Combined Effect of Ultrasound Treatment and a Mix of Krebs Cycle Acids on the Metabolic Processes in Saccharomyces cerevisiae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Materials
2.2. Experiment Settings
2.2.1. Effect of Ultrasonic Treatment on Yeast Fermentation Activity and Non-Viable Cell Count
2.2.2. Effect of Combined Treatment of Yeast with Ultrasound and a Mix of Krebs Cycle Acids on the Glycolysis Enzymes and the Tricarboxylic Acid Cycle
2.2.3. Combined Treatment with Ultrasound and a Mix of Krebs Cycle Acids: Effect on the Assimilation and Synthesis of Nitrogenous Substances
2.3. Research Methods
3. Results and Discussion
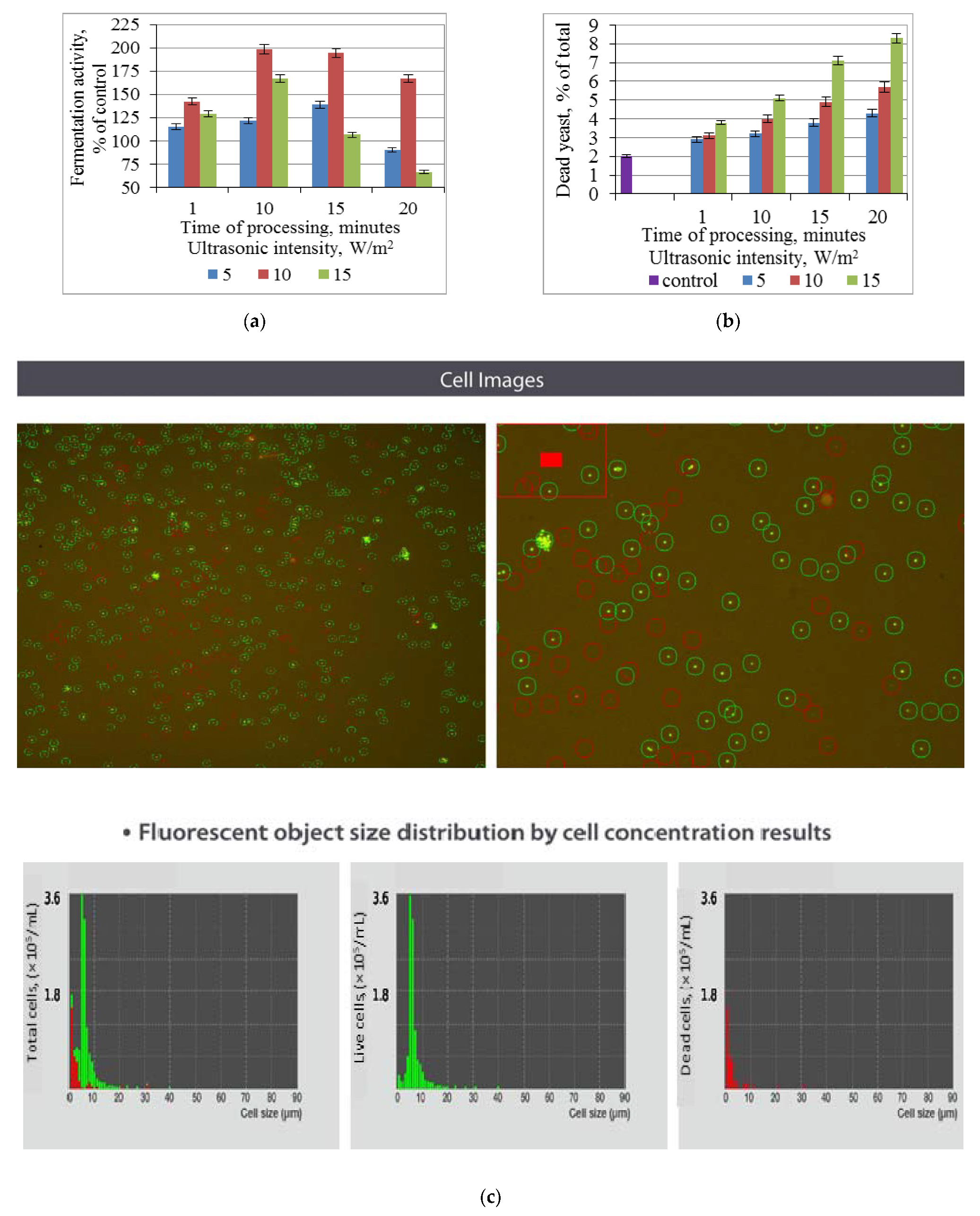
3.1. Effect of Ultrasonic Treatment of Brewer’s Yeast on Fermentation and Non-Viable Cell Count
3.2. Effect of Combined Treatment of Yeast with Ultrasound and a Mix of Krebs Cycle Acids on the Glycolysis Enzymes and the Tricarboxylic Acid Cycle
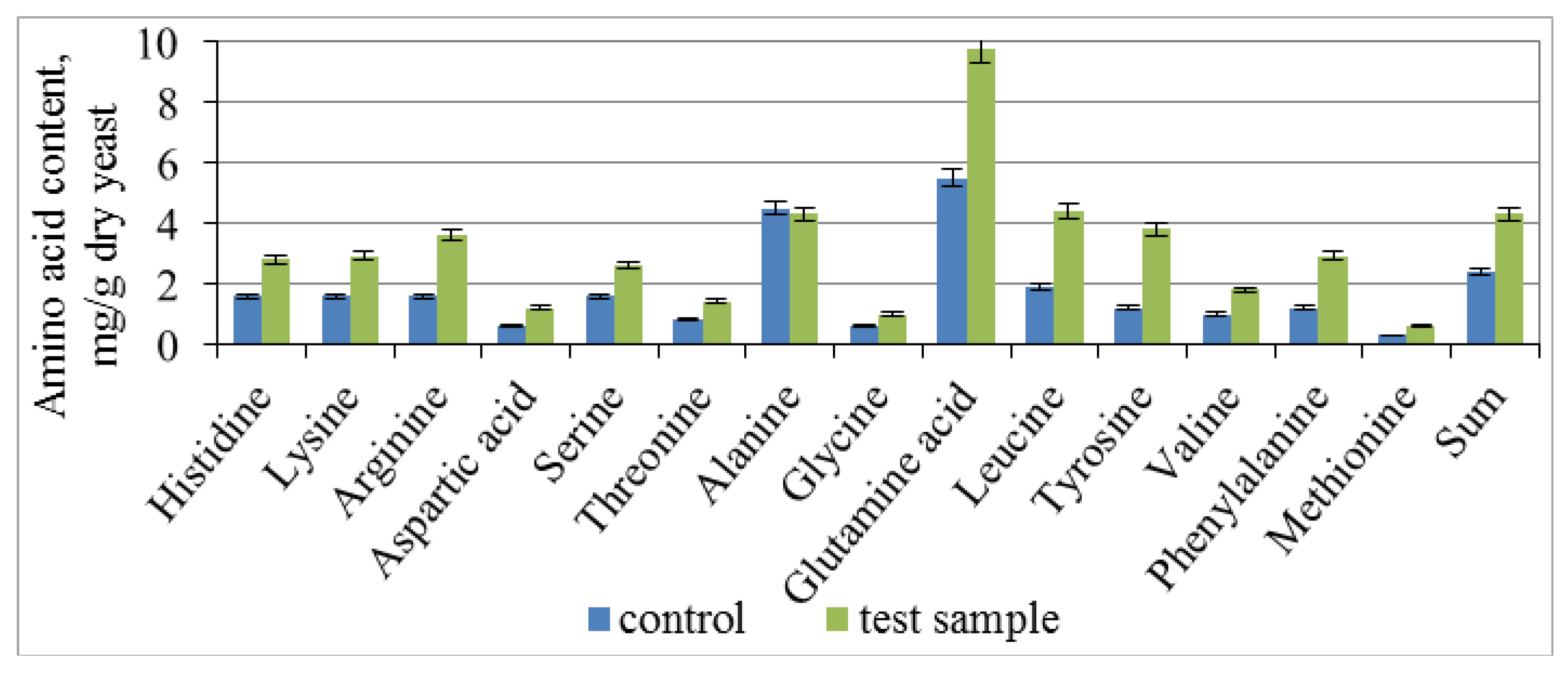
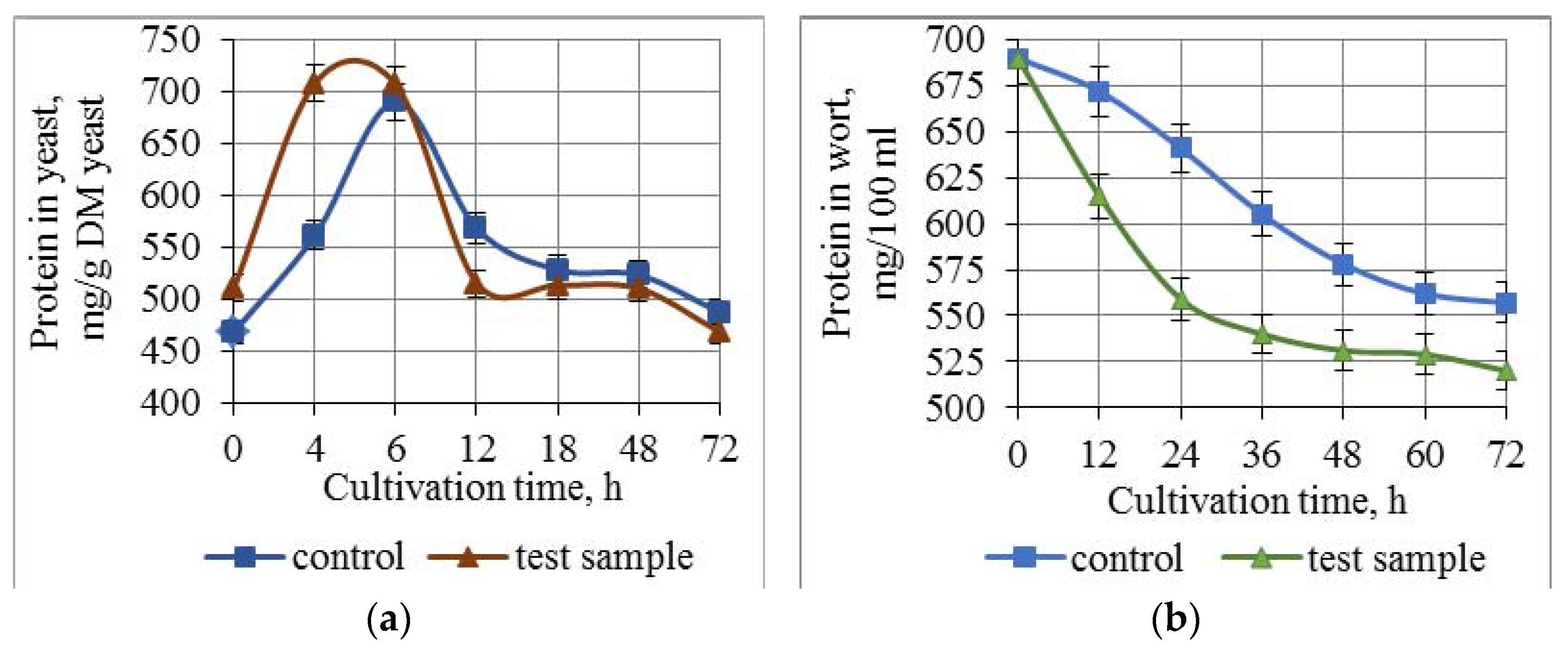
3.3. Effect of Combined Treatment of Yeast with Ultrasound and a Mix of Krebs Cycle Acids on the Assimilation and Synthesis of Nitrogenous Substances
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kurbanova, M.; Voroshilin, R.; Kozlova, O.; Atuchin, V. Effect of Lactobacteria on Bioactive Peptides and Their Sequence Identification in Mature Cheese. Microorganisms 2022, 10, 2068. [Google Scholar] [CrossRef] [PubMed]
- Vesnina, A.; Prosekov, A.; Atuchin, V.; Minina, V.; Ponasenko, A. Tackling Atherosclerosis via Selected Nutrition. Int. J. Mol. Sci. 2022, 23, 8233. [Google Scholar] [CrossRef] [PubMed]
- Kolberg, N.A.; Tikhonova, N.V.; Tikhonov, S.L.; Leontieva, S.A.; Sergeeva, I.Y. Immunotropic Effect of Bursal Peptides on Mice with Experimental Immunodeficiency. Food Process. Tech. Technol. 2022, 52, 296–309. [Google Scholar] [CrossRef]
- Yamada, E.A.; Scarbieri, V.C. Yeast (Saccharomyces cerevisiae) protein concentrate: Preparation, chemical composition, and nutritional and functional properties. J. Agric. Food Chem. 2005, 53, 3931–3936. [Google Scholar] [CrossRef] [PubMed]
- Rimareva, L.V.; Kurbatova, E.I.; Makarova, A.V. Biotechnological aspects of creating food additives with biocorrective action based on microbial biomass. Storage Process. Farm. Prod. 2011, 2, 45–47. [Google Scholar]
- Serba, E.M.; Rachkov, K.V.; Orlova, E.V.; Rimareva, L.V.; Pogorzshelskaya, N.S.; Polyakov, V.A. Fractional composition and fermentolizats functional properties of the yeast biomass. Proc. Samara Sci. Cent. Russ. Acad. Sci. 2013, 15, 1680–1681. [Google Scholar]
- Grishin, D.V.; Podobed, O.V.; Gladilina, Y.A.; Pokrovskaya, M.V.; Aleksandrova, S.S.; Pokrovsky, V.S.; Sokolov, N.N. Bioactive proteins and peptides: Current state and new trends of practical application in the food industry and feed production. Probl. Nutr. 2017, 86, 19–31. [Google Scholar] [CrossRef]
- Serba, E.M.; Rimareva, L.V.; Overchenko, M.B.; Ignatova, N.I.; Shelekhova, N.V.; Pogorzshelskaya, N.S.; Abramova, I.M. Biotechnological aspects of obtaining functional ingredients by the conversion of Saccharomyces cerevisiae 985-T biomass. Biotekhnologiya 2020, 36, 34–41. [Google Scholar] [CrossRef]
- Serba, E.M.; Rimareva, L.V.; Overchenko, M.B.; Ignatova, N.I.; Tadzhibova, P.; Zorin, S.N. Production of peptides and amino acids from microbial biomass in food and feed industries: Biotechnological aspects. Foods Raw Mater. 2020, 8, 268–276. [Google Scholar] [CrossRef]
- Yuraskina, T.V.; Sokolova, E.N.; Fursova, N.A.; Andreeva, S.S.; Serba, E.M. Innovative biotechnological approaches to the production of food ingredients based on enriched microorganisms. Food Ind. 2021, 9, 64–66. [Google Scholar] [CrossRef]
- Pereira, P.R.; Freitas, C.S.; Paschoalin, V.M.F. Saccharomyces cerevisiae biomass as a source of next-generation food preservatives: Evaluating potential proteins as a source of antimicrobial peptides. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4450–4479. [Google Scholar] [CrossRef] [PubMed]
- Jaehrig, S.C.; Rohn, S.; Kroh, L.W.; Wildenauer, F.; Lisdat, F.; Fleischer, L.-G.; Kurz, T. In vitro potential antioxidant activity of (1-3),(1-6)-β-D-glucan and protein fractions from Saccharomyces cerevisiae cell walls. Agric. Food Chem. 2007, 55, 4710–4716. [Google Scholar] [CrossRef]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Agyei, D.; Ongkudon, C.M.; Wei, C.Y.; Chan, A.S.; Danquah, M.K. Bioprocess challenges to the isolation and purification of bioactive peptides. Food Bioprod. Process. 2016, 98, 244–256. [Google Scholar] [CrossRef]
- Branco, P.; Francisco, D.; Monteiro, M.; Almeida, M.G.; Caldeira, J.; Arneborg, N.; Prista, C.; Albergaria, H. Antimicrobial properties and death-inducing mechanisms of saccharomycin, a biocide secreted by Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2017, 101, 159–171. [Google Scholar] [CrossRef]
- Serba, E.M.; Rimareva, L.V.; Kurbatova, E.I.; Volkova, G.S.; Polyakov, V.A.; Varlamov, V.P. The study of the process of enzymatic hydrolysis of yeast biomass to generate food ingredients with the specified fractional composition of protein substances. Probl. Nutr. 2017, 86, 76–83. [Google Scholar] [CrossRef]
- Fakruddin, M.; Hossain, M.N.; Ahmed, M.M. Antimicrobial and antioxidant activities of Saccharomyces cerevisiaeIFST062013, a potential probiotic. BMC Complement. Altern. Med. 2017, 17, 64. [Google Scholar] [CrossRef] [Green Version]
- Agarkova, E.Y.; Kruchinin, A.G. Enzymatic conversion as a method of producing biologically active peptides. Bull. MSTU 2018, 21, 412–419. [Google Scholar] [CrossRef]
- Rizk, Z.; El Rayess, Y.; Ghanem, C.; Mathieu, F.; Taillandier, P.; Nehme, N. Identification of multiple-derived peptides produced by Saccharomyces cerevisiae involved in malolactic fermentation inhibition. FEMS Yeast Res. 2018, 18, foy080. [Google Scholar] [CrossRef] [Green Version]
- Al-Sahlany, S.T.G.; Altemim, A.B.; Al-Manhel, A.J.A.; Niamah, A.K.; Lakhssassi, N.; Ibrahim, S.A. Purification of bioactive peptide with antimicrobial properties produced by Saccharomyces cerevisiae. Foods 2020, 9, 324. [Google Scholar] [CrossRef] [Green Version]
- Tikhonov, S.L.; Tikhonova, N.V.; Kolberg, N.A.; Kudryashov, L.S. Systematization of scientific knowledge about the production technology and mechanism of action of certain biologically active peptides. Agro-Ind. Complex Russ. 2022, 29, 254–261. [Google Scholar] [CrossRef]
- Huang, G.; Chen, S.; Tang, Y.; Dai, C.; Sun, L.; Ma, H.; He, R. Stimulation of low intensity ultrasound on fermentation of skim milk medium for yield of yoghurt peptides by Lactobacillus paracasei. Ultrason. Sonochem. 2019, 51, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Mofa, N.N.; Zhapekova, A.O.; Bakkara, A.E. Ultrasonic processing is an effective method of directed synthesis of nanostructured systems (review). Combust. Plasma Chem. 2021, 19, 67–77. [Google Scholar] [CrossRef]
- Konovalov, S.A. Biochemistry of Yeast; Food Industry: Moscow, Russia, 1980. [Google Scholar]
- Annemuller, G.; Manger, H.-J.; Lietz, P. The Yeast in the Brewery; VLB Berlin: Berlin, Germany, 2011. [Google Scholar]
- Kudryasheva, A.A.; Tikhomirov, A.A. Features of protein biosynthesis by a unicellular organisms wand ways of its regulation. Food Ind. 2016, 7, 40–43. [Google Scholar]
- Starovojtova, O.V.; Sadrieva, A.A.; Mingaleeva, Z.S.; Reshetnik, O.A. Activation of yeast Saccharomyces cerevisiae in the technology of bread preparation. Bull. Kazan Technol. Univ. 2014, 1, 235–237. [Google Scholar]
- Krikunova, L.N.; Rjabova, S.M.; Peschanskaja, V.A.; Urusova, L.M. Influence of succinic acid on the metabolism of yeast Saccharomyces cerevisiae. Beer Drink. 2015, 1, 36–38. [Google Scholar]
- Miller, Y.Y.; Kiseleva, T.F.; Arysheva, I.V. Forming soy malt quality with organic growth promoters. Food Process. Tech. Technol. 2021, 51, 248–259. [Google Scholar] [CrossRef]
- Vereschagin, A.L.; Eremina, V.V.; Zakhareva, Y.I.; Khmeleva, A.N.; Kunets, L.L. Biological activity of ultra-small concentrations of some natural organic acids—Krebs cycle intermediates. Proc. Universities. Appl. Chem. Biotechnol. 2012, 2, 72–75. [Google Scholar]
- Vereshhagin, A.L.; Kunec, L.L. Influence of ultra-low concentrations of intermediates of the Krebs cycle on the growth and development of pure culture Staphylococus aureus. Proc. Universities. Appl. Chem. Biotechnol. 2012, 2, 143–144. [Google Scholar]
- Vereshchagin, A.L.; Nurminsky, V.N.; Eremina, V.V.; Zakharyeva, Y.I.; Ozolina, N.V.; Salyaev, R.K. Influence of a number of dicarboxylic acids in ultra-low concentrations on the barrier function of the membrane of an isolated vacuole. Bull. Irkutsk. State Univ. Ser. Biology. Ecol. 2013, 6, 3–7. [Google Scholar]
- Vereshchagin, A.L.; Tatarnikova, T.V.; Borina, L.L. Effect of Krebs cycle intermediates on the bactericidal activity of ampicillin and chloramphenicol against Staphylococcus aureus and Salmonella typhimurium. Polzunovskiy Vestn. 2015, 2, 128–130. [Google Scholar]
- Borina, L.L.; Vereshchagin, A.L. An effect of Krebs cycle intermediates on the growth and development of Pseudomonas aeruginosa. Proc. Universitets. Appl. Chem. Biotechnol. 2018, 8, 85–91. [Google Scholar] [CrossRef]
- Permyakova, L.V.; Pomozova, V.A.; Vereshchagin, A.L. Use of mix of acids of Krebs cycle in ultralow concentration for activation of culture of beer yeast. Beer Beverages 2018, 1, 20–24. [Google Scholar]
- Vereshchagin, A.L.; Kropotkina, V.V.; Khmeleva, A.N. On the mechanism of growth-stimulating action of ultra-low doses of natural organic acids. Bull. Altai State Agrar. Univ. 2010, 63, 46–48. [Google Scholar]
- Rogov, I.A.; Danilchuk, T.N. The mechanism of biological effects of extremely low doses of oscillatory and wave effects in the field of sound frequencies. Part 1. Biological effects of low-intensity physical impacts in food technologies. Elektron. Obrab. Mater. 2017, 53, 63–69. [Google Scholar] [CrossRef]
- Karpenko, D.V.; Gernet, M.V.; Krjukova, E.V.; Gribkova, I.N.; Nurmukhanbetova, D.E.; Assembayeva, E.K. Acoustic vibration effect on genus Saccaromyces yeast population development. Bull. NAS RK 2019, 4, 103–112. [Google Scholar] [CrossRef]
- Karpenko, D.V.; Karaycheva, A.I. Influence of polyfrequency acoustic impacts on the development of yeast populations. Colloq. J. Chem. Sci. Colloq. J. 2020, 8, 49–51. [Google Scholar] [CrossRef]
- Bodrova, O.J.; Krechetnikova, A.N. Activating and disintegrating effects of ultrasonic treatment of microorganisms. Hist. Sci. Technol. 2006, 4, 51–54. [Google Scholar]
- Chemat, F.; Zill-e-Huma; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–883. [Google Scholar] [CrossRef]
- Vereshchagin, A.L.; Khmeleva, A.N. Effect of Ultrasonic Irradiation and Growth Regulators on the Rhizogene Activity of Plant Objects; AltSTU Publishing House: Biysk, Russia, 2010. [Google Scholar]
- Yaldagard, M.; Mortazavi, S.A.; Tabatabaie, F. Application of ultrasonic waves as a priming technique for accelerating and enhancing the germination of barley seed: Optimization of method by the taguchi approach. J. Inst. Brew. 2008, 114, 14–21. [Google Scholar] [CrossRef]
- Ponomareva, E.I.; Alekhina, N.N.; Skvortsova, O.B. Changing the nutritional value of buckwheat grains when germination with using ultrasound-treated water. J. Izv. Vuzov. Food Technol. 2020, 1, 30–32. [Google Scholar] [CrossRef]
- Del Fresno, J.M.; Morata, A.; Escott, C.; Loira, I.; Cuerda, R.; Suárez-Lepe, J.A. Sonication of yeast biomasses to improve the ageing on lees technique in red wines. Molecules 2019, 24, 635. [Google Scholar] [CrossRef] [PubMed]
- Gavahian, M.; Manyatsi, T.S.; Morata, A.; Tiwari, B.K. Ultrasound-assisted production of alcoholic beverages: From fermentation and sterilization to extraction and aging. Compr. Rev. Food Sci. Food Saf. 2022, 21, 5243–5271. [Google Scholar] [CrossRef] [PubMed]
- Rogov, I.A.; Danilchuk, T.N. The mechanism of biological effects of extremely low doses of oscillatory and wave effects in the field of sound frequencies. Part II. Physicochemical model of the influence of low-intensity physical factors on the activity of hydrolytic enzymes. Elektron. Obrab. Mater. 2017, 53, 70–77. [Google Scholar] [CrossRef]
- Karpenko, D.V.; Kravchenko, V.S.; Shalaginov, K.V. Activation of an amylolytic enzyme preparation by wave action. Beer Drink. 2017, 5, 16–19. [Google Scholar]
- Kaluzhina, O.Y.; Yakovleva, K.S.; Kashapova, R.A.; Chernenkov, E.N.; Chernenkova, A.A.; Bodrov, A.Y. The effect of ultrasound on brewing yeast. Proc. VSUET 2020, 82, 103–109. [Google Scholar] [CrossRef]
- Al Daccache, M.; Koubaa, M.; Salameh, D.; Maroun, R.G.; Louka, N.; Vorobiev, E. Ultrasound-assisted fermentation for cider production from Lebanese apples. Ultrason. Sonochem. 2020, 63, 104952. [Google Scholar] [CrossRef]
- Rakowska, R.; Sadowska, A.; Dybkowska, E.; Świderski, F. Spent yeast as natural source of functional food additives. Rocz Panstw. Zakl. Hig. 2017, 68, 115–121. [Google Scholar]
- Jaeger, A.; Arendt, E.K.; Zannini, E.; Sahin, A.W. Brewer’s spent yeast (BSY), an underutilized brewing by-product. Fermentation 2020, 6, 123. [Google Scholar] [CrossRef]
- Pomozova, V.A.; Permyakova, L.V.; Safonova, E.A.; Artemasov, V.V. Activation of brewer’s yeast. Beer Drink. 2002, 2, 26–27. [Google Scholar]
- Shershenkov, B.S.; Suchkova, E.P. Ultrasonic Modulation of Propionibacterium freudenreichii subsp. shermanii Metabolic Activity in Production of Enriched by B12 Vitamin Food Products. Available online: https://cyberleninka.ru/article/n/ultrazvukovaya-modulyatsiya-metabolicheskoy-aktivnosti-propionibacterium-freudenreichii-subsp-shermanii-pri-poluchenii-pischevyh (accessed on 25 October 2022).
- Vereshchagin, A.L.; Glushkova, Y.I. Influence of Krebs Cycle Intermediates and Physical Factors on Phytotoxicity of Solid Herbicides; AltSTU Publishing House: Biysk, Russia, 2017. [Google Scholar]
- Evstafev, S.N.; Khoang, K.K. Ultrasonic treatment of wheat straw in 1-butyl-3-methylimidazolium chloride. Chem. Plant Raw Mater. 2018, 1, 5–12. [Google Scholar] [CrossRef] [Green Version]
- Davydenko, S.G.; Dedegkayev, A.T.; Meledina, T.V. Development of a new express method of beer physiological influence assessment. Beer Drink. 2012, 5, 20–23. [Google Scholar]
- Klenova, N.A.; Makurina, O.N.; Pisareva, E.V.; Yazykova, M.Y. Special Workshop on the Biochemistry of Animals, Plants and Microorganisms; Educational allowance; Publishing House “S-Print”: Samara, Russia, 2013. [Google Scholar]
- Polygalina, G.V.; Cherednichenko, V.S.; Rimareva, L.V. Determination of Enzyme Activity: A Reference Book for Universities; DeLi Print: Moscow, Russia, 2003. [Google Scholar]
- Wordona, B.A.; Mortimerb, B.; McMaster, L.D. Comparative real-time analysis of Saccharomyces cerevisiae cell viability, injury and death induced by ultrasound (20 kHz) and heat for the application of hurdle technology. Food Res. Int. 2012, 47, 134–139. [Google Scholar] [CrossRef]
- Soro, A.B.; Oliveira, M.; O’Donnell, C.P.; Tiwari, B.K. Ultrasound assisted modulation of yeast growth and inactivation kinetics. Ultrason. Sonochem. 2021, 80, 105819. [Google Scholar] [CrossRef] [PubMed]
- Salogub, E.V.; Bogolyubova, J.S. The study of the influence of ultrasonic treatment on the biosorption properties of yeast. Probl. Mod. Sci. Educ. 2017, 22, 15–17. [Google Scholar]
- Soh, E.Y.; Lim, S.S.; Chew, K.W.; Phuang, X.W.; Ho, V.M.; Chu, K.Y.; Wong, R.; Lee, L.Y.; Tiong, T.J. Valorization of spent brewery yeast biosorbent with sonication-assisted adsorption for dye removal in wastewater treatment. Environ. Res. 2021, 204, 112385. [Google Scholar] [CrossRef]
- Iida, Y.; Tuziuti, T.; Yasui, K.; Kozuka, T.; Towata, A. Protein release from yeast cells as an evaluation method of physical effects in ultrasonic field. Ultrason. Sonochem. 2008, 15, 995–1000. [Google Scholar] [CrossRef]



| Variant | Treatment | Concentration of Krebs Cycle Acids, mol/L | Dose of Krebs Cycle Acids, % to Suspension Volume | Ultrasound Treatment Volume, W/m2 | Treatment Time, min |
|---|---|---|---|---|---|
| Control 1 (C1) | No treatment | - | - | - | - |
| Control 2 (C2) | Krebs cycle acids | 1 × 10−10 | 1 | - | 60 |
| Control 3 (C3) | Ultrasound | - | - | 10 | 10 |
| Test sample 1 (TS1) | Krebs cycle acids + ultrasound | 1 × 10−10 | 1 | 10 | 10 |
| Test sample 2 (TS2) | Krebs cycle acids + ultrasound | 1 × 10−10 | 1 | 10 | 5 |
| Test sample 3 (TS3) | Krebs cycle acids + ultrasound | 1 × 10−10 | 1 | 10 | 3 |
| Enzyme | Activity | |||||
|---|---|---|---|---|---|---|
| C1 | C2 | C3 | TS1 | TS2 | TS3 | |
| Zymase complex, µmol glucose/g DM·min | 85.4 ± 2.3 | 135.7 ± 3.4 | 105.1 ± 2.9 | 118.1 ± 2.6 | 146.3 ± 2.1 | 169.8 ± 3.2 |
| α-glucosidase, µmol maltose/g DM·min | 15.8 ± 0.4 | 36.5 ± 1.3 | 21.0 ± 0.8 | 29.8 ± 0.9 | 43.1 ± 1.5 | 52.0 ± 1.6 |
| β-fructofuranosidase, µmolsucrose/g DM·min | 32.5 ± 0.8 | 47.2 ± 1.6 | 39.2 ± 1.4 | 46.7 ± 1.3 | 62.5 ± 1.6 | 75.4 ± 1.5 |
| Proteolytic activity | Sample | Cultivation time, h | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 12 | 24 | 36 | 48 | 60 | 72 | ||
| extracellular protease, units/10 cm3 × 10−3 | Control | - | 0.63 ± 0.02 | 1.21 ± 0.03 | 1.36 ± 0.03 | 1.45 ± 0.03 | 0.75 ± 0.02 | 0.55 ± 0.02 |
| Test | - | 0.80 ± 0.02 | 1.34 ± 0.03 | 1.61 ± 0.03 | 0.83 ± 0.02 | 0.66 ± 0.02 | 0.60 ± 0.02 | |
| intracellular protease, units/g protein × 10−5 | Control | 9.10 ± 0.35 | 7.83 ± 0.30 | 6.62 ± 0.30 | 8.30 ± 0.35 | 13.23 ± 0.50 | 17.36 ± 0.50 | 11.90 ± 0.44 |
| Test | 12.21 ± 0.40 | 11.50 ± 0.40 | 11.94 ± 0.40 | 13.62 ± 0.50 | 20.55 ± 0.60 | 17.60 ± 0.50 | 12.41 ± 0.40 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Permyakova, L.; Sergeeva, I.; Dolgolyuk, I.; Starovoitova, K.; Atuchin, V.; Vereshchagin, A.; Romanenko, V.; Lashitsky, S. Combined Effect of Ultrasound Treatment and a Mix of Krebs Cycle Acids on the Metabolic Processes in Saccharomyces cerevisiae. Fermentation 2023, 9, 132. https://doi.org/10.3390/fermentation9020132
Permyakova L, Sergeeva I, Dolgolyuk I, Starovoitova K, Atuchin V, Vereshchagin A, Romanenko V, Lashitsky S. Combined Effect of Ultrasound Treatment and a Mix of Krebs Cycle Acids on the Metabolic Processes in Saccharomyces cerevisiae. Fermentation. 2023; 9(2):132. https://doi.org/10.3390/fermentation9020132
Chicago/Turabian StylePermyakova, Larisa, Irina Sergeeva, Irina Dolgolyuk, Kseniya Starovoitova, Victor Atuchin, Alexander Vereshchagin, Vasiliy Romanenko, and Sergey Lashitsky. 2023. "Combined Effect of Ultrasound Treatment and a Mix of Krebs Cycle Acids on the Metabolic Processes in Saccharomyces cerevisiae" Fermentation 9, no. 2: 132. https://doi.org/10.3390/fermentation9020132
APA StylePermyakova, L., Sergeeva, I., Dolgolyuk, I., Starovoitova, K., Atuchin, V., Vereshchagin, A., Romanenko, V., & Lashitsky, S. (2023). Combined Effect of Ultrasound Treatment and a Mix of Krebs Cycle Acids on the Metabolic Processes in Saccharomyces cerevisiae. Fermentation, 9(2), 132. https://doi.org/10.3390/fermentation9020132







