Traditional Fermented Foods: Challenges, Sources, and Health Benefits of Fatty Acids
Abstract
1. Introduction
2. Characteristic and Physiological Influence of Dietary Fatty Acids
2.1. Fatty acid Characteristics
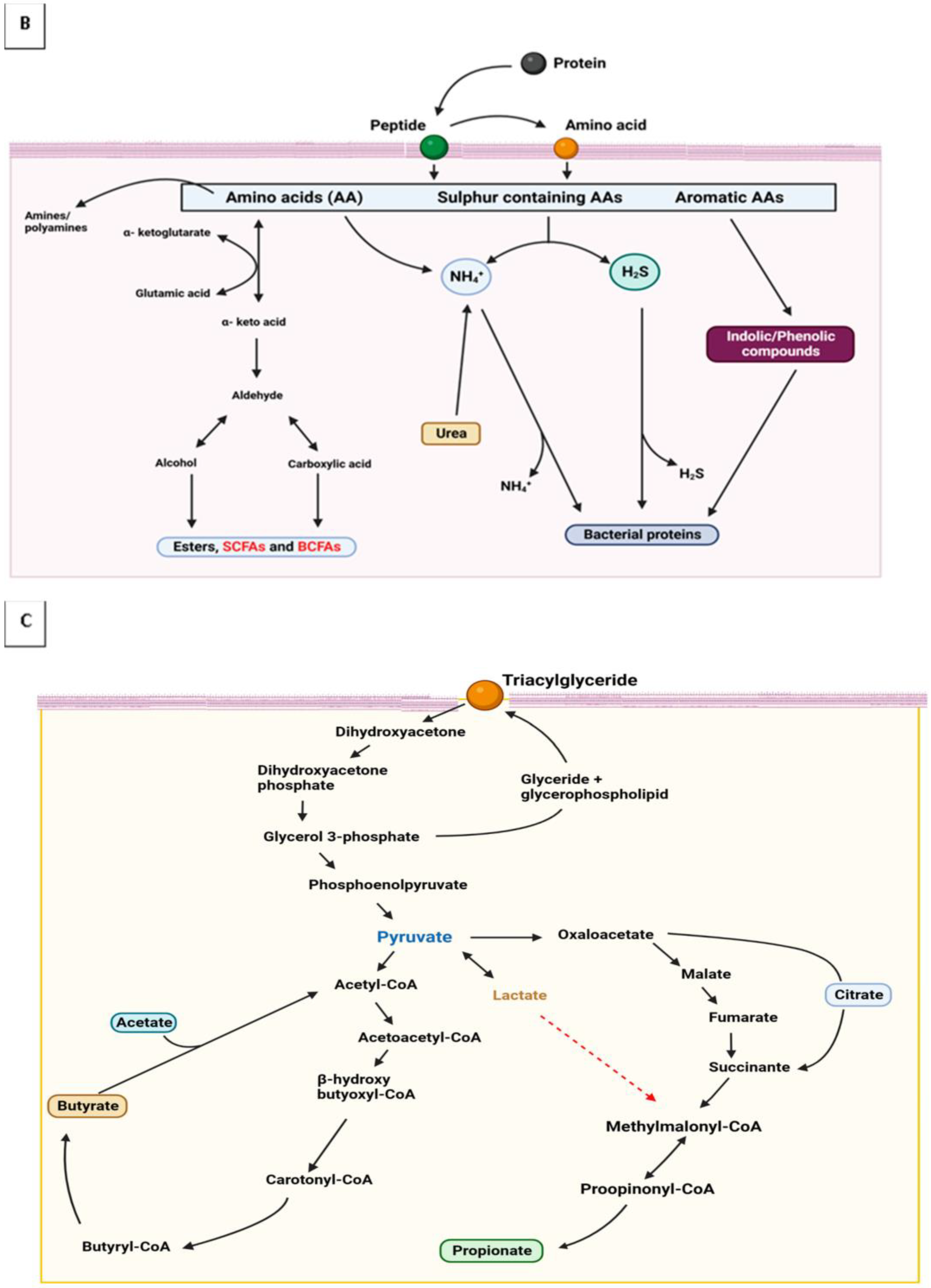
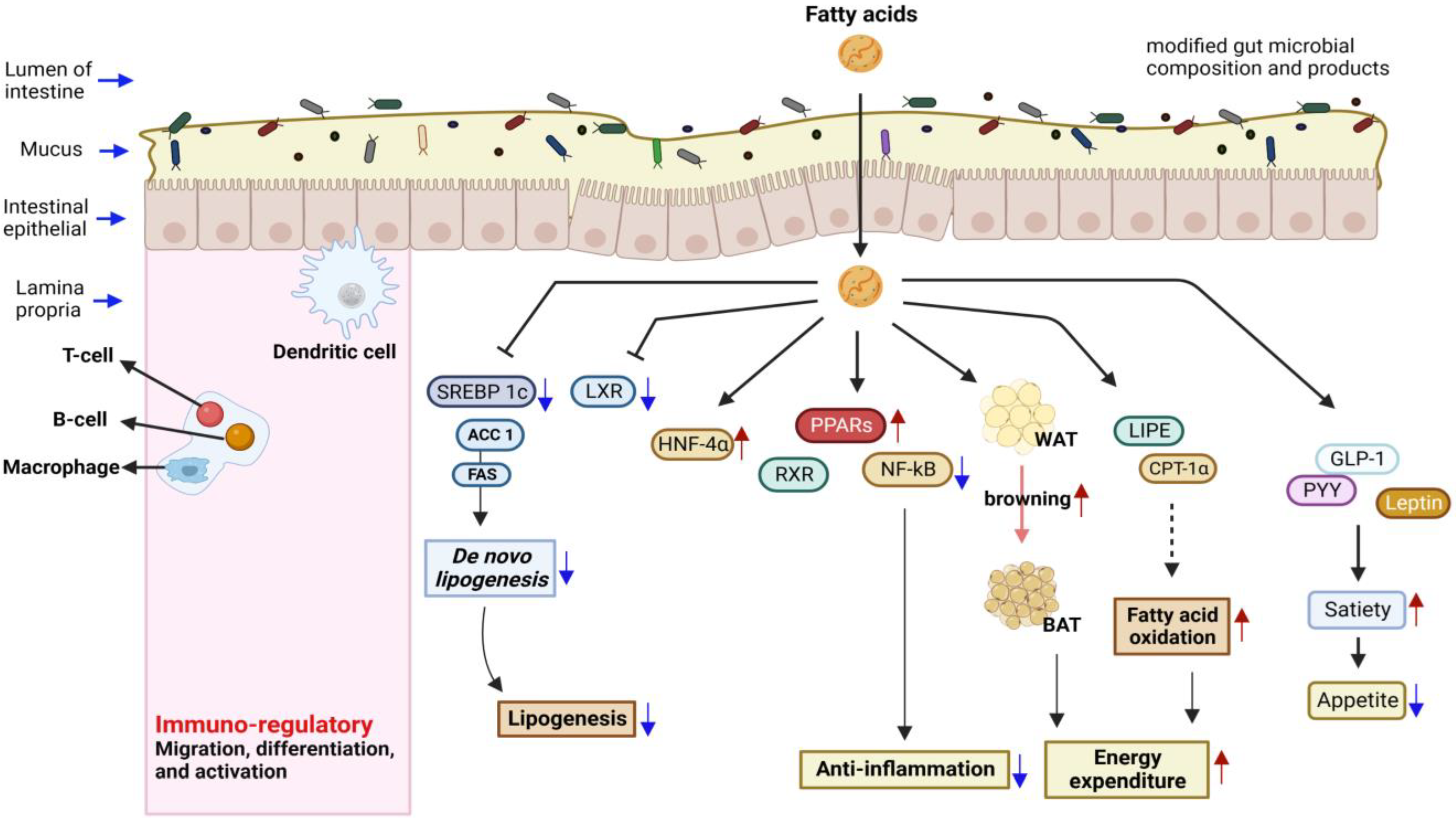
2.2. SCFAs’ Functions and Signaling Patterns
2.3. Fatty Acid Mediators: Gene Expression and Antimicrobial Activity
3. Health Benefits of Dietary Fatty Acids
4. Challenges with Traditional Fermented Foods
5. Regulations and Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial Fermentation and Its Role in Quality Improvement of Fermented Foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Chilton, S.N.; Burton, J.P.; Reid, G. Inclusion of Fermented Foods in Food Guides around the World. Nutrients 2015, 7, 390–404. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut Microbiota Functions: Metabolism of Nutrients and Other Food Components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Feng, R.; Chen, L.; Chen, K. Fermentation Trip: Amazing Microbes, Amazing Metabolisms. Ann. Microbiol. 2018, 68, 717–729. [Google Scholar] [CrossRef]
- Mgbechidinma, C.L.; Adegoke, C.O.; Ogunbanwo, S.T. Lactic Acid Bacteria as Bioactive Potential Against Selected Resistance Candida Species and Pathogenic Bacteria. Int. J. Pharm. Biol. Sci. Arch. 2020, 8, 19–32. [Google Scholar] [CrossRef]
- Bhutia, Y.D.; Ganapathy, V. Short, but Smart: SCFAs Train T Cells in the Gut to Fight Autoimmunity in the Brain. Immunity 2015, 43, 629–631. [Google Scholar] [CrossRef] [PubMed]
- Leeuwendaal, N.K.; Stanton, C.; O’Toole, P.W.; Beresford, T.P. Fermented Foods and the Gut Microbiome. Nutrients 2022, 14, 1527. [Google Scholar] [CrossRef]
- Etok, C.; Akan, O.; Adegoke, A. Bioremediation of Crude Oil Contaminated Soils Using Surfactants and Hydrocarbonoclastic Bacteria. Br. Microbiol. Res. J. 2015, 9, 1–6. [Google Scholar] [CrossRef]
- Mgbechidinma, C.L.; Zheng, G.; Baguya, E.B.; Zhou, H.; Okon, S.U.; Zhang, C. Fatty acid composition and nutritional analysis of waste crude fish oil obtained by optimized milder extraction methods. Environ Eng Res. 2023, 28, 22003. [Google Scholar] [CrossRef]
- Tamang, J.P.; Cotter, P.D.; Endo, A.; Han, N.S.; Kort, R.; Liu, S.Q.; Mayo, B.; Westerik, N.; Hutkins, R. Fermented Foods in a Global Age: East Meets West. Compr. Rev. Food Sci. Food Saf. 2020, 19, 184–217. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Devi, M.K.; Kumar, P.S. Engineering Microbes for Enhancing the Degradation of Environmental Pollutants: A Detailed Review on Synthetic Biology. Environ. Res. 2022, 214, 113868. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; Filannino, P.; Gobbetti, M. Microbial Metabolic Pathways and the “fermented Plant Foods—Human Health” Axis. Foods 2021, 10, 1105. [Google Scholar] [CrossRef] [PubMed]
- Lensch, A.; Duwenig, E.; Dederer, H.G.; Kärenlampi, S.O.; Custers, R.; Borg, A.; Wyss, M. Recombinant DNA in Fermentation Products Is of No Regulatory Relevance. Food Control 2022, 141, 109170. [Google Scholar] [CrossRef]
- Vogel, R.F.; Hammes, W.P.; Habermeyer, M.; Engel, K.; Knorr, D.; Eisenbrand, G. Microbial Food Cultures–Opinion of the Senate Commission on Food Safety (SKLM) of the German Research Foundation (DFG). Mol. Nutr. Food Res. 2011, 55, 654–662. [Google Scholar] [CrossRef]
- Bell, V.; Ferrão, J.; Fernandes, T. Nutritional Guidelines and Fermented Food Frameworks. Foods 2017, 6, 65. [Google Scholar] [CrossRef]
- Nai, C.; Meyer, V. From Axenic to Mixed Cultures: Technological Advances Accelerating a Paradigm Shift in Microbiology. Trends Microbiol. 2018, 26, 538–554. [Google Scholar] [CrossRef]
- Obafemi, Y.D.; Oranusi, S.U.; Ajanaku, K.O.; Akinduti, P.A.; Leech, J.; Cotter, P.D. African Fermented Foods: Overview, Emerging Benefits, and Novel Approaches to Microbiome Profiling. NPJ Sci. Food 2022, 6, 15. [Google Scholar] [CrossRef]
- Olovo, C.; Udoekong, N.; Akan, O. Precision Nutrition, Diet and Gut-Microbiota in Obesity. J. Biotechnol. Bioresearch 2021, 2, 4. [Google Scholar]
- Hesselmar, B.; Hicke-roberts, A.; Wennergren, G. Allergy in Children in Hand Versus Machine Dishwashing. Pediatrics 2015, 135, e590-7. [Google Scholar] [CrossRef]
- Marco, M.L.; Sanders, M.E.; Gänzle, M.; Arrieta, M.C.; Cotter, P.D.; De Vuyst, L.; Hill, C.; Holzapfel, W.; Lebeer, S.; Merenstein, D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on Fermented Foods. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 196–208. [Google Scholar] [CrossRef]
- Mallick, R.; Basak, S.; Duttaroy, A.K. Fatty Acids and Evolving Roles of Their Proteins in Neurological, Cardiovascular Disorders and Cancers. Prog. Lipid Res. 2021, 83, 101116. [Google Scholar] [CrossRef]
- Haghikia, A.; Jörg, S.; Duscha, A.; Berg, J.; Manzel, A.; Waschbisch, A.; Hammer, A.; Lee, D.H.; May, C.; Wilck, N.; et al. Dietary Fatty Acids Directly Impact Central Nervous System Autoimmunity via the Small Intestine. Immunity 2015, 43, 817–829. [Google Scholar] [CrossRef]
- Ferreri, C.; Masi, A.; Sansone, A.; Giacometti, G.; Larocca, A.V.; Menounou, G.; Scanferlato, R.; Tortorella, S.; Rota, D.; Conti, M.; et al. Fatty Acids in Membranes as Homeostatic, Metabolic and Nutritional Biomarkers: Recent Advancements in Analytics and Diagnostics. Diagnostics 2017, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Suryanarayana, L.C.; Chandrashekara, K.A.; Krishnan, P.; Kush, A.; Ravikumar, P. Lactobacillus Plantarum Mediated Fermentation of Psidium Guajava, L. Fruit Extract. J. Biosci. Bioeng. 2015, 119, 430–432. [Google Scholar] [CrossRef]
- Jia, R.; Chen, H.; Chen, H.; Ding, W. Effects of Fermentation with Lactobacillus Rhamnosus GG on Product Quality and Fatty Acids of Goat Milk Yogurt. J. Dairy Sci. 2016, 99, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.H.; Yang, Y.; Wang, Z.; Lawrence, P.; Worobo, R.W.; Brenna, J.T. High Levels of Branched Chain Fatty Acids in Nātto and Other Asian Fermented Foods. Food Chem. 2019, 286, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Mao, B.; Guo, W.; Huang, Z.; Tang, X.; Zhang, Q.; Yang, B.; Zhao, J.; Cui, S.; Zhang, H. Production of Conjugated Fatty Acids in Probiotic-Fermented Walnut Milk with the Addition of Lipase. LWT 2022, 172, 114204. [Google Scholar] [CrossRef]
- Erbaş, M.; Kemal Uslu, M.; Ozgun Erbaş, M.; Certel, M. Effects of Fermentation and Storage on the Organic and Fatty Acid Contents of Tarhana, a Turkish Fermented Cereal Food. J. Food Compos. Anal. 2006, 19, 294–301. [Google Scholar] [CrossRef]
- Asarat, M.; Apostolopoulos, V.; Vasiljevic, T.; Donkor, O. Short-Chain Fatty Acids Produced by Synbiotic Mixtures in Skim Milk Differentially Regulate Proliferation and Cytokine Production in Peripheral Blood Mononuclear Cells. Int. J. Food Sci. Nutr. 2015, 66, 755–765. [Google Scholar] [CrossRef]
- Annunziata, G.; Tenore, G.C.; Ciampaglia, R.; Schisano, C.; Narciso, V.; Maisto, M.; Novellino, E. Short-Time Lactic-Acid Fermentation Improves the Nutraceutical Value of Black Tea Beverage. In Proceedings of the CHIMALI 2018, Italian Food Chemistry Congress, Camerino, Italy, 24–27 September 2018; p. 1. [Google Scholar]
- Uțoiu, E.; Matei, F.; Toma, A.; Diguță, C.F.; Ștefan, L.M.; Mănoiu, S.; Vrăjmașu, V.V.; Moraru, I.; Oancea, A.; Israel-Roming, F.; et al. Bee Collected Pollen with Enhanced Health Benefits, Produced by Fermentation with a Kombucha Consortium. Nutrients 2018, 10, 1365. [Google Scholar] [CrossRef]
- Hu, R.; Zeng, F.; Wu, L.; Wan, X.; Chen, Y.; Zhang, J.; Liu, B. Fermented Carrot Juice Attenuates Type 2 Diabetes by Mediating Gut Microbiota in Rats. Food Funct. 2019, 10, 2935–2946. [Google Scholar] [CrossRef]
- Olšovská, J.; Vrzal, T.; Štěrba, K.; Slabý, M.; Kubizniaková, P.; Čejka, P. The Chemical Profiling of Fatty Acids during the Brewing Process. J. Sci. Food Agric. 2019, 99, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Rawls, J.F. Feeling the Burn: Intestinal Epithelial Cells Modify Their Lipid Metabolism in Response to Bacterial Fermentation Products. Cell Host Microbe 2020, 27, 314–316. [Google Scholar] [CrossRef] [PubMed]
- Roopashree, P.G.; Shetty, S.S.; Suchetha Kumari, N. Effect of Medium Chain Fatty Acid in Human Health and Disease. J. Funct. Foods 2021, 87, 104724. [Google Scholar] [CrossRef]
- Kimura, I.; Ichimura, A.; Ohue-Kitano, R.; Igarashi, M. Free Fatty Acid Receptors in Health and Disease. Physiol. Rev. 2020, 100, 171–210. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Vasu, R.; Zhang, H. The Role of Long-Chain Fatty Acids in Inflammatory Bowel Disease. Mediators Inflamm. 2019, 2019, 8495913. [Google Scholar] [CrossRef]
- Hidalgo, M.A.; Carretta, M.D.; Burgos, R.A. Long Chain Fatty Acids as Modulators of Immune Cells Function: Contribution of FFA1 and FFA4 Receptors. Front. Physiol. 2021, 12, 668330. [Google Scholar] [CrossRef]
- Marrocco, F.; Delli Carpini, M.; Garofalo, S.; Giampaoli, O.; De Felice, E.; Di Castro, M.A.; Maggi, L.; Scavizzi, F.; Raspa, M.; Marini, F.; et al. Short-Chain Fatty Acids Promote the Effect of Environmental Signals on the Gut Microbiome and Metabolome in Mice. Commun. Biol. 2022, 5, 517. [Google Scholar] [CrossRef]
- Venn-Watson, S.K.; Butterworth, C.N. Broader and Safer Clinically-Relevant Activities of Pentadecanoic Acid Compared to Omega-3: Evaluation of an Emerging Essential Fatty Acid across Twelve Primary Human Cell-Based Disease Systems. PLoS ONE 2022, 17, e0268778. [Google Scholar] [CrossRef]
- Weitkunat, K.; Schumann, S.; Nickel, D.; Hornemann, S.; Petzke, K.J.; Schulze, M.B.; Pfeiffer, A.F.H.; Klaus, S. Odd-Chain Fatty Acids as a Biomarker for Dietary Fiber Intake: A Novel Pathway for Endogenous Production from Propionate. Am. J. Clin. Nutr. 2017, 105, 1544–1551. [Google Scholar] [CrossRef]
- Miguel, A.S.; Salgado, M.T.; Rodriguez, M.S.M.; Pachon, J.; Martin, M.A.S.; Lobatoa, P.C.; Pastor, M.R. Role of Butyric Acid in Food and Intestinal Health. Immunol. Infect. 2018, 1, 1–5. [Google Scholar]
- Stachowska, E.; Wisniewska, M.; Dziezyc, A.; Bohatyrewicz, A. Could the Use of Butyric Acid Have a Positive Effect on Microbiota and Treatment of Type 2 Diabetes? Eur. Rev. Med. Pharm. Sci. 2021, 25, 4570–4578. [Google Scholar]
- Coppola, S.; Avagliano, C.; Calignano, A.; Berni Canani, R. The Protective Role of Butyrate against Obesity and Obesity-Related Diseases. Molecules 2021, 26, 682. [Google Scholar] [CrossRef] [PubMed]
- Tengeler, A.C.; Gart, E.; Wiesmann, M.; Arnoldussen, I.A.C.; van Duyvenvoorde, W.; Hoogstad, M.; Dederen, P.J.; Verweij, V.; Geenen, B.; Kozicz, T.; et al. Propionic Acid and Not Caproic Acid, Attenuates Nonalcoholic Steatohepatitis and Improves (Cerebro) Vascular Functions in Obese Ldlr−/−. Leiden Mice. FASEB J. 2020, 34, 9575–9593. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, A.; Baskaran, S.A.; Amalaradjou, M.A.R.; Venkitanarayanan, K. Anticarcinogenic Properties of Medium Chain Fatty Acids on Human Colorectal, Skin and Breast Cancer Cells in Vitro. Int. J. Mol. Sci. 2015, 16, 5014–5027. [Google Scholar] [CrossRef] [PubMed]
- Lemarié, F.; Beauchamp, E.; Legrand, P.; Rioux, V. Revisiting the Metabolism and Physiological Functions of Caprylic Acid (C8:0) with Special Focus on Ghrelin Octanoylation. Biochimie 2016, 120, 40–48. [Google Scholar] [CrossRef]
- Hismiogullari, S.E.; Elyurek, E.; Hismiogullari, A.A.; Sahin, F.; Basalan, M.; Yenice, S. Effects of Caproic and Caprylic Acids on Microbial Growth and Cytotoxicity. J. Anim. Vet. Adv. 2008, 7, 731–735. [Google Scholar]
- Jain, S.; Rai, R.; Singh, D.; Vohora, D. Octanoic Acid a Major Component of Widely Consumed Medium-Chain Triglyceride Ketogenic Diet Is Detrimental to Bone. Sci. Rep. 2021, 11, 7003. [Google Scholar] [CrossRef]
- Shoji, H.; Kunugi, H.; Miyakawa, T. Acute and Chronic Effects of Oral Administration of a Medium-Chain Fatty Acid, Capric Acid, on Locomotor Activity and Anxiety-like and Depression-Related Behaviors in Adult Male C57BL/6J Mice. Neuropsychopharmacol. Reports 2022, 42, 59–69. [Google Scholar] [CrossRef]
- Mett, J.; Müller, U. The Medium-Chain Fatty Acid Decanoic Acid Reduces Oxidative Stress Levels in Neuroblastoma Cells. Sci. Rep. 2021, 11, 6135. [Google Scholar] [CrossRef]
- Damiano, F.; De Benedetto, G.E.; Longo, S.; Giannotti, L.; Fico, D.; Siculella, L.; Giudetti, A.M. Decanoic Acid and Not Octanoic Acid Stimulates Fatty Acid Synthesis in U87MG Glioblastoma Cells: A Metabolomics Study. Front. Neurosci. 2020, 14, 783. [Google Scholar] [CrossRef]
- Warren, E.C.; Dooves, S.; Lugarà, E.; Damstra-Oddy, J.; Schaf, J.; Heine, V.M.; Walker, M.C.; Williams, R.S.B. Decanoic Acid Inhibits MTORC1 Activity Independent of Glucose and Insulin Signaling. Proc. Natl. Acad. Sci. USA 2020, 117, 23617–23625. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Zhang, J.; Xu, B.; Tegomo, A.F.; Sagada, G.; Zheng, L.; Wang, L.; Id, Q.S. Effect of Dietary Supplementation of Lauric Acid on Growth Performance, Antioxidative Capacity, Intestinal Development and Gut Microbiota on Black Sea Bream (Acanthopagrus Schlegelii). PLoS ONE 2022, 17, e0262427. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.T.; Chen, J.W.; Rathod, J.; Jiang, Y.Z.; Tsai, P.J.; Hung, Y.P.; Ko, W.C.; Paredes-Sabja, D.; Huang, I.H. Lauric Acid Is an Inhibitor of Clostridium Difficile Growth in Vitro and Reduces Inflammation in a Mouse Infection Model. Front. Microbiol. 2018, 8, 2635. [Google Scholar] [CrossRef]
- Uday-Kumar, D.; Christopher, V.; Sobarani, D.; Nagendra-Sastry, Y. Lauric Acid as Potential Natural Product in the Treatment of Cardiovascular Disease: A Review. J. Bioanal. Biomed. 2014, 6, 37–39. [Google Scholar] [CrossRef]
- Grünert, S.C.; Wendel, U.; Lindner, M.; Leichsenring, M.; Schwab, K.O.; Vockley, J.; Lehnert, W.; Ensenauer, R. Clinical and Neurocognitive Outcome in Symptomatic Isovaleric Acidemia. Orphanet J. Rare Dis. 2012, 7, 9. [Google Scholar] [CrossRef]
- Szymanska, E.; Jezela-Stanek, A.; Bogdanska, A.; Rokicki, D.; Emczynska-Seliga, E.E.; Pajdowska, M.; Ciara, E.; Tylki-Szymanska, A. Long Term Follow-up of Polish Patients with Isovaleric Aciduria. Clinical and Molecular Delineation of Isovaleric Aciduria. Diagnostics 2020, 10, 738. [Google Scholar] [CrossRef]
- Netto Cândido, T.L.; da Silva, L.E.; Cândido, F.G.; Valente, F.X.; da Silva, J.S.; Gomes Lopes, D.R.; do Carmo Gouveia Peluzio, M.; Mantovani, H.C.; de Cássia Gonçalves Alfenas, R. Effect of the Ingestion of Vegetable Oils Associated with Energy-Restricted Normofat Diet on Intestinal Microbiota and Permeability in Overweight Women. Food Res. Int. 2021, 139, 109951. [Google Scholar] [CrossRef]
- Cho, K.M.; Kim, Y.S.; Lee, M.; Lee, H.Y.; Bae, Y.S. Isovaleric Acid Ameliorates Ovariectomy-Induced Osteoporosis by Inhibiting Osteoclast Differentiation. J. Cell. Mol. Med. 2021, 25, 4287–4297. [Google Scholar] [CrossRef]
- Lee, S.Y.; Rhee, M.S.; Dougherty, R.H.; Kang, D.H. Antagonistic Effect of Acetic Acid and Salt for Inactivating Escherichia Coli O157: H7 in Cucumber Puree. J. Appl. Microbiol. 2010, 108, 1361–1368. [Google Scholar] [CrossRef]
- Valdes, D.S.; So, D.; Gill, P.A.; Kellow, N.J. Effect of Dietary Acetic Acid Supplementation. J. Acad. Nutr. Diet. 2021, 121, 895–914. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Fan, X.; Lu, Y.; Chen, D.; Zhao, Y.; Qi, K. Dietary Acetic Acid Suppress High-Fat Diet-Induced Obesity in Mice by Altering Taurine Conjugated Bile Acids Metabolism. Curr. Res. Food Sci. 2022, 5, 1976–1984. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Meng, L.; Ai, D.; Hou, N.; Li, H.; Shuai, X.; Peng, X. Acetic Acid Alleviates the Inflammatory Response and Liver Injury in Septic Mice by Increasing the Expression of TRIM40. Exp. Ther. Med. 2019, 17, 2789–2798. [Google Scholar] [CrossRef]
- Hernández, M.A.G.; Canfora, E.E.; Jocken, J.W.E.; Blaak, E.E. The Short-Chain Fatty Acid Acetate in Body Weight Control and Insulin Sensitivity. Nutrients 2019, 11, 1943. [Google Scholar] [CrossRef]
- Al-Lahham, S.H.; Peppelenbosch, M.P.; Roelofsen, H.; Vonk, R.J.; Venema, K. Biological Effects of Propionic Acid in Humans; Metabolism, Potential Applications and Underlying Mechanisms. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2010, 1801, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Duscha, A.; Gisevius, B.; Hirschberg, S.; Linker, R.A.; Gold, R.; Haghikia, A.; Duscha, A.; Gisevius, B.; Hirschberg, S.; Yissachar, N.; et al. Propionic Acid Shapes the Multiple Sclerosis Disease Course by an Immunomodulatory Mechanism. Cell 2020, 180, 1067–1080. [Google Scholar] [CrossRef]
- Adler, G.K.; Hornik, E.S.; Murray, G.; Bhandari, S.; Yadav, Y.; Heydarpour, M.; Basu, R.; Garg, R.; Tirosh, A. Acute Effects of the Food Preservative Propionic Acid on Glucose Metabolism in Humans. BMJ Open Diabetes Res. Care 2021, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Al-lahham, S.; Roelofsen, H.; Rezaee, F.; Weening, D.; Hoek, A.; Vonk, R. Propionic Acid Affects Immune Status and Metabolism in Adipose Tissue from Overweight Subjects. Eur. J. Clin. Investig. 2012, 42, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Rioux, V.; Catheline, D.; Bouriel, M.; Legrand, P. Dietary Myristic Acid at Physiologically Relevant Levels Increases the Tissue Content of C20: 5 n-3 and C20: 3 n-6 in the Rat. Reprod. Nutr. Dev. 2005, 45, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.-S.; Shu, G.; Xie, Q.-P.; Zhu, X.-T.; Gao, P.; Zhou, G.-X.; Wang, S.; Wang, L.-N.; Xi, Q.Y.; Zhang, Y.-L.; et al. Myristic Acid (MA) Promotes Adipogenic Gene Expression and the Differentiation of Porcine Intramuscular Adipocyte Precursor Cells. J. Integr. Agric. 2014, 13, 2488–2499. [Google Scholar] [CrossRef]
- Olivieri, O.; Speziali, G.; Castagna, A.; Pattini, P.; Udali, S.; Pizzolo, F.; Liesinger, L.; Gindlhuber, J.; Tomin, T.; Schittmayer, M.; et al. The Positive Association between Plasma Myristic Acid and ApoCIII Concentrations in Cardiovascular Disease Patients Is Supported by the Effects of Myristic Acid in HepG2 Cells. J. Nutr. 2020, 150, 2707–2715. [Google Scholar] [CrossRef]
- de Souza, J.; Strieder-Barboza, C.; Contreras, G.A.; Lock, A.L. Effects of Timing of Palmitic Acid Supplementation during Early Lactation on Nutrient Digestibility, Energy Balance, and Metabolism of Dairy Cows. J. Dairy Sci. 2019, 102, 274–287. [Google Scholar] [CrossRef] [PubMed]
- Palomer, X.; Pizarro-delgado, J.; Barroso, E.; Vázquez-carrera, M. Palmitic and Oleic Acid: The Yin and Yang of Fatty Acids in Type 2 Diabetes Mellitus. Trends Endocrinol. Metab. 2018, 29, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Zhong, Y.; Zhou, S.; Li, Q.Q. Palmitic Acid-Induced Apoptosis in Pancreatic β-Cells Is Increased by Liver X Receptor Agonist and Attenuated by Eicosapentaenoate. In Vivo 2011, 25, 711–718. [Google Scholar] [PubMed]
- Senyilmaz-Tiebe, D.; Pfaff, D.H.; Virtue, S.; Schwarz, K.V.; Fleming, T.; Altamura, S.; Muckenthaler, M.U.; Okun, J.G.; Vidal-puig, A.; Nawroth, P.; et al. Dietary Stearic Acid Regulates Mitochondria in Vivo in Humans. Nat. Commun. 2018, 9, 3129. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Zhao, X.; Siegal, G.P.; Desmond, R.; Hardy, R.W. Dietary Stearic Acid Leads to a Reduction of Visceral Adipose Tissue in Athymic Nude Mice. PLoS ONE 2014, 9, e104083. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Nill, K.; Takechi-Haraya, Y.; Playford, M.P.; Nguyen, D.; Yu, Z.; Pryor, M.; Tang, J.; Rojulpote, K.V.; Mehta, N.N.; et al. Differential Effect of Dietary Supplementation with a Soybean Oil Enriched in Oleic Acid versus Linoleic Acid on Plasma Lipids and Atherosclerosis in LDLR-Deficient Mice. Int. J. Mol. Sci. 2022, 23, 8385. [Google Scholar] [CrossRef]
- Carrillo, C.; Cavia, M.M.; Alonso-Torre, S. Role of Oleic Acid in Immune System: Mechanism of Action, a Review. Nutr. Hosp. 2012, 27, 978–990. [Google Scholar] [CrossRef]
- Schuldt, L.; Brandenstein, K.; Jacobs, C.; Symmank, J. Oleic Acid-Related Anti-Inflammatory Effects in Force-Stressed PdL Fibroblasts Are Mediated by H3 Lysine Acetylation Associated with Altered IL10 Expression. Epigenetics 2022, 17, 1892–1904. [Google Scholar] [CrossRef]
- Teres, S.; Barcelo-Coblijn, G.; Benet, M.; Alvarez, R.; Bressani, R.; Halver, J.E.; Escriba, P.V. Oleic Acid Content Is Responsible for the Reduction in Blood Pressure Induced by Olive Oil. Proc. Natl. Acad. Sci. USA 2008, 105, 13811–13816. [Google Scholar] [CrossRef]
- Farvid, M.S.; Ding, M.; Pan, A.; Sun, Q.; Chiuve, S.E.; Steffen, L.M.; Willett, W.C.; Hu, F.B. Epidemiology and Prevention Dietary Linoleic Acid and Risk of Coronary Heart Disease: A Systematic Review and Meta-Analysis of Prospective. Circulation 2014, 130, 1568–1578. [Google Scholar] [CrossRef]
- Zhao, J.V.; Schooling, M.C. Effect of Linoleic Acid on Ischemic Heart Disease and Its Risk Factors: A Mendelian Randomization Study. BMC Med. 2019, 17, 61. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.Y.; Lemaitre, R.N.; King, I.B.; Song, X.; Psaty, B.M.; Siscovick, D.S.; Mozaffarian, D. And Total and Cause-Specific Mortality. Circulation 2014, 130, 1245–1253. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The Omega-6/Omega-3 Fatty Acid Ratio: Health Implications. Nutrition-Sante 2010, 17, 267–275. [Google Scholar] [CrossRef]
- Bemelmans, W.J.E.; Muskiet, F.A.J.; Feskens, E.J.M.; Vries, J.H.M.; Broer, J.; May, J.F.; Jong, B.M. Associations of Alpha-Linolenic Acid and Linoleic Acid with Risk Factors for Coronary Heart Disease. Eur. J. Clin. Nutr. 2000, 54, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.Y.; Kwon, H.H.; Hong, J.S.; Yoon, J.Y.; Park, M.S.; Jang, M.Y.; Suh, D.H. Effect of Dietary Supplementation with Omega-3 Fatty Acid and Gamma-Linolenic Acid on Acne Vulgaris: A Randomised, Double- Blind, Controlled Trial. Investig. Rep. 2014, 94, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Gara-Ali, M.; Zili, F.; Hosni, K.; Ben Ouada, H.; Ben-Mahrez, K. Lipophilic Extracts of the Thermophilic Cyanobacterium Leptolyngbya Sp. and Chlorophyte Graesiella Sp. and Their Potential Use as Food and Anticancer Agents. Algal Res. 2021, 60, 102511. [Google Scholar] [CrossRef]
- Romero, L.O.; Caires, R.; Nickolls, A.R.; Chesler, A.T.; Cordero-Morales, J.F.; Vásquez, V. A Dietary Fatty Acid Counteracts Neuronal Mechanical Sensitization. Nat. Commun. 2020, 11, 2997. [Google Scholar] [CrossRef] [PubMed]
- Venn-Watson, S.; Lumpkin, R.; Dennis, E.A. Efficacy of Dietary Odd-Chain Saturated Fatty Acid Pentadecanoic Acid Parallels Broad Associated Health Benefits in Humans: Could It Be Essential? Sci. Rep. 2020, 10, 8161. [Google Scholar] [CrossRef]
- Bao-To, N.; Nguyen, Y.T.-K.; Moon, J.Y.; Ediriweera, M.K.; Cho, S.K. Pentadecanoic Acid, an Odd-Chain Fatty Acid, Suppresses the Stemness of MCF-7/SC Human Breast Cancer Stem-Like Cells through JAK2/STAT3 Signaling. Nutrients 2020, 12, 1663. [Google Scholar] [CrossRef]
- Araujo, B.C.; Skrzynska, A.K.; Marques, V.H.; Tinajero, A.; Rio-Zaragoza, O.B.; Viana, M.T.; Mata-Sotres, J.A. Dietary Arachidonic Acid (20:4n-6) Levels and Its Effect on Growth Performance, Fatty Acid Profile, Gene Expression for Lipid Metabolism, and Health Status of Juvenile California Yellowtail (Seriiola Dorsalis). Fishes 2022, 7, 18. [Google Scholar] [CrossRef]
- Sueyasu, T.; Morita, S.; Tokuda, H.; Kaneda, Y.; Rogi, T.; Shibata, H. Dietary Arachidonic Acid Improves Age-Related Excessive Enhancement of the Stress Response. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 2110–2119. [Google Scholar]
- Sambra, V.; Echeverria, F.; Valenzuela, A.; Chouinard-Watkins, R.; Valenzuela, R. Docosahexaenoic and Arachidonic Acids as Neuroprotective Nutrients throughout the Life Cycle. Nutrients 2021, 13, 986. [Google Scholar] [CrossRef] [PubMed]
- Xu, E.; Chen, C.; Fu, J.; Zhu, L.; Shu, J.; Jin, M.; Wang, Y.; Zong, X. Dietary Fatty Acids in Gut Health: Absorption, Metabolism and Function. Anim. Nutr. 2021, 7, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Functional Roles of Fatty Acids and Their Effects on Human Health. J. Parenter. Enter. Nutr. 2015, 39, 18S–32S. [Google Scholar] [CrossRef] [PubMed]
- Okeke, E.S.; Ita, R.E.; Egong, E.J.; Udofia, L.E.; Mgbechidinma, C.L.; Akan, O.D. Metaproteomics Insights into Fermented Fish and Vegetable Products and Associated Microbes. Food Chem. Mol. Sci. 2021, 3, 100045. [Google Scholar] [CrossRef]
- Parvez, S.; Malik, K.A.; Kang, S.A.; Kim, H. Probiotics and Their Fermented Food Products Are Beneficial for Health. J. Appl. Microbiol. 2006, 100, 1171–1185. [Google Scholar] [CrossRef]
- Running, C.A.; Mattes, R.D. Different Oral Sensitivities to and Sensations of Short-, Medium-, and Long-Chain Fatty Acids in Humans. Am. J. Physiol. Gastrointestine Liver Physiol. 2014, 307, 381–389. [Google Scholar] [CrossRef]
- Venegas, D.P.; De La Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Ota, S.; Sakuraba, H. Uptake and Advanced Therapy of Butyrate in Inflammatory Bowel Disease. Immuno 2022, 2, 692–702. [Google Scholar]
- Byndloss, M.X.; Olsan, E.E.; Rivera-chávez, F.; Tiffany, C.R.; Cevallos, S.A.; Lokken, K.L.; Torres, T.P.; Byndloss, A.J.; Faber, F.; Gao, Y.; et al. Microbiota-Activated PPAR-γ-Signaling Inhibits Dysbiotic Enterobacteriaceae Expansion Mariana. Science 2017, 357, 570–575. [Google Scholar] [CrossRef]
- Fellows, R.; Denizot, J.; Stellato, C.; Cuomo, A.; Jain, P.; Stoyanova, E.; Balázsi, S.; Hajnády, Z.; Liebert, A.; Kazakevych, J.; et al. Microbiota Derived Short Chain Fatty Acids Promote Histone Crotonylation in the Colon through Histone Deacetylases. Nat. Commun. 2018, 9, 105. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef] [PubMed]
- Luu, M.; Riester, Z.; Baldrich, A.; Reichardt, N.; Yuille, S.; Busetti, A.; Klein, M.; Wempe, A.; Leister, H.; Raifer, H.; et al. Microbial Short-Chain Fatty Acids Modulate CD8+ T Cell Responses and Improve Adoptive Immunotherapy for Cancer. Nat. Commun. 2021, 12, 4077. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Liu, M.; Lan, Y.; Sun, H.; Mai, K.; Wan, M. Short-Chain Fatty Acids Promote Intracellular Bactericidal Activity in Head Kidney Macrophages from Turbot (Scophthalmus maximus L.) via Hypoxia Inducible Factor-1α. Front. Immunol. 2020, 11, 615536. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, F.; Wu, W.; Sun, M.; Bilotta, A.J.; Yao, S.; Xiao, Y.; Huang, X.; Eaves-Pyles, T.D.; Golovko, G.; et al. GPR43 Mediates Microbiota Metabolite SCFA Regulation of Antimicrobial Peptide Expression in Intestinal Epithelial Cells via Activation of MTOR and STAT3. Mucosal Immunol. 2018, 11, 752–762. [Google Scholar] [CrossRef]
- Zang, J.; Yu, D.; Zhang, P.; Xu, Y.; Xia, W. The Key Enzymes and Flavor Precursors Involved in Formation of Characteristic Flavor Compounds of Low-Salt Fermented Common Carp (Cyprinus carpio L.). LWT 2022, 154, 112806. [Google Scholar] [CrossRef]
- Maleki, N.; Eiteman, M.A. Recent Progress in the Microbial Production of Pyruvic Acid. Fermentation 2017, 3, 8. [Google Scholar] [CrossRef]
- Voidarou, C.; Antoniadou, M.; Rozos, G.; Tzora, A.; Skoufos, I.; Varzakas, T.; Lagiou, A.; Bezirtzoglou, E. Fermentative Foods: Microbiology, Biochemistry, Potential Human Health Benefits and Public Health Issues. Foods 2021, 10, 69. [Google Scholar]
- Pegorier, J.-P.; May, C.L.; Girard, J. Control of Gene Expression by Fatty Acids. J. Nutr. 2004, 134, 2444S–2449S. [Google Scholar] [CrossRef]
- Bravo-Ruiz, I.; Medina, M.Á.; Martínez-Poveda, B. From Food to Genes: Transcriptional Regulation of Metabolism by Lipids and Carbohydrates. Nutrients 2021, 13, 1513. [Google Scholar] [CrossRef] [PubMed]
- Cano-flores, A.; Gómez, J.; Escalona-Torres, I.S.; Velasco-Bejarano, B. Microorganisms as Biocatalysts and Enzyme Sources. In Microorganisms; Blumenberg, M., Shaaban, M., Elgaml, A., Eds.; IntechOpen: London, UK, 2020; pp. 1–33. [Google Scholar]
- Raspor, P.; Goranovic, D. Biotechnological Applications of Acetic Acid Bacteria. Crit. Rev. Biotechnol. 2008, 28, 101–124. [Google Scholar] [CrossRef]
- Gravel, D.; Bell, T.; Barbera, C.; Bouvier, T.; Pommier, T.; Venail, P.; Mouquet, N. Experimental Niche Evolution Alters the Strength of the Diversity–Productivity Relationship. Nature 2011, 469, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Isbell, F.; Cowles, J.M. Biodiversity and Ecosystem Functioning. Rev. Adv. 2014, 45, 471–493. [Google Scholar] [CrossRef]
- Mgbechidinma, C.L.; Akan, O.D.; Zhang, C.; Huang, M.; Linus, N.; Zhu, H.; Wakil, S.M. Integration of Green Economy Concepts for Sustainable Biosurfactant Production—A Review. Bioresour. Technol. 2022, 364, 128021. [Google Scholar] [CrossRef] [PubMed]
- Borchert, E.; Hammerschmidt, K.; Hentschel, U.; Deines, P. Enhancing Microbial Pollutant Degradation by Integrating Eco-Evolutionary Principles with Environmental Biotechnology. Trends Microbiol. 2021, 29, 908–918. [Google Scholar] [CrossRef]
- De Filippis, F.; Parente, E.; Ercolini, D. Recent Past, Present, and Future of the Food Microbiome. Annu. Rev. Food Sci. Technol. 2018, 9, 589–608. [Google Scholar] [CrossRef]
- Oguntoyinbo, F.A. Safety Challenges Associated with Traditional Foods of West Africa. Food Rev. Int. 2014, 30, 338–358. [Google Scholar] [CrossRef]
- Rossi, L.P.R.; Almeida, R.C.C.; Lopes, L.S.; Figueiredo, A.C.L.; Ramos, M.P.P.; Almeida, P.F. Occurrence of Listeria spp. in Brazilian Fresh Sausage and Control of Listeria Monocytogenes Using Bacteriophage P100. Food Control 2011, 22, 954–958. [Google Scholar] [CrossRef]
- Galimberti, A.; Bruno, A.; Agostinetto, G.; Casiraghi, M.; Guzzetti, L.; Labra, M. Fermented Food Products in the Era of Globalization: Tradition Meets Biotechnology Innovations. Curr. Opin. Biotechnol. 2021, 70, 36–41. [Google Scholar] [CrossRef]
- Ramos-Lopez, O.; Alfredo, M.; Milagro, F. Holistic Integration of Omics Tools for Precision Nutrition in Health and Disease. Nutrients 2022, 14, 74. [Google Scholar] [CrossRef] [PubMed]
- Rezac, S.; Kok, C.R.; Heermann, M.; Hutkins, R. Fermented Foods as a Dietary Source of Live Organisms. Front. Microbiol. 2018, 9, 1785. [Google Scholar] [CrossRef] [PubMed]
- Arias-Sánchez, F.I.; Vessman, B.; Mitri, S. Artificially Selecting Microbial Communities: If We Can Breed Dogs, Why Not Microbiomes? PLoS Biol. 2019, 17, e3000356. [Google Scholar] [CrossRef]
- Srinivas, M.; Sullivan, O.O.; Cotter, P.D.; Van Sinderen, D.; Kenny, J.G. The Application of Metagenomics to Study Microbial Communities and Develop Desirable Traits in Fermented Foods. Foods 2022, 11, 3297. [Google Scholar] [CrossRef]
- Laulund, S.; Wind, A.; Derkx, P.M.F.; Zuliani, V. Regulatory and Safety Requirements for Food Cultures. Microorganisms 2017, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Gómez-Sala, B.; O’Connor, E.M.; Kenny, J.G.; Cotter, P.D. Global Regulatory Frameworks for Fermented Foods: A Review. Front. Nutr. 2022, 9, 902642. [Google Scholar] [CrossRef]
- Hu, Z.; Li, M.; Cao, Y.; Akan, O.D.; Guo, T.; Luo, F. Targeting AMPK Signaling by Dietary Polyphenols in Cancer Prevention. Mol. Nutr. Food Res. 2022, 66, e2100732. [Google Scholar] [CrossRef]
- Akan, O.D.; Qin, D.; Guo, T.; Lin, Q.; Luo, F. Sirtfoods: New Concept Foods, Functions, and Mechanisms. Foods 2022, 11, 2955. [Google Scholar] [CrossRef]

| Fermented Food Source | Microbes Involved | Fatty Acid | Reference |
|---|---|---|---|
| Tarhana | Yogurt bacteria and baker’s yeast | Butyric acid 4.6 ± 1.1. | Erbas et al. [28] |
| Guava fruit | Lactobacillus plantarum | Butyrate 17.85 ± 0.68 ng/100 mL; caproate 62.03 ± 0.55 ng/100 mL; caprylate 34.93 ± 0.62 ng/100 mL; caprate 6.97 ± 0.52 ng/100 mL; and laurate 17.97 ± 0.51 ng/100 mL. | Bhat et al. [24] |
| Skim milk | Lactobacilli and Bifidobacteria | B. animalis subsp. lactis + 5% w/v inulin: acetic acid 2.72 mM; propionic acid 0.92 mM; and butyric acid 0.41 mM. B. animalis subsp. lactis + 5% w/v hi-maize: acetic acid 2.11 mM; propionic acid 0.68 mM; and butyric acid 0.24 mM. L. rhamnosus GG + 5% w/v inulin: acetic acid 2.56 mM; propionic acid 0.85 mM; and butyric acid 0.34 mM. L. rhamnosus GG + 5% w/v hi-maize: acetic acid 2.58 mM; propionic acid 0.70 mM; and butyric acid 0.25 mM. | Asarat et al. [29] |
| Goat milk | Lactobacillus rhamnosus GG | Butyric acid 0.75 ± 0.002. | Jia et al. [25] |
| Tea | Lactobacillus spp. | Butyric acid after 180 min – L. Plantarum (1.277 ± 0.024 µg/g); L. acidophilus (1.561 ± 0.033 µg/g); L. rhamnosus (1.014 ± 0.053 µg/g); L. bulgaricus (2.2880 ± 0.031 µg/g). | Annunziata et al. [30] |
| Kombucha with pollen | Kombucha/SCOBY (symbiotic culture of bacteria and yeasts) consortium | Kombucha alone: acetic acid 4.46 ± 0.025 g/L; propionic acid 0.24 ± 0.016 g/L; and butyrate 0.30 ± 0.021 g/L. Kombucha + pollen: acetic acid 3.51 ± 0.11 g/L; propionic acid 0.56 ± 0.041 g/L; and butyrate 1.78 ± 0.054 g/L. | Uțoiu et al. [31] |
| Carrot juice | Lactobacillus rhamnosus GG | Acetic acid 0.42 ± 0.05 mg/mL; propionic acid 0.72 ± 0.09 mg/mL; and butyric acid 0.95 ± 0.09 mg/mL | Hu et al. [32] |
| Beer wort | Yeast | 2.3–8.1 mg/L of butyric, isobutyric, isovaleric, caprylic, and caproic acid. | Olsovska et al. [33] |
| Goat milk | Lactobacillus rhamnosus GG | Lauric acid 3.06 ± 0.06. | Jia et al. [25] |
| Tarhana (traditional Turkish fermented food) | Yogurt bacteria and baker’s yeast | Myristic acid 16.4 ± 1.4; myristoleic acid 0.5 ± 0.0; palmitic acid 40.1 ± 1.0; stearic acid 25.0 ± 1.6; oleic acid 12.7 ± 0.91; and linoleic acid 0.9 ± 0.2. | Erbas et al. [28] |
| Goat milk | Lactobacillus rhamnosus GG | Palmitic acid 24.35 ± 0.01; margaric acid 1.39 ± 0.02; stearic acid 0.23 ± 0.007; C18:1 9.174 ± 0.04; and C18:2 18.04 ± 0.05. | Jia et al. [25] |
| Nātto | NA | Myristic acid 0.05 ± 0.10; pentadecylic acid 0.15 ± 0.08; palmitic acid 0.17 ± 0.12; margaric acid 0.14 ± 0.07; stearic acid <0.003; arachidic acid <0.003; and lignoceric acid <0.003. | Wang et al. [26] |
| Shrimp paste | NA | Myristic acid 0.02 ± 0.01; pentadecylic acid 0.14 ± 0.05; palmitic acid 0.11 ± 0.08; margaric acid 0.50 ± 0.25; stearic acid 0.25 ± 0.10; arachidic acid 0.05 ± 0.07; and lignoceric acid 0.36 ± 0.13. | Wang et al. [26] |
| Fish sauce liquid | NA | Myristic acid <0.003; pentadecylic acid 0.12 ± 0.01; palmitic acid 0.07 ± 0.00; margaric acid 0.09 ± 0.02; stearic acid 0.18 ± 0.02; arachidic acid <0.003; and lignoceric acid <0.003. | Wang et al. [26] |
| Fish sauce (paste-like) | NA | Myristic acid <0.003; pentadecylic acid 0.14 ± 0.00; palmitic acid 0.07 ± 0.00; margaric acid 0.28 ± 0.01; stearic acid 0.11 ± 0.02; arachidic acid <0.003; and lignoceric acid <0.003. | Wang et al. [26] |
| Miso | NA | Myristic acid <0.003; pentadecylic acid 0.20 ± 0.00; palmitic acid 0.04 ± 0.00; margaric acid <0.003; stearic acid 0.04 ± 0.02; arachidic acid <0.003; and lignoceric acid <0.003. | Wang et al. [26] |
| Kimchi | NA | Myristic acid 0.06 ± 0.01; pentadecylic acid 0.04 ± 0.01; palmitic acid 0.12 ± 0.01; margaric acid 0.08 ± 0.00; stearic acid 0.06 ± 0.02; arachidic acid <0.003; and lignoceric acid <0.003. | Wang et al. [26] |
| Douchi | NA | Myristic acid <0.003; pentadecylic acid 0.01 ± 0.01; palmitic acid 0.01 ± 0.00; margaric acid 0.02 ± 0.00; stearic acid <0.003; arachidic acid <0.003; and lignoceric acid <0.003. | Wang et al. [26] |
| Walnut milk | L. plantarum ZS2058, L. casei FZSSZ3-L1, L. rhamnosus JSWX-3-L-2 and B. breve CCFM683 | linoleic acid 63.80 ± 0.08; and linolenic acid 14.70 ± 0.08. | Mao et al. [27] |
| Fatty Acid | Health Benefits | Reference |
|---|---|---|
| Butyric acid (SCFAs) | The main energy source for colonocytes. Stimulates the absorption of sodium and water in the colon. Stimulates the eubiosis of gut microbiota. Induces trophic action in intestinal cells. Anti-obesogenic effect. Inhibits inflammation and carcinogenesis. Promotes colonic defense barrier. Promotes satiety. | Miguel et al. [42] Stachowska et al. [43] Coppola et al. [44] |
| Caproic acid (hexanoic) (MCFAs) | Reverted HFD-induced visual and auditory cortex impairment. Reduced cancer cell viability from 70% to 90% (p < 0.05) compared to controls through by down-regulating cell cycle regulatory genes and up-regulating genes involved in apoptosis. | Tengeler et al. [45] Narayanan et al. [46] |
| Caprylic acid (octanoic) (MCFAs) | The energy source for patients suffering from pancreatic. insufficiency, impaired lymphatic chylomicron transport, and fat malabsorption. Neuroprotective potential against neurodegenerative disorders. Acylates ghrelin (peptide hormone with an orexigenic effect). Anti-microbial (bacteria, viruses, and fungi). Reduces bone weight | Lamarie et al. [47] Hismiogullari et al. [48]. Jian et al. [49] |
| Capric acid (decanoic) (MCFAs) | Energy source. Ameliorates neuropsychiatric disorders. Reduces oxidative stress levels in neuroblastoma cells. Neuroprotective. Suppresses mTORC1 activity independent of glucose and insulin signaling. | Shoji et al. [50] Mett and Muller [51] Dimiano et al. [52] Warrem et al. [53] |
| Lauric acid (dodecanoic) (MCFAs) | Modulated gut microbial composition. Increased anti-oxidative capacity. Inhibited Clostridium difficile growth in vitro. lowers the concentration of very low-density lipoprotein cholesterol (VLDL-c) and elevates total HDL cholesterol in human blood and tissues. | Ullah et al. [54] Yang et al. [55] Uday-Kumar et al. [56] |
| Isovaleric acid (Classical organic acidemias) | Improved neurologic and cognitive outcome. The predominant fatty acid end product of microbial origin is produced from the metabolism of leucine. The branched short-chain fatty acids can have effects on adipocyte lipid and glucose metabolism that can contribute to improved insulin sensitivity in individuals with disturbed metabolism. Ameliorates ovariectomy-induced osteoporosis by inhibiting osteoclast differentiation. | Grünert et al. [57] Szymanska et al. [58] Netto Candido et al. [59] Cho et al. [60] |
| Acetic acid (SCFAs) | Reduced plasma triacylglycerol (TAG) and fasting blood glucose (FBG) concentrations in individuals with type 2 diabetes. Reduced TAG levels in people who are overweight or obese. Restored bile acid homeostasis and suppress hepatic bile acid production. Inhibits intestinal-liver farnesoid X receptor (FXR)-fibroblast growth factor 15 (FGF15)-FGF receptor 4(FGFR4) signaling pathway. Anti-microbial and extended shelf life of cucumber puree (5 to 25oC). Upregulated the expression of TRIM40 which down-regulated TLR expression. Body weight and appetite regulation. | Lee et al. [61] Valdes et al. [62] Wang et al. [63] Yang et al. [64] Hernández et al. [65] |
| Propionic acid (SCFAs) | Reduces HFD-induced body weight gain and systolic pressure. Reversed HFD-induced visual and auditory cortex impairment. Increases glucose transporter type 1-positive cerebral blood vessels. Regulates adipokine production in human adipose tissue. Exacts anti-obesogenic effects through GPCR41 and 43, activates PPARγ and inhibits NF-κB. Altered gut microbiome composition and restored Treg cell/TH17 imbalance. Extends food shelf life, and increases glucagon, norepinephrine, and insulin resistance in humans. Activates the insulin-counterregulatory hormonal network. Down-regulated several inflammatory cytokines and chemokines (TNF-α and CCL5). Increased the expression of lipoprotein lipase and GLUT4, associated with lipogenesis and glucose uptake, respectively. | Tengeler et al. [45] Al-Lahham et al. [66] Duscha et al. [67] Adler et al. [68] Al-Lahham et al. [69] |
| Myristic acid (MCFAs) | High doses increased tissue levels of palmitic acid in rats. Regulates the biosynthesis and metabolism of highly unsaturated fatty acids. Enhanced intramuscular fat content in pork. Increased the expression of peroxisome proliferator-activated receptor-γ (PPARγ) and adipose-related genes, such as glucose transporter 1 (GLUT1), lipoprotein lipase (LPL), adipocyte fatty acid binding protein 4 (FABP4/aP2), fatty acid translocase (FAT), acetyl-CoA carboxylase α (ACCα), adipose triglyceride lipase (ATGL), and fatty acid synthase (FASN) in pigs. Increased plasma TG and ApoCIII concentrations. | Rioux et al. [70] Lu et al. [71] Olivieri et al. [72] |
| Palmitic acid | Increased milk energy output in cows (but disturbed energy balance). Attenuates the insulin signaling pathway through various mechanisms leading to insulin resistance. Induced apoptosis in β-cells is enhanced by T0901317 via the activation of LXRs and is blocked by EPA via the inhibition. Promoted cellular death, inhibiting of SREBP-1c, induced the expression of p27KIP1, transforming growth factor beta 1, and SMAD3 proteins in INS-1 cells. | de Souza et al. [73] Palomer et al. [74] Liang et al. [75] |
| Stearic acid | Influences mitochondrial morphology and functions. Reduces cardiovascular and cancer risk. Reduced serum glucose and increased monocyte chemotactic protein-1 (MCP-1). Induced apoptosis and cytotoxicity in preadipocytes through increased caspase-3 activity, increased Bax gene expression, and decreased cellular inhibitor of apoptosis protein-2 (cIAP2). | Senyilmaz-Tiebe et al. [76] Shen et al. [77] |
| Oleic acid | Suppressed atherosclerotic plaque size in LDLR-KO mice. Decreased the ratio of n-6/n-3 PUFA in the liver. Induces anti-inflammatory activities via inhibited histone acetyltransferases and compressed HPdLFs- increased H3Kac to IL 10 promoter regions. Induced anti-obesogenic effect-reduced body, liver, and epididymal fat weights, and reduced serum triglyceride and leptin levels. Down-regulated mRNA expression of lipogenic genes, proinflammatory cytokines, and upregulated lipid oxidation in the liver. Protected against colon structure damage by increasing the tight junction protein expression, increased Bifidobacteria, and reduced Enterobacteriaceae. Reduces blood pressure by regulating membrane lipid structure (HII phase propensity) via the G protein-mediated signaling | Yang et al. [78] Carrillo et al. [79] Schuldt et al. [80] Teres et al. [81] |
| Linoleic acid | Reduced plasma lipid and cholesterol levels through the regulation of cholesterol metabolism gene. Inversely correlated with cardiovascular disease risk and serum triacylglycerol. Benefits diabetes, lowers total and LDL cholesterols. Regulates the 5-LO pathway in anti-carcinogenesis and anti-tumour activities. Regulates fatty acid metabolism and inflammation via the COX-2 pathway. Contains AA and DHA which are essential for brain development and function. | Yang et al. [78] Farvid et al. [82] Zhao and Schooling [83] Wu et al. [84] Simopoulos [85] Bemelmans et al. [86] Jung et al. [87] |
| Margaric acid | Constitutes a major part of saturated fatty acids dominated in Graesiella sp. with good nutritional properties. Decreases sensory neurons’ mechanical excitability by Inhibiting PIEZO2 channels. Dietary fatty acid counteracts neuronal mechanical sensitization. | Gara-Ali et al. [88] Romero et al. [89] |
| pentadecanoic acid (OCFAs) | Lowers mortality and attenuates inflammation, anaemia, dyslipidaemia, and fibrosis in vivo, potentially by binding to key metabolic regulators and repairing mitochondrial function. Suppressed interleukin-6 (IL-6)-induced JAK2/STAT3 signaling, induced cell cycle arrest at the sub-G1 phase, and promoted caspase-dependent apoptosis in MCF-7/SC. Decreased risk of type 2 diabetes. | Venn-Watson et al. [90] Venn-Watson and Butterworth [40] Bao-To et al. [91] Weitkunat et al. [41] |
| Arachidic acid | Influenced liver and muscle fatty acid profiles, suppressed fatty acid synthase (FAS), and proliferator-activated receptor alpha (PPAR-α) expression. Reduced blood cortisol and glucose via eicosanoids synthesis gene expression and lipid metabolic pathways. Suppressed age-related excessive enhancement of the HPA axis responsiveness. Attenuated age-related reduction in GR translocation into the nucleus in the hippocampus after stress loading. Compose approximately 25% of brain grey matter. Induces neuronal growth and differentiation through the modulation of the physical properties of neuronal membranes, signal transduction associated with G proteins, and gene expression. | Araujo et al. [92] Sueyasu et al. [93] Sambra et al. [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, Y.; Huang, M.; Olovo, C.V.; Mgbechidinma, C.L.; Yang, Y.; Liu, J.; Li, B.; Zhu, M.; Yu, K.; Zhu, H.; et al. Traditional Fermented Foods: Challenges, Sources, and Health Benefits of Fatty Acids. Fermentation 2023, 9, 110. https://doi.org/10.3390/fermentation9020110
Xing Y, Huang M, Olovo CV, Mgbechidinma CL, Yang Y, Liu J, Li B, Zhu M, Yu K, Zhu H, et al. Traditional Fermented Foods: Challenges, Sources, and Health Benefits of Fatty Acids. Fermentation. 2023; 9(2):110. https://doi.org/10.3390/fermentation9020110
Chicago/Turabian StyleXing, Yanxia, Mengzhen Huang, Chinasa V. Olovo, Chiamaka L. Mgbechidinma, Yu Yang, Jing Liu, Bo Li, Mengliu Zhu, Kexue Yu, He Zhu, and et al. 2023. "Traditional Fermented Foods: Challenges, Sources, and Health Benefits of Fatty Acids" Fermentation 9, no. 2: 110. https://doi.org/10.3390/fermentation9020110
APA StyleXing, Y., Huang, M., Olovo, C. V., Mgbechidinma, C. L., Yang, Y., Liu, J., Li, B., Zhu, M., Yu, K., Zhu, H., Yao, X., Bo, L., & Akan, O. D. (2023). Traditional Fermented Foods: Challenges, Sources, and Health Benefits of Fatty Acids. Fermentation, 9(2), 110. https://doi.org/10.3390/fermentation9020110







