Microalgal Feedstock for Biofuel Production: Recent Advances, Challenges, and Future Perspective
Abstract
:1. Introduction
2. Microalgal Biofuels
2.1. Bioethanol
2.2. Biodiesel
2.3. Biocrude Oil
2.4. Pyrolysis Oil
2.5. Bio-Jet Fuel
2.6. Biomethane
| Microalgae Feedstock | Pretreatment Process Parameters | Biomethanation Process Conditions | Biomethane Yield (mL/g VS) | Reference |
|---|---|---|---|---|
| Chlorella pyrenoidosa | Hydrolysis by hydrothermal pretreatment using a parabolic solar thermal system | The feed flow rate of 40 L/h, retention time 30 min and mass fraction 1% | 348 | [116] |
| Chlorella vulgaris | Working vol 2.8 L, pretreated at 85, 55 °C, total hydraulic retention time–6 days. | Mesophilic anaerobic digestion @ 35 °C | 239.3 | [117] |
| Chlamydomonas reinhardtii CC-1690 | Replete Nitrogen and low Nitrogen | 38 °C, hydraulic retention time 20 days, organic loading rate 4 g VS/d | 464 | [118] |
| Chlorella sp. | Pretreated at 60–80 °C, for 5 to 10 min | Mesophilic temperature 35 °C, for 46 d | 252 | [119] |
| Scenedesmus sp. | Nitrogen and Phosphorus depleted | 37 °C | 320 | [120] |
2.7. Biohydrogen
| Microalgae Feedstock | Pretreatment Process Parameters | Fermentation Process Conditions | Biohydrogen Yield | Reference |
|---|---|---|---|---|
| Chlorella vulgaris | Enzymatic, 60 h | pH 7.4, 3 N KOH, 35 °C, 150 rpm | 43 mL g−1 | [140] |
| Mixed microalgae consortia | 121 °C, autoclave | N2 sparging, 220 rpm, 30 °C, 8h | 56.8 mL g−1 VS | [141] |
| Tetraspora sp. | 140 rpm, 36 °C, 7.2 pH | 140 rpm, 36 °C, 29 µmol photons m−1 s−1, 18 h | 512 mL L−1 | [142] |
| Chlorella sp. | 1.5% hydrochloric acid, 121 °C, 20 min | pH 7, 37 °C, 9 g reducing sugar/L | 1276 mL L−1 | [143] |
| Chlamydomonas sp. | 3 h dark adaptation | 25 °C, 4.5–9 h. | 8.73 L kg−1 | [144] |
3. Comparative Net Energy Ratio and Carbon Dioxide Emission from Different Microalgal Biofuels
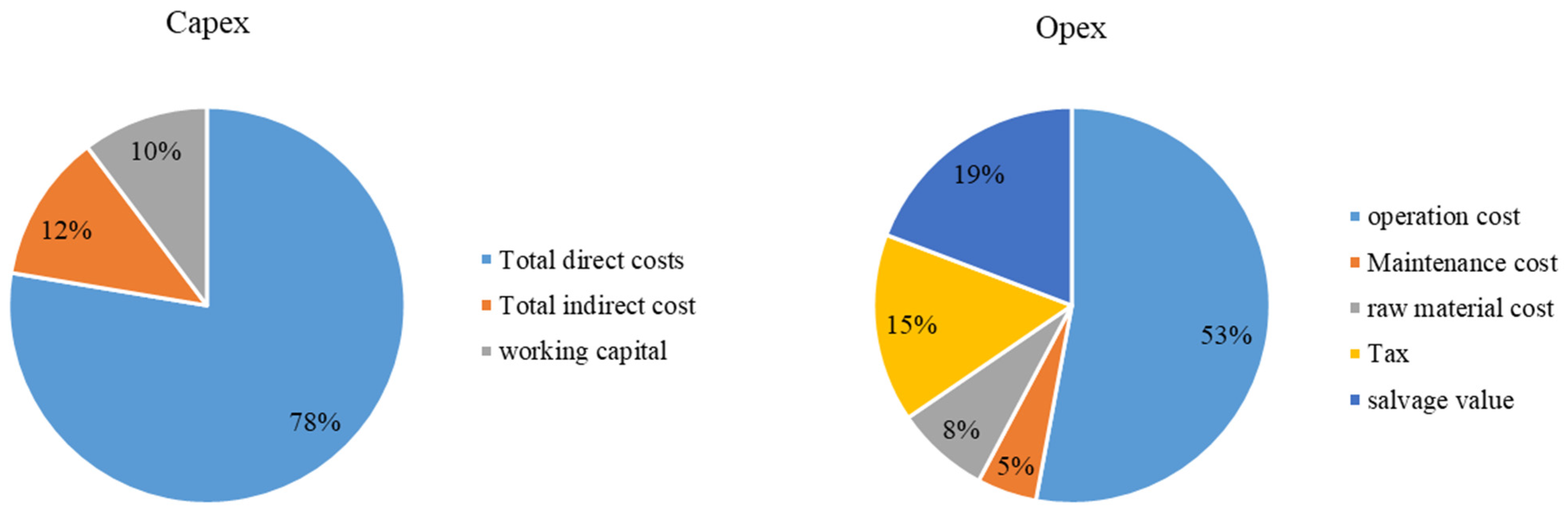
4. Cost Analysis of Various Biofuels Produced from the Microalgal Feedstock
4.1. Microalgal Biodiesel
4.2. Microalgal Biojet Fuel

| Biofuel from Microalgae | Microalgal Strain | Strain Habitat | TEA Model | Production Cost US$ per Unit Fuel | Commercial Fuel Cost US$ per Unit Fuel | Reference |
|---|---|---|---|---|---|---|
| Biodiesel | Nannochloropsis salina | Marine | Simulation model using (ASPEN plus V.12) for 5.4 m3/day biodiesel, cultivation area 153 ha. | 0.77 L−1 | 1.15 L−1 | [159] |
| Biodiesel | Chlorella vulgaris | Freshwater | SAFEER model, 5 to 50 ha scale ORP cultivation of Chlorella vulgaris | 0.8–3.5 L−1 | 1.15 L−1 | [162,163] |
| Bioethanol | Chlorella vulgaris | Freshwater | Model for producing 24.9 m3 ha−1 yr−1 | 19.45 gal−1 | 2.718 gal−1 | [164] |
| Bioethanol | Microalgae | - | Algenol LLC, photobioreactors, 8000 gal/acre/yr | 1.3 gal−1 | 2.718 gal−1 | [165,166] |
| Biohydrogen | Chlamydomonas sp. | Marine | Photobioreactor | 0.57–13.53 kg−1 | 2–8 kg−1 | [167] |
| Biohydrogen | Model microalgae | - | Photobioreactor | 2.13 kg−1 | 2–8 kg−1 | [168,169] |
| Biomethane | Cyanothecae BG0011 | Marine | Modeled at 80,300 kg/h/yr, with biomethane purification using ASPEN V 8.8 | 0.55 m−3 or 14.8/MMbtu | 0.25 to 2.7 m−3 | [170,171] |
| Biomethane | Spirulina sp. | Freshwater | - | 0.3 m−3 | 0.25 to 2.7 m−3 | [172] |
| Biocrude oil | Microalgae powder | ASPEN plus simulation | 2.2 L−1 | 0.48–0.53 L−1 | [173,174] | |
| Biocrude oil | Lipid-extracted algae—Nannochloropsis salina | marine | ASPEN plus for HTL processes | 0.7 L−1 | 0.48–0.53 L−1 | [175] |
| Pyrolysis oil | Chlorella vulgaris | Freshwater | A model to process 2000 metric tonnes of biomass to produce 21.4 million gallons of pyrolysis oil | 1.48–1.8 L−1 | 0.71 L−1 | [176,177] |
| Pyrolysis oil | Microalga | Centrate wastewater | - | 0.58 L−1 | 0.71 L−1 | [178] |
| Biojet fuel | Nannochloropsis sp. | Marine | - | 5.89 L−1 | 0.9 L−1 | [161,179] |
| Biojet fuel | Nannochloropsis sp. | Marine | - | 8.45 L−1 | 0.9 L−1 | [160] |
4.3. Microalgal Biohydrogen
4.4. Microalgal Biomethane
4.5. Microalgal Biocrude Oil
4.6. Microalgal Pyrolysis Oil
4.7. Microalgal Bioethanol
5. Recent Advances, Challenges, and Future Directions for Microalgae-Based Biofuels
5.1. Microalgal Biodiesel
5.2. Microalgal Bioethanol
5.3. Microalgal Biomethane
5.4. Microalgal Biocrude Oil
5.5. Microalgal Biohydrogen
5.6. Microalgal Pyrolysis Oil
5.7. Microalgal Biojet Fuel
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martínez, D.M.; Ebenhack, B.W. Understanding the role of energy consumption in human development through the use of saturation phenomena. Energy Policy 2008, 36, 1430–1435. [Google Scholar] [CrossRef]
- Venkata Subhash, G.; Rajvanshi, M.; Raja Krishna Kumar, G.; Shankar Sagaram, U.; Prasad, V.; Govindachary, S.; Dasgupta, S. Challenges in microalgal biofuel production: A perspective on techno economic feasibility under biorefinery stratagem. Bioresour. Technol. 2022, 343, 126155. [Google Scholar] [CrossRef] [PubMed]
- Plantinga, A.; Scholtens, B. The financial impact of fossil fuel divestment. Clim. Policy 2021, 21, 107–119. [Google Scholar] [CrossRef]
- Djandja, O.S.; Wang, Z.; Chen, L.; Qin, L.; Wang, F.; Xu, Y.; Duan, P. Progress in Hydrothermal Liquefaction of Algal Biomass and Hydrothermal Upgrading of the Subsequent Crude Bio-Oil: A Mini Review. Energy Fuels 2020, 34, 11723–11751. [Google Scholar] [CrossRef]
- Vats, G.; Mathur, R. A net-zero emissions energy system in India by 2050: An exploration. J. Clean. Prod. 2022, 352, 131417. [Google Scholar] [CrossRef]
- Zhai, H.; Gu, B.; Zhu, K.; Huang, C. Feasibility analysis of achieving net-zero emissions in China’s power sector before 2050 based on ideal available pathways. Environ. Impact Assess. Rev. 2023, 98, 106948. [Google Scholar] [CrossRef]
- Nayak, S.; Goveas, L.C.; Selvaraj, R.; Vinayagam, R.; Manickam, S. Advances in the utilisation of carbon-neutral technologies for a sustainable tomorrow: A critical review and the path forward. Bioresour. Technol. 2022, 364, 128073. [Google Scholar] [CrossRef] [PubMed]
- van Loo, S.; Koppejan, J. The Handbook of Biomass Combustion and Co-Firing; Routledge: Oxford, UK, 2012; ISBN 9781136553783. [Google Scholar]
- Qian, X.; Xue, J.; Yang, Y.; Lee, S.W. Thermal properties and combustion-related problems prediction of agricultural crop residues. Energies 2021, 14, 4619. [Google Scholar] [CrossRef]
- Ma, Z.; Cheah, W.Y.; Ng, I.-S.; Chang, J.-S.; Zhao, M.; Show, P.L. Microalgae-based biotechnological sequestration of carbon dioxide for net zero emissions. Trends Biotechnol. 2022, 40, 1439–1453. [Google Scholar] [CrossRef]
- Barthelmie, R.J.; Pryor, S.C. Potential contribution of wind energy to climate change mitigation. Nat. Clim. Chang. 2014, 4, 684–688. [Google Scholar] [CrossRef]
- Ashokkumar, V.; Venkatkarthick, R.; Jayashree, S.; Chuetor, S.; Dharmaraj, S.; Kumar, G.; Chen, W.H.; Ngamcharussrivichai, C. Recent advances in lignocellulosic biomass for biofuels and value-added bioproducts—A critical review. Bioresour. Technol. 2022, 344, 126195. [Google Scholar] [CrossRef]
- Sharma, P.K.; Saharia, M.; Srivstava, R.; Kumar, S.; Sahoo, L. Tailoring microalgae for efficient biofuel production. Front. Mar. Sci. 2018, 5, 382. [Google Scholar] [CrossRef]
- Sathya, A.B.; Thirunavukkarasu, A.; Nithya, R.; Nandan, A.; Sakthishobana, K.; Kola, A.K.; Sivashankar, R.; Tuan, H.A.; Deepanraj, B. Microalgal biofuel production: Potential challenges and prospective research. Fuel 2023, 332, 126199. [Google Scholar] [CrossRef]
- Aniruddha, R.; Rajendran, A.; Sindhu, S. A study on biofuel generation from microalgae species. Mater. Today Proc. 2022, 57, 1660–1665. [Google Scholar] [CrossRef]
- Awogbemi, O.; Kallon, D.V. Von Valorization of agricultural wastes for biofuel applications. Heliyon 2022, 8, e11117. [Google Scholar] [CrossRef]
- Sayre, R. Microalgae: The potential for carbon capture. Bioscience 2010, 60, 722–727. [Google Scholar] [CrossRef]
- Van Den Hende, S.; Vervaeren, H.; Desmet, S.; Boon, N. Bioflocculation of microalgae and bacteria combined with flue gas to improve sewage treatment. N. Biotechnol. 2011, 29, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, M.; Vecchi, V.; Barera, S.; Dall’Osto, L. Biomass from microalgae: The potential of domestication towards sustainable biofactories. Microb. Cell Fact. 2018, 17, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Mitra, D.; van Leeuwen, J.H.; Lamsal, B. Heterotrophic/mixotrophic cultivation of oleaginous Chlorella vulgaris on industrial co-products. Algal Res. 2012, 1, 40–48. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Fact. 2018, 17, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Siddiki, S.Y.A.; Mofijur, M.; Kumar, P.S.; Ahmed, S.F.; Inayat, A.; Kusumo, F.; Badruddin, I.A.; Khan, T.M.Y.; Nghiem, L.D.; Ong, H.C.; et al. Microalgae biomass as a sustainable source for biofuel, biochemical and biobased value-added products: An integrated biorefinery concept. Fuel 2022, 307, 121782. [Google Scholar] [CrossRef]
- Ruiz, J.; Wijffels, R.H.; Dominguez, M.; Barbosa, M.J. Heterotrophic vs autotrophic production of microalgae: Bringing some light into the everlasting cost controversy. Algal Res. 2022, 64, 102698. [Google Scholar] [CrossRef]
- Das, P.; Thaher, M.; AbdulQuadir, M.; Khan, S.; Chaudhary, A.; Al-Jabri, H. Long-term semi-continuous cultivation of a halo-tolerant Tetraselmis sp. using recycled growth media. Bioresour. Technol. 2019, 276, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Thaher, M.I.; Khan, S.; AbdulQuadir, M.; Chaudhary, A.K.; Alghasal, G.; Al-Jabri, H. Comparison of biocrude oil production from self-settling and non-settling microalgae biomass produced in the Qatari desert environment. Int. J. Environ. Sci. Technol. 2019, 16, 7443–7454. [Google Scholar] [CrossRef] [Green Version]
- Al-Jabri, H.; Das, P.; Khan, S.; Thaher, M.; Abdulquadir, M. Treatment of wastewaters by microalgae and the potential applications of the produced biomass—A review. Water 2021, 13, 27. [Google Scholar] [CrossRef]
- Das, P.; Khan, S.; Chaudhary, A.K.; AbdulQuadir, M.; Thaher, M.I.; Al-Jabri, H. Potential Applications of Algae-Based Bio-fertilizer. In Biofertilizers for Sustainable Agriculture and Environment; Giri, B., Prasad, R., Wu, Q.-S., Varma, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 41–65. ISBN 978-3-030-18933-4. [Google Scholar]
- Aljabri, H.; Das, P.; Khan, S.; AbdulQuadir, M.; Thaher, M.; Hawari, A.H.; Al-Shamary, N.M. A study to investigate the energy recovery potential from different macromolecules of a low-lipid marine Tetraselmis sp. biomass through HTL process. Renew. Energy 2022, 189, 78–89. [Google Scholar] [CrossRef]
- Olabi, A.G.; Alami, A.H.; Alasad, S.; Aljaghoub, H.; Sayed, E.T.; Shehata, N.; Rezk, H.; Abdelkareem, M.A. Emerging Technologies for Enhancing Microalgae Biofuel Production: Recent Progress, Barriers, and Limitations. Fermentation 2022, 8, 649. [Google Scholar] [CrossRef]
- Satari, B.; Jaiswal, A.K. Green fractionation of 2G and 3G feedstocks for ethanol production: Advances, incentives and barriers. Curr. Opin. Food Sci. 2021, 37, 1–9. [Google Scholar] [CrossRef]
- Debnath, C.; Bandyopadhyay, T.K.; Bhunia, B.; Mishra, U.; Narayanasamy, S.; Muthuraj, M. Microalgae: Sustainable resource of carbohydrates in third-generation biofuel production. Renew. Sustain. Energy Rev. 2021, 150, 111464. [Google Scholar] [CrossRef]
- Sudhakar, K.; Mamat, R.; Samykano, M.; Azmi, W.H.; Ishak, W.F.W.; Yusaf, T. An overview of marine macroalgae as bioresource. Renew. Sustain. Energy Rev. 2018, 91, 165–179. [Google Scholar] [CrossRef]
- Budiyono; Agustiani, V.; Khoiriyah, L.; Hawali Abdul Matin, H.; Rachmawati, S. Effect of hydrogen peroxide acetic acid pretreatment on kapok (ceiba pentandra) fruit peel waste for bioethanol production using separated hydrolysis and fermentation methods. Mater. Today Proc. 2022, 63, S73–S77. [Google Scholar] [CrossRef]
- Chng, L.M.; Lee, K.T.; Chan, D.J.C. Synergistic effect of pretreatment and fermentation process on carbohydrate-rich Scenedesmus dimorphus for bioethanol production. Energy Convers. Manag. 2017, 141, 410–419. [Google Scholar] [CrossRef]
- Condor, B.E.; de Luna, M.D.G.; Chang, Y.-H.; Chen, J.-H.; Leong, Y.K.; Chen, P.-T.; Chen, C.-Y.; Lee, D.-J.; Chang, J.-S. Bioethanol production from microalgae biomass at high-solids loadings. Bioresour. Technol. 2022, 363, 128002. [Google Scholar] [CrossRef] [PubMed]
- Constantino, A.; Rodrigues, B.; Leon, R.; Barros, R.; Raposo, S. Alternative chemo-enzymatic hydrolysis strategy applied to different microalgae species for bioethanol production. Algal Res. 2021, 56, 102329. [Google Scholar] [CrossRef]
- Jafari Olia, M.S.; Azin, M.; Moazami, N. Application of a statistical design to evaluate bioethanol production from Chlorella S4 biomass after acid—Thermal pretreatment. Renew. Energy 2022, 182, 60–68. [Google Scholar] [CrossRef]
- Tsolcha, O.N.; Patrinou, V.; Economou, C.N.; Dourou, M.; Aggelis, G.; Tekerlekopoulou, A.G. Utilization of biomass derived from cyanobacteria-based agro-industrial wastewater treatment and raisin residue extract for bioethanol production. Water 2021, 13, 486. [Google Scholar] [CrossRef]
- Maity, S.; Mallick, N. Bioprospecting marine microalgae and cyanobacteria as alternative feedstocks for bioethanol production. Sustain. Chem. Pharm. 2022, 29, 100798. [Google Scholar] [CrossRef]
- Harun, R.; Danquah, M.K. Influence of acid pre-treatment on microalgal biomass for bioethanol production. Process Biochem. 2011, 46, 304–309. [Google Scholar] [CrossRef]
- Harun, R.; Danquah, M.K.; Forde, G.M. Microalgal biomass as a fermentation feedstock for bioethanol production. J. Chem. Technol. Biotechnol. 2010, 85, 199–203. [Google Scholar] [CrossRef]
- Sanchez Rizza, L.; Sanz Smachetti, M.E.; Do Nascimento, M.; Salerno, G.L.; Curatti, L. Bioprospecting for native microalgae as an alternative source of sugars for the production of bioethanol. Algal Res. 2017, 22, 140–147. [Google Scholar] [CrossRef]
- Chandra, N.; Shukla, P.; Mallick, N. Role of cultural variables in augmenting carbohydrate accumulation in the green microalga Scenedesmus acuminatus for bioethanol production. Biocatal. Agric. Biotechnol. 2020, 26, 101632. [Google Scholar] [CrossRef]
- Reyimu, Z.; Özçimen, D. Batch cultivation of marine microalgae Nannochloropsis oculata and Tetraselmis suecica in treated municipal wastewater toward bioethanol production. J. Clean. Prod. 2017, 150, 40–46. [Google Scholar] [CrossRef]
- Ngamsirisomsakul, M.; Reungsang, A.; Liao, Q.; Kongkeitkajorn, M.B. Enhanced bio-ethanol production from Chlorella sp. biomass by hydrothermal pretreatment and enzymatic hydrolysis. Renew. Energy 2019, 141, 482–492. [Google Scholar] [CrossRef]
- Hemalatha, M.; Sravan, J.S.; Min, B.; Venkata Mohan, S. Microalgae-biorefinery with cascading resource recovery design associated to dairy wastewater treatment. Bioresour. Technol. 2019, 284, 424–429. [Google Scholar] [CrossRef]
- Huang, X.; Bai, S.; Liu, Z.; Hasunuma, T.; Kondo, A.; Ho, S.H. Fermentation of pigment-extracted microalgal residue using yeast cell-surface display: Direct high-density ethanol production with competitive life cycle impacts. Green Chem. 2020, 22, 153–162. [Google Scholar] [CrossRef]
- Chen, R.; Qin, Z.; Han, J.; Wang, M.; Taheripour, F.; Tyner, W.; O’Connor, D.; Duffield, J. Life cycle energy and greenhouse gas emission effects of biodiesel in the United States with induced land use change impacts. Bioresour. Technol. 2018, 251, 249–258. [Google Scholar] [CrossRef]
- Zahan, K.A.; Kano, M. Biodiesel production from palm oil, its by-products, and mill effluent: A review. Energies 2018, 11, 2132. [Google Scholar] [CrossRef] [Green Version]
- Yahya, M.; Dutta, A.; Bouri, E.; Wadström, C.; Uddin, G.S. Dependence structure between the international crude oil market and the European markets of biodiesel and rapeseed oil. Renew. Energy 2022, 197, 594–605. [Google Scholar] [CrossRef]
- Shah, N.G.; Khan, S.; Mahajani, S.M.; Shah, N.; Shoyeb Khan, S.M.; Shah, N.G.; Khan, S.; Mahajani, S.M. Biodiesel Making from Waste Vegetable Oils: A Case Study of Lab to Plant Technology Transfer. J. Agric. Eng. 2010, 47, 36–42. [Google Scholar]
- Muscat, A.; de Olde, E.M.; de Boer, I.J.M.; Ripoll-Bosch, R. The battle for biomass: A systematic review of food-feed-fuel competition. Glob. Food Sec. 2020, 25, 100330. [Google Scholar] [CrossRef]
- Tien Thanh, N.; Mostapha, M.; Lam, M.K.; Ishak, S.; Kanna Dasan, Y.; Lim, J.W.; Tan, I.S.; Lau, S.Y.; Chin, B.L.F.; Hadibarata, T. Fundamental understanding of in-situ transesterification of microalgae biomass to biodiesel: A critical review. Energy Convers. Manag. 2022, 270, 116212. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Huang, B.-Y.; Chiang, T.-H.; Tang, T.-C. Fuel properties of microalgae (Chlorella protothecoides) oil biodiesel and its blends with petroleum diesel. Fuel 2012, 94, 270–273. [Google Scholar] [CrossRef]
- Huang, J.; Xia, J.; Jiang, W.; Li, Y.; Li, J. Biodiesel production from microalgae oil catalyzed by a recombinant lipase. Bioresour. Technol. 2015, 180, 47–53. [Google Scholar] [CrossRef]
- Mittal, V.; Kumar Ghosh, U. Comparative analysis of two different nanocatalysts for producing biodiesel from microalgae. Mater. Today Proc. 2022, 63, 515–519. [Google Scholar] [CrossRef]
- Cheng, J.; Huang, R.; Li, T.; Zhou, J.; Cen, K. Biodiesel from wet microalgae: Extraction with hexane after the microwave-assisted transesterification of lipids. Bioresour. Technol. 2014, 170, 69–75. [Google Scholar] [CrossRef]
- Wahidin, S.; Idris, A.; Shaleh, S.R.M. Ionic liquid as a promising biobased green solvent in combination with microwave irradiation for direct biodiesel production. Bioresour. Technol. 2016, 206, 150–154. [Google Scholar] [CrossRef]
- Sung, M.; Han, J.I. Ultrasound-assisted in-situ transesterification of wet Aurantiochytrium sp. KRS 101 using potassium carbonate. Bioresour. Technol. 2018, 261, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A.; Esmaeilzadeh, F.; Mowla, D.; Sadatshojaei, E.; Heidari, S.; Wood, D.A. New insights to direct conversion of wet microalgae impregnated with ethanol to biodiesel exploiting extraction with supercritical carbon dioxide. Fuel 2021, 285, 119199. [Google Scholar] [CrossRef]
- Mohamadzadeh Shirazi, H.; Karimi-Sabet, J.; Ghotbi, C. Biodiesel production from Spirulina microalgae feedstock using direct transesterification near supercritical methanol condition. Bioresour. Technol. 2017, 239, 378–386. [Google Scholar] [CrossRef]
- Malekghasemi, S.; Kariminia, H.-R.; Plechkova, N.K.; Ward, V.C.A. Direct transesterification of wet microalgae to biodiesel using phosphonium carboxylate ionic liquid catalysts. Biomass Bioenergy 2021, 150, 106126. [Google Scholar] [CrossRef]
- Im, H.; Lee, H.; Park, M.S.; Yang, J.-W.; Lee, J.W. Concurrent extraction and reaction for the production of biodiesel from wet microalgae. Bioresour. Technol. 2014, 152, 534–537. [Google Scholar] [CrossRef]
- Khan, S.; Gholkar, P.; Shastri, Y.; Shah, N.; Bhartiya, S. Hydrothermal liquefaction of Chlorella sp. for biocrude oil production: Comparison of experimental and modeling results. Int. Agric. Eng. J. 2018, 27, 8–16. [Google Scholar]
- Das, P.; Khan, S.; Thaher, M.I.; AbdulQuadir, M.; Hoekman, S.K.; Al-Jabri, H.; Quadir, M.A.; Chaudhary, A.K.; Thaher, M.I.; Khan, S.; et al. Effect of harvesting methods on the energy requirement of Tetraselmis sp. biomass production and biocrude yield and quality. Bioresour. Technol. 2019, 284, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Al-Naimi, S.; Al-Muftah, A.; Das, P.; Khan, S.; AbdulQuadir, M.; Al-Jabri, H.; Alghasal, G. Biocrude oil and high-value metabolite production potential of the Nitzschia sp. Biomass Convers. Biorefinery 2021. [Google Scholar] [CrossRef]
- Das, P.; Khan, S.; AbdulQuadir, M.; Thaher, M.; Waqas, M.; Easa, A.; Attia, E.S.M.; Al-Jabri, H. Energy recovery and nutrients recycling from municipal sewage sludge. Sci. Total Environ. 2020, 715, 136775. [Google Scholar] [CrossRef]
- Guo, B.; Walter, V.; Hornung, U.; Dahmen, N. Hydrothermal liquefaction of Chlorella vulgaris and Nannochloropsis gaditana in a continuous stirred tank reactor and hydrotreating of biocrude by nickel catalysts. Fuel Process. Technol. 2019, 191, 168–180. [Google Scholar] [CrossRef]
- Shia, Y.-P.; Yu, B.-Y. Development of a rigorous and generalized model on the hydrothermal liquefaction (HTL) process for bio-oil production. Process Saf. Environ. Prot. 2023, 171, 541–554. [Google Scholar] [CrossRef]
- Das, P.; Khan, S.; AbdulQuadir, M.; Thaher, M.I.; Hawari, A.H.; Alshamri, N.; AlGhasal, G.; Al-Jabri, H.M.J. Biocrude oil production from a self-settling marine cyanobacterium, Chroococcidiopsis sp., using a biorefinery approach. Renew. Energy 2023, 203, 1–9. [Google Scholar] [CrossRef]
- Biller, P.; Ross, A.B.; Skill, S.C.; Lea-Langton, A.; Balasundaram, B.; Hall, C.; Riley, R.; Llewellyn, C.A. Nutrient recycling of aqueous phase for microalgae cultivation from the hydrothermal liquefaction process. Algal Res. 2012, 1, 70–76. [Google Scholar] [CrossRef]
- Han, Y.; Hoekman, S.K.; Cui, Z.; Jena, U.; Das, P. Hydrothermal liquefaction of marine microalgae biomass using co-solvents. Algal Res. 2019, 38, 101421. [Google Scholar] [CrossRef]
- Liu, T.; Yang, L.; Jiao, H.; Jin, Z.; Chen, P.; Leng, S.; Zhou, W. Fractional distillation of biocrude from hydrothermal liquefaction of microalgae: Upgrading of fuel properties. Algal Res. 2022, 68, 102888. [Google Scholar] [CrossRef]
- Masoumi, S.; Boahene, P.E.; Dalai, A.K. Biocrude oil and hydrochar production and characterization obtained from hydrothermal liquefaction of microalgae in methanol-water system. Energy 2021, 217, 119344. [Google Scholar] [CrossRef]
- Faeth, J.L.; Savage, P.E. Effects of processing conditions on biocrude yields from fast hydrothermal liquefaction of microalgae. Bioresour. Technol. 2016, 206, 290–293. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Chen, J.; Kandasamy, S.; He, Z. Hydrothermal liquefaction of fresh lemon-peel and Spirulina platensis blending -operation parameter and biocrude chemistry investigation. Energy 2020, 193, 116645. [Google Scholar] [CrossRef]
- Ong, H.C.; Chen, W.H.; Farooq, A.; Gan, Y.Y.; Lee, K.T.; Ashokkumar, V. Catalytic thermochemical conversion of biomass for biofuel production: A comprehensive review. Renew. Sustain. Energy Rev. 2019, 113, 109266. [Google Scholar] [CrossRef]
- Su, G.; Ong, H.C.; Ibrahim, S.; Fattah, I.M.R.; Mofijur, M.; Chong, C.T. Valorisation of medical waste through pyrolysis for a cleaner environment: Progress and challenges. Environ. Pollut. 2021, 279, 116934. [Google Scholar] [CrossRef]
- Tang, Z.; Chen, W.; Chen, Y.; Hu, J.; Yang, H.; Chen, H. Preparation of low-nitrogen and high-quality bio-oil from microalgae catalytic pyrolysis with zeolites and activated carbon. J. Anal. Appl. Pyrolysis 2021, 159, 105182. [Google Scholar] [CrossRef]
- Mustapha, S.I.; Rawat, I.; Bux, F.; Isa, Y.M. Catalytic pyrolysis of nutrient-stressed Scenedesmus obliquus microalgae for high-quality bio-oil production. Renew. Energy 2021, 179, 2036–2047. [Google Scholar] [CrossRef]
- Su, G.; Ong, H.C.; Gan, Y.Y.; Chen, W.-H.; Chong, C.T.; Ok, Y.S. Co-pyrolysis of microalgae and other biomass wastes for the production of high-quality bio-oil: Progress and prospective. Bioresour. Technol. 2022, 344, 126096. [Google Scholar] [CrossRef]
- Wang, S.; Shang, H.; Abomohra, A.E.-F.; Wang, Q. One-step conversion of microalgae to alcohols and esters through co-pyrolysis with biodiesel-derived glycerol. Energy Convers. Manag. 2019, 198, 111792. [Google Scholar] [CrossRef]
- Xu, K.; Li, J.; Zeng, K.; Zhong, D.; Peng, J.; Qiu, Y.; Flamant, G.; Yang, H.; Chen, H. The characteristics and evolution of nitrogen in bio-oil from microalgae pyrolysis in molten salt. Fuel 2023, 331, 125903. [Google Scholar] [CrossRef]
- Kumar, A.; Jamro, I.A.; Yan, B.; Cheng, Z.; Tao, J.; Zhou, S.; Kumari, L.; Li, J.; Aborisade, M.A.; Tafa Oba, B.; et al. Pyrolysis of de-fatted microalgae residue: A study on thermal-kinetics, products’ optimization, and neural network modelling. Fuel 2023, 334, 126752. [Google Scholar] [CrossRef]
- Khodaparasti, M.S.; Khorasani, R.; Tavakoli, O.; Khodadadi, A.A. Optimal Co-pyrolysis of municipal sewage sludge and microalgae Chlorella Vulgaris: Products characterization, synergistic effects, mechanism, and reaction pathways. J. Clean. Prod. 2023, 390, 135991. [Google Scholar] [CrossRef]
- Kumar, A.; Yan, B.; Tao, J.; Li, J.; Kumari, L.; Oba, B.T.; Aborisade, M.A.; Chen, G. Influence of waste plastic on pyrolysis of low-lipid microalgae: A study on thermokinetics, behaviors, evolved gas characteristics, and products distribution. Renew. Energy 2022, 185, 416–430. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, J.; Pan, M.; Hao, Y.; Hu, R.; Xiao, W.; Li, G.; Lyu, T. Valorisation of microalgae residues after lipid extraction: Pyrolysis characteristics for biofuel production. Biochem. Eng. J. 2022, 179, 108330. [Google Scholar] [CrossRef]
- Martinez-Villarreal, S.; Breitenstein, A.; Nimmegeers, P.; Perez Saura, P.; Hai, B.; Asomaning, J.; Eslami, A.A.; Billen, P.; Van Passel, S.; Bressler, D.C.; et al. Drop-in biofuels production from microalgae to hydrocarbons: Microalgal cultivation and harvesting, conversion pathways, economics and prospects for aviation. Biomass Bioenergy 2022, 165, 106555. [Google Scholar] [CrossRef]
- Lam, N.L.; Smith, K.R.; Gauthier, A.; Bates, M.N. Kerosene: A review of household uses and their hazards in low-and middle-income countries. J. Toxicol. Environ. Health Part B Crit. Rev. 2012, 15, 396–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritchie, G.D.; Still, K.R.; Rossi, J.; Bekkedal, M.Y.V.; Bobb, A.J.; Arfsten, D.P. Biological and health effects of exposure to kerosene-based jet fuels and performance additives. J. Toxicol. Environ. Health Part B Crit. Rev. 2003, 6, 357–451. [Google Scholar] [CrossRef]
- Neuling, U.; Kaltschmitt, M. Conversion routes for production of biokerosene—Status and assessment. Biomass Convers. Biorefinery 2015, 5, 367–385. [Google Scholar] [CrossRef]
- Bwapwa, J.K.; Anandraj, A.; Trois, C. Microalgae processing for jet fuel production. Biofuels Bioprod. Biorefining 2018, 12, 522–535. [Google Scholar] [CrossRef]
- Karatzos, S.; Mcmillan, J.; Saddler, J. The Potential and Challenges of “Drop in” Biofuels; IEA Bioenergy: Paris, France, 2014. [Google Scholar]
- Karatzos, S.; van Dyk, J.S.; McMillan, J.D.; Saddler, J. Drop-in biofuel production via conventional (lipid/fatty acid) and advanced (biomass) routes. Part I. Biofuels Bioprod. Biorefining 2017, 11, 344–362. [Google Scholar] [CrossRef]
- Robota, H.J.; Alger, J.C.; Shafer, L. Converting algal triglycerides to diesel and HEFA jet fuel fractions. Energy Fuels 2013, 27, 985–996. [Google Scholar] [CrossRef]
- Bwapwa, J.K.; Anandraj, A.; Trois, C. Possibilities for conversion of microalgae oil into aviation fuel: A review. Renew. Sustain. Energy Rev. 2017, 80, 1345–1354. [Google Scholar] [CrossRef]
- Kim, T.-H.; Lee, K.; Oh, B.-R.; Lee, M.-E.; Seo, M.; Li, S.; Kim, J.-K.; Choi, M.; Chang, Y.K. A novel process for the coproduction of biojet fuel and high-value polyunsaturated fatty acid esters from heterotrophic microalgae Schizochytrium sp. ABC101. Renew. Energy 2021, 165, 481–490. [Google Scholar] [CrossRef]
- Gómez-De la Cruz, A.; Romero-Izquierdo, A.G.; Gutiérrez-Antonio, C.; Gómez-Castro, F.I.; Hernández, S. Modelling of the hydrotreating process to produce renewable aviation fuel from micro-algae oil. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Hillen, L.W.; Pollard, G.; Wake, L.V.; White, N. Hydrocracking of the oils of Botryococcus braunii to transport fuels. Biotechnol. Bioeng. 1982, 24, 193–205. [Google Scholar] [CrossRef]
- Biller, P.; Sharma, B.K.; Kunwar, B.; Ross, A.B. Hydroprocessing of bio-crude from continuous hydrothermal liquefaction of microalgae. Fuel 2015, 159, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Peng, B.; Yao, Y.; Zhao, C.; Lercher, J.A. Towards quantitative conversion of microalgae oil to diesel-range alkanes with bifunctional catalysts. Angew. Chem. Int. Ed. 2012, 51, 2072–2075. [Google Scholar] [CrossRef]
- Poddar, M.K.; Anand, M.; Farooqui, S.A.; Martin, G.J.O.; Maurya, M.R.; Sinha, A.K. Hydroprocessing of lipids extracted from marine microalgae Nannochloropsis sp. over sulfided CoMoP/Al2O3 catalyst. Biomass Bioenergy 2018, 119, 31–36. [Google Scholar] [CrossRef]
- Xu, X.J.; Yan, J.; Yuan, Q.K.; Wang, X.T.; Yuan, Y.; Ren, N.Q.; Lee, D.J.; Chen, C. Enhanced methane production in anaerobic digestion: A critical review on regulation based on electron transfer. Bioresour. Technol. 2022, 364, 128003. [Google Scholar] [CrossRef]
- Kumar, K.; Ghosh, S.; Angelidaki, I.; Holdt, S.L.; Karakashev, D.B.; Morales, M.A.; Das, D. Recent developments on biofuels production from microalgae and macroalgae. Renew. Sustain. Energy Rev. 2016, 65, 235–249. [Google Scholar] [CrossRef]
- Bragança, I.; Sánchez-Soberón, F.; Pantuzza, G.F.; Alves, A.; Ratola, N. Impurities in biogas: Analytical strategies, occurrence, effects and removal technologies. Biomass Bioenergy 2020, 143, 105878. [Google Scholar] [CrossRef]
- Kumar, M.; Sun, Y.; Rathour, R.; Pandey, A.; Thakur, I.S.; Tsang, D.C.W. Algae as potential feedstock for the production of biofuels and value-added products: Opportunities and challenges. Sci. Total Environ. 2020, 716, 137116. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Yi, X.; Yang, F.; Wang, D.; Han, H. Dissimilatory manganese reduction facilitates synergistic cooperation of hydrolysis, acidogenesis, acetogenesis and methanogenesis via promoting microbial interaction during anaerobic digestion of waste activated sludge. Environ. Res. 2023, 218, 114992. [Google Scholar] [CrossRef]
- Shukla, S.K.; Khan, A.; Rao, T.S. Microbial fouling in water treatment plants. In Microbial and Natural Macromolecules; Academic Press: Cambridge, MA, USA, 2021; pp. 589–622. [Google Scholar] [CrossRef]
- Mahata, C.; Das, P.; Khan, S.; Thaher, M.I.A.; Abdul Quadir, M.; Annamalai, S.N.; Al Jabri, H. The Potential of Marine Microalgae for the Production of Food, Feed, and Fuel (3F). Fermentation 2022, 8, 316. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, W.; He, Y.; Zhang, R.; Liu, G. Effect of ammonia on methane production, methanogenesis pathway, microbial community and reactor performance under mesophilic and thermophilic conditions. Renew. Energy 2018, 125, 915–925. [Google Scholar] [CrossRef]
- Ma, J.; Li, L.; Zhao, Q.; Yu, L.; Frear, C. Biomethane production from whole and extracted algae biomass: Long-term performance evaluation and microbial community dynamics. Renew. Energy 2021, 170, 38–48. [Google Scholar] [CrossRef]
- Hu, Y.; Kumar, M.; Wang, Z.; Zhan, X.; Stengel, D.B. Filamentous microalgae as an advantageous co-substrate for enhanced methane production and digestate dewaterability in anaerobic co-digestion of pig manure. Waste Manag. 2021, 119, 399–407. [Google Scholar] [CrossRef]
- Vargas-Estrada, L.; Longoria, A.; Arenas, E.; Moreira, J.; Okoye, P.U.; Bustos-Terrones, Y.; Sebastian, P.J. A Review on Current Trends in Biogas Production from Microalgae Biomass and Microalgae Waste by Anaerobic Digestion and Co-digestion. BioEnergy Res. 2021, 15, 77–92. [Google Scholar] [CrossRef]
- Hu, Y.; Kobayashi, T.; Zhen, G.; Shi, C.; Xu, K.Q. Effects of lipid concentration on thermophilic anaerobic co-digestion of food waste and grease waste in a siphon-driven self-agitated anaerobic reactor. Biotechnol. Rep. 2018, 19, e00269. [Google Scholar] [CrossRef] [PubMed]
- Induchoodan, T.G.; Haq, I.; Kalamdhad, A.S. Factors affecting anaerobic digestion for biogas production: A review. In Advanced Organic Waste Management; Elsevier: Amsterdam, The Netherlands, 2022; pp. 223–233. [Google Scholar] [CrossRef]
- Xiao, C.; Liao, Q.; Fu, Q.; Huang, Y.; Chen, H.; Zhang, H.; Xia, A.; Zhu, X.; Reungsang, A.; Liu, Z. A solar-driven continuous hydrothermal pretreatment system for biomethane production from microalgae biomass. Appl. Energy 2019, 236, 1011–1018. [Google Scholar] [CrossRef]
- Damtie, M.M.; Shin, J.; Jang, H.M.; Cho, H.U.; Wang, J.; Kim, Y.M. Effects of biological pretreatments of microalgae on hydrolysis, biomethane potential and microbial community. Bioresour. Technol. 2021, 329, 124905. [Google Scholar] [CrossRef] [PubMed]
- Klassen, V.; Blifernez-Klassen, O.; Wibberg, D.; Winkler, A.; Kalinowski, J.; Posten, C.; Kruse, O. Highly efficient methane generation from untreated microalgae biomass. Biotechnol. Biofuels 2017, 221, 78–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinnunen, V.; Rintala, J. The effect of low-temperature pretreatment on the solubilization and biomethane potential of microalgae biomass grown in synthetic and wastewater media. Bioresour. Technol. 2016, 221, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Perazzoli, S.; Bruchez, B.M.; Michelon, W.; Steinmetz, R.L.R.; Mezzari, M.P.; Nunes, E.O.; da Silva, M.L.B. Optimizing biomethane production from anaerobic degradation of Scenedesmus spp. biomass harvested from algae-based swine digestate treatment. Int. Biodeterior. Biodegrad. 2016, 109, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Mahata, C.; Ray, S.; Das, D. Optimization of dark fermentative hydrogen production from organic wastes using acidogenic mixed consortia. Energy Convers. Manag. 2020, 219, 113047. [Google Scholar] [CrossRef]
- Nadaleti, W.C.; Lourenço, V.A. A mathematical, economic and energetic appraisal of biomethane and biohydrogen production from Brazilian ethanol plants’ waste: Towards a circular and renewable energy development. Int. J. Hydrogen Energy 2021, 46, 27268–27281. [Google Scholar] [CrossRef]
- Mahata, C.; Das, D. Current Status and Prospects of Biohydrogen Production Process. In Microbial Biotechnology for Renewable and Sustainable Energy; Springer: Berlin/Heidelberg, Germany, 2022; pp. 99–133. [Google Scholar] [CrossRef]
- Das, D.; Veziroǧlu, T.N.; Das, T.; Nejat, D.V.; Das, D. Hydrogen production by biological processes: A survey of literature. Int. J. Hydrogen Energy 2001, 26, 13–28. [Google Scholar] [CrossRef]
- Nagarajan, D.; Lee, D.J.; Kondo, A.; Chang, J.S. Recent insights into biohydrogen production by microalgae—From biophotolysis to dark fermentation. Bioresour. Technol. 2017, 227, 373–387. [Google Scholar] [CrossRef]
- Das, D.; Veziroglu, T.N. Advances in biological hydrogen production processes. Int. J. Hydrogen Energy 2008, 33, 6046–6057. [Google Scholar] [CrossRef]
- Hallenbeck, P.C.; Benemann, J.R. Biological hydrogen production; fundamentals and limiting processes. Int. J. Hydrogen Energy 2002, 27, 1185–1193. [Google Scholar] [CrossRef]
- Nagarajan, D.; Dong, C.D.; Chen, C.Y.; Lee, D.J.; Chang, J.S. Biohydrogen production from microalgae—Major bottlenecks and future research perspectives. Biotechnol. J. 2021, 16, 2000124. [Google Scholar] [CrossRef] [PubMed]
- Melitos, G.; Voulkopoulos, X.; Zabaniotou, A. Waste to Sustainable Biohydrogen Production Via Photo-Fermentation and Biophotolysis − A Systematic Review. Renew. Energy Environ. Sustain. 2021, 6, 45. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Mehariya, S.; Bhatia, R.K.; Kumar, M.; Pugazhendhi, A.; Awasthi, M.K.; Atabani, A.E.; Kumar, G.; Kim, W.; Seo, S.O.; et al. Wastewater based microalgal biorefinery for bioenergy production: Progress and challenges. Sci. Total Environ. 2021, 751, 141599. [Google Scholar] [CrossRef]
- Singh, N.; Sarma, S. Biological routes of hydrogen production: A critical assessment. In Handbook of Biofuels; Academic Press: Cambridge, MA, USA, 2022; pp. 419–434. [Google Scholar] [CrossRef]
- Pandey, A.; Sinha, P.; Pandey, A. Hydrogen production by sequential dark and photofermentation using wet biomass hydrolysate of Spirulina platensis: Response surface methodological approach. Int. J. Hydrogen Energy 2021, 46, 7137–7146. [Google Scholar] [CrossRef]
- Veeravalli, S.S.; Shanmugam, S.R.; Ray, S.; Lalman, J.A.; Biswas, N. Biohydrogen Production from Renewable Resources. In Advanced Bioprocessing for Alternative Fuels, Biobased Chemicals, and Bioproducts; Woodhead Publishing: Sawston, UK, 2019; pp. 289–312. [Google Scholar] [CrossRef]
- Balachandar, G.; Varanasi, J.L.; Singh, V.; Singh, H.; Das, D. Biological hydrogen production via dark fermentation: A holistic approach from lab-scale to pilot-scale. Int. J. Hydrogen Energy 2019, 45, 5202–5215. [Google Scholar] [CrossRef]
- Arras, W.; Hussain, A.; Hausler, R.; Guiot, S.R. Mesophilic, thermophilic and hyperthermophilic acidogenic fermentation of food waste in batch: Effect of inoculum source. Waste Manag. 2019, 87, 279–287. [Google Scholar] [CrossRef]
- Roy, S.; Vishnuvardhan, M.; Das, D. Improvement of hydrogen production by newly isolated Thermoanaerobacterium thermosaccharolyticum IIT BT-ST1. Int. J. Hydrogen Energy 2014, 39, 7541–7552. [Google Scholar] [CrossRef]
- Abreu, A.A.; Karakashev, D.; Angelidaki, I.; Sousa, D.Z.; Alves, M. Biohydrogen production from arabinose and glucose using extreme thermophilic anaerobic mixed cultures. Biotechnol. Biofuels 2012, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Maru, B.T.; López, F.; Kengen, S.W.M.; Constantí, M.; Medina, F. Dark fermentative hydrogen and ethanol production from biodiesel waste glycerol using a co-culture of Escherichia coli and Enterobacter sp. Fuel 2016, 186, 375–384. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Rajesh Banu, J.; Singh, V.; Kumar, G.; Yang, Y.H. Algal biomass to biohydrogen: Pretreatment, influencing factors, and conversion strategies. Bioresour. Technol. 2023, 368, 128332. [Google Scholar] [CrossRef]
- Yun, Y.-M.; Kim, D.-H.; Oh, Y.-K.; Shin, H.-S.; Jung, K.-W. Application of a novel enzymatic pretreatment using crude hydrolytic extracellular enzyme solution to microalgal biomass for dark fermentative hydrogen production. Bioresour. Technol. 2014, 159, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Batista, A.P.; Ambrosano, L.; Graça, S.; Sousa, C.; Marques, P.A.S.S.; Ribeiro, B.; Botrel, E.P.; Castro Neto, P.; Gouveia, L. Combining urban wastewater treatment with biohydrogen production—An integrated microalgae-based approach. Bioresour. Technol. 2015, 184, 230–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maswanna, T.; Lindblad, P.; Maneeruttanarungroj, C. Improved biohydrogen production by immobilized cells of the green alga Tetraspora sp. CU2551 incubated under aerobic condition. J. Appl. Phycol. 2020, 32, 2937–2945. [Google Scholar] [CrossRef]
- Liu, C.-H.; Chang, C.-Y.; Liao, Q.; Zhu, X.; Liao, C.-F.; Chang, J.-S. Biohydrogen production by a novel integration of dark fermentation and mixotrophic microalgae cultivation. Int. J. Hydrogen Energy 2013, 38, 15807–15814. [Google Scholar] [CrossRef]
- Hoshino, T.; Johnson, D.J.; Scholz, M.; Cuello, J.L. Effects of implementing PSI-light on hydrogen production via biophotolysis in Chlamydomonas reinhardtii mutant strains. Biomass Bioenergy 2013, 59, 243–252. [Google Scholar] [CrossRef]
- Singh, H.; Varanasi, J.L.; Banerjee, S.; Das, D. Production of carbohydrate enrich microalgal biomass as a bioenergy feedstock. Energy 2019, 188, 116039. [Google Scholar] [CrossRef]
- Yin, Y.; Chen, Y.; Wang, J. Co-fermentation of sewage sludge and algae and Fe2+ addition for enhancing hydrogen production. Int. J. Hydrogen Energy 2021, 46, 8950–8960. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Banerjee, S.; Banerjee, S.; Singh, V.; Das, D. Sustainable approach for the treatment of poultry manure and starchy wastewater by integrating dark fermentation and microalgal cultivation. J. Mater. Cycles Waste Manag. 2021, 23, 790–803. [Google Scholar] [CrossRef]
- Mahata, C.; Dhar, S.; Ray, S.; Das, D. Flocculation characteristics of anaerobic sludge driven-extracellular polymeric substance (EPS) extracted by different methods on microalgae harvesting for lipid utilization. Biochem. Eng. J. 2021, 167, 107898. [Google Scholar] [CrossRef]
- Jorquera, O.; Kiperstok, A.; Sales, E.A.; Embiruçu, M.; Ghirardi, M.L. Comparative energy life-cycle analyses of microalgal biomass production in open ponds and photobioreactors. Bioresour. Technol. 2010, 101, 1406–1413. [Google Scholar] [CrossRef]
- Razon, L.F.; Tan, R.R. Net energy analysis of the production of biodiesel and biogas from the microalgae: Haematococcus pluvialis and Nannochloropsis. Appl. Energy 2011, 88, 3507–3514. [Google Scholar] [CrossRef]
- Zhang, P.; Feng, L.; Su, B.; Li, X. Microalgae cultivated in wastewater catalytic hydrothermal liquefaction: Effects of process parameter on products and energy balance. J. Clean. Prod. 2022, 341, 130895. [Google Scholar] [CrossRef]
- Pankratz, S.; Kumar, M.; Oyedun, A.O.; Gemechu, E.; Kumar, A. Environmental performances of diluents and hydrogen production pathways from microalgae in cold climates: Open raceway ponds and photobioreactors coupled with thermochemical conversion. Algal Res. 2020, 47, 101815. [Google Scholar] [CrossRef]
- Naaz, F.; Bhattacharya, A.; Pant, K.K.; Malik, A. Investigations on energy efficiency of biomethane/biocrude production from pilot scale wastewater grown algal biomass. Appl. Energy 2019, 254, 113656. [Google Scholar] [CrossRef]
- Gholkar, P.; Shastri, Y.; Tanksale, A. Renewable hydrogen and methane production from microalgae: A techno-economic and life cycle assessment study. J. Clean. Prod. 2021, 279, 123726. [Google Scholar] [CrossRef]
- Al-Jabri, H.; Das, P.; Khan, S.; AbdulQuadir, M.; Thaher, M.I.; Hoekman, K.; Hawari, A.H. A comparison of bio-crude oil production from five marine microalgae–Using life cycle analysis. Energy 2022, 251, 123954. [Google Scholar] [CrossRef]
- Li, G.; Lu, Z.; Zhang, J.; Li, H.; Zhou, Y.; Zayan, A.M.I.; Huang, Z. Life cycle assessment of biofuel production from microalgae cultivated in anaerobic digested wastewater. Int. J. Agric. Biol. Eng. 2020, 13, 241–246. [Google Scholar] [CrossRef] [Green Version]
- Collet, P.; Lardon, L.; Hélias, A.; Bricout, S.; Lombaert-Valot, I.; Perrier, B.; Lépine, O.; Steyer, J.-P.; Bernard, O. Biodiesel from microalgae—Life cycle assessment and recommendations for potential improvements. Renew. Energy 2014, 71, 525–533. [Google Scholar] [CrossRef]
- Hossain, N.; Zaini, J.; Mahlia, T.M.I. Life cycle assessment, energy balance and sensitivity analysis of bioethanol production from microalgae in a tropical country. Renew. Sustain. Energy Rev. 2019, 115, 109371. [Google Scholar] [CrossRef]
- Ianda, T.F.; Kalid, R.D.A.; Rocha, L.B.; Padula, A.D.; Zimmerman, W.B. Techno-economic modeling to produce biodiesel from marine microalgae in sub-Saharan countries: An exploratory study in Guinea-Bissau. Biomass Bioenergy 2022, 158, 106369. [Google Scholar] [CrossRef]
- Klein-Marcuschamer, D.; Turner, C.; Allen, M.; Gray, P.; Dietzgen, R.G.; Gresshoff, P.M.; Hankamer, B.; Heimann, K.; Scott, P.T.; Stephens, E.; et al. Technoeconomic analysis of renewable aviation fuel from microalgae, Pongamia pinnata, and sugarcane. Biofuels Bioprod. Biorefining 2013, 7, 416–428. [Google Scholar] [CrossRef]
- Ewurum, C.E. Techno-Economic Analysis of Micro-Algae Bio-Jet Fuel Production Processes. Ph.D. Thesis, University of Nottingham, Nottingham, UK, 2018. [Google Scholar]
- Amer, L.; Adhikari, B.; Pellegrino, J. Technoeconomic analysis of five microalgae-to-biofuels processes of varying complexity. Bioresour. Technol. 2011, 102, 9350–9359. [Google Scholar] [CrossRef] [PubMed]
- Diesel, F. Available online: https://www.globalpetrolprices.com/USA/diesel_prices/ (accessed on 2 March 2023).
- Hossain, N.; Mahlia, T.M.I.; Zaini, J.; Saidur, R. Techno-economics and Sensitivity Analysis of Microalgae as Commercial Feedstock for Bioethanol Production. Environ. Prog. Sustain. Energy 2019, 38, 13157. [Google Scholar] [CrossRef] [Green Version]
- Rajesh Banu, J.; Preethi; Kavitha, S.; Gunasekaran, M.; Kumar, G. Microalgae based biorefinery promoting circular bioeconomy-techno economic and life-cycle analysis. Bioresour. Technol. 2020, 302, 122822. [Google Scholar] [CrossRef] [PubMed]
- Ethanol. Available online: https://www.globalpetrolprices.com/USA/ethanol_prices/ (accessed on 2 March 2023).
- Amos, W.A. Updated Cost Analysis of Photobiological Hydrogen Production from Chlamydomonas reinhardtii Green Algae: Milestone Completion Report; National Renewable Energy Lab.: Golden, CO, USA, 2004. [Google Scholar]
- Ahmed, S.F.; Rafa, N.; Mofijur, M.; Badruddin, I.A.; Inayat, A.; Ali, M.S.; Farrok, O.; Yunus Khan, T.M. Biohydrogen Production from Biomass Sources: Metabolic Pathways and Economic Analysis. Front. Energy Res. 2021, 9, 753878. [Google Scholar] [CrossRef]
- Hydrogen, G. Available online: https://www.sgh2energy.com/economics (accessed on 2 March 2023).
- Wu, N.; Moreira, C.M.; Zhang, Y.; Doan, N.; Yang, S.; Phlips, E.J.; Svoronos, S.A.; Pullammanappallil, P.C. Techno-Economic Analysis of Biogas Production from Microalgae through Anaerobic Digestion. In Anaerobic Digestion; BoD—Books on Demand: Norderstedt, Germany, 2019. [Google Scholar]
- Methane. Available online: https://www.globalpetrolprices.com/methane_prices/ (accessed on 2 March 2023).
- Bose, A.; O’Shea, R.; Lin, R.; Long, A.; Rajendran, K.; Wall, D.; De, S.; Murphy, J.D. The marginal abatement cost of co-producing biomethane, food and biofertiliser in a circular economy system. Renew. Sustain. Energy Rev. 2022, 169, 112946. [Google Scholar] [CrossRef]
- Masoumi, S.; Dalai, A.K. Techno-economic and life cycle analysis of biofuel production via hydrothermal liquefaction of microalgae in a methanol-water system and catalytic hydrotreatment using hydrochar as a catalyst support. Biomass Bioenergy 2021, 151, 106168. [Google Scholar] [CrossRef]
- Oil, C. Available online: https://www.bloomberg.com/energy (accessed on 2 March 2023).
- Zhu, Y.; Albrecht, K.O.; Elliott, D.C.; Hallen, R.T.; Jones, S.B. Development of hydrothermal liquefaction and upgrading technologies for lipid-extracted algae conversion to liquid fuels. Algal Res. 2013, 2, 455–464. [Google Scholar] [CrossRef]
- Thilakaratne, R.; Wright, M.M.; Brown, R.C. A techno-economic analysis of microalgae remnant catalytic pyrolysis and upgrading to fuels. Fuel 2014, 128, 104–112. [Google Scholar] [CrossRef]
- Oil, H. Fuel. Available online: https://tradingeconomics.com/commodity/heating-oil (accessed on 2 March 2023).
- Xin, C.; Addy, M.M.; Zhao, J.; Cheng, Y.; Cheng, S.; Mu, D.; Liu, Y.; Ding, R.; Chen, P.; Ruan, R. Comprehensive techno-economic analysis of wastewater-based algal biofuel production: A case study. Bioresour. Technol. 2016, 211, 584–593. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://www.iata.org/en/publications/economics/fuel-monitor/ (accessed on 2 March 2023).
- Pathy, A.; Nageshwari, K.; Ramaraj, R.; Pragas Maniam, G.; Govindan, N.; Balasubramanian, P. Biohydrogen production using algae: Potentiality, economics and challenges. Bioresour. Technol. 2022, 360, 127514. [Google Scholar] [CrossRef] [PubMed]
- Zamalloa, C.; Vulsteke, E.; Albrecht, J.; Verstraete, W. The techno-economic potential of renewable energy through the anaerobic digestion of microalgae. Bioresour. Technol. 2011, 102, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Onwudili, J.A.; Sharma, V.; Scaldaferri, C.A.; Hossain, A.K. Production of upgraded fuel blend from fast pyrolysis bio-oil and organic solvent using a novel three-stage catalytic process and its combustion characteristics in a diesel engine. Fuel 2023, 335, 127028. [Google Scholar] [CrossRef]
- Muthukumar, A.; Elayaraja, S.; Ajithkumar, T.T.; Kumaresan, S.; Balasubramanian, T. Biodiesel Production from Marine Microalgae Chlorella Marina and Nannochloropsis Salina. J. Pet. Technol. Altern. Fuels 2012, 3, 58–62. [Google Scholar]
- Gerbens-Leenes, W.; Hoekstra, A.Y.; Van Der Meer, T.H. The water footprint of bioenergy. Proc. Natl. Acad. Sci. USA 2009, 106, 10219–10223. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.R.; Khalekuzzaman, M.; Bin Kabir, S.; Islam, M.B.; Bari, Q.H. Production of light oil-prone biocrude through co-hydrothermal liquefaction of wastewater-grown microalgae and peat. J. Anal. Appl. Pyrolysis 2022, 161, 105423. [Google Scholar] [CrossRef]
- Wu, H.; Li, J.; Liao, Q.; Fu, Q.; Liu, Z. Enhanced biohydrogen and biomethane production from Chlorella sp. with hydrothermal treatment. Energy Convers. Manag. 2020, 205, 112373. [Google Scholar] [CrossRef]
- Wibowo, C.S.; Sugiarto, B.; Zikra, A.; Budi, A.; Mulya, T.; Muchar, M. The effect of bioethanol-varying gasoline blends on performance and emission of SI engine 150 CC. AIP Conf. Proc. 2019, 2062, 020020. [Google Scholar]
- Wiatrowski, M.; Klein, B.C.; Davis, R.W.; Quiroz-Arita, C.; Tan, E.C.D.; Hunt, R.W.; Davis, R.E. Techno-economic assessment for the production of algal fuels and value-added products: Opportunities for high-protein microalgae conversion. Biotechnol. Biofuels Bioprod. 2022, 15, 1–14. [Google Scholar] [CrossRef]
- Mobin, S.; Alam, F. Some Promising Microalgal Species for Commercial Applications: A review. Energy Procedia 2017, 110, 510–517. [Google Scholar] [CrossRef]
- Wijffels, R.H.; Barbosa, M.J.; Eppink, M.H.M. Microalgae for the production of bulk chemicals and biofuels. Biofuels Bioprod. Biorefining 2010, 4, 287–295. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Baldia, A.; Rajput, D.; Kateriya, S.; Babu, V.; Dubey, K.K. Multiomics and optobiotechnological approaches for the development of microalgal strain for production of aviation biofuel and biorefinery. Bioresour. Technol. 2023, 369, 128457. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Jung, J.-M.; Jung, S.; Park, Y.-K.; Tsang, Y.F.; Lin, K.-Y.A.; Choi, Y.-E.; Kwon, E.E. Biodiesel from microalgae: Recent progress and key challenges. Prog. Energy Combust. Sci. 2022, 93, 101020. [Google Scholar] [CrossRef]
- Patil, P.D.; Gude, V.G.; Mannarswamy, A.; Deng, S.; Cooke, P.; Munson-McGee, S.; Rhodes, I.; Lammers, P.; Nirmalakhandan, N. Optimization of direct conversion of wet algae to biodiesel under supercritical methanol conditions. Bioresour. Technol. 2011, 102, 118–122. [Google Scholar] [CrossRef]
- Maneechote, W.; Cheirsilp, B.; Srinuanpan, S.; Pathom-aree, W. Optimizing physicochemical factors for two-stage cultivation of newly isolated oleaginous microalgae from local lake as promising sources of pigments, PUFAs and biodiesel feedstocks. Bioresour. Technol. Rep. 2021, 15, 100738. [Google Scholar] [CrossRef]
- He, J.; Hong, B.; Lu, R.; Zhang, R.; Fang, H.; Huang, W.; Bai, K.; Sun, J. Separation of saturated fatty acids from docosahexaenoic acid-rich algal oil by enzymatic ethanolysis in tandem with molecular distillation. Food Sci. Nutr. 2020, 8, 2234–2241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AS, S. Biodiesel and Polyunsaturated Fatty Acid (PUFA) Potential of MicroalgaeBiomass-A Short Review. Res. Dev. Mater. Sci. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Maity, S.; Mallick, N. Trends and advances in sustainable bioethanol production by marine microalgae: A critical review. J. Clean. Prod. 2022, 345, 131153. [Google Scholar] [CrossRef]
- Zabed, H.M.; Akter, S.; Yun, J.; Zhang, G.; Awad, F.N.; Qi, X.; Sahu, J.N. Recent advances in biological pretreatment of microalgae and lignocellulosic biomass for biofuel production. Renew. Sustain. Energy Rev. 2019, 105, 105–128. [Google Scholar] [CrossRef]
- Jagadevan, S.; Banerjee, A.; Banerjee, C.; Guria, C.; Tiwari, R.; Baweja, M.; Shukla, P. Recent developments in synthetic biology and metabolic engineering in microalgae towards biofuel production. Biotechnol. Biofuels 2018, 11, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Phwan, C.K.; Ong, H.C.; Chen, W.-H.; Ling, T.C.; Ng, E.P.; Show, P.L. Overview: Comparison of pretreatment technologies and fermentation processes of bioethanol from microalgae. Energy Convers. Manag. 2018, 173, 81–94. [Google Scholar] [CrossRef]
- Bose, A.; O’Shea, R.; Lin, R.; Murphy, J.D. A perspective on novel cascading algal biomethane biorefinery systems. Bioresour. Technol. 2020, 304, 123027. [Google Scholar] [CrossRef]
- Wang, P.; Wang, H.; Qiu, Y.; Ren, L.; Jiang, B. Microbial characteristics in anaerobic digestion process of food waste for methane production—A review. Bioresour. Technol. 2018, 248, 29–36. [Google Scholar] [CrossRef]
- Liu, X.; Guo, Y.; Xu, D.; Guan, Q. A review on recent advances in clean microalgal bio-oil production via catalytic hydrothermal deoxygenation. J. Clean. Prod. 2022, 366, 132978. [Google Scholar] [CrossRef]
- Zhang, C.; Zeng, J.; Lin, L.; Peng, Y.; Li, X.; Gong, X. Microalgae liquefaction in ethanol to produce high-quality fuels: Effect of magnetic nanoparticles on nitrogen transformation. Fuel Process. Technol. 2023, 241, 107587. [Google Scholar] [CrossRef]
- Kurade, M.B.; Saha, S.; Salama, E.-S.; Patil, S.M.; Govindwar, S.P.; Jeon, B.-H. Acetoclastic methanogenesis led by Methanosarcina in anaerobic co-digestion of fats, oil and grease for enhanced production of methane. Bioresour. Technol. 2019, 272, 351–359. [Google Scholar] [CrossRef]
- Kim, J.; Kim, W.; Lee, C. Absolute dominance of hydrogenotrophic methanogens in full-scale anaerobic sewage sludge digesters. J. Environ. Sci. 2013, 25, 2272–2280. [Google Scholar] [CrossRef]
- Karan, H.; Roles, J.; Ross, I.L.; Ebrahimi, M.; Rackemann, D.; Rainey, T.; Hankamer, B. Solar biorefinery concept for sustainable co-production of microalgae-based protein and renewable fuel. J. Clean. Prod. 2022, 368, 132981. [Google Scholar] [CrossRef]
- Nagarajan, D.; Chang, J.S.; Lee, D.J. Pretreatment of microalgal biomass for efficient biohydrogen production—Recent insights and future perspectives. Bioresour. Technol. 2020, 302, 122871. [Google Scholar] [CrossRef]
- Feng, H.; Sun, C.; Zhang, C.; Chang, H.; Zhong, N.; Wu, W.; Wu, H.; Tan, X.; Zhang, M.; Ho, S.-H. Bioconversion of mature landfill leachate into biohydrogen and volatile fatty acids via microalgal photosynthesis together with dark fermentation. Energy Convers. Manag. 2022, 252, 115035. [Google Scholar] [CrossRef]
- de Farias Silva, C.E.; Sforza, E.; Bertucco, A. Chapter 3—Enhancing Carbohydrate Productivity in Photosynthetic Microorganism Production: A Comparison Between Cyanobacteria and Microalgae and the Effect of Cultivation Systems. In Advances in Feedstock Conversion Technologies for Alternative Fuels and Bioproducts; Hosseini, M., Ed.; Woodhead Publishing Series in Energy; Woodhead Publishing: Sawston, UK, 2019; pp. 37–67. ISBN 978-0-12-817937-6. [Google Scholar]
- Shanmugam, S.; Hari, A.; Kumar, D.; Rajendran, K.; Mathimani, T.; Atabani, A.E.; Brindhadevi, K.; Pugazhendhi, A. Recent developments and strategies in genome engineering and integrated fermentation approaches for biobutanol production from microalgae. Fuel 2021, 285, 119052. [Google Scholar] [CrossRef]
- Jeevan Kumar, S.P.; Vijay Kumar, G.; Dash, A.; Scholz, P.; Banerjee, R. Sustainable green solvents and techniques for lipid extraction from microalgae: A review. Algal Res. 2017, 21, 138–147. [Google Scholar] [CrossRef]
- Aramkitphotha, S.; Tanatavikorn, H.; Yenyuak, C.; Vitidsant, T. Low sulfur fuel oil from blends of microalgae pyrolysis oil and used lubricating oil: Properties and economic evaluation. Sustain. Energy Technol. Assess. 2019, 31, 339–346. [Google Scholar] [CrossRef]
- Wang, K.; Brown, R.C.; Homsy, S.; Martinez, L.; Sidhu, S.S. Fast pyrolysis of microalgae remnants in a fluidized bed reactor for bio-oil and biochar production. Bioresour. Technol. 2013, 127, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Tzanetis, K.F.; Posada, J.A.; Ramirez, A. Analysis of biomass hydrothermal liquefaction and biocrude-oil upgrading for renewable jet fuel production: The impact of reaction conditions on production costs and GHG emissions performance. Renew. Energy 2017, 113, 1388–1398. [Google Scholar] [CrossRef]
- de Souza, L.M.; Mendes, P.A.S.; Aranda, D.A.G. Oleaginous feedstocks for hydro-processed esters and fatty acids (HEFA) biojet production in southeastern Brazil: A multi-criteria decision analysis. Renew. Energy 2020, 149, 1339–1351. [Google Scholar] [CrossRef]
- Zhu, Z.; Sun, J.; Fa, Y.; Liu, X.; Lindblad, P. Enhancing microalgal lipid accumulation for biofuel production. Front. Microbiol. 2022, 13, 1–11. [Google Scholar] [CrossRef]
- Russell, C.; Rodriguez, C.; Yaseen, M. Microalgae for lipid production: Cultivation, extraction & detection. Algal Res. 2022, 66, 102765. [Google Scholar] [CrossRef]
- Xu, D.; Lin, G.; Guo, S.; Wang, S.; Guo, Y.; Jing, Z. Catalytic hydrothermal liquefaction of algae and upgrading of biocrude: A critical review. Renew. Sustain. Energy Rev. 2018, 97, 103–118. [Google Scholar] [CrossRef]
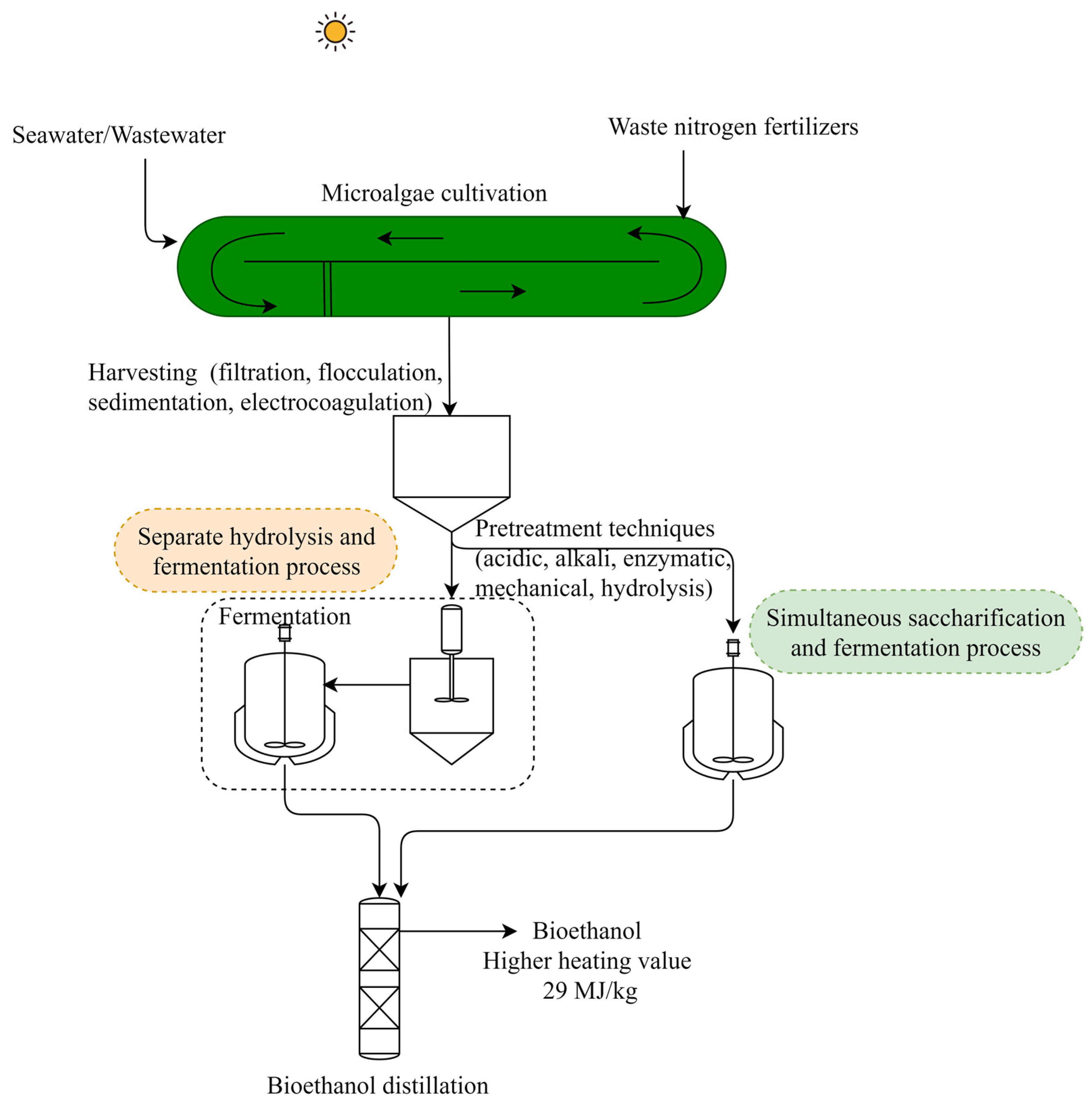
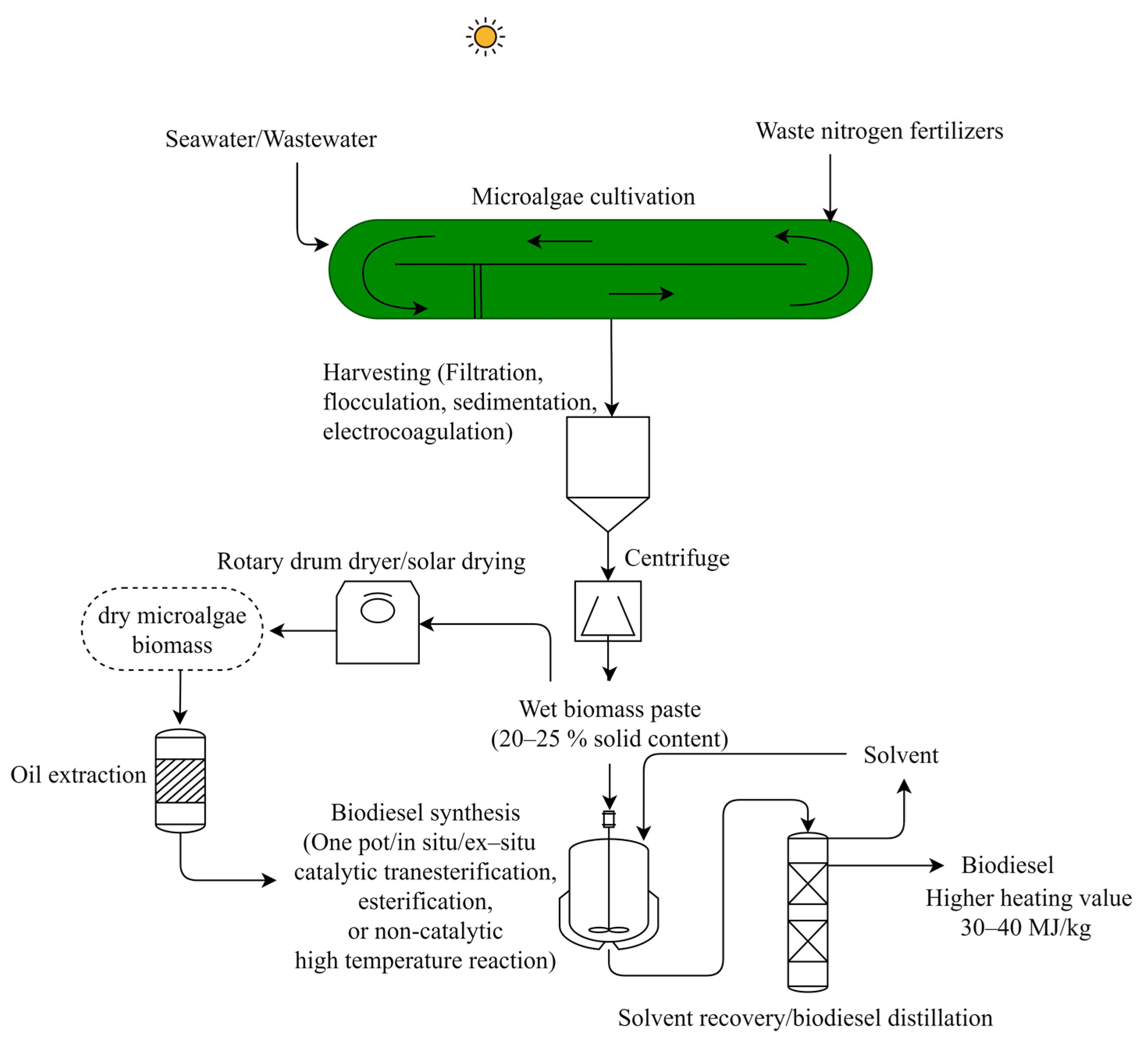










| Microalgae Feedstock | Pretreatment Process Parameters | Fermentation Process Conditions; Microorganism | Bioethanol Yield g g−1 (dry wt Basis) | Reference |
|---|---|---|---|---|
| Scenedesmus dimorphis | Organosolv (2:1), 24 h, 750 rpm, enzymatic hydrolysis | 150 rpm, 34 °C, S. cerevisiae | 0.266 | [34] |
| Chlorella vulgaris | 200 g L−1, H2SO4 at 120 °C for 20 min | 200 rpm, 30 °C; S. cerevisiae | 0.214 | [35] |
| Tetraselmis sp. | Chemo-enzymatic hydrolysis and pH 5, 60 °C | 150 rpm, 30 °C, 48 h, S. cerevisiae | 0.314 | [44] |
| Chlorella sp. | 3% H2SO4, 121 °C for 20 min | 30 °C, 150 rpm, 20 h; S. cerevisiae | 0.4 * | [36] |
| Leptolyngbia sp. | 1.5 N H2SO4, 0.8 bar, 3 h | 250 mL, 30 °C, 4 h, 150 rpm S. cerevisiae | 0.113 | [37] |
| Leptolyngbia valderiana | 1:15 biomass to liquid, H2SO4, MgSO4 at 121 °C for 20 min. | 200 rpm, 80 h, 30 °C; S. cerevisiae | 0.16 | [38] |
| Chlorococum sp. | H2SO4, pH 7, 160 °C | 30 °C, 48 h, 200 rpm | 0.52 | [39] |
| Chlorococum sp. | Supercritical CO2 | 200 rpm, 60 h, 30 °C, S. bayanus | 0.35 | [40] |
| Desmodesmus sp. | 10% solid loading, 120 °C, 30 min, H2SO4 | 28 °C, 120 rpm, 30 h, S. cerevisiae | 0.24 | [41] |
| Scenedesmus acuminatus | 2N H2SO4, autoclaving, pH 5.5 | 200 rpm, 30 °C, 80 h, S. cerevisiae | 0.12 | [42] |
| Tetraselmis sp. | 0.75% Sodium hydroxide for 10 min | 48 h, 30 °C, S. cerevisiae | 0.073 | [43] |
| Microalgae Feedstock | Biomass and Reaction Parameters | Reaction Technique | Biodiesel Conversion (%) | Reference |
|---|---|---|---|---|
| Chlorella pyrenoidosa | Wet biomass with 77% water content, methanol to biomass 8:1. H2SO4, 90 °C, 30 min. | Microwave-assisted | 86 | [57] |
| Nannochloropsis sp. | Wet biomass with 80% water content, solvent system methanol and ionic liquid, 65 °C, 15 min | Microwave-assisted | 36.7 | [58] |
| Aurantiochytrium sp. | Wet biomass with 40% water content, solvent ethanol, catalyst potassium carbonate, 60 °C, 60 min | Ultrasound-assisted | 80 | [59] |
| Nannochloropsis sp. | Wet biomass with 80 % water content, ethanol, and H2SO4, 150 °C, 120 min, 15 MPa | Supercritical carbon dioxide | 25 | [60] |
| Spirulina platensis | Wet biomass with 40 % water content, methanol hexane, 300 °C, 30 min, 67 bar | Supercritical methanol | 99 | [61] |
| Microalgae Feedstock | Solid Content (%) | HTL Processing Conditions | Biocrude Oil Yield (%) | H/C | O/C | References |
|---|---|---|---|---|---|---|
| Tetraselmis sp. | 10 | 275–350 °C, 30 min | 31 | 1.57 | 0.119 | [72] |
| Chlorella sp. | 20 | 320 °C, 1 MPa, 320 °C | 33.8 | 1.5 | 0.28 | [73] |
| Nannochloropsis gaditana | 16.6 | 374 °C, 200 rpm, 239–374 °C, 60 min | 15 | 1.45 | 0.23 | [74] |
| Neochloris sp. | 15 | 350 °C, 60 min | 36 | - | - | [75] |
| Botryococcus sp. | 15 | 350 °C, 60 min | 40 | - | - | [75] |
| Spirulina platensis | 9 | 315 °C, 15 min | 20.96 | 1.36 | 0.17 | [76] |
| Microalgae Feedstock | Pyrolysis Conditions | Bio-Oil Yield (%) | Reference |
|---|---|---|---|
| Spirulina platensis | 400–600 °C, molten salt mixture | 58–60 | [83] |
| Chlorella sorokiniana | 516 °C, 17 min. | 32.3 | [84] |
| Chlorella vulgaris and municipal sewage sludge (MSS) | 520 °C, argon gas | 45.6 | [85] |
| Nannochloropsis sp. | 15 mg sample, heated from 35 to 800 °C | 52.2 | [86] |
| Desmodesmus sp | 350–750 °C, | 41.9 | [87] |
| Microalgae Feedstock | Processing Parameters | Biojet Fuel Yield (%) | Reference |
|---|---|---|---|
| Chlorella sp. lipids | Hydrodeoxygenation, hydrocracking, and hydroisomerization with hydrogen gas at 410 °C and 50 bar pressure in the presence of NiO, MoO3/H-ZSM-5 catalyst | 76 | [98] |
| Botryococcus braunii oil | Hydrocracking at 400 °C and 200 bar pressure with hydrogen gas in the presence of a cobalt-molybdenum catalyst. | 15 | [99] |
| Chlorella sp. | Continuous hydrothermal liquefaction and hydroprocessing at 350 °C, residence time of 1 to 5 min, hydroprocessing in the presence of nickel and cobalt molybdenum catalyst at 405 °C. | 40 | [100] |
| Microalga sp. lipids | Hydrotreated at 260 °C and 40 bar in the presence of Ni/HBeta and HZSM-5 catalyst. | 78 | [101] |
| Nannochloropsis sp. lipids | Hydroprocessed at 375 °C, 5 bar in the presence of cobalt molybdenum aluminum oxide catalyst | 35 | [102] |
| Microalgal Biofuel | Microalgae Strain | Processing Technology | Higher Heating Value (HHV) (MJ/kg) | References |
|---|---|---|---|---|
| Biodiesel | Nannochloropsis salina | Transesterification reaction | 40 | [183] |
| Bioethanol | - | Fermentation | 20.7 | [184] |
| Pyrolytic bio-oil | Scenedesmus obliquus | Noncatalytic pyrolysis | 36.99 | [80] |
| Biocrude oil | Chlorella vulgaris, Chlorella sorokiniana, Scenedesmus simris | Hydrothermal liquefaction | 35.79 | [185] |
| Biomethane | Chlorella sp. | Anaerobic fermentation | 39.8 MJ/m3 | [186] |
| Biohydrogen | Chlorella sp. | Anaerobic fermentation | 12.74 MJ/m3 | [186] |
| Biojet fuel | Schizocytrium sp. | Transesterification, separation of ethyl esters by short path distillation, deoxygenation, and hydrocracking | 46.9 | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, S.; Das, P.; Abdul Quadir, M.; Thaher, M.I.; Mahata, C.; Sayadi, S.; Al-Jabri, H. Microalgal Feedstock for Biofuel Production: Recent Advances, Challenges, and Future Perspective. Fermentation 2023, 9, 281. https://doi.org/10.3390/fermentation9030281
Khan S, Das P, Abdul Quadir M, Thaher MI, Mahata C, Sayadi S, Al-Jabri H. Microalgal Feedstock for Biofuel Production: Recent Advances, Challenges, and Future Perspective. Fermentation. 2023; 9(3):281. https://doi.org/10.3390/fermentation9030281
Chicago/Turabian StyleKhan, Shoyeb, Probir Das, Mohammed Abdul Quadir, Mahmoud Ibrahim Thaher, Chandan Mahata, Sami Sayadi, and Hareb Al-Jabri. 2023. "Microalgal Feedstock for Biofuel Production: Recent Advances, Challenges, and Future Perspective" Fermentation 9, no. 3: 281. https://doi.org/10.3390/fermentation9030281
APA StyleKhan, S., Das, P., Abdul Quadir, M., Thaher, M. I., Mahata, C., Sayadi, S., & Al-Jabri, H. (2023). Microalgal Feedstock for Biofuel Production: Recent Advances, Challenges, and Future Perspective. Fermentation, 9(3), 281. https://doi.org/10.3390/fermentation9030281







