Bioconversion of a Lignocellulosic Hydrolysate to Single Cell Oil for Biofuel Production in a Cost-Efficient Fermentation Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Analysis of Lignocellulosic Hydrolysate
2.1.1. Sugar Analysis
2.1.2. Ash
2.1.3. Elemental Analysis
2.1.4. Quantification of Sulfates
2.2. Strain and Media Composition
2.2.1. Strain
2.2.2. Pretreatment of the Lignocellulosic Hydrolysate
2.2.3. Pre-Culture
2.2.4. Medium Composition for Bioreactor Cultivation
2.3. Fermentation
2.3.1. Fermentation in 1.3 L Bioreactor
2.3.2. Fermentation in 350 mL Bioreactor
2.4. Monitoring of Fermentation
2.4.1. OD600 Measurement
2.4.2. Dry Weight
2.4.3. Lipid Content
2.4.4. Fatty Acid Profile
2.4.5. Statistical Analysis
2.5. Techno-Economic Analysis
3. Results
3.1. Analysis of the Lignocellulosic Hydrolysate
3.2. Pretreatment of the Lignocellulosic Hydrolysate
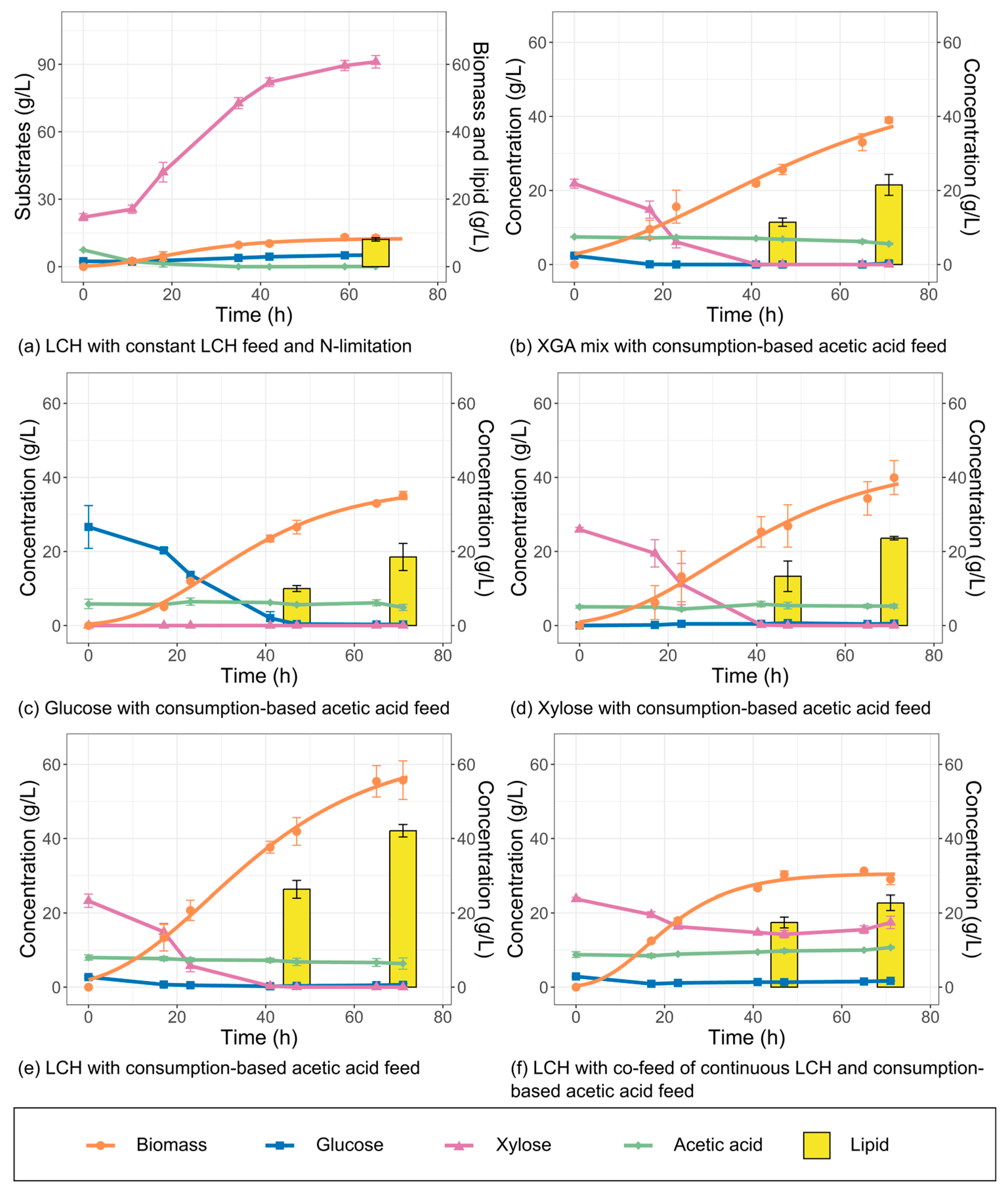
3.3. Nitrogen-Limited Fermentation
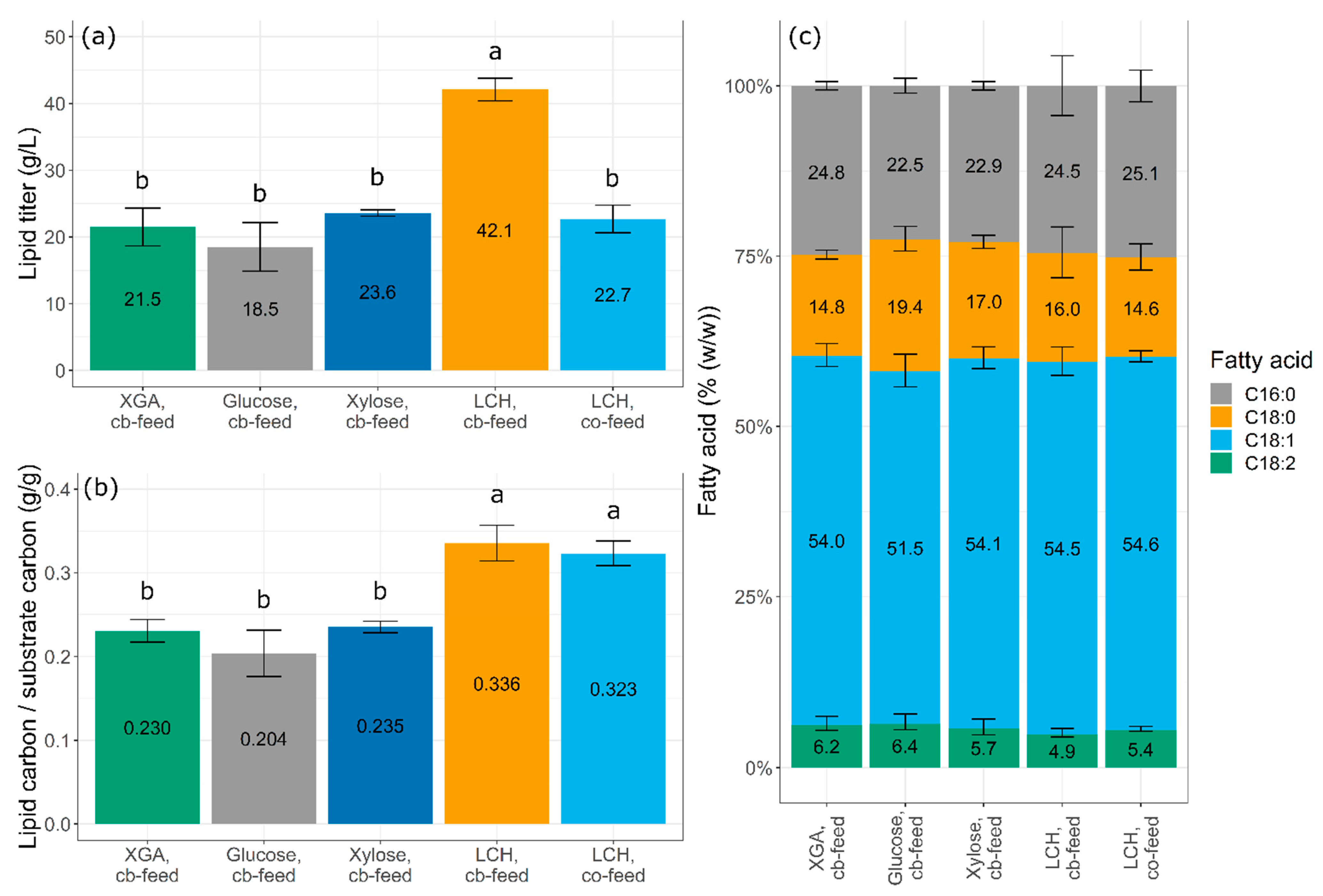
3.4. Acetic Acid-Based Fermentation with Commercial Sugars as Carbon Source
3.5. Acetic Acid-Based Fermentation of Lignocellulosic Hydrolysate at Different Starting Concentrations
3.6. Acetic Acid-Based Fermentation on Lignocellulosic Hydrolysate with Optimized Conditions
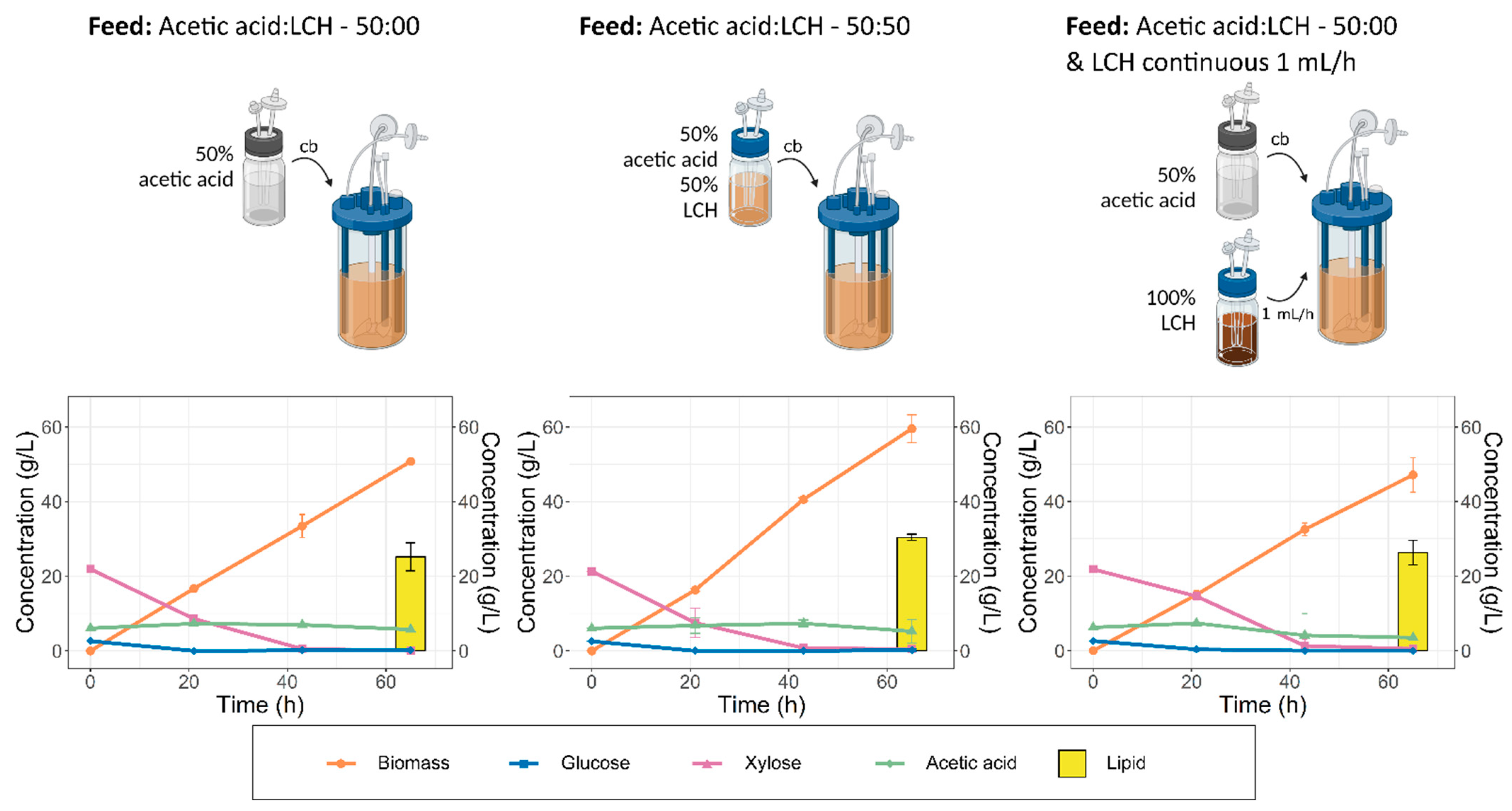
3.7. Screening of Different Strategies for Feeding Lignocellulosic Hydrolysate
3.8. Fermentation with Co-Feeding of Acetic Acid and Lignocellulosic Hydrolysate
3.9. Techno-Economic Analysis
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ge, M.; Friedrich, J.; Vigna, L. 5 Facts about Country & Sector GHG Emissions; World Resources Institute: Washington, DC, USA, 2022; pp. 1–9. [Google Scholar]
- Pörtner, H.-O.; Roberts, D.C.; Adams, H.; Adelekan, I.; Adler, C.; Adrian, R.; Aldunce, P.; Ali, E.; Begum, R.A.; Friedl, B.B.; et al. 2022: Technical Summary; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022; ISBN 9781009157896. [Google Scholar]
- United Nations. “17 Sustainable Development Goals (SDGs)”, Sustainable Development Goals (SDGs), 2022. Available online: https://sdgs.un.org/goals (accessed on 29 September 2022).
- European Commission. REPowerEU Plan; European Commission: Brussels, Belgium, 2022. [Google Scholar]
- European Commission. A Strategic Long-Term Vision for a Prosperous, Modern, Competitive & Climate-Neutral Economy EU Vision for 2050—“A Clean Planet for All”; European Commission: Brussels, Belgium, 2018. [Google Scholar]
- Flach, B.; Lieberz, S.; Bolla, S. EU Biofuels Annual 2019; USDA: Washington, DC, USA, 2019.
- Irena & Ace. Renewable Energy Outlook for ASEAN; Irena & Ace: Masdar City, Abu Dhabi, 2022; ISBN 9789295111288. [Google Scholar]
- Abeln, F.; Chuck, C.J. The history, state of the art and future prospects for oleaginous yeast research. Microb. Cell Fact. 2021, 20, 221. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Chen, X.-F.; Xiong, L.; Chen, X.-D.; Ma, L.L.; Chen, Y. Single cell oil production from low-cost substrates: The possibility and potential of its industrialization. Biotechnol. Adv. 2013, 31, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Agricultural Market Information Company (AMI). Report on Global Market Supply: 2020/2021; Agricultural Market Information Company (AMI): Las Vegas, NV, USA, 2020. [Google Scholar]
- Brandenburg, J. Lipid Production from Lignocellulosic Material by Oleaginous Yeasts. Doctoral Thesis, Swedish University of Agricultural Sciences Uppsala, Uppsala, Sweden, 2021. [Google Scholar]
- Shaigani, P.; Awad, D.; Redai, V.; Fuchs, M.; Haack, M.; Mehlmer, N.; Brueck, T. Oleaginous yeasts- substrate preference and lipid productivity: A view on the performance of microbial lipid producers. Microb. Cell Fact. 2021, 20, 220. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, B.; Teixeira, J.C.; Dragone, G.; Teixeira, J.A. Oleaginous yeasts for sustainable lipid production—From biodiesel to surf boards, a wide range of “green” applications. Appl. Microbiol. Biotechnol. 2019, 103, 3651–3667. [Google Scholar] [CrossRef] [PubMed]
- Banwell, M.G.; Pollard, B.; Liu, X.; Connal, L.A. Exploiting Nature’s Most Abundant Polymers: Developing New Pathways for the Conversion of Cellulose, Hemicellulose, Lignin and Chitin into Platform Molecules (and Beyond). Chem.-Asian J. 2021, 16, 604–620. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Monnappa, A.K.; Mitchell, R.J. Biological activities of lignin hydrolysate-related compounds. BMB Rep. 2012, 45, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Yaguchi, A.; Robinson, A.; Mihealsick, E.; Blenner, M. Metabolism of aromatics by Trichosporon oleaginosus while remaining oleaginous. Microb. Cell Fact. 2017, 16, 206. [Google Scholar] [CrossRef]
- Masri, M.; Garbe, D.; Mehlmer, N.; Brück, T. A sustainable, high-performance process for the economic production of waste-free microbial oils that can replace plant-based equivalents. Energy Environ. Sci. 2019, 12, 2717–2732. [Google Scholar] [CrossRef]
- Yaguchi, A.; Franaszek, N.; O’Neill, K.; Lee, S.; Sitepu, I.; Boundy-Mills, K.; Blenner, M. Identification of oleaginous yeasts that metabolize aromatic compounds. J. Ind. Microbiol. Biotechnol. 2020, 47, 801–813. [Google Scholar] [CrossRef]
- Yu, X.; Zheng, Y.; Dorgan, K.M.; Chen, S. Oil production by oleaginous yeasts using the hydrolysate from pretreatment of wheat straw with dilute sulfuric acid. Bioresour. Technol. 2011, 102, 6134–6140. [Google Scholar] [CrossRef]
- Gong, Z.; Shen, H.; Zhou, W.; Wang, Y.; Yang, X.; Zhao, Z.K. Efficient conversion of acetate into lipids by the oleaginous yeast Cryptococcus curvatus. Biotechnol. Biofuels 2015, 8, 189. [Google Scholar] [CrossRef]
- Gong, Z.; Zhou, W.; Shen, H.; Yang, Z.; Wang, G.; Zuo, Z.; Hou, Y.; Zhao, Z.K. Co-fermentation of acetate and sugars facilitating microbial lipid production on acetate-rich biomass hydrolysates. Bioresour. Technol. 2016, 207, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Saxena, G.; Bharagava, R.N. Bioremediation of Industrial Waste for Environmental Safety; Springer: Singapore, 2020; Volume I, ISBN 9789811318900. [Google Scholar]
- Tiseo, I. Annual production of plastics worldwide from 1950 to 2020 (in million metric tons). Statista 2022, 2020, 22–24. [Google Scholar]
- European Commission. New market niches for the Pulp and Paper Industry waste based on circular economy approaches. In H2020-Eu; European Commission: Brussels, Belgium, 2017; pp. 1–4. [Google Scholar]
- Gottumukkala, L.D.; Haigh, K.; Collard, F.X.; van Rensburg, E.; Görgens, J. Opportunities and prospects of biorefinery-based valorisation of pulp and paper sludge. Bioresour. Technol. 2016, 215, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Liu, Z.; Xie, F.; Bilal, M.; Peng, F. Lignocellulosic biomass to biobutanol: Toxic effects and response mechanism of the combined stress of lignin-derived phenolic acids and phenolic aldehydes to Clostridium acetobutylicum. Ind. Crops Prod. 2021, 170, 113722. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Koutinas, A.A.; Vlysidis, A.; Pleissner, D.; Kopsahelis, N.; Garcia, I.L.; Kookos, I.K.; Papanikolaou, S.; Kwan, T.H.; Lin, C.S.K. Valorization of industrial waste and by-product streams via fermentation for the production of chemicals and biopolymers. Chem. Soc. Rev. 2014, 43, 2587–2627. [Google Scholar] [CrossRef] [PubMed]
- Sixta, H. Lenzinger AG Zellstoffherstellung und Recycling von Roh- und Hilfsstoffen nach dem Lenzinger Mg- Bisulfitverfahren. Lenzing. Ber. 1986, 61, 5–11. [Google Scholar]
- Singh, A.K.; Bilal, M.; Iqbal, H.M.N.; Meyer, A.S.; Raj, A. Bioremediation of lignin derivatives and phenolics in wastewater with lignin modifying enzymes: Status, opportunities and challenges. Sci. Total Environ. 2021, 777, 145988. [Google Scholar] [CrossRef]
- Koul, B.; Yakoob, M.; Shah, M.P. Agricultural waste management strategies for environmental sustainability. Environ. Res. 2021, 206, 112285. [Google Scholar] [CrossRef]
- Nitsos, C.K.; Matis, K.A.; Triantafyllidis, K.S. Optimization of hydrothermal pretreatment of lignocellulosic biomass in the bioethanol production process. ChemSusChem 2013, 6, 110–122. [Google Scholar] [CrossRef]
- Almeida, J.R.M.; Wiman, M.; Heer, D.; Brink, D.P.; Sauer, U.; Hahn-Hägerdal, B.; Lidén, G.; Gorwa-Grauslund, M.F. Physiological and Molecular Characterization of Yeast Cultures Pre-Adapted for Fermentation of Lignocellulosic Hydrolysate. Fermentation 2023, 9, 72. [Google Scholar] [CrossRef]
- Duwe, A.; Tippkötter, N.; Ulber, R. Lignocellulose-biorefinery: Ethanol-focused. Adv. Biochem. Eng. Biotechnol. 2017, 166, 177–215. [Google Scholar]
- Díaz, T.; Fillet, S.; Campoy, S.; Vázquez, R.; Viña, J.; Murillo, J.; Adrio, J.L. Combining evolutionary and metabolic engineering in Rhodosporidium toruloides for lipid production with non-detoxified wheat straw hydrolysates. Appl. Microbiol. Biotechnol. 2018, 102, 3287–3300. [Google Scholar] [CrossRef] [PubMed]
- Nigam, J.N. Ethanol production from hardwood spent sulfite liquor using an adapted strain of Pichia stipitis. J. Ind. Microbiol. Biotechnol. 2001, 26, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Tjørve, K.M.C.; Tjørve, E. The use of Gompertz models in growth analyses, and new Gompertz-model approach: An addition to the Unified-Richards family. PLoS ONE 2017, 12, e0178691. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Intelligen Inc. SuperPro® Designer V10; Intelligen, Inc.: Medford, MA, USA, 2019. [Google Scholar]
- Walas, S.M. Chemical Process Equipment: Selection and Design; Butterworth-Heinemann Reed Publ. Inc.: Oxford, UK, 1990; pp. 1–755. [Google Scholar]
- Humbird, D.; Davis, R.; Tao, L.; Kinchin, C.; Hsu, D.; Aden, A. Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol; National Renewable Energy Lab.: Golden, CO, USA, 2011; pp. 153–170.
- Eurostat Industriestrompreise in Deutschland in den Jahren 2001 bis 2021. Statista. 2022. Available online: https://de.statista.com/statistik/daten/studie/155964/umfrage/entwicklung-der-industriestrompreise-in-deutschland-seit-1995/ (accessed on 18 October 2022).
- Ageitos, J.M.; Vallejo, J.A.; Veiga-Crespo, P.; Villa, T.G. Oily yeasts as oleaginous cell factories. Appl. Microbiol. Biotechnol. 2011, 90, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Bann, W. Acetic Acid/Vinyl Acetate—The Waiting Is The Hardest Part; ResourceWise: Charlotte, NC, USA, 2021. [Google Scholar]
- Alibaba.com. Acetic Acid. 2022. Available online: https://www.alibaba.com/trade/search?fsb=y&IndexArea=product_en&CatId=&tab=all&SearchText=acetic+acid (accessed on 18 October 2022).
- Broda, M.; Yelle, D.J. Bioethanol Production from Lignocellulosic Biomass—Challenges and Solutions. Molecules 2022, 27, 8717. [Google Scholar] [CrossRef]
- Sharma, S.; Tsai, M.L.; Sharma, V.; Sun, P.P.; Nargotra, P.; Bajaj, B.K.; Chen, C.W.; Dong, C. Di Environment Friendly Pretreatment Approaches for the Bioconversion of Lignocellulosic Biomass into Biofuels and Value-Added Products. Environments 2023, 10, 6. [Google Scholar] [CrossRef]
- Chopra, J.; Sen, R. Process optimization involving critical evaluation of oxygen transfer, oxygen uptake and nitrogen limitation for enhanced biomass and lipid production by oleaginous yeast for biofuel application. Bioprocess Biosyst. Eng. 2018, 41, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H. Selective removal of acetic acid from hardwood-spent sulfite liquor using a mutant yeast. Enzym. Microb. Technol. 1996, 19, 94–98. [Google Scholar] [CrossRef]
- Singh, S.; Pandey, D.; Saravanabhupathy, S.; Daverey, A.; Dutta, K.; Arunachalam, K. Liquid wastes as a renewable feedstock for yeast biodiesel production: Opportunities and challenges. Environ. Res. 2022, 207, 112100. [Google Scholar] [CrossRef] [PubMed]
- Van Gerpen, J. Cetane Number Testing of Biodiesel. In Third Liquid Fuel Conference: Liquid Fuel and Industrial Products from Renewable Resources; American Society of Agricultural Engineers: St. Joseph, MI, USA, 1996; pp. 197–206. [Google Scholar]
- Zahan, K.A.; Kano, M. Biodiesel production from palm oil, its by-products, and mill effluent: A review. Energies 2018, 11, 2132. [Google Scholar] [CrossRef]
- Tamilselvan, P.; Sassykova, L.; Bhaskar, K.; Subramanian, S.; Gomathi, K.; Prabhahar, M.; Prakash, S. Effect of Saturated Fatty Acid Composition of Biodiesel on Oxides of Nitrogen and Smoke Emissions. J. Chem. Technol. Metall. 2023, 58, 167–177. [Google Scholar]
- Papanikolaou, S.; Aggelis, G. Lipids of oleaginous yeasts. Part I: Biochemistry of single cell oil production. Eur. J. Lipid. Sci. Technol. 2011, 113, 1031–1051. [Google Scholar] [CrossRef]
- Alibaba_Palm-Oil. 2022. Available online: https://www.alibaba.com/product-detail/Best-Quality-cheap-organic-refined-Crude_60360488487.html?spm=a2700.galleryofferlist.normal_offer.d_title.56f1cfd5ZqfiRs&s=p (accessed on 18 October 2022).
- Brander, M.; Tipper, R.; Hutchison, C.; Davis, G. Consequential and attributional approaches to LCA: A Guide to policy makers with specific reference to greenhouse gas LCA of biofuels. Econ. Press 2008, 44, 1–14. [Google Scholar]
- Alibaba2_Canola-Oil. 2022. Available online: https://www.alibaba.com/trade/search?fsb=y&IndexArea=product_en&CatId=&SearchText=canola+oil&selectedTab=product_en (accessed on 20 October 2022).
- Ullmann, J.; Grimm, D. Algae and their potential for a future bioeconomy, landless food production, and the socio-economic impact of an algae industry. Org. Agric. 2021, 11, 261–267. [Google Scholar] [CrossRef]
- Acetic Acid. Chemical Economics Handbook; IHS Markit, S&P Global: London, UK, 2021; Available online: https://ihsmarkit.com/products/acetic-acid-chemical-economics-handbook.html (accessed on 5 April 2022).
- Helmlinger, E. Celanese Corporation Berichtet Ergebnisse für das Erste Quartal 2021. 2021. Available online: https://www.celanese.de/News-And-Media/2021/April/ergebnisse-fur-das-erste-quartal-2021 (accessed on 17 February 2023).
- Doelle, K.; Bajrami, B. Sodium Hydroxide and Calcium Hydroxide Hybrid Oxygen Bleaching with System. IOP Conf. Ser. Mater. Sci. Eng. 2018, 301, 12136. [Google Scholar] [CrossRef]
- Patil, R.; Genco, J.; Pendse, H.; Van Heiningen, A. Process for producing acetic acid in hardwood kraft pulp mills. Tappi J. 2017, 16, 287–300. [Google Scholar] [CrossRef]
- Kobetičová, K.; Nábělková, J. Effect of wood hemicellulose composition on binding interactions with caffeine. Buildings 2021, 11, 515. [Google Scholar] [CrossRef]
- Sivasamy, A.; Cheah, K.Y.; Fornasiero, P.; Kemausuor, F.; Zinoviev, S.; Miertus, S. Catalytic applications in the production of biodiesel from vegetable oils. ChemSusChem 2009, 2, 278–300. [Google Scholar] [CrossRef] [PubMed]
- Nurchi, C.; Buonvino, S.; Arciero, I.; Melino, S. Sustainable Vegetable Oil-Based Biomaterials: Synthesis and Biomedical Applications. Int. J. Mol. Sci. 2023, 24, 2153. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rerop, Z.S.; Stellner, N.I.; Graban, P.; Haack, M.; Mehlmer, N.; Masri, M.; Brück, T.B. Bioconversion of a Lignocellulosic Hydrolysate to Single Cell Oil for Biofuel Production in a Cost-Efficient Fermentation Process. Fermentation 2023, 9, 189. https://doi.org/10.3390/fermentation9020189
Rerop ZS, Stellner NI, Graban P, Haack M, Mehlmer N, Masri M, Brück TB. Bioconversion of a Lignocellulosic Hydrolysate to Single Cell Oil for Biofuel Production in a Cost-Efficient Fermentation Process. Fermentation. 2023; 9(2):189. https://doi.org/10.3390/fermentation9020189
Chicago/Turabian StyleRerop, Zora S., Nikolaus I. Stellner, Petra Graban, Martina Haack, Norbert Mehlmer, Mahmoud Masri, and Thomas B. Brück. 2023. "Bioconversion of a Lignocellulosic Hydrolysate to Single Cell Oil for Biofuel Production in a Cost-Efficient Fermentation Process" Fermentation 9, no. 2: 189. https://doi.org/10.3390/fermentation9020189
APA StyleRerop, Z. S., Stellner, N. I., Graban, P., Haack, M., Mehlmer, N., Masri, M., & Brück, T. B. (2023). Bioconversion of a Lignocellulosic Hydrolysate to Single Cell Oil for Biofuel Production in a Cost-Efficient Fermentation Process. Fermentation, 9(2), 189. https://doi.org/10.3390/fermentation9020189





