Pyrimidine Biosynthesis and Ribonucleoside Metabolism in Species of Pseudomonas
Abstract
:1. Introduction
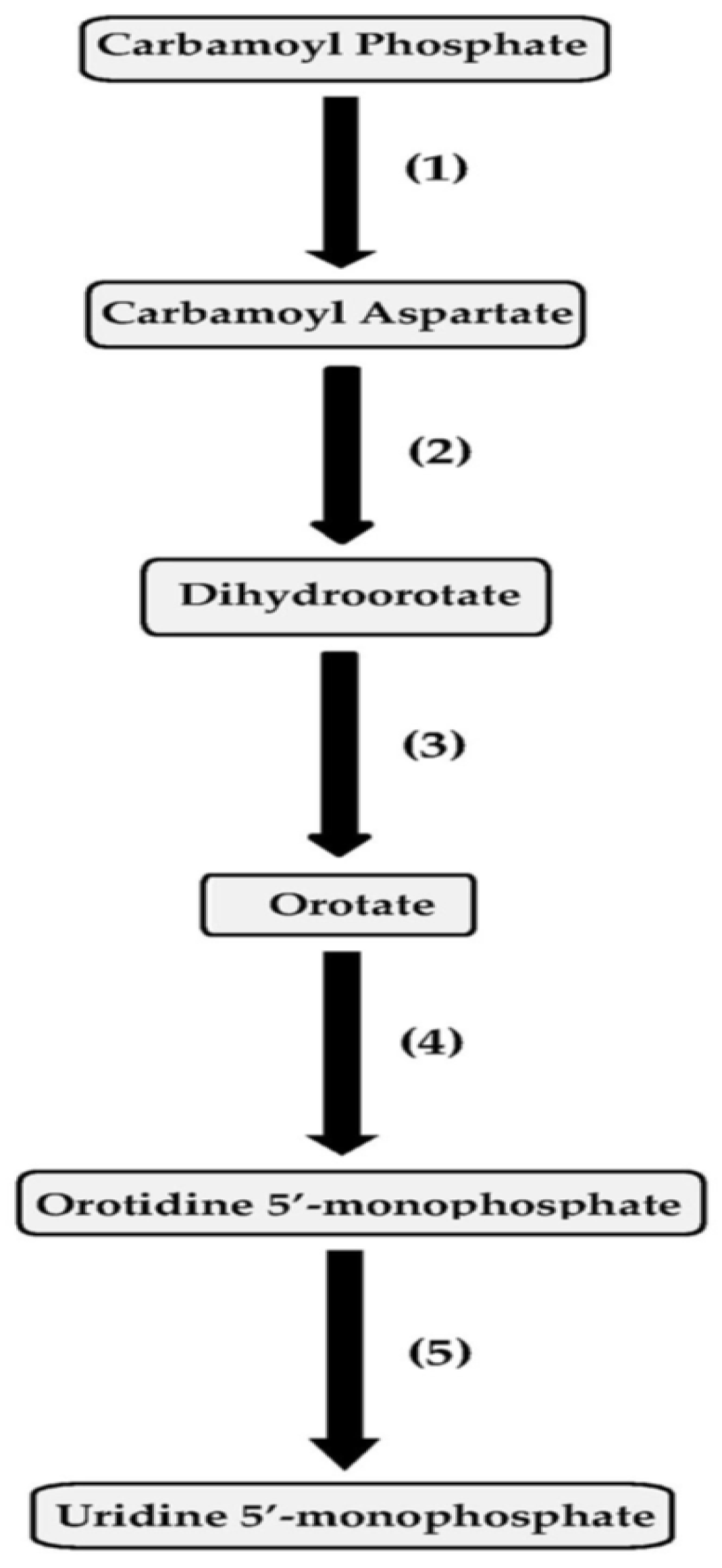
2. Pyrimidine Biosynthesis
2.1. Pseudomonas aeruginosa Homology Group
2.2. Pseudomonas chlororaphis Homology Group
2.3. Pseudomonas fluorescens Homology Group
2.4. Pseudomonas putida Homology Group
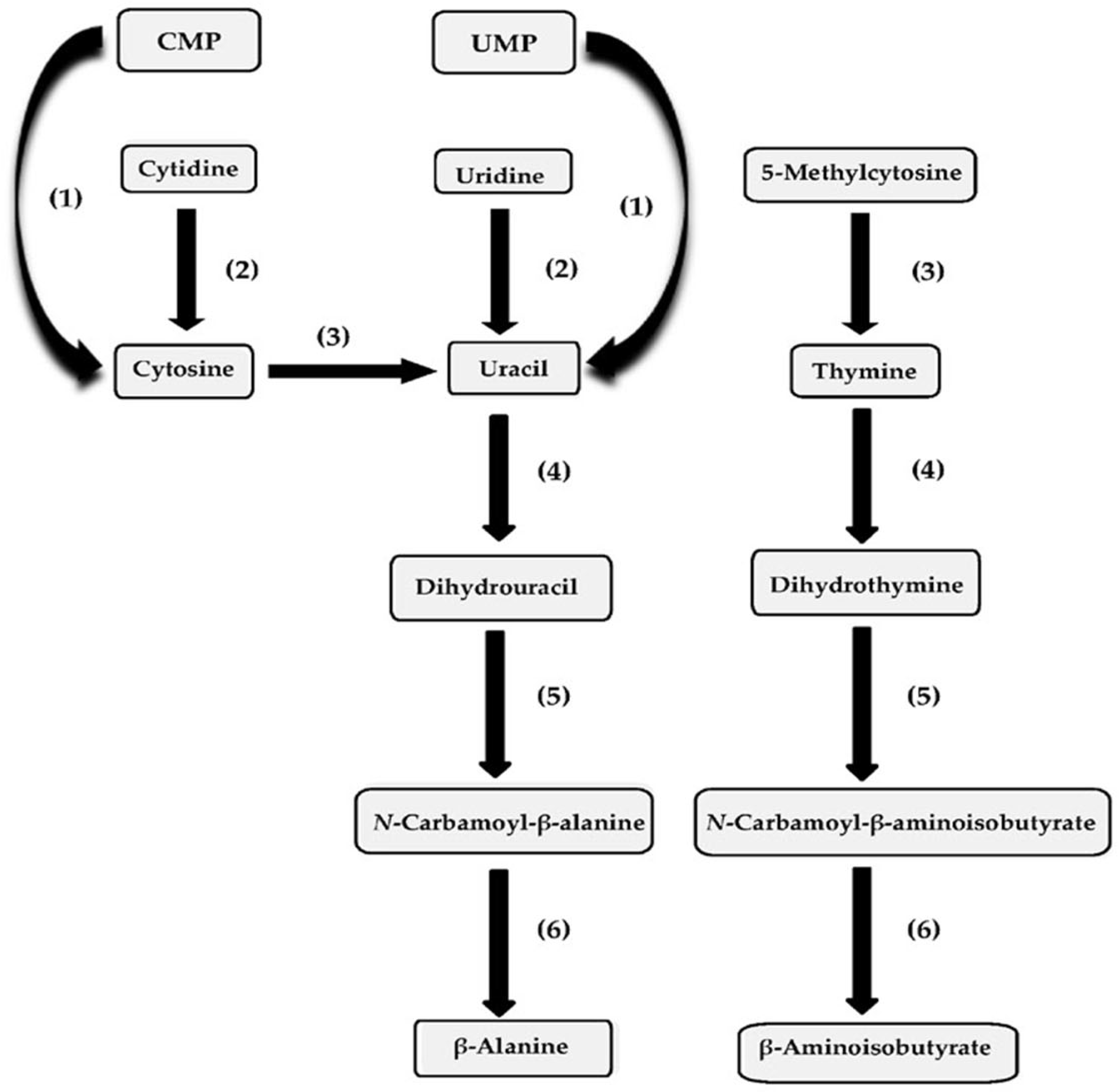
3. Pyrimidine Ribonucleoside Metabolism
3.1. Pseudomonas aeruginosa Homology Group
3.2. Pseudomonas chlororaphis Homology Group
3.3. Pseudomonas fluorescens Homology Group
3.4. Pseudomonas putida Homology Group
4. Pyrimidine Base Catabolism
4.1. Pseudomonas aeruginosa Homology Group
4.2. Pseudomonas chlororaphis Homology Group
4.3. Pseudomonas fluorescens Homology Group
4.4. Pseudomonas putida Homology Group
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yates, R.A.; Pardee, A.B. Pyrimidine biosynthesis in Escherichia coli. J. Biol. Chem. 1956, 221, 743–756. [Google Scholar] [CrossRef]
- Beckwith, J.R.; Pardee, A.B.; Austrian, R.; Jacob, F. Coordination of the synthesis of the enzymes in the pyrimidine pathway of E. coli. J. Mol. Biol. 1962, 5, 618–634. [Google Scholar] [CrossRef] [PubMed]
- Hayward, W.S.; Belser, W.L. Regulation of pyrimidine biosynthesis in Serratia marcescens. Proc. Natl. Acad. Sci. USA 1965, 53, 1483–1489. [Google Scholar] [CrossRef] [PubMed]
- Neuhard, J. Pyrimidine nucleotide metabolism and pathways of thymidine triphosphate biosynthesis in Salmonella typhimurium. J. Bacteriol. 1968, 96, 1519–1527. [Google Scholar] [CrossRef]
- O’Donovan, G.A.; Neuhard, J. Pyrimidine metabolism in microorganisms. Bacteriol. Rev. 1970, 34, 278–343. [Google Scholar] [CrossRef]
- Beck, C.F.; Ingraham, J.L.; Neuhard, J.; Thomassen, E. Metabolism of pyrimidines and pyrimidine nucleosides by Salmonella typhimurium. J. Bacteriol. 1972, 110, 219–228. [Google Scholar] [CrossRef]
- O’Donovan, G.A.; Gerhart, J.C. Isolation and partial characterization of regulatory mutants of the pyrimidine pathway in Salmonella typhimurium. J. Bacteriol. 1972, 109, 1085–1096. [Google Scholar] [CrossRef]
- Schwartz, M.; Neuhard, J. Control of expression of the pyr genes in Salmonella typhimurium: Effects of variations in uridine and cytidine nucleotide pools. J. Bacteriol. 1975, 121, 814–822. [Google Scholar] [CrossRef]
- Kelln, R.A.; Kinahan, J.J.; Foltermann, K.F.; O’Donovan, G.A. Pyrimidine biosynthetic enzymes of Salmonella typhimurium, repressed specifically by growth in the presence of cytidine. J. Bacteriol. 1975, 124, 764–774. [Google Scholar] [CrossRef]
- Jyssum, S. Pyrimidine biosynthesis in Neisseria meningitidis: 2. Regulation of enzyme synthesis. Acta Pathol. Microbiol. Scand. B 1983, 91, 257–260. [Google Scholar] [CrossRef]
- Nowlan, S.F.; Kantrowitz, E.R. Identification of a trans-acting regulatory factor involved in the control of the pyrimidine pathway in E. coli. Mol. Genet. Genom. 1983, 192, 264–271. [Google Scholar] [CrossRef]
- Turnbough, C.L., Jr. Regulation of Escherichia coli aspartate transcarbamylase synthesis by guanosine tetraphosphate and pyrimidine ribonucleoside triphosphates. J. Bacteriol. 1983, 153, 998–1007. [Google Scholar] [CrossRef]
- Bouvier, J.; Patte, J.C.; Stragier, P. Multiple regulatory signals in the control region of the Escherichia coli carAB operon. Proc. Natl. Acad. Sci. USA 1984, 81, 4139–4143. [Google Scholar] [CrossRef]
- Levin, H.L.; Schachman, H.K. Regulation of aspartate transcarbamoylase synthesis in Escherichia coli: Analysis of deletion mutations in the promoter region of the pyrBI operon. Proc. Natl. Acad. Sci. USA 1985, 82, 4643–4647. [Google Scholar] [CrossRef]
- Wilson, H.R.; Chan, P.T.; Turnbough, C.L., Jr. Nucleotide sequence and expression of the pyrC gene of Escherichia coli K-12. J. Bacteriol. 1987, 169, 3051–3058. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.F. Regulation of Salmonella typhimurium pyr gene expression: Effect of changing both purine and pyrimidine nucleotide pools. Microbiology 1989, 135, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Turnbough, C.L., Jr. Multiple control mechanisms for pyrimidine-mediated regulation of pyrBI operon expression in Escherichia coli K-12. J. Bacteriol. 1989, 171, 3337–3342. [Google Scholar] [CrossRef]
- O’Donovan, G.A.; Herlick, S.; Beck, D.E.; Dutta, P.K. UTP/CTP ratio, an important regulatory parameter for ATCase expression. Arch. Microbiol. 1989, 153, 19–25. [Google Scholar] [CrossRef]
- Choi, K.Y.; Zalkin, H.O. Regulation of Escherichia coli pyrC by the purine regulon repressor protein. J. Bacteriol. 1990, 172, 3201–3207. [Google Scholar] [CrossRef]
- Wilson, H.R.; Turnbough, C.L., Jr. Role of the purine repressor in the regulation of pyrimidine gene expression in Escherichia coli K-12. J. Bacteriol. 1990, 172, 3208–3213. [Google Scholar] [CrossRef]
- Baker, K.E.; Ditullio, K.P.; Neuhard, J.; Kelln, R.A. Utilization of orotate as a pyrimidine source by Salmonella typhimurium and Escherichia coli requires the dicarboxylate transport protein encoded by dctA. J. Bacteriol. 1996, 178, 7099–7105. [Google Scholar] [CrossRef] [PubMed]
- Kholti, A.; Charlier, D.; Gigot, D.; Huysveld, N.; Roovers, M.; Glansdorff, N. pyrH-encoded UMP-kinase directly participates in pyrimidine-specific modulation of promoter activity in Escherichia coli. J. Mol. Biol. 1998, 280, 571–582. [Google Scholar] [CrossRef]
- Rodríguez, M.; Good, T.A.; Wales, M.E.; Hua, J.P.; Wild, J.R. Modeling allosteric regulation of de novo pyrimidine biosynthesis in Escherichia coli. J. Theor. Biol. 2005, 234, 299–310. [Google Scholar] [CrossRef]
- Turnbough, C.L., Jr.; Switzer, R.L. Regulation of pyrimidine biosynthetic gene expression in bacteria: Repression without repressors. Microbiol. Mol. Biol. 2008, 72, 266–300. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Terol, G.; Gallego-Jara, J.; Sola-Martínez, R.A.; Ortega, Á.; Martinez Vivancos, A.; Canovas Diaz, M.; de Diego Puente, T. Regulation of the pyrimidine biosynthetic pathway by lysine acetylation of E. coli OPRTase. FEBS J. 2023, 290, 442–464. [Google Scholar] [CrossRef] [PubMed]
- Akberdin, I.R.; Kozlov, K.N.; Kazantsev, F.V.; Fadeev, S.I.; Likhoshvai, V.A.; Khlebodarova, T.M. Impact of negative feedbacks on de novo pyrimidines biosynthesis in Escherichia coli. Int. J. Mol. Sci. 2023, 24, 4806. [Google Scholar] [CrossRef]
- Waleh, N.S.; Ingraham, J.L. Pyrimidine ribonucleoside monophosphokinase and the mode of RNA turnover in Bacillus subtilis. Arch. Microbiol. 1976, 110, 49–54. [Google Scholar] [CrossRef]
- Lerner, C.G.; Stephenson, B.T.; Switzer, R.L. Structure of the Bacillus subtilis pyrimidine biosynthetic (pyr) gene cluster. J. Bacteriol. 1987, 169, 2202–2206. [Google Scholar] [CrossRef]
- Switzer, R.L.; Turner, R.J.; Lu, Y. Regulation of the Bacillus subtilis pyrimidine biosynthetic operon by transcriptional attenuation: Control of gene expression by an mRNA-binding protein. Prog. Nucleic Acid Res. Mol. Biol. 1988, 62, 329–367. [Google Scholar]
- Asahi, S.; Doi, M.; Tsunemi, Y.; Akiyama, S.I. Regulation of pyrimidine nucleotide biosynthesis in cytidine deaminase-negative mutants of Bacillus subtilis. Agric. Biol. Chem. 1989, 53, 97–102. [Google Scholar] [CrossRef]
- Quinn, C.L.; Stephenson, B.T.; Switzer, R.L. Functional organization and nucleotide sequence of the Bacillus subtilis pyrimidine biosynthetic operon. J. Biol. Chem. 1991, 266, 9113–9127. [Google Scholar] [CrossRef] [PubMed]
- Ghim, S.Y.; Neuhard, J. The pyrimidine biosynthesis operon of the thermophile Bacillus caldolyticus includes genes for uracil phosphoribosyltransferase and uracil permease. J. Bacteriol. 1994, 176, 3698–3707. [Google Scholar] [CrossRef]
- Ghim, S.Y.; Nielsen, P.; Neuhard, J. Molecular characterization of pyrimidine biosynthesis genes from the thermophile Bacillus caldolyticus. Microbiology 1994, 140, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.J.; Lu, Y.; Switzer, R.L. Regulation of the Bacillus subtilis pyrimidine biosynthetic (pyr) gene cluster by an autogenous transcriptional attenuation mechanism. J. Bacteriol. 1994, 176, 3708–3722. [Google Scholar] [CrossRef]
- Lu, Y.; Turner, R.J.; Switzer, R.L. Roles of the three transcriptional attenuators of the Bacillus subtilis pyrimidine biosynthetic operon in the regulation of its expression. J. Bacteriol. 1995, 177, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Andersen, P.S.; Martinussen, J.; Hammer, K. Sequence analysis and identification of the pyrKDbF operon from Lactococcus lactis including a novel gene, pyrK, involved in pyrimidine biosynthesis. J. Bacteriol. 1996, 178, 5005–5012. [Google Scholar] [CrossRef]
- Elagöz, A.; Abdi, A.; Hubert, J.C.; Kammerer, B. Structure and organisation of the pyrimidine biosynthesis pathway genes in Lactobacillus plantarum: A PCR strategy for sequencing without cloning. Gene 1996, 182, 37–43. [Google Scholar] [CrossRef]
- Martinussen, J.; Schallert, J.; Andersen, B.; Hammer, K. The pyrimidine operon pyrRPB-carA from Lactococcus lactis. J. Bacteriol. 2001, 183, 2785–2794. [Google Scholar] [CrossRef]
- Thia-Toong, T.L.; Roovers, M.; Durbecq, V.; Gigot, D.; Glansdorff, N.; Charlier, D. Genes of de novo pyrimidine biosynthesis from the hyperthermoacidophilic crenarchaeote Sulfolobus acidocaldarius: Novel organization in a bipolar operon. J. Bacteriol. 2002, 184, 4430–4441. [Google Scholar] [CrossRef]
- Buvelot, H.; Roth, M.; Jaquet, V.; Lozkhin, A.; Renzoni, A.; Bonetti, E.J.; Krause, K.H. Hydrogen peroxide affects growth of S. aureus through downregulation of genes involved in pyrimidine biosynthesis. Front. Immunol. 2021, 12, 673985. [Google Scholar] [CrossRef]
- Chen, S.; He, X.; Qin, Z.; Li, G.; Wang, W.; Nai, Z.; Tian, Y.; Liu, D.; Jiang, X. Loss in the antibacterial ability of a PyrR gene regulating pyrimidine biosynthesis after using CRISPR/Cas9-mediated knockout for metabolic engineering in Lactobacillus casei. Microorganisms 2023, 11, 2371. [Google Scholar] [CrossRef] [PubMed]
- Lovatt, C.J.; Albert, L.S.; Tremblay, G.C. Regulation of pyrimidine biosynthesis in intact cells of Cucurbita pepo. Plant Physiol. 1979, 64, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Jacques, S.; Sung, Z.R. Regulation of pyrimidine and arginine biosynthesis investigated by the use of phaseolotoxin and 5-fluorouracil. Plant Physiol. 1981, 67, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Giermann, N.; Schröder, M.; Ritter, T.; Zrenner, R. Molecular analysis of de novo pyrimidine synthesis in solanaceous species. Plant Mol. Biol. 2002, 50, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Boldt, R.; Zrenner, R. Purine and pyrimidine biosynthesis in higher plants. Physiol. Plant. 2003, 117, 297–304. [Google Scholar] [CrossRef]
- Huang, M.; Graves, L.M. De novo synthesis of pyrimidine nucleotides; emerging interfaces with signal transduction pathways. Cell. Mol. Life Sci. 2003, 60, 321–336. [Google Scholar] [CrossRef]
- Sigoillot, F.D.; Berkowski, J.A.; Sigoillot, S.M.; Kotsis, D.H.; Guy, H.I. Cell cycle-dependent regulation of pyrimidine biosynthesis. J. Biol. Chem. 2003, 278, 3403–3409. [Google Scholar] [CrossRef]
- Evans, D.R.; Guy, H.I. Mammalian pyrimidine biosynthesis: Fresh insights into an ancient pathway. J. Biol. Chem. 2004, 279, 33035–33038. [Google Scholar] [CrossRef]
- Kafer, C.; Zhou, L.; Santoso, D.; Guirgis, A.; Weers, B.; Park, S.; Thornburg, R. Regulation of pyrimidine metabolism in plants. Front. Biosci. Landmark 2004, 9, 1611–1625. [Google Scholar]
- Robertson, B.C.; Jargiello, P.; Blank, J.; Hoffee, P.A. Genetic regulation of ribonucleoside and deoxyribonucleoside catabolism in Salmonella typhimurium. J. Bacteriol. 1970, 102, 628–635. [Google Scholar] [CrossRef]
- Nygaard, P. Nucleoside-catabolizing enzymes in Salmonella typhimurium induction by ribonucleosides. Eur. J. Biochem. 1973, 36, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Mygind, B.; Munch-Petersen, A. Transport of pyrimidine nucleosides in cells of Escherichia coli K 12. Eur. J. Biochem. 1975, 59, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Leer, J.C.; Hammer-Jespersen, K.; Schwartz, M. Uridine phosphorylase from Escherichia coli: Physical and chemical characterization. Eur. J. Biochem. 1977, 75, 217–224. [Google Scholar] [CrossRef]
- Mitchell, A.; Finch, L.R. Enzymes of pyrimidine metabolism in Mycoplasma mycoides subsp. mycoides. J. Bacteriol. 1979, 137, 1073–1080. [Google Scholar] [CrossRef]
- Williams, J.C.; Lee, C.E.; Wild, J.R. Genetic and biochemical characterization of distinct transport systems for uracil, uridine and cytidine in Salmonella typhimurium. Mol. Genet. Genom. 1980, 178, 121–130. [Google Scholar] [CrossRef]
- Andersen, L.; Kilstrup, M.; Neuhard, J. Pyrimidine, purine and nitrogen control of cytosine deaminase synthesis in Escherichia coli K12. Involvement of the glnLG and purR genes in the regulation of codA expression. Arch. Microbiol. 1989, 152, 115–118. [Google Scholar] [CrossRef]
- Kilstrup, M.; Meng, L.M.; Neuhard, J.; Nygaard, P. Genetic evidence for a repressor of synthesis of cytosine deaminase and purine biosynthesis enzymes in Escherichia coli. J. Bacteriol. 1989, 171, 2124–2127. [Google Scholar] [CrossRef]
- Danielsen, S.; Kilstrup, M.; Barilla, K.; Jochimsen, B.; Neuhard, J. Characterization of the Escherichia coli codBA operon encoding cytosine permease and cytosine deaminase. Mol. Microbiol. 1992, 6, 1335–1344. [Google Scholar] [CrossRef]
- Tu, A.H.; Turnbough, C.L., Jr. Regulation of upp expression in Escherichia coli by UTP-sensitive selection of transcriptional start sites coupled with UTP-dependent reiterative transcription. J. Bacteriol. 1997, 179, 6665–6673. [Google Scholar] [CrossRef]
- Hayden, M.S.; Linsley, P.S.; Wallace, A.R.; Marquardt, H.; Kerr, D.E. Cloning, overexpression, and purification of cytosine deaminase from Saccharomyces cerevisiae. Protein Expr. Purif. 1998, 12, 173–184. [Google Scholar] [CrossRef]
- Tiraby, M.; Cazaux, C.; Baron, M.; Drocourt, D.; Reynes, J.-P.; Tiraby, G. Concomitant expression of E. coli cytosine deaminase and uracil phosphoribosyltransferase improves the cytotoxicity of 5-fluorocytosine. FEMS Microbiol. Lett. 1998, 167, 41–49. [Google Scholar] [CrossRef]
- Muse, W.B.; Rosario, C.J.; Bender, R.A. Nitrogen regulation of the codBA (cytosine deaminase) operon from Escherichia coli by the nitrogen assimilation control protein, NAC. J. Bacteriol. 2003, 185, 2920–2926. [Google Scholar] [CrossRef]
- Loh, K.D.; Gyaneshwar, P.; Markenscoff Papadimitriou, E.; Fong, R.; Kim, K.S.; Parales, R.; Zhou, Z.; Inwood, W.; Kustu, S. A previously undescribed pathway for pyrimidine catabolism. Proc. Natl. Acad. Sci. USA 2006, 103, 5114–5119. [Google Scholar] [CrossRef] [PubMed]
- Mihara, H.; Hidese, R.; Yamane, M.; Kurihara, T.; Esaki, N. The iscS gene deficiency affects the expression of pyrimidine metabolism genes. Biochem. Biophys. Res. Commun. 2008, 372, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.; Flörchinger, M.; Kunz, H.-H.; Traub, M.; Wartenberg, R.; Wolfgang Jeblick, W.; Neuhaus, H.E.; Möhlmann, T. Uridine-ribohydrolase is a key regulator in the uridine degradation pathway of Arabidopsis. Plant Cell 2009, 21, 876–891. [Google Scholar] [CrossRef]
- Mach, J. Uridine ribohydrolase and the balance between nucleotide degradation and salvage. Plant Cell 2009, 21, 699. [Google Scholar] [CrossRef]
- Fang, H.; Zhang, C.; Xie, X.; Xu, Q.; Zhou, Y.; Chen, N. Enhanced cytidine production by a recombinant Escherichia coli strain using genetic manipulation strategies. Ann. Microbiol. 2014, 64, 1203–1210. [Google Scholar] [CrossRef]
- García-Bayona, L.; Garavito, M.F.; Lozano, G.L.; Vasquez, J.J.; Myers, K.; Fry, W.E.; Bernal, A.; Zimmerman, B.H.; Restrepo, S. De Novo pyrimidine biosynthesis in the oomycete plant pathogen Phytophthora infestans. Gene 2014, 537, 312–321. [Google Scholar] [CrossRef]
- Garavito, M.F.; Narváez-Ortiz, H.Y.; Zimmermann, B.H. Pyrimidine metabolism: Dynamic and versatile pathways in pathogens and cellular development. J. Genet. Genom. 2015, 42, 195–205. [Google Scholar] [CrossRef]
- Ban, J.; Ljubinka Vitale, L.; Kos, E. Thymine and uracil catabolism in Escherichia coli. Microbiology 1972, 73, 267–272. [Google Scholar] [CrossRef]
- Šimaga, Š.; Kos, E. Properties and regulation of pyrimidine catabolism in Escherichia coli. Int. J. Biochem. 1981, 13, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Matthews, M.M.; Traut, T.W. Regulation of N-carbamoyl-beta-alanine amidohydrolase, the terminal enzyme in pyrimidine catabolism, by ligand-induced change in polymerization. J. Biol. Chem. 1987, 262, 7232–7237. [Google Scholar] [CrossRef] [PubMed]
- Kern, L.; De Montigny, J.; Lacroute, F.; Jund, R. Regulation of the pyrimidine salvage pathway by the FUR1 gene product of Saccharomyces cerevisiae. Curr. Genet. 1991, 19, 333–337. [Google Scholar] [CrossRef] [PubMed]
- West, T.P. Isolation and characterization of an Escherichia coli B mutant strain defective in uracil catabolism. Can. J. Microbiol. 1998, 44, 1106–1109. [Google Scholar] [CrossRef]
- Soong, C.-L.; Ogawa, J.; Sakuradani, E.; Shimizu, S. Barbiturase, a novel zinc-containing amidohydrolase involved in oxidative pyrimidine metabolism. J. Biol. Chem. 2002, 277, 7051–7058. [Google Scholar] [CrossRef]
- Van Kuilenburg, A.B.P.; Meinsma, R.; Beke, E.; Assmann, B.; Ribes, A.; Lorente, I.; Busch, R.; Mayatepek, E.; Nico, G.G.M.; Abeling, N.G.G.M.; et al. β-Ureidopropionase deficiency: An inborn error of pyrimidine degradation associated with neurological abnormalities. Hum. Mol. Genet. 2004, 13, 2793–2801. [Google Scholar] [CrossRef]
- Shimada, T.; Hirao, K.; Kori, A.; Yamamoto, K.; Ishihama, A. RutR is the uracil/thymine-sensing master regulator of a set of genes for synthesis and degradation of pyrimidines. Mol. Microbiol. 2007, 66, 744–757. [Google Scholar] [CrossRef]
- Shimada, T.; Ishihama, A.; Busby, S.J.; Grainger, D.C. The Escherichia coli RutR transcription factor binds at targets within genes as well as intergenic regions. Nucleic Acids Res. 2008, 36, 3950–3955. [Google Scholar] [CrossRef]
- Hidese, R.; Mihara, H.; Kurihara, T.; Nobuyoshi Esaki, N. Escherichia coli dihydropyrimidine dehydrogenase is a novel NAD-dependent heterotetramer essential for the production of 5,6-dihydrouracil. J. Bacteriol. 2011, 193, 989–993. [Google Scholar] [CrossRef]
- Andersson Rasmussen, A.; Kandasamy, D.; Beck, H.; Crosby, S.D.; Björnberg, O.; Schnackerz, K.D.; Piškur, J. Global expression analysis of the yeast Lachancea (Saccharomyces) kluyveri reveals new URC genes involved in pyrimidine catabolism. Eukaryot. Cell 2014, 13, 31–42. [Google Scholar] [CrossRef]
- De Vos, P.; De Ley, J. Intra- and intergeneric similarities of Pseudomonas and Xanthomonas ribosomal ribonucleic acid cistrons. Int. J. Syst. Bacteriol. 1983, 33, 487–509. [Google Scholar] [CrossRef]
- Anzai, Y.; Kim, H.; Park, J.-Y.; Wakabayashi, H.; Oyaizu, H. Phylogenetic affiliation of the pseudomonads based on 16S rRNA sequence. Int. J. Syst. Evol. Microbiol. 2000, 50, 1563–1589. [Google Scholar] [CrossRef] [PubMed]
- Mulet, M.; Lalucat, J.; Garcia-Valdes, E. DNA sequence-based analysis of the Pseudomonas species. Environ. Microbiol. 2010, 12, 1513–1530. [Google Scholar] [CrossRef] [PubMed]
- Isaac, J.H.; Holloway, B.W. Control of pyrimidine biosynthesis in Pseudomonas aeruginosa. J. Bacteriol. 1968, 96, 1732–1741. [Google Scholar] [CrossRef] [PubMed]
- Ralli, P.; Srivastava, A.C.; O’Donovan, G. Regulation of the pyrimidine biosynthetic pathway in a pyrD knockout mutant of Pseudomonas aeruginosa. J. Basic Microbiol. 2007, 47, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Santiago, M.F.; West, T.P. Effect of carbon source on pyrimidine biosynthesis in Pseudomonas alcaligenes ATCC 14909. Microbiol. Res. 2003, 158, 195–199. [Google Scholar] [CrossRef]
- West, T.P. Pyrimidine nucleotide synthesis in Pseudomonas citronellolis. Can. J. Microbiol. 2004, 50, 455–459. [Google Scholar] [CrossRef]
- Santiago, M.F.; West, T.P. Regulation of pyrimidine synthesis in Pseudomonas mendocina. J. Basic Microbiol. 2002, 42, 75–79. [Google Scholar] [CrossRef]
- Santiago, M.F.; West, T.P. Influence of carbon source on pyrimidine synthesis in Pseudomonas mendocina. J. Basic Microbiol. 2003, 43, 534–538. [Google Scholar] [CrossRef]
- West, T.P. Pyrimidine nucleotide synthesis in Pseudomonas nitroreducens and the regulatory role of pyrimidines. Microbiol. Res. 2014, 169, 954–958. [Google Scholar] [CrossRef]
- West, T.P. Control of the pyrimidine biosynthetic pathway in Pseudomonas pseudoalcaligenes. Arch. Microbiol. 1994, 162, 75–79. [Google Scholar] [CrossRef]
- Haugaard, L.E.; West, T.P. Pyrimidine biosynthesis in Pseudomonas oleovorans. J. Appl. Microbiol. 2002, 92, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Saha, R.; Sproer, C.; Beck, B.; Bagley, S. Pseudomonas oleovorans subsp. lubricantis subsp. nov., and reclassification of Pseudomonas pseudoalcaligenes ATCC 17440T as later synonym of Pseudomonas oleovorans ATCC 8062T. Curr. Microbiol. 2010, 60, 294–300. [Google Scholar] [CrossRef]
- West, T.P. Regulation of pyrimidine synthesis in Pseudomonas resinovorans. Lett. Appl. Microbiol. 2005, 40, 473–478. [Google Scholar] [CrossRef]
- Abdurahman, N.; Hughes, L.E. Accumulation of pyrimidine intermediate orotate decreases virulence factor production in Pseudomonas aeruginosa. Curr. Microbiol. 2015, 71, 229–234. [Google Scholar]
- Al Ahmar, R.; Kirby, B.D.; Yua, H.D. Pyrimidine biosynthesis regulates the small-colony variant and mucoidy in Pseudomonas aeruginosa through sigma factor competition. J. Bacteriol. 2019, 201, e00575-18. [Google Scholar] [CrossRef]
- Domakonda, A.; West, T.P. Control of pyrimidine nucleotide formation in Pseudomonas aurantiaca. Arch. Microbiol. 2020, 202, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Bani Ahmad, A.; West, T.P. Regulation of the pyrimidine biosynthetic pathway in the bacterium Pseudomonas chlororaphis. In Pyrimidines and Their Importance; Ward, R.G., Ed.; Nova Press Inc.: Hauppauge, NY, USA, 2023; pp. 191–230. [Google Scholar]
- West, T.P. Control of pyrimidine synthesis in Pseudomonas fragi. Lett. Appl. Microbiol. 2002, 35, 380–384. [Google Scholar] [CrossRef]
- West, T.P. Regulation of pyrimidine formation in Pseudomonas lundensis. Can. J. Microbiol. 2009, 55, 261–268. [Google Scholar] [CrossRef]
- West, T.P. Regulation of pyrimidine nucleotide formation in Pseudomonas taetrolens ATCC 4683. Microbiol. Res. 2004, 159, 29–33. [Google Scholar] [CrossRef]
- Chu, C.P.; West, T.P. Pyrimidine biosynthetic pathway of Pseudomonas fluorescens. J. Gen. Microbiol. 1990, 136, 875–880. [Google Scholar] [CrossRef] [PubMed]
- West, T.P. Effect of carbon source on pyrimidine formation in Pseudomonas fluorescens ATCC 13525. Microbiol. Res. 2005, 160, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Gallie, J.; Libby, E.; Bertels, F.; Remigi, P.; Jendresen, C.B.; Desprat, N.; Buffing, M.F.; Sauer, U.; Beaumont, H.J.E.; Martinussen, J.; et al. Bistability in a metabolic network underpins the de novo evolution of colony switching in Pseudomonas fluorescens. PLoS Biol. 2015, 13, e1002109. [Google Scholar] [CrossRef] [PubMed]
- Murahari, E.C.; West, T.P. The pyrimidine biosynthetic pathway and its regulation in Pseudomonas jessenii. Antonie Leeuwenhoek 2019, 112, 461–469. [Google Scholar] [CrossRef]
- West, T.P. Regulation of the pyrimidine biosynthetic pathway in Pseudomonas mucidolens. Antonie Leeuwenhoek 2005, 88, 181–186. [Google Scholar] [CrossRef] [PubMed]
- West, T.P. Regulation of pyrimidine nucleotide formation in Pseudomonas reptilivora. Lett. Appl. Microbiol. 2004, 38, 81–86. [Google Scholar] [CrossRef]
- West, T.P. Regulation of pyrimidine nucleotide biosynthesis in Pseudomonas synxantha. Antonie Leeuwenhoek 2007, 92, 353–358. [Google Scholar] [CrossRef]
- West, T.P. Pyrimidine biosynthesis in Pseudomonas veronii and its regulation by pyrimidines. Microbiol. Res. 2012, 167, 306–310. [Google Scholar] [CrossRef]
- Condon, S.; Collins, J.K.; O’Donovan, G.A. Regulation of arginine and pyrimidine biosynthesis in Pseudomonas putida. J. Gen. Microbiol. 1976, 92, 375–383. [Google Scholar] [CrossRef]
- Santiago, M.F.; West, T.P. Control of pyrimidine formation in Pseudomonas putida ATCC 17536. Can. J. Microbiol. 2002, 48, 1076–1081. [Google Scholar] [CrossRef]
- Schurr, M.J.; Vickrey, J.F.; Kumar, A.P.; Campbell, A.L.; Cunin, R.; Benjamin, R.C.; Shanley, M.S.; O’Donovan, G.A. Aspartate transcarbamoylase genes of Pseudomonas putida: Requirement for an inactive dihydroorotase for assembly into the dodecameric holoenzyme. J. Bacteriol. 1995, 177, 1753–1759. [Google Scholar] [CrossRef]
- West, T.P. Control of pyrimidine nucleotide formation in Pseudomonas fulva. Antonie Leeuwenhoek 2010, 97, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Chunduru, J.; West, T.P. Pyrimidine nucleotide synthesis in the emerging pathogen Pseudomonas monteilii. Can. J. Microbiol. 2018, 64, 432–438. [Google Scholar] [CrossRef] [PubMed]
- West, T.P. Regulation of pyrimidine formation in Pseudomonas oryzihabitans. J. Basic Microbiol. 2007, 47, 440–443. [Google Scholar] [CrossRef]
- West, T.P. Effect of carbon source on pyrimidine biosynthesis in Pseudomonas oryzihabitans. J. Basic Microbiol. 2010, 50, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Yu, T.; Omata, S. Distribution of enzymes related to cytidine degradation in bacteria. Agric. Biol. Chem. 1976, 40, 1893–1895. [Google Scholar]
- Beck, D.A.; O’Donovan, G.A. Pathways of pyrimidine salvage in Pseudomonas and former Pseudomonas: Detection of recycling enzymes using high-performance liquid chromatography. Curr. Microbiol. 2008, 56, 162–167. [Google Scholar] [CrossRef]
- Shaposhnikov, L.A.; Savin, S.S.; Tishkov, V.I.; Pometun, A.A. Ribonucleoside hydrolases—Structure, functions, physiological role and practical uses. Biomolecules 2023, 13, 1375. [Google Scholar] [CrossRef]
- West, T.P. Degradation of pyrimidine ribonucleosides by Pseudomonas aeruginosa. Antonie Leeuwenhoek 1996, 69, 331–335. [Google Scholar] [CrossRef]
- West, T.P. Utilization of pyrimidine bases and nucleosides by the Pseudomonas stutzeri group. Microbios 1990, 61, 71–81. [Google Scholar]
- West, T.P. Pyrimidine base and ribonucleoside utilization by the Pseudomonas alcaligenes group. Antonie Leeuwenhoek 1991, 59, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Watanabe, T.; Chibata, I. Metabolism of pyrimidine nucleotides in a microorganism. III. Enzymatic production of ribose-5-phosphate from uridine-5′-monophosphate by Pseudomonas oleovorans. Appl. Microbiol. 1971, 22, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.S. Optimization of culture conditions for the production of pyrimidine nucleotide N-ribosidase from Pseudomonas oleovorans. J. Life Sci. 2004, 14, 608–613. [Google Scholar]
- Yu, T.S. Purification and characterization of pyrimidine nucleotide N-ribosidase from Pseudomonas oleovorans. J. Microbiol. Biotechnol. 2005, 15, 573–578. [Google Scholar]
- Gill, R.; West, T.P. Control of a pyrimidine ribonucleotide salvage pathway in Pseudomonas oleovorans. Arch. Microbiol. 2022, 204, 383. [Google Scholar] [CrossRef] [PubMed]
- Terada, M.; Tatibana, M.; Hayaishi, O. Purification and properties of nucleoside hydrolase from Pseudomonas fluorescens. J. Biol. Chem. 1967, 242, 5578–5585. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.P.; West, T.P. Pyrimidine ribonucleoside catabolism in Pseudomonas fluorescens biotype A. Antonie Leeuwenhoek 1990, 57, 253–257. [Google Scholar] [CrossRef]
- West, T.P. Metabolism of pyrimidine bases and nucleosides by Pseudomonas fluorescens biotype F. Microbios 1988, 56, 27–36. [Google Scholar]
- Liu, X.; Wood, P.L.; Parales, J.V.; Parales, R.E. Chemotaxis to pyrimidines and identification of a cytosine chemoreceptor in Pseudomonas putida. J. Bacteriol. 2009, 191, 2909–2916. [Google Scholar] [CrossRef]
- Parales, R.E.; Nesteryuk, V.; Hughes, J.G.; Luu, R.A.; Ditty, J.L. Cytosine chemoreceptor McpC in Pseudomonas putida F1 also detects nicotinic acid. Microbiology 2014, 160, 2661–2669. [Google Scholar] [CrossRef]
- West, T.P. Pyrimidine base catabolism in species of Pseudomonas and Burkholderia. Res. J. Microbiol. 2011, 6, 172–181. [Google Scholar] [CrossRef]
- Kim, S.; West, T.P. Pyrimidine catabolism in Pseudomonas aeruginosa. FEMS Microbiol. Lett. 1991, 77, 175–179. [Google Scholar] [CrossRef]
- West, T.P. Isolation and characterization of a dihydropyrimidine dehydrogenase mutant of Pseudomonas chlororaphis. Arch. Microbiol. 1991, 156, 513–516. [Google Scholar] [CrossRef]
- Santiago, M.F.; West, T.P. Effect of nitrogen source on pyrimidine catabolism by Pseudomonas fluorescens. Microbiol. Res. 1999, 154, 221–224. [Google Scholar] [CrossRef]
- West, T.P. Pyrimidine base catabolism in Pseudomonas putida biotype B. Antonie Leeuwenhoek 2001, 80, 163–167. [Google Scholar] [CrossRef]
- Hidese, R.; Mihara, H.; Kurihara, T.; Esaki, N. Pseudomonas putida PydR, a RutR-like transcriptional regulator, represses the dihydropyrimidine dehydrogenase gene in the pyrimidine reductive catabolic pathway. J. Biochem. 2012, 152, 341–346. [Google Scholar] [CrossRef]
- Matcher, G.F.; Jiwaji, M.; de la Mare, J.; Dorrington, R.A. Complex pathways for regulation of pyrimidine metabolism by carbon catabolite repression and quorum sensing in Pseudomonas putida RU-KM3s. Appl. Microbiol. Biotechnol. 2013, 97, 5993–6007. [Google Scholar] [CrossRef]
| Species | Effect of Uracil Addition on Carbon Source | Pyrimidine Limitation (2 h) on Carbon Source | Reference | ||
|---|---|---|---|---|---|
| Glucose | Succinate | Glucose | Succinate | ||
| P. aeruginosa | AT (0.7); DO (2.2); DD (1.0); OP (0.8); OD (0.7) | ND | AT (3.10); DO (0.4); OP (1.7); OD (1.5) | ND | [85] |
| P. alcaligenes | AT (0.6); DO (1.7); DD (1.0); OP (1.4); OD (1.4) | AT (1.4); DO (1.0); DD (1.1); OP (1.3); OD (1.1) | AT (1.6); DO (2.8); DD (0.7); OP (3.2); OD (3.4) | AT (1.0); DO (0.6); DD (0.7); OP (0.5); OD (1.5) | [86] |
| P. citronellolis | AT (0.6); DO (0.6); DD (1.0); OP (0.8); OD (0.9) | AT (0.5); DO (0.5); DD (0.8); OP (0.9); OD (0.8) | AT (0.3); DO (4.0); DD (6.3); OP (2.5) | AT (1.5); DO (2.1); DD (1.8); OP (2.4) | [87] |
| P. mendocina | AT (0.6); DO (0.7); DD (1.3); OP (0.6); OD (0.7) | AT (0.8); DO (2.2); DD (0.9); OP (0.8); OD (0.6) | AT (0.2); DO (0.8); DD (0.4); OP (3.6); OD (1.5) | AT (0.7); DO (0.6); DD (0.3); OP (0.5); OD (0.6) | [88,89] |
| P. nitroreducens | AT (0.8); DO (0.8); DD (0.6); OP (0.8); OD (0.7) | AT (0.3); DO (0.9); DD (0.3); OP (1.1); OD (0.5) | AT (1.2); DO (10.4); DD (6.0); OP (3.0); OD (1.4) | AT (5.1); DO (4.4); DD (1.8); OP (1.8); OD (1.4) | [90] |
| P. pseudoalcaligenes | ND | AT (0.7); DO (2.2); DD (1.0); OP (0.8); OD (0.7) | ND | AT (0.7); DO (2.2); DD (1.0); OP (0.8); OD (0.7) | [91] |
| P. oleovorans | AT (1.5); DO (2.2); DD (0.6); OP (0.4); OD (0.6) | AT (1.2); DO (2.4); DD (0.7); OP (1.2); OD (1.4) | AT (0.2); DO (4.4); DD (2.6); OP (5.0); OD (2.5) | AT (0.5); DO (0.8); DD (1.4); OP (1.0); OD (1.1) | [92] |
| P. resinovorans | AT (0.6); DO (0.7); DD (0.4); OP (1.0); OD (0.8) | AT (0.5); DO (0.9); DD (1.5); OP (1.0); OD (1.0) | AT (1.5); DO (2.7); DD (5.6); OP (2.2); OD (1.6) | AT (2.5); DO (9.0); DD (1.8); OP (2.5); OD (3.3) | [94] |
| Species | Effect of Uracil Addition on Carbon Source | Pyrimidine Limitation (2 h) on Carbon Source | Reference | ||
|---|---|---|---|---|---|
| Glucose | Succinate | Glucose | Succinate | ||
| P. aurantiaca | AT (1.1); DO (1.7); DD (0.8); OP (0.5); OD (0.7) | AT (0.4); DO (0.5); DD (0.5); OP (0.6); OD (0.4) | AT (0.7); DO (2.0); DD (1.0); OD (16.3) | AT (0.4); DO (1.1); DD (0.5); OD (4.2) | [97] |
| P. chloroaphis | AT (0.3); DO (0.8); DD (1.1); OP (1.9); OD (0.6) | AT (1.1); DO (0.6); DD (0.9); OP (0.8); OD (0.7) | AT (0.2); DO (0.2); DD (0.3); OP (0.6) | AT (5.9); DO (4.5); DD (12.6); OP (1.9) | [98] |
| P. fragi | AT (0.7); DO (0.8); DD (1.5); OP (1.2); OD (1.1) | ND | AT (1.2); DO (2.3); DD (2.5); OP (2.0) | ND | [99] |
| P. lundensis | AT (0.8); DO (0.8); DD (0.7); OP (0.9); OD (1.5) | AT (1.2); DO (1.1); DD (0.4); OP (1.5); OD (1.4) | AT (1.4); DO (3.1); DD (3.1); OP (1.6); OD (4.4) | AT (0.4); DO (1.2); DD (2.0); OP (7.0); OD (3.2) | [100] |
| P. taetrolens | ND | AT (1.2); DO (0.6); DD (0.8); OP (1.0); OD (0.9) | ND | AT (0.7); DO (2.3); DD (0.9); OP (1.6) | [101] |
| Species | Effect of Uracil Addition on Carbon Source | Pyrimidine Limitation (2 h) on Carbon Source | Reference | ||
|---|---|---|---|---|---|
| Glucose | Succinate | Glucose | Succinate | ||
| P. fluorescens | AT (0.8); DO (1.0); DD (1.5); OP (1.2); OD (0.7) | AT (0.9); DO (1.2); DD (1.2); OP (0.5); OD (0.8) | AT (1.2); DO (1.3); OP (0.4); OD (0.9) OD (1.4) | AT (1.0); DO (0.5); OP (0.4); OD (14.0) OD (2.4) | [103] |
| P. jessenii | AT (0.8); DO (1.0); DD (0.9); OP (0.7); OD (0.9) | AT (0.4); DO (0.5); DD (0.4); OP (1.0); OD (0.6) | AT (1.9); DO (1.9); DD (0.8); OP (0.7); OD (1.4) | AT (1.9); DO (2.2); DD (0.8); OP (2.2); OD (1.2) | [105] |
| P. mucidolens | AT (0.7); DO (1.0); DD (0.6); OP (0.6); OD (0.6) | AT (0.9); DO (1.4); DD (1.1); OP (1.3); OD (1.7) | AT (5.8); DO (3.2); DD (2.3); OP (1.4); OD (2.7) | AT (1.0); DO (2.8); DD (2.9); OP (1.4); OD (1.5) | [106] |
| P. reptilivora | AT (1.0); DO (2.8); DD (1.1); OP (0.8); OD (0.9) | AT (0.5); DO (2.4); DD (0.6); OP (1.0); OD (0.8) | DO (5.0); DD (0.7) OP (1.7); OD (2.1) | DO (6.4); DD (0.5) OP (2.5); OD (2.2) | [107] |
| P. synxantha | AT (0.9); DO (1.7); DD (0.9); OP (1.2); OD (0.5) | AT (0.9); DO (1.0); DD (0.3); OP (01.1); OD (0.5) | AT (0.4); DO (0.8); DD (1.3); OP (1.0); OD (1.8) | AT (1.2); DO (2.8); DD (1.6); OP (0.6); OD (1.6) | [108] |
| P. veronii | AT (0.7); DO (0.5); DD (0.9); OP (0.8); OD (1.1) | AT (1.1); DO (1.3); DD (1.3); OP (1.0); OD (2.8) | AT (2.0); DO (2.2); DD (2.4); OP (2.0) | AT (1.3); DO (1.8); DD (1.2); OP (1.4) | [109] |
| Species | Effect of Uracil Addition on Carbon Source | Pyrimidine Limitation (2 h) on Carbon Source | Reference | ||
|---|---|---|---|---|---|
| Glucose | Succinate | Glucose | Succinate | ||
| P. putida | AT (0.7); DO (0.6); DD (0.4); OP (0.7); OD (0.6) | AT (0.2); DO (1.4); DD (1.5); OP (1.3); OD (0.8) | AT (0.4); DO (1.0); DD (0.1); OD (1.8) | AT (0.3); DO (0.6); DD (0.6); OD (0.6) | [111] |
| P. fulva | AT (1.0); DO (0.6); DD (1.0); OP (0.5); OD (0.7) | AT (1.1); DO (0.7); DD (0.5); OP (1.6); OD (1.0) | AT (2.4); DO (5.6); DD (2.6); OP (2.0) | AT (1.0); DO (1.5); DD (0.7); OP (1.3) | [113] |
| P. monteilii | AT (0.7); DO (1.3); DD (0.6); OP (0.4); OD (0.4) | AT (0.2); DO (0.5); DD (0.8); OP (0.7); OD (0.4) | AT (2.4); DO (2.1); DD (4.4); OD (7.3) | AT (1.9); DO (1.7); DD (3.0); OD (3.7) | [114] |
| P. oryzihabitans | AT (1.1); DO (1.0); DD (0.8); OP (0.8); OD (1.0) | AT (1.2); DO (0.9); DD (1.5); OP (1.3); OD (1.2) | AT (0.5); DO (1.1); DD (1.6); OP (1.1) | AT (3.2); DO (3.1); DD (1.3); OP (1.3) | [115,116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
West, T.P. Pyrimidine Biosynthesis and Ribonucleoside Metabolism in Species of Pseudomonas. Fermentation 2023, 9, 955. https://doi.org/10.3390/fermentation9110955
West TP. Pyrimidine Biosynthesis and Ribonucleoside Metabolism in Species of Pseudomonas. Fermentation. 2023; 9(11):955. https://doi.org/10.3390/fermentation9110955
Chicago/Turabian StyleWest, Thomas P. 2023. "Pyrimidine Biosynthesis and Ribonucleoside Metabolism in Species of Pseudomonas" Fermentation 9, no. 11: 955. https://doi.org/10.3390/fermentation9110955
APA StyleWest, T. P. (2023). Pyrimidine Biosynthesis and Ribonucleoside Metabolism in Species of Pseudomonas. Fermentation, 9(11), 955. https://doi.org/10.3390/fermentation9110955







