Cellulosic Fiber Waste Feedstock for Bioethanol Production via Bioreactor-Dependent Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
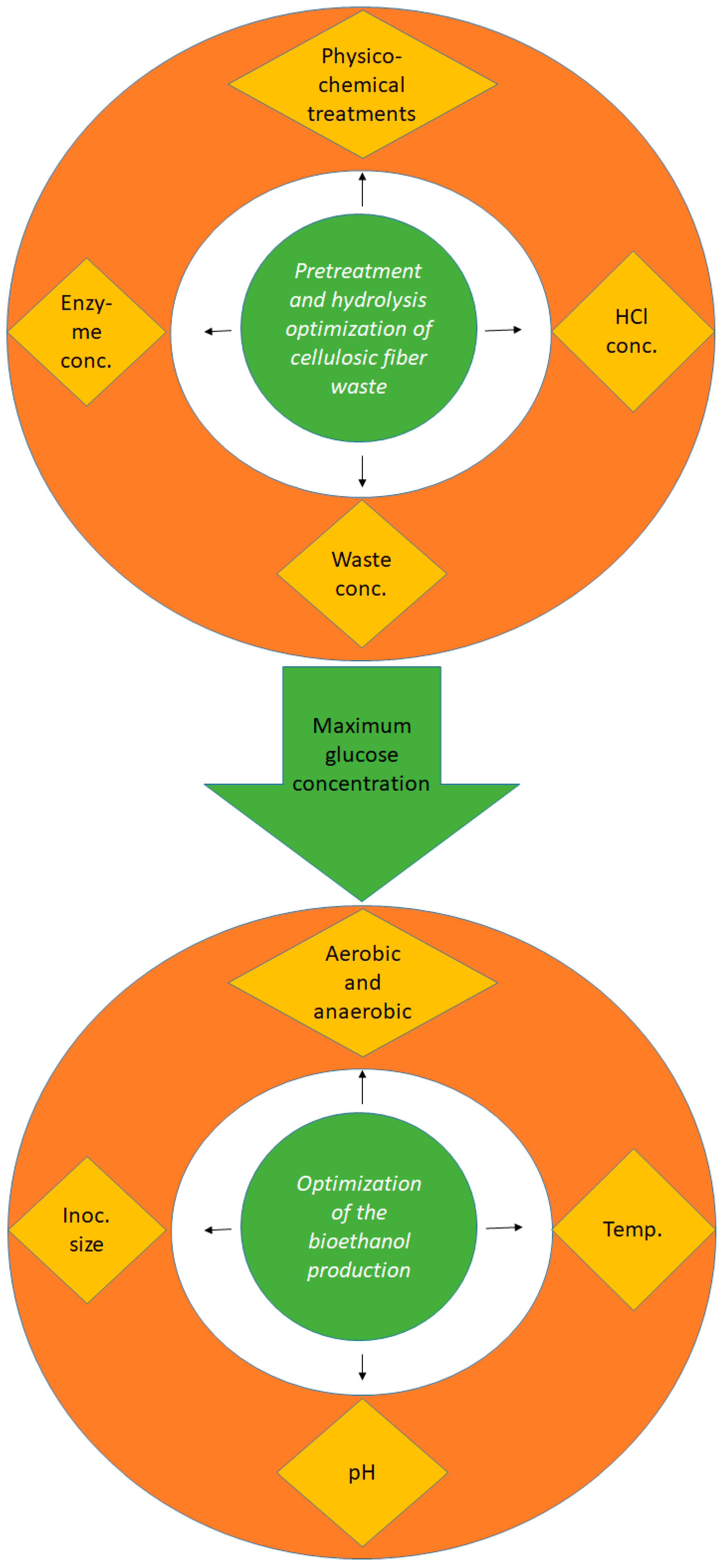
2.2. Optimization of the Conditions Used for the Waste Hydrolysis
2.2.1. Screening of the Optimum Chemical and Physical (Physico-Chemical) Treatments
2.2.2. Optimization of HCl Concentration
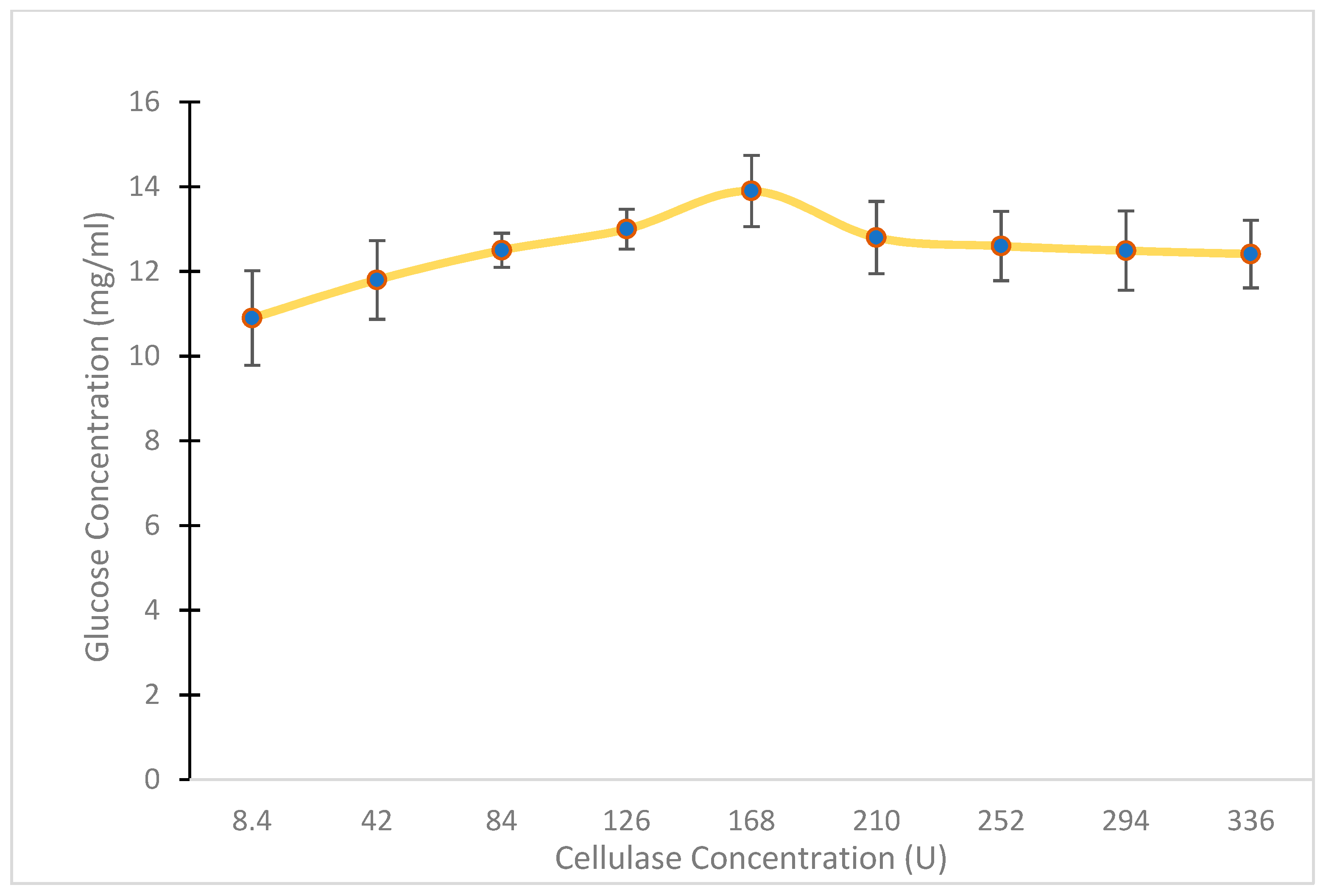
2.2.3. Optimization of Cellulosic Waste Concentration
2.2.4. Mixed Physico-Chemical and Enzymatic Hydrolysis
2.3. Characterization of the Cellulosic Fiber Waste
2.4. Yeast Isolation and Screening for Ethanol Production
2.5. Determination of Glucose and Bioethanol Concentration
2.6. Optimization of the Bioethanol Production Conditions
2.6.1. Effect of Aerobic and Anaerobic Cultivation on Bioethanol Production
2.6.2. Effect of the Cultivation Temperature on the Bioethanol Production
2.6.3. Effect of Medium pH on the Bioethanol Production
2.6.4. Effect of Yeast’s Inoculum Size on Bioethanol Production
2.7. Conversion Yield
2.8. Molecular Identification of the Yeast Isolate (G4)
2.8.1. DNA Extraction
2.8.2. PCR of 23S rRNA Gene
2.8.3. Sequencing and GenBank Submission
2.9. Bioreactor Cultivation
3. Results and Discussion
3.1. Determination of the Optimum Glucose Production Conditions
3.1.1. Determination of the Optimum Physico-Chemical Treatments
3.1.2. Determination of the Optimum HCl Concentration
3.1.3. Optimization of Cellulosic Waste Concentration
3.1.4. Mixed Physico-Chemical and Enzymatic Hydrolysis
3.2. Waste Characterization
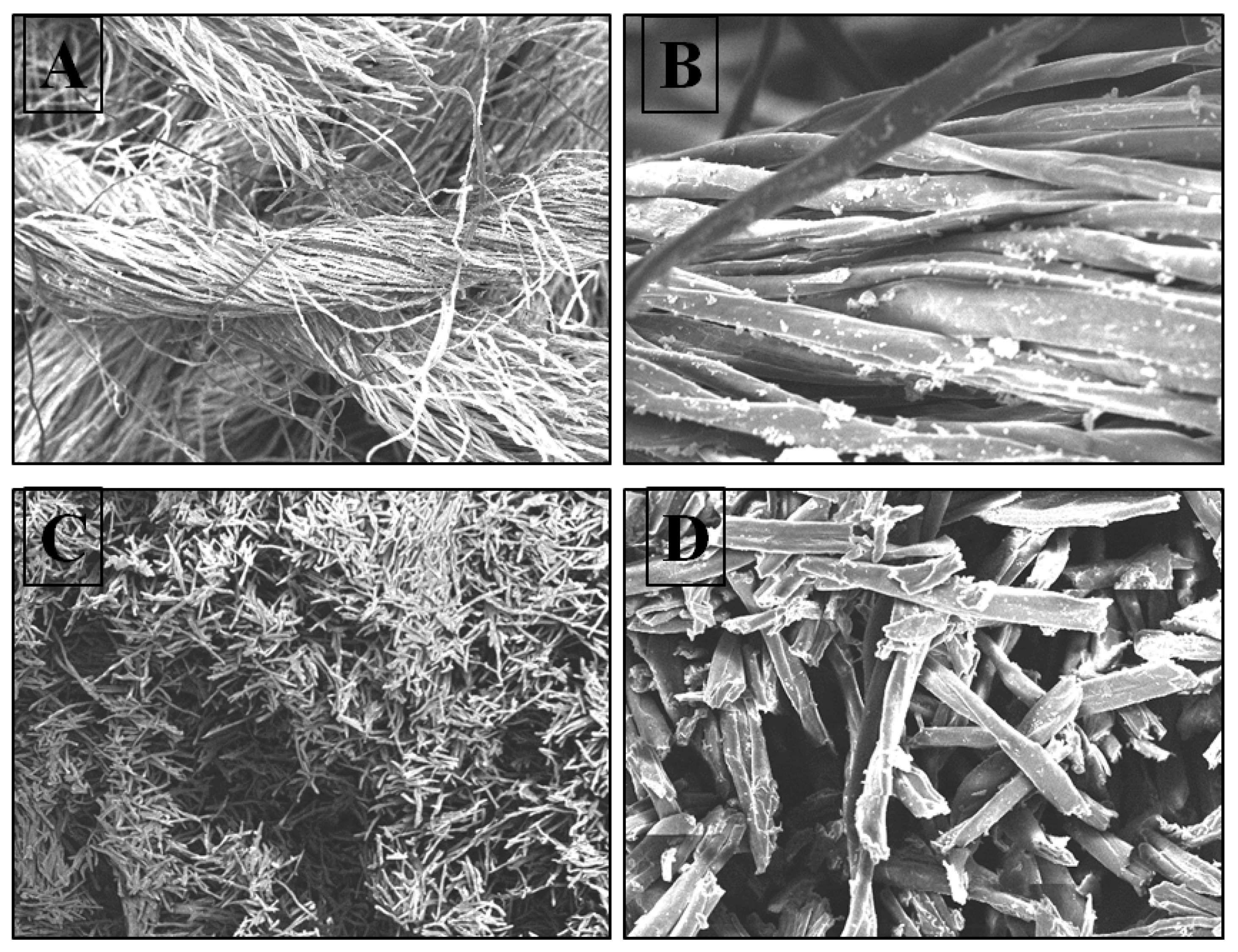
3.2.1. SEM
3.2.2. FTIR Spectroscopic Analysis
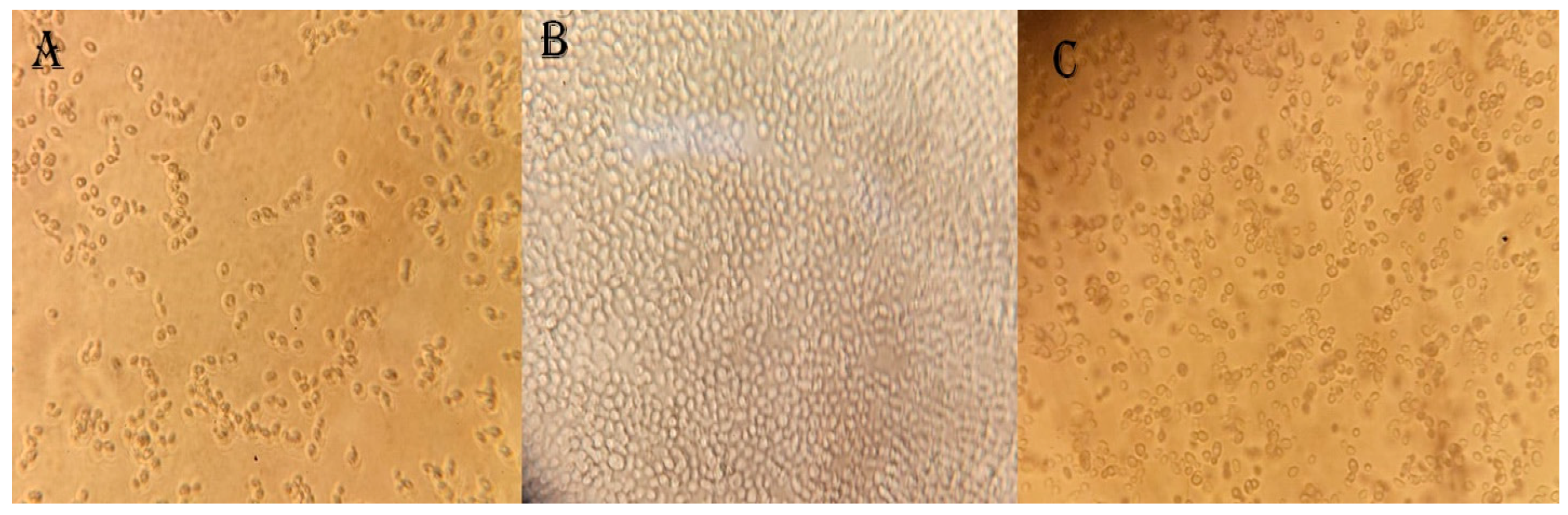
3.3. Yeast Isolation from Different Fruit Samples
3.4. Screening the Bioethanol Production Potential of Isolated Yeast
3.5. Optimization of Bioethanol Production Conditions
3.5.1. Effect of Aerobic and Anaerobic Fermentation
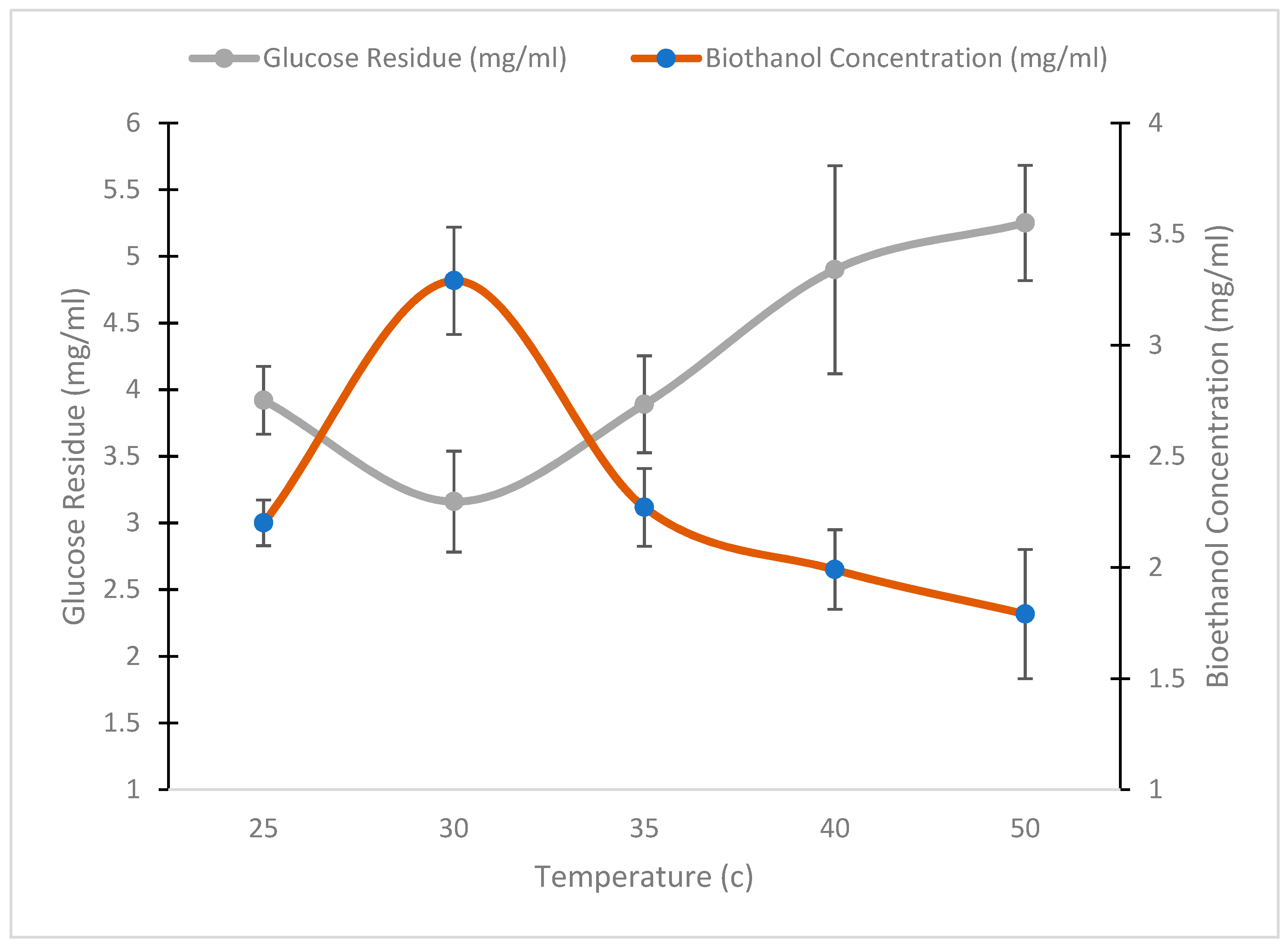
3.5.2. Effect of Cultivation Temperature
3.5.3. Effect of Medium pH
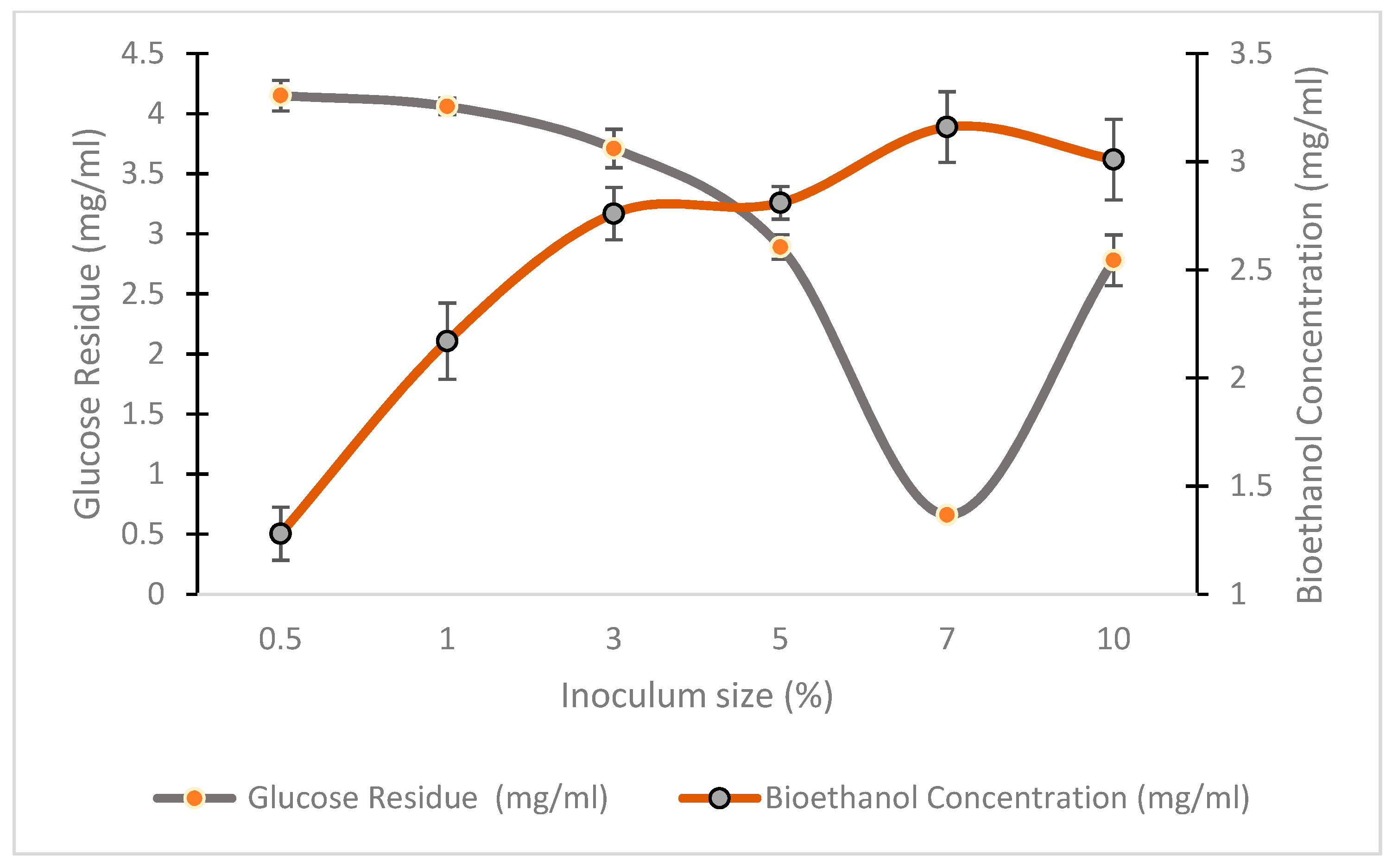
3.5.4. Effect of Inoculum Size
3.6. Molecular Identification of G4 Isolate
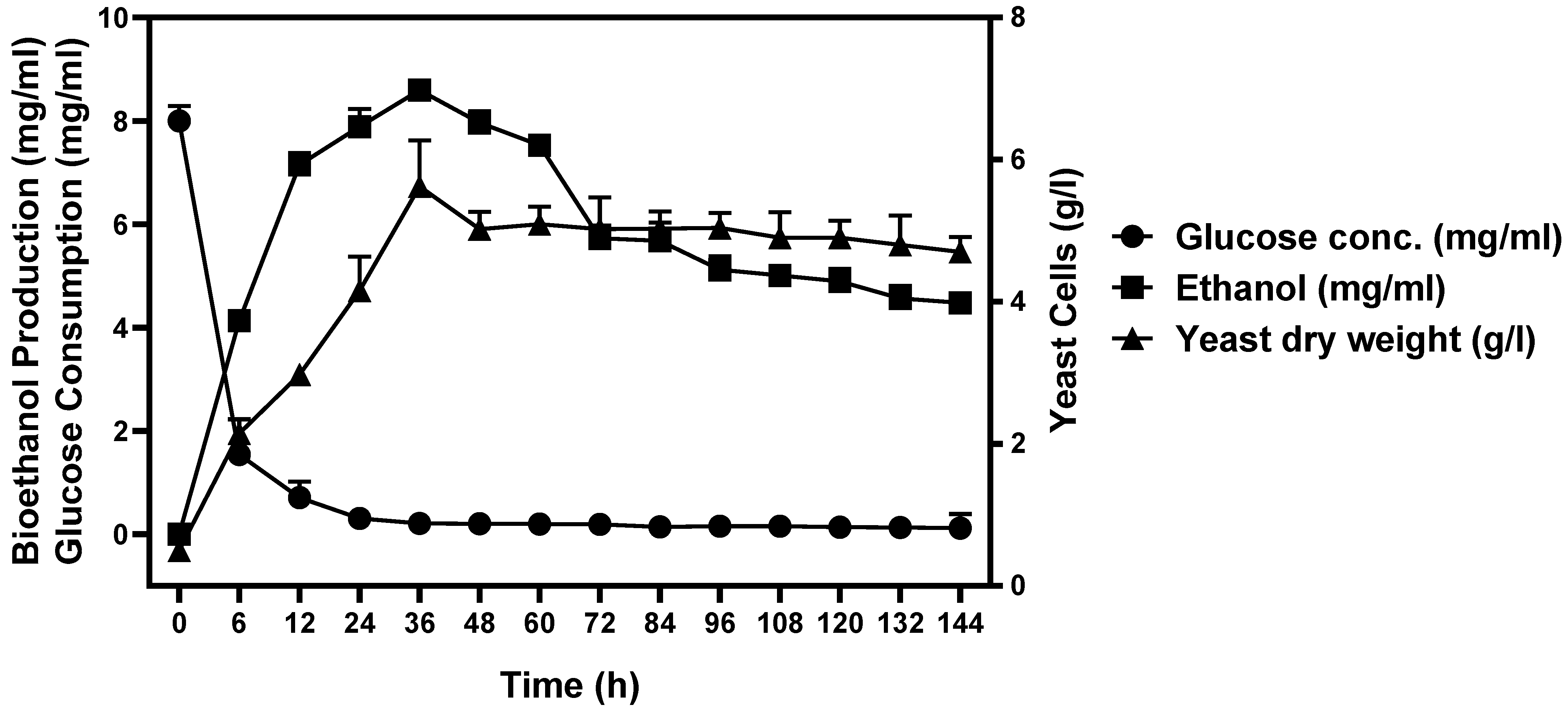
3.7. Bioreactor Cultivation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hussain, A.; Arif, S.M.; Aslam, M. Emerging renewable and sustainable energy technologies: State of the art. Renew. Sustain. Energy Rev. 2017, 71, 12–28. [Google Scholar] [CrossRef]
- Khandaker, S.; Bashar, M.M.; Islam, A.; Hossain, M.T.; Teo, S.H.; Awual, M.R. Sustainable energy generation from textile biowaste and its challenges: A comprehensive review. Renew. Sustain. Energy Rev. 2022, 157, 112051. [Google Scholar] [CrossRef]
- Parry, M.; Arnell, N.; McMichael, T.; Nicholls, R.; Martens, P.; Kovats, S.; Livermore, M.; Rosenzweig, C.; Iglesias, A.; Fischer, G. Millions at risk: Defining critical climate change threats and targets. Glob. Environ. Chang. 2001, 11, 181–183. [Google Scholar] [CrossRef]
- Khan, S.A.; Rashmi; Hussain, M.Z.; Prasad, S.; Banerjee, U. Prospects of biodiesel production from microalgae in India. Renew. Sustain. Energy Rev. 2009, 13, 2361–2372. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, Z.-F.; Zhang, C.-C.; Nan, J.; Ren, N.-Q.; Lee, D.-J.; Chen, C. Advances in pretreatment of lignocellulosic biomass for bioenergy production: Challenges and perspectives. Bioresour. Technol. 2021, 343, 126123. [Google Scholar] [CrossRef]
- Nieves, D.C.; Alanís, M.J.R.; Quiroz, R.D.L.C.; Ruiz, H.A.; Iqbal, H.M.; Parra-Saldívar, R. Current status and future trends of bioethanol production from agro-industrial wastes in Mexico. Renew. Sustain. Energy Rev. 2019, 102, 63–74. [Google Scholar] [CrossRef]
- Gandam, P.K.; Chinta, M.L.; Gandham, A.P.; Pabbathi, N.P.P.; Konakanchi, S.; Bhavanam, A.; Atchuta, S.R.; Baadhe, R.R.; Bhatia, R.K. A New Insight into the Composition and Physical Characteristics of Corncob—Substantiating Its Potential for Tailored Biorefinery Objectives. Fermentation 2022, 8, 704. [Google Scholar] [CrossRef]
- Melendez, J.R.; Mátyás, B.; Hena, S.; Lowy, D.A.; El Salous, A. Perspectives in the production of bioethanol: A review of sustainable methods, technologies, and bioprocesses. Renew. Sustain. Energy Rev. 2022, 160, 112260. [Google Scholar] [CrossRef]
- Szulczyk, K.R.; Ziaei, S.M.; Zhang, C. Environmental ramifications and economic viability of bioethanol production in Malaysia. Renew. Energy 2021, 172, 780–788. [Google Scholar] [CrossRef]
- Jarunglumlert, T.; Bampenrat, A.; Sukkathanyawat, H.; Prommuak, C. Enhanced Energy Recovery from Food Waste by Co-Production of Bioethanol and Biomethane Process. Fermentation 2021, 7, 265. [Google Scholar] [CrossRef]
- Sarris, D.; Papanikolaou, S. Biotechnological production of ethanol: Biochemistry, processes and technologies. Eng. Life Sci. 2016, 16, 307–329. [Google Scholar] [CrossRef] [Green Version]
- Branco, R.H.R.; Serafim, L.S.; Xavier, A.M.R.B. Second Generation Bioethanol Production: On the Use of Pulp and Paper Industry Wastes as Feedstock. Fermentation 2018, 5, 4. [Google Scholar] [CrossRef] [Green Version]
- Zeghlouli, J.; Christophe, G.; Ogura, K.; Sawamura, N.; Guendouz, A.; El Modafar, C.; Michaud, P.; Delattre, C. High-Pressure Water Jet System Treatment of Argan Nut Shell and Enzymatic Hydrolysis for Bioethanol Production. Fermentation 2022, 8, 627. [Google Scholar] [CrossRef]
- Rosen, Y.; Mamane, H.; Gerchman, Y. Immersed ozonation of agro-wastes as an effective pretreatment method in bioethanol production. Renew. Energy 2021, 174, 382–390. [Google Scholar] [CrossRef]
- Garlapati, V.K.; Chandel, A.K.; Kumar, S.J.; Sharma, S.; Sevda, S.; Ingle, A.P.; Pant, D. Circular economy aspects of lignin: Towards a lignocellulose biorefinery. Renew. Sustain. Energy Rev. 2020, 130, 109977. [Google Scholar] [CrossRef]
- Derman, E.; Abdulla, R.; Marbawi, H.; Sabullah, M.K.; Gansau, J.A.; Ravindra, P. Simultaneous saccharification and fermentation of empty fruit bunches of palm for bioethanol production using a microbial consortium of S. cerevisiae and T. harzianum. Fermentation 2022, 8, 295. [Google Scholar] [CrossRef]
- Widiarti, N.; Ni’Mah, Y.L.; Bahruji, H.; Prasetyoko, D. Development of CaO from Natural Calcite as a Heterogeneous Base Catalyst in the Formation of Biodiesel: Review. J. Renew. Mater. 2019, 7, 915–939. [Google Scholar] [CrossRef]
- Wong, K.H.; Tan, I.S.; Foo, H.C.Y.; Chin, L.M.; Cheah, J.R.N.; Sia, J.K.; Tong, K.T.X.; Lam, M.K. Third-generation bioeth-anol and L-lactic acid production from red macroalgae cellulosic residue: Prospects of Industry 5.0 algae. Energy Convers. Manag. 2022, 253, 115155. [Google Scholar] [CrossRef]
- Jusakulvijit, P.; Bezama, A.; Thrän, D. An Integrated Assessment of GIS-MCA with Logistics Analysis for an Assess-ment of a Potential Decentralized Bioethanol Production System Using Distributed Agricultural Residues in Thailand. Sustainability 2022, 14, 9885. [Google Scholar] [CrossRef]
- Chen, J.-C.; Guo, J.-S. Improving the conversion efficiency of waste cotton to bioethanol by microwave hydrolysis technology. Sustain. Environ. Res. 2013, 23, 333–339. [Google Scholar]
- Gupta, R.; Sharma, K.K.; Kuhad, R.C. Separate hydrolysis and fermentation (SHF) of Prosopis juliflora, a woody substrate, for the production of cellulosic ethanol by Saccharomyces cerevisiae and Pichia stipitis-NCIM. Bioresour. Technol. 2009, 100, 1214–1220. [Google Scholar] [CrossRef]
- Salam, M.; Pondith, P.C.; Islam, A.; Khan, M.R.; Uddin, M.R.; Islam, M. Conversion of Cellulosic waste into fermentable sugar: Process optimization. J. Chem. Eng. 2013, 28, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Abu-Saied, M.A.; Taha, T.H.; Elnaggar, E.M.; Amer, R.A.; Mansy, A.E.; Elkady, G.M. Green production of bioethanol from cellulosic fiber waste and its separation using polyacrylonitrile-co-poly methyl acrylate membrane. Cellulose 2018, 25, 6621–6644. [Google Scholar] [CrossRef]
- Kumar, H.M.; Kumar, K.H.; Yogananda, S.; Swamy, H.V.K.; Nagaraja, T.; Kumar, S.S.; Dalawai, N. Isolation and characterization of yeast strains for bio-ethanol production. J. Pure Appl. Microbiol. 2015, 9, 2435–2441. [Google Scholar]
- Sayyad, S.; Chaudhari, S.; Panda, B. Quantitative determination of ethanol in arishta by using UV-visible spectro-photometer. Pharm. Biol. Eval. 2015, 2, 204–207. [Google Scholar]
- Ünal, M.Ü.; Chowdhury, G.; Şener, A. Effect of temperature and nitrogen supplementation on bioethanol produc-tion from waste bread, watermelon and muskmelon by Saccharomyces cerevisiae. Biofuels 2020, 13, 395–399. [Google Scholar] [CrossRef]
- Ogbonda, K.H.; Kiin-Kabari, D.B. Effect of temperature and pH on ethanol production by a Blastomyces species isolated from the intestine of oil palm weevil (Rhynchophorus palmarum, coleoptera). Afr. J. Biotechnol. 2013, 12, 588–591. [Google Scholar]
- Kusmiyati; Shitophyta, L.M. The effect of yeast weight and temperature on ethanol production from sorghum and iles-iles flour. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2015; p. 030019. [Google Scholar] [CrossRef]
- De Jong, R.J.E. Gosselink, Lignocellulose-based chemical products. In Bioenergy Research: Advances and Applications; Elsevier: Amsterdam, The Netherlands, 2014; pp. 277–313. [Google Scholar]
- Taha, R.H.; Taha, T.H.; Elsherif, M.A.; Mansy, A. Successive Application of Physicochemical and Enzymatic Treat-ments of Office Paper Waste for the Production of Bioethanol with Possible Using of Carbon Dioxide as an Indicator for the Determination of the Bioethanol Concentration. J. Biobased Mater. Bioenergy 2021, 15, 790–798. [Google Scholar] [CrossRef]
- Taha, R.H.; Taha, T.H.; Abu-Saied, M.; Mansy, A.; Elsherif, M.A. Maximization of the bioethanol concentration pro-duced through the cardboard waste fermentation by using ethylenediamine-modifying poly (acrylonitrile-co-methyl acrylate) membrane. Biomass Convers. Biorefinery 2022, 1–19. [Google Scholar] [CrossRef]
- Taha, R.H.; Taha, T.H.; Abu-Saied, M.; Mansy, A.; Elsherif, M.A. Successful production of bioethanol from olive waste residues followed by its purification using poly (acrylonitrile-co-methylacrylate)/polymethylmethaacrylate membrane. Biomass Convers. Biorefinery 2022, 1–15. [Google Scholar] [CrossRef]
- Vasić, K.; Knez, Ž.; Leitgeb, M. Bioethanol Production by Enzymatic Hydrolysis from Different Lignocellulosic Sources. Molecules 2021, 26, 753. [Google Scholar] [CrossRef] [PubMed]
- Irfanullah, N.R.; Arain, M.B.; Shah, N. Conversion of cotton to glucose by base hydrolysis using various hydrolytic conditions. Pakhtunkhwa J. Life Sci. 2014, 2, 125–137. [Google Scholar]
- Nwosu-Obieogu, K.; Aguele, F.O.; Agu, C.M.; Onyekwulu, C.S.; Akatobi, K.N. Optimisation of the hydrolysis of melon seed (Citrullus lanatus) husk with hydrochloric acid using response surface methodology. Indian Chem. Eng. 2021, 64, 293–302. [Google Scholar] [CrossRef]
- Tippkötter, N.; Duwe, A.-M.; Wiesen, S.; Sieker, T.; Ulber, R. Enzymatic hydrolysis of beech wood lignocellulose at high solid contents and its utilization as substrate for the production of biobutanol and dicarboxylic acids. Bioresour. Technol. 2014, 167, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Ostadjoo, S.; Hammerer, F.; Dietrich, K.; Dumont, M.-J.; Friscic, T.; Auclair, K. Efficient Enzymatic Hydrolysis of Biomass Hemicellulose in the Absence of Bulk Water. Molecules 2019, 24, 4206. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Zhang, C.; Tong, S.; Cui, Z.; Liu, P. Enhanced Enzymatic Hydrolysis and Structural Features of Corn Stover by NaOH and Ozone Combined Pretreatment. Molecules 2018, 23, 1300. [Google Scholar] [CrossRef] [Green Version]
- Zeghlouli, J.; Christophe, G.; Guendouz, A.; El Modafar, C.; Belkamel, A.; Michaud, P.; Delattre, C. Optimization of bio-ethanol production from enzymatic treatment of Argan pulp feedstock. Molecules 2021, 26, 2516. [Google Scholar] [CrossRef]
- Barampouti, E.M.; Grammatikos, C.; Stoumpou, V.; Malamis, D.; Mai, S. Emerging Synergies on the Co-treatment of Spent Coffee Grounds and Brewer’s Spent Grains for Ethanol Production. Waste Biomass Valorization 2021, 13, 877–891. [Google Scholar] [CrossRef]
- Khenblouche, A.; Bechki, D.; Gouamid, M.; Charradi, K.; Segni, L.; Hadjadj, M.; Boughali, S. Extraction and characterization of cellulose microfibers from Retama raetam stems. Polímeros 2019, 29. [Google Scholar] [CrossRef]
- Afsharpour, M.; Rad, F.T.; Malekian, H. New cellulosic titanium dioxide nanocomposite as a protective coating for preserving paper-art-works. J. Cult. Heritage 2011, 12, 380–383. [Google Scholar] [CrossRef]
- Neto, W.P.F.; Silvério, H.A.; Dantas, N.O.; Pasquini, D. Extraction and characterization of cellulose nanocrystals from agro-industrial residue—Soy hulls. Ind. Crops Prod. 2012, 42, 480–488. [Google Scholar] [CrossRef]
- Elerian, A.F.; Abu-Saied, M.A.; Abd-Elnaim, G.H.; Elnaggar, E.M. Development of polymer electrolyte membrane based on poly(Vinyl Chloride)/graphene oxide modified with zirconium phosphate for fuel cell applications. J. Polym. Res. 2023, 6, 30. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Dragone, G.; Guimarães, P.M.; Silva, J.P.A.; Carneiro, L.M.; Roberto, I.C.; Vicente, A.; Domingues, L.; Teixeira, J.A. Technological trends, global market, and challenges of bio-ethanol production. Biotechnol. Adv. 2010, 28, 817–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azhar, S.H.M.; Abdulla, R.; Jambo, S.A.; Marbawi, H.; Gansau, J.A.; Faik, A.A.M.; Rodrigues, K.F. Yeasts in sustainable bioethanol production: A review. Biochem. Biophys. Rep. 2017, 10, 52–61. [Google Scholar]
- Liu, R.; Shen, F. Impacts of main factors on bioethanol fermentation from stalk juice of sweet sorghum by immobilized Saccharomyces cerevisiae (CICC 1308). Bioresour. Technol. 2008, 99, 847–854. [Google Scholar] [CrossRef]
- Betiku, E.; Taiwo, A.E. Modeling and optimization of bioethanol production from breadfruit starch hydrolyzate vis-à-vis response surface methodology and artificial neural network. Renew. Energy 2015, 74, 87–94. [Google Scholar] [CrossRef]
- Tsafrakidou, P.; Manthos, G.; Zagklis, D.; Mema, J.; Kornaros, M. Assessment of substrate load and process pH for bioethanol production—Development of a kinetic model. Fuel 2022, 313, 123007. [Google Scholar] [CrossRef]
- Joginder, S.D.; Ashok, K.; Sunil, K.T. Bioethanol production from starchy part of tuberous plant (potato) using Sac-charomyces cerevisiae MTCC-170. Afr. J. Microbiol. Res. 2013, 7, 5253–5260. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, H.; Gan, M.; Jin, Y.; Gao, X.; Chen, Q.; Guan, J.; Wang, Z. Application of simultaneous saccharification and fermentation (SSF) from viscosity reducing of raw sweet potato for bioethanol production at laboratory, pilot and industrial scales. Bioresour. Technol. 2011, 102, 4573–4579. [Google Scholar] [CrossRef]
- Devi, A.; Bajar, S.; Kour, H.; Kothari, R.; Pant, D.; Singh, A. Lignocellulosic biomass valorization for bioethanol production: A circular bioeconomy approach. Bioenergy Res. 2022, 1–22. [Google Scholar] [CrossRef]
- Trejo, M.; Bhuyar, P.; Unpaprom, Y.; Dussadee, N.; Ramaraj, R. Advancement of fermentable sugars from fresh elephant ear plant weed for efficient bioethanol production. Environ. Dev. Sustain. 2022, 24, 7377–7387. [Google Scholar] [CrossRef] [PubMed]







| Sample | Residual Glucose Conc. (mg/mL) | Bioethanol Conc. (mg/mL) |
|---|---|---|
| AP1 | 0.16 | 4.48 |
| B2 | 0.38 | 4.23 |
| G4 | 0.11 | 4.68 |
| Conditions | Residual Glucose Conc. (mg/mL) | Bioethanol Conc. (mg/mL) |
|---|---|---|
| Aerobic | 2.90 | 3.82 |
| Anaerobic | 2.22 | 4.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansy, A.E.; El-Desouky, E.; El-Gendi, H.; Abu-Saied, M.A.; Taha, T.H.; Amer, R.A. Cellulosic Fiber Waste Feedstock for Bioethanol Production via Bioreactor-Dependent Fermentation. Fermentation 2023, 9, 176. https://doi.org/10.3390/fermentation9020176
Mansy AE, El-Desouky E, El-Gendi H, Abu-Saied MA, Taha TH, Amer RA. Cellulosic Fiber Waste Feedstock for Bioethanol Production via Bioreactor-Dependent Fermentation. Fermentation. 2023; 9(2):176. https://doi.org/10.3390/fermentation9020176
Chicago/Turabian StyleMansy, Ahmed E., Eman El-Desouky, Hamada El-Gendi, Mohamed A. Abu-Saied, Tarek H. Taha, and Ranya A. Amer. 2023. "Cellulosic Fiber Waste Feedstock for Bioethanol Production via Bioreactor-Dependent Fermentation" Fermentation 9, no. 2: 176. https://doi.org/10.3390/fermentation9020176







