Development of a Simple and Robust Kinetic Model for the Production of Succinic Acid from Glucose Depending on Different Operating Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism
2.2. Culture Media
2.3. Cultivation Conditions
2.4. Analytical Methods
3. Results and Discussion
3.1. Development of a Simple Kinetic Model
3.2. Kinetic Study Based on the Initial Biomass Concentration
3.3. Kinetic Study Based on the CO2 Flow
3.4. Kinetic Study Based on the Stirring Speed
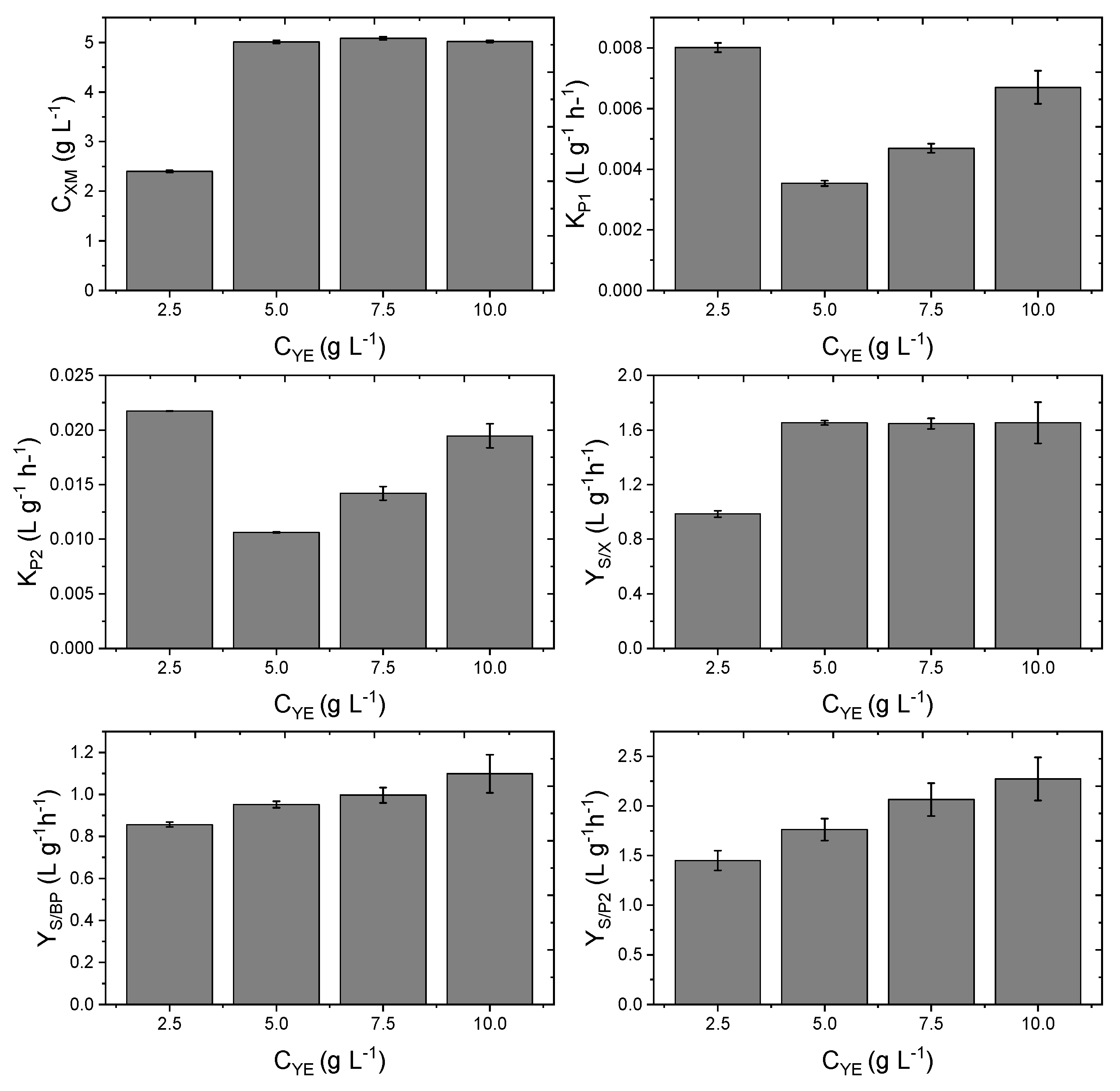
3.5. Kinetic Study Based on the Yeast Extract Concentration
3.6. Kinetic Estimation of the Stages of a Fed-Batch Type Operation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kover, A.; Kraljić, D.; Marinaro, R.; Rene, E.R. Processes for the valorization of food and agricultural wastes to value-added products: Recent practices and perspectives. Syst. Microbiol. Biomanufacturing 2022, 2, 50–66. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Awasthi, A.K.; Lukk, T.; Tuohy, M.G.; Gong, L.; Nguyen-Tri, P.; Goddard, A.D.; Bill, R.M.; Nayak, S.; et al. Lignocellulosic biorefineries: The current state of challenges and strategies for efficient commercialization. Renew. Sustain. Energy Rev. 2021, 148, 111258. [Google Scholar] [CrossRef]
- Saxena, R.K.; Saran, S.; Isar, J.; Kaushik, R. Production and Applications of Succinic Acid. In Current Developments in Biotechnology and Bioengineering: Production, Isolation and Purification of Industrial Products; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 601–630. ISBN 9780444636621. [Google Scholar]
- Pateraki, C.; Patsalou, M.; Vlysidis, A.; Kopsahelis, N.; Webb, C.; Koutinas, A.A.; Koutinas, M. Actinobacillus succinogenes: Advances on succinic acid production and prospects for development of integrated biorefineries. Biochem. Eng. J. 2016, 112, 285–303. [Google Scholar] [CrossRef]
- Shen, N.; Li, S.; Qin, Y.; Jiang, M.; Zhang, H. Optimization of succinic acid production from xylose mother liquor (XML) by Actinobacillus succinogenes using response surface methodology. Biotechnol. Biotechnol. Equip. 2022, 36, 439–447. [Google Scholar] [CrossRef]
- Banger, G.; Kaya, K.; Omwene, P.; Shakoory, S.; Karagündüz, A.; Keskinler, B.; Nikerel, E. Delactosed Whey Permeate as Substrate for Succinic Acid Fermentation by Actinobacillus succinogenes. Waste Biomass-Valorization 2021, 12, 5481–5489. [Google Scholar] [CrossRef]
- Escanciano, I.A.; Wojtusik, M.; Esteban, J.; Ladero, M.; Santos, V.E. Modeling the succinic acid bioprocess: A review. Fermentation 2022, 8, 368. [Google Scholar] [CrossRef]
- Salma, A.; Djelal, H.; Abdallah, R.; Fourcade, F.; Amrane, A. Well Knowledge of the Physiology of Actinobacillus succinogenes to improve succinic acid production. Appl. Microbiol. 2021, 1, 304–328. [Google Scholar] [CrossRef]
- Sillaparassamee, O.; Chinwetkitvanich, S.; Kanchanasuta, S.; Pisutpaisal, N.; Champreda, V. Metabolic flux analysis on succinic acid production from crude glycerol by Actinobacillus succinogenes. Biomass-Convers. Biorefinery 2021, 1–12. [Google Scholar] [CrossRef]
- Luthfi, A.A.I.; Jahim, J.M.; Harun, S.; Tan, J.P.; Manaf, S.F.A.; Shah, S.S.M. Kinetics of the bioproduction of succinic acid by Actinobacillus succinogenes from oil palm lignocellulosic hydrolysate in a bioreactor. Bioresources 2018, 13, 8279–8294. [Google Scholar] [CrossRef]
- Salvachúa, D.; Mohagheghi, A.; Smith, H.; Bradfield, M.F.A.; Nicol, W.; Black, B.A.; Biddy, M.J.; Dowe, N.; Beckham, G.T. Succinic acid production on xylose-enriched biorefinery streams by Actinobacillus succinogenes in batch fermentation. Biotechnol. Biofuels 2016, 9, 28. [Google Scholar] [CrossRef] [Green Version]
- Ferone, M.; Raganati, F.; Olivieri, G.; Salatino, P.; Marzocchella, A. Biosuccinic acid from lignocellulosic-based hexoses and pentoses by Actinobacillus succinogenes: Characterization of the conversion process. Appl. Biochem. Biotechnol. 2017, 183, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Pateraki, C.; Almqvist, H.; Ladakis, D.; Lidén, G.; Koutinas, A.A.; Vlysidis, A. Modelling succinic acid fermentation using a xylose based substrate. Biochem. Eng. J. 2016, 114, 26–41. [Google Scholar] [CrossRef]
- Omwene, P.I.; Yağcıoğlu, M.; Öcal-Sarihan, Z.B.; Ertan, F.; Keris-Sen, Ü.D.; Karagunduz, A.; Keskinler, B. Batch fermentation of succinic acid from cheese whey by Actinobacillus succinogenes under variant medium composition. 3 Biotech 2021, 11, 389. [Google Scholar] [CrossRef]
- Bumyut, A.; Champreda, V.; Singhakant, C.; Kanchanasuta, S. Effects of immobilization of Actinobacillus succinogenes on efficiency of bio-succinic acid production from glycerol. Biomass-Convers. Biorefinery 2020, 12, 643–654. [Google Scholar] [CrossRef]
- Kuglarz, M.; Rom, M. Influence of carbon dioxide and nitrogen source on sustainable production of succinic acid from miscanthus hydrolysates. Int. J. Environ. Sci. Dev. 2019, 10, 362–367. [Google Scholar] [CrossRef] [Green Version]
- Xi, Y.-L.; Chen, K.-Q.; Li, J.; Fang, X.-J.; Zheng, X.-Y.; Sui, S.-S.; Jiang, M.; Wei, P. Optimization of culture conditions in CO2 fixation for succinic acid production using Actinobacillus succinogenes. J. Ind. Microbiol. Biotechnol. 2011, 38, 1605–1612. [Google Scholar] [CrossRef]
- Zou, W.; Zhu, L.-W.; Li, H.-M.; Tang, Y.-J. Significance of CO2 donor on the production of succinic acid by Actinobacillus succinogenes ATCC 55618. Microb. Cell Factories 2011, 10, 87. [Google Scholar] [CrossRef] [Green Version]
- Herselman, J.; Bradfield, M.F.; Vijayan, U.; Nicol, W. The effect of carbon dioxide availability on succinic acid production with biofilms of Actinobacillus succinogenes. Biochem. Eng. J. 2017, 117, 218–225. [Google Scholar] [CrossRef] [Green Version]
- Amulya, K.; Kopperi, H.; Mohan, S.V. Tunable production of succinic acid at elevated pressures of CO2 in a high pressure gas fermentation reactor. Bioresour. Technol. 2020, 309, 123327. [Google Scholar] [CrossRef]
- Vigato, F.; Angelidaki, I.; Woodley, J.M.; Alvarado-Morales, M. Dissolved CO2 profile in bio-succinic acid production from sugars-rich industrial waste. Biochem. Eng. J. 2022, 187, 108602. [Google Scholar] [CrossRef]
- Filippi, K.; Papapostolou, H.; Alexandri, M.; Vlysidis, A.; Myrtsi, E.D.; Ladakis, D.; Pateraki, C.; Haroutounian, S.A.; Koutinas, A. Integrated biorefinery development using winery waste streams for the production of bacterial cellulose, succinic acid and value-added fractions. Bioresour. Technol. 2021, 343, 125989. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Park, S.O.; Yeon, J.Y.; Chun, G.-T. Development of a cell-recycled continuous fermentation process for enhanced production of succinic acid by high-yielding mutants of Actinobacillus succinogenes. Biotechnol. Bioprocess Eng. 2020, 26, 125–136. [Google Scholar] [CrossRef]
- Jokodola, E.O.; Narisetty, V.; Castro, E.; Durgapal, S.; Coulon, F.; Sindhu, R.; Binod, P.; Banu, J.R.; Kumar, G.; Kumar, V. Process optimisation for production and recovery of succinic acid using xylose-rich hydrolysates by Actinobacillus succinogenes. Bioresour. Technol. 2021, 344, 126224. [Google Scholar] [CrossRef]
- Ercole, A.; Raganati, F.; Salatino, P.; Marzocchella, A. Continuous succinic acid production by immobilized cells of Actinobacillus succinogenes in a fluidized bed reactor: Entrapment in alginate beads. Biochem. Eng. J. 2021, 169, 107968. [Google Scholar] [CrossRef]
- Mokwatlo, S.C.; Nicol, W. Structure and cell viability analysis of Actinobacillus succinogenes biofilms as biocatalysts for succinic acid production. Biochem. Eng. J. 2017, 128, 134–140. [Google Scholar] [CrossRef] [Green Version]
- Cao, W.; Wang, Y.; Luo, J.; Yin, J.; Xing, J.; Wan, Y. Succinic acid biosynthesis from cane molasses under low pH by Actinobacillus succinogenes immobilized in luffa sponge matrices. Bioresour. Technol. 2018, 268, 45–51. [Google Scholar] [CrossRef]
- Escanciano, I.A.; Ladero, M.; Santos, V.E. On the succinic acid production from xylose by growing and resting cells of Actinobacillus succinogenes: A comparison. Biomass-Convers. Biorefinery 2022, 1–14. [Google Scholar] [CrossRef]
- Shen, N.; Zhang, H.; Qin, Y.; Wang, Q.; Zhu, J.; Li, Y.; Jiang, M.-G.; Huang, R. Efficient production of succinic acid from duckweed (Landoltia punctata) hydrolysate by Actinobacillus succinogenes GXAS137. Bioresour. Technol. 2018, 250, 35–42. [Google Scholar] [CrossRef]
- Gonzales, T.A.; de Carvalho Silvello, M.A.; Duarte, E.R.; Santos, L.O.; Alegre, R.M.; Goldbeck, R. Optimization of anaerobic fermentation of Actinobacillus succinogenes for increase the succinic acid production. Biocatal. Agric. Biotechnol. 2020, 27, 101718. [Google Scholar] [CrossRef]
- Tan, J.P.; Jahim, J.M.; Wu, T.Y.; Harun, S.; Mumtaz, T. Use of corn steep liquor as an economical nitrogen source for biosuccinic acid production by Actinobacillus succinogenes. IOP Conf. Ser. Earth Environ. Sci. 2016, 36, 012058. [Google Scholar] [CrossRef]
- Xi, Y.-L.; Chen, K.-Q.; Dai, W.-Y.; Ma, J.-F.; Zhang, M.; Jiang, M.; Wei, P.; Ouyang, P.-K. Succinic acid production by Actinobacillus succinogenes NJ113 using corn steep liquor powder as nitrogen source. Bioresour. Technol. 2013, 136, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Chen, K.; Liu, Z.; Wei, P.; Ying, H.; Chang, H. Succinic acid production by Actinobacillus succinogenes using spent brewer′s yeast hydrolysate as a nitrogen source. Appl. Biochem. Biotechnol. 2009, 160, 244–254. [Google Scholar] [CrossRef]
- Delgado-Noboa, J.; Bernal, T.; Soler, J.; Peña, J. Kinetic modeling of batch bioethanol production from CCN-51 Cocoa Mucilage. J. Taiwan Inst. Chem. Eng. 2021, 128, 169–175. [Google Scholar] [CrossRef]
- Louasté, B.; Eloutassi, N. Succinic acid production from whey and lactose by Actinobacillus succinogenes 130Z in batch fermentation. Biotechnol. Rep. 2020, 27, e00481. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Dai, W.; Xi, Y.; Wu, M.; Kong, X.; Ma, J.; Zhang, M.; Chen, K.; Wei, P. Succinic acid production from sucrose by Actinobacillus succinogenes NJ113. Bioresour. Technol. 2014, 153, 327–332. [Google Scholar] [CrossRef]
- Lin, S.K.C.; Du, C.; Koutinas, A.; Wang, R.; Webb, C. Substrate and product inhibition kinetics in succinic acid production by Actinobacillus succinogenes. Biochem. Eng. J. 2008, 41, 128–135. [Google Scholar] [CrossRef]
- Guarnieri, M.T.; Chou, Y.-C.; Salvachúa, D.; Mohagheghi, A.; John, P.C.S.; Peterson, D.J.; Bomble, Y.J.; Beckham, G.T. Metabolic engineering of Actinobacillus succinogenes provides insights into succinic acid biosynthesis. Appl. Environ. Microbiol. 2017, 83, e00996-17. [Google Scholar] [CrossRef] [Green Version]
- Lubsungneon, J.; Srisuno, S.; Rodtong, S.; Boontawan, A. Nanofiltration coupled with vapor permeation-assisted esterification as an effective purification step for fermentation-derived succinic acid. J. Membr. Sci. 2014, 459, 132–142. [Google Scholar] [CrossRef]
- Cao, W.; Wang, Y.; Luo, J.; Yin, J.; Xing, J.; Wan, Y. Effectively converting carbon dioxide into succinic acid under mild pressure with Actinobacillus succinogenes by an integrated fermentation and membrane separation process. Bioresour. Technol. 2018, 266, 26–33. [Google Scholar] [CrossRef]
- Rigaki, A.; Webb, C.; Theodoropoulos, C. Double substrate limitation model for the bio-based production of succinic acid from glycerol. Biochem. Eng. J. 2019, 153, 107391. [Google Scholar] [CrossRef]
- Zhang, W.; Tao, Y.; Wu, M.; Xin, F.; Dong, W.; Zhou, J.; Gu, J.; Ma, J.; Jiang, M. Adaptive evolution improves acid tolerance and succinic acid production in Actinobacillus succinogenes. Process. Biochem. 2020, 98, 76–82. [Google Scholar] [CrossRef]
- Wan, C.; Li, Y.; Shahbazi, A.; Xiu, S. Succinic Acid Production from Cheese Whey using Actinobacillus succinogenes 130 Z. Appl. Biochem. Biotechnol. 2007, 145, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Anwar, N.A.K.K.; Hassan, N.; Yusof, N.M.; Idris, A. High-titer bio-succinic acid production from sequential alkalic and metal salt pretreated empty fruit bunch via simultaneous saccharification and fermentation. Ind. Crop. Prod. 2021, 166, 113478. [Google Scholar] [CrossRef]
- Argun, H.; Kargi, F.; Kapdan, I.K.; Oztekin, R. Batch dark fermentation of powdered wheat starch to hydrogen gas: Effects of the initial substrate and biomass concentrations. Int. J. Hydrogen Energy 2008, 33, 6109–6115. [Google Scholar] [CrossRef]
- Eker, S.; Sarp, M. Hydrogen gas production from waste paper by dark fermentation: Effects of initial substrate and biomass concentrations. Int. J. Hydrogen Energy 2017, 42, 2562–2568. [Google Scholar] [CrossRef]
- Ding, M.-Z.; Tian, H.-C.; Cheng, J.-S.; Yuan, Y.-J. Inoculum size-dependent interactive regulation of metabolism and stress response of Saccharomyces cerevisiae revealed by comparative metabolomics. J. Biotechnol. 2009, 144, 279–286. [Google Scholar] [CrossRef]
- Almqvist, H.; Pateraki, C.; Alexandri, M.; Koutinas, A.; Lidén, G. Succinic acid production by Actinobacillus succinogenes from batch fermentation of mixed sugars. J. Ind. Microbiol. Biotechnol. 2016, 43, 1117–1130. [Google Scholar] [CrossRef]
- Corona-González, R.I.; Miramontes-Murillo, R.; Arriola-Guevara, E.; Guatemala-Morales, G.; Toriz, G.; Pelayo-Ortiz, C. Immobilization of Actinobacillus succinogenes by adhesion or entrapment for the production of succinic acid. Bioresour. Technol. 2014, 164, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ochoa, F.; Gomez, E. Bioreactor scale-up and oxygen transfer rate in microbial processes: An overview. Biotechnol. Adv. 2009, 27, 153–176. [Google Scholar] [CrossRef]
- Garcia-Ochoa, F.; Gomez, E.; Santos, V.E.; Merchuk, J.C. Oxygen uptake rate in microbial processes: An overview. Biochem. Eng. J. 2010, 49, 289–307. [Google Scholar] [CrossRef]
- Garcia-Ochoa, F.; Gomez, E.; Santos, V.E. Fluid dynamic conditions and oxygen availability effects on microbial cultures in STBR: An overview. Biochem. Eng. J. 2020, 164, 107803. [Google Scholar] [CrossRef]
- Ventrone, M.; Schiraldi, C.; Squillaci, G.; Morana, A.; Cimini, D. Chestnut shells as waste material for succinic acid production from Actinobacillus succinogenes 130Z. Fermentation 2020, 6, 105. [Google Scholar] [CrossRef]
- Patsalou, M.; Chrysargyris, A.; Tzortzakis, N.; Koutinas, M. A biorefinery for conversion of citrus peel waste into essential oils, pectin, fertilizer and succinic acid via different fermentation strategies. Waste Manag. 2020, 113, 469–477. [Google Scholar] [CrossRef]
- Kanchanasuta, S.; Champreda, V.; Pisutpaisal, N.; Singhakant, C. Optimization of bio-succinic fermentation process from crude glycerol by Actinobacillus succinogenes. Environ. Eng. Res. 2021, 26, 200121. [Google Scholar] [CrossRef]
- Bradfield, M.F.A.; Mohagheghi, A.; Salvachúa, D.; Smith, H.; Black, B.A.; Dowe, N.; Beckham, G.T.; Nicol, W. Continuous Succinic Acid Production by Actinobacillus succinogenes on Xylose-Enriched Hydrolysate. Biotechnol. Biofuels 2015, 8, 181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradfield, M.F.A.; Nicol, W. Continuous succinic acid production from xylose by Actinobacillus succinogenes. Bioprocess Biosyst. Eng. 2016, 39, 233–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlysidis, A.; Binns, M.; Webb, C.; Theodoropoulos, C. Glycerol utilisation for the production of chemicals: Conversion to succinic acid, a combined experimental and computational study. Biochem. Eng. J. 2011, 58, 1–11. [Google Scholar] [CrossRef]
- Song, H.; Jang, S.H.; Park, J.M.; Lee, S.Y. Modeling of batch fermentation kinetics for succinic acid production by Mannheimia succiniciproducens. Biochem. Eng. J. 2008, 40, 107–115. [Google Scholar] [CrossRef]
- Li, Q.; Wang, D.; Wu, Y.; Yang, M.; Li, W.; Xing, J.; Su, Z. Kinetic evaluation of products inhibition to succinic acid producers Escherichia coli NZN111, AFP111, BL21, and Actinobacillus succinogenes 130ZT. J. Microbiol. 2010, 48, 290–296. [Google Scholar] [CrossRef]
- Corona-González, R.I.; Bories, A.; González-Álvarez, V.; Pelayo-Ortiz, C. Kinetic study of succinic acid production by Actinobacillus succinogenes ZT-130. Process. Biochem. 2008, 43, 1047–1053. [Google Scholar] [CrossRef]
- Bevilaqua, D.B.; Montipó, S.; Pedroso, G.B.; Martins, A.F. Sustainable succinic acid production from rice husks. Sustain. Chem. Pharm. 2015, 1, 9–13. [Google Scholar] [CrossRef]
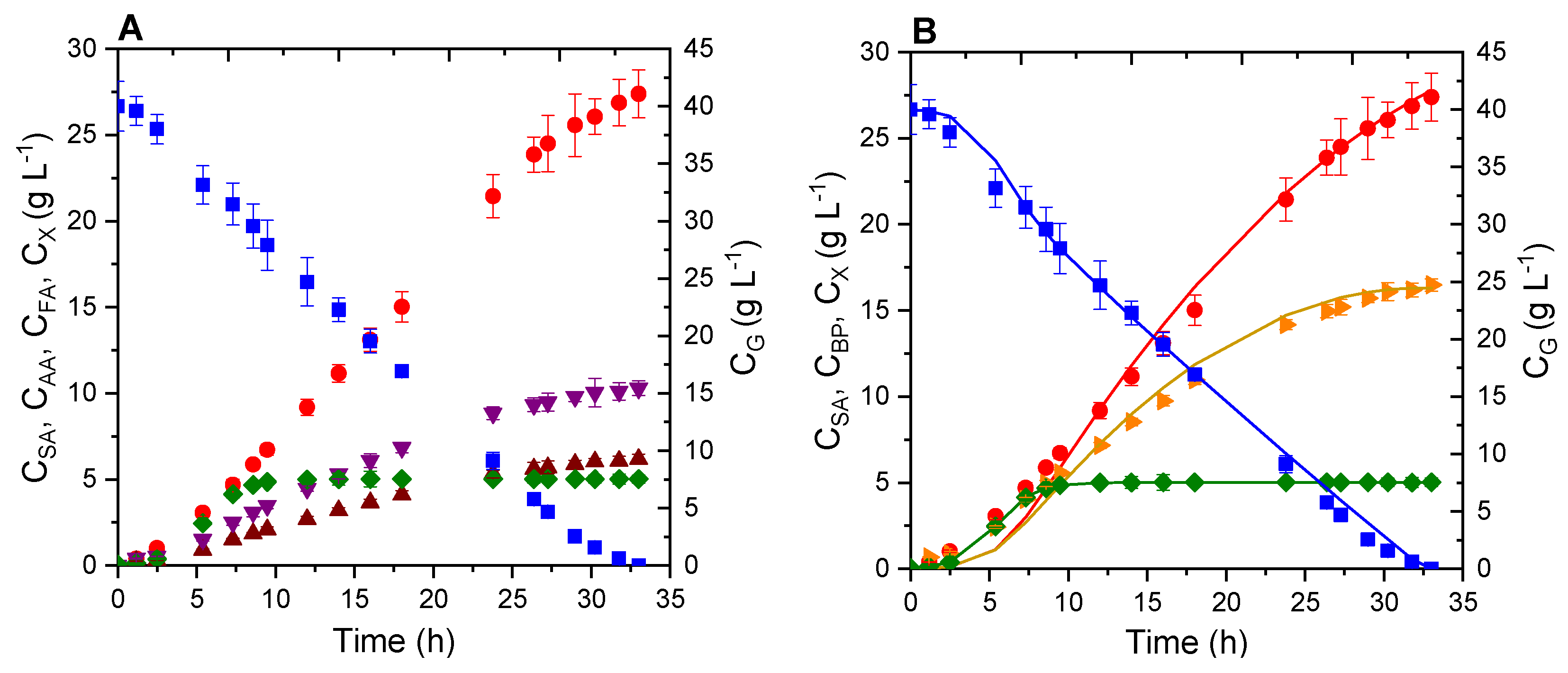





| Run | Type of Operation | Cbiomass (g·L−1) | Agitation (rpm) | CO2 Flow (L·min−1) | CYE (g·L−1) | CSA (g·L−1) | YSA (g·g−1) | PSA (g·L−1·h−1) | SSA (g·g−1) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Batch | 0.05 | 300 | 0.1 | 10 | 27.4 | 0.68 | 0.83 | 0.62 |
| 2 | Batch | 0.075 | 300 | 0.1 | 10 | 28.5 | 0.71 | 0.96 | 0.64 |
| 3 | Batch | 0.1 | 300 | 0.1 | 10 | 28.3 | 0.70 | 0.76 | 0.66 |
| 4 | Batch | 0.05 | 300 | 0.5 | 10 | 27.6 | 0.69 | 0.84 | 0.63 |
| 5 | Batch | 0.05 | 300 | 1 | 10 | 26.1 | 0.65 | 0.81 | 0.63 |
| 6 | Batch | 0.05 | 150 | 0.1 | 10 | 23.6 | 0.59 | 0.72 | 0.61 |
| 7 | Batch | 0.05 | 200 | 0.1 | 10 | 26.4 | 0.66 | 0.78 | 0.62 |
| 8 | Batch | 0.05 | 250 | 0.1 | 10 | 28.5 | 0.71 | 0.84 | 0.62 |
| 9 | Batch | 0.05 | 300 | 0.1 | 2.5 | 23.8 | 0.59 | 0.48 | 0.68 |
| 10 | Batch | 0.05 | 300 | 0.1 | 5 | 26.8 | 0.66 | 0.53 | 0.66 |
| 11 | Batch | 0.05 | 300 | 0.1 | 7.5 | 28.9 | 0.72 | 0.58 | 0.64 |
| 12 | Fed-batch | 0.05 | 300 | 0.1 | 10 | 39.7 | 0.67 | 0.72 | 0.69 |
| Run | Type of | Cbiomass | Agitation | CO2 Flow | CYE | Cxm ± Error | Kp1 ± Error | Kp2 ± Error | μ ± Error | YS/P1 ± Error | YS/P2 ± Error | YS/BP ± Error | YS/X ± Error | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Operation | (g·L−1) | (rpm) | (L·min−1) | (g·L−1) | (gX·L−1) | (L‧g−1‧h−1) | (L‧g−1‧h−1) | (h−1) | (g‧g−1) | (g‧g−1) | (g‧g−1) | (g‧g−1) | |||||||||||||||||
| 1 | Batch | 0.05 | 300 | 0.1 | 10 | 5.02 | ± | 0.02 | 0.007 | ± | 0.001 | 0.019 | ± | 0.001 | 0.85 | ± | 0.04 | 0.24 | ± | 0.02 | 2.27 | ± | 0.22 | 1.10 | ± | 0.09 | 1.65 | ± | 0.15 |
| 2 | Batch | 0.075 | 300 | 0.1 | 10 | 5.81 | ± | 0.03 | 0.009 | ± | 0.001 | 0.013 | ± | 0.001 | 0.85 | ± | 0.03 | 0.24 | ± | 0.02 | 2.20 | ± | 0.19 | 1.08 | ± | 0.07 | 1.63 | ± | 0.16 |
| 3 | Batch | 0.1 | 300 | 0.1 | 10 | 6.79 | ± | 0.08 | 0.004 | ± | 0.000 | 0.008 | ± | 0.001 | 0.85 | ± | 0.02 | 0.24 | ± | 0.02 | 2.25 | ± | 0.19 | 1.09 | ± | 0.08 | 1.63 | ± | 0.16 |
| 4 | Batch | 0.05 | 300 | 0.5 | 10 | 5.07 | ± | 0.03 | 0.008 | ± | 0.001 | 0.018 | ± | 0.001 | 0.85 | ± | 0.01 | 0.25 | ± | 0.02 | 2.27 | ± | 0.22 | 1.09 | ± | 0.09 | 1.66 | ± | 0.16 |
| 5 | Batch | 0.05 | 300 | 1 | 10 | 5.07 | ± | 0.03 | 0.008 | ± | 0.001 | 0.018 | ± | 0.001 | 0.85 | ± | 0.01 | 0.25 | ± | 0.02 | 2.27 | ± | 0.22 | 1.09 | ± | 0.09 | 1.66 | ± | 0.16 |
| 6 | Batch | 0.05 | 150 | 0.1 | 10 | 5.09 | ± | 0.09 | 0.004 | ± | 0.001 | 0.019 | ± | 0.001 | 0.85 | ± | 0.04 | 0.26 | ± | 0.02 | 2.30 | ± | 0.21 | 1.10 | ± | 0.08 | 1.56 | ± | 0.13 |
| 7 | Batch | 0.05 | 200 | 0.1 | 10 | 5.05 | ± | 0.06 | 0.006 | ± | 0.001 | 0.018 | ± | 0.001 | 0.85 | ± | 0.06 | 0.26 | ± | 0.02 | 2.27 | ± | 0.18 | 1.04 | ± | 0.07 | 1.54 | ± | 0.11 |
| 8 | Batch | 0.05 | 250 | 0.1 | 10 | 5.00 | ± | 0.05 | 0.008 | ± | 0.001 | 0.018 | ± | 0.001 | 0.85 | ± | 0.05 | 0.24 | ± | 0.02 | 2.31 | ± | 0.18 | 1.07 | ± | 0.09 | 1.60 | ± | 0.13 |
| 9 | Batch | 0.05 | 300 | 0.1 | 2.5 | 2.40 | ± | 0.02 | 0.008 | ± | 0.001 | 0.022 | ± | 0.001 | 0.85 | ± | 0.05 | 0.24 | ± | 0.02 | 1.45 | ± | 0.10 | 0.86 | ± | 0.01 | 0.98 | ± | 0.02 |
| 10 | Batch | 0.05 | 300 | 0.1 | 5 | 5.01 | ± | 0.03 | 0.004 | ± | 0.001 | 0.011 | ± | 0.001 | 0.85 | ± | 0.03 | 0.25 | ± | 0.01 | 1.76 | ± | 0.11 | 0.95 | ± | 0.02 | 1.65 | ± | 0.02 |
| 11 | Batch | 0.05 | 300 | 0.1 | 7.5 | 5.08 | ± | 0.03 | 0.005 | ± | 0.001 | 0.014 | ± | 0.001 | 0.85 | ± | 0.04 | 0.24 | ± | 0.01 | 2.06 | ± | 0.17 | 1.00 | ± | 0.04 | 1.65 | ± | 0.04 |
| 12 | Fed-batch cycle 1 | 0.05 | 300 | 0.1 | 10 | 5.02 | ± | 0.02 | 0.007 | ± | 0.001 | 0.019 | ± | 0.001 | 0.85 | ± | 0.04 | 0.24 | ± | 0.02 | 2.27 | ± | 0.22 | 1.10 | ± | 0.09 | 1.65 | ± | 0.15 |
| 12 | Fed-batch cycle 1 | 0.05 | 300 | 0.1 | 10 | 5.02 | ± | 0.02 | 0.007 | ± | 0.001 | 0.019 | ± | 0.001 | 0.85 | ± | 0.04 | 0.24 | ± | 0.02 | 2.27 | ± | 0.22 | 0.59 | ± | 0.02 | 1.65 | ± | 0.15 |
| 12 | Fed-batch cycle 1 | 0.05 | 300 | 0.1 | 10 | 5.02 | ± | 0.02 | 0.004 | ± | 0.001 | 0.005 | ± | 0.000 | 0.85 | ± | 0.04 | 0.24 | ± | 0.02 | 2.27 | ± | 0.22 | 0.37 | ± | 0.01 | 1.65 | ± | 0.15 |
| Run | Type of | Cbiomass | Agitation | CO2 Flow | CYE | F95 | RMSE | SSR | VE |
|---|---|---|---|---|---|---|---|---|---|
| Operation | (g·L-1) | (rpm) | (L·min-1) | (g·L-1) | % | ||||
| 1 REF. | Batch | 0.05 | 300 | 0.1 | 10 | 41,242 | 0.67 | 6.47 | 99.5 |
| 2 | Batch | 0.075 | 300 | 0.1 | 10 | 13,660 | 1.09 | 11.26 | 98.2 |
| 3 | Batch | 0.1 | 300 | 0.1 | 10 | 40,640 | 1.00 | 10.04 | 98.7 |
| 4 | Batch | 0.05 | 300 | 0.5 | 10 | 8457 | 1.14 | 12.17 | 98.5 |
| 5 | Batch | 0.05 | 300 | 1 | 10 | 8457 | 1.14 | 12.17 | 98.5 |
| 6 | Batch | 0.05 | 150 | 0.1 | 10 | 19,384 | 0.92 | 9.01 | 99.0 |
| 7 | Batch | 0.05 | 200 | 0.1 | 10 | 19,684 | 1.03 | 9.99 | 98.6 |
| 8 | Batch | 0.05 | 250 | 0.1 | 10 | 11,751 | 1.07 | 11.70 | 98.5 |
| 9 | Batch | 0.05 | 300 | 0.1 | 2.5 | 5037 | 1.19 | 14.29 | 97.3 |
| 10 | Batch | 0.05 | 300 | 0.1 | 5 | 22,441 | 1.10 | 11.57 | 98.9 |
| 11 | Batch | 0.05 | 300 | 0.1 | 7.5 | 17,270 | 1.00 | 6.32 | 98.9 |
| 12 | Fed-batch cycle 1 | 0.05 | 300 | 0.1 | 10 | 41,242 | 0.67 | 6.47 | 99.5 |
| 12 | Fed-batch cycle 1 | 0.05 | 300 | 0.1 | 10 | 13,512 | 1.05 | 8.97 | 98.4 |
| 12 | Fed-batch cycle 1 | 0.05 | 300 | 0.1 | 10 | 24,175 | 0.95 | 6.09 | 99.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escanciano, I.A.; Ladero, M.; Santos, V.E.; Blanco, Á. Development of a Simple and Robust Kinetic Model for the Production of Succinic Acid from Glucose Depending on Different Operating Conditions. Fermentation 2023, 9, 222. https://doi.org/10.3390/fermentation9030222
Escanciano IA, Ladero M, Santos VE, Blanco Á. Development of a Simple and Robust Kinetic Model for the Production of Succinic Acid from Glucose Depending on Different Operating Conditions. Fermentation. 2023; 9(3):222. https://doi.org/10.3390/fermentation9030222
Chicago/Turabian StyleEscanciano, Itziar A., Miguel Ladero, Victoria E. Santos, and Ángeles Blanco. 2023. "Development of a Simple and Robust Kinetic Model for the Production of Succinic Acid from Glucose Depending on Different Operating Conditions" Fermentation 9, no. 3: 222. https://doi.org/10.3390/fermentation9030222
APA StyleEscanciano, I. A., Ladero, M., Santos, V. E., & Blanco, Á. (2023). Development of a Simple and Robust Kinetic Model for the Production of Succinic Acid from Glucose Depending on Different Operating Conditions. Fermentation, 9(3), 222. https://doi.org/10.3390/fermentation9030222








