Abstract
Dilute acid hydrolysis of lignocellulosic biomass generates inhibitors in the hydrolysate which hamper yeast metabolism and the fermentation process. Therefore, understanding the effect of these compounds on the performance of microorganisms becomes essential to achieve improved product yields. In this study, the effect of acetic acid, furfural, and hydroxymethylfurfural was evaluated on yeast growth and fermentation efficiency. Various parameters for the pretreatment of rice straw, such as an acid catalyst, and its concentration and residence time, were optimized for the maximum liberation of sugars in the hydrolysate. Further, the yeast strains Candida tropicalis and Meyerozyma caribbica were adapted for the tolerance of inhibitors at higher concentrations. A comparative analysis was carried out using un-adapted and adapted strains of Candida tropicalis and Meyerozyma caribbica for xylitol production. The findings of this study revealed that sulfuric acid (1.25% v/v) at 121 ரC for 30 min can efficiently convert rice straw xylan to xylose, with the release of 16.07 g/L xylose in the hydrolysate. Further, the adaptation results showed an increase of 76.42% and 69.33% in xylose assimilation by C. tropicalis and M. caribbica, respectively. The xylitol production with the adapted C. tropicalis was increased by 7.54% to 28.03 g/L xylitol. However, the xylitol production with the adapted M. caribbica was increased by 8.33%, yielding 26.02 g/L xylitol in the non-detoxified hydrolysate when compared to the un-adapted strains. Repeated batch fermentation was carried out for seven batches, and xylitol was found to be efficiently produced by the yeasts during five successive batches without any significant loss in the xylitol yield. Moreover, the results suggest that M. caribbica is a promising microorganism for the transformation of rice straw-derived xylose to xylitol.
1. Introduction
Lignocellulosic biomass is abundantly available as low-cost, renewable, and sustainable energy source rich in carbohydrates (cellulose and hemicellulose) and non-carbohydrate fractions. In the present global scenario, tremendous efforts have been made to efficiently utilize the carbohydrate fraction of biomass to foster the bioeconomy. The bioeconomy, as a broad spectrum, offers a wide range of opportunities for the conversion of biomass to value-added compounds, such as biofuels, biochemicals, and bioenergy [1]. Among various forms of lignocellulosic biomass, rice is the second-largest crop grown in India, with a production rate of 103 million tonnes. The global paddy production is escalating significantly with the increase in demand and population. The rising demand for rice is a cause of concern, because the rice harvesting process generates a significant amount of waste in the form of straws and husks [2]. Rice straw is mainly composed of cellulose (35%), hemicellulose (18%), and lignin (15%) [2,3]. These macromolecules can be converted to valuable biomolecules such as ethanol, xylitol, furfural, phenolic, etc. [3,4].
Xylitol, a naturally occurring pentitol, is classified as one of the most versatile platform chemicals that can be obtained from biomass. It is used as a crucial ingredient in numerous products manufactured by the food, pharmaceutical, and odontological industries, due to its low glycemic index, insulin-independent metabolism, and anti-cariogenic nature [5]. The demand for xylitol has steadily risen over the years, and its global market share is projected to expand from an estimated value of USD 1190.12 million in 2021 to USD 1475.87 million by the 2030s, at a CAGR of about 6% [6]. Commercially, the demand for xylitol is fulfilled by the chemical hydrogenation of xylose at high pressure and temperature in the presence of chemical catalysts such as Ni, Ru, Pt, and Pl. However, this process is eco-unfriendly, energy-intensive, and expensive, as it requires toxic catalysts, extreme reaction conditions, and a series of complicated purification procedures to separate the xylitol from other by-products formed during the production process [7,8]. The biotechnological route of xylitol production using microorganisms, such as yeasts and bacteria, serves as a promising alternative to the chemical hydrogenation process due to several advantages, such as their environment-friendly nature, low energy requirement, high product yield, and relatively easier process [5,9].
Among different microorganisms, yeasts such as Candida and Debaromyces are regarded as efficient xylitol producers, due to their high xylose assimilation rates and xylitol productivity [10]. Candida tropicalis has been an extensively investigated yeast for xylitol production due to its ability to utilize pentoses, high tolerance to inhibitors, and propensity to thrive in all kinds of biomass-derived hemicellulosic hydrolysates [1,2,3,4,5,11]. Moreover, Meyerozyma caribbica, a mesophilic yeast that is part of the same family as Candida, i.e., Debaromycetaceae, is gaining popularity in biorefineries, due to its capability to utilize a variety of carbon sources from lignocellulosic biomass [12,13]. According to recent studies, it is reported to be an efficient pentose-fermenting yeast, with xylitol as the main product of pentose metabolism [14,15].
One of the key aspects of xylitol production via the biotechnological route is the pretreatment of biomass for the solubilization of hemicellulosic sugars. Acid hydrolysis is frequently used for the pretreatment of biomass because of its low cost, high reaction rates, and ability to recover approximately 70–95% of hemicellulosic sugars [16]. However, the process also generates inhibitory compounds, such as acetic acid, furfural, and 5-hydroxymethylfurfural, which adversely affect the growth of microorganisms and thereby reduce the overall fermentation performance [17,18]. Several approaches have been proposed in the literature for mitigating the toxicity of these inhibitors on microorganisms, such as the adaptation of microbial strains, entrapment of microbial cells, and detoxification of hydrolysates by activated charcoal, ion-exchange resins, and over-liming [8,19,20,21]. The adaptation of microorganisms has been described as an efficient method for increasing the natural tolerance of microorganisms by pre-exposing them to non-lethal concentrations of inhibitors. As compared to un-adapted strains, the use of adapted strains can increase fermentation yields and productivity, even in high concentrations of inhibitors [22]. The implementation of adaptation strategies has been reported in previous studies for ethanol production [23,24].
Keeping the above points in view, the present study was aimed at assessing the efficacy of a minimally explored yeast, M. caribbica, in comparison to the most widely used yeast, C. tropicalis, for conversion of rice straw-derived xylose to xylitol. The effect of inhibitors on xylitol production was studied in the presence of acetic acid, furfural, and hydroxymethyl furfural. Furthermore, in order to enhance the natural tolerance of yeasts towards inhibitory compounds in the hydrolysate, an adaptation strategy was employed. Xylitol production by both un-adapted and adapted strains of M. caribbica and C. tropicalis was carried out.
2. Materials and Methods
2.1. Chemicals and Biomass
All the chemicals and media used in the present study were of analytical or commercial grade and procured from Sigma-Aldrich, Co., Denmark, and HIMEDIA laboratories, Mumbai, India unless specified otherwise. Rice straw (RS) biomass was collected from local fields adjoining the Center of Innovative and Applied Bioprocessing, Mohali, India. The biomass was shredded with a shredder, followed by oven-drying at 70 °C for 48 h. The compositional analysis of the dried RS used was the same as suggested in a previous study [25].
2.2. Microorganisms Used
Two in-house isolated strains, including Candida tropicalis OK165575 [25] and Meyerozyma caribbica MZ057612 [26], were used to evaluate their efficiency for xylitol production from D-xylose, as well as xylose derived from the RS hemicellulosic hydrolysate. The yeast strains were maintained on yeast extract 10 g/L, peptone 20 g/L, and xylose 20 g/L (YPX) agar plates and stored at 4 °C.
2.3. Optimization of Dilute Acid Pre-Treatment of RS
The acid hydrolysis of the RS was carried out to solubilize the maximum concentration of fermentable sugars in the hydrolysate under different parameters, such as acid catalysts (HCl, H2SO4 HNO3, CH3COOH, H3BO3, and H3PO4), acid concentration (0.5%, 1%, 1.25%, 1.5%, and 2%), and residence time (15 to 60 min), in an autoclave. First, the shredded RS biomass was mixed with an acidic solution in 1:10 (solid-to-liquid ratio) at room temperature. The slurry prepared was then introduced to the reaction vessel and pre-treatment was carried out at a temperature of 121 °C and contact time of 30 min. The biomass was also treated with hot water, without adding the reagent, with the same conditions of temperature and time, to serve as the control set. The solid and liquid fractions were separated by centrifugation at 8000 rpm for 15 min. Further, the supernatant was neutralized and analyzed for the presence of sugars (glucose, xylose, and arabinose) and inhibitory compounds (acetic acid, furfural, and hydroxymethylfurfural) using HPLC.
2.4. Large-Scale Pre-Treatment of RS
The best-chosen parameters obtained after optimization were used to carry out the final hydrolysis. One kilogram of RS was mixed with an acidic solution and autoclaved at the optimized process conditions. The vessel containing the hydrolyzed biomass was cooled immediately to terminate undesirable reactions. Subsequently, the solid and liquid fractions were separated using a muslin cloth, followed by vacuum filtration with Whatman filter paper no. 1 (pore size = 11 microns). The filtrate obtained was analyzed by HPLC to determine the content of reducing sugar and fermentation inhibitors. The hydrolysate was stored at 4 °C for further use.
2.5. Preparation of RS-Derived Fermentation Medium
The RS hydrolysate was separated into two different fractions for the preparation of detoxified and non-detoxified hydrolysates. The detoxified hydrolysate was prepared by using activated charcoal. The activated charcoal 2.0% (w/v) was added to the hydrolysate and incubated at 30 °C, 200 rpm, for 1 h. The activated charcoal was removed by vacuum filtration using Whatman filter paper no. 1. Further, the pH of the hydrolysate was adjusted to 5.5 using calcium oxide. However, the non-detoxified hydrolysate medium of pH 5.5 was obtained by using CaO. The neutralized hydrolysates were filtered using vacuum filtration, and the clear filtrates obtained were then analyzed for the estimation of fermentable sugars and inhibitors. The hydrolysates were concentrated using a vacuum evaporator at 70 °C to reach a xylose concentration > 50 g/L, an appropriate concentration for efficient conversion by the isolates into xylitol, as optimized [1,2,3,4,5,25].
2.6. Inoculum Preparation
The inocula of the experimental yeasts were prepared by aseptically transferring a loopful of cells from YPX agar plates to 20 mL of YPX broth medium in 100 mL conical flasks. The flasks were then incubated at 30 °C and 150 rpm for 24 h in a rotary shaker.
2.7. Effect of Toxic Inhibitors on Fermentation Efficiency of C. tropicalis and M. caribbica
The ability of both yeasts to tolerate inhibitory compounds during xylitol fermentation was evaluated. The fermentation medium was composed of ammonium sulphate 1 g/L, yeast extract 1 g/L, magnesium sulphate heptahydrate 0.08 g/L, disodium orthophosphate 0.2 g/L, potassium dihydrogen phosphate 0.2 g/L, glucose 6 g/L, and xylose 50 g/L. Both the yeasts were inoculated in the fermentation medium containing the aforementioned components and synthetic inhibitory compounds, such as furfural (1–5 g/L), HMF (1–5 g/L), and acetic acid (2–10 g/L), which were present in the acidic hydrolysate as a result of degradation. The acetic acid was added to the medium at different pH values (4.5, 5.5, and 6.5) to assess its effect on the microorganism in different conditions. All the fermentation experiments were carried out in 250 mL Erlenmeyer flasks containing 100 mL of medium, followed by incubation at 30 °C, 150 rpm for 72 h in a rotary shaker. The samples were withdrawn periodically to estimate the xylose consumption and xylitol production.
2.8. Adaptive Evolution of Yeasts in RS Hemicellulosic Hydrolysate
The ability to produce xylitol by adapted yeasts was further assessed in hydrolysate containing biomass-derived xylose. The fermentation experiments were performed in 500 mL Erlenmeyer flasks with 200 mL non-detoxified and detoxified RS hydrolysate, supplemented with the same medium components used in the previous experiments. The cultures were incubated at 30 °C, 150 rpm for 24 h in a rotary shaker. The yeast cells, which were separated by centrifugation at 8000 rpm for 15 min, were transferred to a fresh fermentation medium. The samples were withdrawn periodically after every 24 h to estimate the xylitol production and xylose consumption.
2.9. Evaluation of Fermentation Potential of Un-Adapted and Adapted Yeasts
The ability of un-adapted and adapted strains of C. tropicalis and M. caribbica to convert commercial xylose and RS-derived xylose to xylitol was evaluated by carrying out fermentation in 500 mL Erlenmeyer flasks with 200 mL of medium. The pH of the medium was set at 5.5 by using 1 mol·L−1 HCl or 5 mol·L−1 NaOH, followed by sterilization at 121 °C for 15 min. The fermentation media were inoculated with 5% (v/v) yeast cultures and incubated at 30 °C, 150 rpm for 120 h in a rotary shaker. Samples were withdrawn at regular time intervals to estimate the xylose consumption and xylitol production.
2.10. Repeated Batch Fermentation
The usage of yeast cells without preparing fresh inoculum was evaluated by transferring 5% (v/v) broth from each preceding fermentation batch to the successive batches of fermentation. The test was repeated for up to 7 batches of fermentation, and samples were withdrawn periodically to estimate the concentrations of xylose and xylitol. Each batch of fermentation lasted about 72 h.
2.11. Analytical Methods
The moisture content of the biomass at various stages of pre-treatment was analyzed by an MA 35 moisture analyzer. The reducing sugars, inhibitory compounds, and xylitol were estimated by HPLC (Agilent Technologies, 1260 Infinity), fitted with a reverse-phase BIORAD Aminex HPX-87H column (300 × 7.8 mm), with 5 mM sulfuric acid as the mobile phase, a 0.55 mL/min flow rate, and 60 °C column temperature.
2.12. Statistical Analysis
All the experiments were repeated at least three times, and the data were analyzed by using GraphPad Prism software version 5. The error bar was calculated from the mean of three replicates. The significant differences were calculated by two-way ANOVA using Bonferroni post-tests at p < 0.0001.
3. Results and Discussion
3.1. Effect of Inhibitors on Xylitol Production
The optimum process parameters for RS pretreatment to extract the maximum concentration of xylose from the RS biomass were 1.25% (v/v) H2SO4 concentration, 121 °C hydrolysis temperature, and 30 min residence time with a solid-to-liquid ratio of 1:10 (g/mL), as observed from Figure 1.
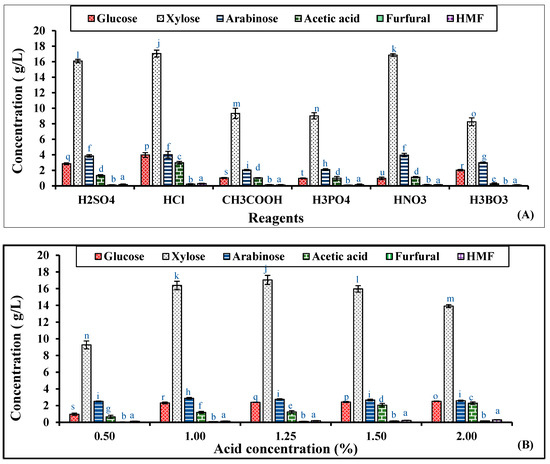
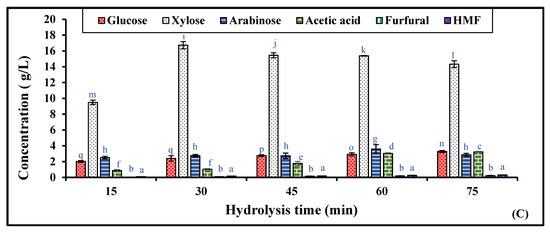
Figure 1.
Optimization of RS hydrolysis by varying different (A) reagents, (B) sulfuric acid concentrations (0.5 to 2.0%), and (C) residence times of rice straw hydrolysis by 1.25% sulfuric acid (15 to 75 min). Significant differences were calculated by two-way ANOVA using Bonferroni post-tests and are indicated by small letters. At different hydrolysis conditions, the bars with different lowercase letters represent significant differences. Bars with the same letters are not significantly different, according to p < 0.0001.
The pretreatment of the lignocellulosic biomass generated inhibitory compounds such as acetic acid, furfural, and hydroxymethyl furfural (Table 1). These inhibitors influence the efficiency of the fermentation process performed by using a microbial platform [27]. Also, such byproducts have an adverse effect on the growth of microbes, making them inefficient for utilizing sugars, ultimately resulting in a poor yield of products. Therefore, the selection of microbes with a natural tolerance to inhibitory compounds is generally recommended to increase fermentation yields. Hence, the tolerance to different by-products, such as HMF, acetic acid, and furfural, of the isolated yeasts, C. tropicalis and M. caribbica, was evaluated. The efficiency of fermentation by measuring the xylitol yield in the presence of these inhibitors was estimated by withdrawing a sample every 24 h during the fermentation.

Table 1.
Chemical composition of detoxified and non-detoxified rice straw hydrolysate obtained by sulfuric acid treatment.
The key enzyme involved in the production of xylitol from xylose during the fermentation process involving yeasts is xylose reductase (XR). During xylitol production, XR requires a continuous supply of NADPH. The maintenance of the NADPH level in the yeast for xylitol production by XR is mainly determined by an efficient recycling system, including formate dehydrogenase and glucose dehydrogenase [5,10,12,15]. Hence, the involvement of such enzymes is very crucial for xylitol production with yeasts. The robustness of such enzymes in the yeast, along with the appropriate conditions and biomass types, decides the xylitol production.
3.1.1. Effect of Acetic Acid on Xylitol Production
Acetic acid is a compound formed in the hydrolysate as a result of the breakdown of the hemi-acetyl linkage. Acetic acid is present in higher concentrations compared to HMF and furfural during biomass hydrolysis [28]. An acetic acid accumulation above the threshold levels causes a drop in the intracellular pH, as well as in the fermentation broth. These high levels of acetic acid are supposed to slow the growth rate of microbes, resulting in incomplete cycles of fermentation. Therefore, the tolerance of yeasts towards acetic acid was evaluated in a synthetic medium containing variable concentrations of acetic acid (2–10 g/L) at different pH values (4.5, 5.5, and 6.5). The effect of the variable pH values of the medium was analyzed to see the reported decrease in the toxic undissociated form of acetic acid with an increase in pH. The pKa of acetic acid is 4.75, and, therefore, at lower pH, protonated acetic acid is predominant, and, at higher pH, deprotonated acetic acid is predominant [29]. The results of the present study revealed that the toxic nature of acetic acid was alleviated with an increase in pH from 4.5 to 6.5. The highest xylitol yield and xylose assimilation (99%) were observed at pH 5.5 with both yeast strains. The xylose consumption was not affected by acetic acid at 2–10 g/L concentration. However, it was observed from the results that the xylitol yield started declining with an increase in the acetic acid concentration from 2 to 10 g/L. This decrease in the xylitol yield might be due to the diversion of xylose flux in the yeast to energy generation [19]. Inhibitors such as acetic acid might be affecting the activity of xylose reductase (XR). The acetic acid concentration (2 g/L) at pH 5.5 decreased the xylitol production rate to 4.18% and 4.90% in comparison to the control medium (without acetic acid) during fermentation by C. tropicalis and M. caribbica, respectively (Table 2). Further, it was observed that the xylitol production was the maximum at pH 5.5 compared to pH 4.5 and 6.5, which might be due to the decrease in toxicity of acetic acid, or pH 5.5 might be the optimum pH for the yeast metabolism and activity of xylose reductase (XR) enzyme. Moreover, it was observed that the fermentation time was increased at pH 6.5. This increase in incubation time might be responsible for salt build-up by neutralization, or an unfavorable pH for XR enzyme activity. Both C. tropicalis and M. caribbica strains were able to tolerate acetic acid up to the concentration of 10 g/L at pH 5.5 and 6.5, with significantly higher xylitol yields in comparison to the yields obtained at pH 4.5. It was observed that the acetic acid at a 10 g/L concentration and at pH 4.5 turns out to be toxic for both the yeasts, as observed in terms of poor growth and xylitol yields. However, at pH 5.5, the yeast C. tropicalis was able to tolerate 10 g/L acetic acid with 29.76 g/L xylitol titer, while M. caribbica exhibited low xylitol titer (12.04 g/L), due to the diversion of the xylose flux to energy generation. Similarly, in another study, a high cell density of Candida sojae JCM 1644 was observed in a medium supplemented with 3% acetic acid, whereas the cell density was decreased when the concentration of acetic acid was increased to 5%, indicating the sensitivity of the strain to a high acetic acid concentration [30]. In the present study, the xylose consumption and xylitol production were not found to be affected when the acetic acid concentration was 2 g/L and the pH was 4.5. However, when the concentration of acetic acid was increased further, the xylitol production was decreased significantly at pH 4.5 during fermentation by both yeasts (Table 2). Similar observations have also been reported during fermentation by using C. tropicalis and C. gulliermondii [31,32].

Table 2.
Effect of acetic acid concentration in rice straw hydrolysate on xylitol production at different pH values by Candida tropicalis and Meyerozyma caribbica.
3.1.2. Effect of Furfural and Hydroxymethylfurfural on Xylitol Production
It has been reported in previous studies that furfural and HMF break down the DNA and inhibit the protein and RNA synthesis mechanism of the microorganisms, thereby affecting their growth and fermentation efficiency [33,34]. Therefore, the effect of furfural and HMF on xylitol production was also evaluated by varying the concentrations of furfural (1–4 g/L) and HMF (1–4 g/L) in the fermentation medium. To study the synergistic effect of both inhibitors on the yeasts, the medium was supplemented with 2 g/L acetic acid, along with furfural at different concentrations. The maximum xylitol production was observed at 1 g/L furfural by both C. tropicalis and M. caribbica, which was decreased upon further increasing the furfural concentration. When the concentration of furfural was increased beyond 1 g/L to 4 g/L, a gradual decrease in xylitol yield (16.28%) was observed during fermentation by C. tropicalis, whereas, in the case of M. caribbica, a 23.13% decrease in xylitol yield was observed (Figure 2a). Moreover, the growth of the yeasts was not completely inhibited at a 4 g/L furfural concentration, which might be due to the capability of the yeasts to reduce the inhibitory effect of furfural by converting it to less-toxic compounds, such as furoic acid or furfuryl alcohol [35]. Similarly, 22.64 g/L of xylitol titre and 0.44 g/g of xylitol yield were reported after the adaptive evolution of Geotrichum sp. in sugarcane bagasse hydrolysate. Geotrichum sp. was able to tolerate 6 g/L of furfural, along with glucose and xylose as the carbon source [36]. In another study, a furfural concentration of 500 m g/L was reported for the highest inhibition of cell growth of Pichia stiptis NCIM 3497 and poor xylitol yields [29].
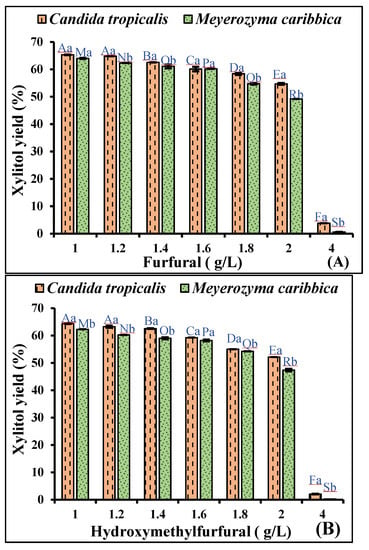
Figure 2.
Effect of inhibitory compounds (A) furfural, (B) hydroxymethylfurfural (HMF) on xylitol fermentation efficiency of C. tropicalis and M. caribbica in rice straw hydrolysate. Significant differences were calculated by two-way ANOVA using Bonferroni post-tests and are indicated by small and capital letters. In different concentrations of furfural and HMF, bars with different uppercase letters are significantly different, while bars with lowercase letters represent a significant difference in xylitol production by the two yeasts. Different letters denote a significant difference between the two, and the same letters on the bar denote no significant difference between the two at p < 0.0001.
Further, fermentation by both yeasts in the medium containing acetic acid at 2 g/L, furfural at 1 g/L, and HMF at variable concentrations (1–4 g/L) was carried out to evaluate the synergistic effect of these three inhibitors on the process of fermentation. A noteworthy effect of HMF was seen on both C. tropicalis and M. caribbica, with a gradual decrease in the xylitol yield. When the concentration of HMF was increased from 1 to 2 g/L, the xylitol yield by C. tropicalis was decreased to 18.95%, whereas a decrease of 23.90% was seen in the xylitol production efficiency of M. caribbica. A further decline in xylitol yield was seen during fermentation by both yeasts when the medium was supplemented with 4 g/L HMF, and, ultimately, a complete inhibition of microbial growth in the medium was observed (Figure 2b). Similarly, the synergistic effects of low, medium, and high concentrations of HMF, furfural, and acetic acid were analyzed on xylose consumption and xylitol production by Pichia stiptis NCIM 3497. The authors have observed 11.5% and 50% reductions in xylitol yield in the presence of the lower and moderate concentrations of inhibitors, respectively, in comparison to the control. However, 66.67% and 67.57% reductions in the xylitol yield were observed in the presence of a higher concentration of furfural, HMF, and acetic acid at 48 and 72 h of fermentation, respectively [29]. Similarly, a high concentration of HMF, furfural, and acetic acid, above 3 g/L, was reported to negatively affect xylitol production by Rhodotorula mucilaginosa PTD3 I [37].
3.2. Adaptation of Yeasts in RS Hydrolysate
The increase in the concentration of inhibitors led to a decrease in the xylitol yield, which could be due to the slow sugar assimilation by the yeasts in the presence of inhibitors [38]. Therefore, the cells were allowed to adapt in the non-detoxified RS hydrolysate to increase the consumption of sugars in the presence of inhibitors. Both the yeasts, C. tropicalis and M. caribbica, were inoculated in the non-detoxified hydrolysate comprising xylose and glucose, along with certain inhibitory compounds (Table 1). The cells were transferred from the previous batch to the next successive batch every 24 h, and a significant increase in xylose assimilation was observed in each cycle. The lowest xylose consumption rate was 1.40 g/L/h and 1.50 g/L/h in the first cycle (I), and the highest was 2.47 g/L/h and 2.54 g/L/h in the last cycle (V) of fermentation by M. caribbica (Figure 3a) and C. tropicalis (Figure 3b), respectively. Similarly, the rate of xylose consumption has been observed to increase during fermentation in sugarcane bagasse hemicellulosic hydrolysate with adapted cells in comparison to un-adapted Candida guilliermondii yeast cells [1].
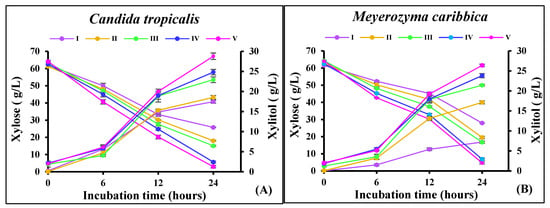
Figure 3.
Xylose assimilation and xylitol production profiles in repeated batch fermentation by (A) C. tropicalis, (B) M. caribbica in rice straw hydrolysate medium. First cycle (I), second cycle (II), third cycle (III), fourth cycle (IV), and fifth cycle (V).
3.3. Comparative Assessment of Fermentation in RS Hydrolysate with Un-Adapted and Adapted Yeasts
Fermentative tests were carried out by shake flask fermentation using both adapted and un-adapted strains of C. tropicalis and M. caribbica. A high concentration of xylitol 28.03 g/L, with a 0.57 g/g xylitol yield and productivity of 0.29 g/L/h, was observed during fermentation by the adapted strain of C. tropicalis in non-detoxified hydrolysate compared to the un-adapted strain (26.32 g/L xylitol; 0.53 g/g xylitol yield; 0.21 g/L/h xylitol productivity). The xylitol yield increased by 7.54% and 3.38% during fermentation by adapted C. tropicalis in non-detoxified and detoxified RS hydrolysate, respectively (Figure 4a,b). However, the maximum xylitol concentration of 26.02 g/L, with 0.52 g/g of xylitol yield and productivity of 0.21 g/L/h, was observed during fermentation by the adapted strain of M. caribbica in the non-detoxified hydrolysate, in comparison to the un-adapted strain (23.38 g/L xylitol; 0.48 g/g xylitol yield; 0.19 g/L/h xylitol productivity). There were 8.33% and 7.40% increases in xylitol yield with the adapted strain of M. caribbica during fermentation in the non-detoxified and detoxified RS hydrolysates, respectively (Figure 4c,d). In a similar study, a low xylitol concentration of 17.33 g/L and 0.44 g/g xylitol yield was reported from steam-pretreated acid-catalyzed sugarcane bagasse hydrolysate using M. caribbica JA9 [39], while a 0.54 g/g xylitol yield was obtained from detoxified corn cob hydrolysate after 120 h [40]. The xylitol yield from the corn hydrolysate was lower than the xylitol yield of the present study. In a study on the exploration of five different yeasts for xylitol production from sugarcane bagasse hydrolysate, Meyerozyma caribbica could utilize only 89% of the xylose, and residual sugar was present at the end of fermentation, whereas a complete utilization of xylose was observed during fermentation by C. tropicalis. Contrarily, in the present study, both the yeasts were highly competitive and completely utilized the xylose by the end of the fermentation. The higher xylitol yield in the present study could be due to the RS hydrolysate containing a lower concentration of glucose, and therefore the alcohol dehydrogenase activity was not expressed, favoring high xylitol productivity [40].
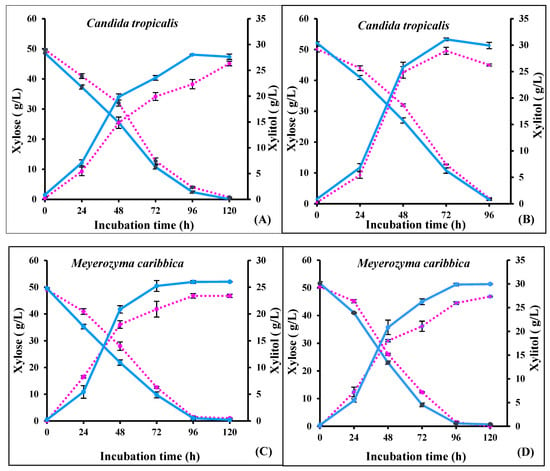
Figure 4.
Fermentation profile of un-adapted (dotted line) and adapted (solid line) strains of C. tropicalis in (A) non-detoxified rice straw hydrolysate, and (B) detoxified rice straw hydrolysate; and M. caribbica in (C) non-detoxified rice straw hydrolysate, and (D) detoxified rice straw hydrolysate.
A comparative analysis of the performance of the yeasts for xylitol production on rice straw and other biomasses is presented in Table 3. The analysis suggests the higher potential of the identified yeasts for xylitol production, at least from rice straw biomass.

Table 3.
Comparison of xylitol production by different strains.
3.4. Repeated Batch Fermentation in Non-Detoxified and Detoxified Hydrolysate
Repeated batch fermentation was carried out to omit the need for fresh seed culture preparation. The fermentation was carried out by inoculating 5% (v/v) broth from the preceding batch to the successive batch for up to seven cycles in both non-detoxified and detoxified RS hydrolysates. The results of the repeated batch fermentation showed that the cells can be used for up to five batches, with each batch lasting for 72 h, without any significant effect on the efficiency of the yeast for xylitol production. During fermentation in non-detoxified media, significant declines of 24.62% and 25.97% were seen in the xylitol yield after the fifth to seventh cycles of fermentation by C. tropicalis and M. caribbica, respectively (Figure 5a). However, in the detoxified hydrolysate, declines of 21.79% and 29.68% in xylitol efficiency were observed after the fifth to seventh cycles of fermentation by C. tropicalis and M. caribbica, respectively (Figure 5b). Similarly, D-lactic acid has been produced by Sporolactobacillus sp. using repeated fermentation for up to four batches, with each batch lasting for 48 h. Consistent lactic acid production was observed in the first three batches, and a slight decrease of 3.29% was observed in the fourth batch of fermentation [44]. Also, repeated fed-batch fermentation was conducted for xylitol production by using C. magnoliae for three cycles in a synthetic D-xylose-based medium. The researchers observed a constant xylitol yield of 0.727 g/g, 0.719 g/g, and 0.720 g/g in the first, second, and third batches of fermentation, respectively [45]. The simultaneous production of poly-3-hydroxybutyrate, xylitol, and xylonic acid was reported with xylose-rich sugar mixtures by a wild strain of Burkholderia sacchari [46].
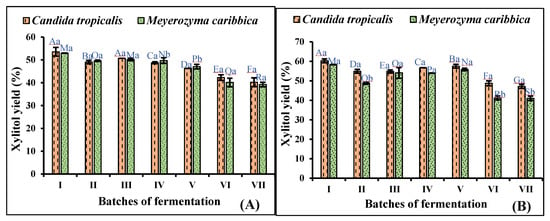
Figure 5.
Repeated batch fermentation of adapted strains of C. tropicalis and M. caribbica in (A) non-detoxified rice straw hydrolysate, and (B) detoxified rice straw hydrolysate. Significant differences were calculated by two-way ANOVA using Bonferroni post-tests and are indicated by small and capital letters. In different batches, bars with different uppercase letters are significantly different, while bars with different lowercase letters represent significant differences in xylitol production by the two yeasts. Different letters denote significant difference between the two, and the same letter on the bar denotes no significant difference between the two at p < 0.0001.
4. Conclusions
The presence of inhibitors in biomass hydrolysates has been observed to be a major barrier, hindering the production of xylitol by microbial fermentation. Understanding the effect of such inhibitors on yeast growth is an important step to overcoming this difficulty during fermentation. The present study was performed to elucidate the fermentative performance of C. tropicalis and M. caribbica for xylitol production in rice straw (RS) biomass hydrolysate, with the formation of toxic inhibitors during the process. A high concentration of inhibitors was found to severely inhibit the growth of yeast, resulting in poor xylitol yields. In order to enhance the xylitol yield, an adaptation strategy was employed to increase the natural tolerance of yeasts in the presence of a high concentration of inhibitors. Hence, adapted strains of C. tropicalis and M. caribbica were developed, documenting the better tolerance to inhibitors when compared to the un-adapted strain. The rate of xylose assimilation after adaptation was increased to 76.42% and 69.33% in C. tropicalis and M. caribbica, respectively. Moreover, repeated batch fermentation studies were carried out to omit the need for seed culture preparation, and it was found that the culture can be used for up to five batches of fermentation. Further, the findings of the present study indicate that the M. caribbica strain is a promising yeast, which could be used for xylitol production from a low-cost substrate. Importantly, the high xylitol yield with M. caribbica was close to the yield obtained from biomass hydrolysate by using C. tropicalis, even without employing optimized process conditions for xylitol fermentation.
Author Contributions
Conceptualization, S.K.Y., S.K. and P.G.; methodology, S.K.Y., S.K. and P.G.; formal analysis, S.K.Y., S.K. and P.G.; investigation, S.K. and P.G.; data curation, S.K. and P.G.; writing—original draft preparation, S.K. and P.G.; writing—review and editing, S.K.Y; supervision, S.K.Y.; funding acquisition, S.K.Y. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful to the CEO of CIAB for his continuous support and motivation. The authors express sincere gratitude to the Department of Biotechnology, Government of India for providing financial support in the form of the flagship project (BT/CIAB-Flagship/2018) to SKY, CIAB, Mohali.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare that there is no conflict of interest.
References
- Bianchini, I.D.A.; Sene, L.; da Cunha, M.A.A.; Felipe, M.D.G.D.A. Short-term adaptation strategy improved xylitol production by Candida guilliermondii on sugarcane bagasse hemicellulosic hydrolysate. Bioenergy Res. 2022, 15, 1182–1194. [Google Scholar] [CrossRef]
- Logeswaran, J.; Shamsuddin, A.H.; Silitonga, A.S.; Mahlia, T.M.I. Prospect of using rice straw for power generation: A review. Environ. Sci. Pollut. Res. 2020, 27, 25956–25969. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.; Mathew, A.K.; Sindhu, R.; Pandey, A.; Binod, P. Potential of rice straw for bio-refining: An overview. Bioresour. Technol. 2016, 215, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Zhao, M.M.; Zhou, Z.W.; Huang, T.; Chen, X.L.; Wang, Y. Isolation of cellulose with ionic liquid from steam exploded rice straw. Ind. Crops Prod. 2011, 33, 734–738. [Google Scholar] [CrossRef]
- Hernandez-Perez, A.F.; de Arruda, P.V.; Sene, L.; da Silva, S.S.; Chandel, A.K.; de Almeida Felipe, M.D.G. Xylitol bioproduction: State-of-the-art, industrial paradigm shift, and opportunities for integrated biorefineries. Crit. Rev. Biotechnol. 2019, 39, 924–943. [Google Scholar] [CrossRef] [PubMed]
- Global Xylitol Market Size/Share Worth 1475.87 Million by 2030 at a 6% CAGR: Custom Market Insights. Available online: https://www.globenewswire.com/ (accessed on 20 November 2022).
- Zhang, B.; Ren, L.; Zhao, Z.; Zhang, S.; Xu, D.; Zeng, X.; Li, F. High-temperature xylitol production through simultaneous co-utilization of glucose and xylose by engineered Kluyveromyces marxianus. Biochem. Eng. J. 2021, 165, 107820. [Google Scholar] [CrossRef]
- Kumar, V.; Krishania, M.; Sandhu, P.; Ahluwalia, P.; Gnansounou, E.; Sangwan, R.S. Efficient detoxification of corn cob hydrolysate with ion-exchange resin for enhanced xylitol production by Candida tropicalis MTCC 6192. Bioresour. Technol. 2017, 251, 416–419. [Google Scholar] [CrossRef]
- Prakash, G.; Varma, A.J.; Prabhune, A.; Shouche, Y.; Rao, M. Microbial production of xylitol from d-xylose and sugarcane bagasse hemicellulose using newly isolated thermotolerant yeast Debaryomyces hansenii. Bioresour. Technol. 2011, 102, 3304–3308. [Google Scholar] [CrossRef]
- Mohamad, N.L.; Mustapa Kamal, S.M.; Mokhtar, M.N. Xylitol biological production: A review of recent studies. Food Rev. Int. 2015, 31, 74–89. [Google Scholar] [CrossRef]
- Jia, H.; Shao, T.; Zhong, C.; Li, H.; Jiang, M.; Zhou, H.; Wei, P. Evaluation of xylitol production using corncob hemicellulosic hydrolysate by combining tetrabutylammonium hydroxide extraction with dilute acid hydrolysis. Carbohydr. Polym. 2016, 151, 676–683. [Google Scholar] [CrossRef]
- Tadioto, V.; Milani, L.M.; Barrilli, É.T.; Baptista, C.W.; Bohn, L.; Dresch, A.; Harakava, R.; Fogolari, O.; Mibielli, G.M.; Bender, J.P.; et al. Analysis of glucose and xylose metabolism in new indigenous Meyerozyma caribbica strains isolated from corn residues. World J. Microbiol. Biotechnol. 2022, 38, 35. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.R.; Dhanarajan, G.; Sarkarb, D.; Sen, R. Multi-fold enhancement in sustainable production of biomass, lipids and biodiesel from oleaginous yeast: An artificial neural network-genetic algorithm approach. Sustain. Energy Fuels 2020, 4, 6075–6084. [Google Scholar] [CrossRef]
- Moremi, M.E.; Van Rensburg, E.L.J.; La Grange, D.C. The improvement of bioethanol production by pentose-fermenting yeasts isolated from herbal preparations, the gut of dung beetles, and marula wine. Int. J. Microbiol. 2020, 2020, 5670936. [Google Scholar] [CrossRef] [PubMed]
- Sukpipat, W.; Komeda, H.; Prasertsan, P.; Asano, Y. Purification and characterization of xylitol dehydrogenase with L-arabitol dehydrogenase activity from the newly isolated pentose-fermenting yeast Meyerozyma caribbica 5XY2. J. Biosci. Bioeng. 2016, 123, 20–27. [Google Scholar] [CrossRef]
- Arcaño, Y.D.; Valmaña García, O.D.; Mandelli, D.; Carvalho, W.A.; Pontes, L.A.M. Xylitol: A review on the progress and challenges of its production by chemical route. Catal. Today 2020, 344, 2–14. [Google Scholar] [CrossRef]
- Silva-Fernandes, T.; Santos, J.C.; Hasmann, F.; Rodrigues, R.; Filho, H.I.; Felipe, M. Biodegradable alternative for removing toxic compounds from sugarcane bagasse hemicellulosic hydrolysates for valorization in biorefineries. Bioresour. Technol. 2017, 243, 384–392. [Google Scholar] [CrossRef]
- Ur-Rehman, S.; Mushtaq, Z.; Zahoor, T.; Jamil, A.; Murtaza, M.A. Xylitol: A review on bioproduction, application, health benefits, and related safety issues. Crit. Rev. Food Sci. Nutr. 2015, 55, 1514–1528. [Google Scholar] [CrossRef]
- Yewale, T.; Panchwagh, S.; Rajagopalan, S.; Dhamole, P.B.; Jain, R. Enhanced xylitol production using immobilized Candida tropicalis with non-detoxified corn cob hemicellulosic hydrolysate. 3 Biotech 2016, 6, 75. [Google Scholar] [CrossRef]
- Zahed, O.; Jouzani, G.S.; Abbasalizadeh, S.; Khodaiyan, F.; Tabatabaei, M. Continuous co-production of ethanol and xylitol from rice straw hydrolysate in a membrane bioreactor. Folia Microbiol. 2015, 61, 179–189. [Google Scholar] [CrossRef]
- Rao, R.S.; Jyothi, C.P.; Prakasham, R.S.; Sarma, P.N.; Rao, L.V. Xylitol production from corn fiber and sugarcane bagasse hydrolysates by Candida tropicalis. Bioresour. Technol. 2006, 97, 1974–1978. [Google Scholar] [CrossRef]
- Costa Nogueira, C.D.; Araújo Padilha, C.E.D.; Medeiros Dantas, J.M.D.; Macedo de Medeiros, F.G.; Araújo Guilherme, A.D.; Santana Souza, D.F.D.; dos Santos, E.S. In-situ detoxification strategies to boost bio-alcohol production from lignocellulosic biomass. Renew. Energ. 2021, 180, 914–936. [Google Scholar] [CrossRef]
- Zhang, P.; Wells, Y.; Liang, J.; Love, D.; Parker, A.; Botella, C. Effect of diluted hydrolysate as yeast propagation medium on ethanol production. Bioresour. Technol. 2019, 271, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Landaeta, R.; Aroca, G.; Acevedo, F.; Teixeira, J.A.; Mussatto, S.I. Adaptation of a flocculent Saccharomyces cerevisiae strain to lignocellulosic inhibitors by cell recycle batch fermentation. Appl. Energy 2013, 102, 124–130. [Google Scholar] [CrossRef]
- Kaur, S.; Guleria, P.; Sidana, A.; Yadav, S.K. Efficient process for xylitol production from nitric acid pretreated rice straw derived pentosans by Candida tropicalis GS18. Biomass Bioenergy 2022, 166, 106618. [Google Scholar] [CrossRef]
- Pooja; Purohit, A.; Kaur, S.; Yadav, S.K. Identification of a yeast Meyerozyma caribbica M72 from mahua flower for efficient transformation of rice straw into ethanol. Biomass Conv. Bioref. 2021, 1–13. [Google Scholar] [CrossRef]
- Sharma, B.; Larroche, C.; Dussap, C.G. Comprehensive assessment of 2G bioethanol production. Bioresour. Technol. 2020, 31, 123630. [Google Scholar] [CrossRef]
- Bhavana, B.K.; Mudliar, S.N.; Bokade, V.V.; Debnath, S. Effect of furfural, acetic acid and 5-hydroxymethylfurfural on yeast growth and xylitol fermentation using Pichia stipitis NCIM 3497. Biomass Convers. Biorefinery 2022, 1–15. [Google Scholar] [CrossRef]
- Ping, Y.; Ling, H.Z.; Song, G.; Ge, J.P. Xylitol production from non-detoxified corncob hemicellulose acid hydrolysate by Candida tropicalis. Biochem. Eng. J. 2013, 75, 86–91. [Google Scholar] [CrossRef]
- Pant, S.; Ritika; Prakash, A.; Kuila, A. Integrated production of ethanol and xylitol from Brassica juncea using Candida sojae JCM 1644. Bioresour. Technol. 2022, 351, 126903. [Google Scholar] [CrossRef]
- Cheng, K.K.; Zhang, J.A.; Ling, H.Z.; Ping, W.X.; Huang, W.; Ge, J.P.; Xu, J.M. Optimization of pH and acetic acid concentration for bioconversion of hemicellulose from corncobs to xylitol by Candida tropicalis. Biochem. Eng. J. 2009, 43, 203–207. [Google Scholar] [CrossRef]
- Lima, L.H.A.; Felipe, M.G.A.; Vitolo, M.; Torres, F.A.G. Effect of acetic acid present in bagasse hydrolysate on the activities of xylose reductase and xylitol dehydrogenase in C. guilliermondii. Appl. Microbiol. Biotechnol. 2004, 65, 734–738. [Google Scholar] [CrossRef]
- Modig, T.; Lidén, G.; Taherzadeh, M.J. Inhibition effects of furfural on alcohol dehydrogenase, aldehyde dehydrogenase and pyruvate dehydrogenase. Biochem. J. 2002, 363, 769–776. [Google Scholar] [CrossRef]
- Liu, Z.L.; Ma, M.; Song, M. Evolutionarily engineered ethanologenic yeast detoxifies lignocellulosic biomass conversion inhibitors by reprogrammed pathways. Mol. Genet. Genom. 2009, 282, 233–244. [Google Scholar]
- Horvath, I.S.; Franzen, C.J.; Taherzadeh, M.J.; Niklasson, C.; Liden, G. Effects of furfural on the respiratory metabolism of Saccharomyces cerevisiae in glucose-limited chemostats. Appl. Environ. Microbiol. 2003, 69, 4076–4086. [Google Scholar] [CrossRef]
- Matos, Í.T.S.R.; do Carmo, E.J.; de Assunc, E.N.; de Almeida, R.A.; Soares, V.M.; Filho, S.A. Xylitol production and furfural consumption by a wild type Geotrichum sp. Electron. J. Biotechnol. 2016, 24, 21–25. [Google Scholar] [CrossRef][Green Version]
- Bura, R.; Vajzovic, A.; Doty, S.L. Novel endophytic yeast Rhodotorula mucilaginosa strain PTD3 I: Production of xylitol and ethanol. J. Ind. Microbiol. Biotechnol. 2012, 39, 1003–1011. [Google Scholar] [CrossRef]
- Perna, M.D.; Bastos, R.G.; Ceccato-Antonini, S.R. Single and combined effects of acetic acid, furfural, and sugars on the growth of the pentose-fermenting yeast Meyerozyma guilliermondii. 3 Biotech 2018, 8, 119. [Google Scholar] [CrossRef]
- Trichez, D.; Steindorff, A.S.; Soares, C.E.; Formighieri, E.F.; Almeida, J.R. Physiological and comparative genomic analysis of new isolated yeasts Spathaspora sp. JA1 and Meyerozyma caribbica JA9 reveal insights into xylitol production. FEMS Yeast Res. 2019, 19, 34. [Google Scholar] [CrossRef]
- Nagarajan, A.; Thulasinathan, B.; Arivalagan, P.; Alagarsamy, A.; Muthuramalingam, J.B.; Thangarasu, S.D.; Thangavel, K. Particle size influence on the composition of sugars in corncob hemicellulose hydrolysate for xylose fermentation by Meyerozyma caribbica. Bioresour. Technol. 2021, 340, 125677. [Google Scholar] [CrossRef]
- Ko, J.K.; Enkh-Amgalan, T.; Gong, G.; Um, Y.; Lee, S.M. Improved bioconversion of lignocellulosic biomass by Saccharomyces cerevisiae engineered for tolerance to acetic acid. GCB Bioenergy 2020, 12, 90–100. [Google Scholar] [CrossRef]
- Pramasari, D.A.; Oktaviani, M.; Thontowi, A.; Purnawan, A.; Ermawar, R.A.; Sondari, D.; Ningrum, R.S.; Laksana, R.P.B.; Lianawati, A.; Fahrezi, M.Z.M.; et al. The use of hemicellulose acid hydrolysate for hydrolysis of sugarcane trash and its fermentation for producing xylitol. Ind. Crops Prod. 2023, 193, 116163. [Google Scholar] [CrossRef]
- Tiwari, S.; Jadhav, R.; Avchar, R.; Lanjekar, V.; Datar, M.; Baghela, A. Nectar yeast community of tropical flowering plants and assessment of their osmotolerance and xylitol-producing potential. Curr. Microbiol. 2021, 79, 28. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Wang, L.; Li, F.; Hua, D.; Ma, C.; Ma, Y.; Xu, P. Kinetics of d-lactic acid production by Sporolactobacillus sp. strain CASD using repeated batch fermentation. Bioresour. Technol. 2010, 101, 6499–6505. [Google Scholar] [CrossRef] [PubMed]
- Sirisansaneeyakul, S.; Wannawilai, S.; Chisti, Y. Repeated fed-batch production of xylitol by Candida magnoliae TISTR 5663. J. Chem. Technol. Biotechnol. 2012, 88, 1121–1129. [Google Scholar] [CrossRef]
- Raposo, R.S.; de Almeida, M.C.M.D.; de Oliveira, M.C.M.A.; da Fonseca, M.M.; Cesario, M.T. A Burkholderia sacchari cell factory: Production of poly-3-hydroxybutyrate, xylitol and xylonic acid from xylose-rich sugar mixtures. New Biotechnol. 2017, 34, 12–22. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).