Valorization of Spent Brewer’s Yeast for the Production of High-Value Products, Materials, and Biofuels and Environmental Application
Abstract
:1. Introduction
2. Systematic Review Strategy
3. Brewer’s Yeast: Types and Characteristics
Chemical Composition of Spent Brewer’s Yeast
4. Valorization of Spent Brewer’s Yeast
4.1. Emerging Trends in the Use of Spent Brewer’s Yeast
4.1.1. β-Glucans
4.1.2. Proteins, Peptides, and Amino Acids
4.1.3. 5′-Nucleotides
4.1.4. Acids
4.1.5. Material Production
4.1.6. Biofuel Production and Environmental Application
5. Potential Processing Flow of the Main Components of Spent Brewer’s Yeast Cells
5.1. β-Glucan Production
5.2. Mannoprotein Production
5.3. Chitin Production
5.4. Glycogen Production
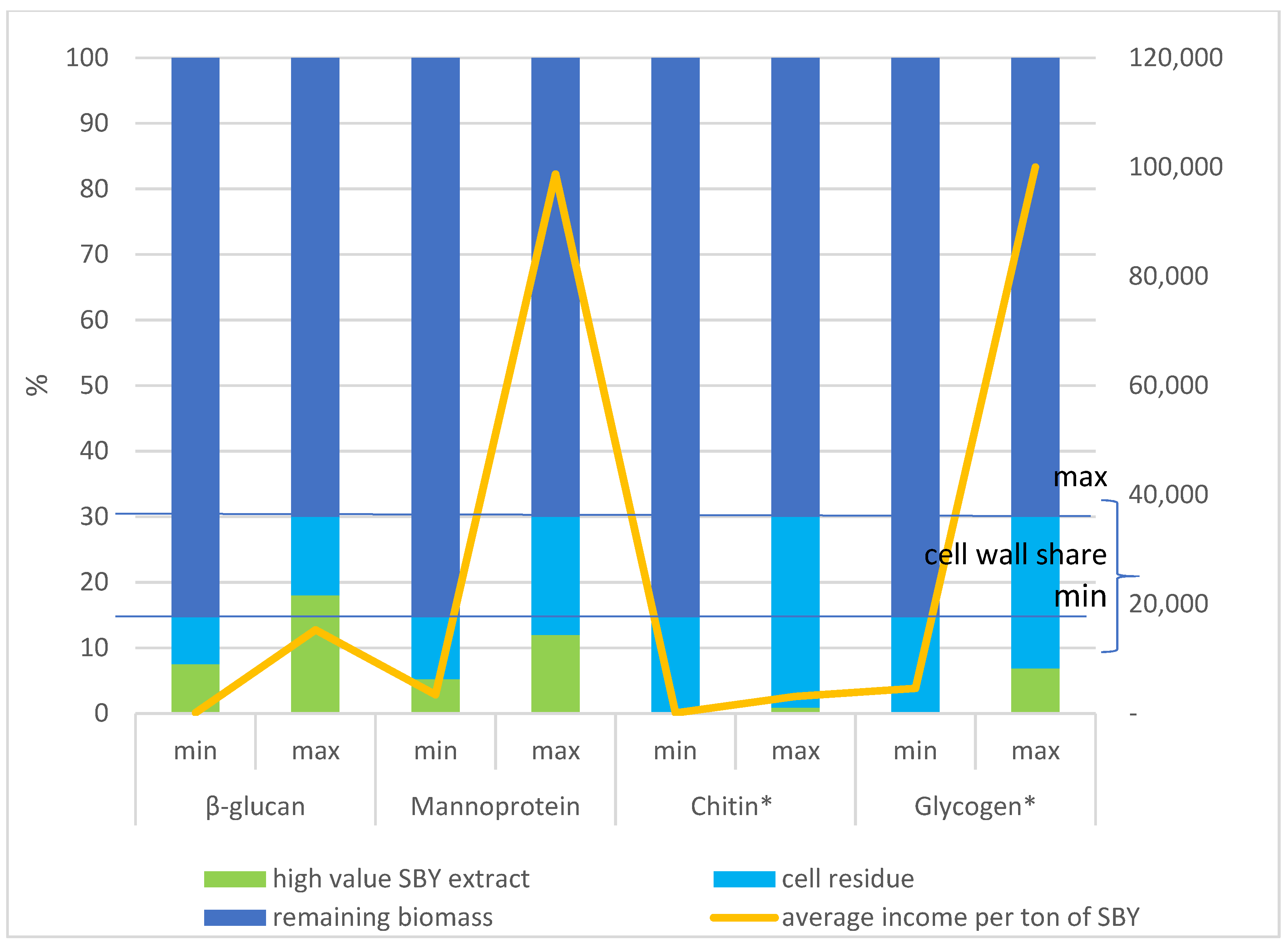
6. Economic Perspective of Cascade Use of Spent Brewer’s Yeast
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rachwał, K.; Waśko, A.; Gustaw, K.; Polak-Berecka, M. Utilization of brewery wastes in food Industry. PeerJ 2020, 8, e9427. [Google Scholar] [CrossRef] [PubMed]
- Statista. Beer Production Worldwide from 1998 to 2021. Available online: https://www.statista.com/statistics/270275/worldwide-beer-production/ (accessed on 13 February 2023).
- ReportLinker. Brewer’s Spent Yeast Market Research Report by Type, Application, Region—Global Forecast to 2027—Cumulative Impact of COVID-19. Available online: https://www.globenewswire.com/news-release/2022/06/08/2458532/0/en/Brewer-s-Spent-Yeast-Market-Research-Report-by-Type-Application-Region-Global-Forecast-to-2027-Cumulative-Impact-of-COVID-19.html (accessed on 16 January 2023).
- Avramia, I.; Amariei, S. Spent brewer’s yeast as a source of insoluble β-glucans. Int. J. Mol. Sci. 2021, 22, 825. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, A.; Arendt, E.K.; Zannini, E.; Sahin, A.W. Brewer’s spent yeast (bsy), an underutilized brewing by-product. Fermentation 2020, 6, 123. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Ferreira, C.; Pereira, J.O.; Pintado, M.E.; Carvalho, A.P. Spent brewer’s yeast (Saccharomyces cerevisiae) as a potential source of bioactive peptides: An overview. Int. J. Biol. Macromol. 2022, 208, 1116–1126. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Ferreira, C.; Pereira, J.O.; Pintado, E.M.; Carvalho, A.P. Valorisation of protein-rich extracts from spent brewer’s yeast (Saccharomyces cerevisiae): An overview. Biomass Conv. Bioref. 2022, 1–23. [Google Scholar] [CrossRef]
- Puligundla, P.; Mok, C.; Park, S. Advances in the valorization of spent brewer’s yeast. Innov. Food Sci. Emerg. Technol. 2020, 62, 102350. [Google Scholar] [CrossRef]
- Schlabitz, C.; Lehn, D.N.; de Souza, C.F.V. A review of Saccharomyces cerevisiae and the applications of its byproducts in dairy cattle feed: Trends in the use of residual brewer’s yeast. J. Clean. Prod. 2021, 332, 130059. [Google Scholar] [CrossRef]
- Rakowska, R.; Sadowska, A.; Dybkowska, E.; Swiderski, F. Spent yeast as natural source of functional food additives. Rocz. Państwowego Zakładu Hig. 2017, 68, 115–121. [Google Scholar]
- European Comission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions: A New Circular Economy Action Plan For a Cleaner and More Competitive Europe; Publication Office of the European Union: Luxembourg, 2020. [Google Scholar]
- Moher, D. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264. [Google Scholar] [CrossRef] [Green Version]
- Capece, A.; Romaniello, R.; Siesto, G.; Romano, P. Conventional and non-conventional yeasts in beer production. Fermentation 2018, 4, 38. [Google Scholar] [CrossRef] [Green Version]
- Viana, A.C.; Pimentel, T.C.; Borges do Vale, R.; Clementino, L.S.; Januario Ferreira, E.T.; Magnani, M.; dos Santos Lima, M. American pale Ale craft beer: Influence of brewer’s yeast strains on the chemical composition and antioxidant capacity. LWT 2021, 152, 112317. [Google Scholar] [CrossRef]
- Brányik, T.; Silva, D.P.; Baszczyňski, M.; Lehnert, R.; Almeida e Silva, J.B. A review of methods of low alcohol and alcohol-free beer production. J. Food Eng. 2012, 108, 493–506. [Google Scholar] [CrossRef]
- Kalayu, G. Serial re-pitching: Its effect on yeast physiology, fermentation performance, and product quality. Ann. Microbiol. 2019, 69, 787–796. [Google Scholar] [CrossRef]
- Marson, G.V.; de Castro, R.J.S.; Belleville, M.P.; Hubinger, M.D. Spent brewer’s yeast as a source of high added value molecules: A systematic review on its characteristics, processing and potential applications. World J. Microbiol. Biotechnol. 2020, 36, 95. [Google Scholar] [CrossRef]
- Lasanta, C.; Durán-Guerrero, E.; Díaz, A.B.; Castro, R. Influence of fermentation temperature and yeast type on the chemical and sensory profile of handcrafted beers. J. Sci. Food Agric. 2021, 101, 1174–1181. [Google Scholar] [CrossRef]
- WYEAST. Wyeast Classic Culture Collection. Yeast & Cultures. Wyeast Laboratories, Inc. Available online: https://wyeastlab.com/yeast-cultures/ (accessed on 28 October 2022).
- Bertolo, A.P.; Biz, A.P.; Kempka, A.P.; Rigo, E.; Cavalheiro, D. Yeast (Saccharomyces cerevisiae): Evaluation of cellular disruption processes, chemical composition, functional properties and digestibility. J. Food Sci. Technol. 2019, 56, 3697–3706. [Google Scholar] [CrossRef]
- Marson, G.V.; Saturno, R.P.; Comunian, T.A.; Consoli, L.; da Costa Machado, M.T.; Hubinger, M.D. Maillard conjugates from spent brewer’s yeast by-product as an innovative encapsulating material. Int. Food Res. J. 2020, 136, 109365. [Google Scholar] [CrossRef]
- Mathias, T.R.D.S.; Alexandre, V.M.F.; Cammarota, M.C.; de Mello, P.P.M.; Sérvulo, E.F.C. Characterization and determination of brewer’s solid wastes composition. J. Inst. Brew. 2015, 121, 400–404. [Google Scholar] [CrossRef] [Green Version]
- Jacob, F.F.; Striegel, L.; Rychlik, M.; Hutzler, M.; Methner, F.J. Yeast extract production using spent yeast from beer manufacture: Influence of industrially applicable disruption methods on selected substance groups with biotechnological relevance. Eur. Food Res. Technol. 2019, 245, 1169–1182. [Google Scholar] [CrossRef]
- Vieira, E.F.; Carvalho, J.; Pinto, E.; Cunha, S.; Almeida, A.A.; Ferreira, I.M.P.L.V.O. Nutritive value, antioxidant activity and phenolic compounds profile of brewer’s spent yeast extract. J. Food Compos. Anal. 2016, 52, 44–51. [Google Scholar] [CrossRef]
- Onofre, S.B.; Bertoldo, I.C.; Abatti, D.; Refosco, D. Chemical composition of the biomass of Saccharomyces cerevisiae- (Meyen ex EC Hansen, 1883) yeast obtained from the beer manufacturing process. Int. J. Adv. Eng. Res. Sci. 2017, 5, 264258. [Google Scholar] [CrossRef]
- Chollom, P.F.; Agbo, B.E.; Doma, D.U.; Okojokwu, J.O.; Yisa, A.G. Nutritional value of spent brewers’ yeast (Saccharomyces cerevisiae): A potential replacement for soya bean in poultry feed formulation. Researcher 2017, 9, 70–74. [Google Scholar]
- León-González, M.E.; Gómez-Mejía, E.; Rosales-Conrado, N.; Madrid-Albarrán, Y. Residual brewing yeast as a source of polyphenols: Extraction, identification and quantification by chromatographic and chemometric tools. Food Chem. 2018, 267, 246–254. [Google Scholar] [CrossRef]
- Marson, G.V.; da Costa Machado, M.T.; de Castro, R.J.S.; Hubinger, M.D. Sequential hydrolysis of spent brewer’s yeast improved its physico-chemical characteristics and antioxidant properties: A strategy to transform waste into added-value biomolecules. Process Biochem. 2019, 84, 91–102. [Google Scholar] [CrossRef]
- LIFE YEAST. LIFE16 ENV/ES/000158. Available online: https://webgate.ec.europa.eu/life/publicWebsite/index.cfm?fuseaction=search.dspPage&n_proj_id=6265 (accessed on 19 January 2023).
- Da Silva Guedes, J.; Pimentel, T.C.; Diniz-Silva, H.T.; Tayse da Cruz Almeida, E.; Tavares, J.F.; Leite de Souza, E.; Magnani, M. Protective effects of β-glucan extracted from spent brewer yeast during freeze-drying, storage and exposure to simulated gastrointestinal conditions of probiotic lactobacilli. LWT 2019, 116, 108496. [Google Scholar] [CrossRef]
- Chotigavin, N.; Sriphochanart, W.; Yaiyen, S.; Kudan, S. Increasing the production of β-glucan from Saccharomyces carlsbergensis RU01 by using tannic acid. Appl. Biochem. Biotechnol. 2021, 193, 2591–2601. [Google Scholar] [CrossRef]
- Martins, Z.E.; Pinho, O.; Ferreira, I.M.P.L.V.O. Impact of new ingredients obtained from brewer’s spent yeast on bread characteristics. Int. J. Food Sci. Technol. 2018, 55, 1966–1971. [Google Scholar] [CrossRef]
- Silva Araújo, V.B.; da Melo, A.N.F.; de Costa, A.G.; Castro-Gomez, R.H.; Madruga, M.S.; Souza, E.L.; de Magnani, M. Followed extraction of β-glucan and mannoprotein from spent brewer’s yeast (Saccharomyces uvarum) and application of the obtained mannoprotein as a stabilizer in mayonnaise. Innov. Food Sci. Emerg. Technol. 2014, 23, 164–170. [Google Scholar] [CrossRef]
- De Melo, A.N.F.; de Souza, E.L.; da Silva Araujo, V.B.; Magnani, M. Stability, nutritional and sensory characteristics of French salad dressing made with mannoprotein from spent brewer’s yeast. LWT 2015, 62, 771–774. [Google Scholar] [CrossRef]
- Bryant, R.W.; Cohen, S.D. Characterization of Hop Acids in Spent Brewer’s Yeast from Craft and Multinational Sources. J. Am. Soc. Brew. Chem. 2015, 73, 159–164. [Google Scholar] [CrossRef]
- Jiang, M.; Chen, K.; Liu, Z.; Wei, P.; Ying, H.; Chang, H. Succinic acid production by Actinobacillus succinogenes using spent brewer’s yeast hydrolysate as a nitrogen source. Appl. Biochem. Biotechnol. 2009, 160, 244–254. [Google Scholar] [CrossRef]
- Manurung, A.R.; Koentjoro, M.P.; Isdiantoni Ekawati, I.; Alami, N.H.; Prasetyo, E.N. Enzymatic conversion of Brewer’s Spent Yeast as raw material for glutamic acid production. AIP Conf. Proc. 2021, 2330, 070012. [Google Scholar] [CrossRef]
- Jacob, F.F.; Striegel, L.; Rychlik, M.; Hutzler, M.; Methner, F.J. Spent yeast from brewing processes: A biodiverse starting material for yeast extract production. Fermentation 2019, 5, 51. [Google Scholar] [CrossRef] [Green Version]
- Vieira, E.; Brandão, T.; Ferreira, I.M. Evaluation of brewer’s spent yeast to produce flavor enhancer nucleotides: Influence of serial repitching. J. Agric. Food Chem. 2013, 61, 8724–8729. [Google Scholar] [CrossRef]
- Boonyeun, P.; Shotipruk, A.; Prommuak, C.; Suphantharika, M.; Muangnapoh, C. Enhancement of amino acid production by two-step autolysis of spent brewer’s yeast. Chem. Eng. Commun. 2011, 198, 1594–1602. [Google Scholar] [CrossRef]
- Jacob, F.F.; Hutzler, M.; Methner, F.J. Comparison of various industrially applicable disruption methods to produce yeast extract using spent yeast from top-fermenting beer production: Influence on amino acid and protein content. Eur. Food Res. Technol. 2019, 245, 95–109. [Google Scholar] [CrossRef]
- Varelas, V.; Liouni, M.; Calokerinos, A.C.; Nerantzis, E.T. An evaluation study of different methods for the production of β-D-glucan from yeast biomass. Drug Test Anal. 2015, 46, 48–55. [Google Scholar] [CrossRef]
- Petravić-Tominac, V.; Zechner-Krpan, V.; Berković, K.; Galović, P.; Herceg, Z.; Srečec, S.; Špoljarić, I. Rheological properties, water-holding and oil-binding capacities of particulate β-glucans isolated from spent brewer’s yeast by three different procedures. Food Technol. Biotechno. 2011, 49, 56–64. Available online: https://hrcak.srce.hr/65578 (accessed on 28 October 2022).
- Worrasinchai, S.; Suphantharika, M.; Pinjai, S.; Jamnong, P. β-Glucan prepared from spent brewer’s yeast as a fat replacer in mayonnaise. Food Hydrocoll. 2006, 20, 68–78. [Google Scholar] [CrossRef]
- Raikos, V.; Grant, S.B.; Hayes, H.; Ranawana, V. Use of β-glucan from spent brewer’s yeast as a thickener in skimmed yogurt: Physicochemical, textural, and structural properties related to sensory perception. J. Dairy Sci. 2018, 101, 5821–5831. [Google Scholar] [CrossRef] [Green Version]
- Thammakiti, S.; Suphantharika, M.; Phaesuwan, T.; Verduyn, C. Preparation of spent brewer’s yeast beta-glucans for potential applications in the food industry. Int. J. Food Sci. 2004, 39, 21–29. [Google Scholar] [CrossRef]
- Ascencio, J.J.; Philippini, R.R.; Gomes, F.M.; Pereira, F.M.; da Silva, S.S.; Kumar, V.; Chandel, A.K. Comparative highly efficient production of β-glucan by Lasiodiplodia theobromae CCT 3966 and its multiscale characterization. Fermentation 2021, 7, 108. [Google Scholar] [CrossRef]
- Liepins, J.; Kovačova, E.; Shvirksts, K.; Grube, M.; Rapoport, A.; Kogan, G. Drying enhances immunoactivity of spent brewer’s yeast cell wall β-d-glucans. J. Biotechnol. 2015, 206, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, Q.; Wu, X.; Algharib, S.A.; Gong, F.; Hu, J.; Luo, W.; Zhou, M.; Pan, Y.; Yan, Y.; et al. Structure, preparation, modification, and bioactivities of β-glucan and mannan from yeast cell wall: A review. Int. J. Biol. Macromol. 2021, 73, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Amorim, M.; Marques, C.; Pereira, J.O.; Guardão, L.; Martins, M.J.; Osório, H.; Moura, D.; Calhau, C.; Pinheiro, H.; Pintado, M. Antihypertensive effect of spent brewer yeast peptides. Process Biochem. 2019, 76, 213–218. [Google Scholar] [CrossRef]
- Vélez-Erazo, E.M.; Saturno, R.P.; Marson, G.V.; Hubinger, M.D. Spent brewer’s yeast proteins and cell debris as innovative emulsifiers and carrier materials for edible oil microencapsulation. Int. Food Res. J. 2021, 140, 109853. [Google Scholar] [CrossRef]
- Gonzales, T.A.; Carvalho Silvello, M.A.; de Duarte, E.R.; Santos, L.O.; Alegre, R.M.; Goldbeck, R. Optimization of anaerobic fermentation of Actinobacillus succinogenes for increase the succinic acid production. Biocatal. Agric. Biotechnol. 2020, 27, 101718. [Google Scholar] [CrossRef]
- Chen, K.-Q.; Li, J.; Ma, J.-F.; Jiang, M.; Wei, P.; Liu, Z.-M.; Ying, H.-J. Succinic acid production by Actinobacillus succinogenes using hydrolysates of spent yeast cells and corn fiber. Bioresour. Technol. 2011, 102, 1704–1708. [Google Scholar] [CrossRef]
- Yilmaz, C.; Gökmen, V. Neuroactive compounds in foods: Occurrence, mechanism and potential health effects. Int. Food Res. J. 2019, 128, 108744. [Google Scholar] [CrossRef]
- Pejin, J.; Radosavljević, M.; Kocić-Tanackov, S.; Marković, R.; Djukić-Vuković, A.; Mojović, L. Use of spent brewer’s yeast in L-(+) lactic acid fermentation. J. Inst. Brew. 2019, 125, 357–363. [Google Scholar] [CrossRef]
- Dadkhodazade, E.; Khanniri, E.; Khorshidian, N.; Hosseini, S.M.; Mortazavian, A.M.; Moghaddas Kia, E. Yeast cells for encapsulation of bioactive compounds in food products: A review. Biotechnol. Prog. 2021, 37, e3138. [Google Scholar] [CrossRef]
- Grgić, J.; Šelo, G.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Role of the encapsulation in bioavailability of phenolic compounds. Antioxidants 2020, 9, 923. [Google Scholar] [CrossRef]
- Yantcheva, N.S.; Karashanova, D.B.; Georgieva, B.C.; Vasileva, I.N.; Stoyanova, A.S.; Denev, P.N.; Dinkova, R.H.; Ognyanov, M.H.; Slavov, A.M. Characterization and application of spent brewer’s yeast for silver nanoparticles synthesis. Bulg. Chem. Commun. 2019, 51, 173–177. [Google Scholar]
- Krutyakov, Y.A.; Kudrinskiy, A.A.; Olenin, A.Y.; Lisichkin, G.V. Synthesis and properties of silver nanoparticles: Advances and prospects. Russ. Chem. Rev. 2008, 77, 233–257. [Google Scholar] [CrossRef]
- Zupančič, G.D.; Panjičko, M.; Zelić, B. Biogas production from brewer’s yeast using an anaerobic sequencing batch reactor. Food Technol. Biotechn. 2017, 55, 187–196. [Google Scholar] [CrossRef]
- Kawa-Rygielska, J.; Pietrzak, W. Ethanol fermentation of very high gravity (VHG) maize mashes by Saccharomyces cerevisiae with spent brewer’s yeast supplementation. Biomass Bioenergy 2014, 60, 50–57. [Google Scholar] [CrossRef]
- Mosai, A.K. Simultaneous recovery of Pd(II), Ir(III) and Rh(III) from aqueous solutions by spent brewer’s yeast-functionalised zeolite using flow-through column mode. Miner. Eng. 2021, 163, 106770. [Google Scholar] [CrossRef]
- Pinto, M.; Coelho, E.; Nunes, A.; Brandão, T.; Coimbra, M.A. Valuation of brewers spent yeast polysaccharides: A structural characterization approach. Carbohydr. Polym. 2015, 116, 215–222. [Google Scholar] [CrossRef]
- Stewart, G.G. The structure and function of the yeast cell wall, plasma membrane and periplasm. In Brewing and distilling yeasts. The Yeast Handbook.; Springer: Cham, Switzerland, 2017; pp. 55–75. [Google Scholar] [CrossRef]
- Lesage, G.; Bussey, H. Cell wall assembly in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2006, 70, 317–343. [Google Scholar] [CrossRef] [Green Version]
- Wilson, W.A.; Boyer, M.P.; Davis, K.D.; Burke, M.; Roach, P.J. The subcellular localization of yeast glycogen synthase is dependent upon glycogen content. Can. J. Microbiol. 2010, 56, 408–420. [Google Scholar] [CrossRef] [Green Version]
- Bzducha-Wróbel, A.; Błażejak, S.; Kawarska, A.; Stasiak-Różańska, L.; Gientka, I.; Majewska, E. Evaluation of the efficiency of different disruption methods on yeast cell wall preparation for β-glucan isolation. Molecules 2014, 19, 20941–20961. [Google Scholar] [CrossRef] [PubMed]
- Koubaa, M.; Imatoukene, N.; Drévillon, L.; Vorobiev, E. Current insights in yeast cell disruption technologies for oil recovery: A review. Chem. Eng. Process. 2020, 150, 107868. [Google Scholar] [CrossRef]
- Liu, D.; Ding, L.; Sun, J.; Boussetta, N.; Vorobiev, E. Yeast cell disruption strategies for recovery of intracellular bio-active compounds—A review. Innov. Food Sci. Emerg. Technol. 2016, 36, 181–192. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 1–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eur-LEX. Provedbena Odluka Komisije (EU) 2017/2078 od 10. Studenoga 2017. o Odobravanju Proširenja Uporabe Beta-Glukana iz Kvasca kao novog Sastojka Hrane u Skladu s Uredbom (EZ) br. 258/97 Europskog Parlamenta i Vijeća, 77–80. Available online: https://eur-lex.europa.eu/eli/dec_impl/2017/2078/oj (accessed on 20 November 2021).
- Murugesan, R.; Orsat, V. Spray drying for the production of nutraceutical ingredients—A review. Food Bioprocess Technol. 2012, 5, 3–14. [Google Scholar] [CrossRef]
- Zechner-Krpan, V.; Petravić-Tominac, V.; Galović, P.; Galović, V.; Filipović-Grčić, J.; Srečec, S. Application of different drying methods on β-glucan isolated from spent brewer’s yeast using alkaline procedure. Agric. Conspec. Sci. 2010, 75, 45–50. [Google Scholar]
- Li, J.; Karboune, S. A comparative study for the isolation and characterization of mannoproteins from Saccharomyces cerevisiae yeast cell wall. Int. J. Biol. Macromol. 2018, 119, 654–661. [Google Scholar] [CrossRef]
- Faustino, M.; Durão, J.; Pereira, C.F.; Oliveira, A.S.; Pereira, J.O.; Pereira, A.M.; Ferreira, C.; Pintado, M.E.; Carvalho, A.P. Comparative analysis of mannans extraction processes from spent yeast Saccharomyces cerevisiae. Foods 2022, 11, 3753. [Google Scholar] [CrossRef]
- Faustino, M.; Durão, J.; Pereira, C.F.; Pintado, M.E.; Carvalho, A.P. Mannans and mannan oligosaccharides (MOS) from Saccharomyces cerevisiae–A sustainable source of functional ingredients. Carbohydr. Polym. 2021, 272, 118467. [Google Scholar] [CrossRef]
- Abo Elsoud, M.M.; El Kady, E.M. Current trends in fungal biosynthesis of chitin and chitosan. Bull Natl. Res. Cent. 2019, 43, 59. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, C.; Silva, S.; Van Voorst, F.; Aguiar, C.; Kielland-Brandt, M.C.; Brandt, A.; Lucas, C. Absence of Gup1p in Saccharomyces cerevisiae results in defective cell wall composition, assembly, stability and morphology. FEMS Yeast Res. 2006, 6, 1027–1038. [Google Scholar] [CrossRef] [Green Version]
- Kaczmarek, M.B.; Struszczyk-Swita, K.; Li, X.; Szczęsna-Antczak, M.; Daroch, M. Enzymatic Modifications of chitin, chitosan, and chitooligosaccharides. Front. Bioeng. Biotechnol. 2019, 7, 243. [Google Scholar] [CrossRef] [Green Version]
- Aklujkar, P.P.; Sankh, S.N.; Arvindekar, A.U. A Simplified Method for the Isolation and Estimation of Cell Wall Bound Glycogen in Saccharomyces cerevisiae. J. Inst. Brew. 2008, 114, 205–208. [Google Scholar] [CrossRef]
- Chen, Y.; Futcher, B. Assaying glycogen and trehalose in yeast. Bio-Protocl 2017, 7, e2371. [Google Scholar] [CrossRef]
- Alibaba. Available online: https://www.alibaba.com/product-detail/High-Protein-Inactive-Dry-Yeast-Animal_10000007203840.html (accessed on 14 January 2023).
- Made in China. Available online: https://cdaohebio.en.made-in-china.com/product/KSRmgoPUnFcb/China-Manufacturer-Supply-Best-Price-Animal-Feed-Yeast-Crude-Protein-40-55-.html (accessed on 14 January 2023).
- Made in China. Available online: https://feedadditive.en.made-in-china.com/product/cZqfxPdvXaAW/China-Affordable-Yeast-Powder-50-60-Animal-Feed-Protein.html (accessed on 14 January 2023).
- Made in China. Available online: https://tessinlife.en.made-in-china.com/product/cnVRepuTTEkr/China-Food-Additives-CAS-9012-72-0-Yeast-Beta-1-3-D-Glucan-Water-Insoluble-70-.html (accessed on 14 January 2023).
- Made in China. Available online: https://tessinlife.en.made-in-china.com/product/mQtUHWjAVEcF/China-Yeast-Extract-Powder-Food-Grade-Beta-Glucan-Powder-80-.html (accessed on 14 January 2023).
- Made in China. Available online: https://xahnbmic.en.made-in-china.com/product/JOCGTIAERmWu/China-Top-Quality-Yeast-Beta-Glucan-Beta-Glucan-Yeast.html (accessed on 14 January 2023).
- Made in China. Available online: https://www.made-in-china.com/productdirectory.do?word=Beta+Glucan+yeast+Price&file=&searchType=0&subaction=hunt&style=b&mode=and&code=0&comProvince=nolimit&order=0&isOpenCorrection=1&org=top (accessed on 14 January 2023).
- Resolvent Supply. Available online: https://www.resolventsupply.com/winemaking-supplies-price-list.htm (accessed on 14 January 2023).
- Carolina Wine Supply. Available online: https://carolinawinesupply.com/products/mannofeel (accessed on 14 January 2023).
- Alibaba. Available online: https://angelyeast.en.alibaba.com/product/60705977125-218419497/Angel_MP60_Yeast_Extract_Mannoprotein_for_Wine_Fermentation_stability.html (accessed on 14 January 2023).
- Chemical Book. Available online: https://www.chemicalbook.com/Price/Chitin.htm (accessed on 14 January 2023).
- Chemical Book. Available online: https://www.chemicalbook.com/Price/GLYCOGEN.htm (accessed on 14 January 2023).
- Đurđević, D.; Hulenić, I.; Kulišić, B. Degradation of lignocellulosic complex through production of struvite from digestate. Waste Biomass Valor. 2020, 11, 2559–2566. [Google Scholar] [CrossRef]
- Montgomery, L.; Bochmann, G. Pretreatment of Feedstock for Enhanced Biogas Production; IEA Bioenergy: Dublin, Ireland, 2014. [Google Scholar]
- Carrere, H.; Antonopoulou, G.; Afes, R.; Passos, F.; Battimelli, A.; Lyberatos, G.; Ferrer, I. Review of feedstock pretreatment strategies for improved anaerobic digestion: From lab-scale research to full-scale application. Bioresour. Technol. 2016, 199, 386–397. [Google Scholar] [CrossRef]
- Strobl, M.; Keymer, U. Biogasausbeute mobil, bayerische landesanstalt für landwirtschaft, München. Available online: http://www.lfl.bayern.de/appl/biogas/ausbeute/ (accessed on 12 December 2022).

| Type of Beer | Species | Attenuation | Fermentation Temperature | Flocculation |
|---|---|---|---|---|
| American Ales (Pale Ale, IPA, NEIPA, Porter, and Stout) | Saccharomyces cerevisiae | 73–77% | 16–22 °C | Low to Medium |
| United Kingdom Ales (Bitter, Pale Ale, IPA, Porter, and Stout) | Saccharomyces cerevisiae | 67–71% | 18–22 °C | High |
| German Kölsch | Saccharomyces pastorianus | 73–77% | 13–21 °C | Low |
| German Wheat | Saccharomyces cerevisiae | 70–76% | 18–24 °C | Low |
| German Alt | Saccharomyces cerevisiae | 73–77% | 13–20 °C | Low |
| Bohemian Lager | Saccharomyces pastorianus | 70–74% | 10–14 °C | Medium to High |
| German Lager | Saccharomyces pastorianus | 73–77% | 7–20 °C | Low to Medium |
| Belgian Abbey Ale | Saccharomyces cerevisiae | 74–78% | 18–26 °C | Medium |
| Belgian Sour (Lambic, Gueze, and Flanders Red) | Brettanomyces bruxellensis | 78–84% | 16–24 °C | Medium |
| Belgian Wit | Saccharomyces cerevisiae | 72–76% | 17–24 °C | Medium |
| Compound (%DW *) | Min–Max Values | References |
|---|---|---|
| Crude protein | 40.00–74.30 | [4,20,21,22,23,24,25,26,28] |
| Total sugars | 37.80–43.50 | [21,28] |
| Ash | 1.74–14.53 | [20,21,22,23,24,25,26,28] |
| Total carbohydrates | 12.90–59.00 | [4,20,23,24,25] |
| Lipids | 0.67–4.64 | [4,20,23,24,25,26] |
| Fibers | 4.31–6.60 | [21,26] |
| RNA | 1.90–8.12 | [21,23,24,25] |
| Amino acids (g/100 g protein) | ||
| Lysine | 2.93–6.73 | [24,25,26] |
| Leucine | 3.51–8.75 | [24,25,26] |
| Isoleucine | 3.23–5.37 | [24,25,26] |
| Threonine | 2.60–6.09 | [24,25,26] |
| Tryptophan | 0.00–0.96 | [24,25,26] |
| Valine | 4.94–6.07 | [24,25,26] |
| Histidine | 2.78–11.9 | [24,25,26] |
| Methionine | 2.28–3.12 | [24,25,26] |
| Cysteine | 1.24–2.19 | [24,25,26] |
| Tyrosine | 2.15–4.12 | [24,25,26] |
| Glutamic acid | 8.56–15.00 | [24,25,26] |
| Aspartic acid | 5.98–11.98 | [24,25,26] |
| Serine | 4.60–5.75 | [24,25,26] |
| Proline | 2.65–5.11 | [24,25,26] |
| Alanine | 6.89–9.29 | [24,25,26] |
| Glycine | 3.69–5.23 | [24,25,26] |
| Arginine | 4.02–6.00 | [24,25,26] |
| Asparagin | 2.00 | [24] |
| Glutamine | 3.13 | [24] |
| Phenylalanine | 3.01–5.57 | [24,25,26] |
| Fatty acids (%biomass lipid fraction) | ||
| Caprylic | 0.29 | [25] |
| Capric | 6.26 | |
| Lauric | 1.26 | |
| Myristic | 0.78 | |
| Myristoleic | 0.39 | |
| Palmitic | 34.33 | |
| Palmitoleic | 2.99 | |
| Stearic | 9.56 | |
| Oleic | 11.02 | |
| Linoleic | 4.37 | |
| Linolenic | 0.63 | |
| Polyphenols (mg/gDW) | ||
| Gallic acid | 0.23–0.55 | [24,27] |
| p-coumaric acid | 0.03–0.34 | [24,27] |
| Rutin | 0.06–0.10 | [24,27] |
| Ferulic acid | 0.05–0.09 | [24,27] |
| Naringin | 0.66 | [24,27] |
| Quercetin | 0.01 | [24,27] |
| Kaempferol | 0.03 | [24,27] |
| Protocatechuic acid | 0.13 | [24] |
| Catechin | 0.59 | [24] |
| Cinnamic acid | 0.01 | [24] |
| Elements (mg/100 gDW) | ||
| Phosphorus | 17.31–3213.60 | [23,25] |
| Potassium | 14.21–9148.00 | [23,24,25] |
| Sodium | 9.13–1228.00 | [23,24,25] |
| Magnesium | 9.13–1228.00 | [23,24,25] |
| Aluminum | 3.02–695.50 | [23,25] |
| Calcium | 0.75–1.12 | [23,24,25] |
| Iron | 0.87–27.10 | [23,24,25] |
| Selenium | 0.03–25.12 | [24,25] |
| Manganese | 0.56–14.98 | [23,24,25] |
| Lead | 10.11 | [25] |
| Chromium | 0.02–9.05 | [24,25] |
| Nickel | 8.23 | [25] |
| Lithium | 6.13 | [25] |
| Zinc | 4.89–22.59 | [23,24,25] |
| Copper | 0.36–7.84 | [23,24,25] |
| Vanadium | 0.56 | [25] |
| Cadmium | 0.45 | [25] |
| Cobalt | 0.03–0.07 | [24,25] |
| Molybdenum | 0.003 | [24] |
| Boron | 0.64 | [23] |
| Barium | 0.35 | [23] |
| Strontium | 1.07 | [23] |
| Vitamins (mg/100gDW) | ||
| Nicotinic acid (B3) | 77.20–94.19 | [23,24] |
| Pyridoxine (B6) | 4.86–55.10 | [23,24] |
| Folic acid (B9) | 3.01–4.52 | [23,24] |
| Riboflavin (B2) | 0.33–2.16 | [23,24] |
| Cyanocobalamin (B12) | 0.18–0.26 | [23,24] |
| Thiamine (B1) | 6.88 | [23] |
| Pantothenic acid (B5) | 20.36 | [23] |
| Biotin (B7) | 113.92 | [23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeko-Pivač, A.; Habschied, K.; Kulisic, B.; Barkow, I.; Tišma, M. Valorization of Spent Brewer’s Yeast for the Production of High-Value Products, Materials, and Biofuels and Environmental Application. Fermentation 2023, 9, 208. https://doi.org/10.3390/fermentation9030208
Zeko-Pivač A, Habschied K, Kulisic B, Barkow I, Tišma M. Valorization of Spent Brewer’s Yeast for the Production of High-Value Products, Materials, and Biofuels and Environmental Application. Fermentation. 2023; 9(3):208. https://doi.org/10.3390/fermentation9030208
Chicago/Turabian StyleZeko-Pivač, Anđela, Kristina Habschied, Biljana Kulisic, Ingo Barkow, and Marina Tišma. 2023. "Valorization of Spent Brewer’s Yeast for the Production of High-Value Products, Materials, and Biofuels and Environmental Application" Fermentation 9, no. 3: 208. https://doi.org/10.3390/fermentation9030208
APA StyleZeko-Pivač, A., Habschied, K., Kulisic, B., Barkow, I., & Tišma, M. (2023). Valorization of Spent Brewer’s Yeast for the Production of High-Value Products, Materials, and Biofuels and Environmental Application. Fermentation, 9(3), 208. https://doi.org/10.3390/fermentation9030208











