, Lachnospiraceae_NK4A136_group, Oscillospiraceae, Desulfovibrio ↓ Muribaculaceae and Alloprevotella ↑ | [67] | ||
| Milk kefir had a free radical scavenging activity of 76.640.42% In the colon: SOD and CAT ↑ Brain butyrate and propionate ↑ Fecal butyrate ↑ Lachnospiraceae and Lachnoclostridium ↑ Relative abundance of Firmicutes ↑ Proteobacteria and Epsilonbacteraeota ↓ | [71] | ||
| Bone health | Prevented estrogen-deficiency-induced bone loss Bone volume/total volume ↑ Bone mineral density ↑ Trabecular thickness ↑ Trabecular number↑ Average cortical elastic moduli, hardness ↑ Trabecular separation ↓ Type I collagen levels ↓ | [40] | |
| Antidiabetic | Insulin ↓, HOMA-IR ↓ | [38] | |
| Serum glucose ↓ HbA1c ↓ | [72] | ||
| Cognitive function | Improvement in performance in the MMSE Improvement in the memory test | [37] | |
| Hypocholesterolemic | Serum LDL-C ↓ LDL-C/HDL-C ratio ↓ Serum HDL-C ↑ | [39] | |
| Lactic acid bacteria | Immunomodulatory | Mucins (MUC-1 and MUC-2) and IgA gene expression ↑ | [39] |
| Antioxidant | Lactiplantibacillus plantarum MA2 had antioxidant potential | [47] | |
| Organic acids | Antimicrobial | Milk fermentation with kefir grains antagonizes Bacillus cereus through the organic acids (lactic acid and acetic acid) produced during fermentation | [73] |
| Escherichia coli, Salmonella, and Bacillus Cereus pathogenic strains’ growths were inhibited This related to the concentration of lactic acid | [74] | ||
| Bioactive peptides | Antihypertensive | ACE activity inhibition | [75] |
| Peptides defined in kefir have previously shown an ACE inhibiting effect | [76] | ||
| ACE inhibitory activity | [77] | ||
| Antifibrosis | Kidney cells Relative expression of α-SMA) ↓ Relative expression of ET-1 ↓ Relative expression of MMCP-1) ↓ Kidney tissues Protein expression of ET-1 ↓ Protein expression of α-SMA ↓ | [78] | |
| Anti-inflammatory | Pro-inflammatory cytokines ↓ | [34] | |
| NF-kB protein expression ↓ TGF-β protein expression ↓ NLPR3 protein expression | [78] | ||
| Antioxidant | Total antioxidant capacity of the FRAP ↑ | [41] | |
| ABTS and DPPH radical scavenging activity | [79] | ||
| ROS production ↓ Lipid peroxidation ↓ | [34] | ||
| Renal effects: SOD activity ↑ ROS activity ↓ | [78] | ||
| Antimicrobial | Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, Klebsiella pneumoniae ATCC 29665, Bacillus subtilis ATCC 6633, Bacillus cereus ATCC 33019, and Staphylococcus aureus ATCC 6538 growths were inhibited | [79] | |
| Increasing the outer and inner membrane permeability of Escherichia coli, causing damage to the cell membrane, and promoting intracellular material leakage | [80] | ||
| Neuromodulation | Neurodegeneration index ↓ Acetylcholinesterase activity ↓ Lower amyloid content | [41] | |
| Bone health | Preventing menopausal osteoporosis Trabecular number ↑ Trabecular bone volume ↑ Trabecular thickness ↑ Average cortical elastic moduli, hardness ↑ Bone mineral density ↑ Trabecular separation ↓ | [81] | |
| Microbiota modulation | Restored the abundances of Alloprevotella, Parasutterella, Anaerostipes, Ruminococcus_1, Romboutsia, and Streptococcus genera | [81] | |
| Polysaccharide | |||
| Kefiran | Anticancer | MCF7 cancer cells ↓, PBMC ↑ | [49] |
| Anti-proliferative effect on HeLa and HepG2 Cell viability of HeLa and HepG2 ↓ | [50] | ||
| Anti-inflammatory and immunomodulatory roles | Proinflammatory cytokines (NF-kB, IL-1β, TNF-α) ↓ Overexpression of TLR4 ↓ | [51] | |
| BALB/c mice Small intestine: IgA, IL-10, IL-6, IL-12 ↑ Serum: IL-4, IL-6, IL-10 ↑ Intestinal fluid: IL-4, IL-12 ↑ Large intestine: IgA, IgG, IL-6, IL-10, IL-4, IFN, TNF ↑ | [52] | ||
| Inhibition percentage of nitric oxide radical production | [53] | ||
| Antioxidant | Scavenging of superoxide and hydroxyl radicals | [53] | |
| DPPH free radicals scavenging activity ↑ | [54] | ||
| Lipid peroxide of βVLDL ↓ | [55] | ||
| Microbiota modulation | Intestinal Bifidobacteria ↑ | [42] | |
| Bifidobacterium bifidum PRL2010 ↑ | [48] | ||
| Exopolysaccharide | Anticancer | Antitumor activity against colon cancer HT-29 cells Upregulate the expression of Cyto-c, BAD, BAX, caspase3, caspase8, and caspase9 Downregulate BCl-2 | [56] |
| Anti-inflammatory and immunomodulatory roles | Cell viability of the RAW264.7 cells ↑ NO concentration ↑ TNF-α, IL-1β concentration ↑ iNOS concentration ↑ Proliferation and phagocytosis are increased to combat infection and inflammation | [57] | |
| Dose-dependent effects: Cell viability of the RAW264.7 cells ↑ NO concentration ↑ TNF-α, IL-1β concentration ↑ Enhanced the proliferation, phagocytosis | [58] | ||
| Dose-dependent effects: NO concentration ↑ TNF-α, IL-6, IL-1β, IL-10 concentration ↑ Increasing the activity of acid phosphatase Enhancing macrophages’ phagocytosis Viability of macrophages | [59] | ||
| Antioxidant | GPx 21.55%, SOD 33.14%, CAT 61.09% Total antioxidant capacity 38.18% MDA ↓ | [61] | |
Certain scavenging activities:
| [60] | ||
| Microbiota modulation | The abundance of Flexispira ↓ The abundances of Blautia and Butyricicoccus ↑ Content of SCFA ↑ Content of NO ↓ | [61] | |
| Total SCFA ↑ Propionic acid and Butyric acid ↑ Proportion of the genera Victivallis, Acidaminococcus, and Comamonas ↑ Proportion of Enterobacteria ↓ | [62] | ||
| The abundance of the phyla Bacteroidetes, Verrucomicrobia, and Proteobacteria ↑ The abundance of the Firmicutes and Actinobacteria ↓ The enhanced abundance of Akkermansia spp. in feces | [63] | ||
| Anti-obesity | Lower intracellular lipid accumulation Epididymal adipose tissue weight 19% ↓ Body weight gain ↓ VLDL-C 36% ↓ | [63] | |
| Fermented Vegetables | Microorganism | Health Effects | Specific Effects | References |
|---|---|---|---|---|
| Kimchi | ||||
| Weissella cibaria JW15 | Anti-inflammatory | Proinflammatory cytokines (IL-1β, IL-6, TNF-α) ↓ Nitric oxide, prostaglandin E2, COX-2 ↓ IκB-α degradation and MAPKs, NF-κB activation ↓ | [239] | |
| Lactiplantibacillus plantarum LB5 (LPLB5) | Antioxidant Anti-inflammatory Antibacterial | Proinflammatory cytokines (IL-1β, IL-6, TNF-α) ↓ Anti-inflammatory cytokines (IL-4, IL-10, IFN-γ) ↑ Escherichia coli O157:H7 Pseudomonas aeruginosa, Listeria monocytogenes, and Staphylococcus aureus ↓ ABTS radical scavenging activity ↑ | [240] | |
| Lactiplantibacillus plantarum LRCC5314 | Anti-inflammatory Anti-stress | TNF-α, IL-1β, IFN-γ, NO ↓ Cortisol concentration ↓ Adipocytes: TG concentration ↓ Adipogenesis-related genes, adiponectin, FAS, PPAR/γ, and C/EBPα, TNF-α, IL-6↓ | [241] | |
| Lactiplantibacillus plantarum 200655 | Neuroprotective | BDNF expression and concentration ↑ BDNF and tyrosine hydroxylase mRNA expression ↑ Apoptosis-related Bax/Bcl-2 ratio ↓ Caspase-3 activity ↓ | [242] | |
| Lactobacillus sakei | Anti-obesity | Body fat mass ↓ Abdominal visceral fat↓ Waist circumference ↓ | [243] | |
| Levilactobacillus brevis KU15153 | Antioxidant Antimicrobial | Escherichia coli ATCC 25922, L. monocytogenes ATCC 15313, S. Typhimurium P99, and S. aureus KCCM 11335 ↓ DPPH radical scavenging activity ↑ | [244] | |
| Levilactobacillus brevis KU15147 | Antioxidant Immune enhancing | NO production, iNOS, TNF-α ↓ Radical scavenging activity of DPPH 38.56% Radical scavenging activity of ABTS 22.30% β-carotene bleaching inhibitory activity 23.82% | [245] | |
| Lactiplantibacillus plantarum LRCC5310 Lactiplantibacillus plantarum LRCC5314 | Antidiabetic Anti-inflammatory | Serum insulin ↑ Fasting blood glucose ↓ Upregulating expression of GLUT 4 and adiponectin TNF-α, IL-6 ↓ Downregulation of Ccl2 and leptin expression Serum corticosterone ↓ mRNA levels of stress-related genes (Npy, Y2r) ↓ | [246] | |
| Sauerkraut | ||||
| Exopolysaccharides from Lacticaseibacillus paracasei | Antioxidant | Total antioxidant capacity 76.34% Hydrogen peroxide scavenging activity 68.65% DPPH free radical scavenging activity 60.31% | [248] | |
| Exopolysaccharides from Lacticaseibacillus Casei | Antioxidant Immunomodulatory | Showed dose-dependent effects: Hydrogen peroxide scavenging activity, DPPH free radical scavenging activity, superoxide radicals scavenging activity In macrophages: TNF-α, ROS production ↑ NF-κB p65 expression ↑ Expression of the c-jun protein ↑ | [249] | |
| Lacticaseibacillus casei NA-2 | Antibacterial | Inhibit the growth of Bacillus cereus, Staphylococcus aureus, Escherichia coli O157:H7, and Salmonella typhimurium | [250] | |
| Lactiplantibacillus plantarum | Antimicrobial | Escherichia coli O157 and Shigella flexneri CMCC(B) ↓ | [251] |
| Fermented Foods | Specific Foods | Certain Bioactive Compounds | Effects of Health | References |
|---|---|---|---|---|
| Kidney beans | White and dark kidney beans | - | Cecal short-chain fatty acid levels (acetate, butyrate, and propionate), colon crypt height, and MUC1 and Relmβ mRNA expression ↑ Genes of TLR4, MUC1-3, Relmβ ↑ Expressions of IL-6, IFNγ, IL-1β, MCP-1, and TNFα ↓ Levels of serum for IL-17A, TNFα, IL-6, IL-1β, and IFNγ ↓ | [360] |
| Kidney bean fermented broth | - | With this diet, level of blood lipids (ALT, AST, TG) in hyperlipidemia ↓ With this diet, serum HDL in hyperlipidemia ↑ Firmicutes/Bacterioidetes ratio and pathogenic bacteria ↓ Beneficial bacteria ↑ | [361] | |
| Soybean | Fermented soybean dried extracts | Isoflavin β | 12-O-tetradecanoylphorbol-13-acetate (TPA-)-induced biochemical alterations in skin ↓ GSH depletion ↓ | [362] |
| Fermented soybean paste | Histamine Tyramine | Increased hepatic expression of IL-1β and PARP-1 ↓ Elevated blood plasma levels of MAO-A, AST/ALT, and CRP ↓ | [363] | |
| Fermented soybean products | - | IgE immunoreactivity ↓ | [364] | |
| Fermented mung bean Fermented soybean | - | Having cytotoxicity activities opposite to breast cancer MCF-7 cells by arresting the G0/G1 phase, followed by apoptosis Vaibility and the proliferation of splenocyte ↑ Levels of serum for IL-2 and IFN-γ ↑ | [365] | |
| Fermented soybean | Aqueous extract of Hawaijar | Glucose uptake, G6P production, and expressions of pPI3K, pAKT, pAMPK, and GLUT4 ↑ | [366] | |
| Fermented soybean | Isoflavone (genistein and daidzein) | Level of progesterone ↑ | [367] | |
| Alcohol-fermented soybean | - | p38, iNOS mRNA, JNK, and TNF-α in mouse peritoneal macrophages ↑ | [368] | |
| Soybean fermented by Lacticaseibacillus paracasei TK1501 | Lipoteichoic acid (LTA) Peptidoglycan (PGN) | Via lipoteichoic acid (LTA): Serum IL-4 and colonic TGF-β1 expression ↑, serum IL-1β and colonic IFN-γ expression ↓, intestinal inflammation ↓, mRNA levels of MUC2 ↑ Via peptidoglycan (PGN): Serum TNF-α and colonic IFN-γ ↓, colonic TGF-β1 expression ↑, mRNA levels of MUC2 ↑ | [369] | |
| Soybean fermented by Bacillus subtilis | Menaquinone-7, daidzin, genistein, glycitin, and nattokinase | AChE activity within hippocampus ↑ Protein carbonyl contents in hippocampus ↓ Activity of reduced glutathione, catalase, superoxide dismutase in hippocampus ↑ | [370] | |
| Black soybean fermented by Bacillus subtilis | - | Expression of aging biomarkers (hepatic p16INK4A and GLB1) ↓ Hepatic 8-hydoxy-2′-deoxyguanosine (8-oxodG) ↓ Hepatic levels of IL-6, MCP-1, and IL-10 levels in elder mice ↓ Beneficial microbiomes (Alistipes, Anaeroplasma, Coriobacteriaceae UCG002, and Parvibacter spp.) ↑ | [371] | |
| Black soybean and fermented black soybean broth | - | Antioxidative effect by inhibiting power and ferrous ion chelating ↑ Detroit 551 cell viability ↑ | [372] | |
| Fermented soy permeate | Isoflavones and α-galactooligosaccharides | Muscle glycogen content ↑ | [373] | |
| Chungkookjang | Genistin Daidzein | DNA fragmentation ↓ Viability of splenocytes and thymocytes ↑ Apoptosis of splenocytes and thymocytes ↓ | [374] | |
| Cheonggukjang | Intact isoflavones (genistein, daidzein, and glycitein) Equol 7-glucuronide Genistein, 3-hydroxygenistein, and 4′-sulfate | Intact isoflavones (genistein, daidzein, glycitein), 3-hydroxygenistein, genistein 4′-sulfate, and equol 7-glucuronide promote osteoblastogenesis via increased ALP activity, 3-hydroxygenistein inhibits osteoclast formation via decreased bone resorption activity | [375] | |
| Cheonggukjang | - | NF-kB and MAPK activation, IL-4 mRNA expression, IgE expression, and IL-31 mRNA expression in atopic dermatitis ↓ | [376] | |
| Doenjang Cheonggukjang | - | Activation of redox-sensitive NF-kB ↓ iNOs levels, COX-2 ↓ | [377] | |
| Doenjang Cheonggukjang | - | Th1-mediated immune responses ↑ Level of IFN-γ ↑ Level of IL-4 ↓ Resistance to Listeria monocytogenes infection ↑ | [378] | |
| Doenjang | - | Fecal lipopolysaccharide levels ↓ The amount of Ruminococcaceae, Bifidobacteria, Lachnospiraceae, and Firmicutes ↓ The amount of Odoribacter_f and Bacterioidetes ↑ β-glucuronidase and NF-kB activity ↓ TNF- α expression ↓ IL-10 expression ↑ Occludin ↑ | [379] | |
| Cheonggukjang (natto) | Nattokinase | Digestion of fibrin ↑ Digestion of plasmin substrate (H-D-Val-Leu-Lys-Pna (s-2251)) ↑ | [380] | |
| Natto | Natto extract (Heated-natto extract or Unheated-natto extract) | Heated-natto extract, degradation of Glycoprotein D of BHV-1 Degradation of SARS-CoV-2 receptor-binding domain Unheated-natto extract, inhibition of anti-BHV-1 activity by serine protease inhibitor | [381] | |
| Natto | Vitamin K Phytoestrogens | Vitamin K, bone health ↑ Phytoestrogens, menopausal disorder, osteoporosis, breast cancer risk ↓ | [382] | |
| Natto | Vitamin K2 | Maintaining bone stiffness | [383] | |
| Natto | - | In women aged under 60 years, dementia risk ↓ | [384] | |
| Miso | Lipopolysaccharide-neutralizing protein | PGD2 production via macrophage cells ↓ | [385] | |
| Fermented soybeans | C-miso (a) S10-miso (b) S9O1-miso (c) | Antioxidant effects: For unheated forms: a > b > c For heated forms: a > b > c Antimutagenicity effects: For unheated forms: a = b > c For heated forms: a > b > c | [386] | |
| Miso soup, fermented soybeans, houba-miso | Isoflavone | Hot flush severity↓ | [387] | |
| Miso soup, natto, and soybeans | - | Attenuated arterial stiffness via brachial–ankle pulse wave velocity ↓ | [388] | |
| Miso and natto | Isoflavones | Blood pressure ↓ | [389] | |
| Low-salt O-miso | - | Serum cholesterol ↓ Serum and liver TBARS value ↓ Serum GSH-Px and hepatic catalase ↑ | [390] | |
| Soybean koji | - | Increase in mRNA expression incident to lipogenic genes and weightiness of white adipose tissue ↓ Serum levels of triglyceride, low-density lipoprotein cholesterol, and total cholesterol ↓ Serum levels of high-density lipoprotein cholesterol ↑ Lipid accumulation in the white adipose tissue and liver ↓ | [391] | |
| Soy Meju | Tetragenococcus halophilus EFEL7002 | May adhere to Caco-2 cells Protective effect against H2O2-induced epithelial damage Antioxidant activity in human intestine Anti-inflammatory effects by inhibiting NO synthase within RAW 264.7 cells mRNA expressions of IL-6, IL-10, and IL-1β ↑ | [392] | |
| Thua-Nao | Daidzein Genistein | MCF-7 and HEK293 cancer cell growth ↓ Amount of viable HepG2 cells ↓ | [393] | |
| Fermented soy beverage | - | Levels of LDL cholesterol and total cholesterol ↓ | [394] | |
| Soy milk | Soy milk powder | Isoflavones 3-HAA | TG accumulation and total cholesterol within liver under oxidative stress ↓ | [395] |
| Fermented soy milk via Enterococcus faecalis VB43 | Reduction in conglycinin (7S) and glycinin (11S) | Enterococcus faecalis VB43-fermented soy milk may cause less severe allergy reactions in susceptible people | [396] | |
| Red bean | Tempeh (fermented red bean via Rhizopus and Lactobacillus) | Anthocyanin and GABA | ROS, pCREB, and iNOS expressions ↓ BDNF expression ↑ | [397] |
| Fermented Foods | Certain Bioactive Compounds | Effects of Health | References |
|---|---|---|---|
| Jalebi | Lapidilactobacillus bayanensis Bacillota Candida glabrata Lapidilactobacillus dextrinicus Pichia kudriavzevii Pediococcus stilesii Wickerhamomyces anomalus Gluconobacter japonicus | Probiotic functions | [414] |
| Ogi | Combination with tigernuts and sesame seeds | Antioxidant activity ↑ α-glucosidase enzyme inhibitory activity ↑ | [415] |
| Borde | Lactic acid bacteria strains (WS07, AM15, and AM20) Yeast strains (WS15, AA19, AM18, and AM23) | Cholesterol lowering ability ↑ | [416] |
| Kunu-zaki | Limosilactobacillus fermentum Leuconostoc citreum Weissella confusa | Anti-fungal activity | [417] |
| Kounou | Flavonoids Polyphenols | Antioxidant activity | [418] |
| Bozai (Boza) | Bacteriocin LF-BZ532 | Antimicrobial spectrum opposite to both Gram-positive and Gram-negative bacteria | [419] |
| Fermented cereal pastes | Lactobacillus | Serum and hepatic cholesterol levels ↓ Ratio of LDL-C to HDL-C ↓ Hepatic LDL receptor and CYP7A1 gene expressions ↑ Activity of superoxide dismutase ↑ Count of coliform and Clostridium perfringens in feces ↓ | [420] |
| Kunu-zaki Ogi | Lactiplantibacillus plantarum ULAG11 Lactiplantibacillus plantarum ULAG24 | Exclusion of Salmonella enterica LT2 via adherence of L. plantarum ULAG24 to HT29 cell line ↑ Stimulation of IFNγ and IL-10 via L. plantarum ULAG24 ↑ Expression of amylase via L. plantarum ULAG11 ↑ | [421] |
| Fermented quinoa and wheat | Bifidobacterium breve Bifidobacterium longum | ACE-inhibition activities ↑ Antioxidant activities ↑ Cytotoxicity activities against Caco-2 cell line ↑ | [422] |
| Fermented barley | Lactobacillus | Hepatic superoxide dismutase activity ↑ Improvement in intestinal microbiota dysbiosis ↑ Bacteroidetes ↑ Firmicutes/Bacteroidetes ratio ↓ | [423] |
| Fermented quinoa flour | Pleurotus ostreatus | ACE-I inhibitory ↑ | [424] |
| Togwa | Lactic acid | Campylobacter spp., Salmonella spp., ETEC and Shigella spp. ↓ | [425] |
| Fermented rye | - | Romboutsia↑ Bilophila↓ Fecal acetic acid ↑ | [426] |
| Fermented Tartary buckwheat | Monascus purpureus | Liver glycogen content ↑ SOD activity ↑ CAT activity ↑ | [427] |
| Fermented pearl millet flour | Aspergillus sojae | Antioxidant activity ↑ DNA damage protection activity ↑ | [428] |
| Fermented sorghum | Pediococcus acidilactici OHFR1 | Muribaculum, Parabacteroides, and Phocaeicola ↑ Oscillibcater, Acetatifactor, and Acetivibrio↓ | [429] |
| Bhaati Jaanr | - | Proliferation of colon adenocarcinoma cell lines (HT29 and SW480) ↓ Expression of IL-1β, COX-2, IL-6, and TNF-α ↓ | [13] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deveci, G.; Çelik, E.; Ağagündüz, D.; Bartkiene, E.; Rocha, J.M.F.; Özogul, F. Certain Fermented Foods and Their Possible Health Effects with a Focus on Bioactive Compounds and Microorganisms. Fermentation 2023, 9, 923. https://doi.org/10.3390/fermentation9110923
Deveci G, Çelik E, Ağagündüz D, Bartkiene E, Rocha JMF, Özogul F. Certain Fermented Foods and Their Possible Health Effects with a Focus on Bioactive Compounds and Microorganisms. Fermentation. 2023; 9(11):923. https://doi.org/10.3390/fermentation9110923
Chicago/Turabian StyleDeveci, Gülsüm, Elif Çelik, Duygu Ağagündüz, Elena Bartkiene, João Miguel F. Rocha, and Fatih Özogul. 2023. "Certain Fermented Foods and Their Possible Health Effects with a Focus on Bioactive Compounds and Microorganisms" Fermentation 9, no. 11: 923. https://doi.org/10.3390/fermentation9110923
APA StyleDeveci, G., Çelik, E., Ağagündüz, D., Bartkiene, E., Rocha, J. M. F., & Özogul, F. (2023). Certain Fermented Foods and Their Possible Health Effects with a Focus on Bioactive Compounds and Microorganisms. Fermentation, 9(11), 923. https://doi.org/10.3390/fermentation9110923











