Effect of Blue LED Light on Bioemulsifier Production in Bioreactor by Aureobasidium pullulans LB83 in Solid State Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism and Inoculum Preparation
2.2. Alkaline Pretreatment of Sugarcane Bagasse
2.3. Evaluation of Different LED Light Wavelengths on SSF Bioemulsifier Production in Erlenmeyer Flasks
2.4. Solid-State Fermentation in Erlenmeyer Flasks under Blue LED Light
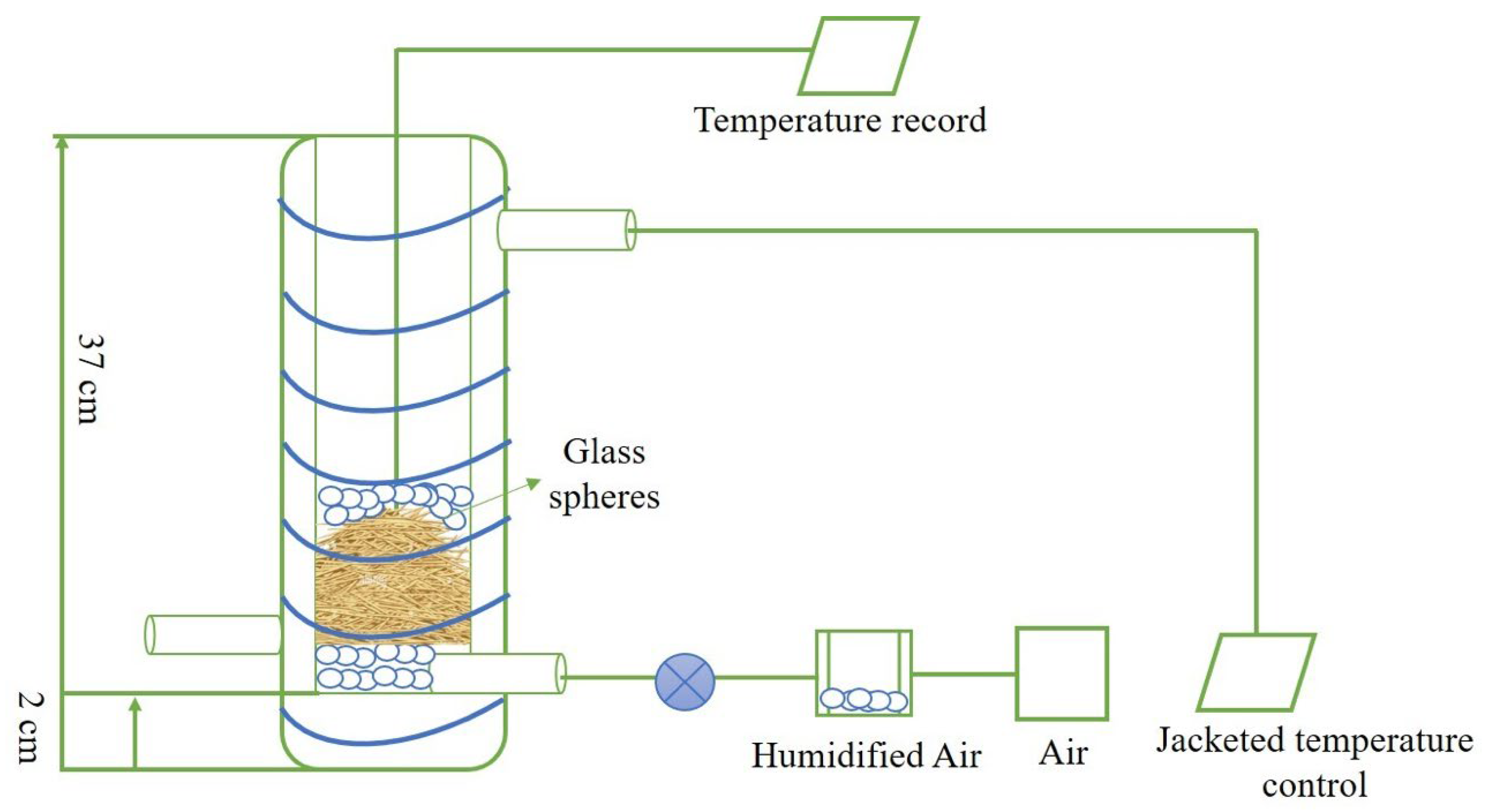
2.5. Evaluation of Light Influence on Solid-State Fermentation in Packed Bed Column
2.6. Extraction and Recovery of Biosurfactant
2.7. Emulsification Index
2.8. Enzymatic Activities
2.8.1. Analysis of Endoglucanase Activity
2.8.2. Analysis of Exoglucanase Activity
2.9. Scanning Electron Micrograph
2.10. Statistical Analysis
3. Results
3.1. Screening of LED Lights for Bioemulsifier Production by A. pullulans LB83 in Erlenmeyer Flasks
3.2. Time Course of BE Production by A. pullulans on PSB under the Influence of Blue LED Light in Erlenmeyer Flasks
3.3. Determination of the Enzymatic Activity from SSF under the Influence of Blue LED Light in Erlenmeyer Flasks
3.4. Bioemulsifier Production by A. pullulans on PSB under the Light Influence in Packed Bed Bioreactor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uzoigwe, C.; Burgess, J.G.; Ennis, C.J.; Rahman, P.K.S.M. Bioemulsifiers are not biosurfactants and require different screening approaches. Front. Microbiol. 2015, 6, 245. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.N.S.; Rodríguez, D.M.; Campos-Takaki, G.M.; da Silva Andrade, R.F. Biosurfactant and bioemulsifier as promising molecules produced by Mucor hiemalis isolated from Caatinga soil. Electron. J. Biotechnol. 2020, 47, 51–58. [Google Scholar] [CrossRef]
- Jiao, H.; Song, X.; Lai, C.; Fang, H.; Song, Y.; Zhu, J. Progress in preparation of cellulase from lignocellulose using fungi. Biotechnol. Bioprocess Eng. 2021, 26, 871–886. [Google Scholar] [CrossRef]
- Satpute, S.K.; Banat, I.M.; Dhakephalkar, P.K.; Banpurkar, A.G.; Chopade, B.A. Biosurfactants, bioemulsifiers and exopolysaccharides from marine microorganisms. Biotechnol. Adv. 2010, 28, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Peele, K.A.; Kodali, V.P. Emulsifying activity of a biosurfactant produced by a marine bacterium. 3 Biotech 2016, 6, 3247. [Google Scholar] [CrossRef]
- Das, S. Structural and mechanical characterization of biofilm-associated bacterial polymer in the emulsification of petroleum hydrocarbon. 3 Biotech 2021, 11, 239. [Google Scholar] [CrossRef]
- Thraeib, J.Z.; Altemimi, A.B.; Al-Manhel, A.J.A.; Abedelmaksoud, T.G.; El-Maksoud, A.A.A.; Madankar, C.S.; Cacciola, F. Production and Characterization of a Bioemulsifier Derived from Microorganisms with Potential Application in the Food Industry. Life 2022, 12, 924. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Chen, L.Y.; Tian, Z.J.; Sun, Y.; JLiu, J.B.; Huang, L. Characterization and application of a novel bioemulsifier in crude oil degradation by Acinetobacter beijerinckii ZRS. J. Basic Microbiol. 2015, 56, 184–195. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Hamishehkar, H.; Khezerlou, A.; Azizi-Lalabadi, M.; Azadi, Y.; Nattagh-Eshtivani, E.; Fasihi, M.; Ghavami, A.; Aynehchi, A.; Ehsani, A. Bioemulsifiers Derived from Microorganisms: Applications in the Drug and Food Industry. Adv. Pharm. Bull. 2018, 8, 191–199. [Google Scholar] [CrossRef]
- Marques, N.P.; de Cassia Pereira, J.; Gomes, E.; da Silva, R.; Araújo, A.R.; Ferreira, H.; Rodrigues, A.; Dussán, K.J.; Bocchini, D.A. Cellulases and xylanases production by endophytic fungi by solid state fermentation using lignocellulosic substrates and enzymatic saccharification of pretreated sugarcane bagasse. Ind. Crops Prod. 2018, 122, 66–75. [Google Scholar] [CrossRef]
- Santamaria-Echart, A.; Fernandes, I.P.; Silva, S.C.; Rezende, S.C.; Colucci, G.; Dias, M.M.; Barreiro, M.F. New trends in natural emulsifiers and emulsion technology for the food industry. In Food Additives, 1st ed.; Prieto, M.A., Otero, P., Eds.; IntechOpen: London, UK, 2021; Volume 1, pp. 1–31. [Google Scholar]
- Harrison, R.G.; Todd, P.W.; Rudge, S.R.; Petrides, D.P. Bioseparations Science and Engineering, 2nd ed.; Oxford University Press: New York, NY, USA, 2003. [Google Scholar]
- Mohanty, S.S.; Koul, Y.; Varjani, S.; Pandey, A.; Ngo, H.H.; Chang, J.-S.; Wong, J.W.C.; Bui, X.-T. A critical review on various feedstocks as sustainable substrates for biosurfactants production: A way towards cleaner production. Microb. Cell Factories 2021, 20, 120. [Google Scholar] [CrossRef] [PubMed]
- Ohno, A.; Ano, T.; Shoda, M. Production of antifungal antibiotic, iturin in a solid-state fermentation by Bacillus subtilis NB22 using wheat bran as a substrate. Biotechnol. Lett 1992, 14, 817–822. [Google Scholar] [CrossRef]
- Das, K.; Mukherjee, A.K. Comparison of lipopeptide biosurfactants production by Bacillus subtilis strains in submerged and solid-state fermentation systems using a cheap carbon source: Some industrial applications of biosurfactants. Process Biochem. 2007, 42, 1191–1199. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, F.; Wei, Z.; Ran, W.; Shen, Q. The usage of rice straw as a major substrate for the production of surfactin by Bacillus amyloliquefaciens XZ-173 in solid-state fermentation. J. Environ. Manag. 2013, 127, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Nalini, S.; Parthasarathi, R. Production and characterization of rhamnolipids produced by Serratia rubidaea SNAU02 under solid-state fermentation and its application as biocontrol agent. Bioresour. Technol. 2014, 173, 231–238. [Google Scholar] [CrossRef]
- Pandey, A. Solid-state fermentation. Biochem. Eng. J. 2003, 13, 81–84. [Google Scholar] [CrossRef]
- Chilakamarry, C.R.; Sakinah, A.M.; Zularisam, A.W.; Sirohi, R.; Khilji, I.A.; Ahmad, N.; Pandey, A. Advances in solid-state fermentation for bioconversion of agricultural wastes to value-added products: Opportunities and challenges. Bioresour. Technol. 2022, 343, 126065. [Google Scholar] [CrossRef]
- El Sheikha, A.F.; Ray, R.C. Bioprocessing of horticultural wastes by solid-state fermentation into value-added/innovative bioproducts: A review. Food Rev. Int. 2022, 39, 3009–3065. [Google Scholar] [CrossRef]
- Abdullah, A.; Hamid, H. Production of cellulase by A. niger and T. reesei under solid state fermentation using bagasse as substrate. J. Bioresour. Environ. Sci. 2022, 1, 8–11. [Google Scholar] [CrossRef]
- Bernardi, B.; Wendland, J. Homologous recombination: A GRAS yeast genome editing tool. Fermentation 2020, 6, 57. [Google Scholar] [CrossRef]
- Singh, N.; Gaur, S. GRAS Fungi: A New Horizon in Safer Food Product. Fungi in Sustainable Food Production; Springer: Berlin/Heidelberg, Germany, 2021; pp. 27–37. [Google Scholar]
- Manitchotpisit, P.; Leathers, T.D.; Peterson, S.W.; Kurtzman, C.P.; Li, X.-L.; Eveleigh, D.E.; Lotrakul, P.; Prasongsuk, S.; Dunlap, C.A.; Vermillion, K.E.; et al. Multilocus phylogenetic analyses, pullulan production and xylanase activity of tropical isolates of Aureobasidium pullulans. Mycol. Res. 2009, 113, 1107–1120. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, I.K.; Yun, B.S. A Novel Biosurfactant Produced by Aureobasidium pullulans L3-GPY from a Tiger Lily Wild Flower, Lilium lancifolium Thunb. PLoS ONE 2015, 10, e0122917. [Google Scholar] [CrossRef] [PubMed]
- Saur, K.M.; Brumhard, O.; Scholz, K.; Hayen, H.; Tiso, T. A pH shift induces high-titer liamocin production in Aureobasidium pullulans. Appl. Microbiol. Biotechnol. 2019, 103, 4741–4752. [Google Scholar] [CrossRef] [PubMed]
- Leathers, T.D. Biotechnological production and applications of pullulan. Appl. Microbiol. Biotechnol. 2003, 62, 468–473. [Google Scholar] [CrossRef]
- Chi, Z.; Wang, F.; Chi, Z.; Yue, L.; Liu, G.; Zhang, T. Bioproducts from Aureobasidium pullulans, a biotechnologically important yeast. Appl. Microbiol. Biotechnol. 2009, 82, 793–804. [Google Scholar] [CrossRef]
- Garay, L.A.; Sitepu, I.R.; Cajka, T.; Xu, J.; Teh, H.E.; German, J.B.; Pan, Z.; Dungan, S.R.; Block, D.E.; Boundy-Mills, K.L. Extracellular fungal polyol lipids: A new class of potential high value lipids. Biotechnol. Adv. 2018, 36, 397–414. [Google Scholar] [CrossRef]
- Kang, X.X.; Jia, S.L.; Wei, X.; Zhang, M.; Liu, G.L.; Hu, Z.; Chi, Z.; Chi, Z.M. Liamocins biosynthesis, its regulation in Aureobasidium spp, and their bioactivities. Crit. Rev. Biotechnol. 2022, 42, 93–105. [Google Scholar] [CrossRef]
- Wang, P.; Jia, S.L.; Liu, G.L.; Chi, Z.; Chi, Z.M. Aureobasidium spp. and their applications in biotechnology. Process Biochem. 2022, 116, 72–83. [Google Scholar] [CrossRef]
- Hilares, R.T.; Orsi, C.A.; Ahmed, M.A.; Marcelino, P.F.; Menegatti, C.R.; da Silva, S.S.; dos Santos, J.C. Low-melanin containing pullulan production from sugarcane bagasse hydrolysate by Aureobasidium pullulans in fermentations assisted by light-emitting diode. Bioresour. Technol. 2017, 230, 76–81. [Google Scholar] [CrossRef]
- Brumano, L.P.; Antunes, F.A.F.; Souto, S.G.; dos Santos, J.C.; Venus, J.; Schneider, R.; da Silva, S.S. Biosurfactant production by Aureobasidium pullulans in stirred tank bioreactor: New approach to understand the influence of important variables in the process. Bioresour. Technol. 2017, 243, 264–272. [Google Scholar] [CrossRef]
- Kitamoto, D.; Ikegami, T.; Suzuki, G.T.; Sasaki, A.; Takeyama, Y.-I.; Idemoto, Y.; Koura, N.; Yanagishita, H. Microbial conversion of n-alkanes into glycolipid biosurfactants, mannosylerythritol lipids, by Pseudozyma (Candida antarctica). Biotechnol. Lett. 2001, 23, 1709–1714. [Google Scholar] [CrossRef]
- Sluiter, A.B.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass NREL/TP-510-42618 2008; US National Renewable Energy Laboratory: Golden, CO, USA, 2008.
- Cooper, D.G.; Goldenberg, B.G. Surface-active agents from two Bacillus Species. Appl. Environ. Microbiol. 1987, 53, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Taniguchi, M.; Matsuno, R.; Kamikubo, T. Purification and properties of cellulases from Eupenicillium javanicum. J. Ferment. Technol. 1981, 59, 177–183. [Google Scholar]
- Wood, T.M.; Bhat, K.M. Methods for measuring cellulase activities. Methods Enzym. 1988, 160, 87–112. [Google Scholar]
- Baral, N.R.; Shah, A. Microbial inhibitors: Formation and effects on acetone-butanol-ethanol fermentation of lignocellulosic biomass. Appl. Microbiol. Biotechnol. 2014, 98, 9151–9172. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, R.; Jaiswal, A.K. A comprehensive review on pre-treatment strategy for lignocellulosic food industry waste: Challenges and opportunities. Bioresour. Technol. 2016, 199, 92–102. [Google Scholar] [CrossRef]
- Medic, J.; Atkinson, C.; Hurburgh, C.R. Current knowledge in soybean composition. J. Am. Oil Chem. Soc. 2014, 91, 363–384. [Google Scholar] [CrossRef]
- Luepongpattana, S.; Thaniyavarn, J.; Morikawa, M. Production of massoia lactone by Aureobasidium pullulans YTP6-14 isolated from the Gulf of Thailand and its fragrant biosurfactant properties. J. Appl. Microbiol. 2017, 123, 1488–1497. [Google Scholar] [CrossRef]
- Krzemińska, I.; Nosalewicz, A.; Reszczyńska, E.; Pawlik-Skowrońska, B. Enhanced light-induced biosynthesis of fatty acids suitable for biodiesel production by the yellow-green alga Eustigmatos magnus. Energies 2020, 13, 6098. [Google Scholar] [CrossRef]
- Li, W.; Xu, X.; Fujibayashi, M.; Niu, Q.; Tanaka, N.; Nishimura, O. Response of microalgae to elevated CO2 and temperature: Impact of climate change on freshwater ecosystems. Environ. Sci. Pollut. Res. 2016, 23, 19847–19860. [Google Scholar] [CrossRef]
- Pereira, S.; Zille, A.; Micheletti, E.; Moradas-Ferreira, P.; De Philippis, R.; Tamagnini, P. Complexity of cyanobacterial exopolysaccharides: Composition, structures, inducing factors and putative genes involved in their biosynthesis and assembly. FEMS Microbiol. Rev. 2009, 33, 917–941. [Google Scholar] [CrossRef]
- Rossi, F.; De Philippis, R. Exocellular Polysaccharides in Microalgae and Cyanobacteria: Chemical Features, Role and Enzymes and Genes Involved in Their Biosynthesis. In The Physiology of Microalgae; Borowitzka, M.A., Beardall, J., Raven, J.A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 565–590. [Google Scholar]
- Fattom, A.; Shilo, M. Phormidium J-1 bioflocculant: Production and activity. Arch. Microbiol. 1984, 139, 421–426. [Google Scholar] [CrossRef]
- Fattom, A.; Shilo, M. Production of emulcyan by Phormidium J-1: Its activity and function. FEMS Microbiol. Lett 1985, 31, 3–9. [Google Scholar] [CrossRef]
- Muñoz, R.; Guieysse, B.; Mattiasson, B. Phenanthrene biodegradation by an algal-bacterial consortium in two-phase partitioning bioreactors. Appl. Microbiol. Biotechnol. 2003, 61, 261–267. [Google Scholar] [CrossRef]
- You, T.; Barnett, S.M. Effect of light quality on production of extracellular polysaccharides and growth rate of Porphyridium cruentum. Biochem. Eng. J. 2004, 19, 251–258. [Google Scholar] [CrossRef]
- Liqin, S.; Wang, C.; Lei, S. Effects of Light Regime on Extracellular Polysaccharide Production by Porphyridium cruentum Cultured in Flat Plate Photobioreactors. In Proceedings of the 2nd International Conference on Bioinformatics and Biomedical Engineering (ICBBE ’08), Shanghai, China, 16–18 May 2008; pp. 1488–1491. [Google Scholar]
- Clément-Larosière, B.; Lopes, F.; Gonçalves, A.; Taidi, B.; Benedetti, M.; Minier, M.; Pareau, D. Carbon dioxide biofixation by Chlorella vulgaris at different CO2 concentrations and light intensities. Eng. Life Sci. 2014, 14, 509–519. [Google Scholar] [CrossRef]
- Lee, J.K.; Jung, H.M.; Kim, S.Y. 1,8-dihydroxynaphthalene (DHN)-melanin biosynthesis inhibitors increase erythritol production in Torula corallina, and DHN-melanin inhibits erythrose reductase. Appl. Environ. Microbiol. 2003, 6, 3427–3434. [Google Scholar] [CrossRef] [PubMed]
- Engelberg, D.; Perlman, R.; Levitzki, A. Transmembrane signaling in Saccharomyces cerevisiae as a model for signaling in metazoans: State of the art after 25years. Cells Signal. 2014, 26, 2865–2878. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.-J.; Liu, G.-L.; Chi, Z.; Gao, Z.-C.; Hu, Z.; Chi, Z.-M. Genetic evidences for the core biosynthesis pathway, regulation, transport and secretion of liamocins in yeast-like fungal cells. Biochem. J. 2020, 477, 887–903. [Google Scholar] [CrossRef]
- Verghese, J.; Abrams, J.; Wang, Y.; Morano, K.A. Biology of the heat shock response and protein chaperones: Budding yeast (Saccharomyces cerevisiae) as a Model System. Microbiol. Mol. Biol. Rev. 2012, 76, 115–158. [Google Scholar] [CrossRef]
- Chi, Z.; Kong, C.C.; Wang, Z.Z.; Wang, Z.; Liu, G.L.; Hu, Z.; Chi, Z.M. The signaling pathways involved in metabolic regulation and stress responses of the yeast-like fungi Aureobasidium spp. Biotechnol. Adv. 2022, 55, 107898. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Liu, G.L.; Wang, S.J.; Chi, Z.M.; Chi, Z. Pullulan biosynthesis in yeast-like fungal cells is regulated by the transcriptional activator Msn2 and cAMP-PKA signaling pathway. Int. J. Biol. Macromol. 2020, 157, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Peñalver, P.; Castillejos, M.; Koh, A.; Gross, R.; Sánchez, A.; Font, X.; Gea, T. Production and characterization of sophorolipids from stearic acid by solid-state fermentation, a cleaner alternative to chemical surfactants. J. Clean. Prod. 2018, 172, 2735–2747. [Google Scholar] [CrossRef]
- Velioglu, Z.; Urek, R.O. Concurrent biosurfactant and ligninolytic enzyme production by Pleurotus spp. in Solid-State Fermentation. Appl. Biochem. Biotechnol. 2014, 174, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Gea, T.; Sánchez, A.; Font, X. Agro-wastes and inert materials as supports for the production of biosurfactants by solid-state fermentation. Waste Biomass Valorizat. 2021, 12, 1963–1976. [Google Scholar] [CrossRef]
- Leathers, T.D.; Price, N.P.J.; Manitchotpisit, P.; Bischoff, K.M. Production of anti-streptococcal liamocins from agricultural biomass by Aureobasidium pullulans. World J. Microbiol. Biotechnol. 2016, 32, 199. [Google Scholar] [CrossRef]
- Kudanga, T.; Mwenje, E. Extracellular cellulase production by tropical isolates of Aureobasidium pullulans. Can. J. Microbiol. 2005, 51, 773–776. [Google Scholar] [CrossRef]
- Kumar, D.; Murthy, G.S. Stochastic molecular model of enzymatic hydrolysis of cellulose for ethanol production. Biotechnol. Biofuels 2013, 6, 63. [Google Scholar] [CrossRef]
- Leite, R.S.R.; Bocchini, D.A.; Martins, E.D.S.; Silva, D.; Gomes, E.; da Silva, R. Production of cellulolytic and hemicellulolytic enzymes from Aureobasidium pullulans on solid state fermentation. Appl. Biochem. Biotechnol. 2007, 137, 281–288. [Google Scholar]
- Vieira, M.M.; Kadoguchi, E.; Segato, F.; da Silva, S.S.; Chandel, A.K. Production of cellulases by Aureobasidium pullulans LB83: Optimization, characterization, and hydrolytic potential for the production of cellulosic sugars. Prep. Biochem. Biotechnol. 2021, 51, 153–163. [Google Scholar] [CrossRef]
- Zhang, Y.-H.P.; Lynd, L.R. Toward an aggregated understanding of enzymatic hydrolysis of cellulose: Noncomplexed cellulase systems. Biotechnol. Bioeng. 2004, 88, 797–824. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Sun, C.; Liu, R.; Yin, R.; Wu, X. Comparison of the effects of five pretreatment methods on enhancing the enzymatic digestibility and ethanol production from sweet sorghum bagasse. Bioresour. Technol. 2012, 111, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Liao, A.-M.; Zhang, J.; Yang, Z.-L.; Huang, J.-H.; Pan, L.; Hou, Y.-C.; Li, X.-X.; Zhao, P.-H.; Dong, Y.-Q.; Hu, Z.-Y.; et al. Structural, Physicochemical, and Functional Properties of Wheat Bran Insoluble Dietary Fiber Modified with Probiotic Fermentation. Front. Nutr. 2022, 9, 803440. [Google Scholar] [CrossRef] [PubMed]




| Bioreactor | EI24% Kerosene | EI24% Soybean Oil | BE Yield (g BE g−1 Substrate) | Endoglucanase (Ug−1) | Exoglucanase (Ug−1) |
|---|---|---|---|---|---|
| Without LED light | 29.5 ± 0.6 a | 36.9 ± 0.8 a | 0.09 ± 0.6 a | 2.94 ± 0.02 a | 0.45 ± 0.00 a |
| White LED light | 32.5 ± 0.7 b | 41.1 ± 0.8 b | 0.13 ± 0.5 b | 3.25 ± 0.02 b | 0.48 ± 0.00 b |
| Blue LED light | 45.7 ± 0.4 c | 55.0 ± 0.9 c | 0.20 ± 0.3 c | 3.87 ± 0.02 c | 0.53 ± 0.00 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubio-Ribeaux, D.; Costa, R.A.M.d.; Pereira, R.M.; Marcelino, P.R.F.; Casciatori, F.P.; Santos, J.C.d.; Silva, S.S.d. Effect of Blue LED Light on Bioemulsifier Production in Bioreactor by Aureobasidium pullulans LB83 in Solid State Fermentation. Fermentation 2023, 9, 946. https://doi.org/10.3390/fermentation9110946
Rubio-Ribeaux D, Costa RAMd, Pereira RM, Marcelino PRF, Casciatori FP, Santos JCd, Silva SSd. Effect of Blue LED Light on Bioemulsifier Production in Bioreactor by Aureobasidium pullulans LB83 in Solid State Fermentation. Fermentation. 2023; 9(11):946. https://doi.org/10.3390/fermentation9110946
Chicago/Turabian StyleRubio-Ribeaux, Daylin, Rogger Alessandro Mata da Costa, Renan Murbach Pereira, Paulo Ricardo Franco Marcelino, Fernanda Perpétua Casciatori, Júlio César dos Santos, and Silvio Silvério da Silva. 2023. "Effect of Blue LED Light on Bioemulsifier Production in Bioreactor by Aureobasidium pullulans LB83 in Solid State Fermentation" Fermentation 9, no. 11: 946. https://doi.org/10.3390/fermentation9110946
APA StyleRubio-Ribeaux, D., Costa, R. A. M. d., Pereira, R. M., Marcelino, P. R. F., Casciatori, F. P., Santos, J. C. d., & Silva, S. S. d. (2023). Effect of Blue LED Light on Bioemulsifier Production in Bioreactor by Aureobasidium pullulans LB83 in Solid State Fermentation. Fermentation, 9(11), 946. https://doi.org/10.3390/fermentation9110946







