Effect of Dioscorea Opposite Waste Supplementation on Antioxidant Capacity, Immune Response and Rumen Microbiome in Weaned Lambs
Abstract
1. Introduction
2. Materials and Methods
2.1. Location and Ethical Considerations
2.2. Animals, Diets and Management
2.3. Diet Sample Collection and Analysis
2.4. Blood Sample Collection and Measurements
2.5. Rumen Fluid Sampling
2.6. DNA Extraction, Library Preparation, and Metagenome Sequencing
2.7. Assembly, Gene Prediction and Gene Catalogue
2.8. Taxonomy Profiles
2.9. Statistical Analysis
3. Results
3.1. Routine Plasma Biochemical Indicators
3.2. Plasma Immune Parameters
3.3. Plasma Antioxidant Index
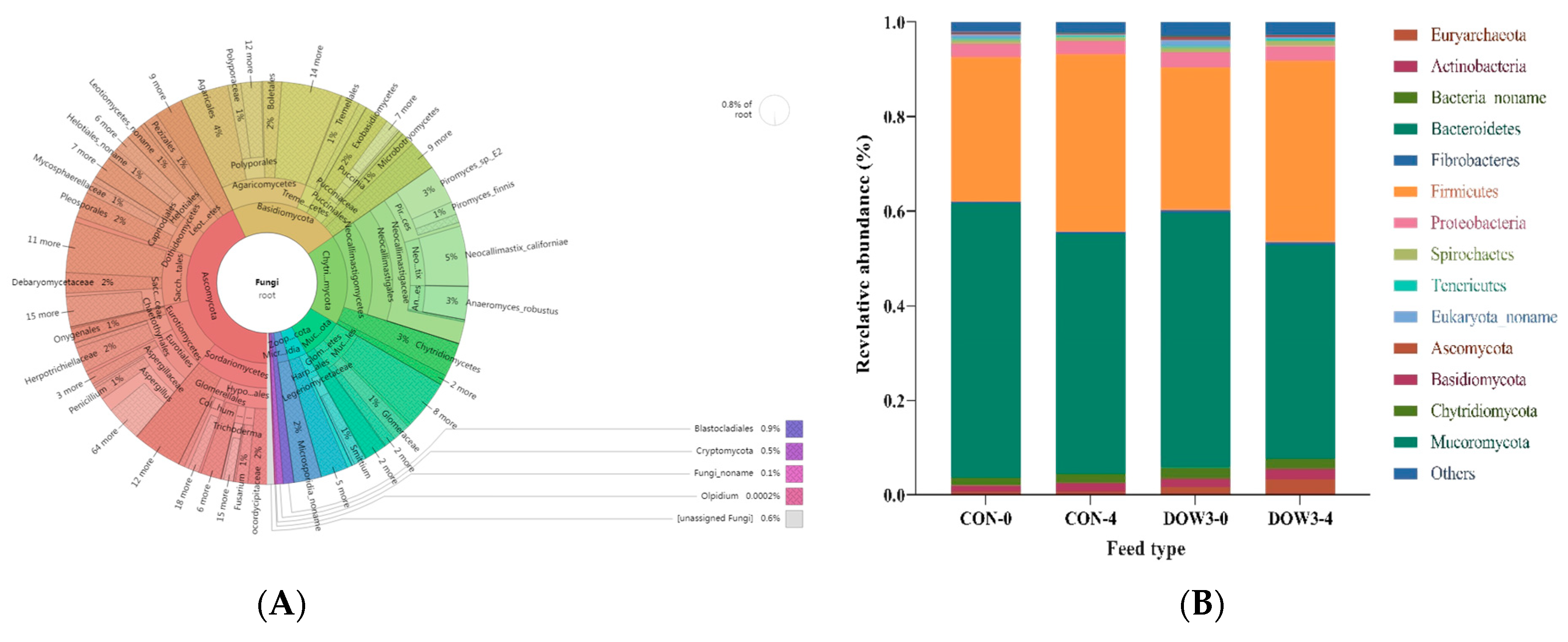
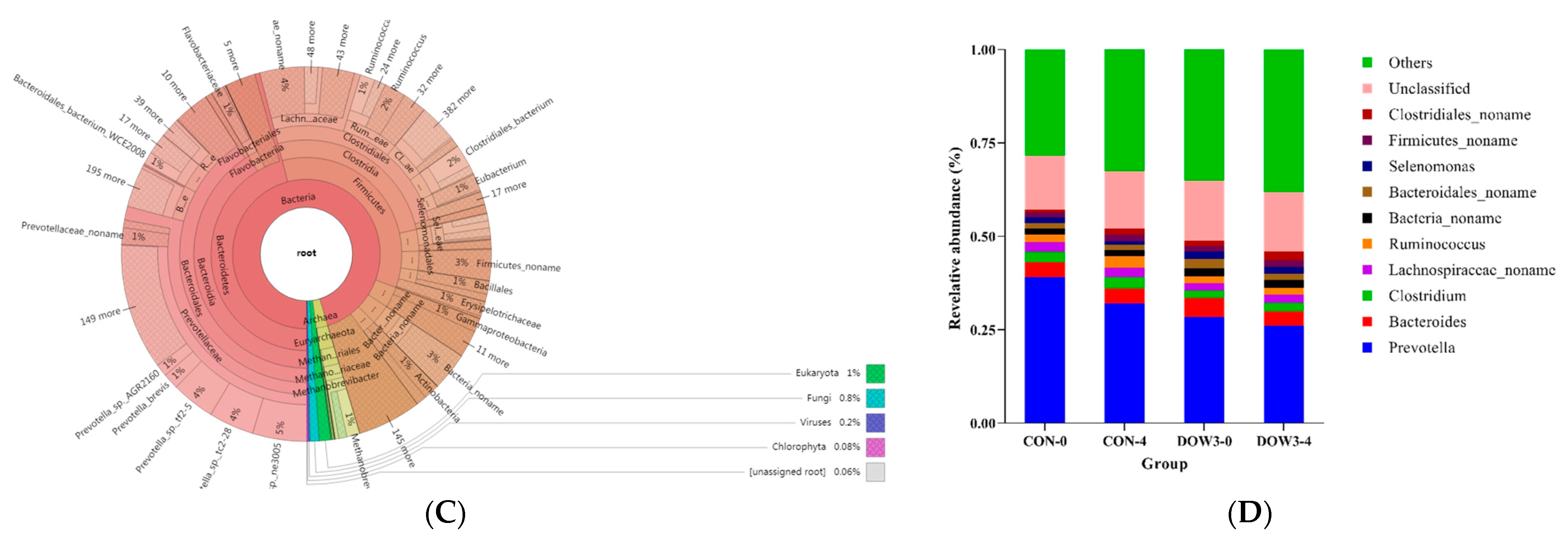
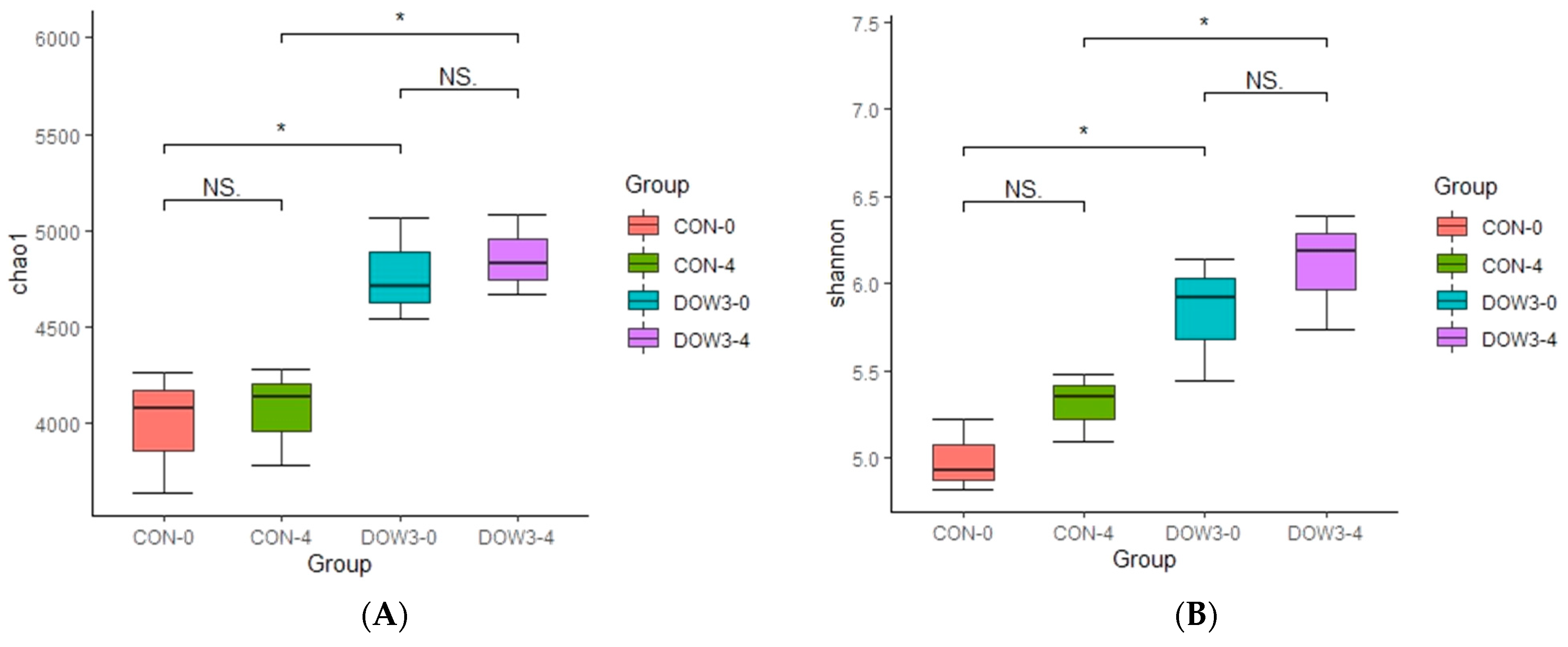
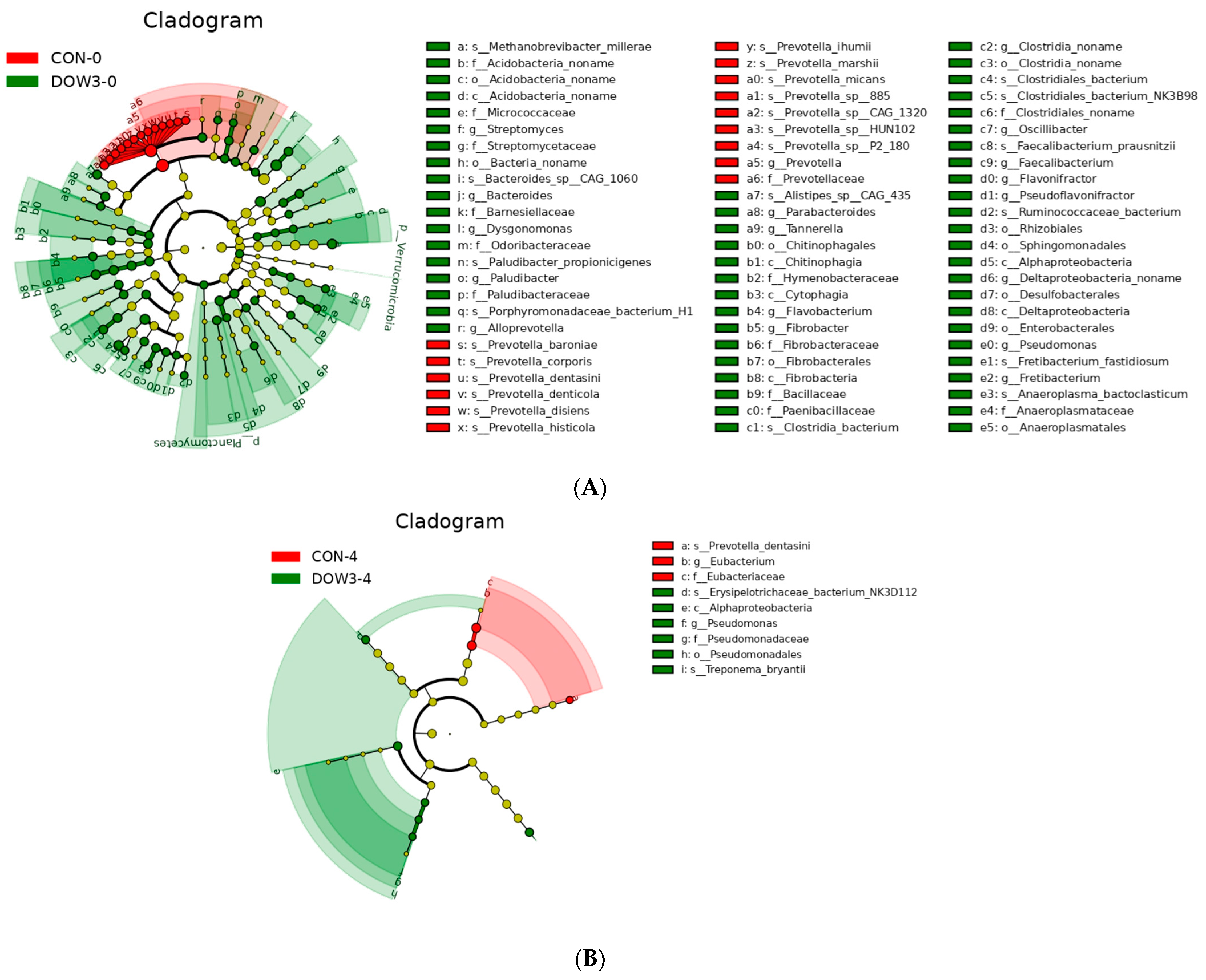
3.4. Rumen Microorganism Profiles
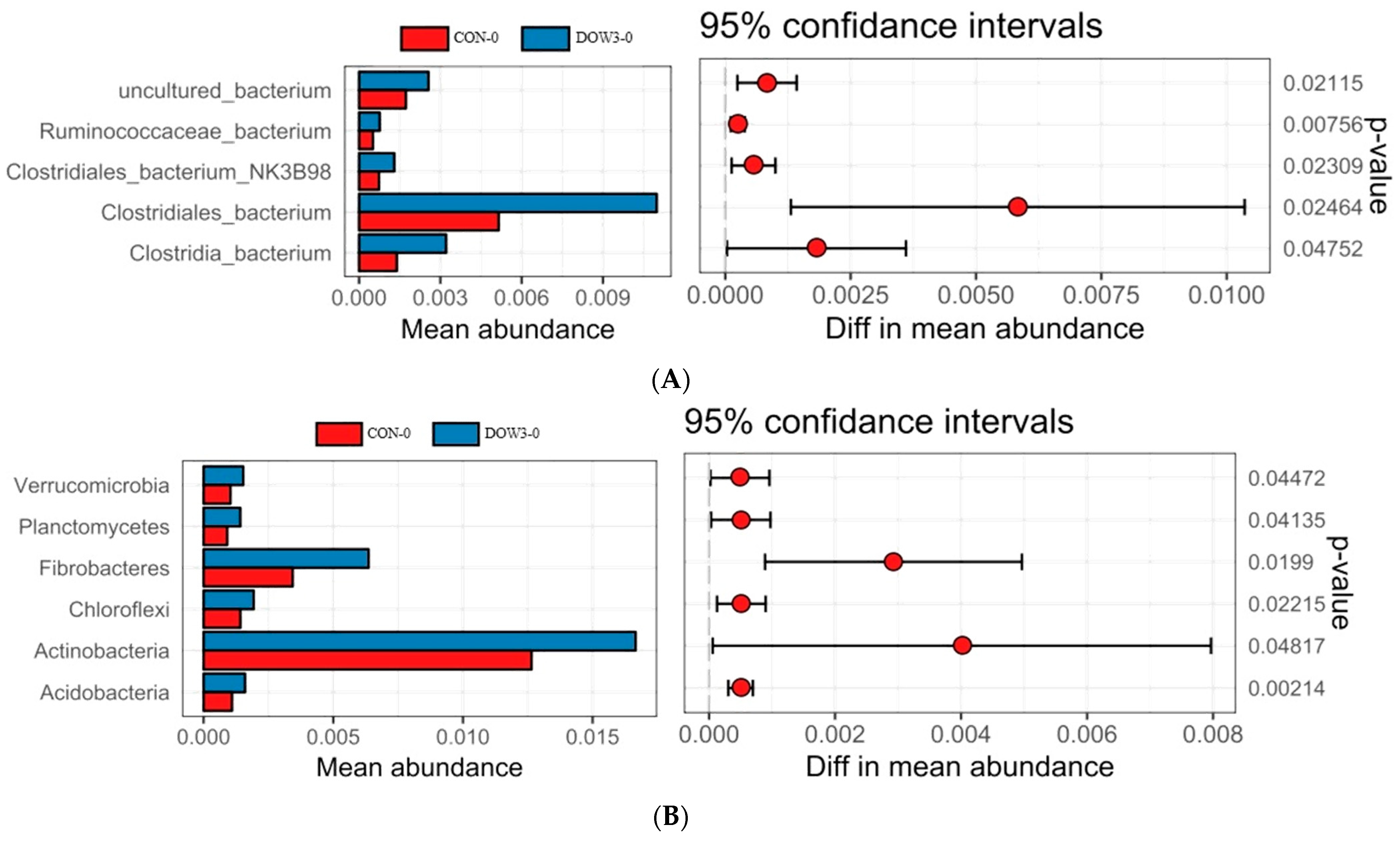
3.5. Composition of Rumen Microbiota Structure
3.6. Mining of Rumen Metagenome for Identification of Cellulolytic Enzymes
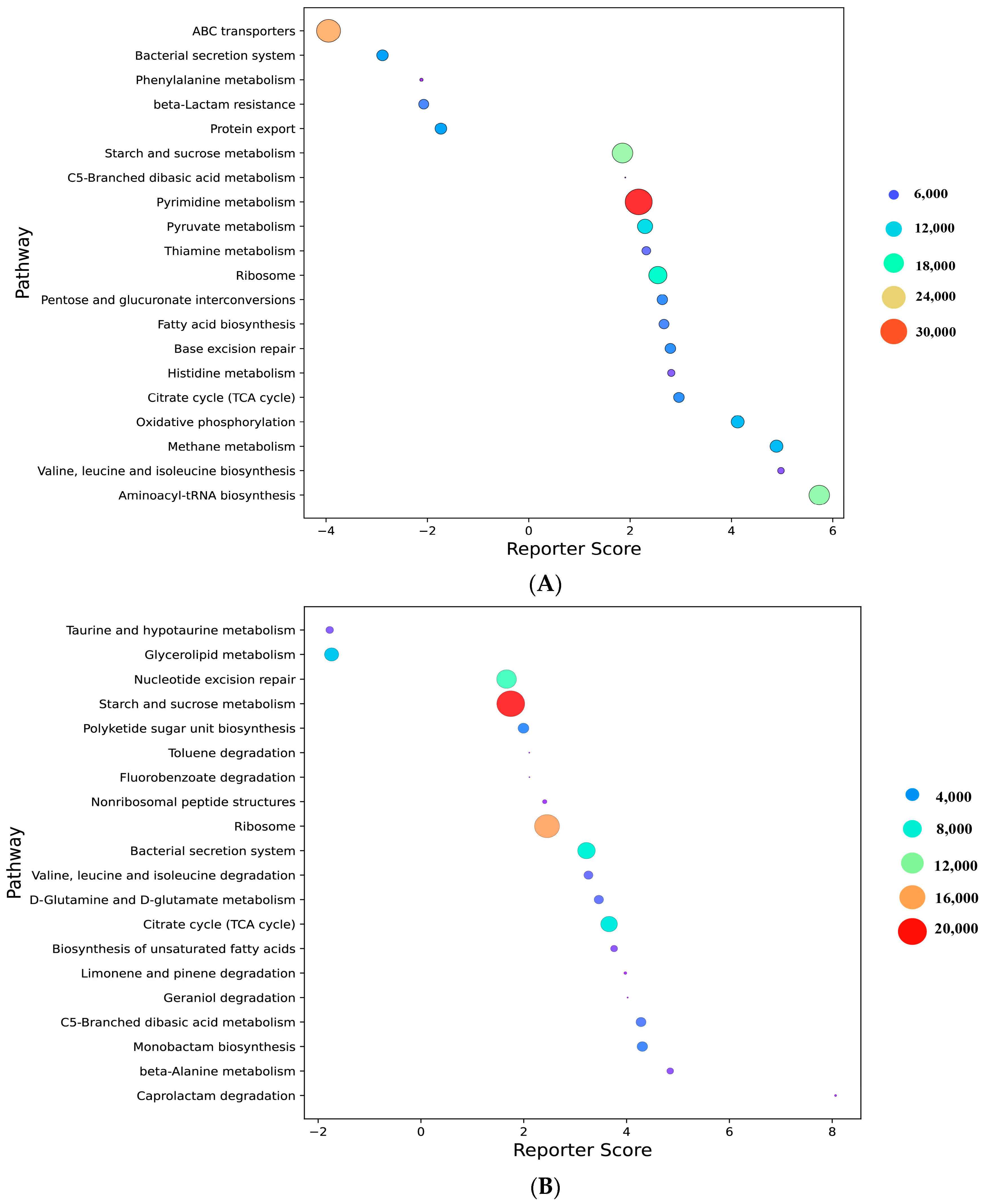
3.7. Metabolic Pathway Analysis of Different Feed Types
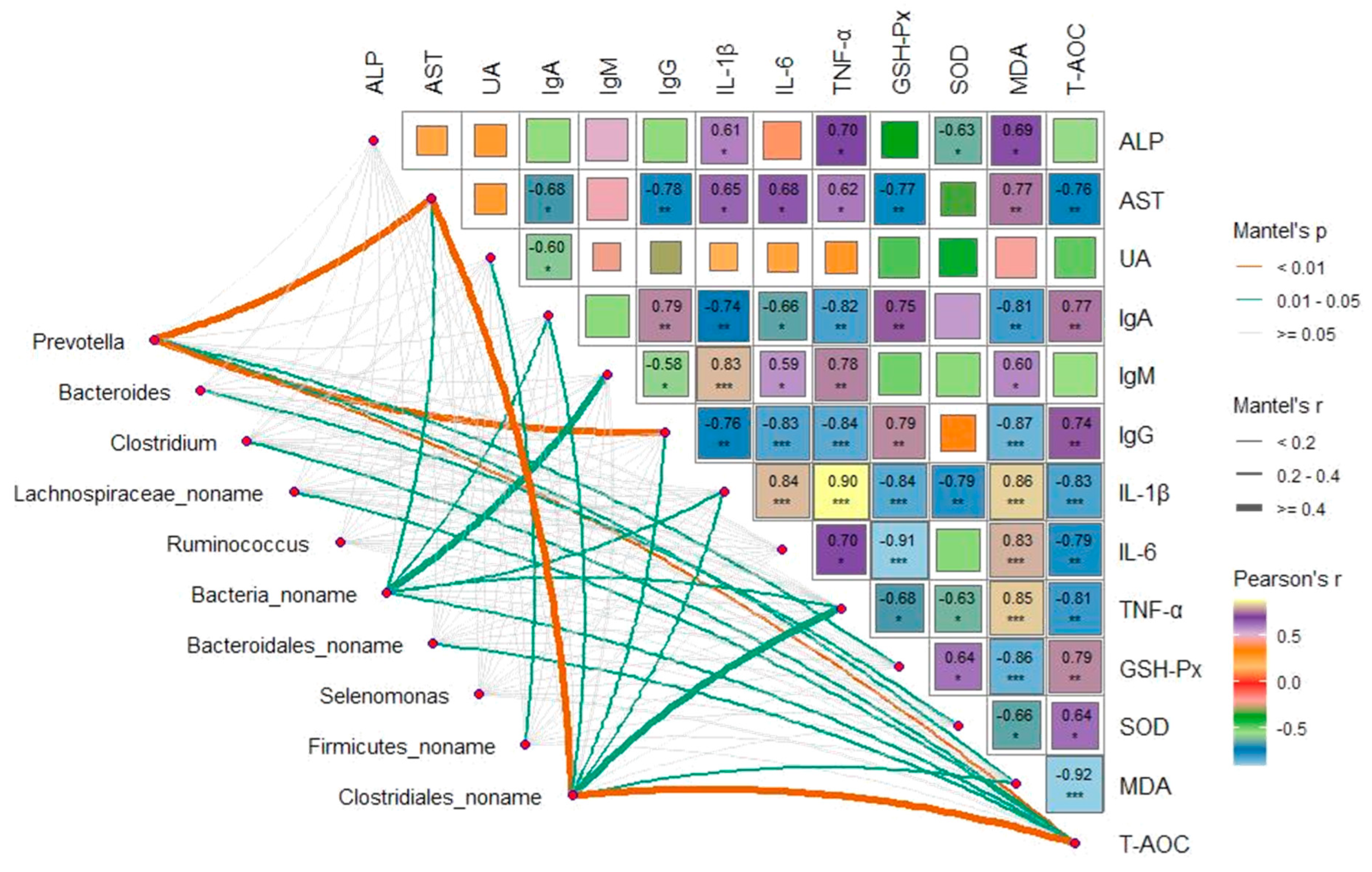
3.8. Correlations Analysis between Bacterial Genera and Plasma Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Shonka-Martin, B.; Heins, B.; Hansen, L. Three-breed rotational crossbreds of Montbéliarde, Viking Red, and Holstein compared with Holstein cows for feed efficiency, income over feed cost, and residual feed intake. J. Dairy Sci. 2019, 102, 3661–3673. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.C.; Wang, X.K.; Li, D.X.; Lin, Y.L.; Yang, F.Y.; Ni, K.K. Impact of wilting and additives on fermentation quality and carbohydrate composition of mulberry silage. Asian-Australas. J. Anim. Sci. 2020, 33, 254. [Google Scholar] [CrossRef]
- He, L.; Zhou, W.; Wang, Y.; Wang, C.; Chen, X.; Zhang, Q. Effect of applying lactic acid bacteria and cellulase on the fermentation quality, nutritive value, tannins profile and in vitro digestibility of Neolamarckia cadamba leaves silage. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1429–1436. [Google Scholar] [CrossRef]
- Salehi, B.; Sener, B.; Kilic, M.; Sharifi-Rad, J.; Naz, R.; Yousaf, Z.; Mudau, F.N.; Fokou, P.V.T.; Ezzat, S.M.; El Bishbishy, M.H. Dioscorea plants: A genus rich in vital nutra-pharmaceuticals—A review. Iran. J. Pharm. Res. 2019, 18, 68. [Google Scholar] [CrossRef]
- Shujun, W.; Jinglin, Y.; Hongyan, L.; Weiping, C. Characterisation and preliminary lipid-lowering evaluation of starch from Chinese yam. Food Chem. 2008, 108, 176–181. [Google Scholar] [CrossRef]
- Shen, L.; Xu, J.; Luo, L. Predicting the potential global distribution of diosgenin-contained Dioscorea species. Chin. Med.-UK 2018, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Zhong, H.; Hu, B. Agro-ecological suitability assessment of Chinese Medicinal Yam under future climate change. Environ. Geochem. Health 2020, 42, 987–1000. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.R.; Lee, J.S.; Lee, C.H.; Kim, J.Y.; Kim, S.D.; Nam, D.H. Effect of ethanol extract of dried Chinese yam (Dioscorea batatas) flour containing dioscin on gastrointestinal function in rat model. Arch. Pharm. Res. 2006, 29, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Das, G.; Shin, H.-S.; Patra, J.K. Dioscorea spp.(a wild edible tuber): A study on its ethnopharmacological potential and traditional use by the local people of Similipal Biosphere Reserve, India. Front. Pharmacol. 2017, 8, 52. [Google Scholar] [CrossRef]
- Zhang, C.Q.; Chen, X.; Ding, K. Structural characterization of a galactan from Dioscorea opposita Thunb. and its bioactivity on selected Bacteroides strains from human gut microbiota. Carbohydr. Polym. 2019, 218, 299–306. [Google Scholar] [CrossRef]
- Sharma, L.N.; Bastakoti, R. Ethnobotany of Dioscorea L. with emphasis on food value in Chepang communities in Dhading district, central Nepal. Bot. Orient. J. Plant Sci. 2009, 6, 12–17. [Google Scholar] [CrossRef]
- Kumar, S.; Mahanti, P.; Rath, S.K.; Patra, J.K. Qualitative phytochemical analysis and antibacterial activity of Dioscorea alata L.: A nutraceutical tuber crops of rural odisha. J. Altern. Med. Res. 2017, 3, 122. [Google Scholar]
- Kong, X.; Zhang, Y.; Wu, X.; Yin, Y.; Tan, Z.; Feng, Y.; Yan, F.; Bo, M.; Huang, R.; Li, T. Fermentation characterization of Chinese yam polysaccharide and its effects on the gut microbiota of rats. Int. J. Microbiol. 2009, 2009, 598152. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Gurjar, S.; Pandey, D.K. Medicinal aspect of saponins shows their wide range of pharmacological/biological activities. Pharmacologyonline 2010, 2, 579–584. [Google Scholar]
- Burkill, H.M. The Useful Plants of West Tropical Africa; University Press of Virginia: Charlottesville, VA, USA, 1995; pp. 1–3. [Google Scholar]
- Zhou, C.; Wu, Y.; Zhang, Y.; Yan, Y. The manufacture and utilization of Chinese yam. Anhui Agric. Sci. Bull. 2004, 10, 65–66. [Google Scholar]
- Baah, F.; Maziya-Dixon, B.; Asiedu, R.; Oduro, I.; Ellis, W. Nutritional and biochemical composition of D. alata (Dioscorea spp.) tubers. J. Food Agric. Environ. 2009, 7, 373–378. [Google Scholar]
- Mayan Hong, J.Y. Optimization of Ultrasonic-assisted Extraction of Polysaccharides from Chinese Yam Peel and Its Antioxidant Activity. Food Res. Dev. 2016, 37, 34–38. (In Chinese) [Google Scholar]
- Lee, S.C.; Tsai, C.C.; Chen, J.C.; Lin, J.G.; Lin, C.C.; Hu, M.L.; Lu, S. Effects of Chinese yam on hepato-nephrotoxicity of acetaminophen in rats. Acta Pharmacol. Sin. 2002, 23, 503–508. [Google Scholar]
- Samonte, R.F. Utilization of Raw Wild Yam (Dioscorea dumetorum) As Feed Supplement for Broiler Chickens. J. Nat. Allied Sci. 2019, 3, 28–35. [Google Scholar]
- Meng, X.; Hu, W.; Wu, S. Chinese yam peel enhances the immunity of the common carp (Cyprinus carpio L.) by improving the gut defence barrier and modulating the intestinal microflora. Fish Shellfish. Immunol. 2019, 95, 528–537. [Google Scholar] [CrossRef]
- Doko Allou, S.; Farougou, S.; Hountondji, F. Impact of prophylactic measures and the use of local food resources on the viability and growth of pre-weaning lambs in Djougou, in the northern region of Benin. J. Anim. Plant Sci. 2013, 19, 2933–2940. Available online: https://www.researchgate.net/publication/258341187 (accessed on 15 February 2023).
- Yashim, S.; Haniel, B.; Makama, S.; Adama, H. Growth Performance, nutrient digestibility and nitrogen balance of red Sokoto bucks fed Irish potato (Solanum tuberosum L.) peels as a replacement for maize offal. J. Anim. Prod. Res. 2016, 28, 245–253. [Google Scholar]
- Zhang, Y.; Shi, H.; Yun, Y. The Effect of Anthocyanins from Dioscorea alata L. on Antioxidant Properties of Perinatal Hainan Black Goats and Its Possible Mechanism in the Mammary Gland. Animals 2022, 12, 3320. [Google Scholar] [CrossRef] [PubMed]
- NRC—National Research Council. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids; National Academy of Science: Washintgton, DC, USA, 2007; 347p. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Bradstreet R, B. Kjeldahl method for organic nitrogen. Anal. Chem. 1954, 26, 185–187. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Oliveira, M.A.; Alves, S.P.; Santos-Silva, J.; Bessa, R.J. Effects of clays used as oil adsorbents in lamb diets on fatty acid composition of abomasal digesta and meat. Anim. Feed Sci. Technol. 2016, 213, 64–73. [Google Scholar] [CrossRef]
- Do, D.T.; Lam, D.H.; Nguyen, T.; Mai, T.T.P.; Phan, L.T.M.; Vuong, H.T.; Nguyen, D.V.; Linh, N.T.T.; Hoang, M.N.; Mai, T.P.; et al. Utilization of response surface methodology in optimization of polysaccharides extraction from vietnamese red ganoderma lucidum by ultrasound-assisted enzymatic method and examination of bioactivities of the extract. Sci. World J. 2021, 1, 11. [Google Scholar] [CrossRef]
- Zhao, K.; Huo, B.; Shen, X. Studies on antioxidant capacity in selenium-deprived the Choko yak in the Shouqu prairie. Biol. Trace Elem. Res. 2021, 199, 3297–3302. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Li, D.; Liu, C.-M.; Luo, R.; Sadakane, K.; Lam, T.W. MEGAHIT: An ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef]
- Zhu, W.; Lomsadze, A.; Borodovsky, M. Ab initio gene identification in metagenomic sequences. Nucleic Acids Res. 2010, 38, e132. [Google Scholar] [CrossRef]
- Li, W.; Godzik, A. Cd-hit: A fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 2006, 22, 1658–1659. [Google Scholar] [CrossRef] [PubMed]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Menzel, P.; Ng, K.L.; Krogh, A. Fast and sensitive taxonomic classification for metagenomics with Kaiju. Nat. Commun. 2016, 7, 11257. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhang, H.; Zhang, P.; Zhang, G.; Cai, Y.; Wang, Q.; Zhou, Z.; Ding, Y.; Zubair, M. Effect of substrate load on anaerobic fermentation of rice straw with rumen liquid as inoculum: Hydrolysis and acidogenesis efficiency, enzymatic activities and rumen bacterial community structure. Waste Manag. 2021, 124, 235–243. [Google Scholar] [CrossRef]
- Geng, A.; Zhang, Y.; Zhang, J.; Zeng, L.; Chang, C.; Wang, H.; Yan, Z.; Chu, Q.; Liu, H. Effects of light regime on the hatching performance, body development and serum biochemical indexes in Beijing You Chicken. Poultry Sci. 2021, 100, 101270. [Google Scholar] [CrossRef]
- Ma, J.; Wang, C.; Wang, Z.; Cao, G.; Hu, R.; Wang, X.; Zou, H.; Kang, K.; Peng, Q.; Xue, B. Active dry yeast supplementation improves the growth performance, rumen fermentation, and immune response of weaned beef calves. Anim. Nutr. 2021, 7, 1352–1359. [Google Scholar] [CrossRef]
- Vranković, L.; Aladrović, J.; Ljubić, B.B.; Pipal, I.; Prvanović-Babić, N.; Mašek, T.; Stojević, Z. Blood biochemical parameters of bone metabolism in cows and calves kept in a beef suckler system during the early postpartum period. Livest. Sci. 2018, 211, 8–13. [Google Scholar] [CrossRef]
- Amat, N.; Amat, R.; Abdureyim, S.; Hoxur, P.; Osman, Z.; Mamut, D.; Kijjoa, A. Aqueous extract of dioscorea opposita thunb normalizes the hypertension in 2K1C hypertensive rats. BMC Complement. Altern. Med. 2014, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, X.; Liu, C.; Li, J. The degradation, antioxidant and antimutagenic activity of the mucilage polysaccharide from Dioscorea opposita. Carbohydr. Polym. 2016, 150, 227–231. [Google Scholar] [CrossRef]
- Chen, G.L.; Wei, W.; Xu, S.Y. Effect and mechanism of total saponin of Dioscorea on animal experimental hyperuricemia. Am. J. Chin. Med. 2006, 34, 77–85. [Google Scholar] [CrossRef]
- Xu, L.Q.; Zhao, X.X.; Wang, P.X.; Yang, J.; Yang, Y.M. Multidisciplinary treatment of a patient with necrotizing fasciitis caused by Staphylococcus aureus: A case report. World J. Clin. Cases 2019, 7, 3595. [Google Scholar] [CrossRef]
- Fu, L.; Sun, M.; Dong, W.; Zhang, G.; Han, D.; Zang, J.; Liu, H. Effects of compound of hawthorn (Crataegus pinnatifida) and Chinese yam (Dioscorea opposita Thunb.) extracts on growth performance, intestinal health, and immune function in weaned pigs. Anim. Sci. J. 2022, 93, e13790. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Li, R.; Xu, L.; Yin, L.; Xu, Y.; Han, X.; Peng, J. Neuroprotective effect of dioscin on the aging brain. Molecules 2019, 24, 1247. [Google Scholar] [CrossRef]
- Bandyopadhyay, B.; Mitra, P.K.; Mandal, V.; Mandal, N.C. Novel fructooligosaccharides of Dioscorea alata L. tuber have prebiotic potentialities. Eur. Food Res. Technol. 2021, 247, 3099–3112. [Google Scholar] [CrossRef]
- Lombardi, P.; Musco, N.; Cutrignelli, M.I.; Mollica, M.P.; Trinchese, G.; Calabro, S.; Tudisco, R.; Grossi, M.; Mastellone, V.; Vassalotti, G.; et al. The association of aloe and beta-carotene supplementation improves oxidative stress and inflammatory state inn pregnant buffalo cows. Buffalo Bull. 2017, 36, 497–503. [Google Scholar]
- Akhalaya, M.Y.; Platonov, A.; Baizhumanov, A. Short-term cold exposure improves antioxidant status and general resistance of animals. Bull. Exp. Biol. Med. 2006, 141, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Marin, D.; Pistol, G.; Gras, M.; Palade, M.; Taranu, I. A comparison between the effects of ochratoxin A and aristolochic acid on the inflammation and oxidative stress in the liver and kidney of weanling piglets. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2018, 391, 1147–1156. [Google Scholar] [CrossRef]
- Yang, M.; Chen, R.; Song, Y.; Zhou, Y.; Liu, Q.; Zhuang, S. Effects of dietary betaine supplementation on growth performance, meat quality, muscle fatty acid composition and antioxidant ability in slow-growing broiler chickens. Br. Poult. Sci. 2021, 63, 351–359. [Google Scholar] [CrossRef]
- Sakthidevi, G.; Mohan, V. Total phenolic, flavonoid contents and in vitro antioxidant activity of Dioscorea alata L. tuber. J. Pharm. Sci. Res. 2013, 5, 115–119. [Google Scholar]
- Ju, Y.; Xue, Y.; Huang, J.; Zhai, Q.; Wang, X.-H. Antioxidant Chinese yam polysaccharides and its pro-proliferative effect on endometrial epithelial cells. Int. J. Biol. Macromol. 2014, 66, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, X.; Wu, J. Rumen-protected glucose supplementation in transition dairy cows shifts fermentation patterns and enhances mucosal immunity. Anim. Nutr. 2021, 7, 1182–1188. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Wu, J.; Wang, M. Rhubarb supplementation promotes intestinal mucosal innate immune homeostasis through modulating intestinal epithelial microbiota in goat kids. J. Agric. Food Chem. 2018, 66, 1047–1057. [Google Scholar] [CrossRef]
- Zhang, N.; Liang, T.; Jin, Q.; Shen, C.; Zhang, Y.; Jing, P. Chinese yam (Dioscorea opposita Thunb.) alleviates antibiotic-associated diarrhea, modifies intestinal microbiota, and increases the level of short-chain fatty acids in mice. Food Res. Int. 2019, 122, 191–198. [Google Scholar] [CrossRef]
- Aswani, M.A.; Kathade, S.A.; Nisar, A.; Anand, P.K.; Kunchiraman, B.N.; Jagtap, S.D. Prebiotic Profiling of Indigenous selected dioscorea Spp. Using In-Vitro Techniques. Biosci. Biotechnol. Res. Asia 2022, 19, 387–394. [Google Scholar] [CrossRef]
- Gharechahi, J.; Zahiri, H.S.; Noghabi, K.A.; Salekdeh, G.H. In-depth diversity analysis of the bacterial community resident in the camel rumen. Syst. Appl. Microbiol. 2015, 38, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J.; Bayer, E.A.; Rincon, M.T.; Lamed, R.; White, B.A. Polysaccharide utilization by gut bacteria: Potential for new insights from genomic analysis. Nat. Rev. Microbiol. 2008, 6, 121–131. [Google Scholar] [CrossRef]
- Jewell, K.A.; Scott, J.J.; Adams, S.M.; Suen, G. A phylogenetic analysis of the phylum Fibrobacteres. Syst. Appl. Microbiol. 2013, 36, 376–382. [Google Scholar] [CrossRef]
- Thoetkiattikul, H.; Mhuantong, W.; Laothanachareon, T.; Tangphatsornruang, S.; Pattarajinda, V.; Eurwilaichitr, L.; Champreda, V. Comparative analysis of microbial profiles in cow rumen fed with different dietary fiber by tagged 16S rRNA gene pyrosequencing. Curr. Microbiol. 2013, 67, 130–137. [Google Scholar] [CrossRef]
- Liang, J.; Raza, S.H.A.; Kou, S.; Chen, C.; Yao, M.; Wu, Y.; Wang, S.; Ma, X.; Zhang, W.-J.; Nie, C. Effect of Clostridium butyricum on plasma immune function, antioxidant activity and metabolomics of weaned piglets. Livest. Sci. 2020, 241, 104267. [Google Scholar] [CrossRef]
- Bohra, V.; Dafale, N.A.; Purohit, H.J. Understanding the alteration in rumen microbiome and CAZymes profile with diet and host through comparative metagenomic approach. Arch. Microbiol. 2019, 201, 1385–1397. [Google Scholar] [CrossRef]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The Carbohydrate-Active EnZymes database (CAZy): An expert resource for glycogenomics. Nucleic Acids Res. 2009, 37, D233–D238. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.D.; Auffret, M.D.; Warr, A.; Wiser, A.H.; Press, M.O.; Langford, K.W.; Liachko, I.; Snelling, T.J.; Dewhurst, R.J.; Walker, A.W. Assembly of 913 microbial genomes from metagenomic sequencing of the cow rumen. Nat. Commun. 2018, 9, 870. [Google Scholar] [CrossRef]
- Jose, V.L.; Appoothy, T.; More, R.P.; Arun, A.S. Metagenomic insights into the rumen microbial fibrolytic enzymes in Indian crossbred cattle fed finger millet straw. AMB Express 2017, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Lairson, L.; Henrissat, B.; Davies, G.; Withers, S. Glycosyltransferases: Structures, functions, and mechanisms. Annu. Rev. Biochem. 2008, 77, 521–555. [Google Scholar] [CrossRef] [PubMed]
- Boraston, A.B.; Bolam, D.N.; Gilbert, H.J.; Davies, G.J. Carbohydrate-binding modules: Fine-tuning polysaccharide recognition. Biochem. J. 2004, 382, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Krause, D.O.; Denman, S.E.; Mackie, R.I.; Morrison, M.; Rae, A.L.; Attwood, G.T.; McSweeney, C.S. Opportunities to improve fiber degradation in the rumen: Microbiology, ecology, and genomics. FEMS Microbiol. Rev. 2003, 27, 663–693. [Google Scholar] [CrossRef]
- Wang, L.J.; Zhang, G.N.; Xu, H.J.; Xin, H.S.; Zhang, Y.G. Metagenomic Analyses of Microbial and Carbohydrate-Active Enzymes in the Rumen of Holstein Cows Fed Different Forage-to-Concentrate Ratios. Front. Microbiol. 2019, 10, 649. [Google Scholar] [CrossRef]




| Items | Treatment | |||
|---|---|---|---|---|
| CON | DOW1 | DOW2 | DOW3 | |
| Ingredient | ||||
| Peanut Vine | 23.06 | 23.22 | 23.22 | 23.22 |
| DOW | 0.00 | 10.00 | 15.00 | 20.00 |
| Corn grain | 40.65 | 35.56 | 31.85 | 27.00 |
| Soybean meal | 14.97 | 9.74 | 8.61 | 8.46 |
| Concentrate supplement | 15.10 | 15.26 | 15.10 | 15.10 |
| Premix | 4.00 | 4.00 | 4.00 | 4.00 |
| NH4Cl | 0.74 | 0.74 | 0.74 | 0.74 |
| Baking Soda | 0.74 | 0.74 | 0.74 | 0.74 |
| Salt | 0.74 | 0.74 | 0.74 | 0.74 |
| Chemical Composition | ||||
| ME, MJ/kg | 12.01 | 12.01 | 11.80 | 11.90 |
| CP | 16.70 | 16.7 | 16.7 | 16.7 |
| Ca, g/kg | 4.58 | 4.40 | 4.42 | 4.50 |
| P, g/kg | 2.84 | 2.29 | 2.55 | 3.08 |
| Chemical Composition | Concentration |
|---|---|
| Dry matter, % as fresh matter | 12 |
| Crude protein | 11.8 |
| ADF | 15.9 |
| NDF | 25.43 |
| Starch | 52.5 |
| EE | 2.8 |
| Ash | 2.62 |
| Polysaccharides | 20.39 |
| DE, MJ/kg | 0.79 |
| Ca | 0.41 |
| P | 0.41 |
| K | 0.09 |
| Item | Treatments | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | DOW1 | DOW2 | DOW3 | T | L | Q | ||
| ALT, U/L | 17.33 | 14.67 | 14.25 | 12.00 | 0.551 | 1.701 | 0.06 | 0.674 |
| ALP, U/L | 188.00 a | 156.67 ab | 149.75 b | 142.75 b | 11.08 | 0.041 | 0.035 | 0.207 |
| AST, U/L | 104.25 a | 99.67 a | 93.75 a | 75.00 b | 5.473 | 0.015 | 0.002 | 0.538 |
| GGT, U/L | 77.40 | 69.40 | 72.40 | 71.80 | 4.260 | 0.613 | 0.543 | 0.418 |
| TBIL | 0.460 | 0.260 | 0.280 | 0.380 | 0.079 | 0.284 | 0.752 | 0.079 |
| TP, g/L | 79.40 | 81.20 | 83.20 | 86.20 | 3.129 | 0.478 | 0.127 | 0.927 |
| ALB, g/L | 28.20 | 26.20 | 29.60 | 26.75 | 1.393 | 0.353 | 0.753 | 0.562 |
| TG, g/L | 0.650 | 0.560 | 0.700 | 0.636 | 0.071 | 0.619 | 0.821 | 0.907 |
| Cre, μmol/L | 170.5 | 174.2 | 175.0 | 174.6 | 1.037 | 4.051 | 0.554 | 0.588 |
| UA, μmol/L | 301.0 | 298.5 | 278.3 | 271.25 | 9.835 | 0.219 | 0.049 | 0.706 |
| GLU, mmol/L | 6.010 | 5.790 | 5.322 | 5.154 | 0.263 | 0.116 | 0.079 | 0.966 |
| HDL, mmol/L | 2.870 | 2.776 | 2.728 | 2.510 | 0.164 | 0.474 | 0.151 | 0.828 |
| LDL, mmol/L | 2.706 | 2.594 | 2.428 | 2.398 | 0.202 | 0.680 | 0.274 | 0.658 |
| CHO, mmol/L | 4.252 | 4.098 | 3.830 | 3.672 | 0.225 | 0.298 | 0.071 | 0.701 |
| Item | Treatments | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | DOW1 | DOW2 | DOW3 | T | L | Q | ||
| IgA (μg/mL) | 359.1 c | 408.0 b | 448.2 a | 470.3 a | 11.01 | <0.001 | <0.001 | 0.014 |
| IgM (mg/mL) | 2.049 b | 2.227 b | 2.704 a | 2.789 a | 0.070 | <0.001 | <0.001 | 0.011 |
| IgG (mg/mL) | 29.07 c | 38.19 b | 40.07 b | 55.81 a | 1.591 | <0.001 | <0.001 | 0.641 |
| IL-1β (pg/mL) | 63.67 a | 50.42 b | 37.82 c | 36.39 c | 2.077 | <0.001 | <0.001 | <0.001 |
| IL-6 (pg/mL) | 95.95 a | 84.08 a | 44.46 b | 36.38 b | 3.83 | <0.001 | <0.001 | 0.007 |
| TNF-α (pg/mL) | 132.3 a | 93.70 b | 92.16 b | 57.01 c | 4.20 | <0.001 | <0.001 | 0.104 |
| Item | Treatments | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | DOW1 | DOW2 | DOW3 | T | L | Q | ||
| GSH-Px (nmol/mL) | 116.7 c | 149.5 b | 246.8 a | 268.4 a | 8.542 | <0.001 | <0.001 | 0.001 |
| SOD (U/mL) | 138.2 c | 250.7 b | 279.6 b | 389.4 a | 16.86 | <0.001 | 0.001 | <0.001 |
| MDA (nmol/mL) | 3.893 a | 3.101 b | 2.517 c | 1.627 d | 0.142 | <0.001 | <0.001 | 0.116 |
| T-AOC (μmol/mL) | 0.307 d | 0.391 c | 0.455 b | 0.513 a | 0.013 | <0.001 | <0.001 | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, R.; Guo, Y.; Zhang, S.; Hao, Q.; Duan, C.; Wang, Y.; Ji, S.; Yan, H.; Zhang, Y.; Liu, Y. Effect of Dioscorea Opposite Waste Supplementation on Antioxidant Capacity, Immune Response and Rumen Microbiome in Weaned Lambs. Fermentation 2023, 9, 256. https://doi.org/10.3390/fermentation9030256
Yang R, Guo Y, Zhang S, Hao Q, Duan C, Wang Y, Ji S, Yan H, Zhang Y, Liu Y. Effect of Dioscorea Opposite Waste Supplementation on Antioxidant Capacity, Immune Response and Rumen Microbiome in Weaned Lambs. Fermentation. 2023; 9(3):256. https://doi.org/10.3390/fermentation9030256
Chicago/Turabian StyleYang, Ruochen, Yunxia Guo, Shuo Zhang, Qinghong Hao, Chunhui Duan, Yong Wang, Shoukun Ji, Hui Yan, Yingjie Zhang, and Yueqin Liu. 2023. "Effect of Dioscorea Opposite Waste Supplementation on Antioxidant Capacity, Immune Response and Rumen Microbiome in Weaned Lambs" Fermentation 9, no. 3: 256. https://doi.org/10.3390/fermentation9030256
APA StyleYang, R., Guo, Y., Zhang, S., Hao, Q., Duan, C., Wang, Y., Ji, S., Yan, H., Zhang, Y., & Liu, Y. (2023). Effect of Dioscorea Opposite Waste Supplementation on Antioxidant Capacity, Immune Response and Rumen Microbiome in Weaned Lambs. Fermentation, 9(3), 256. https://doi.org/10.3390/fermentation9030256




