Chemical Profile and Hematoprotective Activity of Artisanal Jabuticaba (Plinia jabuticaba) Wine and Derived Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Jabuticaba Wine Sample
2.2. Partitioning Procedures
2.3. Sample Preparation and Solid Phase Microextraction (SPME)
2.4. Volatile Compounds Analysis by GC–MS
2.5. Odor Activity Value
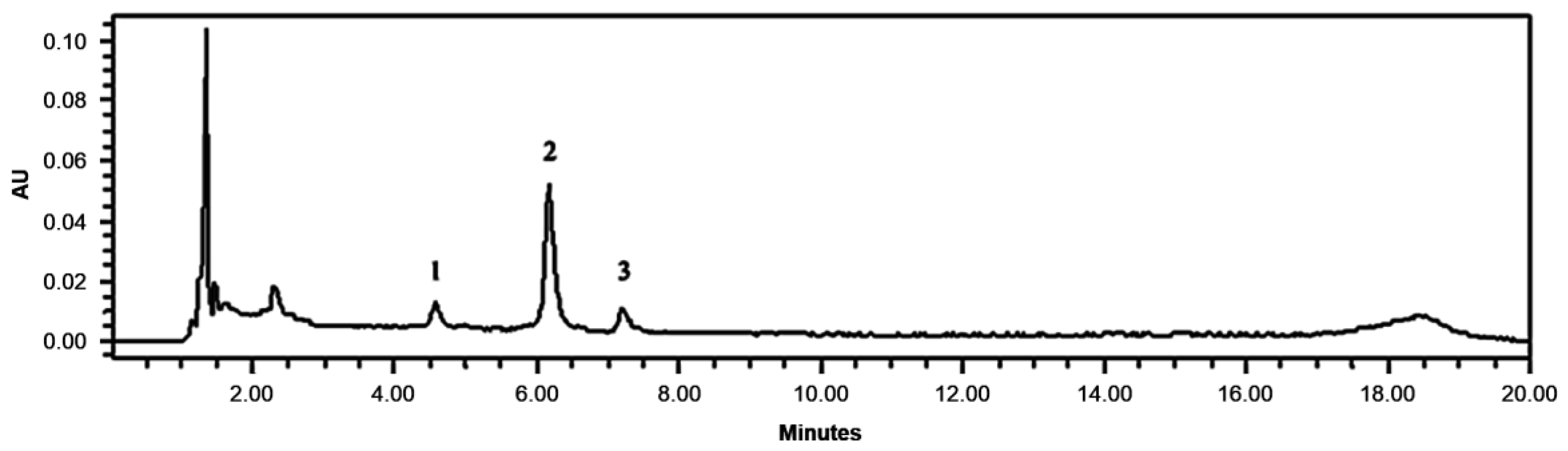
2.6. Anthocyanins Analysis by HPLC
2.7. Total Phenolic and Flavonoids Contents
2.8. Evaluation of Antioxidant Capacity
2.9. AAPH-Induced Oxidative Stress against RBC
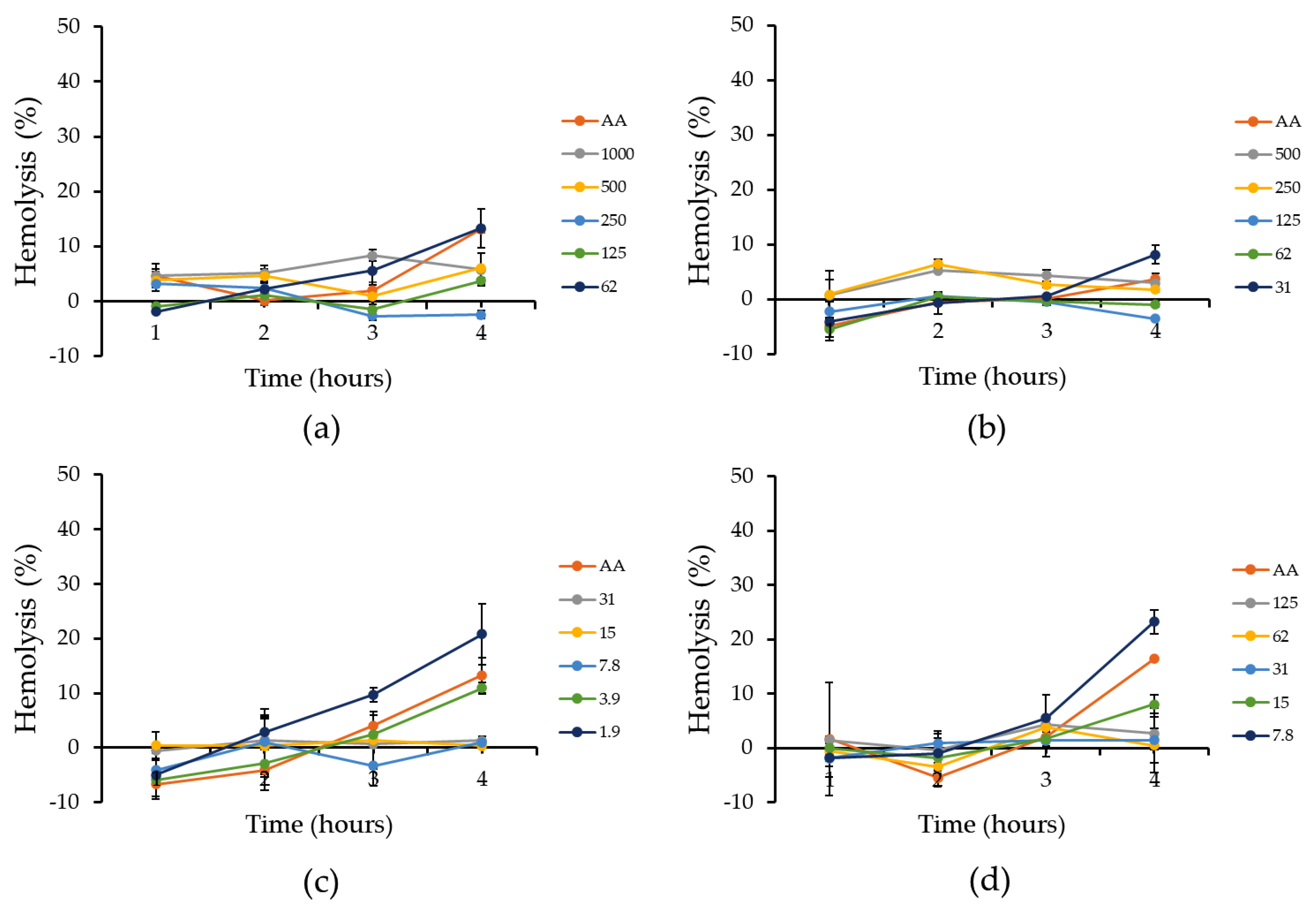
2.10. Hematoprotective Activity Assay
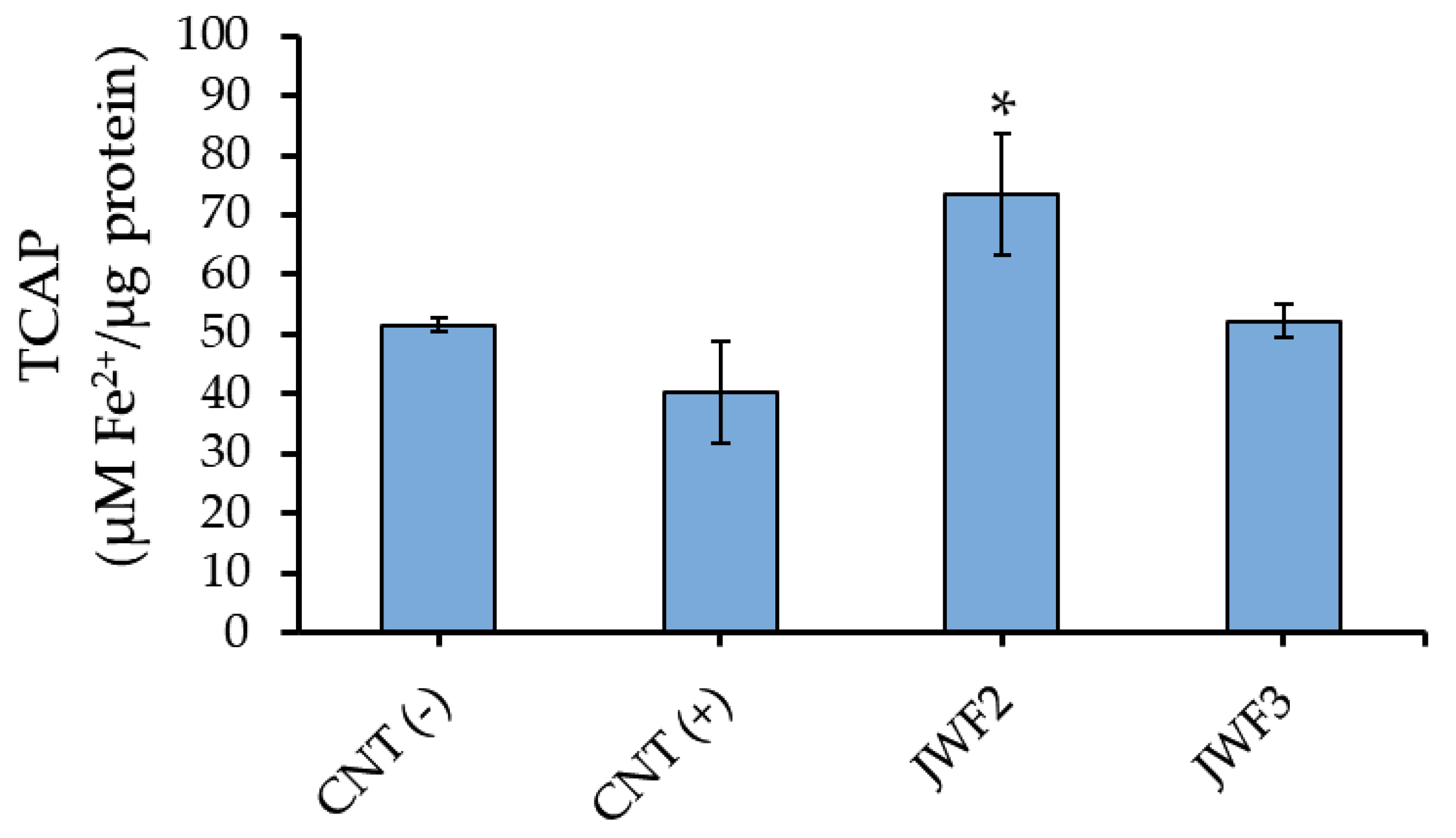
2.11. Total Cellular Antioxidant Power (TCAP)
2.12. Liquid Chromatography-Mass Spectrometry Analysis
2.13. Data Processing and Annotation
2.14. Statistical Analysis
3. Results and Discussion
3.1. Volatile Compounds
| Compound | Compound Content (ppb) | Odor descriptor * | Odor Threshold ** (ppb) | OAV |
|---|---|---|---|---|
| Alcohols | ||||
| Ethanol | 447.3 ± 8.03 | Fruity [29] | 100,000 [29] | <0.01 |
| Isoamyl alcohol | 459.5 ± 9.95 | Fusel, whiskey, malt, burnt [30] | 30,000 [30] | 0.02 |
| 1-Dodecanol | 5.2 ± 0.61 | Raw carrot, medicinal [38] | 730 [39] | 0.01 |
| 1-Tridecanol | 8.3 ± 1.82 | - | - | - |
| Pentadecanol | 5.0 ± 0.62 | - | - | - |
| N-Tetradecanol | 57.4 ± 2.90 | Cinnamon [31] | - | - |
| Hexadecanol | 24.1 ± 4.46 | flower, wax [31] | 750 [40] | 0.03 |
| 1-Octadecanol | 30.6 ± 4.40 | - | 870 | 0.04 |
| 1-Eicosanol | 5.6 ± 1.35 | - | - | |
| α-Methylbenzyl alcohol | 77.6 ± 4.01 | Floral, honey, rose [31] | 750 [41] | 0.10 |
| Aldehydes and ketones | ||||
| Nonanal | 2.0 ± 3.39 | Floral [42] | 1 [42] | 2.0 |
| Decanal | 6.7 ± 0.52 | Orange skin-like, floral [43] | 2 [43] | 3.3 |
| α-Hexylcinnamaldehyde | 9.9 ± 0.61 | Fresh, floral, herbal [44] | 50 [44] | 0.2 |
| 5,9,13-Trimethyl-4,8,12-tetradecatrienal | 29.7 ± 7.22 | - | - | - |
| Dodecanal | 1.0 ± 1.74 | Fatty, citrus-like [43] | 2 [43] | 0.5 |
| Hexadecanal | 1.3 ± 2.18 | leathery, burnt rubber [1] | 75 [40] | 0.02 |
| (4E,8E,12E,16E)-4,8,13,17,21-Pentamethyl-4,8,12,16,20-docosapentaenal | 24.0 ± 4.60 | - | - | - |
| 3-Octanone | 3.1 ± 5.41 | Buttery, herbal, moldy [31] | 28 [31] | 0.1 |
| Neryl acetone | 40.5 ± 3.34 | - | - | - |
| Esters | ||||
| Ethyl acetate | 364.5 ± 5.59 | - | 5000 [29] | 0.07 |
| Diethyl succinate | 85.4 ± 3.17 | Fruity, watermelon [45] | 200 [45] | 0.4 |
| Ethyl octanoate | 24.8 ± 3.20 | Burnt, beer [32] | 5 [32] | 5.0 |
| Phenethyl acetate | 4.9 ± 0.46 | Rose, floral, fruity, sweet [45] | 250 [45] | 0.02 |
| Ethyl decanoate | 8.8 ± 0.74 | Grape, floral, soapy [30] | 6300 [29] | <0.01 |
| Ethyl 3-methylbutyl butanedioate | 19.8 ± 2.39 | - | - | - |
| Ethyl cinnamate | 4.6 ± 0.56 | Strawberry, fruity, honey, cinnamon [46] | 1.1 [46] | 4.2 |
| Ethyl dodecanoate | 5.0 ± 0.85 | - | 5900 [29] | <0.01 |
| Methyl palmitate | 7.4 ± 0.36 | Ester-like [31] | 2000 [47] | <0.01 |
| Isopropyl myristate | 8.9 ± 1.53 | Faintly oily, fatty [48] | - | - |
| 2-Octyl benzoate | 4.1 ± 3.61 | - | - | - |
| 2-Methylbutyl salicylate | 7.8 ± 0.35 | - | - | - |
| 1-(4-Isopropylphenyl)-2-methylpropyl acetate | 25.2 ± 2.06 | - | - | - |
| Nonyl 2-methylpropanoate | 2.8 ± 4.85 | - | - | - |
| Hydrocarbons | ||||
| Tetradecane | 1.1 ± 1.83 | - | - | - |
| Hexadecane | 14.3 ± 3.21 | - | - | - |
| Phytane | 36.9 ± 2.95 | - | - | - |
| Carboxylic acid | ||||
| Octanoic acid | 19.0 ± 3.20 | Fatty acid, cheese [45] | 500 [45] | 0.04 |
| Decanoic acid | 4.2 ± 0.52 | Rancid fat [30] | 10,000 [29] | <0.01 |
| Tetradecanoic acid | 13.6 ± 3.87 | - | 10,000 [41] | <0.01 |
| Pentadecanoic acid | 20.3 ± 12.78 | - | 10,000 [29] | <0.01 |
| 4-Octylbenzoic acid | 32.2 ± 7.88 | - | - | - |
| Terpenes | ||||
| 1,8-Cineole | 35.0 ± 3.13 | Camphor, cool, eucalyptol, minty [31] | 1.3 [29] | 26.9 |
| Isomethyl-β-ionone | 5.7 ± 2.35 | - | - | - |
| β-Selinene | 13.7 ± 4.33 | Herbal [31] | - | - |
| Juniper camphor | 2.9 ± 2.73 | - | - | - |
3.2. Anthocyanin Content of Jabuticaba Wine
3.3. Phenolic and Flavonoid Contents and Antioxidant Capacity
3.4. Hematoprotective Effect of Jabuticaba Wine Extracts
3.5. Chemical Profile of Jabuticaba Wine Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethical Statement
References
- Chen, Y.; Yin, H.; Wu, X.; Shi, X.; Qi, K.; Zhang, S. Comparative analysis of the volatile organic compounds in mature fruits of 12 Occidental pear (Pyrus communis L.) cultivars. Sci. Hortic. 2018, 240, 239–248. [Google Scholar] [CrossRef]
- Plagemann, I.; Krings, U.; Berger, R.G.; Marostica Jr, M.R. Volatile constituents of jabuticaba (Myrciaria jaboticaba (Vell.) O. Berg) fruits. J. Essent. Oil Res. 2012, 24, 45–51. [Google Scholar] [CrossRef]
- Paula, D.G.C.D.; Vasconcelos, C.M.; Pereira, A.F.; Quintão, A.L.D.C.; Chaves, J.O.; Parreiras, P.M.; Menezes, C.C. Maceration time over antioxidant activity, volatile compounds and temporal dominant sensation of fermented jabuticaba. Food Sci. Technol. 2022, 42. [Google Scholar] [CrossRef]
- De Sá, L.Z.M.; Castro, P.F.; Lino, F.M.; Bernardes, M.J.; Viegas, J.C.; Dinis, T.C.; Santana, M.J.; Romao, W.; Vaz, B.G.; Lião, L.M.; et al. Antioxidant potential and vasodilatory activity of fermented beverages of jabuticaba berry (Myrciaria jaboticaba). J. Funct. Foods 2014, 8, 169–179. [Google Scholar] [CrossRef]
- Morales, P.; Barros, L.; Dias, M.I.; Santos-Buelga, C.; Ferreira, I.C.; Asquieri, E.R.; Berrios, J.D.J. Non-fermented and fermented jabuticaba (Myrciaria cauliflora Mart.) pomaces as valuable sources of functional ingredients. Food Chem. 2016, 208, 220–227. [Google Scholar] [CrossRef] [PubMed]
- De Souza, A.C.; Fernandes, A.C.; Silva, M.S.; Schwan, R.F.; Dias, D.R. Antioxidant activities of tropical fruit wines. J. Inst. Brew. 2018, 124, 492–497. [Google Scholar] [CrossRef]
- Zhang, J.; Stanley, R.A.; Adaim, A.; Melton, L.D.; Skinner, M.A. Free radical scavenging and cytoprotective activities of phenolic antioxidants. Mol. Nutr. Food Res. 2006, 50, 996–1005. [Google Scholar] [CrossRef]
- Vona, R.; Gambardella, L.; Cittadini, C.; Straface, E.; Pietraforte, D. Biomarkers of oxidative stress in metabolic syndrome and associated diseases. Oxid. Med. Cell. Longev. 2019, 2019, 8267234. [Google Scholar] [CrossRef]
- Bissinger, R.; Bhuyan, A.A.M.; Qadri, S.M.; Lang, F. Oxidative stress, eryptosis and anemia: A pivotal mechanistic nexus in systemic diseases. FEBS J. 2019, 286, 826–854. [Google Scholar] [CrossRef]
- Scott, M.D.; Van Den Berg, J.J.; Repka, T.; Rouyer-Fessard, P.; Hebbel, R.P.; Beuzard, Y.; Lubin, B.H. Effect of excess alpha-hemoglobin chains on cellular and membrane oxidation in model beta-thalassemic erythrocytes. J. Clin. Investig. 1993, 91, 1706–1712. [Google Scholar] [CrossRef]
- Tedesco, I.; Russo, M.; Russo, P.; Iacomino, G.; Russo, G.L.; Carraturo, A.; Faruolo, C.; Moio, L.; Palumbo, R. Antioxidant effect of red wine polyphenols on red blood cells. J. Nutr. Biochem. 2000, 11, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Pazzini, C.E.; Colpo, A.C.; Poetini, M.R.; Pires, C.F.; de Camargo, V.B.; Mendez, A.S.; Azevedo, M.L.; Soares, J.C.; Folmer, V. Effects of red wine tannat on oxidative stress induced by glucose and fructose in erythrocytes in vitro. Int. J. Med. Sci. 2015, 12, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, I.; Spagnuolo, C.; Russo, G.L.; Russo, M.; Cervellera, C.; Moccia, S. The pro-oxidant activity of red wine polyphenols induces an adaptive antioxidant response in human erythrocytes. Antioxidants 2021, 10, 800. [Google Scholar] [CrossRef] [PubMed]
- Macedo, E.H.; Santos Jr, G.C.; Santana, M.N.; Jesus, E.F.; de Araújo, U.B.; Anjos, M.J.; Pinheiro, A.S.; Carneiro, C.S.; Rodrigues, I.A. Unveiling the physicochemical properties and chemical profile of artisanal jabuticaba wines by bromatological and NMR-based metabolomics approaches. LWT 2021, 146, 111371. [Google Scholar] [CrossRef]
- Ghiselli, A.; Nardini, M.; Baldi, A.; Scaccini, C. Antioxidant activity of different phenolic fractions separated from an italian red wine. J. Agric. Food Chem. 1998, 46, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.R.; Ramos, A.S.; Amaral, A.C.F.; Ferreira, J.L.P.; Carneiro, C.S.; Silva, J.R.A. Evaluation of Amazon fruits: Chemical and nutritional studies on Borojoa sorbilis. J. Sci. Food Agric. 2018, 98, 3943–3952. [Google Scholar] [CrossRef]
- Wang, X.; Xie, K.; Zhuang, H.; Ye, R.; Fang, Z.; Feng, T. Volatile flavor compounds, total polyphenolic contents and antioxidant activities of a China gingko wine. Food Chem. 2015, 182, 41–46. [Google Scholar] [CrossRef]
- Gouvêa, A.C.; Melo, A.; Santiago, M.C.; Peixoto, F.M.; Freitas, V.; Godoy, R.L.; Ferreira, I.M. Identification and quantification of anthocyanins in fruits from Neomitranthes obscura (DC.) N. Silveira an endemic specie from Brazil by comparison of chromatographic methodologies. Food Chem. 2015, 185, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Aires, E.; Barreira, J.C.M.; Estevinho, L.M. Antioxidant activity of Portuguese honey samples: Different contributions of the entire honey and phenolic extract. Food Chem. 2009, 114, 1438–1443. [Google Scholar] [CrossRef]
- Rufino, M.S.M.; Alves, R.E.; Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef]
- Mensor, L.L.; Menezes, F.S.; Leitão, G.G.; Reis, A.S.; Santos, T.C.D.; Coube, C.S.; Leitão, S.G. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef] [PubMed]
- López-Alarcón, C.; Fuentes-Lemus, E.; Figueroa, J.D.; Dorta, E.; Schöneich, C.; Davies, M.J. Azocompounds as generators of defined radical species: Contributions and challenges for free radical research. Free Radic. Biol. Med. 2020, 160, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Korivi, M.; Lin, M.K.; Chang, H.C.; Wu, C.R.; Lee, M.S.; Chen, W.T.; Hseu, Y.C. Antihemolytic and antioxidant properties of pearl powder against 2,2’-azobis(2-amidinopropane) dihydrochloride-induced hemolysis and oxidative damage to erythrocyte membrane lipids and proteins. J. Food Drug. Anal. 2017, 25, 898–907. [Google Scholar] [CrossRef]
- Olson, B.J.; Markwell, J. Assays for determination of protein concentration. Curr. Protoc. Pharmacol. 2007, 38, A.3A.1–A.3A.29. [Google Scholar] [CrossRef]
- Bolanos de la Torre, A.A.; Henderson, T.; Nigam, P.S.; Owusu-Apenten, R.K. A universally calibrated microplate ferric reducing antioxidant power (FRAP) assay for foods and applications to Manuka honey. Food Chem. 2015, 174, 119–123. [Google Scholar] [CrossRef]
- Guo, X.; Ho, C.T.; Wan, X.; Zhu, H.; Liu, Q.; Wen, Z. Changes of volatile compounds and odor profiles in Wuyi rock tea during processing. Food Chem. 2021, 341, 128230. [Google Scholar] [CrossRef]
- Jeleń, H.H.; Majcher, M.; Dziadas, M. Microextraction techniques in the analysis of food flavor compounds: A review. Anal Chim Acta. 2012, 738, 13–26. [Google Scholar] [CrossRef]
- Pino, J.A.; Mesa, J. Contribution of volatile compounds to mango (Mangifera indica L.) aroma. Flavour Fragr. J. 2006, 21, 207–213. [Google Scholar] [CrossRef]
- Malherbe, S.; Tredoux, A.G.J.; Nieuwoudt, H.H.; du Toit, M. Comparative metabolic profiling to investigate the contribution of O. oeni MLF starter cultures to red wine composition. J. Ind. Microbiol. Biotechnol. 2011, 39, 477–494. [Google Scholar] [CrossRef]
- FEMA. Flavors Extracts Manufacturers Association. Available online: https://www.femaflavor.org/ (accessed on 3 October 2022).
- Gómez-Míguez, M.J.; Cacho, J.F.; Ferreira, V.; Vicario, I.M.; Heredia, F.J. Volatile components of Zalema white wines. Food Chem. 2007, 100, 1464–1473. [Google Scholar] [CrossRef]
- Sanz, C.; Olias, J.M.; Perez, A.G. Aroma biochemistry of fruits and vegetables. In Proceedings-Phytochemical Society of Europe; Oxford University Press Inc: Oxford, UK, 1996; Volume 41, pp. 125–156. [Google Scholar]
- Echeverría, G.; Graell, J.; López, M.L.; Lara, I. Volatile production, quality and aroma-related enzyme activities during maturation of ‘Fuji’apples. Postharvest Biol. Technol. 2004, 31, 217–227. [Google Scholar] [CrossRef]
- Zohre, D.; Erten, H. The influence of Kloeckera apiculata and Candida pulcherrima yeasts on wine fermentation. Process. Biochem. 2002, 38, 319–324. [Google Scholar] [CrossRef]
- Juan, C.; Jianquan, K.; Junni, T.; Zijian, C.; Ji, L. The profile in polyphenols and volatile compounds in alcoholic beverages from different cultivars of mulberry. J. Food Sci. 2012, 77, C430–C436. [Google Scholar] [CrossRef] [PubMed]
- Hirst, M.B.; Richter, C.L. Review of aroma formation through metabolic pathways of Saccharomyces cerevisiae in beverage fermentations. Am. J. Enol. Vitic. 2016, 67, 4. [Google Scholar] [CrossRef]
- Ahmed, E.M.; Dennison, R.A.; Dougherty, R.H.; Shaw, P.E. Flavor and odor thresholds in water of selected orange juice components. J. Agric. Food Chem. 1978, 26, 187–191. [Google Scholar] [CrossRef]
- Varlet, V.; Knockaert, C.; Prost, C.; Serot, T. Comparison of odor-active volatile compounds of fresh and smoked salmon. J. Agri. Food Chem. 2006, 54, 3391–3401. [Google Scholar] [CrossRef] [PubMed]
- Pino, J.A.; Quijano, C.E. Study of the volatile compounds from plum (Prunus domestica L. cv. Horvin) and estimation of their contribution to the fruit aroma. Ciênc. Tecnol. Aliment. 2012, 32, 76–83. [Google Scholar] [CrossRef]
- Leffingwell, J.C.; Leffingwell, D. GRAS flavor chemicals-detection thresholds. Perfum. flavor. 1991, 16, 1–19. [Google Scholar]
- Pino, J.A.; Bent, L. Odour-active compounds in guava (Psidium guajava L. cv. Red Suprema). J. Sci. Food Agric. 2013, 93, 3114–3120. [Google Scholar] [CrossRef]
- Nobrega, I.C.; Ataíde, C.S.; Moura, O.M.; Livera, A.V.; Menezes, P.H. Volatile constituents of cooked bullfrog (Rana catesbeiana) legs. Food Chem. 2007, 102, 186–191. [Google Scholar] [CrossRef]
- Shi, J.; Tong, G.; Yang, Q.; Huang, M.; Ye, H.; Liu, Y.; Wu, J.; Zhang, J.; Sun, X.; Zhao, D. Characterization of key aroma compounds in tartary buckwheat (Fagopyrum tataricum Gaertn.) by means of sensory-directed flavor analysis. J. Agric. Food Chem. 2021, 69, 11361–11371. [Google Scholar] [CrossRef]
- Chang, E.H.; Jung, S.M.; Hur, Y.Y. Changes in the aromatic composition of grape cv. Cheongsoo wine depending on the degree of grape ripening. Food Sci. Biotechnol. 2014, 23, 1761–1771. [Google Scholar] [CrossRef]
- Gómez García-Carpintero, E.; Sánchez-Palomo, E.; González Viñas, M.A. Volatile composition of Bobal red wines subjected to alcoholic/malolactic fermentation with oak chips. LWT 2014, 55, 586–594. [Google Scholar] [CrossRef]
- Buttery, R.G.; Turnbaugh, J.G.; Ling, L.C. Contribution of volatiles to rice aroma. J. Agric. Food Chem. 1988, 36, 1006–1009. [Google Scholar] [CrossRef]
- Joshi, R.; Gulati, A. Fractionation and identification of minor and aroma-active constituents in Kangra orthodox black tea. Food Chem. 2015, 167, 290–298. [Google Scholar] [CrossRef]
- Inada, K.O.P.; Oliveira, A.A.; Revorêdo, T.B.; Martins, A.B.N.; Lacerda, E.C.Q.; Freire, A.S.; Braz, B.F.; Santelli, R.E.; Torres, A.G.; Perrone, D.; et al. Screening of the chemical composition and occurring antioxidants in jabuticaba (Myrciaria jaboticaba) and jussara (Euterpe edulis) fruits and their fractions. J. Funct. Foods 2015, 17, 422–433. [Google Scholar] [CrossRef]
- Da Silva, F.L.; Escribano-Bailón, M.T.; Alonso, J.J.P.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. Anthocyanin pigments in strawberry. LWT 2007, 40, 374–382. [Google Scholar] [CrossRef]
- Quatrin, A.; Pauletto, R.; Maurer, L.H.; Minuzzi, N.; Nichelle, S.M.; Carvalho, J.F.C.; Junior, M.M.; Rodrigues, E.; Bochi, V.C.; Emanuelli, T. Characterization and quantification of tannins, flavonols, anthocyanins and matrix-bound polyphenols from jaboticaba fruit peel: A comparison between Myrciaria trunciflora and M. jaboticaba. J. Food Compos. Anal. 2019, 78, 59–74. [Google Scholar] [CrossRef]
- Fernandes, I.A.A.; Maciel, G.M.; Maroldi, W.V.; Bortolini, D.G.; Pedro, A.C.; Haminiuk, C.W.I. Bioactive compounds, health-promotion properties and technological applications of Jabuticaba: A literature overview. Meas. Food 2022, 8, 100057. [Google Scholar] [CrossRef]
- Geraldi, M.V.; Cazarin, C.B.B.; Dias-Audibert, F.L.; Pereira, G.A.P.; Carvalho, G.G.; Kabuki, D.Y.; Catharino, R.R.; Pastore, G.M.; Behrens, M.C.; Júnior Marostica, M.R. Influence of high isostatic pressure and thermal pasteurization on chemical composition, color, antioxidant properties and sensory evaluation of jabuticaba juice. LWT 2021, 139, 110548. [Google Scholar] [CrossRef]
- Santos, R.O.; Trindade, S.C.; Maurer, L.H.; Bersch, A.M.; Sautter, C.K.; Penna, N.G. Physicochemical, Antioxidant and Sensory Quality of Brazilian Blueberry Wine. An. Acad. Bras. Cienc. 2016, 88, 1557–1568. [Google Scholar] [CrossRef]
- Lenquiste, S.A.; da Silva Marineli, R.; Moraes, É.A.; Dionísio, A.P.; de Brito, E.S.; Junior, M.R. Jaboticaba peel and jaboticaba peel aqueous extract shows in vitro and in vivo antioxidant properties in obesity model. Food Res. Int. 2015, 77, 162–170. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. Free radical scavenging capacity and inhibition of lipid oxidation of wines, grape juices and related polyphenolic constituents. Food Res. Int. 1999, 32, 407–412. [Google Scholar] [CrossRef]
- Hacke, A.C.M.; Valério, T.P.; Cubo, M.F.; Lima, D.; Pessôa, C.A.; Vellosa, J.C.R.; Pereira, R.P. Antioxidant capacity of Myrciaria cauliflora seed extracts by spectrophotometric, biochemical, and electrochemical methods and its protective effect against oxidative damage in erythrocytes. J. Food Biochem. 2022, 46, e14222. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Liu, K.; Zhao, S.; Chen, F. Changes in antioxidant activity of peptides identified from brown rice hydrolysates under different conditions and their protective effects against AAPH-induced oxidative stress in human erythrocytes. ACS Omega 2020, 5, 12751–12759. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.N.; Wu, W.J.; Sun, C.Z.; Liu, H.F.; Chen, W.B.; Zhan, Q.P.; Lei, Z.G.; Xin, X.; Ma, J.J.; Yao, K.; et al. Antioxidant and anti-inflammatory capacity of ferulic acid released from wheat bran by solid-state fermentation of Aspergillus niger. Biomed. Environ. Sci. 2019, 32, 11–21. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, T.; Jiang, J.; Wong, Y.S.; Yang, F.; Zheng, W. Selenium-containing allophycocyanin purified from selenium-enriched Spirulina platensis attenuates AAPH-induced oxidative stress in human erythrocytes through inhibition of ROS generation. J. Agric. Food Chem. 2011, 59, 8683–8690. [Google Scholar] [CrossRef]
- Takebayashi, J.; Kaji, H.; Ichiyama, K.; Makino, K.; Gohda, E.; Yamamoto, I.; Tai, A. Inhibition of free radical-induced erythrocyte hemolysis by 2-O-substituted ascorbic acid derivatives. Free Radic. Biol. Med. 2007, 43, 1156–1164. [Google Scholar] [CrossRef]
- Leonard, W.; Zhang, P.; Ying, D.; Adhikari, B.; Fang, Z. Fermentation transforms the phenolic profiles and bioactivities of plant-based foods. Biotechnol. Adv. 2021, 49, 107763. [Google Scholar] [CrossRef] [PubMed]
- Keshavarzi, A.; Seifikar, S.; Ranjbar, A.; Khiripour, N.; Ghaleiha, A.; Soltaniyan, A.; Rafieemehr, H. Effect of fresh red grape juice and grape fermentative product on oxidative-stress in human erythrocytes in vitro. Arch. Biotechnol. Biomed. 2022, 6, 001–006. [Google Scholar] [CrossRef]
- Ferreira, P.R.; Pinheiro, F.D.A.; Duarte, M.D.S.; da Silva, W.; dos Reis, N.R.; Marques, D.B.D.; Bastos, D.S.S.; Fontes, E.A.F. Effect of jaboticaba and pequi extracts on gene expression of antioxidant enzymes in C2C12 mouse muscle cells. Res. Soc. Dev. 2021, 10, e375101018864. [Google Scholar] [CrossRef]
- Alezandro, M.R.; Dubé, P.; Desjardins, Y.; Lajolo, F.M.; Genovese, M.I. Comparative study of chemical and phenolic compositions of two species of jaboticaba: Myrciaria jaboticaba (Vell.) Berg and Myrciaria cauliflora (Mart.) O. Berg. Food Res. Int. 2013, 54, 468–477. [Google Scholar] [CrossRef]
- De Andrade Neves, N.; Stringheta, P.C.; da Silva, I.F.; García-Romero, E.; Gómez-Alonso, S.; Hermosín-Gutiérrez, I. Identification and quantification of phenolic composition from different species of Jabuticaba (Plinia spp.) by HPLC-DAD-ESI/MSn. Food Chem. 2021, 355, 129605. [Google Scholar] [CrossRef]
- Anesini, C.; Turner, S.; Cogoi, L.; Filip, R. Study of the participation of caffeine and polyphenols on the overall antioxidant activity of mate (Ilex paraguariensis). LWT 2012, 45, 299–304. [Google Scholar] [CrossRef]
| Sample | ABTS (EC50 µg·mL−1) | DPPH (EC50 µg·mL−1) |
|---|---|---|
| JWE | 3.89 (±0.22) a | 30.17 (±8.89) a |
| JWF1 | 3.30 (±0.11) a,b | 18.85 (±4.96) b |
| JWF2 | 2.15 (±0.65) b | 5.18 (±3.58) c |
| JWF3 | 1.22 (±0.10) b | 6.20 (±2.06) c |
| Standard Trolox | 1.05 (±0.26) b | 3.05 (±0.19) c |
| Compounds | RT (min) | Ionization Mode | Experimental Mass | Absolute Area (Relative %) | ||||
|---|---|---|---|---|---|---|---|---|
| JWE | JWF1 | JWF2 | JWF3 | JWR | ||||
| Citric acid | 13.53 | [M-H]− | 191.020 | 7.8 × 107 (23.8) | 1.7 × 108 (51.8) | 3.9 × 105 (0.1) | 5.8 × 107 (17.7) | 2.2 × 107 (6.7) |
| Cyanidin | 12.60 | [M+H]+ | 287.0526 | 1.7 × 109 (98.6) | 9.1 × 106 (0.5) | - | 1.4 × 107 (0.8) | 8.2 × 105 (0.1) |
| Cyanidin-3-O-glucoside | 13.86 | [M+H]+ | 449.1052 | 5.8 × 105 (0.5) | - | - | - | 1.1 × 108 (99.5) |
| Delphinidin | 12.29 | [M+H]+ | 303.0499 | 3.4 × 106 (0.4) | 8.4 × 108 (96.5) | - | 2.7 × 107 (3.1) | - |
| Ellagic acid | 11.84 | [M-H]− | 300.9989 | 1.1 × 108 (59.7) | - | 1.3 × 106 (0.7) | 7.0 × 106 (3.8) | 6.6 ×107 (35.8) |
| Ellagic acid hexose | 11.70 | [M-H]− | 481.0620 | 6.6 × 108 (99.6) | - | - | - | 2.6 × 106 (0.4) |
| Gallic acid | 12.66 | [M-H]− | 169.0143 | 3.3 × 108 (12.9) | 3.7 × 107 (14.5) | 1.5 × 107 (0.6) | 3.7 × 108 (1.4) | 1.8 × 109 (70.5) |
| Glucose/ Fructose | 12.19 | [M-H]− | 179.0563 | 6.1 × 107 (89.2) | - | 3.4 × 106 (5.0) | - | 4.0 × 106 (5.8) |
| Guaijaverin | 11.69 | [M-H]− | 433.0797 | 2.1 × 108 (90.8) | - | 2.3 × 106 (1.0) | 9.0 × 105 (0.4) | 1.8 × 107 (7.8) |
| Isomyricitrin | 11.44 | [M-H]− | 479.0856 | 8.7 × 107 (92.6) | - | 8.3 × 105 (0.9) | - | 6.1 × 106 (6.5) |
| isso-oenothein C | 11.26 | [M-H]− | 783.0702 | 1.7 × 108 (99.9) | - | - | - | 1.3 × 105 (0.1) |
| Isoquercitrin | 11.86 | [M-H]− | 463.0884 | 8.5 × 107 (99.3) | - | 4.6 × 105 (0.5) | - | 1.7 × 105 (0.1) |
| Lactose/sucrose | 10 | [M-H]− | 341.1090 | 8.1 × 107 (95.3) | - | 2.6 × 106 (3.1) | - | 1.4 × 106 (1.6) |
| Malic acid | 11.80 | [M-H]− | 133.0143 | 2.8 × 108 (53.1) | - | 4.5 × 106 (0.8) | 3.1 × 106 (0.6) | 2.4 × 108 (45.5) |
| Monogalloyl glucose | 11.93 | [M-H]− | 331.0660 | 4.3 × 108 (84.2) | - | 1.3 × 105 (0.1) | 2.6 × 105 (0.1) | 8.0 × 107 (15.7) |
| Myricetin | 11.55 | [M+H]+ | 319.0423 | 1.3 ×109 (75.6) | 4.2 × 108 (24.4) | - | - | - |
| Quinic acid | 12.83 | [M-H]− | 191.0562 | 9.0 × 108 (72.1) | 1.7 × 108 (13.6) | 6.7 × 106 (0.5) | 2.0 × 106 (0.2) | 1.7 × 108 (13.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, R.; Silva, M.V.T.; Gomes, B.A.; Macedo, E.H.B.C.; Santana, M.N.; Amaral, A.C.F.; Silva, J.R.A.; Corrêa, P.G.; Godoy, R.L.O.; Santiago, M.C.P.A.; et al. Chemical Profile and Hematoprotective Activity of Artisanal Jabuticaba (Plinia jabuticaba) Wine and Derived Extracts. Fermentation 2023, 9, 157. https://doi.org/10.3390/fermentation9020157
Lima R, Silva MVT, Gomes BA, Macedo EHBC, Santana MN, Amaral ACF, Silva JRA, Corrêa PG, Godoy RLO, Santiago MCPA, et al. Chemical Profile and Hematoprotective Activity of Artisanal Jabuticaba (Plinia jabuticaba) Wine and Derived Extracts. Fermentation. 2023; 9(2):157. https://doi.org/10.3390/fermentation9020157
Chicago/Turabian StyleLima, Raissa, Marcos Vinicius T. Silva, Brendo A. Gomes, Ellis Helena B. C. Macedo, Michele N. Santana, Ana Claudia F. Amaral, Jefferson R. A. Silva, Pollyane G. Corrêa, Ronoel Luiz O. Godoy, Manuela Cristina P. A. Santiago, and et al. 2023. "Chemical Profile and Hematoprotective Activity of Artisanal Jabuticaba (Plinia jabuticaba) Wine and Derived Extracts" Fermentation 9, no. 2: 157. https://doi.org/10.3390/fermentation9020157





