The Effects of Nanoparticles- Zerovalent Iron on Sustainable Biomethane Production through Co-Digestion of Olive Mill Wastewater and Chicken Manure
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrates
2.2. Analytical Methods
2.3. The Hydrolysis and Acidification Rate, and VS Destruction
2.4. Nanoparticles- Zerovalent Iron Preparation
2.5. Experimental Setup
3. Results
3.1. Influence of NP-ZVIs on the AcoD Process
3.1.1. Effects of Dose and Size of NP-ZVIs on the Hydrolysis Percentage
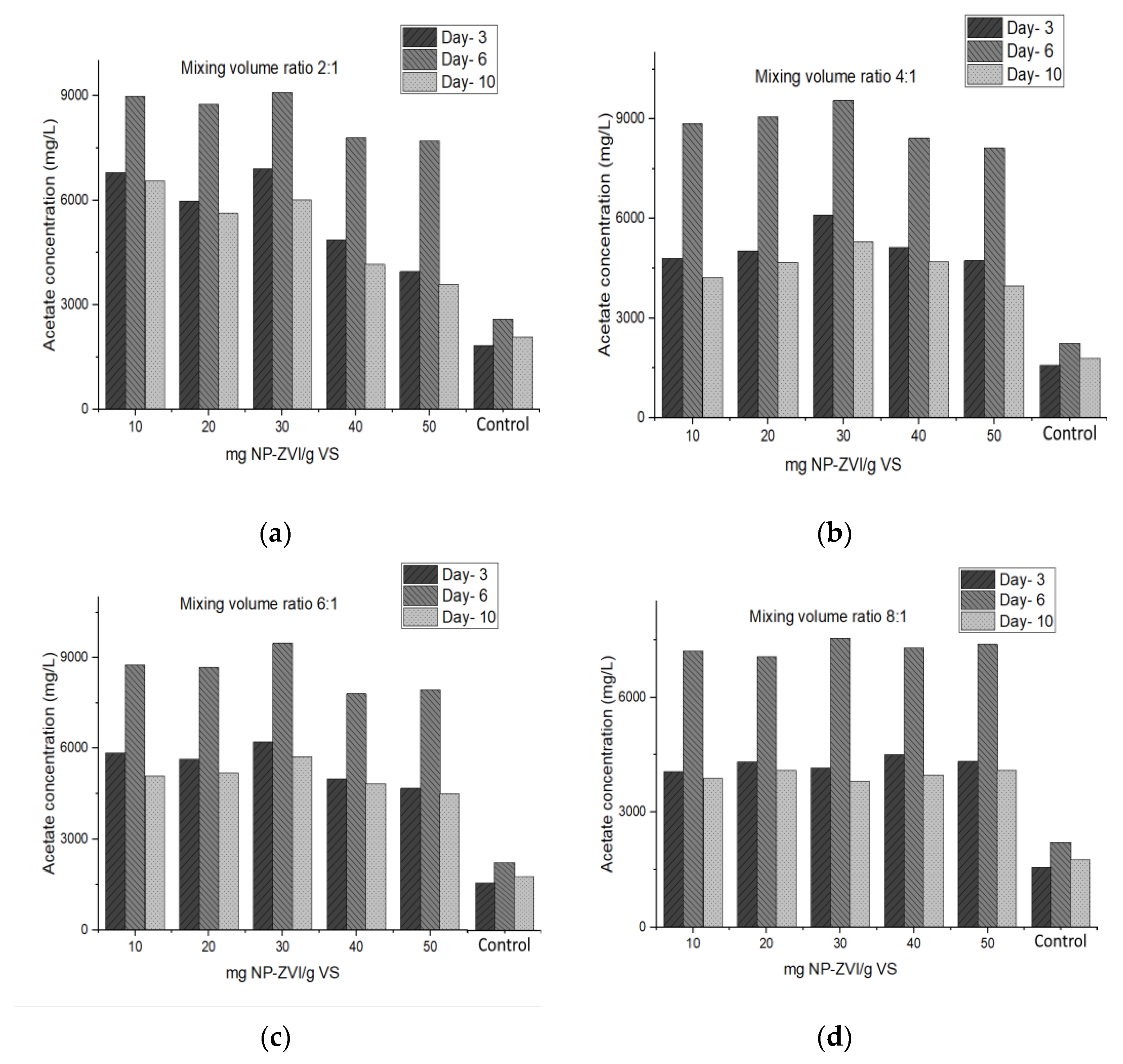
3.1.2. Effects of Dose and Size of NP-ZVIs on the Percentage of Acidification
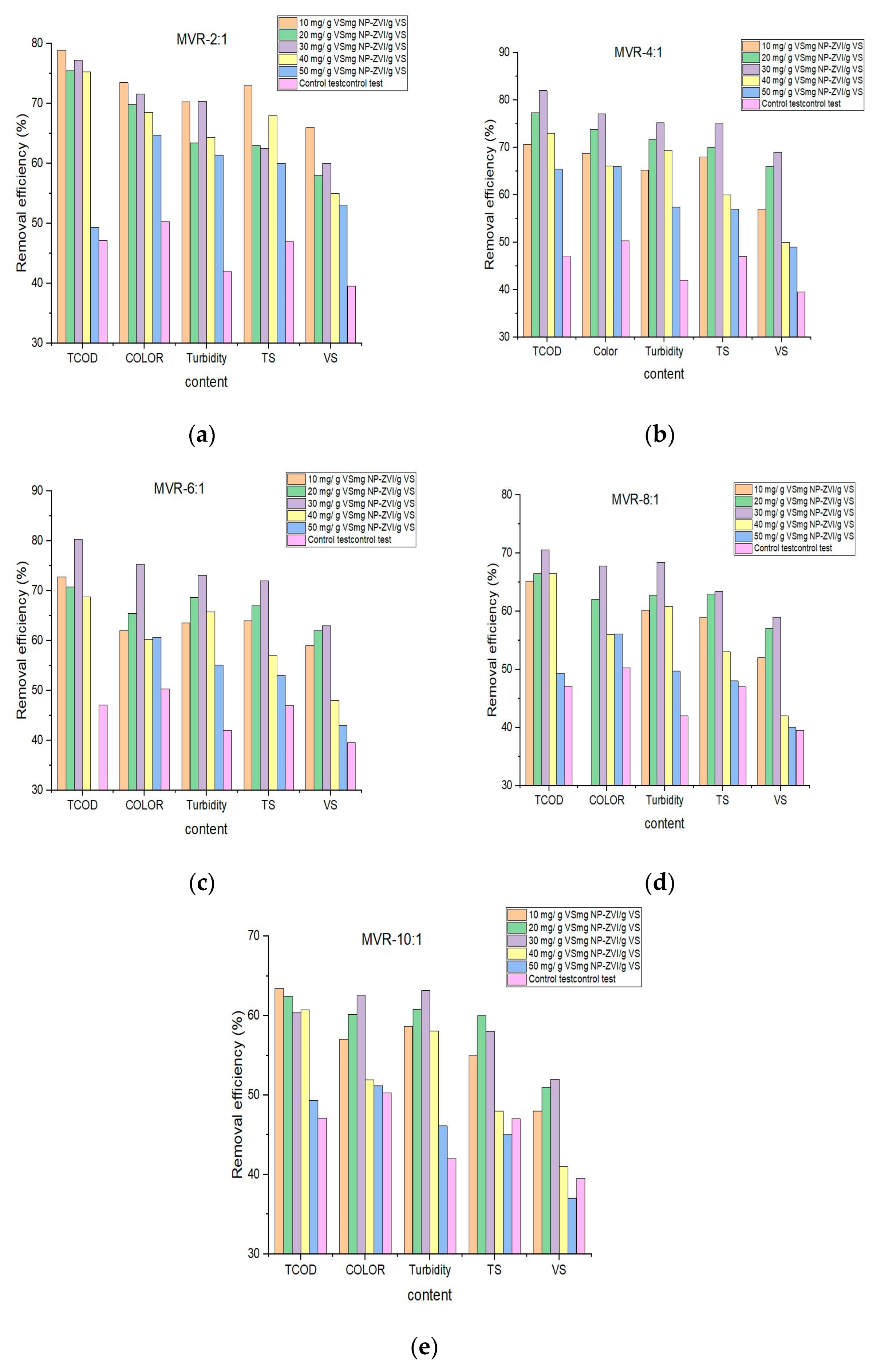
3.1.3. Effects of Dose and Size NP-ZVI on the Removal Contaminant and Biological Treatment
3.1.4. Effects of Dose and Size of NP-ZVI on the Biogas Yield and Methane Content
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Santamouris, M. Energy Consumption and Environmental Quality of the Building Sector. In Minimizing Energy Consumption, Energy Poverty and Global and Local Climate Change in the Built Environment: Innovating to Zero; Elsevier: Amsterdam, The Netherlands, 2019; pp. 29–64. [Google Scholar] [CrossRef]
- Alrawashde, K.; Talat, N.; Alshorman, A. Multi Stage Flashing Small Scale Plant Combined CHP Plant Driven by Biogas Plant. Int. J. Appl. Sci. Technol. 2017, 7, 51–59. [Google Scholar]
- IEA. World Energy Outlook; IEA: Paris, France, 2019; Chapter 7; pp. 328–333. Available online: https://www.iea.org/reports/world-energy-outlook-2019 (accessed on 5 September 2022).
- Al-Samrraie, L.A.; Alrawashdeh, K.A.B.; Al-Issa, H.A.; Shakhatreh, S.; Hussien, A.A.; Qasem, I. Improve Heavy Metals and Pollutants Removal from the Pharmaceuticals Wastewater Using Washingtonia Robusta: New Extractisssson Process. Civ. Environ. Eng. 2022, 18, 340–349. [Google Scholar] [CrossRef]
- Bkoor Alrawashdeh, K.A.; Gul, E.; Yang, Q.; Yang, H.; Bartocci, P.; Fantozzi, F. Effect of Heavy Metals in the Performance of Anaerobic Digestion of Olive Mill Waste. Processes 2020, 8, 1146. [Google Scholar] [CrossRef]
- Gul, E.; Campana, P.E.; Chandrasekaran, A.; Subbiah, S.; Yang, H.; Yang, Q.; Yan, J.; Li, H.; Desideri, U.; Barelli, L.; et al. Perspectives and State of the Art in Producing Solar Fuels and Chemicals from CO2. In Advanced Technology for the Conversion of Waste into Fuels and Chemicals; Elsevier: Amsterdam, The Netherlands, 2021; pp. 181–219. [Google Scholar] [CrossRef]
- Jurgutis, L.; Slepetiene, A.; Volungevicius, J.; Amaleviciute-Volunge, K. Biogas Production from Chicken Manure at Different Organic Loading Rates in a Mesophilic Full Scale Anaerobic Digestion Plant. Biomass Bioenergy 2020, 141, 105693. [Google Scholar] [CrossRef]
- Al bkoor Alrawashdeh, K. Anaerobic Co-Digestion Efficiency under the Stress Exerted by Different Heavy Metals Concentration: An Energy Nexus Analysis. Energy Nexus 2022, 7, 100099. [Google Scholar] [CrossRef]
- Al bkoor Alrawashdeh, K. Improving Anaerobic Co-Digestion of Sewage Sludge with Thermal Dried Olive Mill Wastewater. Waste Biomass Valorization 2018, 10, 2213–2219. [Google Scholar] [CrossRef]
- Bkoor Alrawashdeh, K.A.; Al-Essa, A.H. Anaerobic Co-Digestion Mill Wastewater—Activated Sludge Effect of Aerobic Pretreatment on the Performance of OMW Anaerobic Digestion. Waste Biomass Valorization 2020, 11, 4781–4788. [Google Scholar] [CrossRef]
- Liao, X.; Li, H.; Cheng, Y.; Chen, N.; Li, C.; Yang, Y. Process Performance of High-Solids Batch Anaerobic Digestion of Sewage Sludge. Environ. Technol. 2014, 35, 2652–2659. [Google Scholar] [CrossRef]
- Azbar, N.; Keskin, T.; Yuruyen, A. Enhancement of Biogas Production from Olive Mill Effluent (OME) by Co-Digestion. Biomass Bioenergy 2008, 32, 1195–1201. [Google Scholar] [CrossRef]
- Al Bkoor Alrawashdeh, K.; Al-Zboon, K.K.; Rabadi, S.A.; Gul, E.; AL-Samrraie, L.A.; Ali, R.; Al-Tabbal, J.A. Impact of Iron Oxide Nanoparticles on Sustainable Production of Biogas through Anaerobic Co-Digestion of Chicken Waste and Wastewater. Front. Chem. Eng. 2022, 4, 974546. [Google Scholar] [CrossRef]
- Yuan, T.; Bian, S.; Ko, J.H.; Liu, J.; Shi, X.; Xu, Q. Exploring the Roles of Zero-Valent Iron in Two-Stage Food Waste Anaerobic Digestion. Waste Manag. 2020, 107, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Domrongpokkaphan, V.; Phalakornkule, C.; Khemkhao, M. In-Situ Methane Enrichment of Biogas from Anaerobic Digestion of Palm Oil Mill Effluent by Addition of Zero Valent Iron (ZVI). Int. J. Hydrogen Energy 2021, 46, 30976–30987. [Google Scholar] [CrossRef]
- Zhang, Y.; Jing, Y.; Quan, X.; Liu, Y.; Onu, P. A Built-in Zero Valent Iron Anaerobic Reactor to Enhance Treatment of Azo Dye Wastewater. Water Sci. Technol. 2011, 63, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Peng, S.; Xie, B.; Yang, X.; Sun, S.; Yao, H.; Li, D. Influence Characteristics and Mechanism of Organic Carbon on Denitrification, N2O Emission and NO2− Accumulation in the Iron [Fe(0)]-Oxidizing Supported Autotrophic Denitrification Process. Chem. Eng. J. 2020, 393, 124736. [Google Scholar] [CrossRef]
- Tchobanoglous, G.; Angelakis, A.N. Technologies for Wastewater Treatment Appropriate for Reuse: Potential for Applications in Greece. Water Sci. Technol. 1996, 33, 15–24. [Google Scholar] [CrossRef]
- Konadu-Amoah, B.; Hu, R.; Ndé-Tchoupé, A.I.; Gwenzi, W.; Noubactep, C. Metallic Iron (Fe0)-Based Materials for Aqueous Phosphate Removal: A Critical Review. J. Environ. Manag. 2022, 315, 115157. [Google Scholar] [CrossRef]
- Alrawashdeh, K.A.B.; Al-Samrraie, L.A.; Al Issa, H.A.; Qasem, I.; Hussien, A.A.; Al-Zboon, K.K.; Damseh, R.A.; Gul, E. Prediction and Optimization of Biogas Production from OMW Digestion Using Fenton Pre-Treatment Process with Particle Swarm Optimization. Int. J. Des. Nat. Ecodynamics 2022, 17, 157–168. [Google Scholar] [CrossRef]
- Kassab, G.; Khater, D.; Odeh, F.; Shatanawi, K.; Halalsheh, M.; Arafah, M.; van Lier, J.B. Impact of Nanoscale Magnetite and Zero Valent Iron on the Batch-Wise Anaerobic Co-Digestion of Food Waste and Waste-Activated Sludge. Water 2020, 12, 1283. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, C.; Hou, J.; Wang, P.; You, G.; Miao, L.; Lv, B.; Yang, Y.; Zhang, F. Application of zero valent iron coupling with biological process for wastewater treatment: A review. Rev. Environ. Sci. Bio/Technol. 2017, 16, 667–693. [Google Scholar] [CrossRef]
- Boontian, N. Effect of Zero Valent Iron (ZVI) in Wastewater Treatment: A Review. Appl. Mech. Mater. 2015, 775, 180–184. [Google Scholar] [CrossRef]
- Hu, R.; Cui, X.; Xiao, M.; Qiu, P.; Lufingo, M.; Gwenzi, W.; Noubactep, C. Characterizing the Suitability of Granular Fe0 for the Water Treatment Industry. Processes 2019, 7, 652. [Google Scholar] [CrossRef]
- Lufingo, M.; Ndé-Tchoupé, A.I.; Hu, R.; Njau, K.N.; Noubactep, C. A Novel and Facile Method to Characterize the Suitability of Metallic Iron for Water Treatment. Water 2019, 11, 2465. [Google Scholar] [CrossRef]
- Lien, H.-L.; Zhang, W.-X. Nanoscale Pd/Fe Bimetallic Particles: Catalytic Effects of Palladium on Hydrodechlorination. Appl. Catal. B: Environ. 2007, 77, 110–116. [Google Scholar] [CrossRef]
- Lizama, A.C.; Figueiras, C.C.; Gaviria, L.A.; Pedreguera, A.Z.; Ruiz Espinoza, J.E. Nanoferrosonication: A Novel Strategy for Intensifying the Methanogenic Process in Sewage Sludge. Bioresour. Technol. 2019, 276, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Mouftahi, M.; Tlili, N.; Hidouri, N.; Bartocci, P.; Bkoor Alrawashdeh, K.A.; Gul, E.; Liberti, F.; Fantozzi, F. Biomethanation Potential (BMP) Study of Mesophilic Anaerobic Co-Digestion of Abundant Bio-Wastes in Southern Regions of Tunisia. Processes 2020, 9, 48. [Google Scholar] [CrossRef]
- Pan, X.; Lv, N.; Cai, G.; Zhou, M.; Wang, R.; Li, C.; Ning, J.; Li, J.; Li, Y.; Ye, Z.; et al. Carbon- and Metal-Based Mediators Modulate Anaerobic Methanogenesis and Phenol Removal: Focusing on Stimulatory and Inhibitory Mechanism. J. Hazard. Mater. 2021, 420, 126615. [Google Scholar] [CrossRef]
- Baniamerian, H.; Isfahani, P.G.; Tsapekos, P.; Alvarado-Morales, M.; Shahrokhi, M.; Vossoughi, M.; Angelidaki, I. Application of Nano-Structured Materials in Anaerobic Digestion: Current Status and Perspectives. Chemosphere 2019, 229, 188–199. [Google Scholar] [CrossRef]
- Jiang, W.; Dionysiou, D.D.; Kong, M.; Liu, Z.; Sui, Q.; Lyu, S. Utilization of Formic Acid in Nanoscale Zero Valent Iron-Catalyzed Fenton System for Carbon Tetrachloride Degradation. Chem. Eng. J. 2020, 380, 122537. [Google Scholar] [CrossRef]
- Wang, S.; Yuan, R.; Liu, C.; Zhou, B. Effect of Fe2+ Adding Period on the Biogas Production and Microbial Community Distribution during the Dry Anaerobic Digestion Process. Process Saf. Environ. Prot. 2020, 136, 234–241. [Google Scholar] [CrossRef]
- WANG, L.; ZHOU, Q.; LI, F. Avoiding Propionic Acid Accumulation in the Anaerobic Process for Biohydrogen Production. Biomass Bioenergy 2006, 30, 177–182. [Google Scholar] [CrossRef]
- Flaherty, D.W. Direct Synthesis of H2O2 from H2 and O2 on Pd Catalysts: Current Understanding, Outstanding Questions, and Research Needs. ACS Catal. 2018, 8, 1520–1527. [Google Scholar] [CrossRef]
- Huang, Y.-X.; Guo, J.; Zhang, C.; Hu, Z. Hydrogen Production from the Dissolution of Nano Zero Valent Iron and Its Effect on Anaerobic Digestion. Water Res. 2016, 88, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chang, J.; Wang, H.; Liu, Y.; Zhang, X.; Liang, P.; Huang, X. Enhancing Direct Interspecies Electron Transfer in Syntrophic-Methanogenic Associations with (Semi)Conductive Iron Oxides: Effects and Mechanisms. Sci. Total Environ. 2019, 695, 133876. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Yu, S.; Xu, S.; Fang, W.; Liu, J.; Li, H. Effect of Fe0 Addition on Volatile Fatty Acids Evolution on Anaerobic Digestion at High Organic Loading Rates. Waste Manag. 2018, 71, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Al bkoor Alrawashdeh, K.; Pugliese, A.; Slopiecka, K.; Pistolesi, V.; Massoli, S.; Bartocci, P.; Bidini, G.; Fantozzi, F. Codigestion of Untreated and Treated Sewage Sludge with the Organic Fraction of Municipal Solid Wastes. Fermentation 2017, 3, 35. [Google Scholar] [CrossRef]
- Alrawashdeh, K.A.B.; Slopiecka, K.; Alshorman, A.A.; Bartocci, P.; Fantozzi, F. Pyrolytic Degradation of Olive Waste Residue (OWR) by TGA: Thermal Decomposition Behavior and Kinetic Study. J. Energy Power Eng. 2017, 11, 497–510. [Google Scholar] [CrossRef]
- Goulding, D.A.; Fox, P.F.; O’Mahony, J.A. Milk Proteins: An Overview. In Milk Proteins; Elsevier: Amsterdam, The Netherlands, 2020; pp. 21–98. [Google Scholar] [CrossRef]
- Abdelsalam, E.; Samer, M.; Attia, Y.A.; Abdel-Hadi, M.A.; Hassan, H.E.; Badr, Y. Effects of Co and Ni Nanoparticles on Biogas and Methane Production from Anaerobic Digestion of Slurry. Energy Convers. Manag. 2017, 141, 108–119. [Google Scholar] [CrossRef]
- APHA. Standard Methods Committee of the American Public Health Association, American Water Works Association, and Water Environment Federation. 4500-p phosphorus. In Standard Methods for the Examination of Water and Wastewater; Lipps, W.C., Baxter, T.E., Braun-Howland, E., Eds.; APHA Press: Washington, DC, USA, 2018. [Google Scholar] [CrossRef]
- Boe, K.; Batstone, D.J.; Angelidaki, I. An Innovative Online VFA Monitoring System for the Anerobic Process, Based on Headspace Gas Chromatography. Biotechnol. Bioeng. 2007, 96, 712–721. [Google Scholar] [CrossRef]
- Ahuja, N.; Ansari, A.; Rajput, R.; Singh, P. Synthesis and Characterization of Zero Valent Iron Nanoparticles for Textile Wastewater Treatment. Pollution 2020, 6, 773–783. [Google Scholar] [CrossRef]
- Song, H.; Clarke, W.P.; Blackall, L.L. Concurrent Microscopic Observations and Activity Measurements of Cellulose Hydrolyzing and Methanogenic Populations during the Batch Anaerobic Digestion of Crystalline Cellulose. Biotechnol. Bioeng. 2005, 91, 369–378. [Google Scholar] [CrossRef]
- Switzenbaum, M.S.; Farrell, J.B.; Pincince, A.B. Relationship between the van Kleeck and Mass-Balance Calculation of Volatile Solids Loss. Water Environ. Res. 2003, 75, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Ismail, Z.Z.; Talib, A.R. Assessment of anaerobic co-digestion of agro wastes for biogas recovery: A bench scale application to date palm wastes. Energy Environ. 2014, 5, 591–600. [Google Scholar]
- Cheah, Y.-K.; Vidal-Antich, C.; Dosta, J.; Mata-Álvarez, J. Volatile Fatty Acid Production from Mesophilic Acidogenic Fermentation of Organic Fraction of Municipal Solid Waste and Food Waste under Acidic and Alkaline PH. Environ. Sci. Pollut. Res. 2019, 26, 35509–35522. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Kang, H.-J.; Park, K.-H.; Park, H.-D. Direct Interspecies Electron Transfer via Conductive Materials: A Perspective for Anaerobic Digestion Applications. Bioresour. Technol. 2018, 254, 300–311. [Google Scholar] [CrossRef]
- Hamza, R.A.; Zaghloul, M.S.; Iorhemen, O.T.; Sheng, Z.; Tay, J.H. Optimization of Organics to Nutrients (COD:N:P) Ratio for Aerobic Granular Sludge Treating High-Strength Organic Wastewater. Sci. Total Environ. 2019, 650, 3168–3179. [Google Scholar] [CrossRef]
- Shubair, T.; Eljamal, O.; Matsunaga, N. Evaluation of Nanoscale Zero Valent Iron Particles for the Removal of Cesium from Aqueous Solutions. IOP Conf. Ser. Mater. Sci. Eng. 2018, 454, 012104. [Google Scholar] [CrossRef]
- Pullin, H.; Springell, R.; Parry, S.; Scott, T. The Effect of Aqueous Corrosion on the Structure and Reactivity of Zero-Valent Iron Nanoparticles. Chem. Eng. J. 2017, 308, 568–577. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Li, Y.; Quan, X.; Zhao, Z. Comparing the Mechanisms of ZVI and Fe3O4 for Promoting Waste-Activated Sludge Digestion. Water Res. 2018, 144, 126–133. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, Y.; Tan, D.; Zhao, Z.; Zhao, H.; Quan, X. Roles of Magnetite and Granular Activated Carbon in Improvement of Anaerobic Sludge Digestion. Bioresour. Technol. 2018, 249, 666–672. [Google Scholar] [CrossRef]
- Zhang, Q.-L.; Liu, Y.; Ai, G.-M.; Miao, L.-L.; Zheng, H.-Y.; Liu, Z.-P. The Characteristics of a Novel Heterotrophic Nitrification–Aerobic Denitrification Bacterium, Bacillus Methylotrophicus Strain L7. Bioresour. Technol. 2012, 108, 35–44. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Yu, Q.; Zhang, Y. Ferroferric Oxide Triggered Possible Direct Interspecies Electron Transfer between Syntrophomonas and Methanosaeta to Enhance Waste Activated Sludge Anaerobic Digestion. Bioresour. Technol. 2018, 250, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, Z.; Xu, R.; Xiang, Y.; Jia, M.; Hu, J.; Zheng, Y.; Xiong, W.; Cao, J. Enhanced Mesophilic Anaerobic Digestion of Waste Sludge with the Iron Nanoparticles Addition and Kinetic Analysis. Sci. Total Environ. 2019, 683, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, J.; Shin, S.G.; Hwang, S.; Lee, C. Continuous Fermentation of Food Waste Leachate for the Production of Volatile Fatty Acids and Potential as a Denitrification Carbon Source. Bioresour. Technol. 2016, 207, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Conductive Materials in Anaerobic Digestion: From Mechanism to Application. Bioresour. Technol. 2020, 298, 122403. [CrossRef]
- Aboudi, K.; Álvarez-Gallego, C.J.; Romero-García, L.I. Semi-Continuous Anaerobic Co-Digestion of Sugar Beet Byproduct and Pig Manure: Effect of the Organic Loading Rate (OLR) on Process Performance. Bioresour. Technol. 2015, 194, 283–290. [Google Scholar] [CrossRef]
- Pan, X.; Zhao, L.; Li, C.; Angelidaki, I.; Lv, N.; Ning, J.; Cai, G.; Zhu, G. Deep Insights into the Network of Acetate Metabolism in Anaerobic Digestion: Focusing on Syntrophic Acetate Oxidation and Homoacetogenesis. Water Res. 2021, 190, 116774. [Google Scholar] [CrossRef]
- Wu, D.; Zheng, S.; Ding, A.; Sun, G.; Yang, M. Performance of a Zero Valent Iron-Based Anaerobic System in Swine Wastewater Treatment. J. Hazard. Mater. 2015, 286, 1–6. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, J.; Zhao, Y.; Zhao, X.; Zheng, Z.; Wen, B.; Cui, Z.; Wang, X. A New Perspective of Using Sequential Extraction: To Predict the Deficiency of Trace Elements during Anaerobic Digestion. Water Res. 2018, 140, 335–343. [Google Scholar] [CrossRef]
- Xu, R.; Xu, S.; Zhang, L.; Florentino, A.P.; Yang, Z.; Liu, Y. Impact of Zero Valent Iron on Blackwater Anaerobic Digestion. Bioresour. Technol. 2019, 285, 121351. [Google Scholar] [CrossRef]
- Dong, D.; Aleta, P.; Zhao, X.; Choi, O.K.; Kim, S.; Lee, J.W. Effects of Nanoscale Zero Valent Iron (NZVI) Concentration on the Biochemical Conversion of Gaseous Carbon Dioxide (CO2) into Methane (CH4). Bioresour. Technol. 2019, 275, 314–320. [Google Scholar] [CrossRef]
- Zhu, X.; Thi Le, T.; Du, J.; Tiantian Xu, T.; Cui, Y.; Ling, H.; Hoon Kim, S.H. Novel core-shell sulfidated nano-Fe(0) particles for chromate sequestration: Promoted electron transfer and Fe(II) production. Chemosphere 2021, 284, 131379. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-P.; Li, X.-Q.; Zhang, W.-X.; Wang, H.P. A Method for the Preparation of Stable Dispersion of Zero-Valent Iron Nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2007, 308, 60–66. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Y.; Quan, X.; Chen, S. Enhanced Anaerobic Digestion of Waste Activated Sludge Digestion by the Addition of Zero Valent Iron. Water Res. 2014, 52, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.H.; Abdulaziz, M. The Effect of Different Zero-Valent Iron Sources on Biogas Production from Waste Sludge Anaerobic Digestion. J. Biotechnol. Res. 2016, 2, 59–67. [Google Scholar]


| Mixing Volume Ratio (NaBH4:FeSO4.7H2O) | mg NP-ZVI/ g VS | ||||
|---|---|---|---|---|---|
| 10 | 20 | 30 | 40 | 50 | |
| 2:1 | A10 | A20 | A30 | A40 | A50 |
| 4:1 | B10 | B20 | B30 | B40 | B50 |
| 6:1 | C10 | C20 | C30 | C40 | C50 |
| 8:1 | D10 | D20 | D30 | D40 | D50 |
| 10:1 | E10 | E20 | E30 | E40 | E50 |
| Substrate | Total Solids, TS (%) | Volatile Solids, VS (%) | Ash (%) | Moisture (%) | Fixed Carbone (%) | Phenols (gl −1) | TCOD (gl −1) | BOD5 (gl −1) | pH |
|---|---|---|---|---|---|---|---|---|---|
| OMWW | 7.12 ± 0.96 | 2.92 ± 0.52 | 0.39 ± 0.09 | 92.88 ± 0.97 | 3.81 ± 0.8 | 4.21 ± 0.15 | 53.79 ± 2.04 | 8.43 ± 3.56 | 5.08 ± 1.32 |
| CM | 27.65 ± 2.82 | 5.12 ± 1.02 | 2.31 ± 0.21 | 72.35 ± 2.05 | 20.22 ± 2.7 | 6.83 ± 0.65 | 9.05 ± 1.96 | 7.21 ± 1.08 | 6.39 ± 0.18 |
| Inoculum | 2.51 ± 0.62 | 0.53 ± 0.06 | 0.51 ± 0.07 | 97.49 ± 1.04 | 1.47 ± 0.2 | 2.95 ± 1.36 | 21.82 ± 2.91 | 3.62 ± 2.13 | 6.75 ± 0.95 |
| Mixing Volume Ratio (NaBH4:FeSO4.7H2O) | Hydrolysis (%) | ||||
|---|---|---|---|---|---|
| 10 mg/g VS | 20 mg/g VS | 30 mg/g VS | 40 mg/g VS | 50 mg/g VS | |
| 2:1 | 75.25 | 73.9 | 74.13 | 69.84 | 65.18 |
| 4:1 | 76.88 | 77.13 | 82.32 | 72.08 | 71.77 |
| 6:1 | 73.52 | 74.37 | 78.60 | 65.93 | 72.83 |
| 8:1 | 61.63 | 58.75 | 63.52 | 60.39 | 60.82 |
| 10:1 | 54.61 | 56.91 | 57.26 | 57.67 | 64.65 |
| Mixing Volume Ratio (NaBH4:FeSO4.7H2O) | Biogas (Nm3/kg.VS) | ||||
|---|---|---|---|---|---|
| 10 mg/g VS | 20 mg/g VS | 30 mg/g VS | 40 mg/g VS | 50 mg/g VS | |
| 2:1 | 0.311 ± 0.015 | 0.28 ± 0.051 | 0.282 ± 0.023 | 0.182 ± 0.011 | 0.128 ± 0.002 |
| 4:1 | 0.32 ± 0.046 | 0.335 ± 0.013 | 0.389 ± 0.005 | 0.158 ± 0.004 | 0.107 ± 0.019 |
| 6:1 | 0.264 ± 0.027 | 0.305 ± 0.01 | 0.356 ± 0.07 | 0.136 ± 0.013 | 0.083 ± 0.008 |
| 8:1 | 0.237 ± 0.014 | 0.23 ± 0.021 | 0.245 ± 0.022 | 0.105 ± 0.034 | 0.064 |
| 10:1 | 0.222 ± 0.006 | 0.228 ± 0.015 | 0.238 ± 0.019 | 0.106 ± 0.009 | 0.055 ± 0.015 |
| Mixing Volume Ratio (NaBH4:FeSO4.7H2O) | Methane (%) | ||||
|---|---|---|---|---|---|
| 10 mg/g VS | 20 mg/g VS | 30 mg/g VS | 40 mg/g VS | 50 mg/g VS | |
| 2:1 | 66.47 | 65.08 | 67.08 | 52.43 | 50.05 |
| 4:1 | 67.35 | 66.89 | 68.34 | 49.05 | 46.22 |
| 6:1 | 65.78 | 66.92 | 67.79 | 46.42 | 45.18 |
| 8:1 | 63.11 | 64.88 | 65.93 | 45.38 | 45.1 |
| 10:1 | 56.47 | 59.15 | 65.05 | 44.93 | 42.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bkoor Alrawashdeh, K.A.; Al-Zboon, K.K.; Al-Tabbal, J.A.; AL-Samrraie, L.A.; Al Bsoul, A.; Damseh, R.A.; Khasawneh, A.; Dessouky, Y.; Tonbol, K.; Ali, B.M.; et al. The Effects of Nanoparticles- Zerovalent Iron on Sustainable Biomethane Production through Co-Digestion of Olive Mill Wastewater and Chicken Manure. Fermentation 2023, 9, 183. https://doi.org/10.3390/fermentation9020183
Bkoor Alrawashdeh KA, Al-Zboon KK, Al-Tabbal JA, AL-Samrraie LA, Al Bsoul A, Damseh RA, Khasawneh A, Dessouky Y, Tonbol K, Ali BM, et al. The Effects of Nanoparticles- Zerovalent Iron on Sustainable Biomethane Production through Co-Digestion of Olive Mill Wastewater and Chicken Manure. Fermentation. 2023; 9(2):183. https://doi.org/10.3390/fermentation9020183
Chicago/Turabian StyleBkoor Alrawashdeh, Khalideh Al, Kamel K. Al-Zboon, Jalal A. Al-Tabbal, La’aly A. AL-Samrraie, Abeer Al Bsoul, Rebhi A. Damseh, Ayat Khasawneh, Yasser Dessouky, Kareem Tonbol, Bassma M. Ali, and et al. 2023. "The Effects of Nanoparticles- Zerovalent Iron on Sustainable Biomethane Production through Co-Digestion of Olive Mill Wastewater and Chicken Manure" Fermentation 9, no. 2: 183. https://doi.org/10.3390/fermentation9020183
APA StyleBkoor Alrawashdeh, K. A., Al-Zboon, K. K., Al-Tabbal, J. A., AL-Samrraie, L. A., Al Bsoul, A., Damseh, R. A., Khasawneh, A., Dessouky, Y., Tonbol, K., Ali, B. M., & Youssef, E. E. (2023). The Effects of Nanoparticles- Zerovalent Iron on Sustainable Biomethane Production through Co-Digestion of Olive Mill Wastewater and Chicken Manure. Fermentation, 9(2), 183. https://doi.org/10.3390/fermentation9020183