Investigating Yeast–Lactobacilli Interactions through Co-Culture Growth and Metabolite Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of Yeasts and Bacteria on Differential Media
2.2. Genomic DNA Extraction and Quantification
2.3. Library Preparation and Sequencing
2.4. Sequencing Data Processing
2.5. Taxonomic Identification of Isolated Strains
2.6. Physiological Characterization
- 37 °C in yeast extract + peptone + dextrose 2%;
- 30 °C pH 2,5 in yeast extract + peptone + dextrose 2%;
- 30 °C pH 4,5 in yeast extract + peptone + dextrose 2%;
- 30 °C in yeast extract + peptone + dextrose 2%;
- 30 °C in yeast extract + peptone + lactic acid 1%;
- 30 °C in yeast extract + peptone + sucrose 2%;
- 30 °C in yeast extract + peptone + lactose 2%.
- 37 °C in LB;
- 37 °C in MRS + L-cysteine 0.05%;
- 37 °C in LB + dextrose 2%;
- 37 °C in MRS + L-cysteine 0.05% + dextrose 2%;
- 37 °C in LB + lactic acid 1%;
- 37 °C in MRS + L-cysteine 0.05% + lactic acid 1%;
- 37 °C in LB + sucrose 2%;
- 37 °C in MRS + L-cysteine 0.05% + sucrose 2%;
- 37 °C in LB + lactose 2%;
- 37 °C in MRS + L-cysteine 0.05% + lactose 2%;
- 30 °C in LB;
- 30 °C in MRS + L-cysteine 0.05%.
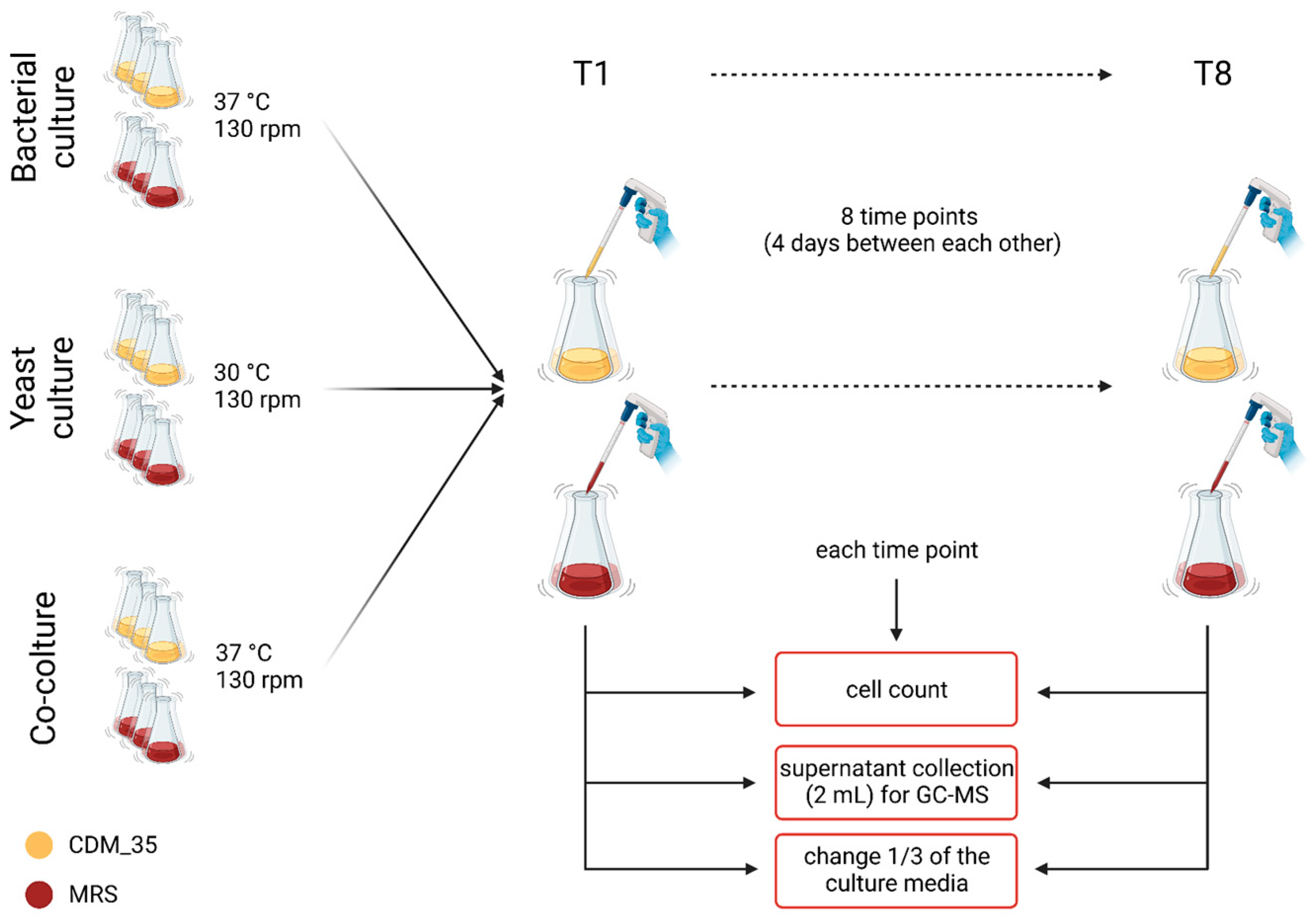
2.7. Cell Cultures
2.7.1. Co-Culture Setup
2.7.2. Cell Counts
2.7.3. Lab Media Growth of the Kefir Community
2.8. Chemical Analysis
2.8.1. Chemicals
2.8.2. Fatty Acids Quantification
2.9. Culture Data Analysis
3. Results
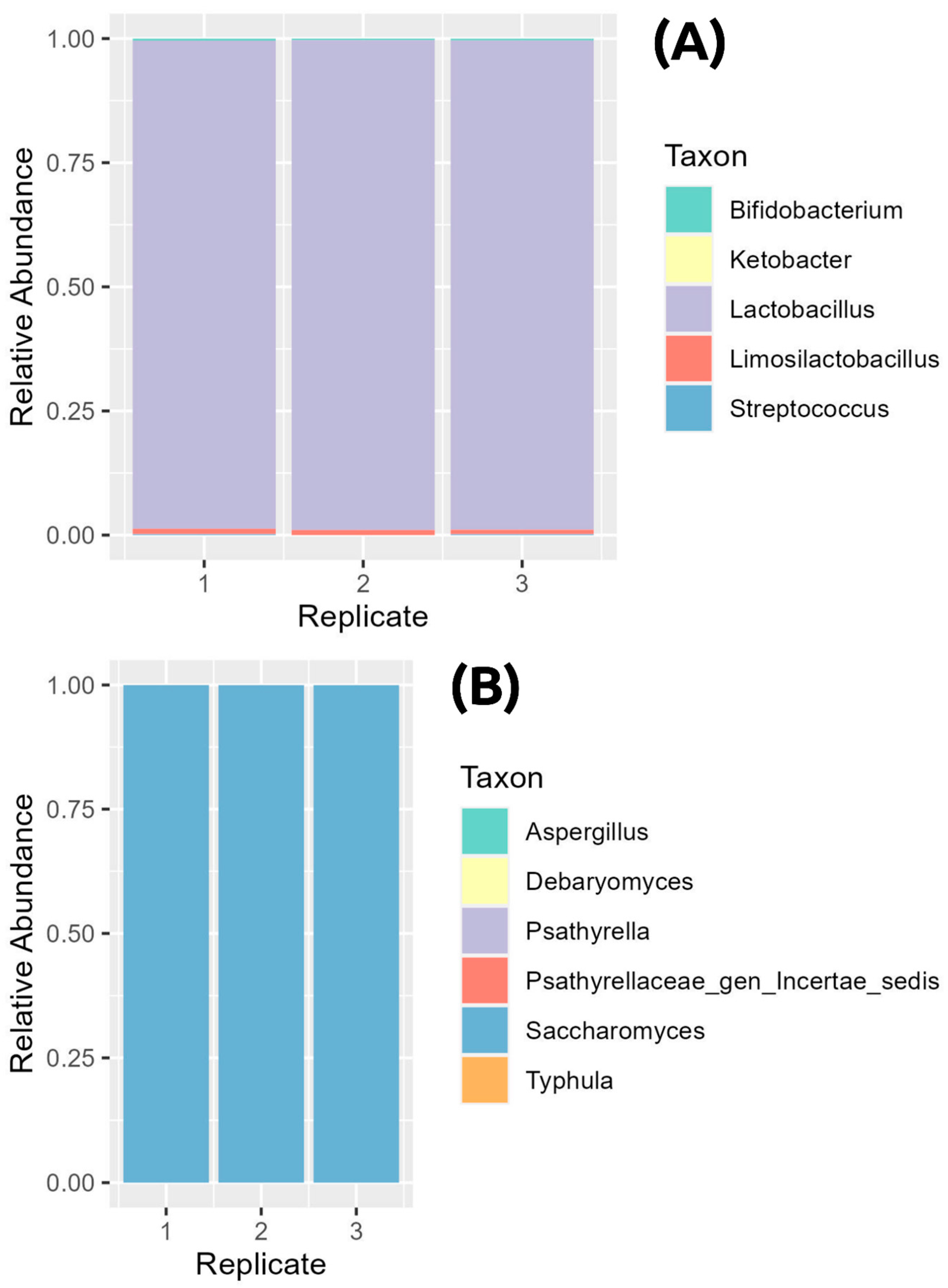
3.1. Metabarcoding of Kefir Samples
3.2. Strain Isolation and Sequencing
3.3. Physiological Characterization
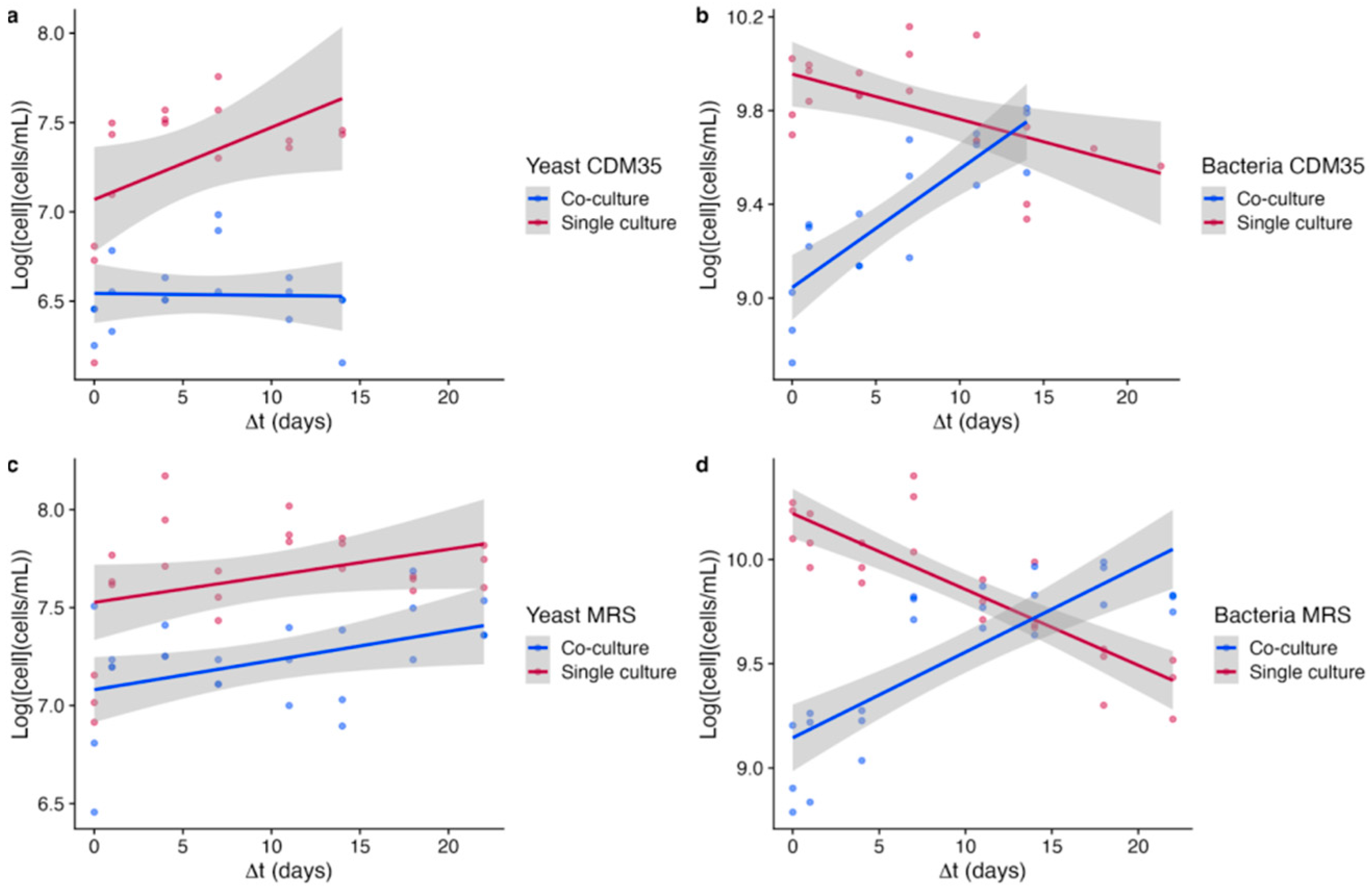
3.4. Dynamics of Cell Growths
3.5. GC-MS Analysis
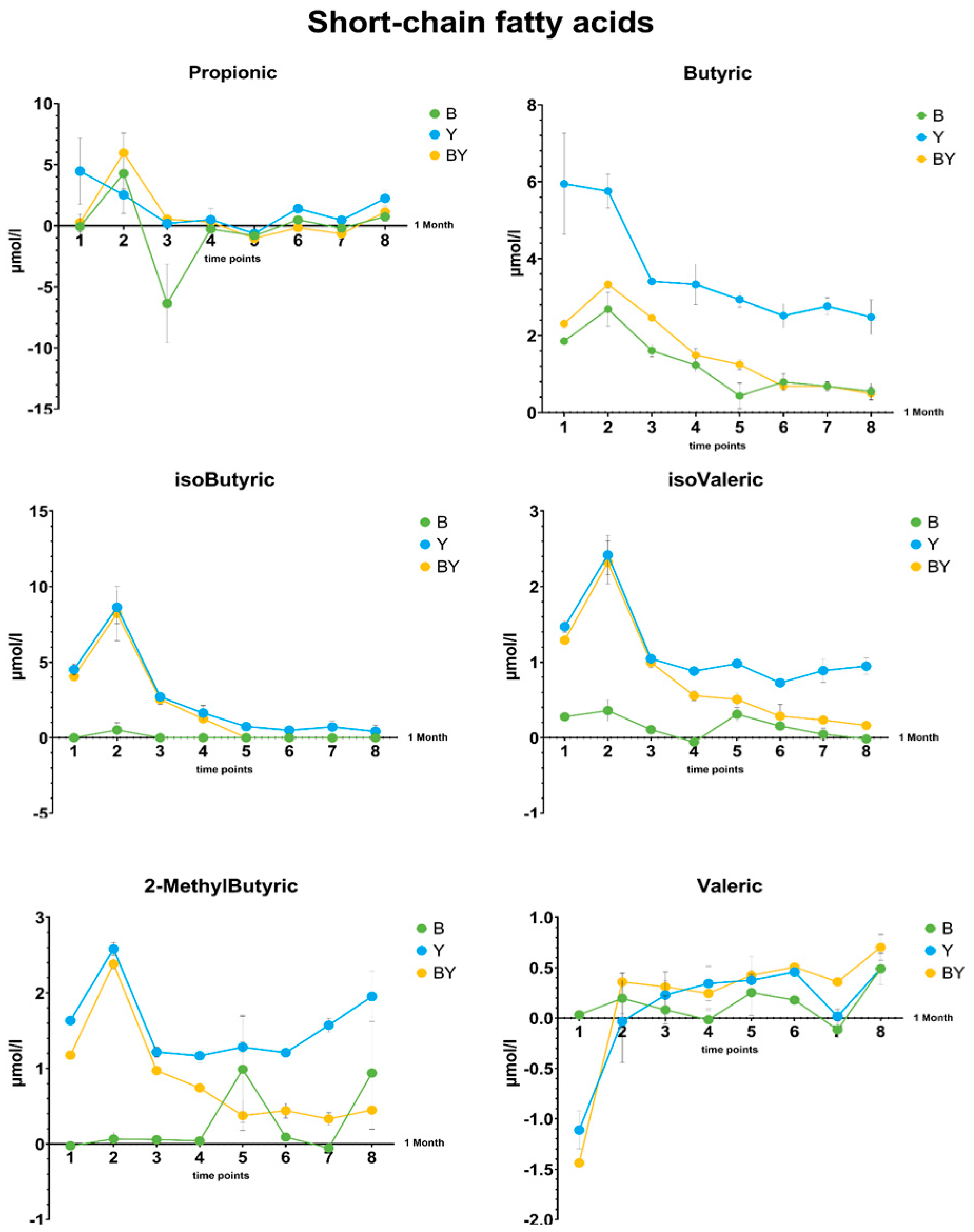
3.5.1. Fatty Acids Production in Rich Media by the Kefir Community
3.5.2. Differences in Short-Chain Fatty Acid Production between Single and Co-Cultures in Selective Media
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kruger, S.; Ilmer, M.; Kobold, S.; Cadilha, B.L.; Endres, S.; Ormanns, S.; Schuebbe, G.; Renz, B.W.; D’Haese, J.G.; Schloesser, H.; et al. Advances in Cancer Immunotherapy 2019—Latest Trends. J. Exp. Clin. Cancer Res. 2019, 38, 268. [Google Scholar] [CrossRef]
- Ponomarova, O.; Gabrielli, N.; Sévin, D.C.; Mülleder, M.; Zirngibl, K.; Bulyha, K.; Andrejev, S.; Kafkia, E.; Typas, A.; Sauer, U.; et al. Yeast Creates a Niche for Symbiotic Lactic Acid Bacteria through Nitrogen Overflow. Cell Syst. 2017, 5, 345–357.e6. [Google Scholar] [CrossRef] [PubMed]
- Seth, E.C.; Taga, M.E. Nutrient Cross-Feeding in the Microbial World. Front. Microbiol. 2014, 5, 350. [Google Scholar] [CrossRef] [PubMed]
- Gobbetti, M.; Corsetti, A.; Rossi, J. The Sourdough Microflora. Interactions between Lactic Acid Bacteria and Yeasts: Metabolism of Amino Acids. World J. Microbiol. Biotechnol. 1994, 10, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Qvirist, L.A.; De Filippo, C.; Strati, F.; Stefanini, I.; Sordo, M.; Andlid, T.; Felis, G.E.; Mattarelli, P.; Cavalieri, D. Isolation, Identification and Characterization of Yeasts from Fermented Goat Milk of the Yaghnob Valley in Tajikistan. Front. Microbiol. 2016, 7, 1690. [Google Scholar] [CrossRef]
- Kazou, M.; Grafakou, A.; Tsakalidou, E.; Georgalaki, M. Zooming into the Microbiota of Home-Made and Industrial Kefir Produced in Greece Using Classical Microbiological and Amplicon-Based Metagenomics Analyses. Front. Microbiol. 2021, 12, 621069. [Google Scholar] [CrossRef]
- Tenorio-Salgado, S.; Castelán-Sánchez, H.G.; Dávila-Ramos, S.; Huerta-Saquero, A.; Rodríguez-Morales, S.; Merino-Pérez, E.; Roa de la Fuente, L.F.; Solis-Pereira, S.E.; Pérez-Rueda, E.; Lizama-Uc, G. Metagenomic Analysis and Antimicrobial Activity of Two Fermented Milk Kefir Samples. MicrobiologyOpen 2021, 10, e1183. [Google Scholar] [CrossRef]
- Liu, S.; Lu, S.-Y.; Qureshi, N.; Enshasy, H.A.E.; Skory, C.D. Antibacterial Property and Metagenomic Analysis of Milk Kefir. Probiotics Antimicrob. Proteins 2022, 14, 1170–1183. [Google Scholar] [CrossRef]
- Kalamaki, M.S.; Angelidis, A.S. High-Throughput, Sequence-Based Analysis of the Microbiota of Greek Kefir Grains from Two Geographic Regions. Food Technol. Biotechnol. 2020, 58, 138–146. [Google Scholar] [CrossRef]
- Serafini, F.; Turroni, F.; Ruas-Madiedo, P.; Lugli, G.A.; Milani, C.; Duranti, S.; Zamboni, N.; Bottacini, F.; van Sinderen, D.; Margolles, A.; et al. Kefir Fermented Milk and Kefiran Promote Growth of Bifidobacterium Bifidum PRL2010 and Modulate Its Gene Expression. Int. J. Food Microbiol. 2014, 178, 50–59. [Google Scholar] [CrossRef]
- Plessas, S.; Nouska, C.; Mantzourani, I.; Kourkoutas, Y.; Alexopoulos, A.; Bezirtzoglou, E. Microbiological Exploration of Different Types of Kefir Grains. Fermentation 2017, 3, 1. [Google Scholar] [CrossRef]
- Bourrie, B.C.T.; Willing, B.P.; Cotter, P.D. The Microbiota and Health Promoting Characteristics of the Fermented Beverage Kefir. Front. Microbiol. 2016, 7, 647. [Google Scholar] [CrossRef]
- Cheirsilp, B.; Shoji, H.; Shimizu, H.; Shioya, S. Interactions between Lactobacillus Kefiranofaciens and Saccharomyces Cerevisiae in Mixed Culture for Kefiran Production. J. Biosci. Bioeng. 2003, 96, 279–284. [Google Scholar] [CrossRef]
- Farnworth, E.R.; Mainville, I. Kefir—A Fermented Milk Product; Routledge Handbooks Online: London, UK, 2008; ISBN 978-1-4200-5326-5. [Google Scholar]
- McSweeney, P.L.H.; McNamara, J.P. Encyclopedia of Dairy Sciences, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2022; ISBN 978-0-12-818767-8. [Google Scholar]
- Rea, M.C.; Lennartsson, T.; Dillon, P.; Drinan, F.D.; Reville, W.J.; Heapes, M.; Cogan, T.M. Irish Kefir-like Grains: Their Structure, Microbial Composition and Fermentation Kinetics. J. Appl. Bacteriol. 1996, 81, 83–94. [Google Scholar] [CrossRef]
- Tamime, A. Fermented Milks: A Historical Food with Modern Applications–a Review. Eur. J. Clin. Nutr. 2002, 56, S2–S15. [Google Scholar] [CrossRef] [PubMed]
- Lopitz-Otsoa, F.; Rementeria, A.; Elguezabal, N.; Garaizar, J. Kefir: A Symbiotic Yeasts-Bacteria Community with Alleged Healthy Capabilities. Rev. Iberoam. Micol. 2006, 23, 67–74. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Encyclopedia of Dairy Sciences—2nd Edition. Available online: https://shop.elsevier.com/books/encyclopedia-of-dairy-sciences/fuquay/978-0-12-374402-9 (accessed on 1 September 2023).
- Pessione, E. Lactic Acid Bacteria Contribution to Gut Microbiota Complexity: Lights and Shadows. Front. Cell. Infect. Microbiol. 2012, 2, 86. [Google Scholar] [CrossRef]
- Samuel, B.S.; Shaito, A.; Motoike, T.; Rey, F.E.; Backhed, F.; Manchester, J.K.; Hammer, R.E.; Williams, S.C.; Crowley, J.; Yanagisawa, M.; et al. Effects of the Gut Microbiota on Host Adiposity Are Modulated by the Short-Chain Fatty-Acid Binding G Protein-Coupled Receptor, Gpr41. Proc. Natl. Acad. Sci. USA 2008, 105, 16767–16772. [Google Scholar] [CrossRef]
- Matsuki, T.; Pédron, T.; Regnault, B.; Mulet, C.; Hara, T.; Sansonetti, P.J. Epithelial Cell Proliferation Arrest Induced by Lactate and Acetate from Lactobacillus Casei and Bifidobacterium Breve. PLoS ONE 2013, 8, e63053. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and Functional Importance in the Gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Kaisar, M.M.M.; Pelgrom, L.R.; van der Ham, A.J.; Yazdanbakhsh, M.; Everts, B. Butyrate Conditions Human Dendritic Cells to Prime Type 1 Regulatory T Cells via Both Histone Deacetylase Inhibition and G Protein-Coupled Receptor 109A Signaling. Front. Immunol. 2017, 8, 1429. [Google Scholar] [CrossRef] [PubMed]
- Schulthess, J.; Pandey, S.; Capitani, M.; Rue-Albrecht, K.C.; Arnold, I.; Franchini, F.; Chomka, A.; Ilott, N.E.; Johnston, D.G.W.; Pires, E.; et al. The Short Chain Fatty Acid Butyrate Imprints an Antimicrobial Program in Macrophages. Immunity 2019, 50, 432–445.e7. [Google Scholar] [CrossRef] [PubMed]
- Alimolaei, M.; Golchin, M. An Efficient DNA Extraction Method for Lactobacillus Casei, a Difficult-to-Lyse Bacterium. Int. J. Enteric Pathog. 2016, 4, e32472. [Google Scholar] [CrossRef]
- Shaffer, J.P.; Carpenter, C.S.; Martino, C.; Salido, R.A.; Minich, J.J.; Bryant, M.; Sanders, K.; Schwartz, T.; Humphrey, G.; Swafford, A.D.; et al. A Comparison of Six DNA Extraction Protocols for 16S, ITS and Shotgun Metagenomic Sequencing of Microbial Communities. BioTechniques 2022, 73, 34–46. [Google Scholar] [CrossRef]
- Takahashi, S.; Tomita, J.; Nishioka, K.; Hisada, T.; Nishijima, M. Development of a Prokaryotic Universal Primer for Simultaneous Analysis of Bacteria and Archaea Using Next-Generation Sequencing. PLoS ONE 2014, 9, e105592. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols; Elsevier: Amsterdam, The Netherlands, 1990; pp. 315–322. ISBN 978-0-12-372180-8. [Google Scholar]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet. J. 2011, 17, 10. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Robeson, M.S.; O’Rourke, D.R.; Kaehler, B.D.; Ziemski, M.; Dillon, M.R.; Foster, J.T.; Bokulich, N.A. RESCRIPt: Reproducible Sequence Taxonomy Reference Database Management. PLOS Comput. Biol. 2021, 17, e1009581. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Kõljalg, U.; Larsson, K.-H.; Abarenkov, K.; Nilsson, R.H.; Alexander, I.J.; Eberhardt, U.; Erland, S.; Høiland, K.; Kjøller, R.; Larsson, E.; et al. UNITE: A Database Providing Web-Based Methods for the Molecular Identification of Ectomycorrhizal Fungi. New Phytol. 2005, 166, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Stevens, M.H.H.; Oksanen, M.J.; Suggests, M.A.S.S. The vegan package. Community Ecol. Package 2007, 10, 719. [Google Scholar]
- Rainey, F.A.; Ward-Rainey, N.; Kroppenstedt, R.M.; Stackebrandt, E. The Genus Nocardiopsis Represents a Phylogenetically Coherent Taxon and a Distinct Actinomycete Lineage: Proposal of Nocardiopsaceae Fam. Nov. Int. J. Syst. Bacteriol. 1996, 46, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Moens, F.; Duysburgh, C.; van den Abbeele, P.; Morera, M.; Marzorati, M. Lactobacillus Rhamnosus GG and Saccharomyces Cerevisiae Boulardii Exert Synergistic Antipathogenic Activity in Vitro against Enterotoxigenic Escherichia coli. Benef. Microbes 2019, 10, 923–935. [Google Scholar] [CrossRef]
- Niccolai, E.; Baldi, S.; Ricci, F.; Russo, E.; Nannini, G.; Menicatti, M.; Poli, G.; Taddei, A.; Bartolucci, G.; Calabrò, A.S.; et al. Evaluation and Comparison of Short Chain Fatty Acids Composition in Gut Diseases. World J. Gastroenterol. 2019, 25, 5543–5558. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Use R! Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Browne, H.P.; Neville, B.A.; Forster, S.C.; Lawley, T.D. Transmission of the Gut Microbiota: Spreading of Health. Nat. Rev. Microbiol. 2017, 15, 531–543. [Google Scholar] [CrossRef]
- Lebeer, S.; Vanderleyden, J.; De Keersmaecker, S.C.J. Genes and Molecules of Lactobacilli Supporting Probiotic Action. Microbiol. Mol. Biol. Rev. 2008, 72, 728–764. [Google Scholar] [CrossRef]
- Rezac, S.; Kok, C.R.; Heermann, M.; Hutkins, R. Fermented Foods as a Dietary Source of Live Organisms. Front. Microbiol. 2018, 9, 1785. [Google Scholar] [CrossRef]
- Raimondi, S.; Amaretti, A.; Gozzoli, C.; Simone, M.; Righini, L.; Candeliere, F.; Brun, P.; Ardizzoni, A.; Colombari, B.; Paulone, S.; et al. Longitudinal Survey of Fungi in the Human Gut: ITS Profiling, Phenotyping, and Colonization. Front. Microbiol. 2019, 10, 1575. [Google Scholar] [CrossRef]
- Di Paola, M.; Rizzetto, L.; Stefanini, I.; Vitali, F.; Massi-Benedetti, C.; Tocci, N.; Romani, L.; Ramazzotti, M.; Lionetti, P.; De Filippo, C.; et al. Comparative Immunophenotyping of Saccharomyces Cerevisiae and Candida Spp. Strains from Crohn’s Disease Patients and Their Interactions with the Gut Microbiome. J. Transl. Autoimmun. 2020, 3, 100036. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.; Gasbarrini, A.; Mele, M. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Lian, W.-S.; Wang, F.-S.; Chen, Y.-S.; Tsai, M.-H.; Chao, H.-R.; Jahr, H.; Wu, R.-W.; Ko, J.-Y. Gut Microbiota Ecosystem Governance of Host Inflammation, Mitochondrial Respiration and Skeletal Homeostasis. Biomedicines 2022, 10, 860. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.V.D.; Camargo, M.R.d.; Russo, E.; Amedei, A. Role of Diet and Gut Microbiota on Colorectal Cancer Immunomodulation. World J. Gastroenterol. 2018, 25, 151–162. [Google Scholar] [CrossRef]
| Sample | Species | Seq. ID Gene Bank | Query Cover | Percentage Identity | Query Length |
|---|---|---|---|---|---|
| KYP1 | Pichia kudriavzevii | MT539198.1 | 100% | 100% | 430 |
| KYP3 | Saccharomyces cerevisiae | CP006391.1 | 100% | 99.25% | 804 |
| KLB1 | Pichia kudriavzevii | MT539198.1 | 99% | 99.76% | 424 |
| KLB3L | Saccharomyces cerevisiae | CP006391.1 | 99% | 99.52% | 823 |
| KRSAN1 | Saccharomyces cerevisiae | CP006391.1 | 100% | 99.49% | 780 |
| KEA1 | Pichia kudriavzevii | MT539198.1 | 100% | 99.30% | 426 |
| KEA2 | Saccharomyces cerevisiae | CP006391.1 | 99% | 99.51% | 823 |
| KMRA1 | Pichia kudriavzevii | MT539198.1 | 99% | 99.76% | 424 |
| KMRA2L | Saccharomyces cerevisiae | CP006391.1 | 96% | 98.50% | 828 |
| Sample | Species | Seq. ID Gene Bank | Query Cover | Percentage Identity | Query Length |
|---|---|---|---|---|---|
| KLB3B | Lacticaseibacillus rhamnosus | CP053619.1 | 100% | 99.93% | 1494 |
| KMRA2B | Limosilactobacillus fermentum | CP047584.1 | 98% | 99.48% | 1494 |
| KRSAN2 | Lactobacillus helveticus | CP031018.1 | 99% | 99.47% | 1518 |
| KRSAN3 | Limosilactobacillus fermentum | CP047584.1 | 99% | 99.60% | 1522 |
| KRSAN4 | Lacticaseibacillus rhamnosus | CP053619.1 | 100% | 99.67% | 1508 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nenciarini, S.; Reis-Costa, A.; Pallecchi, M.; Renzi, S.; D’Alessandro, A.; Gori, A.; Cerasuolo, B.; Meriggi, N.; Bartolucci, G.L.; Cavalieri, D. Investigating Yeast–Lactobacilli Interactions through Co-Culture Growth and Metabolite Analysis. Fermentation 2023, 9, 933. https://doi.org/10.3390/fermentation9110933
Nenciarini S, Reis-Costa A, Pallecchi M, Renzi S, D’Alessandro A, Gori A, Cerasuolo B, Meriggi N, Bartolucci GL, Cavalieri D. Investigating Yeast–Lactobacilli Interactions through Co-Culture Growth and Metabolite Analysis. Fermentation. 2023; 9(11):933. https://doi.org/10.3390/fermentation9110933
Chicago/Turabian StyleNenciarini, Stefano, Ana Reis-Costa, Marco Pallecchi, Sonia Renzi, Aldo D’Alessandro, Agnese Gori, Benedetta Cerasuolo, Niccolò Meriggi, Gian Luca Bartolucci, and Duccio Cavalieri. 2023. "Investigating Yeast–Lactobacilli Interactions through Co-Culture Growth and Metabolite Analysis" Fermentation 9, no. 11: 933. https://doi.org/10.3390/fermentation9110933
APA StyleNenciarini, S., Reis-Costa, A., Pallecchi, M., Renzi, S., D’Alessandro, A., Gori, A., Cerasuolo, B., Meriggi, N., Bartolucci, G. L., & Cavalieri, D. (2023). Investigating Yeast–Lactobacilli Interactions through Co-Culture Growth and Metabolite Analysis. Fermentation, 9(11), 933. https://doi.org/10.3390/fermentation9110933






