Effect of Laccase Detoxification on Bioethanol Production from Liquid Fraction of Steam-Pretreated Olive Tree Pruning
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material and Steam Explosion Pretreatment
2.2. Enzymes
2.3. Microorganisms and Media
2.4. Enzymatic Hydrolysis
2.5. Laccase Detoxification
2.6. Fermentation
2.7. Analytical Methods
3. Results and Discussion
3.1. Steam Explosion
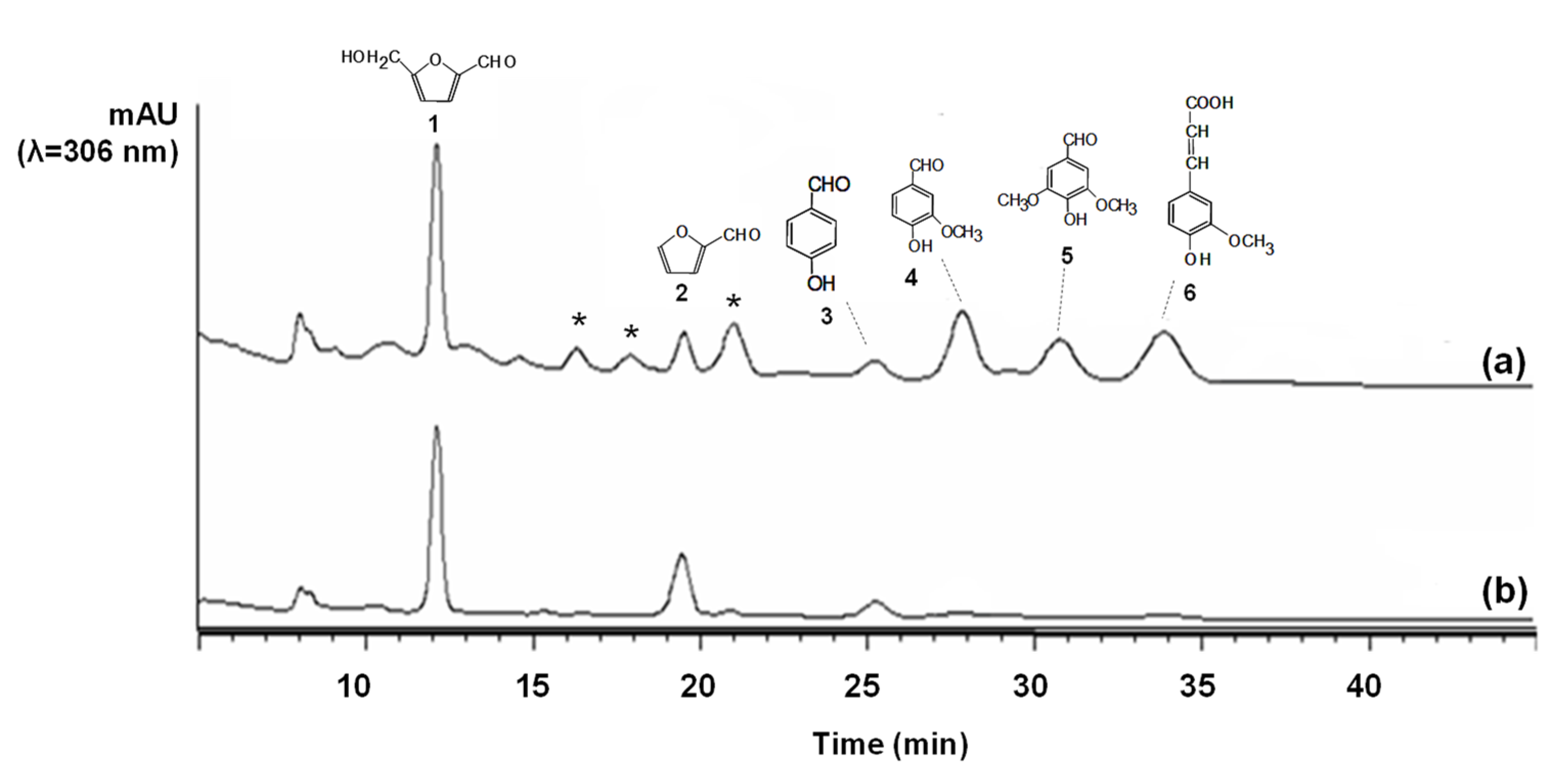
3.2. Laccase Detoxification
3.3. Fermentation
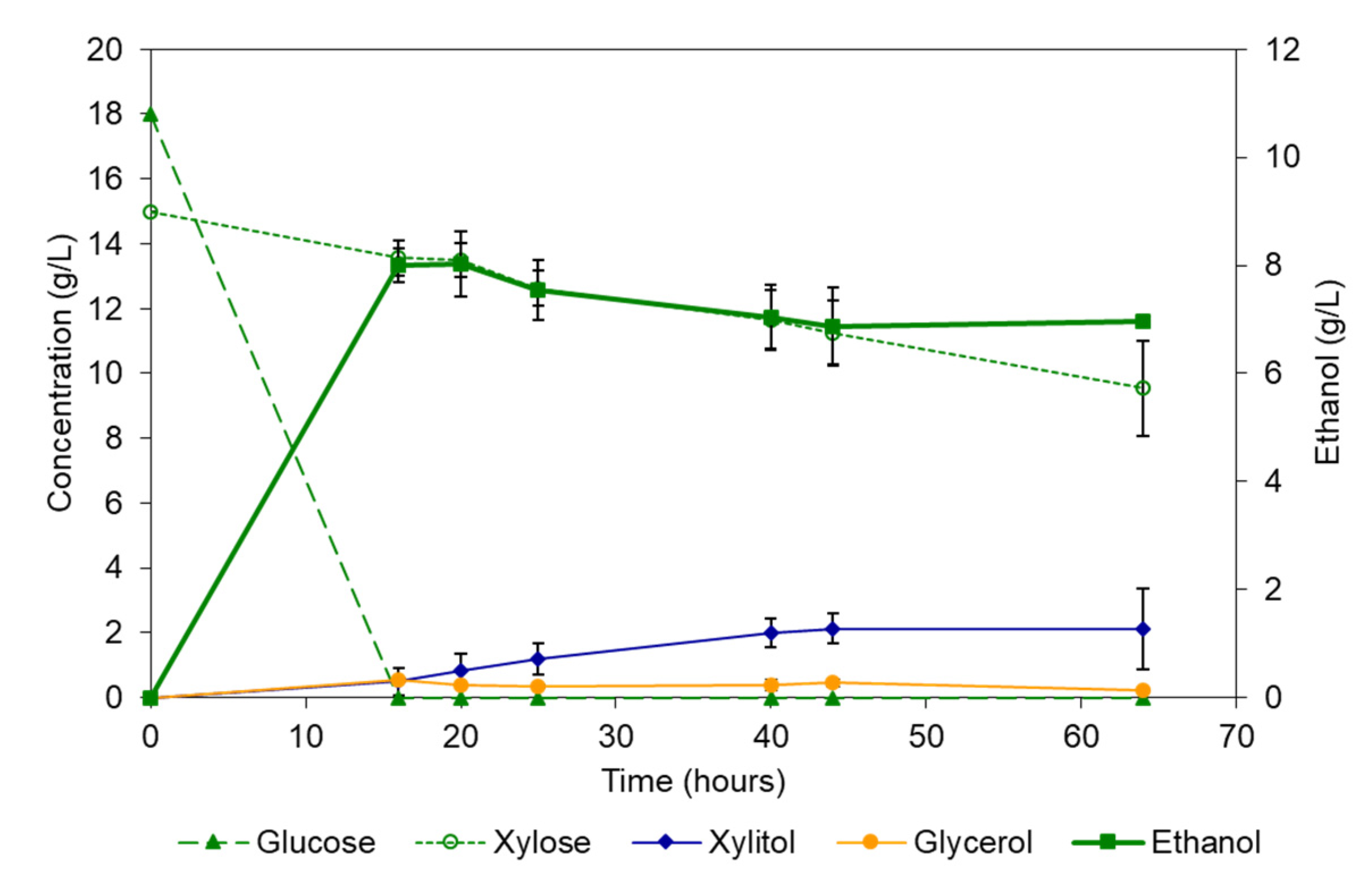
3.3.1. Fermentation of Synthetic Medium
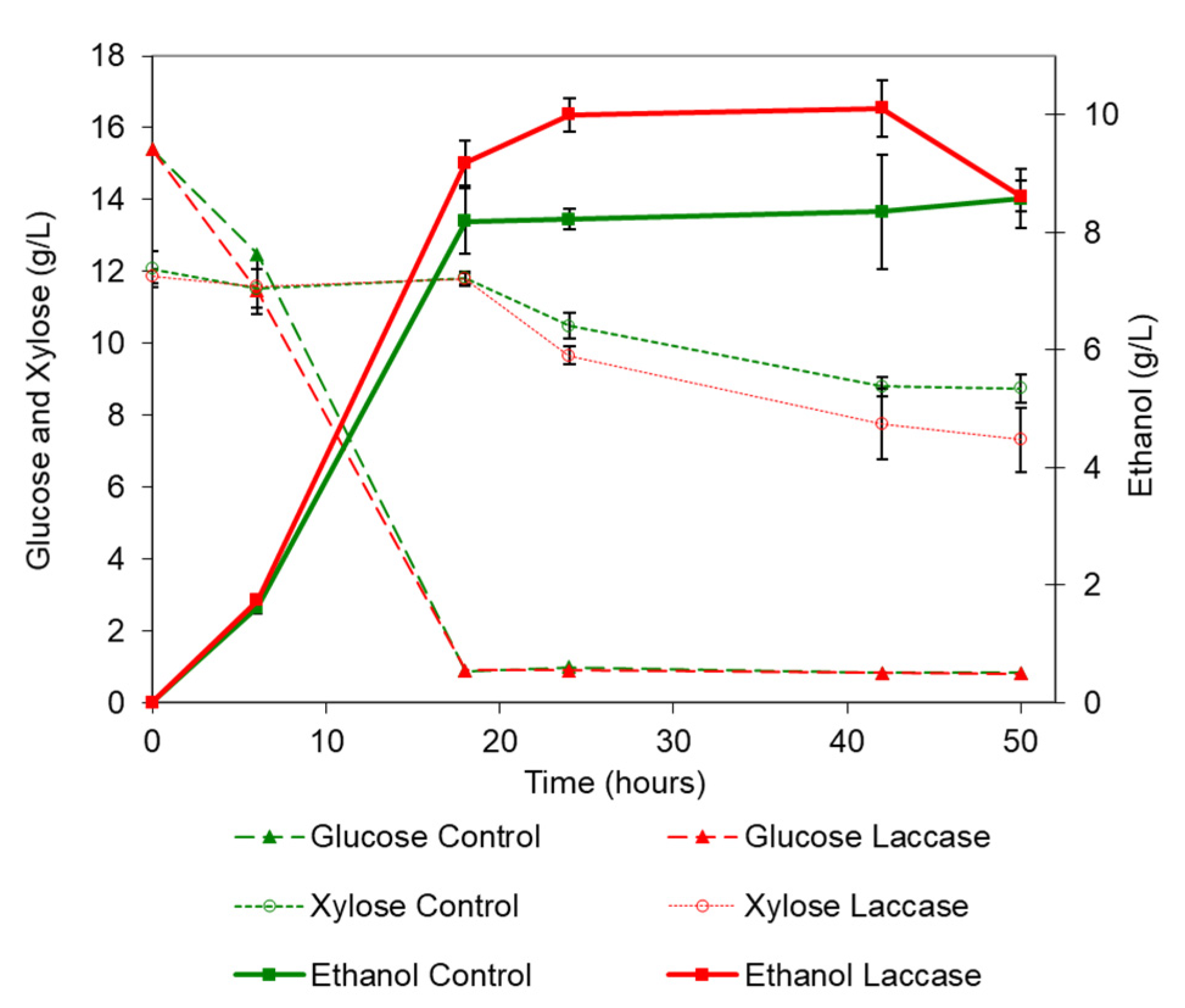
3.3.2. Fermentation of Laccase Liquid Fraction Enzymatic Hydrolysates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Comission, E. A European Green Deal. European Commission. 2019. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM%3A2019%3A640%3AFIN (accessed on 30 January 2023).
- FAOSTAT. Crops. 2021. Available online: http://www.fao.org/faostat (accessed on 30 January 2023).
- Contreras, M.M.; Romero, I.; Moya, M.; Castro, E. Olive-derived biomass as a renewable source of value-added products. Process Biochem. 2020, 97, 45–56. [Google Scholar] [CrossRef]
- Gutiérrez-Sánchez, M.; Espinosa, E.; Bascón-Villegas, I.; Pérez-Rodríguez, F.; Carrasco, E.; Rodríguez, A. Production of Cellulose Nanofibers from Olive Tree Harvest—A Residue with Wide Applications. Agronomy 2020, 10, 696. [Google Scholar] [CrossRef]
- Requejo, A.; Rodríguez, A.; Colodette, J.L.; Gomide, J.L.; Jiménez, L. TCF bleaching sequence in kraft pulping of olive tree pruning residues. Bioresour. Technol. 2012, 117, 117–123. [Google Scholar] [CrossRef]
- Mamaní, A.; Maturano, Y.; Mestre, V.; Montoro, L.; Gassa, L.; Deiana, C.; Sardella, F. Valorization of olive tree pruning. Application for energy storage and biofuel production. Ind. Crops Prod. 2021, 173, 114082. [Google Scholar] [CrossRef]
- Mateo, S.; Puentes, J.G.; Moya, A.J.; Sánchez, S. Ethanol and xylitol production by fermentation of acid hydrolysate from olive pruning with Candida tropicalis NBRC 0618. Bioresour. Technol. 2015, 190, 1–6. [Google Scholar] [CrossRef] [PubMed]
- BP Statistical Review of World Energy 2020, 68 p. Available online: https://www.bp.com (accessed on 27 October 2022).
- Posada, J.A.; Patel, A.D.; Roes, A.; Blok, K.; Faaij, A.P.C.; Patel, M.K. Potential of bioethanol as a chemical building block for biorefineries: Preliminary sustainability assessment of 12 bioethanol-based products. Bioresour. Technol. 2013, 135, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, G.; Acharya, A.; Poudel, D.K.; Aryal, B.; Gyawali, N.; Niraula, P.; Phuyal, S.R.; Prakriti Budhathoki, P.; Bk, G.; Parajuli, N. Recent advances in bioethanol production from lignocellulosic biomass. Int. J. Green Energy 2021, 18, 731–744. [Google Scholar] [CrossRef]
- Wagle, A.; Angove, M.J.; Mahara, A.; Wagle, A.; Mainali, B.; Martins, M.; Goldbeck, R.; Paudel, S.R. Multi-stage pre-treatment of lignocellulosic biomass for multi-product biorefinery: A review. Sustain. Energy Technol. Assess. 2022, 19, 101702. [Google Scholar] [CrossRef]
- Zhou, M.; Thian, X. Development of different pretreatments and related technologies for efficient biomass conversion of lignocellulose. Int. J. Biol. Macromol. 2022, 202, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, L.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef]
- Guo, H.; Zhao, Y.; Chang, J.-S.; Lee, D.-J. Inhibitor formation and detoxification during lignocellulose biorefinery: A review. Bioresour. Technol. 2022, 361, 127666. [Google Scholar] [CrossRef]
- Malhotra, M.; Suman, S.K. Laccase-mediated delignification and detoxification of lignocellulosic biomass: Removing obstacles in energy generation. Environ. Sci. Pollut. Res. 2021, 28, 58929–58944. [Google Scholar] [CrossRef] [PubMed]
- Tramontina, R.; Brenelli, L.B.; Sodré, V.; Franco Cairo, J.P.; Medeiros Travália, B.; Yoshimi Egawa, V.; Goldbeck, R.; Marcio Squina, R. Enzymatic removal of inhibitory compounds from lignocellulosic hydrolysates for biomass to bioproducts applications. World J. Microbiol. Biotechnol. 2020, 36, 166. [Google Scholar] [CrossRef] [PubMed]
- Giardina, P.; Faraco, V.; Pezzella, C.; Piscitelli, A.; Vanhulle, S.; Sannia, G. Laccases: A never-ending story. Cell Mol. Life Sci. 2010, 67, 369–385. [Google Scholar] [CrossRef]
- Jurado, M.; Prieto, A.; Martínez-Alcalá, A.; Martínez, A.T.; Martínez, M.J. Laccase detoxification of steam-exploded wheat straw for second generation bioethanol. Bioresour. Technol. 2009, 100, 6378–6384. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.D.; Ibarra, D.; Mialon, A.; Ballesteros, M. A bacterial laccase for enhancing saccharification and ethanol fermentation of steam-pretreated biomass. Fermentation 2016, 2, 11. [Google Scholar] [CrossRef]
- De la Torre, M.; Martín-Sampedro, R.; Fillat, Ú.; Eugenio, M.E.; Blánquez, A.; Hernández, M.; Arias, M.E.; Ibarra, D. Comparison of the eficiency of bacterial and fungal laccases in delignifcation and detoxifcation of steam pretreated lignocellulosic. J. Ind. Microbiol. Biotechnol. 2017, 44, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Martín, J.; Martínez-Bernal, C.; Zamorano, L.S.; Reyes-Sosa, F.M.; Díez García, B. Inhibition of enzymatic hydrolysis of pretreated corn stover and sugar cane straw by laccases. Process Biochem. 2018, 67, 88–91. [Google Scholar] [CrossRef]
- Kuhad, R.C.; Gupta, R.; Khasa, Y.P.; Singh, A.; Zhang, Y.-H.P. Bioethanol production from pentose sugars: Current status and future prospects. Renew. Sustain. Energy Rev. 2011, 15, 4950–4962. [Google Scholar] [CrossRef]
- Favaro, F.; Jansen, T.; van Zyl, W.H. Exploring industrial and natural Saccharomyces cerevisiae strains for the bio-based economy from biomass: The case of bioethanol. Crit. Rev. Biotechnol. 2019, 39, 800–816. [Google Scholar] [CrossRef]
- Sonderegger, M.; Jeppsson, H.; Larsson, C.; Gorwa-Grauslund, M.F.; Boles, E.; Olsson, L.; Spencer-Martins, I.; Hahn-Hägerdal, B.; Sauer, U. Fermentation performance of engineered and evolved xylose-Fermenting Saccharomyces cerevisiae strains. Biotechnol. Bioeng. 2004, 87, 90–98. [Google Scholar] [CrossRef]
- Tomás-Pejó, E.; Ballesteros, M.; Oliva, J.M.; Olsson, L. Adaptation of the xylose fermenting yeast Saccharomyces cerevisiae F12 for improving ethanol production in different fed-batch SSF processes. J. Ind. Microbiol. Biotechnol. 2010, 37, 1211–1220. [Google Scholar] [CrossRef]
- Ballesteros, I.; Ballesteros, M.; Cara, C.; Sáez, F.; Castro, E.; Manzanares, P.; Negro, M.J.; Oliva, J.M. Effect of water extraction on sugars recovery from steam exploded olive tree pruning. Bioresour. Technol. 2011, 102, 6611–6616. [Google Scholar] [CrossRef] [PubMed]
- Verduyn, C.; Postma, E.; Scheffers, W.A.; van Dijken, J.P. Physiology of Saccharomyces cerevisiae in anaerobic glucose-limited chemostat cultures. J. Gen. Microbiol. 1990, 136, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.D.; Ibarra, D.; Alvira, P.; Tomás-Pejó, E.; Ballesteros, M. Exploring laccase and mediators behavior during saccharification and fermentation of steam-exploded wheat straw for bioethanol production. J. Chem. Technol. Biotechnol. 2016, 91, 1816–1825. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, Y.; Ren, X.; Lau, A.; Rezaei, H.; Takada, M.; Bi, X.; Sohansanj, S. Steam explosion of lignocellulosic biomass for multiple advanced bioenergy processes: A review. Renew. Sust. Energ. Rev. 2022, 154, 111871. [Google Scholar] [CrossRef]
- Ilanidis, D.; Stagge, S.; Jönsson, L.J.; Martín, C. Hydrothermal pretreatment of wheat straw: Effects of temperature and acidity on byproduct formation and inhibition of enzymatic hydrolysis and ethanolic fermentation. Agronomy 2021, 11, 487. [Google Scholar] [CrossRef]
- Díaz, M.J.; Moya, M.; Castro, E. Bioethanol production from steam-exploded barley straw by co-Fermentation with Escherichia coli SL100. Agronomy 2022, 12, 874. [Google Scholar] [CrossRef]
- Hemansi; Saini, J.K. Enhanced cellulosic ethanol production via fed-batch simultaneous saccharification and fermentation of sequential dilute acid-alkali pretreated sugarcane bagasse. Bioresour. Technol. 2023, 372, 128671. [Google Scholar] [CrossRef] [PubMed]
- Parawira, W.; Tekere, M. Biotechnological strategies to overcome inhibitors in lignocellulose hydrolysates for ethanol production: Review. Crit. Rev. Biotechnol. 2011, 31, 20–31. [Google Scholar] [CrossRef]
- Kolb, M.; Sieber, V.; Amann, M.; Faulstich, M.; Schieder, M. Removal of monomer delignification products by laccase from Trametes versicolor. Bioresour. Technol. 2012, 104, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Schneider, W.D.; Fontana, R.C.; Baudel, H.M.; Siqueira, F.G.; Rencoret, J.; Gutiérrez, A.; Eugenio, L.I.; Prieto, A.; Martínez, M.J.; Martínez, A.T.; et al. Lignin degradation and detoxification of eucalyptus wastes by on-site manufacturing fungal enzymes to enhance second-generation ethanol yield. Appl. Energy 2020, 262, 114493. [Google Scholar] [CrossRef]
- Kalyani, D.; Dhiman, S.S.; Kim, H.; Jeya, M.; Kim, I.-W.; Lee, J.-K. Characterization of a novel laccase from the isolated Coltricia perennis and its application to detoxification of biomass. Process Biochem. 2010, 47, 671–678. [Google Scholar] [CrossRef]
- Esteves, P.J.; Milagres, A.M.F.; Qian, X.; Chandel, A.K.; Wickramasinghe, S.R.; Silva, S.S.; Carvalho, W. Simplified configuration for conversion of sugars from sugarcane bagasse into ethanol. Bioresour. Technol. Rep. 2021, 6, 100835. [Google Scholar] [CrossRef]
- Chandel, A.K.; Kapoor, R.K.; Singh, A.; Kuhad, R.C. Detoxification of sugarcane bagasse hydrolysate improves ethanol production by Candida shehatae NCIM 3501. Bioresour. Technol. 2007, 98, 1947–1950. [Google Scholar] [CrossRef]
- Moreno, A.D.; Tomás-Pejó, E.; Ibarra, D.; Ballesteros, M.; Olsson, L. Fed-batch SSCF using steam-exploded wheat straw at high dry matter consistencies and a xylose-fermenting Saccharomyces cerevisiae strain: Effect of laccase supplementation. Biotechnol. Biofuels 2013, 6, 160. [Google Scholar] [CrossRef]
- Kapoor, R.K.; Rajan, K.; Carrier, D.J. Applications of Trametes versicolor crude culture filtrates in detoxification of biomass pretreatment hydrolyzates. Bioresour. Technol. 2015, 189, 99–106. [Google Scholar] [CrossRef]
- Tramontina, R.; Brenelli, L.B.; Sousa, A.; Alves, R.; Arenas, A.M.Z.; Nascimento, V.M.; Rabelo, S.C.; Freitas, S.; Ruller, R.; Squina, F.M. Designing a cocktail containing redox enzymes to improve hemicellulosic hydrolysate fermentability by microorganisms. Enzym. Microb. Technol. 2020, 135, 109490. [Google Scholar] [CrossRef] [PubMed]
- Meinander, N.Q.; Boels, I.; Hahn-Hägerdal, B. Fermentation of xylose/glucose mixtures by metabolically engineered Saccharomyces cerevisiae strains expressing XYL1 and XYL2 from Pichia stipitis with and without overexpression of TAL1. Bioresour. Technol. 1999, 68, 79–87. [Google Scholar] [CrossRef]
- Meinander, N.Q.; Hahn-H/igerdal, B. Fed-batch xylitol production with two recombinant Saccharomyces cerevisiae strains expressing XYL1 at different levels, using glucose as a cosubstrate: A comparison of production parameters and strain stability. Biotechnol. Bioeng. 1997, 54, 391–399. [Google Scholar] [CrossRef]
- Moysés, D.N.; Reis, V.C.B.; Almeida, J.R.M.d.; Moraes, L.M.P.d.; Torres, F.A.G. Xylose fermentation by Saccharomyces cerevisiae: Challenges and prospects. Int. J. Mol. Sci. 2016, 17, 207. [Google Scholar] [CrossRef] [PubMed]
- Batt, C.A.; Caryallo, S.; Easson, D.D., Jr.; Akedo, M.; Sinskey, A.J. Direct evidence for a xylose metabolic pathway in Saccharomyces cerevisiae. Biotechnol. Bioeng. 1986, 28, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, A.P.; Ballesteros, I.; Sáez, F.; Perotti, N.I.; Martínez, M.A.; Negro, M.J. Integral process assessment of sugarcane agricultural crop residues conversion to ethanol. Bioresour. Technol. 2018, 260, 241–247. [Google Scholar] [CrossRef]
- Tomás-Pejó, E.; Oliva, J.M.; Ballesteros, M.; Olsson, L. Comparison of SHF and SSF processes from steam-exploded wheat straw for ethanol production by xylose-fermenting and robust glucose-fermenting Saccharomyces cerevisiae strains. Biotechnol. Bioeng. 2008, 100, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Klinke, H.B.; Thomsen, A.B.; Ahring, B.K. Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl. Microbiol. Biotechnol. 2004, 66, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Wahlbom, C.F.; Hahn-Hägerdal, B. Furfural, 5-hydroxymethyl furfural and acetoin act as external electron acceptors during anaerobic fermentation of xylose in recombinant Saccharomyces cerevisiae. Biotechnol. Bioeng. 2002, 17, 172–178. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Palmqvist, E.; Nilvebrant, N.-O.; Hahn-Hägerdal, B. Detoxification of wood hydrolysates with laccase and peroxidase from the white-rot fungus Trametes versicolor. Appl. Microbiol. Biotechnol. 1998, 49, 691–697. [Google Scholar] [CrossRef]
- Larsson, S.; Reimann, A.; Nilvebrant, N.-O.-; Jönsson, L.J. Comparison of different methods for the detoxification of lignocellulose hydrolyzates of spruce. Appl. Microbiol. Biotechnol. 1999, 77–79, 91–103. [Google Scholar] [CrossRef]
- Martín, C.; Galbe, M.; Wahlbom, C.F.; Hahn-Hägerdal, B.; Jönsson, L. Ethanol production from enzymatic hydrolysates of sugarcane bagasse using recombinant xylose-utilising Saccharomyces cerevisiae. Enzym. Microb. Technol. 2002, 31, 274–282. [Google Scholar] [CrossRef]


| Monomeric form | Total | |
|---|---|---|
| Sugars (g/L) | ||
| Glucose | 2.0 | 17.3 |
| Xylose | 1.5 | 14.5 |
| Galactose | 0.7 | 3.1 |
| Arabinose | 1.7 | 2.6 |
| Mannose | nd | 1.1 |
| Inhibitors (g/L) | ||
| Furfural | 0.6 | |
| 5-HMF | 0.2 | |
| Formic acid | 1.0 | |
| Acetic acid | 4.0 | |
| Vanillin | 0.01 | |
| Syringaldehyde | 0.02 | |
| p-Coumaric acid | 0.003 | |
| Control | Laccase | |
|---|---|---|
| Inhibitors (g/L) | ||
| Furfural | 0.466 ± 0.033 | 0.436 ± 0.011 |
| 5-HMF | 0.139 ± 0.051 | 0.120 ± 0.032 |
| Formic acid | 1.014 ± 0.022 | 0.926 ± 0.012 |
| Acetic acid | 3.878 ± 0.041 | 3.778 ± 0.023 |
| Vanillin | 0.093 ± 0.015 | 0.001 ± 0.002 * |
| Syringaldehyde | 0.015 ± 0.001 | 0.000 ± 0.000 * |
| p-Coumaric acid | 0.003 ± 0.001 | 0.000 ± 0.001 * |
| Strain | ||
|---|---|---|
| S. cerevisiae Ethanol Red | S. cerevisiae F12 | |
| Residual sugars | ||
| Glucose (g/L) | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Xylose (g/L) | 9.5 ± 1.4 | 2.1 ± 0.7 |
| EtOHmax (g/L) | 8.0 ± 0.6 | 10.7 ± 0.0 |
| Xylitolmax (g/L) | 2.1 ± 1.3 | 1.2 ± 0.8 |
| Glycerolmax (g/L) | 0.6 ± 0.1 | 1.3 ± 0.1 |
| YE/S (g/g) | 0.24 | 0.32 |
| YE/ET (%) | 47.5 | 63.5 |
| Sample | EtOHmax (g/L) | YE/S (g/g) | YE/ET (%) | QE (g/L h) | |
|---|---|---|---|---|---|
| S. cerevisiae | Control | 6.8 ± 0.4 | 0.21 | 41.8 | 0.12 |
| Laccase | 8.0 ± 0.2 | 0.25 | 49.7 | 0.22 | |
| S. cerevisiae F12 | Control | 8.6 ± 0.5 | 0.27 | 52.9 | 0.43 |
| Laccase | 10.1 ± 0.5 | 0.32 | 62.4 | 0.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibarra, D.; Eugenio, M.E.; Alvira, P.; Ballesteros, I.; Ballesteros, M.; Negro, M.J. Effect of Laccase Detoxification on Bioethanol Production from Liquid Fraction of Steam-Pretreated Olive Tree Pruning. Fermentation 2023, 9, 214. https://doi.org/10.3390/fermentation9030214
Ibarra D, Eugenio ME, Alvira P, Ballesteros I, Ballesteros M, Negro MJ. Effect of Laccase Detoxification on Bioethanol Production from Liquid Fraction of Steam-Pretreated Olive Tree Pruning. Fermentation. 2023; 9(3):214. https://doi.org/10.3390/fermentation9030214
Chicago/Turabian StyleIbarra, David, María E. Eugenio, Pablo Alvira, Ignacio Ballesteros, Mercedes Ballesteros, and María J. Negro. 2023. "Effect of Laccase Detoxification on Bioethanol Production from Liquid Fraction of Steam-Pretreated Olive Tree Pruning" Fermentation 9, no. 3: 214. https://doi.org/10.3390/fermentation9030214
APA StyleIbarra, D., Eugenio, M. E., Alvira, P., Ballesteros, I., Ballesteros, M., & Negro, M. J. (2023). Effect of Laccase Detoxification on Bioethanol Production from Liquid Fraction of Steam-Pretreated Olive Tree Pruning. Fermentation, 9(3), 214. https://doi.org/10.3390/fermentation9030214





