Influence of Heat Treatment and Solid-State Fermentation on the Lignocellulosic Fractions of Substrates Supporting Lentinula edodes (Berk.) Pegler Cultivation: Implications for Commercial Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of the Materials
2.2. Substrate Preparation and Heat Treatment
2.3. Cropping Trial
2.4. Sampling and Analysis of the Lignocellulosic Fractions
2.5. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Yield Performance of the Shiitake Strains
3.2. Degradation of the Lignocellulosic Components of Substrate
3.3. Correlation between Yield Parameters and Substrate Utilization Pattern
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Royse, D.J.; Baars, J.; Qi, T. Current overview of mushroom production in the world. In Edible and Medicinal Mushrooms: Technology and Applications; Zied, D.C., Ed.; John Wiley & Sons: New York, NY, USA, 2017. [Google Scholar]
- Singh, M.; Kamal, S.; Sharma, V.P. Species and Region-wise Mushroom Production in Leading Mushroom Producing Countries-China, Japan, USA, Canada and India. Mushroom Res. 2021, 30, 99–108. [Google Scholar] [CrossRef]
- Chihara, G. Medicinal aspects of lentinan isolated from Lentinus edodes (Berk.) Sing. In Mushroom Biology and Mushroom Products, Proceedings of the First International Conference on Mushroom Biology and Mushroom Products, 23–26 August 1993, Hong Kong, China; Chang, S.T., Buswell, J.A., Chiu, S.W., Eds.; The Chinese University of Hong Kong Press: Hong Kong, China, 1993; pp. 261–266. [Google Scholar]
- Wasser, S.; Weis, A. Medicinal properties of substances occurring in higher basidiomycetes mushrooms: Current perspectives. Int. J. Med. Mushrooms 1999, 1, 31–62. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.P.; Annepu, S.K. Advancement in medicinal mushroom research. In New Age Herbals; Springer: Singapore, 2018; pp. 151–162. [Google Scholar] [CrossRef]
- Luo, X. Progress of xiang-gu (shiitake) cultivation in China. In Science and Cultivation of Edible and Medicinal Fungi; Rinker, D.L., Royse, D.J., Eds.; Penn State University Press: University Park, PA, USA, 2004; pp. 317–322. [Google Scholar]
- Royse, D.J. Effect of spawn run time and substrate nutrition on yield and size of the shiitake mushroom. Mycologia 1985, 77, 756–762. [Google Scholar] [CrossRef]
- Miller, M.W.; Jong, S.C. Commercial cultivation of shiitake sawdust filled plastic bags. In Cultivating Edible Fungi: Proceeding of the International Symposiums on Scientific and Technical Aspects of Cultivating Edible Fungi, State College, PA, USA, 15–17 July 1986; Wuest, P.J., Royse, D.J., Beelman, R.B., Eds.; Elsevier: Amsterdam, The Netherlands, 1987; pp. 421–426. [Google Scholar]
- Royse, D.J.; Bahler, B.D. Yield and size of shiitake as influenced by synthetic log diameter and genotype. Mushroom J. Trop. 1989, 9, 109–113. [Google Scholar]
- Delpech, P.; Olivier, J.M. Cultivation of shiitake on straw-based pasteurized substrates. Mushroom Sci. 1991, 13, 523–528. [Google Scholar]
- Rinker, D.L. The influence of heat treatment, genotype and other cultural practices on the production of shiitake mushrooms on sawdust. In Science and Cultivation of Edible Fungi; Maher, M.J., Ed.; MushWorld: Seoul, Republic of Korea, 1991; pp. 497–502. [Google Scholar]
- Mata, G.; Delpech, P.; Savoie, J.M. Selection of strains of Lentinula edodes and Lentinula boryana adapted for efficient mycelial growth on wheat straw. Rev. Iberoam. Micol. 2001, 18, 118–122. [Google Scholar]
- Gaitan-Hernandez, R.; Esqueda, M.; Gutierrez, A.; Sanchez, A.; Beltran-García, M. Bioconversion of agro-wastes by Lentinula edodes: The high potential of viticulture residues. Appl. Microbiol. Biotechnol. 2006, 71, 432–439. [Google Scholar] [CrossRef]
- Royse, D.J.; Sanchez, J.E. Ground wheat straw as a substitute for portions of oak wood chips used in shiitake (Lentinula edodes) substrate formulae. Bioresour. Technol. 2007, 98, 2137–2141. [Google Scholar] [CrossRef]
- Philippoussis, A.; Diamantopoulou, P.; Israilides, C. Productivity of agricultural residues used for the cultivation of the medicinal fungus Lentinula edodes. Int. Bio-Deterior. Biodegrad. 2007, 59, 216–219. [Google Scholar] [CrossRef]
- Annepu, S.K.; Sharma, V.P.; Kumar, S.; Barh, A.; Banayal, S.; Kamal, S. Enzyme profile of shiitake mushroom strains grown on wheat straw. Indian J. Hortic. 2018, 75, 475–481. [Google Scholar] [CrossRef]
- Rossi, I.H.; Monteiro, A.C.; Machado, J.O.; Andrioli, J.C.; Barbosa, J.C. Shiitake (Lentinula edodes) production on sterilized bagasse substrate enriched with rice bran and sugarcane molasses. Braz. J. Microbiol. 2003, 34, 66–71. [Google Scholar] [CrossRef] [Green Version]
- Curvetto, N.R.; González-Matute, R.; Figlas, D.; Delmastro, S. Shiitake bag cultivation. Chapter 4: Sunflower seed hulls. In Mushroom Growers Handbook 2—Shiitake Cultivation; Mushworld-Heineart Inc.: Seoul, Republic of Korea, 2005; pp. 119–124. [Google Scholar]
- Gaitan-Hernandez, R.; Esqueda, M.; Gutierrez, A.; Beltran-García, M. Quantitative changes in the biochemical composition of lignocellulosic residues during the vegetative growth of Lentinula edodes. Braz. J. Microbiol. 2011, 42, 30–40. [Google Scholar] [CrossRef] [Green Version]
- Eira, F.C.; Meirelles, W.F.; Meirelles, L.D. Shiitake production in corncob substrates. Rev. Bras. De Milho E Sorgo 2005, 4, 141–148. [Google Scholar] [CrossRef]
- Atila, F. Compositional changes in lignocellulosic content of some agro-wastes during the production cycle of shiitake mushroom. Sci. Hortic. 2019, 245, 263–268. [Google Scholar] [CrossRef]
- Gao, S.; Huang, Z.; Feng, X.; Bian, Y.; Huang, W.; Liu, Y. Bioconversion of rice straw agro residues by Lentinula edodes and evaluation of non-volatile taste compounds in mushrooms. Sci. Rep. 2020, 10, 1814. [Google Scholar] [CrossRef] [Green Version]
- Pire, D.G.; Wright, J.E.; Alberto, E. Cultivation of shiitake using sawdust from widely available local woods in Argentina. Mycol. Appl. Int. 2001, 13, 87–91. [Google Scholar]
- Atila, F. Cultivation and Utilization of Shiitake Mushroom. In Medicinal Plants. Sustainable Development and Biodiversity; Ekiert, H.M., Ramawat, K.G., Arora, J., Eds.; Springer: Cham, Switzerland, 2021; p. 28. [Google Scholar] [CrossRef]
- Annepu, S.K.; Sharma, V.P.; Barh, A.; Kumar, S.; Shirur, M.; Kamal, S. Effects of genotype and growing substrate on bio-efficiency of gourmet and medicinal mushroom, Lentinula edodes (Berk.) Pegler. Bangladesh J. Bot. 2019, 48, 129–138. [Google Scholar] [CrossRef]
- Goering, H.K.; Van-Soest, P.J. Forage Fibre Analyses (Apparatus, Reagents, Procedures and Some Applications); Agricultural Handbook No. 379; Agricultural Research Service, USDA: Washington, DC, USA, 1970. [Google Scholar]
- Sharma, V.P.; Annepu, S.K.; Barh, A.; Shirur, M.; Kamal, S. Genetic divergence and cluster analysis in shiitake genotypes based on yield related traits with commercial breeding significance to shorten the production period. Int. J. Veg. Sci. 2018, 24, 424–431. [Google Scholar] [CrossRef]
- Kirchhoff, B.; Lelley, J. Investigations of shiitake (Lentinus edodes (Berk.) Sing.) bag-log cultivation to increase the yield in Germany. In Science and Cultivation of Edible Fungi, Proceedings of the 13th International Congress on the Science and Cultivation of Edible Fungi, Dublin, Ireland, 1–6 September 1991; Maher, M.J., Ed.; A.A. Balkema: Rotterdam, The Netherlands, 1991; pp. 509–516. [Google Scholar]
- Przybylowicz, P.; Donoghue, J. Shiitake Grower’s Handbook: The Art and Science of Mushroom Cultivation; Kendall/Hunt Publishing Company: Dubuque, IA, USA, 1988. [Google Scholar]
- Diehle, D.A.; Royse, D.J. Effect of substrate heat treatment on biological efficiency (BE) and size of a selected line of Lentinula edodes. In Science and Cultivation of Edible Fungi, Proceedings of the 13th International Congress on the Science and Cultivation of Edible Fungi, Dublin, Ireland, 1–6 September 1991; Maher, M.J., Ed.; A.A. Balkema: Rotterdam, The Netherlands, 1991; pp. 521–571. [Google Scholar]
- Philippoussis, A.; Diamantopoulou, P.; Zervakis, G. Correlation of the properties of several lignocellulosic substrates to the crop performance of the shiitake mushroom Lentinus edodes. World J. Microbiol. Biotechnol. 2003, 19, 551–557. [Google Scholar] [CrossRef]
- Jonathan, S.G.; Fasidi, I.O.; Ajayi, A.O.; Adegeye, O. Biodegradation of Nigerian wood wastes by Pleurotus tuber-regium (Fries) Singer. Bioresour. Technol. 2008, 99, 807–811. [Google Scholar] [CrossRef]
- Peksen, A.; Yakupoglu, G.; Yakupoglu, T.; Gulser, C.; Ozturk, E.; Ozdemir, N. Changes in chemical compositions of substrates before and after Ganoderma lucidum cultivation. World J. Microbiol. Biotechnol. 2011, 27, 637–642. [Google Scholar] [CrossRef]
- Atila, F. Lignocellulosic and proximate based compositional changes in substrates during cultivation of Hericium erinaceus mushroom. Sci. Hortic. 2019, 258, 108779. [Google Scholar] [CrossRef]
- Morais, M.H.; Ramos, A.C.; Matos, N.; Oliveira, E.J.S. Production of shiitake mushroom (Lentinula edodes) on lignocellulosic residues. Food Sci. Technol. Int. 2000, 6, 123–128. [Google Scholar] [CrossRef]
- Myoson, E.; Verachtert, H. Growth of high fungi on wheat straw and their impact on the digestibility of the substrate. Appl. Microbiol. Biotechnol. 1991, 36, 421–424. [Google Scholar] [CrossRef]
- Mao, L.; van Arkel, J.; Hendriks, W.H.; Cone, J.W.; de Vos, R.C.H.; Sonnenberg, A.S.M. Assessing the nutritional quality of fungal treated wheat straw: Compounds formed after treatment with Ceriporiopsis subvermispora and Lentinula edodes. Anim. Feed. Sci. Technol. 2021, 276, 114924. [Google Scholar] [CrossRef]
- Hatakka, A.; Hammel, K.E. Fungal biodegradation of lignocelluloses. In Industrial Applications. The Mycota (A Comprehensive Treatise on Fungi as Experimental Systems for Basic and Applied Research); Hofrchter, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 10, pp. 319–340. [Google Scholar]
- Nishimura, H.; Kamiya, A.; Nagata, T.; Katahira, M.; Watanabe, T. Direct evidence for α ether linkage between lignin and carbohydrates in wood cell walls. Sci. Rep. 2018, 8, 6538. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, C. Lignocellulosic residues: Biodegrdataion and bioconversion by fungi. Biotechnol. Adv. 2009, 27, 185–194. [Google Scholar] [CrossRef]
- Chaudhary, K.; Mittal, S.L.; Tauro, B. Control of cellulose hydrolysis by fungi. Biotechnol. Lett. 1985, 7, 455. [Google Scholar] [CrossRef]
- Merritt, M.W.; Jong, S.C. Partial pyrolysis of wood. Ind. Eng. Chem. 1943, 35, 297–301. [Google Scholar] [CrossRef]
- Kilpatrick, M.; Murray, D.J.; Ward, F. Influence of substrate formulation and autoclave treatment on Lentinula edodes production. In Science and Cultivation of Edible Fungi, Proceedings of the 15th International Congress on the Science and Cultivation of Edible Fungi, Maastricht, The Netherlands, 15–19 May 2000; Van Griensven, L.J.L.D., Ed.; A.A. Balkema: Rotterdam, The Netherlands, 2000; pp. 803–810. [Google Scholar]
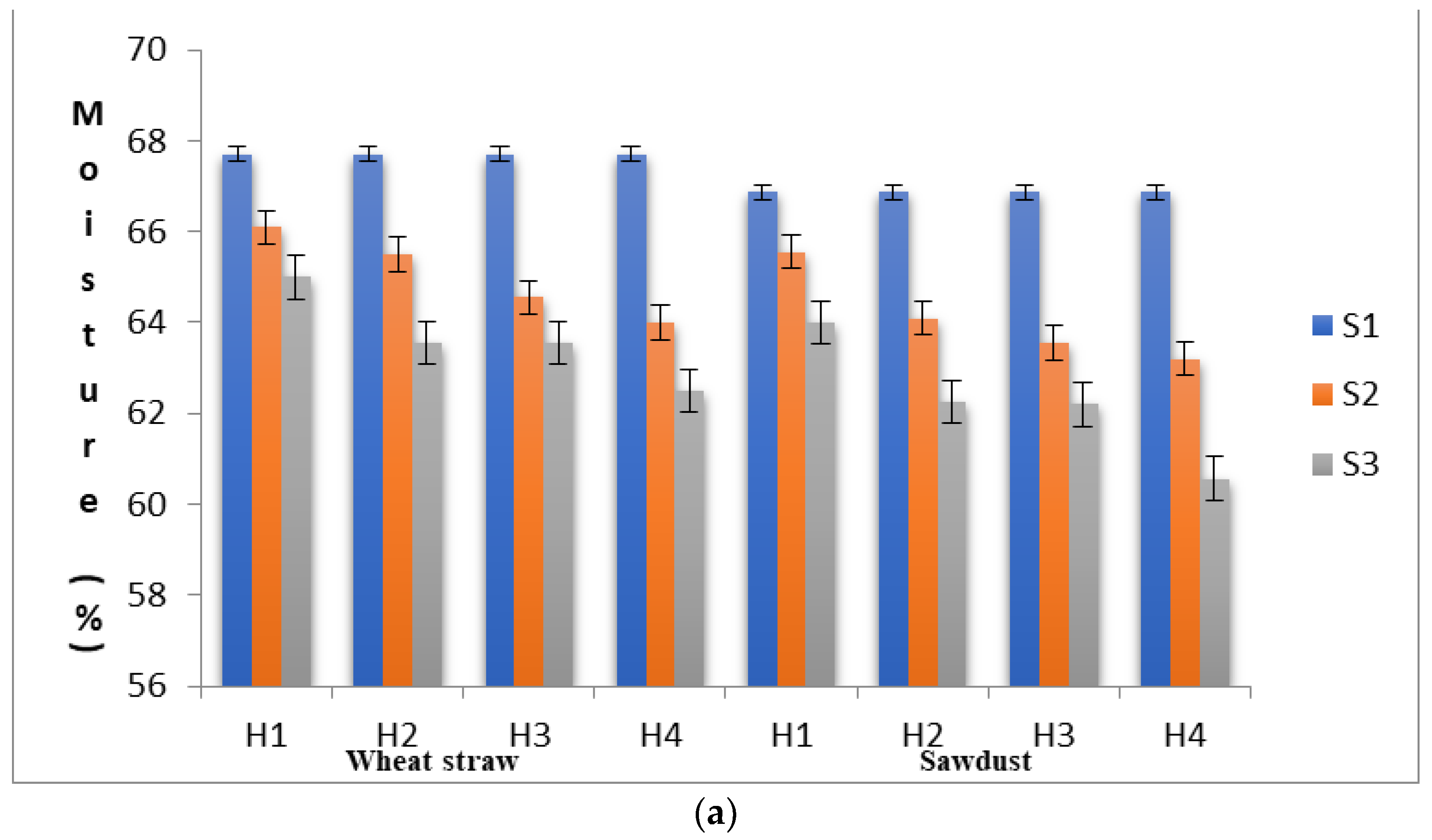
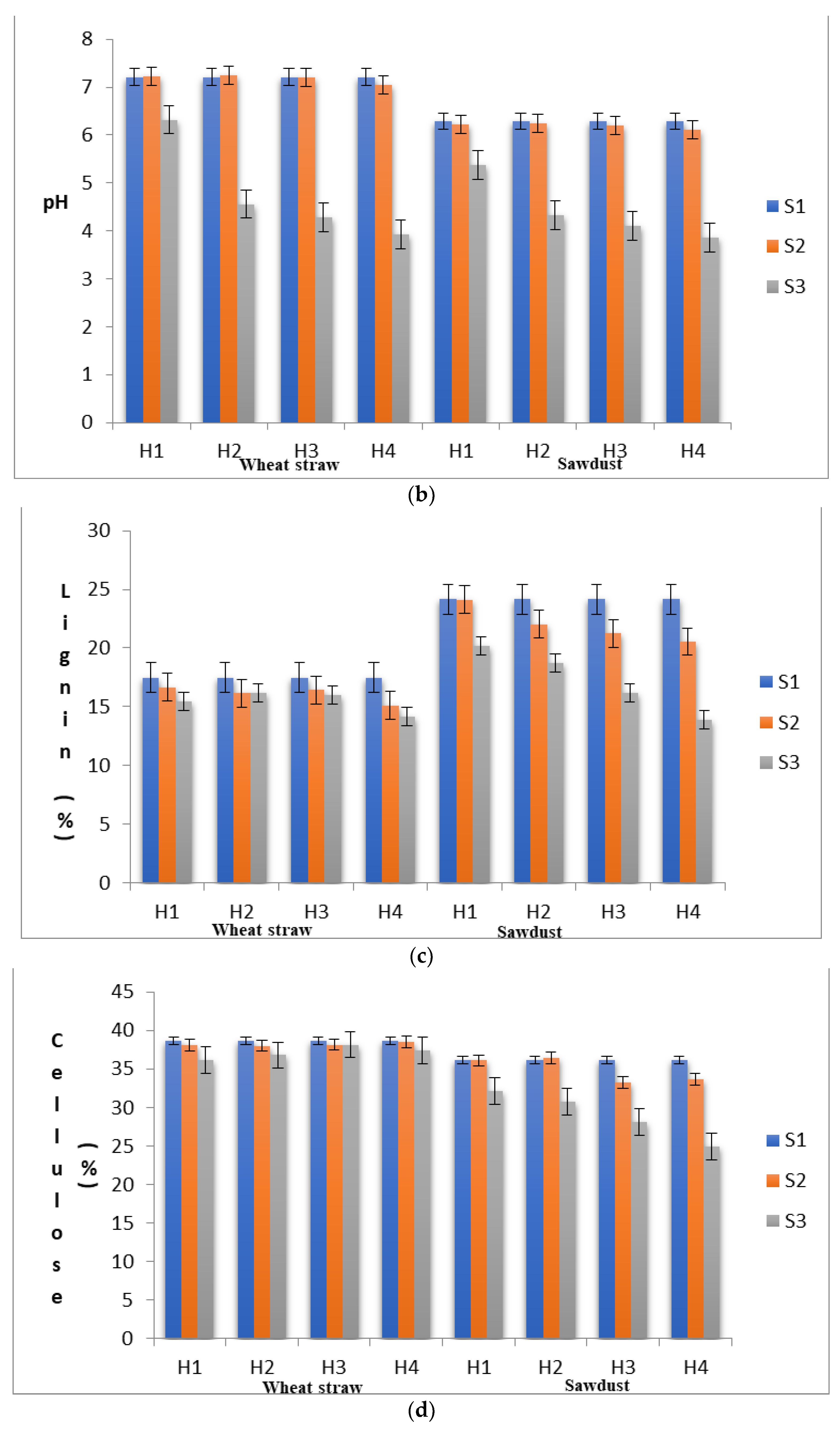

| Strains | Substrate | Days to First Harvest | Total Yield (g) | BE (%) | Production Rate | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H1 | H2 | H3 | H4 | H1 | H2 | H3 | H4 | H1 | H2 | H3 | H4 | H1 | H2 | H3 | H4 | ||
| DMRO-35 | WS | - | - | 98.50 | 97.00 | - | - | 46.30 | 65.29 | - | - | 8.81 | 12.43 | - | - | 0.08 | 0.12 |
| SD | - | - | 87.50 | 82.75 | - | - | 56.20 | 83.75 | - | - | 10.70 | 15.95 | - | - | 0.10 | 0.16 | |
| DMRO-51 | WS | 81.5 | 84.5 | 94.25 | 87.00 | 23.78 | 28.20 | 67.26 | 90.02 | 4.53 | 5.37 | 12.81 | 17.14 | 0.05 | 0.05 | 0.11 | 0.16 |
| SD | - | 91.50 | 95.00 | 73.12 | - | 51.20 | 76.15 | 100.4 | - | 9.75 | 14.50 | 19.13 | - | 0.09 | 0.13 | 0.21 | |
| DMRO-388s | WS | 54.5 | 58.75 | 59.00 | 59.25 | 42.84 | 43.15 | 141.5 | 201.0 | 8.16 | 8.22 | 26.95 | 38.28 | 0.13 | 0.11 | 0.38 | 0.53 |
| SD | - | 60.75 | 62.75 | 60.00 | - | 72.15 | 154.4 | 222.2 | - | 13.74 | 29.41 | 42.32 | - | 0.19 | 0.36 | 0.54 | |
| DMRO-297 | WS | - | - | 83.00 | 75.50 | - | - | 41.07 | 76.13 | - | - | 7.80 | 14.50 | - | - | 0.08 | 0.17 |
| SD | - | - | 86.00 | 101.5 | - | - | 66.29 | 83.12 | - | - | 12.75 | 15.83 | - | - | 0.12 | 0.14 | |
| DMRO-410 | WS | - | 89.0 | 82.50 | 76.50 | - | 56.85 | 68.55 | 110.5 | - | 10.83 | 13.05 | 21.05 | - | 0.11 | 0.14 | 0.23 |
| SD | - | 87.50 | 90.75 | 72.12 | - | 60.60 | 85.05 | 140.0 | - | 11.54 | 16.20 | 26.68 | - | 0.12 | 0.15 | 0.29 | |
| DMRO-412 | WS | - | - | 93.75 | 94.75 | - | - | 58.57 | 95.76 | - | - | 11.15 | 18.24 | - | - | 0.11 | 0.17 |
| SD | - | - | 100.0 | 81.87 | - | - | 64.87 | 135.3 | - | - | 12.35 | 25.77 | - | - | 0.10 | 0.26 | |
| CD | WS | 2.28 | 4.37 | 5.46 | 3.87 | 6.80 | 11.69 | 16.59 | 14.85 | 1.29 | 2.22 | 3.16 | 2.82 | 0.02 | 0.03 | 0.06 | 0.03 |
| SD | - | 4.69 | 4.00 | 2.03 | - | 11.37 | 12.68 | 12.45 | - | 2.16 | 2.41 | 2.37 | - | 0.02 | 0.02 | 0.02 | |
| SE (m) | WS | 0.76 | 1.46 | 1.82 | 1.29 | 2.27 | 3.90 | 5.50 | 4.96 | 0.43 | 0.74 | 1.05 | 0.94 | 0.01 | 0.01 | 0.02 | 0.01 |
| SD | - | 1.56 | 1.33 | 0.68 | - | 3.80 | 4.23 | 4.15 | - | 0.72 | 0.80 | 0.79 | - | 0.01 | 0.01 | 0.01 | |
| Sampling Stage | Heat Treatments | pH | Lignin | Cellulose | Hemicellulose | Cellulose: Lignin |
|---|---|---|---|---|---|---|
| After heat treatment (S2) | H1 | 0.14 | −4.75 | −1.50 | −0.58 | 3.20 |
| H2 | 0.42 | −7.50 | −1.76 | −4.23 | 5.99 | |
| H3 | −0.14 | −6.01 | −1.37 | −1.04 | 4.72 | |
| H4 | −2.36 | −13.52 | −0.47 | −6.63 | 14.85 | |
| At the time of sporophore induction (S3) (DMRO-388s) | H1 | −12.34 | −11.51 | −6.51 | −14.39 | 5.43 |
| H2 | −36.75 | −7.33 | −4.91 | −23.05 | 2.40 | |
| H3 | −40.64 | −8.59 | −1.37 | −31.80 | 7.67 | |
| H4 | −45.63 | −19.01 | −3.23 | −34.99 | 19.24 |
| Sampling Stage | Heat Treatments | pH | Lignin | Cellulose | Hemicellulose | Cellulose: Lignin |
|---|---|---|---|---|---|---|
| After heat treatment (S2) | H1 | −1.11 | −0.21 | −0.14 | −3.55 | −0.14 |
| H2 | −0.79 | −8.90 | 0.69 | −2.43 | 10.30 | |
| H3 | −1.43 | −12.01 | −8.02 | 7.93 | 4.31 | |
| H4 | −2.86 | −14.95 | −6.94 | −8.34 | 9.19 | |
| At the time of sporophore induction (S3)(DMRO-388s) | H1 | −14.47 | −16.56 | −11.07 | −0.37 | 6.37 |
| H2 | −31.32 | −22.57 | −15.08 | 6.77 | 9.45 | |
| H3 | −34.66 | −33.13 | −22.13 | −8.19 | 16.20 | |
| H4 | −38.63 | −42.44 | −31.12 | −20.56 | 19.42 |
| Days to First Harvest | BE (%) | Production Rate | ||||
|---|---|---|---|---|---|---|
| WS | SD | WS | SD | WS | SD | |
| pH | 0.987 * | −0.312 | 0.733 * | 0.999 * | 0.706 * | 0.999 * |
| Lignin | 0.095 | −0.228 | 0.711 * | 0.999 * | 0.718 * | −0.998 * |
| Cellulose | −0.781 * | −0.330 | −0.740 * | 0.992 * | −0.733 * | 0.998 * |
| Hemi cellulose | 0.882 * | −0.210 | 0.912 * | 0.998 * | 0.897 * | 0.992 * |
| Cellulose: lignin | 0.378 | −0.065 | 0.910 * | 0.989 * | 0.913 * | 0.976 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Annepu, S.K.; Sharma, V.P.; Barh, A.; Kamal, S.; Shirur, M.; Kumar, S.; Bairwa, R.K.; Gupta, S.; Gupta, M.; Dutta, U.; et al. Influence of Heat Treatment and Solid-State Fermentation on the Lignocellulosic Fractions of Substrates Supporting Lentinula edodes (Berk.) Pegler Cultivation: Implications for Commercial Production. Fermentation 2023, 9, 130. https://doi.org/10.3390/fermentation9020130
Annepu SK, Sharma VP, Barh A, Kamal S, Shirur M, Kumar S, Bairwa RK, Gupta S, Gupta M, Dutta U, et al. Influence of Heat Treatment and Solid-State Fermentation on the Lignocellulosic Fractions of Substrates Supporting Lentinula edodes (Berk.) Pegler Cultivation: Implications for Commercial Production. Fermentation. 2023; 9(2):130. https://doi.org/10.3390/fermentation9020130
Chicago/Turabian StyleAnnepu, Sudheer Kumar, Ved Prakash Sharma, Anupam Barh, Shwet Kamal, Mahantesh Shirur, Satish Kumar, Rakesh Kumar Bairwa, Sachin Gupta, Moni Gupta, Upma Dutta, and et al. 2023. "Influence of Heat Treatment and Solid-State Fermentation on the Lignocellulosic Fractions of Substrates Supporting Lentinula edodes (Berk.) Pegler Cultivation: Implications for Commercial Production" Fermentation 9, no. 2: 130. https://doi.org/10.3390/fermentation9020130
APA StyleAnnepu, S. K., Sharma, V. P., Barh, A., Kamal, S., Shirur, M., Kumar, S., Bairwa, R. K., Gupta, S., Gupta, M., Dutta, U., Summuna, B., Gupta, D., & Kumar, R. (2023). Influence of Heat Treatment and Solid-State Fermentation on the Lignocellulosic Fractions of Substrates Supporting Lentinula edodes (Berk.) Pegler Cultivation: Implications for Commercial Production. Fermentation, 9(2), 130. https://doi.org/10.3390/fermentation9020130





