Optimal Enzymatic Hydrolysis of Sweet Lupine Protein towards Food Ingredients
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design via Taguchi Methodology
2.2. Enzymatic Hydrolysis of SLPC
2.3. The Degree of Hydrolysis
2.4. Sensory Evaluation—Scaling Method
2.5. Techno-Functional Properties
2.5.1. Water Binding Capacity (WBC)
2.5.2. The Emulsification Activity (AE)
2.5.3. The Stability of the Emulsion (SE)
2.6. Identification of the Molecular Mass Distribution
2.7. Statistical Analysis
3. Results and Discussions
3.1. The Degree of Hydrolysis
3.2. Sensory Analysis
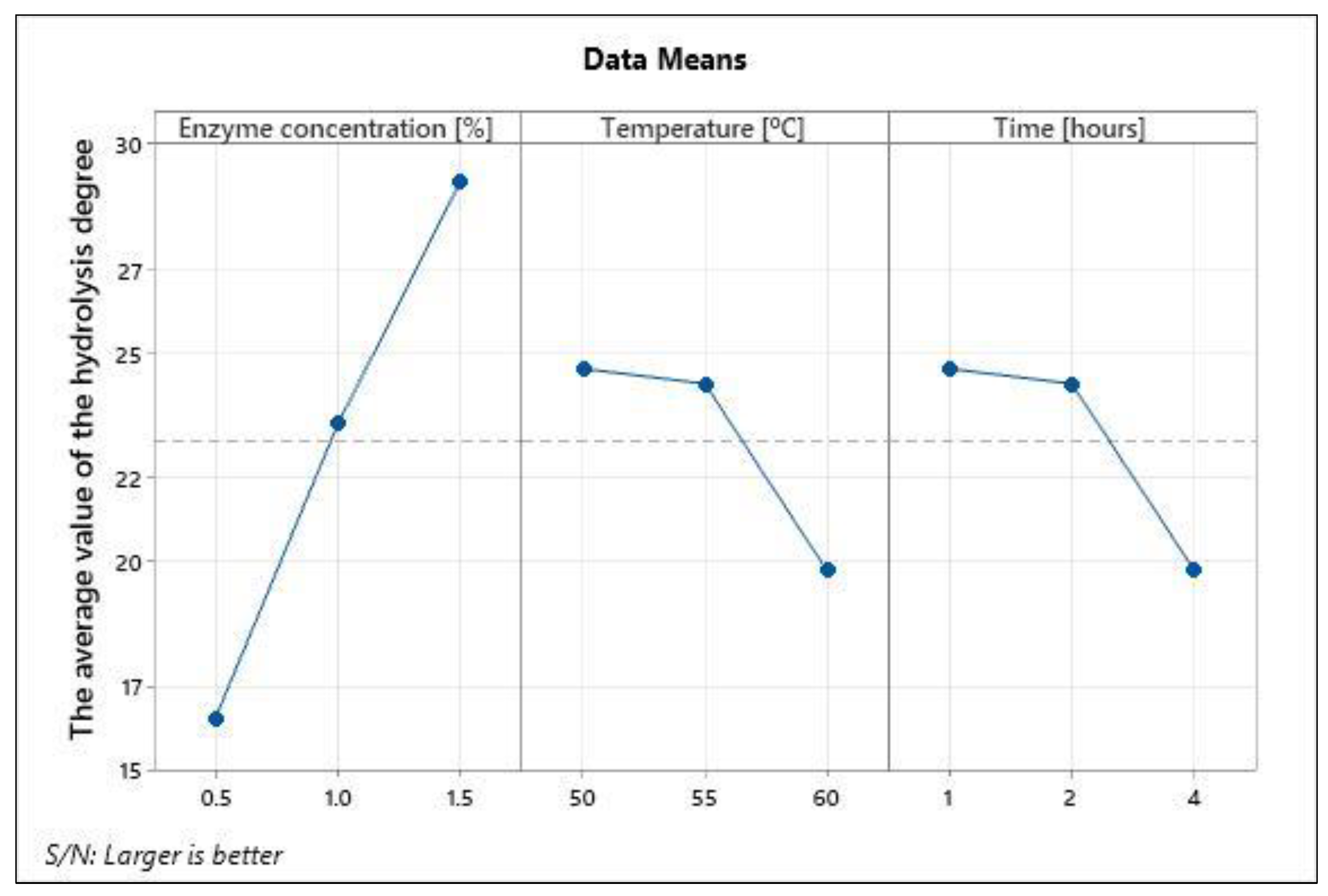
3.3. Optimization of Hydrolysis Parameters by Taguchi Methodology
3.3.1. Range Analysis
3.3.2. S/N Ratio Analysis
3.3.3. Analysis of Variance (ANOVA)
3.4. Techno-Functional Properties
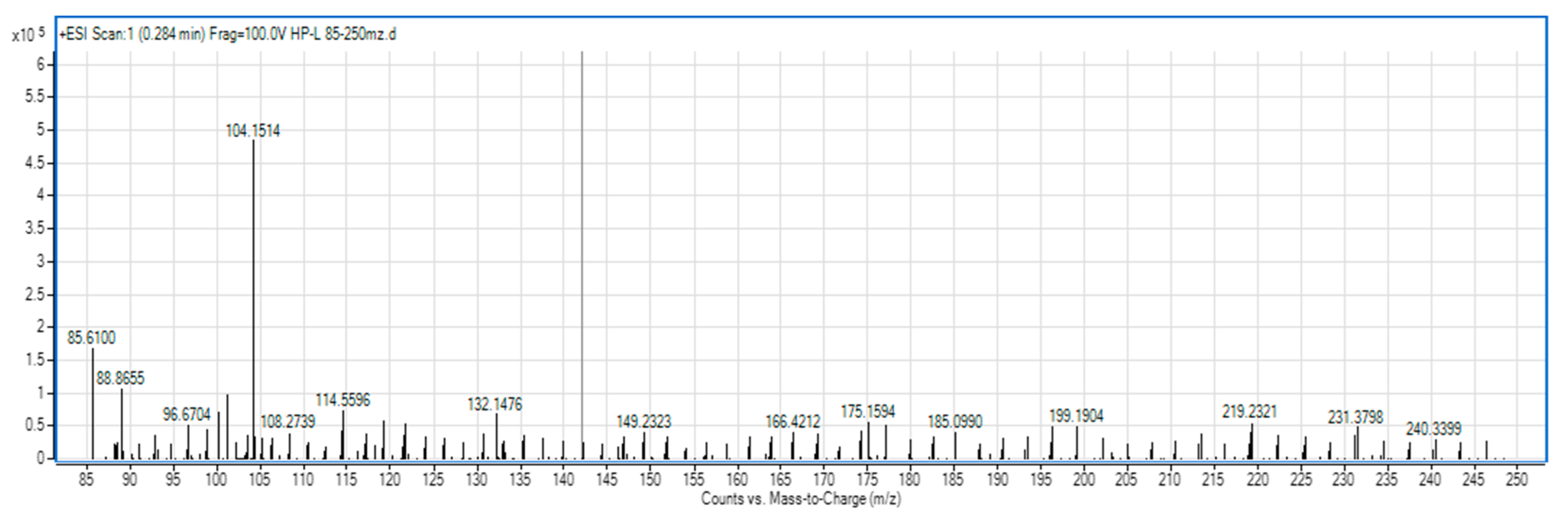
3.5. Determination of the Molecular Mass Distribution
4. Conclusions
5. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ismail, B.P.; Senaratne-Lenagala, L.; Stube, A.; Brackenridge, A. Protein demand: Review of plant and animal proteins used in alternative protein product development and production. Anim. Front. 2020, 10, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Ciocirlan, V. Flora ilustrată a României: Pteridophyta et Spermatophyta; Ceres: Bucharest, Romania, 2000. [Google Scholar]
- Villarino, C.B.J.; Jayasena, V.; Coorey, R.; Chakrabarti-Bell, S.; Johnson, S.K. Nutritional, health, and technological functionality of lupin flour addition to bread and other baked products: Benefits and challenges. Crit. Rev. Food Sci. 2016, 56, 835–857. [Google Scholar] [CrossRef] [PubMed]
- Arnoldi, A.; Boschin, G.; Zanoni, C.; Lammi, C. The health benefits of sweet lupin seed flours and isolated proteins. J. Funct. Foods 2015, 18, 550–563. [Google Scholar] [CrossRef]
- Cortés-Avendaño, P.; Tarvainen, M.; Suomela, J.P.; Glorio-Paulet, P.; Yang, B.; Repo-Carrasco-Valencia, R. Profile and content of residual alkaloids in ten ecotypes of Lupinus mutabilis Sweet after the aqueous debittering process. Plant Foods Hum. Nutr. 2020, 75, 184–191. [Google Scholar] [CrossRef]
- Duranti, M.; Consonni, A.; Magni, C. The major proteins of lupin seed: Characterisation and molecular properties for use as functional and nutraceutical ingredients. Trends Food Sci. Technol. 2008, 19, 624–633. [Google Scholar] [CrossRef]
- Piornos, J.A.; Burgos-Díaz, C.; Ogura, T. Functional and physicochemical properties of a protein isolate from AluProt-CGNA: A novel protein-rich lupin variety (Lupinus luteus). Food Res. Int. 2015, 76, 719–724. [Google Scholar] [CrossRef]
- Boukid, F.; Pasqualone, A. Lupine (Lupinus spp.) proteins: Characteristics, safety and food applications. Eur. Food Res. Technol. 2022, 248, 345–356. [Google Scholar] [CrossRef]
- Cruz-Chamorro, I.; Álvarez-Sánchez, N.; del Carmen Millán-Linares, M.; del Mar Yust, M.; Pedroche, J.; Millán, F.; Carrillo-Vico, A. Lupine protein hydrolysates decrease the inflammatory response and improve the oxidative status in human peripheral lymphocytes. Food Res. Int. 2019, 126, 108585. [Google Scholar] [CrossRef]
- Boschin, G.; Scigliuolo, G.M.; Resta, D.; Arnoldi, A. ACE-inhibitory activity of enzymatic protein hydrolysates from lupin and other legumes. Food Chem. 2014, 145, 34–40. [Google Scholar] [CrossRef]
- Burgos-Díaz, C.; Piornos, J.A.; Wandersleben, T.; Ogura, T.; Hernández, X.; Rubilar, M. Emulsifying and foaming properties of different protein fractions obtained from a novel lupin variety AluProt-CGNA® (Lupinus luteus). J. Food Sci. 2016, 81, C1699–C1706. [Google Scholar] [CrossRef]
- Schlegel, K.; Sontheimer, K.; Hickisch, A.; Wani, A.A.; Eisner, P.; Schweiggert-Weisz, U. Enzymatic hydrolysis of lupin protein isolates—Changes in the molecular weight distribution, technofunctional characteristics, and sensory attributes. Food Sci. Nutr. 2019, 7, 2747–2759. [Google Scholar] [CrossRef] [PubMed]
- Vogelsang-O’Dwyer, M.; Bez, J.; Petersen, I.L.; Joehnke, M.S.; Detzel, A.; Busch, M.; Zannini, E. Techno-functional, nutritional and environmental performance of protein isolates from blue lupin and white lupin. Foods 2020, 9, 230. [Google Scholar] [CrossRef] [PubMed]
- Karami, Z.; Akbari-Adergani, B. Bioactive food derived peptides: A review on correlation between structure of bioactive peptides and their functional properties. J. Food Technol. 2019, 56, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Kuesten, C.; Hu, C. Functional foods and protein supplementation. In Handbook of Eating and Drinking; Meiselman, H.L., Ed.; Springer: Cham, Switzerland, 2020; pp. 941–964. [Google Scholar] [CrossRef]
- Martínez, M.I.L.; Miguel, M.; Rimón, M.G. Protein and sport: Alternative sources and strategies for bioactive and sustainable sports nutrition. Front. Nutr. 2022, 9, 926043. [Google Scholar] [CrossRef]
- Boschin, G.; Scigliuolo, G.M.; Resta, D.; Arnoldi, A. Optimization of the enzymatic hydrolysis of lupin (Lupinus) proteins for producing ACE-inhibitory peptides. J. Agric. Food Chem. 2014, 62, 1846–1851. [Google Scholar] [CrossRef]
- Zaky, A.A.; Simal-Gandara, J.; Eun, J.B.; Shim, J.H.; Abd El-Aty, A.M. Bioactivities, applications, safety, and health benefits of bioactive peptides from food and by-products: A review. Front. Nutr. 2021, 8, 815640. [Google Scholar] [CrossRef]
- Meinlschmidt, P.; Schweiggert-Weisz, U.; Brode, V.; Eisner, P. Enzyme assisted degradation of potential soy protein allergens with special emphasis on the technofunctionality and the avoidance of a bitter taste formation. LWT-Food Sci. Technol. 2016, 68, 707–716. [Google Scholar] [CrossRef]
- Rogers, L.D.; Overall, C.M. Proteolytic post-translational modification of proteins: Proteomic tools and methodology. Mol. Cell. Proteom. 2013, 12, 3532–3542. [Google Scholar] [CrossRef]
- Shu, G.; Zhang, B.; Zhang, Q.; Wan, H.; Li, H. Effect of temperature, pH, enzyme to substrate ratio, substrate concentration and time on the antioxidative activity of hydrolysates from goat milk casein by alcalase. Acta Univ. Cibiniensis. Ser. E Food Technol. 2016, 20, 29–38. [Google Scholar] [CrossRef]
- Shuai, X.; Gao, L.; Geng, Q.; Li, T.; He, X.; Chen, J.; Liu, C.; Dai, T. Effects of Moderate Enzymatic Hydrolysis on Structure and Functional Properties of Pea Protein. Foods 2022, 11, 2368. [Google Scholar] [CrossRef]
- Islam, M.; Huang, Y.; Islam, S.; Fan, B.; Tong, L.; Wang, F. Influence of the Degree of Hydrolysis on Functional Properties and Antioxidant Activity of Enzymatic Soybean Protein Hydrolysates. Molecules 2022, 27, 6110. [Google Scholar] [CrossRef] [PubMed]
- Yampakdee, S.; Benjakul, S.; Kristinsson, H.G.; Kishimura, H. Antioxidant and sensory properties of protein hydrolysate derived from Nile tilapia (Oreochromis niloticus) by one-and two-step hydrolysis. J. Food Sci. Technol. 2015, 52, 3336–3349. [Google Scholar] [CrossRef]
- Idowu, A.T.; Benjakul, S.; Sinthusamran, S.; Sookchoo, P.; Kishimura, H. Protein hydrolysate from salmon frames: Production, characteristics and antioxidative activity. J. Food Biochem. 2018, 43, e12734. [Google Scholar] [CrossRef] [PubMed]
- Peter, A.S.; Hymavathi, T.V. Recent developments with debittering of protein hydrolysates. Asian J. Food Agro-Ind. 2011, 4, 365–381. [Google Scholar]
- Arteaga, V.G.; Guardia, M.A.; Muranyi, I.; Eisner, P.; Schweiggert-Weisz, U. Effect of enzymatic hydrolysis on molecular weight distribution, techno-functional properties and sensory perception of pea protein isolates. Innov. Food Sci. Emerg. Technol. 2020, 65, 102449. [Google Scholar] [CrossRef]
- Puh, F.; Brezočnik, M.; Jurković, Z.; Cukor, G.; Perinic, M.; Sekulić, M. Multi-Response Optimization of Turning Parameters Using the Grey-Based Taguchi Method. In Proceedings of the 19th International Research/Expert Conference “Trends in the Development of Machinery and Associated Technology” TMT 2015, Barcelona, Spain, 22–23 July 2015. [Google Scholar]
- Fraley, S.; Oom, M.; Terrien, B.; Zalewski, J. Design of experiments via Taguchi methods: Orthogonal arrays. In Chemical Engineering Process Dynamics and Controls Open Textbook; University of Michigan: Ann Arbor, MI, USA, 2007. [Google Scholar]
- Kaewka, K.; Therakulkait, C.R.; Cadwallader, K. Extract of preparation conditions on composition and sensory aroma characteristics of acid hydrolyzed rice bran protein concentrate. J. Cereal. Sci. 2009, 50, 56–60. [Google Scholar] [CrossRef]
- Ogonda, L.A.; Muge, E.K.; Mbatia, B.; Mulaa, F.J. Optimization of alcalase hydrolysis conditions for production of dagaa (Rastrineobola argentea) hydrolysate with antioxidative properties. Ind. Chem. 2017, 3, 1–6. [Google Scholar] [CrossRef]
- Rutherfurd, S.M. Methodology for determining degree of hydrolysis of proteins in hydrolysates: A Review. J. AOAC Int. 2010, 93, 1515–1522. [Google Scholar] [CrossRef]
- Lawless, H.T.; Heymann, H. Sensory Evaluation of Food: Principles and Practices; Springer: New York, NY, USA, 2010. [Google Scholar]
- Lawless, H.T. Laboratory Exercises for Sensory Evaluation; Springer: New York, NY, USA, 2012. [Google Scholar]
- Lim, J. Hedonic scaling: A review of methods and theory. Food Qual. Prefer. 2011, 22, 733–747. [Google Scholar] [CrossRef]
- Wichchukit, S.; O’Mahony, M. The 9-point hedonic and unstructured line hedonic scales: An alternative analysis with more relevant effect sizes for preference. Food Qual. Prefer. 2022, 99, 104575. [Google Scholar] [CrossRef]
- Daroub, H.; Olabi, A.; Toufeili, I. Designing and testing of an Arabic version of the hedonic scale for use in acceptability tests. Food Qual. Prefer. 2010, 21, 33–43. [Google Scholar] [CrossRef]
- Schlegel, K.; Sontheimer, K.; Eisner, P.; Schweiggert-Weisz, U. Effect of enzyme-assisted hydrolysis on protein pattern, techno-functional, and sensory properties of lupin protein isolates using enzyme combinations. Food Sci. Nutr. 2020, 8, 3041–3051. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, K.; Lidzba, N.; Ueberham, E.; Eisner, P.; Schweiggert-Weisz, U. Fermentation of Lupin Protein Hydrolysates—Effects on Their Functional Properties, Sensory Profile and the Allergenic Potential of the Major Lupin Allergen Lup an 1. Foods 2021, 10, 281. [Google Scholar] [CrossRef] [PubMed]
- Spellman, D.; O’Cuinn, G.; FitzGerald, R.J. Bitterness in Bacillus proteinase hydrolysates of whey proteins. Food Chem. 2009, 114, 440–446. [Google Scholar] [CrossRef]
- Rodriguez-Ambriz, S.L.; Martinez-Ayala, A.L.; Millan, F.; Davila-Ortiz, G. Composition and functional properties of Lupinus campestris protein isolates. Plant Foods Hum. Nutr. 2005, 60, 99–107. [Google Scholar] [CrossRef]
- Fekria, A.M.; Isam, A.M.A.; Suha, O.A.; Elfadil, E.B. Nutritional and functional characterization of defatted seed cake flour of two Sudanese groundnuts (Arachis hypogaea) cultivars. Int. Food Res. J. 2012, 19, 629–637. [Google Scholar]
- Thakur, M.; Nanda, V. Exploring the physical, functional, thermal, and textural properties of bee pollen from different botanical origins of India. J. Food Process Eng. 2018, 43, e12935. [Google Scholar] [CrossRef]
- Seo, W.H.; Lee, H.G.; Baek, H.H. Evaluation of bitterness in enzymatic hydrolysates of soy protein isolate by taste dilution analysis. J. Food Sci. 2008, 73, S41–S46. [Google Scholar] [CrossRef]
- Chiang, K.T.; Chang, F.P.; Tsai, T.C. Optimum design parameters of Pin-Fin heat sink using the grey-fuzzy logic based on the orthogonal arrays. Int. J. Heat Mass Tran. 2006, 33, 744–752. [Google Scholar] [CrossRef]
- Tizazu, H.; Emire, S.A. Chemical composition, physiochemical and functional properties of lupin (Lupinus albus) seeds grown in Ethiopia. Afr. J. Food Agric. Nutr. Dev. 2010, 10, 3029–3046. [Google Scholar] [CrossRef]
- Yin, S.-W.; Tang, C.-T.; Cao, J.-S.; Hun, E.-K.; Wen, Q.-B.; Yang, X.-Q. Effects of limited enzymatic hydrolysis with trypsin on the functional properties of hemp (Cannabis sativa L.) protein isolate. Food Chem. 2008, 106, 1004–1013. [Google Scholar] [CrossRef]
- Prima-Hartley, V.G.B.; Gray, D.A.; Taylor, A.J. Relationship between the activity of soybean lipoxygenase 1 and the physicochemical characteristics of model food emulsion systems. J. Agric. Food Chem. 2000, 48, 3190–3197. [Google Scholar] [CrossRef]
- Aluko, R.E.; Mofolasayo, O.A.; Watts, B.M. Emulsifying and foaming properties of commercial yellow pea (Pisum sativa) seed flours. J. Agric. Food Chem. 2009, 57, 9793–9800. [Google Scholar] [CrossRef] [PubMed]
- Castilla, J.C.; Hernandez-Alvarez, A.J.; Jimenez-Martınez, C.; Gutierrez-Lopez, G.F.; Davila-Ortiz, G. Use of proteomics and peptidomics methods in food bioactive peptide science and engineering. Food Eng. Rev. 2012, 4, 224–243. [Google Scholar] [CrossRef]
- Lahrichi, S.L.; Affolter, M.; Zolezzi, I.S.; Panchaud, A. Food peptidomics: Large scale analysis of small bioactive peptides—A pilot study. J. Proteom. 2013, 88, 83–91. [Google Scholar] [CrossRef] [PubMed]


| Levels | Parameters | ||
|---|---|---|---|
| (A) Enzyme Concentration (%) | (B) Temperature (°C) | (C) Time (Hours) | |
| (1) | 0.5 | 50 | 1 |
| (2) | 1 | 55 | 2 |
| (3) | 1.5 | 60 | 4 |
| Run | A | B | C |
|---|---|---|---|
| R1 | 0.5 | 50 | 1 |
| R2 | 0.5 | 55 | 2 |
| R3 | 0.5 | 60 | 4 |
| R4 | 1 | 55 | 2 |
| R5 | 1 | 60 | 4 |
| R6 | 1 | 50 | 1 |
| R7 | 1.5 | 60 | 4 |
| R8 | 1.5 | 50 | 1 |
| R9 | 1.5 | 55 | 2 |
| Run | Nitrogen Content from the Supernatant (%) | DH (%) |
|---|---|---|
| R1 | 0.067 ± 0.008 | 6.03 ± 0.76 |
| R2 | 0.080 ± 0.010 | 7.20 ± 0.95 |
| R3 | 0.069 ± 0.003 | 6.27 ± 0.29 |
| R4 | 0.163 ± 0.008 | 14.74 ± 0.81 |
| R5 | 0.094 ± 0.008 | 8.52 ± 0.80 |
| R6 | 0.273 ± 0.005 | 24.61 ± 0.41 |
| R7 | 0.191 ± 0.004 | 17.23 ± 0.28 |
| R8 | 0.364 ± 0.005 | 32.80 ± 0.47 |
| R9 | 0.450 ± 0.010 | 40.54 ± 0.92 |
| Run | Parameters | DH (%) | IB | ||
|---|---|---|---|---|---|
| A | B | C | |||
| R1 | 0.5 | 50 | 1 | 6.03 ± 0.76 | 1.75 ± 0.106 |
| R2 | 0.5 | 55 | 2 | 7.20 ± 0.95 | 2.50 ± 0.040 |
| R3 | 0.5 | 60 | 4 | 6.27 ± 0.29 | 1.88 ± 0.049 |
| R4 | 1 | 55 | 2 | 14.74 ± 0.81 | 3.25 ± 0.070 |
| R5 | 1 | 60 | 4 | 8.52 ± 0.80 | 2.50 ± 0.100 |
| R6 | 1 | 50 | 1 | 24.61 ± 0.41 | 3.50 ± 0.070 |
| R7 | 1.5 | 60 | 4 | 17.23 ± 0.28 | 3.00 ± 0.049 |
| R8 | 1.5 | 50 | 1 | 32.80 ± 0.47 | 3.75 ± 0.049 |
| R9 | 1.5 | 55 | 2 | 40.54 ± 0.92 | 3.95 ± 0.028 |
| K (1)—DH | 6.50 | 21.14 | 12.66 | ||
| K (2)—DH | 15.95 | 20.82 | 16.17 | ||
| K (3)—DH | 30.19 | 10.67 | 23.80 | ||
| K (1)—IB | 2.05 | 3.00 | 2.67 | ||
| K (2)—IB | 3.09 | 3.23 | 2.92 | ||
| K (3)—IB | 3.56 | 2.45 | 3.10 | ||
| R—DH | 23.69 | 10.47 | 11.14 |
| Parameter | DF | Seq SS | Adj SS | Adj MS | F | p | IP (%) |
|---|---|---|---|---|---|---|---|
| The concentration of enzyme (%) | 2 | 247.836 | 247.836 | 123.918 | 102.95 | 0.010 | 77.06 |
| Temperature (°C) | 2 | 43.315 | 43.315 | 21.657 | 17.99 | 0.053 | 13.46 |
| Time (h) | 2 | 28.011 | 28.011 | 14.005 | 11.64 | 0.079 | 8.71 |
| Residual error | 2 | 2.407 | 2.407 | 1.204 | |||
| Total | 8 | 321.568 |
| Techno-Functional Parameters | SLPC | SLPC Hydrolysate |
|---|---|---|
| WBC (g/g) | 0.78 ± 0.021 | 1.45 ± 0.07 |
| AE (%) | 48.18 ± 0.14 | 50.91 ± 0.25 |
| SE (%) | 51.92 ± 0.25 | 92 ± 0.106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasarin, D.; Lavric, V.; Enascuta, C.E.; Ghizdareanu, A.-I.; Matei, C.B. Optimal Enzymatic Hydrolysis of Sweet Lupine Protein towards Food Ingredients. Fermentation 2023, 9, 203. https://doi.org/10.3390/fermentation9030203
Pasarin D, Lavric V, Enascuta CE, Ghizdareanu A-I, Matei CB. Optimal Enzymatic Hydrolysis of Sweet Lupine Protein towards Food Ingredients. Fermentation. 2023; 9(3):203. https://doi.org/10.3390/fermentation9030203
Chicago/Turabian StylePasarin, Diana, Vasile Lavric, Cristina Emanuela Enascuta, Andra-Ionela Ghizdareanu, and Catalin Bogdan Matei. 2023. "Optimal Enzymatic Hydrolysis of Sweet Lupine Protein towards Food Ingredients" Fermentation 9, no. 3: 203. https://doi.org/10.3390/fermentation9030203
APA StylePasarin, D., Lavric, V., Enascuta, C. E., Ghizdareanu, A.-I., & Matei, C. B. (2023). Optimal Enzymatic Hydrolysis of Sweet Lupine Protein towards Food Ingredients. Fermentation, 9(3), 203. https://doi.org/10.3390/fermentation9030203









