Valorization of Delonix regia Pods for Bioethanol Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biomass Collection and Estimation of Moisture Contents
2.2. Fourier-Transform Infrared Spectroscopy Assay
2.3. Optimization of Variables by RSM and Statistical Analysis
2.4. Acid Hydrolysis
2.5. Estimation of Reducing Sugars
2.6. Determination of Lignin Contents
2.7. Enzymatic Hydrolysis
2.8. Media and Inoculum Preparation
2.9. Fermentation and Quantification of Ethanol
3. Results and Discussion
3.1. Estimation of Moisture Contents
3.2. Chemical Analysis of D. regia Pods by FTIR
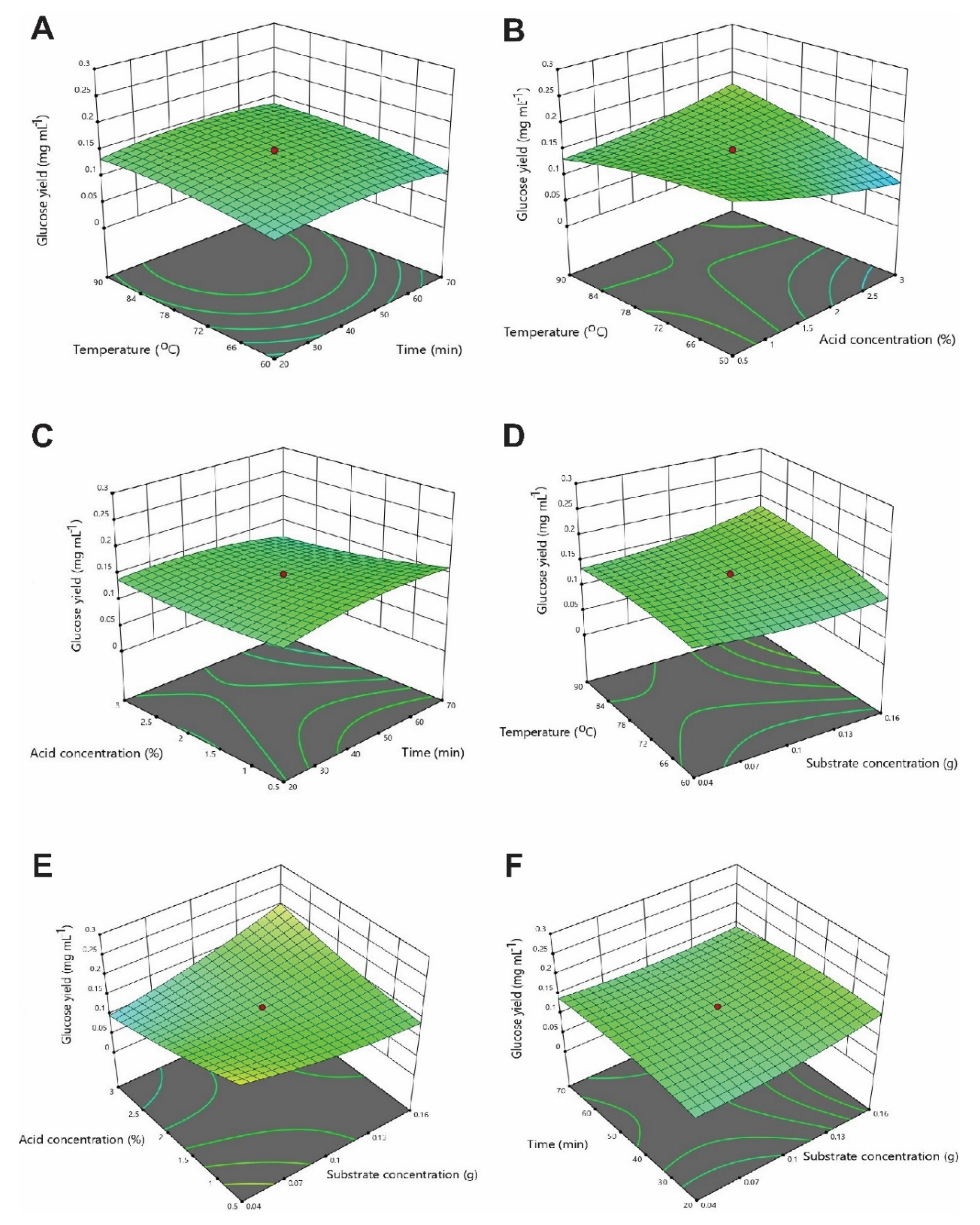
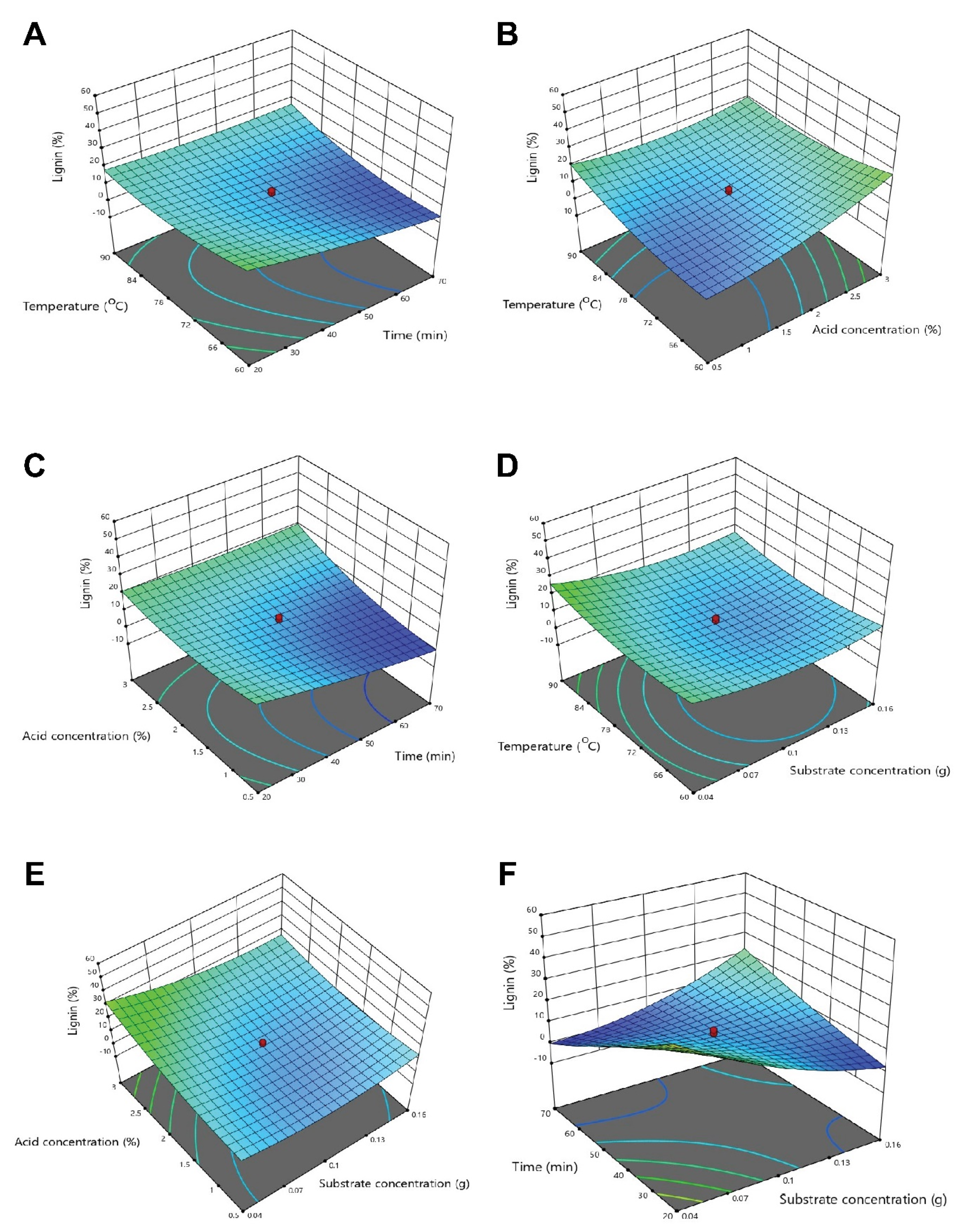
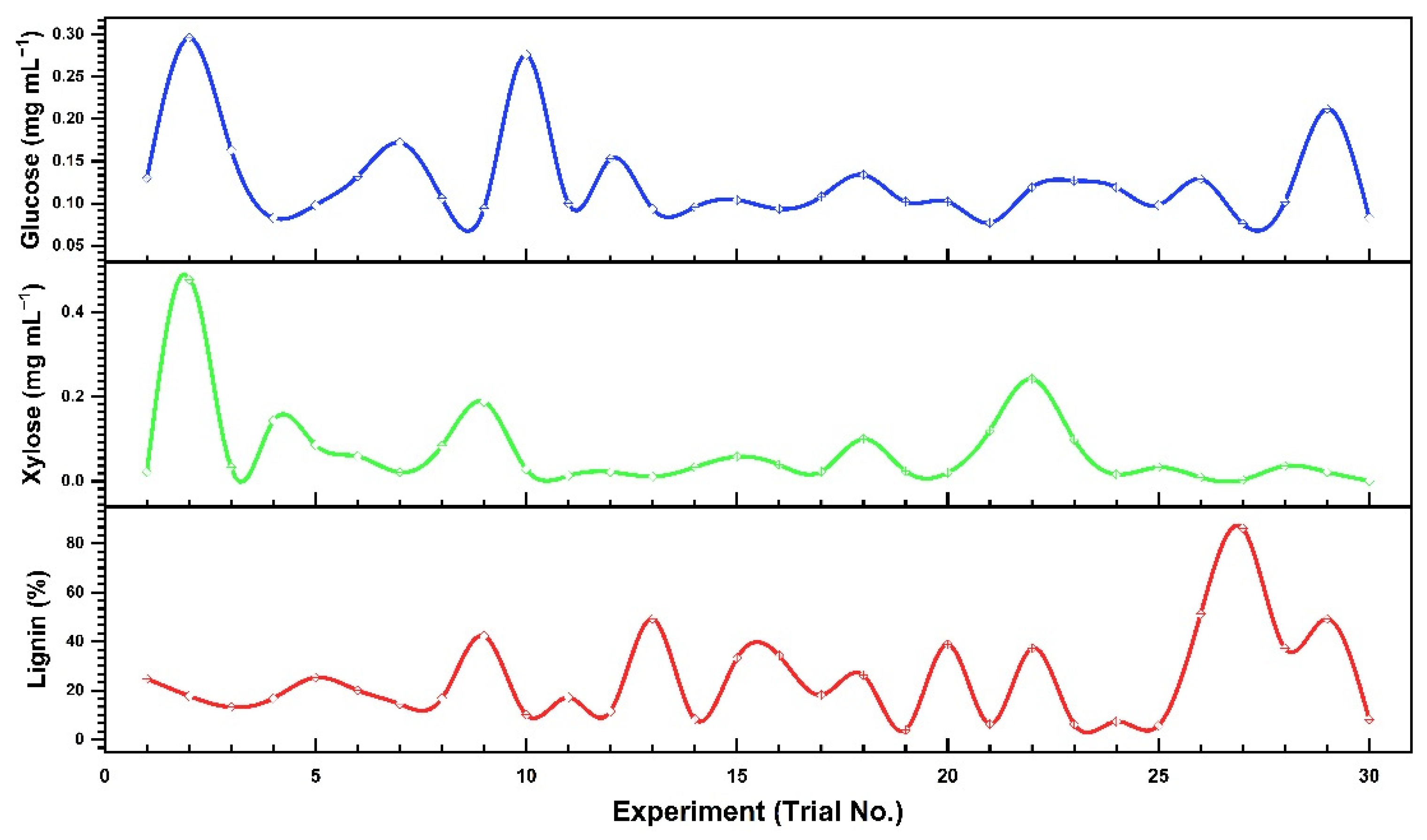
3.3. Optimization of Physicochemical Parameters by RSM
3.3.1. Optimization of Interactive Variables for Glucose Production
3.3.2. Optimization of Interactive Variables for Xylose Production
3.3.3. Optimization of Interactive Variables for Delignification
3.4. Experimental Testing and Optimal Experimental Values
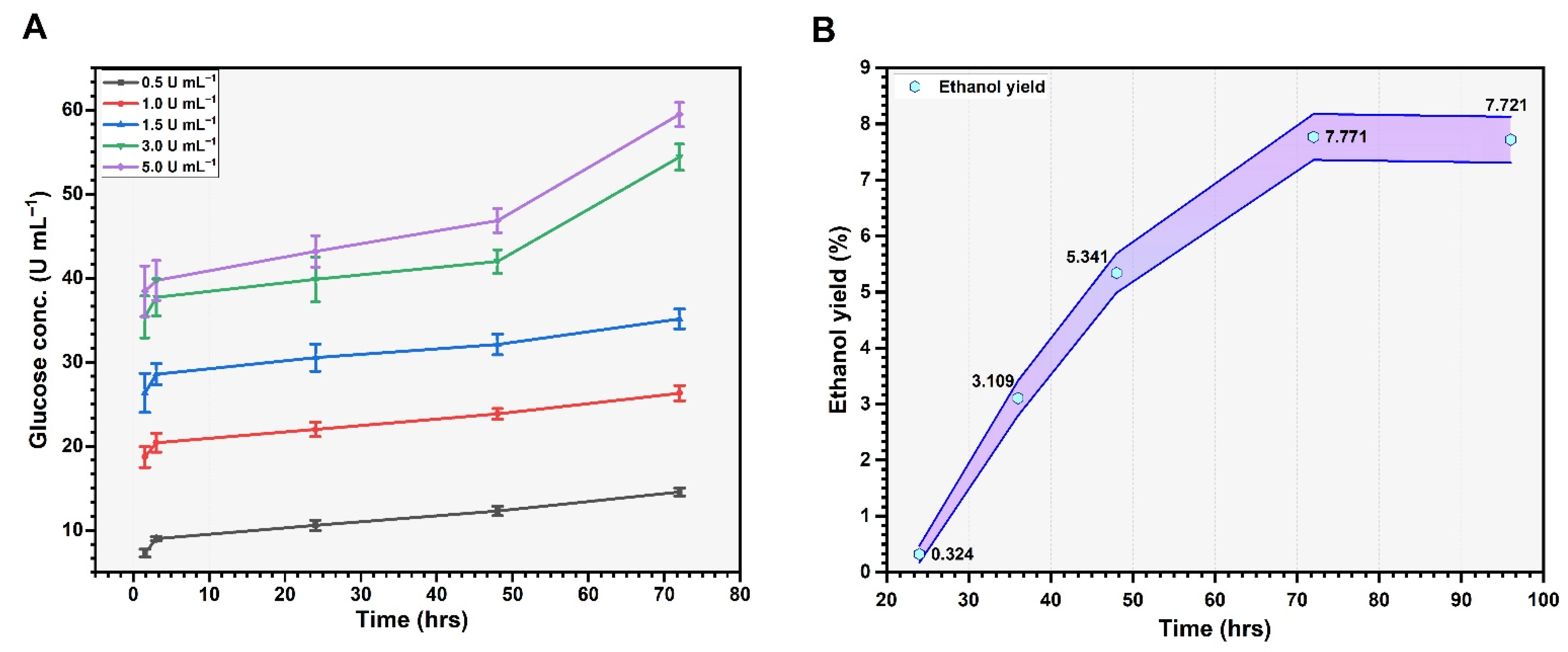
3.5. Enzymatic Hydrolysis and Bioethanol Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dessie, W.; Luo, X.; Wang, M.; Feng, L.; Liao, Y.; Wang, Z.; Yong, Z.; Qin, Z. Current advances on waste biomass transformation into value-added products. Appl. Microbiol. Biotechnol. 2020, 104, 4757–4770. [Google Scholar] [CrossRef]
- Tripathi, C.; Baredar, P.; Tripathi, L. Air pollution in Delhi:Biomass energy and suitable environmental policies are sustainable pathways for health safety. Curr. Sci. 2019, 117, 1153. [Google Scholar] [CrossRef]
- Casabar, J.T.; Unpaprom, Y.; Ramaraj, R. Fermentation of pineapple fruit peel wastes for bioethanol production. Biomass Convers. Biorefinery 2019, 9, 761–765. [Google Scholar] [CrossRef]
- Pasha, M.K.; Dai, L.; Liu, D.; Guo, M.; Du, W. An overview to process design, simulation and sustainability evaluation of biodiesel production. Biotechnol. Biofuels 2021, 14, 129. [Google Scholar] [CrossRef] [PubMed]
- Balat, M. Global bio-fuel processing and production trends. Energy Explor. Exploit. 2007, 25, 195–218. [Google Scholar] [CrossRef]
- Pant, S.; Ritika; Kuila, A. Chapter 8—Pretreatment of lignocellulosic biomass for bioethanol production. In Advanced Biofuel Technologies; Tuli, D., Kasture, S., Kuila, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 177–194. [Google Scholar] [CrossRef]
- Rodionova, M.V.; Poudyal, R.S.; Tiwari, I.; Voloshin, R.A.; Zharmukhamedov, S.K.; Nam, H.G.; Zayadan, B.K.; Bruce, B.D.; Hou, H.J.M.; Allakhverdiev, S.I. Biofuel production: Challenges and opportunities. Int. J. Hydrog. Energy. 2017, 42, 8450–8461. [Google Scholar] [CrossRef]
- Sarris, D.; Matsakas, L.; Aggelis, G.; Koutinas, A.A.; Papanikolaou, S. Aerated vs non-aerated conversions of molasses and olive mill wastewaters blends into bioethanol by Saccharomyces cerevisiae under non-aseptic conditions. Ind. Crops Prod. 2014, 56, 83–93. [Google Scholar] [CrossRef]
- Shah, S.H.; Raja, I.A.; Rizwan, M.; Rashid, N.; Mahmood, Q.; Shah, F.A.; Pervez, A. Potential of microalgal biodiesel production and its sustainability perspectives in Pakistan. Renew. Sust. Energ. Rev. 2018, 81, 76–92. [Google Scholar] [CrossRef]
- Chew, K.W.; Chia, S.R.; Show, P.L.; Ling, T.C.; Arya, S.S.; Chang, J.-S. Food waste compost as an organic nutrient source for the cultivation of Chlorella Vulgaris. Bioresour. Technol. 2018, 267, 356–362. [Google Scholar] [CrossRef]
- Hans, M.; Lugani, Y.; Chandel, A.K.; Rai, R.; Kumar, S. Production of first- and second-generation ethanol for use in alcohol-based hand sanitizers and disinfectants in India. Biomass Convers. Biorefinery 2021, 1–18. [Google Scholar] [CrossRef]
- Lin, Y.; Tanaka, S. Ethanol fermentation from biomass resources: Current state and prospects. Appl. Microbiol. Biotechnol. 2006, 69, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Ishola, M.M.; Babapour, A.B.; Gavitar, M.N.; Brandberg, T.; Taherzadeh, M.J. Effect of high solids loading on bacterial contamination in lignocellulosic ethanol production. Bioresources 2013, 8, 4429–4439. [Google Scholar] [CrossRef] [Green Version]
- Surendhiran, D.; Sirajunnisa, A.R. Role of genetic engineering in bioethanol production from algae. In Bioethanol Production from Food Crops; Ray, R.C., Ramachandran, S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 361–381. [Google Scholar] [CrossRef]
- Edgardo, A.; Carolina, P.; Manuel, R.; Juanita, F.; Baeza, J. Selection of thermotolerant yeast strains Saccharomyces cerevisiae for bioethanol production. Enzyme Microb. Technol. 2008, 43, 120–123. [Google Scholar] [CrossRef]
- Kumar, A.; Priyadarshinee, R.; Roy, A.; Dasgupta, D.; Mandal, T. Current techniques in rice mill effluent treatment: Emerging opportunities for waste reuse and waste-to-energy conversion. Chemosphere 2016, 164, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Broda, M.; Yelle, D.J.; Serwańska, K. Bioethanol production from lignocellulosic biomass-challenges and solutions. Molecules 2022, 27, 8717. [Google Scholar] [CrossRef]
- Scully, S.M.; Orlygsson, J. Recent advances in second generation ethanol production by thermophilic bacteria. Energies 2015, 8, 1–30. [Google Scholar] [CrossRef] [Green Version]
- McMillan, J.D. Pretreatment of lignocellulosic biomass. In Enzymatic Conversion of Biomass for Fuels Production; American Chemical Society: Washington, DC, USA, 1994; Volume 566, pp. 292–324. [Google Scholar]
- Esteghlalian, A.; Hashimoto, A.G.; Fenske, J.J.; Penner, M.H. Modeling and optimization of the dilute-sulfuric-acid pretreatment of corn stover, poplar and switchgrass. Bioresour. Technol. 1997, 59, 129–136. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef]
- De Oliveira Costa, A.; Silva, L.A.S.; Duarte, I.M.; Machado, M.; da Silva, G.Z.; da Silva, D.F.P.; da Costa Netto, A.P.; Rocha, D.I. Shoot proliferation and in vitro organogenesis from shoot apex and cotyledonary explants of royal poinciana (Delonix regia), an ornamental leguminous tree. Trees 2020, 34, 189–197. [Google Scholar] [CrossRef]
- Gilman, E.F.; Watson, D.G.; Klein, R.; Koeser, A.; Hilbert, D.; McLean, D. Delonix regia: Royal Poinciana. In The Institute of Food and Agricultural Sciences (IFAS); University of Florida: Gainesville, FL, USA, 2019. [Google Scholar]
- Mirahmadi, K.; Kabir, M.; Jeihanipour, A.; Karimi, K.; Taherzadeh, M. Alkaline pretreatment of spruce and birch to improve bioethanol and biogas production. Bioresources 2010, 5, 928–938. [Google Scholar]
- Anwar, Z.; Gulfraz, M.; Imran, M.; Asad, M.J.; Shafi, A.; Anwar, P.; Qureshi, R. Optimization of dilute acid pretreatment using response surface methodology for bioethanol production from cellulosic biomass of rice polish. Pak. J. Bot. 2012, 44, 169–176. [Google Scholar]
- Minitab, LLC. Minitab. Available online: https://www.minitab.com (accessed on 25 November 2022).
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of structural carbohydrates and lignin in biomass. Lab. Anal. Proced. 2008, 1617, 1–15. [Google Scholar]
- Anwar, Z.; Gulfraz, M.; Asad, M.J.; Imran, M.; Akram, Z.; Mehmood, S.; Rehman, A.; Anwar, P.; Sadiq, A. Bioethanol productions from rice polish by optimization of dilute acid pretreatment and enzymatic hydrolysis. Afr. J. Biotech. 2012, 11, 992–998. [Google Scholar]
- Imran, M.; Hussain, A.; Anwar, Z.; Zeeshan, N.; Yaseen, A.; Akmal, M.; Idris, M. Immobilization of fungal cellulase on calcium alginate and xerogel matrix. Waste Biomass Valorization 2020, 11, 1229–1237. [Google Scholar] [CrossRef]
- Ghose, T.K. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, X.; Tan, T. An novel immobilization method of Saccharomyces cerevisiae to sorghum bagasse for ethanol production. J. Biotechnol. 2007, 129, 415–420. [Google Scholar] [CrossRef]
- Bennett, C. Spectrophotometric acid dichromate method for the determination of ethyl alcohol. Am. J. Med. Technol. 1971, 37, 217–220. [Google Scholar]
- Comitre, A.L.D.; Reis, B.F. Automatic multicommutated flow system for ethanol determination in alcoholic beverages by spectrophotometry. Lab Rob. Autom. 2000, 12, 31–36. [Google Scholar] [CrossRef]
- Sugumaran, P.; Susan, V.P.; Ravichandran, P.; Seshadri, S. Production and characterization of activated carbon from banana empty fruit bunch and Delonix regia fruit pod. J. Sust. Energy Environ. 2012, 3, 125–132. [Google Scholar]
- Olugbenga, O.; James, O.; Lukman, B.; Olayinka, O.; Olusegun, O. Delonix regia seeds and pods: Characterization and its potential as a feedstock for thermochemical conversion. J. Appl. Chem. 2020, 13, 39–49. [Google Scholar]
- Adapa, P.; Tabil, L.; Schoenau, G. Compaction characteristics of barley, canola, oat and wheat straw. Biosyst. Eng. 2009, 104, 335–344. [Google Scholar] [CrossRef]
- Mani, S.; Tabil, L.G.; Sokhansanj, S. Effects of compressive force, particle size and moisture content on mechanical properties of biomass pellets from grasses. Biomass Bioenerg. 2006, 30, 648–654. [Google Scholar] [CrossRef]
- Jiménez, L.; Pérez, I.; de la Torre, M.J.; López, F.; Ariza, J. Use of formaldehyde for making wheat straw cellulose pulp. Bioresour. Technol. 2000, 72, 283–288. [Google Scholar] [CrossRef]
- Ghaly, A.E.; Al-Taweel, A. Physical and thermochemical properties of cereal straws. Energy Sources 1990, 12, 131–145. [Google Scholar] [CrossRef]
- Liang, C.; Das, K.C.; McClendon, R.W. The influence of temperature and moisture contents regimes on the aerobic microbial activity of a biosolids composting blend. Bioresour. Technol. 2003, 86, 131–137. [Google Scholar] [CrossRef]
- Pommier, S.; Chenu, D.; Quintard, M.; Lefebvre, X. Modelling of moisture-dependent aerobic degradation of solid waste. Waste Manag. 2008, 28, 1188–1200. [Google Scholar] [CrossRef] [PubMed]
- Asadieraghi, M.; Wan Daud, W.M.A. Characterization of lignocellulosic biomass thermal degradation and physiochemical structure: Effects of demineralization by diverse acid solutions. Energy Convers. Manag. 2014, 82, 71–82. [Google Scholar] [CrossRef]
- Zhuang, J.; Li, M.; Pu, Y.; Ragauskas, A.J.; Yoo, C.G. Observation of potential contaminants in processed biomass using fourier transform infrared spectroscopy. Appl. Sci. 2020, 10, 4345. [Google Scholar] [CrossRef]
- Tucker, M.P.; Nguyen, Q.A.; Eddy, F.P.; Kadam, K.L.; Gedvilas, L.M.; Webb, J.D. Fourier transform infrared quantitative analysis of sugars and lignin in pretreated softwood solid residues. Appl. Biochem. Biotechnol. 2001, 91, 51–61. [Google Scholar] [CrossRef]
- Awoyale, A.A.; Lokhat, D. Experimental determination of the effects of pretreatment on selected Nigerian lignocellulosic biomass in bioethanol production. Sci. Rep. 2021, 11, 557. [Google Scholar] [CrossRef]
- Li, M.; Han, G.; Song, Y.; Jiang, W.; Zhang, Y. Structure, composition, and thermal properties of cellulose fibers from pueraria lobata treated with a combination of steam explosion and laccase mediator system. BioResources 2016, 11, 6854–6866. [Google Scholar] [CrossRef] [Green Version]
- Onoji, S.E.; Iyuke, S.E.; Igbafe, A.I.; Nkazi, D.B. Rubber seed oil: A potential renewable source of biodiesel for sustainable development in sub-Saharan Africa. Energy Convers. Manag. 2016, 110, 125–134. [Google Scholar] [CrossRef]
- Oh, S.Y.; Yoo, D.I.; Shin, Y.; Kim, H.C.; Kim, H.Y.; Chung, Y.S.; Park, W.H.; Youk, J.H. Crystalline structure analysis of cellulose treated with sodium hydroxide and carbon dioxide by means of X-ray diffraction and FTIR spectroscopy. Carbohydr. Res. 2005, 340, 2376–2391. [Google Scholar] [CrossRef] [PubMed]
- Boukir, A.; Fellak, S.; Doumenq, P. Structural characterization of Argania spinosa Moroccan wooden artifacts during natural degradation progress using infrared spectroscopy (ATR-FTIR) and X-Ray diffraction (XRD). Heliyon 2019, 5, e02477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodirlau, R.; Teaca, C. Fourier transform infrared spectroscopy and thermal analysis of lignocellulose fillers treated with organic anhydrides. Rom. J. Phys. 2009, 54, 93–104. [Google Scholar]
- Sun, S.-L.; Wen, J.-L.; Ma, M.-G.; Sun, R.-C.; Jones, G.L. Structural features and antioxidant activities of degraded lignins from steam exploded bamboo stem. Ind. Crops Prod. 2014, 56, 128–136. [Google Scholar] [CrossRef]
- Colom, X.; Carrillo, F.; Nogués, F.; Garriga, P. Structural analysis of photodegraded wood by means of FTIR spectroscopy. Polym. Degrad. Stab. 2003, 80, 543–549. [Google Scholar] [CrossRef]
- Ezhumalai, S.; Thangavelu, V. Kinetic and optimization studies on the bioconversion of lignocellulosic material into ethanol. Bioresources 2010, 5, 1879–1894. [Google Scholar]
- Akkaravathasinp, S.; Narataruksa, P.; Prapainainar, C. Optimization of semi-batch reactive distillation using response surface method: Case study of esterification of acetic acid with methanol in a process simulation. Appl. Sci. Eng. Prog. 2019, 12, 209–215. [Google Scholar] [CrossRef]
- Khan, Y.; Munir, H.; Anwar, Z. Optimization of process variables for enhanced production of urease by indigenous Aspergillus niger strains through response surface methodology. Biocatal. Agric. Biotechnol. 2019, 20, 101202. [Google Scholar] [CrossRef]
- Nadeem, F.; Mehmood, T.; Anwar, Z.; Saeed, S.; Bilal, M.; Meer, B. Optimization of bioprocess steps through response surface methodology for the production of immobilized lipase using Chaetomium globosum via solid-state fermentation. Biomass Convers. Biorefinery 2021, 1–12. [Google Scholar] [CrossRef]
- Chawla, S.K.; Goyal, D. Optimization of pre-treatment using RSM on wheat straw and production of Lactic scid using thermotolerant, inhibitor tolerant and xylose utilizing Bacillus sonorenesis strain DGS15. Res. Sq. 2022. preprint. [Google Scholar] [CrossRef]
- Ifeanyichukwu, E. Bioethanol Production: An Overview. In Bioethanol Technologies; Freddie, I., Ed.; IntechOpen: Rijeka, Croatia, 2020; Chapter 1. [Google Scholar] [CrossRef]
- Expert, D. Version 7.0.0. Stat-Ease, Design Expert Inc., Minneapolis, USA. Available online: https://www.statease.com/software/design-expert/ (accessed on 12 November 2022).
- Chatkaew, C.; Panakkal, E.J.; Rodiahwati, W.; Kirdponpattara, S.; Chuetor, S.; Sriariyanun, M.; Cheenkachorn, K. Effect of sodium hydroxide pretreatment on released sugar yields from pomelo peels for biofuel production. E3S Web Conf. 2021, 302, 02015. [Google Scholar] [CrossRef]
- Gao, X.; Kumar, R.; Singh, S.; Simmons, B.A.; Balan, V.; Dale, B.E.; Wyman, C.E. Comparison of enzymatic reactivity of corn stover solids prepared by dilute acid, AFEX™, and ionic liquid pretreatments. Biotechnol. Biofuels 2014, 7, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kshirsagar, S.D.; Waghmare, P.R.; Loni, P.C.; Patil, S.A.; Govindwar, S.P. Dilute acid pretreatment of rice straw, structural characterization and optimization of enzymatic hydrolysis conditions by response surface methodology. RSC Adv. 2015, 5, 46525–46533. [Google Scholar] [CrossRef]
- Galbe, M.; Zacchi, G. A review of the production of ethanol from softwood. Appl. Microbiol. Biotechnol. 2002, 59, 618–628. [Google Scholar] [CrossRef]
- Brodeur, G.; Yau, E.; Badal, K.; Collier, J.; Ramachandran, K.B.; Ramakrishnan, S. Chemical and physicochemical pretreatment of lignocellulosic biomass: A review. Enzyme Res. 2011, 2011, 787532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Zhao, J.; Xu, F.; Li, Y. Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog. Energy Combust. Sci. 2014, 42, 35–53. [Google Scholar] [CrossRef]
- Rasmussen, H.; Sørensen, H.R.; Meyer, A.S. Formation of degradation compounds from lignocellulosic biomass in the biorefinery: Sugar reaction mechanisms. Carbohydr. Res. 2014, 385, 45–57. [Google Scholar] [CrossRef]
- Tang, J.; Chen, K.; Huang, F.; Xu, J.; Li, J. Characterization of the pretreatment liquor of biomass from the perennial grass, Eulaliopsis binata, for the production of dissolving pulp. Bioresour. Technol. 2013, 129, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.B.; Lundin, M.; Lennartsson, P.R.; Taherzadeh, M.J. Optimizing dilute phosphoric acid pretreatment of wheat straw in the laboratory and in a demonstration plant for ethanol and edible fungal biomass production using Neurospora intermedia. J. Chem. Technol. Biotechnol. 2017, 92, 1256–1265. [Google Scholar] [CrossRef] [Green Version]
- Parveen, H.; Tewari, L.; Pradhan, D.; Chaudhary, P. Comparative study of diverse pretreatment approaches to degrade lignin from Bambusa balcooa. BioResources 2022, 17, 5578–5599. [Google Scholar] [CrossRef]
- Kadivar, M.; Gauss, C.; Stanislas, T.T.; Ahrar, A.J.; Charca, S.; Savastano, H. Effect of bamboo species and pre-treatment method on physical and mechanical properties of bamboo processed by flattening-densification. Mater. Chem. Phys. 2022, 291, 126746. [Google Scholar] [CrossRef]
- Morais, W.G.; Pacheco, T.F.; Corrêa, P.S.; Martins, A.A.; Mata, T.M.; Caetano, N.S. Acid pretreatment of sugarcane biomass to obtain hemicellulosic hydrolisate rich in fermentable sugar. Energy Rep. 2020, 6, 18–23. [Google Scholar] [CrossRef]
- Sritrakul, N.; Nitisinprasert, S.; Keawsompong, S. Evaluation of dilute acid pretreatment for bioethanol fermentation from sugarcane bagasse pith. Agric. Nat. Resour. 2017, 51, 512–519. [Google Scholar] [CrossRef]
- Aboagye, D.; Banadda, N.; Kambugu, R.; Seay, J.; Kiggundu, N.; Zziwa, A.; Kabenge, I. Glucose recovery from different corn stover fractions using dilute acid and alkaline pretreatment techniques. J. Ecol. Environ. 2017, 41, 26. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhou, P.; Lv, X.; Xiao, W.; Gong, Y.; Lin, J.; Liu, Z. Use of sugarcane bagasse with different particle sizes to determine the relationship between physical properties and enzymatic hydrolysis. BioResources 2016, 11, 4745–4757. [Google Scholar] [CrossRef]
- Mardawati, E.; Putri, A.V.; Yuliana, T.; Rahimah, S.; Nurjanah, S.; Hanidah, I. Effects of substrate concentration on bioethanol production from oil palm empty fruit bunches with simultaneous saccharification and fermentation (SSF). IOP Conf. Ser. Earth Environ. Sci. 2019, 230, 12079. [Google Scholar] [CrossRef]
- Schwald, W.; Breuil, C.; Brownell, H.H.; Chan, M.; Saddler, J.M. Assessment of pretreatment conditions to obtain fast complete hydrolysis on high substrate concentrations. Appl. Biochem. Biotechnol. 1989, 20, 29–44. [Google Scholar] [CrossRef]
- Aliberti, A.; Ventorino, V.; Robertiello, A.; Galasso, M.; Blaiotta, G.; Comite, E.; Faraco, V.; Pepe, O. Effect of cellulase, substrate concentrations, and configuration processes on cellulosic ethanol production from pretreated Arundo donax. BioResources 2017, 12, 5321–5342. [Google Scholar] [CrossRef] [Green Version]
- Hendriks, A.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef]
- Roslan, A.; Yee, P.; Shah, U.; Aziz, S.; Hassan, M. Production of bioethanol from rice straw using cellulase by local Aspergillus sp. Int. J. Agric. Res. 2011, 6, 188–193. [Google Scholar] [CrossRef] [Green Version]
- Satimanont, S.; Luengnaruemitchai, A.; Wongkasemjit, S. Effect of temperature and time on dilute acid pretreatment of corn cobs. Int. J. Chem. Biol. Eng. 2012, 6, 333–337. [Google Scholar]
- Cui, Z.; Wan, C.; Shi, J.; Sykes, R.W.; Li, Y. Enzymatic digestibility of corn stover fractions in response to fungal pretreatment. Ind. Eng. Chem. Res. 2012, 51, 7153–7159. [Google Scholar] [CrossRef]
- De Carvalho, D.M.; Colodette, J.L. Comparative study of acid hydrolysis of lignin and polysaccharides in biomasses. BioResources 2017, 12, 6907–6923. [Google Scholar] [CrossRef]
- Lee, J.W.; Gwak, K.S.; Park, J.Y.; Park, M.J.; Choi, D.H.; Kwon, M.; Choi, I.G. Biological pretreatment of softwood Pinus densiflora by three white rot fungi. J. Microbiol. 2007, 45, 485–491. [Google Scholar]
- Yan, X.; Wang, Z.; Zhang, K.; Si, M.; Liu, M.; Chai, L.; Liu, X.; Shi, Y. Bacteria-enhanced dilute acid pretreatment of lignocellulosic biomass. Bioresour. Technol. 2017, 245, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Thite, V.S.; Nerurkar, A.S. Valorization of sugarcane bagasse by chemical pretreatment and enzyme mediated deconstruction. Sci. Rep. 2019, 9, 15904. [Google Scholar] [CrossRef] [Green Version]
- Sannigrahi, P.; Kim, D.H.; Jung, S.; Ragauskas, A. Pseudo-lignin and pretreatment chemistry. Energy Environ. Sci. 2011, 4, 1306–1310. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, Z.; Wang, T.; Zhang, Q.; Ma, L. Effect of the temperature on the dissolution of corn straw in ethanol solution. RSC Adv. 2016, 6, 102306–102314. [Google Scholar] [CrossRef]
- Soares, I.B.; Mendes, K.C.S.; Benachour, M.; Abreu, C.A.M. Evaluation of the effects of operational parameters in the pretreatment of sugarcane bagasse with diluted sulfuric acid using analysis of variance. Chem. Eng. Commun. 2017, 204, 1369–1390. [Google Scholar] [CrossRef]
- Patri, A.S.; McAlister, L.; Cai, C.M.; Kumar, R.; Wyman, C.E. CELF significantly reduces milling requirements and improves soaking effectiveness for maximum sugar recovery of Alamo switchgrass over dilute sulfuric acid pretreatment. Biotechnol. Biofuels 2019, 12, 177. [Google Scholar] [CrossRef]
- McIntosh, S.; Vancov, T. Optimisation of dilute alkaline pretreatment for enzymatic saccharification of wheat straw. Biomass Bioenerg. 2011, 35, 3094–3103. [Google Scholar] [CrossRef]
- Gao, J.; Anderson, D.; Levie, B. Saccharification of recalcitrant biomass and integration options for lignocellulosic sugars from Catchlight Energy’s sugar process (CLE Sugar). Biotechnol. Biofuels 2013, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Karmakar, B.; Samanta, S.; Halder, G. Delonix regia heterogeneous catalyzed two-step biodiesel production from Pongamia pinnata oil using methanol and 2-propanol. J. Clean. Prod. 2020, 255, 120313. [Google Scholar] [CrossRef]
- Saha, B.C.; Nichols, N.N.; Qureshi, N.; Cotta, M.A. Comparison of separate hydrolysis and fermentation and simultaneous saccharification and fermentation processes for ethanol production from wheat straw by recombinant Escherichia coli strain FBR5. Appl. Microbiol. Biotechnol. 2011, 92, 865–874. [Google Scholar] [CrossRef]
- Palmarola-Adrados, B.; Chotěborská, P.; Galbe, M.; Zacchi, G. Ethanol production from non-starch carbohydrates of wheat bran. Bioresour. Technol. 2005, 96, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Favaro, L.; Basaglia, M.; van Zyl, W.H.; Casella, S. Using an efficient fermenting yeast enhances ethanol production from unfiltered wheat bran hydrolysates. Appl. Energy 2013, 102, 170–178. [Google Scholar] [CrossRef]
- Siqueira, P.F.; Karp, S.G.; Carvalho, J.C.; Sturm, W.; Rodríguez-León, J.A.; Tholozan, J.-L.; Singhania, R.R.; Pandey, A.; Soccol, C.R. Production of bio-ethanol from soybean molasses by Saccharomyces cerevisiae at laboratory, pilot and industrial scales. Bioresour. Technol. 2008, 99, 8156–8163. [Google Scholar] [CrossRef] [PubMed]
- Nimbkar, N.; Ghanekar, A.; Joseph, R. Development of improved cultivars and management practices in sweet sorghum as a source for ethanol, Technology and applications for alternative uses of sorghum. In Proceedings of the National Seminar, Parbhani, India, 2–3 February 1987; Marathwada Agricultural University: Maharashtra, India, 1987; pp. 180–188. [Google Scholar]
- Buaban, B.; Inoue, H.; Yano, S.; Tanapongpipat, S.; Ruanglek, V.; Champreda, V.; Pichyangkura, R.; Rengpipat, S.; Eurwilaichitr, L. Bioethanol production from ball milled bagasse using an on-site produced fungal enzyme cocktail and xylose-fermenting Pichia Stipitis. J. Biosci. Bioeng. 2010, 110, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Markou, G.; Angelidaki, I.; Nerantzis, E.; Georgakakis, D. Bioethanol production by carbohydrate-enriched biomass of Arthrospira (Spirulina) platensis. Energies 2013, 6, 3937–3950. [Google Scholar] [CrossRef]
- Ho, S.-H.; Huang, S.-W.; Chen, C.-Y.; Hasunuma, T.; Kondo, A.; Chang, J.-S. Bioethanol production using carbohydrate-rich microalgae biomass as feedstock. Bioresour. Technol. 2013, 135, 191–198. [Google Scholar] [CrossRef] [PubMed]
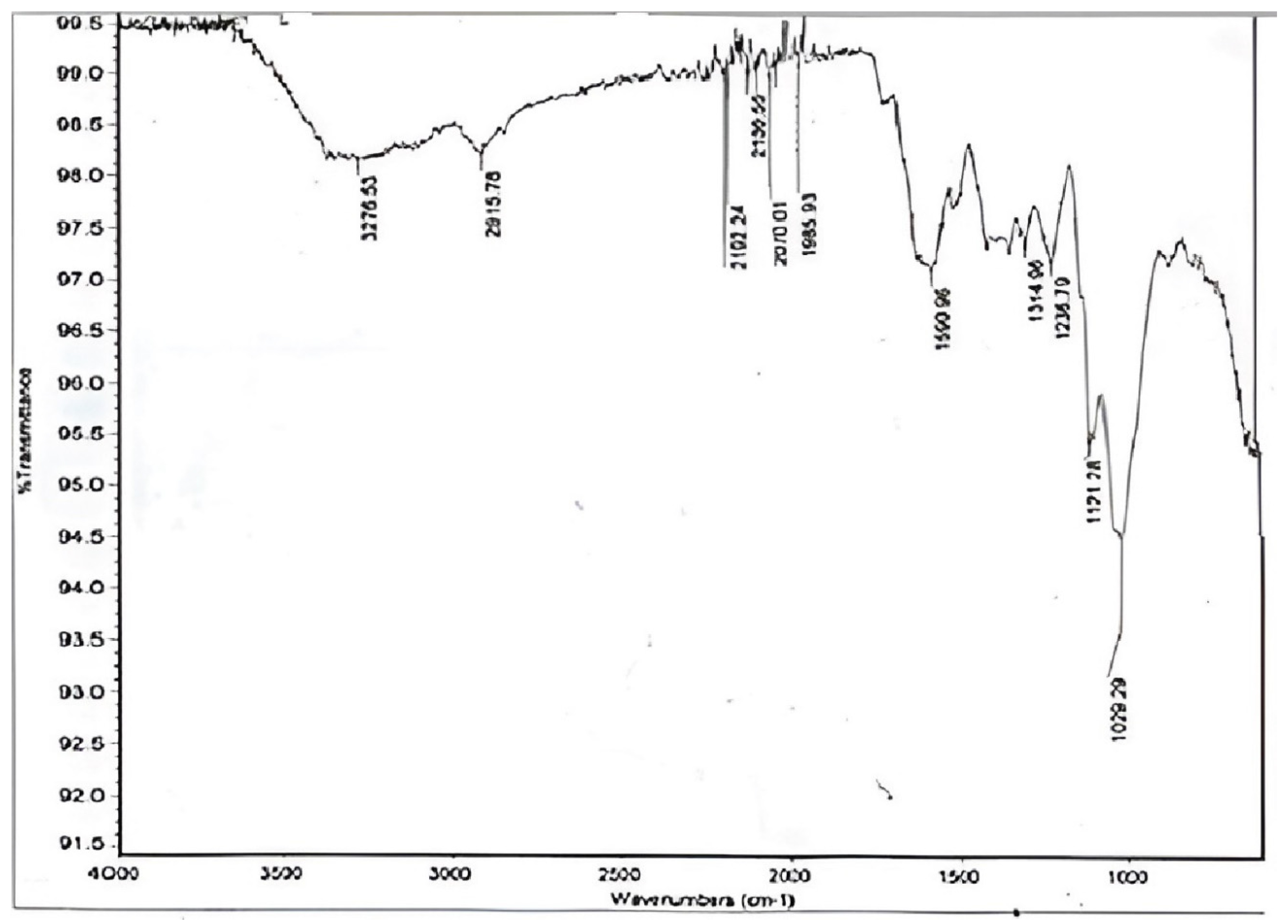

| Sr. No. | Wave Number (cm−1) | Functional Groups | Bond |
|---|---|---|---|
| 1 | 3276.53 | Alcohol | O-H |
| 2 | 2915.78 | Alkane | C-H |
| 3 | 2192.24 | Alkynes | C≡C |
| 4 | 2135.55 | Alkynes | C≡C |
| 5 | 2070.01 | Alkynes | C≡C |
| 6 | 1935.93 | Nitriles | C≡N |
| 7 | 1590.96 | Alkenes | C=C |
| 8 | 1314.96 | Carbonyl | C=O |
| 9 | 1236.79 | Amines | N-H and C=O (Hemicellulose) |
| 10 | 1121.28 | Sulfoxide | S=O |
| 11 | 1029.29 | Halogen | C-F, C-H (guaiacyl unit of lignin) and C-O (primary alcohol and cellulose) |
| Sr. No | Variables | Un-Coded Values | ||||
|---|---|---|---|---|---|---|
| 1 | Amount of substrate (g) | 0.02 | 0.04 | 0.1 | 0.16 | 0.22 |
| 2 | Acid (%) | 0.025 | 0.5 | 1.75 | 3 | 4.25 |
| 3 | Temperature (°C) | 45 | 60 | 75 | 90 | 100 |
| 4 | Time (minutes) | 10 | 20 | 45 | 70 | 90 |
| Coded values | ||||||
| −α | −1 | 0 | +1 | +α | ||
| Trial No. | Substrate (g) | Time (min) | Acid conc. (%) | Temperature (°C) | Glucose (mg·mL−1) | Xylose (mg·mL−1) | Insoluble Lignin (%) |
|---|---|---|---|---|---|---|---|
| 1 | 0.16 | 20 | 0.5 | 90 | 0.130 | 0.022 | 24.768 |
| 2 | 0.16 | 70 | 3.0 | 90 | 0.296 | 0.477 | 17.783 |
| 3 | 0.04 | 20 | 0.5 | 90 | 0.163 | 0.033 | 13.212 |
| 4 | 0.16 | 70 | 3.0 | 90 | 0.083 | 0.143 | 16.783 |
| 5 | 0.16 | 20 | 3.0 | 60 | 0.098 | 0.086 | 25.269 |
| 6 | 0.16 | 70 | 0.5 | 60 | 0.132 | 0.059 | 20.096 |
| 7 | 0.04 | 20 | 0.5 | 60 | 0.172 | 0.021 | 14.485 |
| 8 | 0.16 | 70 | 3.0 | 60 | 0.106 | 0.084 | 16.792 |
| 9 | 0.04 | 70 | 3.0 | 60 | 0.094 | 0.188 | 42.291 |
| 10 | 0.10 | 45 | 1.75 | 75 | 0.276 | 0.028 | 10.094 |
| 11 | 0.04 | 20 | 3.0 | 60 | 0.100 | 0.013 | 17.185 |
| 12 | 0.16 | 20 | 0.5 | 60 | 0.153 | 0.022 | 11.467 |
| 13 | 0.10 | 45 | 1.75 | 45 | 0.0935 | 0.011 | 49.292 |
| 14 | 0.22 | 45 | 1.75 | 75 | 0.0956 | 0.033 | 8.271 |
| 15 | 0.10 | 10 | 1.75 | 75 | 0.104 | 0.058 | 33.261 |
| 16 | 0.10 | 45 | 4.25 | 75 | 0.0935 | 0.039 | 34.231 |
| 17 | 0.10 | 45 | 1.75 | 75 | 0.108 | 0.023 | 18.184 |
| 18 | 0.02 | 45 | 1.75 | 75 | 0.134 | 0.1 | 26.291 |
| 19 | 0.10 | 95 | 1.75 | 75 | 0.102 | 0.024 | 3.91 |
| 20 | 0.16 | 20 | 3.0 | 90 | 0.102 | 0.02 | 38.849 |
| 21 | 0.16 | 70 | 0.5 | 90 | 0.077 | 0.12 | 6.292 |
| 22 | 0.16 | 20 | 3.0 | 90 | 0.119 | 0.242 | 37.292 |
| 23 | 0.10 | 45 | 1.75 | 100 | 0.127 | 0.099 | 6.246 |
| 24 | 0.10 | 45 | 0.25 | 75 | 0.119 | 0.016 | 7.332 |
| 25 | 0.10 | 70 | 3.0 | 90 | 0.098 | 0.033 | 5.651 |
| 26 | 0.16 | 70 | 0.5 | 60 | 0.129 | 0.009 | 51.192 |
| 27 | 0.10 | 45 | 1.75 | 100 | 0.076 | 0.003 | 86.192 |
| 28 | 0.10 | 70 | 1.75 | 75 | 0.102 | 0.036 | 37.242 |
| 29 | 0.04 | 70 | 0.5 | 60 | 0.212 | 0.022 | 49.209 |
| 30 | 0.16 | 70 | 0.5 | 60 | 0.083 | 0.001 | 8.207 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, Z.; Siddiqua, A.; Anwar, Z.; Munir, M. Valorization of Delonix regia Pods for Bioethanol Production. Fermentation 2023, 9, 289. https://doi.org/10.3390/fermentation9030289
Iqbal Z, Siddiqua A, Anwar Z, Munir M. Valorization of Delonix regia Pods for Bioethanol Production. Fermentation. 2023; 9(3):289. https://doi.org/10.3390/fermentation9030289
Chicago/Turabian StyleIqbal, Zafar, Adarsh Siddiqua, Zahid Anwar, and Muhammad Munir. 2023. "Valorization of Delonix regia Pods for Bioethanol Production" Fermentation 9, no. 3: 289. https://doi.org/10.3390/fermentation9030289
APA StyleIqbal, Z., Siddiqua, A., Anwar, Z., & Munir, M. (2023). Valorization of Delonix regia Pods for Bioethanol Production. Fermentation, 9(3), 289. https://doi.org/10.3390/fermentation9030289







