Improving the Nutritional Value of Plant Protein Sources as Poultry Feed through Solid-State Fermentation with a Special Focus on Peanut Meal—Advances and Perspectives
Abstract
:1. Introduction
2. The Application of PNM as Poultry Feed
| Years | Species | Control Diet | PNM Proportion | Production Performance | Other Effects | Reference | |
|---|---|---|---|---|---|---|---|
| 1946 | Broilers | Comb White Leghorn broilers | Corn-soybean | 50% substitute soybean meal | No differences | — | Heuser et al. [16] |
| 1959 | Broilers | Vantress × White Plymouth broilers | Corn-soybean | 50% substitute soybean meal | Decreased BW | — | Douglas and Harms [18] |
| 1989 | Broilers | Hybro broilers | Corn-soybean | 10% of diet | Decreased BW | — | EL Boushy and Raterink [19] |
| 1989 | Broilers | Hybro broilers | Corn-soybean | 15% of diet | Decreased BW | — | EL Boushy and Raterink [19] |
| 2001 | Broilers | Ross 208 broilers | Corn-soybean | 10% of diet | No differences | — | Costa et al. [20] |
| 2001 | Broilers | Ross 208 broilers | Corn-soybean | 20% of diet | No differences | — | Costa et al. [20] |
| 2001 | Broilers | Ross 208 broilers | Corn-soybean | 32% of diet | Decreased BW; increased F:G ratio | — | Costa et al. [20] |
| 2009 | Broilers | Vencob broilers | Corn-soybean | 25% substitute soybean meal | Increased BW | — | Ghadge et al. [25] |
| 2009 | Broilers | Vencob broilers | Corn-soybean | 50% substitute soybean meal | Increased BW; decreased F:G ratio | — | Ghadge et al. [25] |
| 2009 | Broilers | Vencob broilers | Corn-soybean | 75% substitute soybean meal | Increased BW; decreased F:G ratio | — | Ghadge et al. [25] |
| 2009 | Broilers | Vencob broilers | Corn-soybean | 100% substitute soybean meal | Increased BW; decreased F:G ratio | — | Ghadge et al. [25] |
| 2016 | Broilers | Lohman broilers | Corn-soybean | 50% substitute soybean meal | No differences | — | Ata [26] |
| 2016 | Broilers | Lohman broilers | Corn-soybean | 100% substitute soybean meal | Increased BW | — | Ata [26] |
| 2020 | Broilers | Ross 708 broilers | Corn-soybean | 12% of diet | Decreased BW, carcass and breast meat yields | Increased PUFA in breast meat | Toomer et al. [23,24] |
| 2022 | Broilers | Cobb 500 broilers | Corn-soybean | 10% of diet | No differences | Decreased serum TC, TG and LDL-cholesterol | Saleh et al. [17] |
| 2003 | Hens | Hyline W-36 White Leghorn hens | Corn-soybean | 3.8 g/hen per d | Decreased egg weight (first 6 weeks) | — | Pesti et al. [21] |
| 2013 | Hens | Rugao laying hens | Corn-soybean | 5.3% substitute soybean meal | No differences | Decreased egg yolk cholesterol content | Lu et al. [27] |
| 2013 | Hens | Rugao laying hens | Corn-soybean | 10.6% substitute soybean meal | No differences | Decreased egg yolk cholesterol content | Lu et al. [27] |
| 2013 | Hens | Rugao laying hens | Corn-soybean | 15.9% substitute soybean meal | No differences | — | Lu et al. [27] |
| 2022 | Ducks | Longyan laying ducks | Corn-soybean | 25% substitute soybean meal | No differences | Decreased serum GSH | Xia et al. [22] |
| 2022 | Ducks | Longyan laying ducks | Corn-soybean | 50% substitute soybean meal | Decreased feed intake | Decreased serum GSH | Xia et al. [22] |
| 2022 | Ducks | Longyan laying ducks | Corn-soybean | 75% substitute soybean meal | Decreased feed intake | Decreased serum GSH | Xia et al. [22] |
| 2022 | Ducks | Longyan laying ducks | Corn-soybean | 100% substitute soybean meal | Decreased FI and egg weight; increased F:G ratio | Decreased serum GSH; increased serum MDA | Xia et al. [22] |
3. The Limiting Factors of PNM as Poultry Feed
3.1. Imbalance of Amino Acid Composition
3.2. Phytate
3.3. Risk of Aflatoxin Pollution
3.4. Other Limiting Factors
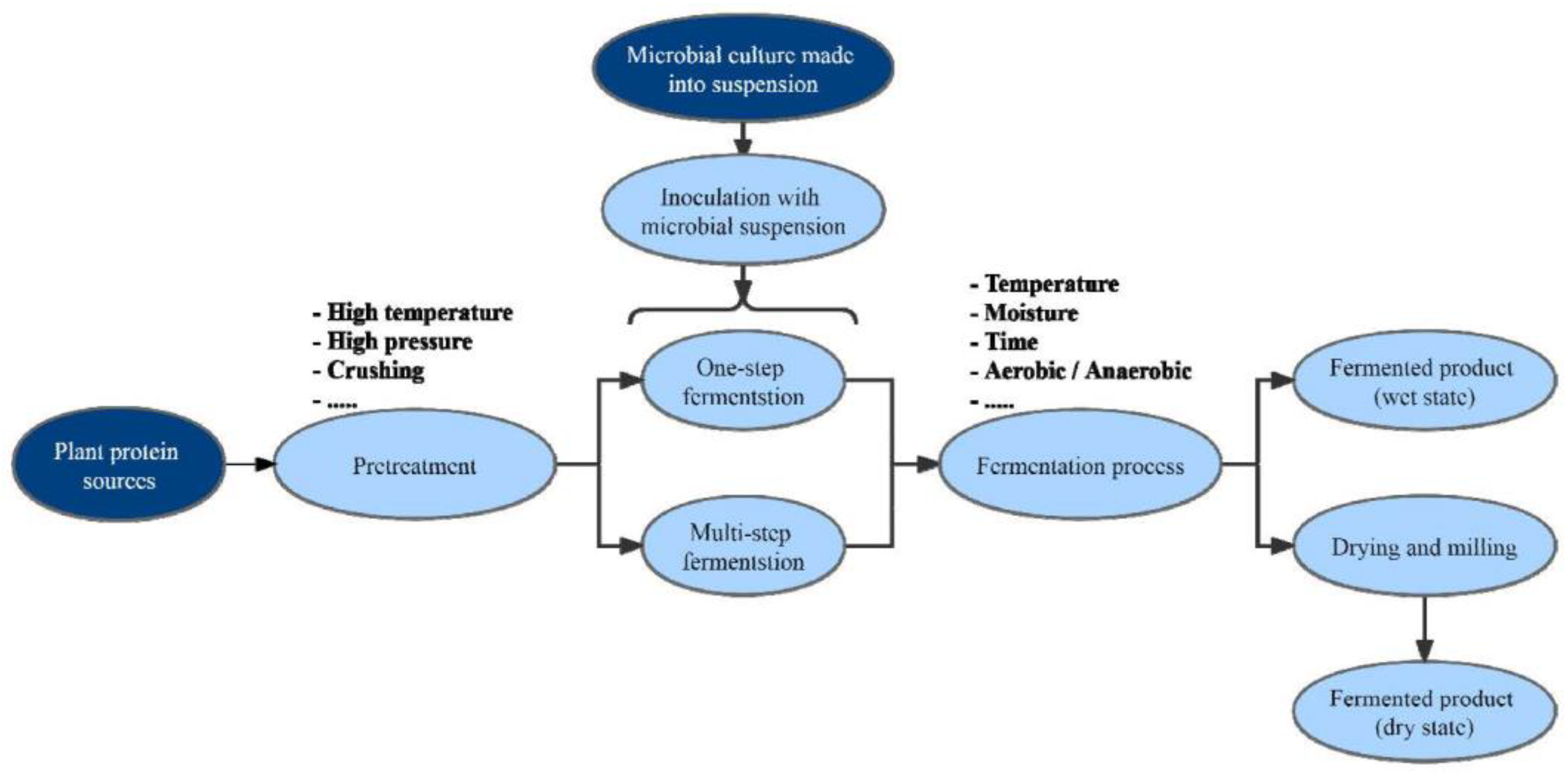
4. Solid-State Fermented Plant Protein Sources in the Diets of Poultry
| Years | Substrates | Microorganisms | Animals Applied | Nutritional Improvement/Beneficial Effects | Reference |
|---|---|---|---|---|---|
| 1998 | Soybean meal | Aspergillus usamii | Broilers | Decreased phytate phosphorus (complete degradation). Fermented soybean meal improved BW, retained phosphorus and femoral phosphorus in broilers | Hirabayashi et al. [13] |
| 2006 | Soybean meal | Aspergillus niger | Broilers | Fermented soybean meal improved BW, ileum villi length and width in broilers | Mathivanan et al. [72] |
| 2007 | Soybean meal | Aspergillus oryzae | Broilers | Fermented soybean meal improved ADG, FI, serum phosphorus, IgM and IgA; decreased serum urea nitrogen in broilers | Feng et al. [73] |
| 2016 | Soybean meal | Bacillus amyloliquefaciens | — | Decreased trypsin inhibitor, raffinose and stachyose; increased antioxidant activity and metal-chelating ability | Chi and Cho [74] |
| 2016 | Soybean meal | Bacillus subtilis | — | Decreased trypsin inhibitor and β-conglycinin | Seo and Cho [75] |
| 2020 & 2022 | Soybean meal | Bacillus amyloliquefaciens, Lactobacillus acidophilus and Saccharomyces cerevisiae | Broilers | Decreased glycinin and β-conglycinin; increased CP and TCA-SP. Fermented soybean meal improved energy digestibility and SID of amino acids in broilers | Li et al. [14,76] |
| 2023 | Soybean meal | Bacillus spp. yeast, Lactobacillus spp. and Clostridium spp. | Laying hens | Increased the CP, amino acids and organic acids; decreased NDF and ADF. Fermented soybean meal improved the laying performance, egg quality, intestinal barrier function and follicle development in hens | Lu et al. [67] |
| 2001 | Rapeseed meal | Rhizopus oligosporus | — | Increased nitrogen and protein contents; decreased glucosinolates, thiooxazolidones, phytate and CF | Vig and Walia [77] |
| 2016 | Rapeseed meal | Bacillus subtilis, Candida utilis and Enterococcus faecalis | Broilers | Increase CP and small peptides; decreased CF, glucosinolate, isothiocyanate, tannin and phytate. Fermented rapeseed meal improved antioxidant level and intestinal morphology of broilers | Hu et al. [15] |
| 2017 | Rapeseed meal | Lactobacillus acidophilus, Bacillus subtilis, and Aspergillus niger | Broilers | Fermented rapeseed meal decreased colonization of Salmonela and Typhimurium; improved growth performance | Ashayerizadeh et al. [78] |
| 2019 | Rapeseed meal | Bacillus licheniformis, Yeast and Lactobacillus | Broilers | Improved the sensory properties, CP, lactic acid and total amino acid; decreased glucosinolate and NDF. Fermented rapeseed meal improved productivity performances of broilers | Wang et al. [69] |
| 2022 | Rapeseed meal | Bacillus subtilis | Japanese quail | No significant differences were found | Wengerska et al. [79] |
| 2022 | Rapeseed meal | Bacillus subtilis and Aspergillus niger | Laying hens | Increased lactic acid bacteria and CP; decreased pH, dry matter, CF and anti-nutritional factors. Fermented rapeseed meal improved egg production and egg mass in hens | Taheri et al. [80] |
| 2012 | Cottonseed meal | Bacillus subtilis | Broilers | Decreased free gossypol. Fermented cottonseed meal improved growth performance and immunity in broilers | Tang et al. [81] |
| 2017 | Cottonseed meal | Bacillus subtilis, Aspergillus niger and Aspergillus oryzae | Broilers | Decreased CF and free gossypol; increased CP and lactic acid bacteria. Fermented cottonseed meal improved intestinal development and growth performance; decreased abdominal fat yield in broilers | Jazi et al. [82] |
| 2019 | Cottonseed meal | Candida tropicalis | Broilers | Fermented cottonseed meal decreased abdominal fat yield and subcutaneous fat thickness in broilers | Niu et al. [83] |
4.1. Soybean Meal
4.2. Rapeseed Meal
4.3. Cottonseed Meal
5. Improving the Nutritional Value of PNM through Solid-State Fermentation
5.1. Bio-Transformation and Bio-Conversion of PNM
5.2. Selection of Strains for Solid-State Fermentation of PNM
5.3. Framework for Future Research
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United States Department of Agriculture (USDA). Peanut 2022 World Production. Available online: https://ipad.fas.usda.gov/cropexplorer/cropview/commodityView.aspx?cropid=2221000 (accessed on 27 December 2022).
- Fletcher, S.M.; Shi, Z. An Overview of World Peanut Markets. Peanuts Genet. Process. Util. 2016, 1, 267–287. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, J.; Du, F. Potential Use of Peanut By-Products in Food Processing: A Review. J. Food Sci. Technol. 2012, 49, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Sales, J.M.; Resurreccion, A.V.A. Maximising Resveratrol and Piceid Contents in UV and Ultrasound Treated Peanuts. Food Chem. 2009, 117, 674–680. [Google Scholar] [CrossRef]
- Arrutia, F.; Binner, E.; Williams, P.; Waldron, K.W. Oilseeds beyond Oil: Press Cakes and Meals Supplying Global Protein Requirements. Trends Food Sci. Technol. 2020, 100, 88–102. [Google Scholar] [CrossRef]
- Batal, A.; Dale, N.; Café, M. Nutrient Composition of Peanut Meal. J. Appl. Poult. Res. 2005, 14, 254–257. [Google Scholar] [CrossRef]
- Gupta, Y.P. Anti-Nutritional and Toxic Factors in Food Legumes: A Review. Plant Foods Hum. Nutr. 1987, 37, 201–228. [Google Scholar] [CrossRef]
- Tola, M.; Kebede, B. Occurrence, Importance and Control of Mycotoxins: A Review. Cogent Food Agric. 2016, 2, 1191103. [Google Scholar] [CrossRef]
- Driggers, J.C.; Tarver, F.R. High Protein Peanut Oil Meal with Fish Meal-Fish Solubles Blend in Broiler Diets. Poult. Sci. 1958, 37, 1107–1111. [Google Scholar] [CrossRef]
- Shi, A.; Liu, H.; Liu, L.; Hu, H.; Wang, Q.; Adhikari, B. Isolation, Purification and Molecular Mechanism of a Peanut Protein-Derived ACE-Inhibitory Peptide. PLoS ONE 2014, 9, e111188. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Teng, D.; Wang, X.; Guan, Q.; Mao, R.; Hao, Y.; Wang, J. Enhancement of Nutritional and Antioxidant Properties of Peanut Meal by Bio-Modification with Bacillus Licheniformis. Appl. Biochem. Biotechnol. 2016, 180, 1227–1242. [Google Scholar] [CrossRef]
- Olukomaiya, O.; Fernando, C.; Mereddy, R.; Li, X.; Sultanbawa, Y. Solid-State Fermented Plant Protein Sources in the Diets of Broiler Chickens: A Review. Anim. Nutr. 2019, 5, 319. [Google Scholar] [CrossRef]
- Hirabayashi, M.; Matsui, T.; Yano, H.; Nakajima, T. Fermentation of Soybean Meal with Aspergillus Usamii Reduces Phosphorus Excretion in Chicks. Poult. Sci. 1998, 77, 552–556. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Li, C.; Chang, W.; Cai, H.; Liu, G. Effects of Fermentation on the Apparent Metabolizable Energy and Standardized Ileal Digestibility of Amino Acids in Soybean Meal Fed to Broiler Chickens. Fermentation 2022, 9, 23. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Y.; Li, A.; Wang, Z.; Zhang, X.; Yun, T.; Qiu, L.; Yin, Y. Effects of Fermented Rapeseed Meal on Antioxidant Functions, Serum Biochemical Parameters and Intestinal Morphology in Broilers. Food Agric. Immunol. 2016, 27, 182–193. [Google Scholar] [CrossRef]
- Heuser, G.F.; Norris, L.C.; McGinnis, J. Vegetable Protein Concentrates Fed Alone and in Combination with Soybean Oil Meal and Fish Meal as the Chief Supplementary Protein in Chick Starting Rations. Poult. Sci. 1946, 25, 130–136. [Google Scholar] [CrossRef]
- Saleh, A.A.; Nahla, A.; Amber, K.; Badawi, N.; Aboelenin, S.M.; Alzawqari, M.H.; Albogami, S.; Abdel-Moneim, A.M.E.; Soliman, M.M.; Shukry, M. Effect of Dietary Incorporation of Peanut and Linseed Meals with or without Enzyme Mixture on Physiological Performance of Broilers. Saudi J. Biol. Sci. 2022, 29, 103291. [Google Scholar] [CrossRef]
- Douglas, C.R.; Harms, R.H. Peanut Oil Meal as a Source of Protein in Broiler Diets. Poult. Sci. 1959, 38, 786–790. [Google Scholar] [CrossRef]
- El Boushy, A.R.; Raterink, R. Replacement of Soybean Meal by Cottonseed Meal and Peanut Meal or Both in Low Energy Diets for Broilers. Poult. Sci. 1989, 68, 799–804. [Google Scholar] [CrossRef]
- Costa, E.F.; Miller, B.R.; Pesti, G.M.; Bakalli, R.I.; Ewing, H.P. Studies on Feeding Peanut Meal as a Protein Source for Broiler Chickens. Poult. Sci. 2001, 80, 306–313. [Google Scholar] [CrossRef]
- Pesti, G.M.; Bakalli, R.I.; Driver, J.P.; Sterling, K.G.; Hall, L.E.; Bell, E.M. Comparison of Peanut Meal and Soybean Meal as Protein Supplements for Laying Hens. Poult. Sci. 2003, 82, 1274–1280. [Google Scholar] [CrossRef]
- Xia, W.G.; Abouelezz, K.F.M.; Makled, M.N.; Wang, S.; Chen, W.; Zhang, Y.N.; Elokil, A.A.; Wang, S.L.; Huang, X.B.; Li, K.C.; et al. Effects of Dietary Substitution of Peanut Meal for Soybean Meal on Egg Production, Egg Quality, Oxidative Status, and Yolk Fatty Acid Profile in Laying Ducks. Animal 2022, 16, 100652. [Google Scholar] [CrossRef] [PubMed]
- Toomer, O.T.; Livingston, M.; Wall, B.; Sanders, E.; Vu, T.; Malheiros, R.D.; Livingston, K.A.; Carvalho, L.V.; Ferket, P.R.; Dean, L.L. Feeding High-Oleic Peanuts to Meat-Type Broiler Chickens Enhances the Fatty Acid Profile of the Meat Produced. Poult. Sci. 2020, 99, 2236. [Google Scholar] [CrossRef] [PubMed]
- Toomer, O.T.; Sanders, E.; Vu, T.C.; Malheiros, R.D.; Redhead, A.K.; Livingston, M.L.; Livingston, K.A.; Carvalho, L.V.; Ferket, P.R. The Effects of High-Oleic Peanuts as an Alternative Feed Ingredient on Broiler Performance, Ileal Digestibility, Apparent Metabolizable Energy, and Histology of the Intestine. Transl. Anim. Sci. 2020, 4, txaa137. [Google Scholar] [CrossRef] [PubMed]
- Ghadge, V.N.; Upase, B.T.; Patil, P.V. Effect of Replacing Groundnut Cake by Soybean Meal on Performance of Broilers. Vet. World 2009, 2, 183–184. [Google Scholar]
- Ata, M. The Impact of Partial and Total Replacement of Soybean with Peanut Meal on Broilers Performance. J. Nat. Sci. Res. 2016, 6, 77–81. [Google Scholar]
- Lu, J.; Wang, K.H.; Tong, H.B.; Shi, S.R.; Wang, Q. Effects of Graded Replacement of Soybean Meal by Peanut Meal on Performance, Egg Quality, Egg Fatty Acid Composition and Cholesterol Content in Laying Hens. Arch. Für Geflügelkd. 2013, 77, 43–45. [Google Scholar]
- National Research Council. Nutrient Requirements of Poultry, 9th ed.; National Academies Press: Washington, DC, USA, 1994. [CrossRef]
- Zampiga, M.; Laghi, L.; Petracci, M.; Zhu, C.; Meluzzi, A.; Dridi, S.; Sirri, F. Effect of Dietary Arginine to Lysine Ratios on Productive Performance, Meat Quality, Plasma and Muscle Metabolomics Profile in Fast-Growing Broiler Chickens. J. Anim. Sci. Biotechnol. 2018, 9, 79. [Google Scholar] [CrossRef] [Green Version]
- Waldroup, P.W.; Harms, R.H. Amino Acid Supplementation of Peanut Meal Diets for Broiler Chicks. Poult. Sci. 1963, 42, 652–657. [Google Scholar] [CrossRef]
- Robbins, K.R. Threonine Requirement of the Broiler Chick as Affected by Protein Level and Source. Poult. Sci. 1987, 66, 1531–1534. [Google Scholar] [CrossRef]
- Zhang, Y.; Parsons, C.M. Effects of Overprocessing on the Nutritional Quality of Peanut Meal. Poult. Sci. 1996, 75, 514–518. [Google Scholar] [CrossRef]
- Chinese Feed Database News Web Center. Tables of Feed Composition and Nutritive Values in China, 31st ed. Available online: http://www.chinafeeddata.org.cn/admin/Zylist/index?type=cfdb3&year=2020 (accessed on 20 March 2023).
- Cowieson, A.J.; Sorbara, J.O.B.; Pappenberger, G.; Abdollahi, M.R.; Ravindran, V. Toward Standardized Amino Acid Matrices for Exogenous Phytase and Protease in Corn–Soybean Meal–Based Diets for Broilers. Poult. Sci. 2020, 99, 3196. [Google Scholar] [CrossRef]
- Cangussu, A.S.R.; Aires Almeida, D.; Aguiar, R.W.D.S.; Bordignon-Junior, S.E.; Viana, K.F.; Barbosa, L.C.B.; Cangussu, E.W.D.S.; Brandi, I.V.; Portella, A.C.F.; Dos Santos, G.R.; et al. Characterization of the Catalytic Structure of Plant Phytase, Protein Tyrosine Phosphatase-Like Phytase, and Histidine Acid Phytases and Their Biotechnological Applications. Enzym. Res. 2018, 2018, 8240698. [Google Scholar] [CrossRef] [Green Version]
- Nyman, M.E.; Bjorck, I.M. In Vivo Effects of Phytic Acid and Polyphenols on the Bioavailability of Polysaccharides and Other Nutrients. J. Food Sci. 1989, 54, 1332–1335. [Google Scholar] [CrossRef]
- Ren, H.; Li, T.; Wan, H.; Yue, J. Optimization of Extraction Condition for Phytic Acid from Peanut Meal by Response Surface Methodology. Resour. Technol. 2017, 3, 226–231. [Google Scholar] [CrossRef]
- Cowieson, A.J.; Acamovic, T.; Bedford, M.R. The Effects of Phytase and Phytic Acid on the Loss of Endogenous Amino Acids and Minerals from Broiler Chickens. Br. Poult. Sci. 2007, 45, 101–108. [Google Scholar] [CrossRef]
- Hunt, J.R.; Vanderpool, R.A. Apparent Copper Absorption from a Vegetarian Diet. Am. J. Clin. Nutr. 2001, 74, 803–807. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.K.; Gangoliya, S.S.; Singh, N.K. Reduction of Phytic Acid and Enhancement of Bioavailable Micronutrients in Food Grains. J. Food Sci. Technol. 2015, 52, 676–684. [Google Scholar] [CrossRef] [Green Version]
- Bohn, L.; Meyer, A.S.; Rasmussen, S.K. Phytate: Impact on Environment and Human Nutrition. A Challenge for Molecular Breeding. J. Zhejiang Univ. Sci. B 2008, 9, 165–191. [Google Scholar] [CrossRef] [Green Version]
- Babatunde, O.O.; Jendza, J.A.; Ader, P.; Xue, P.; Adedokun, S.A.; Adeola, O. Response of Broiler Chickens in the Starter and Finisher Phases to 3 Sources of Microbial Phytase. Poult. Sci. 2020, 99, 3997. [Google Scholar] [CrossRef]
- Walk, C.L.; Addo-Chidie, E.K.; Bedford, M.R.; Adeola, O. Evaluation of a Highly Soluble Calcium Source and Phytase in the Diets of Broiler Chickens. Poult. Sci. 2012, 91, 2255–2263. [Google Scholar] [CrossRef]
- Babatunde, O.O.; Cowieson, A.J.; Wilson, J.W.; Adeola, O. The Impact of Age and Feeding Length on Phytase Efficacy during the Starter Phase of Broiler Chickens. Poult. Sci. 2019, 98, 6742–6750. [Google Scholar] [CrossRef] [PubMed]
- Driver, J.P.; Atencio, A.; Edwards, H.M.; Pesti, G.M. Improvements in Nitrogen-Corrected Apparent Metabolizable Energy of Peanut Meal in Response to Phytase Supplementation. Poult. Sci. 2006, 85, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Stevens, A.J.; Saunders, C.N.; Spence, J.B.; Newham, A.G. Investigations into “Diseases” of Turkey Poults. Vet. Rec. 1960, 72, 627–628. [Google Scholar]
- Kana, J.R.; Gnonlonfin, B.G.J.; Harvey, J.; Wainaina, J.; Wanjuki, I.; Skilton, R.A.; Teguia, A. Assessment of Aflatoxin Contamination of Maize, Peanut Meal and Poultry Feed Mixtures from Different Agroecological Zones in Cameroon. Toxins 2013, 5, 884. [Google Scholar] [CrossRef]
- Chen, Y.C.; Liao, C.D.; Lin, H.Y.; Chiueh, L.C.; Shih, D.Y.C. Survey of Aflatoxin Contamination in Peanut Products in Taiwan from 1997 to 2011. J. Food Drug Anal. 2013, 21, 247–252. [Google Scholar] [CrossRef] [Green Version]
- Yu, B.; Jiang, H.; Pandey, M.K.; Huang, L.; Huai, D.; Zhou, X.; Kang, Y.; Varshney, R.K.; Sudini, H.K.; Ren, X.; et al. Identification of Two Novel Peanut Genotypes Resistant to Aflatoxin Production and Their SNP Markers Associated with Resistance. Toxins 2020, 12, 156. [Google Scholar] [CrossRef] [Green Version]
- Arias, R.S.; Sobolev, V.S.; Massa, A.N.; Orner, V.A.; Walk, T.E.; Ballard, L.L.; Simpson, S.A.; Puppala, N.; Scheffler, B.E.; de Blas, F.; et al. New Tools to Screen Wild Peanut Species for Aflatoxin Accumulation and Genetic Fingerprinting. BMC Plant Biol. 2018, 18, 170. [Google Scholar] [CrossRef] [Green Version]
- Taguchi, K.; Takaku, M.; Egner, P.A.; Morita, M.; Kaneko, T.; Mashimo, T.; Kensler, T.W.; Yamamoto, M. Generation of a New Model Rat: Nrf2 Knockout Rats Are Sensitive to Aflatoxin B1 Toxicity. Toxicol. Sci. 2016, 152, 40. [Google Scholar] [CrossRef] [Green Version]
- Rajput, S.A.; Zhang, C.; Feng, Y.; Wei, X.T.; Khalil, M.M.; Rajput, I.R.; Baloch, D.M.; Shaukat, A.; Rajput, N.; Qamar, H.; et al. Proanthocyanidins Alleviates AflatoxinB1-Induced Oxidative Stress and Apoptosis through Mitochondrial Pathway in the Bursa of Fabricius of Broilers. Toxins 2019, 11, 157. [Google Scholar] [CrossRef] [Green Version]
- Jiang, M.; Peng, X.; Fang, J.; Cui, H.; Yu, Z.; Chen, Z. Effects of Aflatoxin B1 on T-Cell Subsets and MRNA Expression of Cytokines in the Intestine of Broilers. Int. J. Mol. Sci. 2015, 16, 6945–6959. [Google Scholar] [CrossRef] [Green Version]
- Bedoya-Serna, C.M.; Michelin, E.C.; Massocco, M.M.; Carrion, L.C.S.; Godoy, S.H.S.; Lima, C.G.; Ceccarelli, P.S.; Yasui, G.S.; Rottinghaus, G.E.; Sousa, R.L.M.; et al. Effects of Dietary Aflatoxin B1 on Accumulation and Performance in Matrinxã Fish (Brycon cephalus). PLoS ONE 2018, 13, e0201812. [Google Scholar] [CrossRef] [Green Version]
- Fowler, J.; Li, W.; Bailey, C. Effects of a Calcium Bentonite Clay in Diets Containing Aflatoxin When Measuring Liver Residues of Aflatoxin B1 in Starter Broiler Chicks. Toxins 2015, 7, 3455. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Commission Regulation (EU) No 165/2010 of 26 February 2010 Amending Regulation (EC) No 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs as Regards Aflatoxins. Off. J. Eur. Union 2010, 50, 8–12. [Google Scholar]
- General Administration of Quality Supervision. Hygienical Standard for Feeds. In Inspection and Quarantine tPsRoC (GAQSIQ) GB/T 13078-2017; Standards Press of China: Beijing, China, 2017. [Google Scholar]
- Ding, X.; Li, P.; Bai, Y.; Zhou, H. Aflatoxin B1 in Post-Harvest Peanuts and Dietary Risk in China. Food Control 2012, 23, 143–148. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Manual on the Application of the HACCP System in Mycotoxin Prevention and Control; FAO: Rome, Italy, 2001. [Google Scholar]
- Bueno, D.J.; Casale, C.H.; Pizzolitto, R.P.; Salvano, M.A.; Oliver, G. Physical Adsorption of Aflatoxin B1 by Lactic Acid Bacteria and Saccharomyces Cerevisiae: A Theoretical Model. J. Food Prot. 2007, 70, 2148–2154. [Google Scholar] [CrossRef]
- Gao, X.; Ma, Q.; Zhao, L.; Lei, Y.; Shan, Y.; Ji, C. Isolation of Bacillus Subtilis: Screening for Aflatoxins B1, M1, and G1 Detoxification. Eur. Food Res. Technol. 2011, 232, 957–962. [Google Scholar] [CrossRef]
- Xu, L.; Ahmed, M.F.E.; Sangare, L.; Zhao, Y.; Selvaraj, J.N.; Xing, F.; Wang, Y.; Yang, H.; Liu, Y. Novel Aflatoxin-Degrading Enzyme from Bacillus Shackletonii L7. Toxins 2017, 9, 36. [Google Scholar] [CrossRef]
- Alberts, J.F.; Gelderblom, W.C.A.; Botha, A.; van Zyl, W.H. Degradation of Aflatoxin B(1) by Fungal Laccase Enzymes. Int. J. Food Microbiol. 2009, 135, 47–52. [Google Scholar] [CrossRef]
- Motomura, M.; Toyomasu, T.; Mizuno, K.; Shinozawa, T. Purification and Characterization of an Aflatoxin Degradation Enzyme from Pleurotus Ostreatus. Microbiol. Res. 2003, 158, 237–242. [Google Scholar] [CrossRef]
- Brock, P.M.; Murdock, C.C.; Martin, L.B. The History of Ecoimmunology and Its Integration with Disease Ecology. Integr. Comp. Biol. 2014, 54, 353–362. [Google Scholar] [CrossRef] [Green Version]
- Talcott, S.T.; Duncan, C.E.; Del Pozo-Insfran, D.; Gorbet, D.W. Polyphenolic and Antioxidant Changes during Storage of Normal, Mid, and High Oleic Acid Peanuts. Food Chem. 2005, 89, 77–84. [Google Scholar] [CrossRef]
- Lu, Z.; Zeng, N.; Jiang, S.; Wang, X.; Yan, H.; Gao, C. Dietary Replacement of Soybean Meal by Fermented Feedstuffs for Aged Laying Hens: Effects on Laying Performance, Egg Quality, Nutrient Digestibility, Intestinal Health, Follicle Development, and Biological Parameters in a Long-Term Feeding Period. Poult. Sci. 2023, 102, 102478. [Google Scholar] [CrossRef] [PubMed]
- Couri, S.; Da Costa Terzi, S.; Saavedra Pinto, G.A.; Pereira Freitas, S.; Augusto Da Costa, A.C. Hydrolytic Enzyme Production in Solid-State Fermentation by Aspergillus Niger 3T5B8. Process Biochem. 2000, 36, 255–261. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Wei, F.; Liu, X.; Yi, C.; Zhang, Y. Improvement of the Nutritional Value, Sensory Properties and Bioavailability of Rapeseed Meal Fermented with Mixed Microorganisms. LWT 2019, 112, 108238. [Google Scholar] [CrossRef]
- Rérat, A. Nutritional Value of Protein Hydrolysis Products (Oligopeptides and Free Amino Acids) as a Consequence of Absorption and Metabolism Kinetics. Arch. Tierernahr. 1995, 48, 23–36. [Google Scholar] [CrossRef]
- Li, C.; Zhang, B.; Wang, X.; Pi, X.; Wang, X.; Zhou, H.; Mai, K.; He, G. Improved Utilization of Soybean Meal through Fermentation with Commensal Shewanella sp. MR-7 in Turbot (Scophthalmus maximus L.). Microb. Cell Fact. 2019, 18, 214. [Google Scholar] [CrossRef] [Green Version]
- Mathivanan, R.; Selvaraj, P.; Nanjappan, K. Feeding of Fermented Soybean Meal on Broiler Performance. Int. J. Poult. Sci. 2006, 5, 868–872. [Google Scholar]
- Feng, J.; Liu, X.; Xu, Z.R.; Liu, Y.Y.; Lu, Y.P. Effects of Aspergillus Oryzae 3.042 Fermented Soybean Meal on Growth Performance and Plasma Biochemical Parameters in Broilers. Anim. Feed Sci. Technol. 2007, 134, 235–242. [Google Scholar] [CrossRef]
- Chi, C.H.; Cho, S.J. Improvement of Bioactivity of Soybean Meal by Solid-State Fermentation with Bacillus Amyloliquefaciens versus Lactobacillus Spp. and Saccharomyces Cerevisiae. LWT-Food Sci. Technol. 2016, 68, 619–625. [Google Scholar] [CrossRef]
- Seo, S.H.; Cho, S.J. Changes in Allergenic and Antinutritional Protein Profiles of Soybean Meal during Solid-State Fermentation with Bacillus Subtilis. LWT 2016, 70, 208–212. [Google Scholar] [CrossRef]
- Li, Y.; Guo, B.; Li, C.; Wang, W.; Wu, Z.; Liu, G.; Cai, H. Isolation of a Highly Efficient Antigenic-Protein-Degrading Bacillus Amyloliquefaciens and Assessment of Its Safety. Animals 2020, 10, 1144. [Google Scholar] [CrossRef]
- Pal Vig, A.; Walia, A. Beneficial Effects of Rhizopus Oligosporus Fermentation on Reduction of Glucosinolates, Fibre and Phytic Acid in Rapeseed (Brassica napus) Meal. Bioresour. Technol. 2001, 78, 309–312. [Google Scholar] [CrossRef]
- Ashayerizadeh, A.; Dastar, B.; Shams Shargh, M.; Sadeghi Mahoonak, A.; Zerehdaran, S. Fermented Rapeseed Meal Is Effective in Controlling Salmonella Enterica Serovar Typhimurium Infection and Improving Growth Performance in Broiler Chicks. Vet. Microbiol. 2017, 201, 93–102. [Google Scholar] [CrossRef]
- Wengerska, K.; Czech, A.; Knaga, S.; Drabik, K.; Próchniak, T.; Bagrowski, R.; Gryta, A.; Batkowska, J. The Quality of Eggs Derived from Japanese QuailfFed with the Fermented and Non-Fermented Rapeseed Meal. Foods 2022, 11, 2492. [Google Scholar] [CrossRef]
- Taheri, M.; Dastar, B.; Ashayerizadeh, O.; Mirshekar, R. The Effect of Fermented Rapeseed Meal on Production Performance, Egg Quality and Hatchability in Broiler Breeders after Peak Production. Br. Poult. Sci. 2022, 12, 1–9. [Google Scholar] [CrossRef]
- Tang, J.W.; Sun, H.; Yao, X.H.; Wu, Y.F.; Wang, X.; Feng, J. Effects of Replacement of Soybean Meal by Fermented Cottonseed Meal on Growth Performance, Serum Biochemical Parameters and Immune Function of Yellow-Feathered Broilers. Asian-Australas. J. Anim. Sci. 2012, 25, 393–400. [Google Scholar] [CrossRef] [Green Version]
- Jazi, V.; Boldaji, F.; Dastar, B.; Hashemi, S.R.; Ashayerizadeh, A. Effects of Fermented Cottonseed Meal on the Growth Performance, Gastrointestinal Microflora Population and Small Intestinal Morphology in Broiler Chickens. Br. Poult. Sci. 2017, 58, 402–408. [Google Scholar] [CrossRef]
- Niu, J.L.; Zhang, J.; Wei, L.Q.; Zhang, W.J.; Nie, C.X. Effect of Fermented Cottonseed Meal on the Lipid-Related Indices and Serum Metabolic Profiles in Broiler Chickens. Animals 2019, 9, 930. [Google Scholar] [CrossRef] [Green Version]
- Domagalski, J.M.; Kollipara, K.P.; Bates, A.H.; Brandon, D.L.; Friedman, M.; Hymowitz, T. Nulls for the Major Soybean Bowman-Birk Protease Inhibitor in the Genus Glycine. Crop Sci. 1992, 32, 1502–1505. [Google Scholar] [CrossRef]
- Liener, I.E. Implications of Antinutritional Components in Soybean Foods. Crit. Rev. Food Sci. Nutr. 1994, 34, 31–67. [Google Scholar] [CrossRef]
- Wang, C.; Shi, C.; Su, W.; Jin, M.; Xu, B.; Hao, L.; Zhang, Y.; Lu, Z.; Wang, F.; Wang, Y.; et al. Dynamics of the Physicochemical Characteristics, Microbiota, and Metabolic Functions of Soybean Meal and Corn Mixed Substrates during Two-Stage Solid-State Fermentation. mSystems 2020, 5, e00501-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, K.J.; Lee, C.H.; Sung, W.K. Aspergillus Oryzae GB-107 Fermentation Improves Nutritional Quality of Food Soybeans and Feed Soybean Meals. J. Med. Food 2004, 7, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Jazi, V.; Mohebodini, H.; Ashayerizadeh, A.; Shabani, A.; Barekatain, R. Fermented Soybean Meal Ameliorates Salmonella Typhimurium Infection in Young Broiler Chickens. Poult. Sci. 2019, 98, 5648–5660. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Kim, T.H.; Lee, S.K.; Chang, K.H.; Cho, S.J.; Lee, K.W.; An, B.K. The Use of Fermented Soybean Meals during Early Phase Affects Subsequent Growth and Physiological Response in Broiler Chicks. Asian-Australas. J. Anim. Sci. 2016, 29, 1287–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.; Liu, J.; Chen, J.; Pirzado, S.A.; Li, Y.; Cai, H.; Liu, G. Effects of Fermentation on Standardized Ileal Digestibility of Amino Acids and Apparent Metabolizable Energy in Rapeseed Meal Fed to Broiler Chickens. Animals 2020, 10, 1774. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, M.K.; Mishra, A.S. Glucosinolates in Animal Nutrition: A Review. Anim. Feed Sci. Technol. 2007, 132, 1–27. [Google Scholar] [CrossRef]
- Xu, F.Z.; Zeng, X.G.; Ding, X.L. Effects of Replacing Soybean Meal with Fermented Rapeseed Meal on Performance, Serum Biochemical Variables and Intestinal Morphology of Broilers. Asian-Australas. J. Anim. Sci. 2012, 25, 1734–1741. [Google Scholar] [CrossRef]
- He, T.; Zhang, H.J.; Wang, J.; Wu, S.G.; Yue, H.Y.; Qi, G.H. Application of Low-Gossypol Cottonseed Meal in Laying Hens’ Diet. Poult. Sci. 2015, 94, 2456–2463. [Google Scholar] [CrossRef]
- Zhao, F.; Zheng, M.; Xu, X. Microbial Conversion of Agro-Processing Waste (Peanut Meal) to Rhamnolipid by Pseudomonas Aeruginosa: Solid-State Fermentation, Water Extraction, Medium Optimization and Potential Applications. Bioresour. Technol. 2023, 369, 128426. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, B.; Li, F.; Xu, K.; Ma, C.; Tao, F.; Li, Q.; Xu, P. Highly Efficient Production of D-Lactate by Sporolactobacillus sp. CASD with Simultaneous Enzymatic Hydrolysis of Peanut Meal. Appl. Microbiol. Biotechnol. 2011, 89, 1009–1017. [Google Scholar] [CrossRef]
- Shen, N.; Qin, Y.; Wang, Q.; Liao, S.; Zhu, J.; Zhu, Q.; Mi, H.; Adhikari, B.; Wei, Y.; Huang, R. Production of Succinic Acid from Sugarcane Molasses Supplemented with a Mixture of Corn Steep Liquor Powder and Peanut Meal as Nitrogen Sources by Actinobacillus Succinogenes. Lett. Appl. Microbiol. 2015, 60, 544–551. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Lu, X.; Zhang, H.; Wang, L.; Guo, X.; Qi, X.; Qian, H. Isolation and Identification of an Antioxidant Peptide Prepared from Fermented Peanut Meal Using Bacillus Subtilis Fermentation. Int. J. Food Prop. 2014, 17, 1237–1253. [Google Scholar] [CrossRef]
- Hariharan, S.; Patti, A.; Arora, A. Functional Proteins from Biovalorization of Peanut Meal: Advances in Process Technology and Applications. Plant Foods Hum. Nutr. 2023, 78, 13–24. [Google Scholar] [CrossRef]
- Wei, M.; Tang, L.; Yang, Q.; Yu, L. Membrane Separation and Antioxidant Activity Research of Peanut Peptides. Appl. Mech. Mater. 2012, 209–211, 2009–2012. [Google Scholar] [CrossRef]
- Su, G.; Zheng, L.; Cui, C.; Yang, B.; Ren, J.; Zhao, M. Characterization of Antioxidant Activity and Volatile Compounds of Maillard Reaction Products Derived from Different Peptide Fractions of Peanut Hydrolysate. Food Res. Int. 2011, 44, 3250–3258. [Google Scholar] [CrossRef]
- Wang, N.F.; Yan, Z.; Li, C.Y.; Jiang, N.; Liu, H.J. Antioxidant Activity of Peanut Flour Fermented by Lactic Acid Bacteria. J. Food Biochem. 2011, 35, 1514–1521. [Google Scholar] [CrossRef]
- White, B.L.; Oakes, A.J.; Shi, X.; Price, K.M.; Lamb, M.C.; Sobolev, V.S.; Sanders, T.H.; Davis, J.P. Development of a Pilot-Scale Process to Sequester Aflatoxin and Release Bioactive Peptides from Highly Contaminated Peanut Meal. LWT-Food Sci. Technol. 2013, 51, 492–499. [Google Scholar] [CrossRef]
- Štursová, M.; Žifčáková, L.; Leigh, M.B.; Burgess, R.; Baldrian, P. Cellulose Utilization in Forest Litter and Soil: Identification of Bacterial and Fungal Decomposers. FEMS Microbiol. Ecol. 2012, 80, 735–746. [Google Scholar] [CrossRef]
- Teusink, B.; Smid, E.J. Modelling Strategies for the Industrial Exploitation of Lactic Acid Bacteria. Nat. Rev. Microbiol. 2006, 4, 46–56. [Google Scholar] [CrossRef]
- Li, C.; Wang, S.; Chen, S.; Wang, X.; Deng, X.; Liu, G.; Chang, W.; Beckers, Y.; Cai, H. Screening and Characterization of Pediococcus Acidilactici LC-9-1 toward Selection as a Potential Probiotic for Poultry with Antibacterial and Antioxidative Properties. Antioxidants 2023, 12, 215. [Google Scholar] [CrossRef]
- Forestier, C.; De Champs, C.; Vatoux, C.; Joly, B. Probiotic Activities of Lactobacillus Casei Rhamnosus: In Vitro Adherence to Intestinal Cells and Antimicrobial Properties. Res. Microbiol. 2001, 152, 167–173. [Google Scholar] [CrossRef] [PubMed]

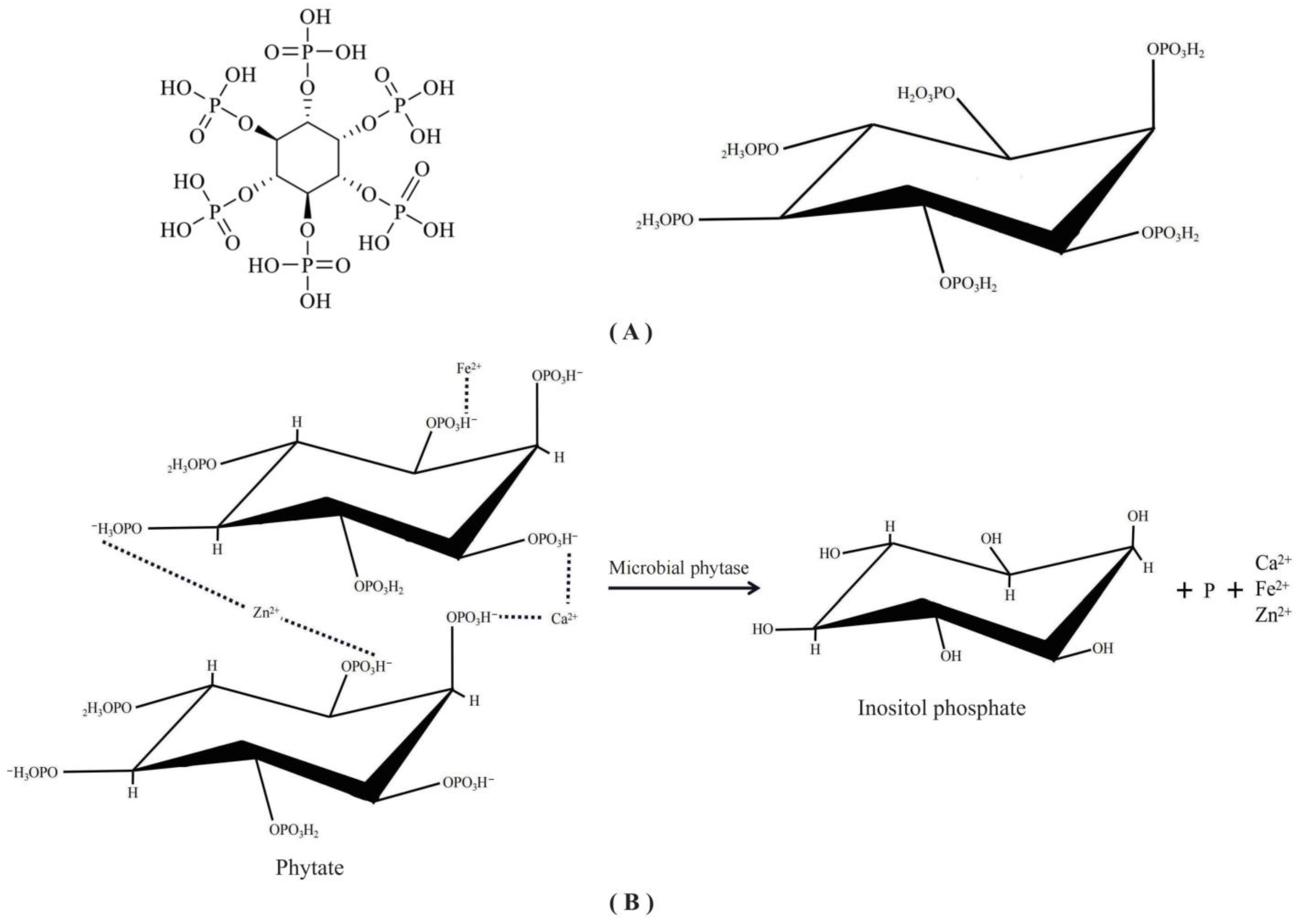
| Items | PNM | Soybean Meal | ||
|---|---|---|---|---|
| Zhang’s Report [32] (USA) | Chinese Feed Database [33] (China) | Cowieson’s Report [34] (New Zealand) | Chinese Feed Database [33] (China) | |
| CP, % | 47.8 | 47.8 | 48.2 | 47.9 |
| Essential amino acids | ||||
| Methionine | 0.47 | 0.41 | 0.66 | 0.68 |
| Lysine | 1.66 | 1.40 | 2.98 | 2.99 |
| Threonine | 1.31 | 1.11 | 2.02 | 1.85 |
| Arginine | 5.90 | 4.88 | 3.68 | 3.43 |
| Isoleucine | 1.60 | 1.25 | 2.11 | 2.10 |
| Leucine | 3.10 | 2.50 | 3.52 | 3.57 |
| Valine | 1.91 | 1.36 | 2.32 | 2.26 |
| Histidine | 1.10 | 0.88 | 1.22 | 1.22 |
| Phenylalanine | 2.35 | 1.92 | 2.38 | 2.33 |
| Non-essential amino acid | ||||
| Glycine | 2.88 | — | 2.17 | — |
| Serine | 2.56 | — | 2.72 | — |
| Proline | 2.12 | — | 2.37 | — |
| Alanine | 1.91 | — | 2.11 | — |
| Asparagine | 6.36 | — | 5.91 | — |
| Glutamine | 10.15 | — | 8.44 | — |
| Cysteine | 0.69 | 0.40 | 0.65 | 0.73 |
| Tyrosine | 1.42 | 1.39 | 1.47 | 1.57 |
| Years | Preparation Method | Strain/Enzyme | Objective | Characteristic | Reference |
|---|---|---|---|---|---|
| 2016 | Fermentation | Bacillus licheniformis | Enhancement of nutritional and antioxidant properties | The nutritional properties and antioxidant capacity of PNM were enhanced | Yang et al. [11] |
| 2011 | Fermentation | Bifidobacterium longum Lactobacillus casei Lactobacillus acidophilus Lactobacillus plantarum | Produce fermented peanut flour | Antioxidant activity was increased | Wang et al. [101] |
| 2012 | Fermentation | Aspergillus oryzae Aspergillus niger | Produce antioxidant peptides | Peptide fraction of 3–10 kDa showed the highest antioxidant activity | Wei et al. [99] |
| 2013 | Fermentation | Bacillus subtilis | Produce antioxidant peptides | High antioxidant peptide activity was obtained | Zhang et al. [97] |
| 2015 | Fermentation | Actinobacillus succinogenes | Produce succinic acid | PNM can be used as an efficient and economical source of nitrogen | Shen et al. [96] |
| 2023 | Fermentation | Pseudomonas aeruginosa | Produce rhamnolipid | Produced rhamnolipid exhibited good physicochemical and antimicrobial activities | Zhao et al. [94] |
| 2010 | Hydrolysis | Sporolactobacillus sp. | Produce D-lactate | High D-lactate production | Wang et al. [95] |
| 2011 | Hydrolysis | Crude enzyme obtained from Aspergillus oryzae | Produce antioxidant peptides | Peptide fraction of 1–3 kDa showed the highest antioxidant activity | Su et al. [100] |
| 2013 | Hydrolysis | Alcalase from Bacillus licheniformis | Produce bioactive peptides | Bioactive peptides have a potential benefit for blood pressure regulation | White et al. [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Li, S.; Zhu, Y.; Chen, S.; Wang, X.; Deng, X.; Liu, G.; Beckers, Y.; Cai, H. Improving the Nutritional Value of Plant Protein Sources as Poultry Feed through Solid-State Fermentation with a Special Focus on Peanut Meal—Advances and Perspectives. Fermentation 2023, 9, 364. https://doi.org/10.3390/fermentation9040364
Li C, Li S, Zhu Y, Chen S, Wang X, Deng X, Liu G, Beckers Y, Cai H. Improving the Nutritional Value of Plant Protein Sources as Poultry Feed through Solid-State Fermentation with a Special Focus on Peanut Meal—Advances and Perspectives. Fermentation. 2023; 9(4):364. https://doi.org/10.3390/fermentation9040364
Chicago/Turabian StyleLi, Chong, Shuzhen Li, Yanbin Zhu, Si Chen, Xiaoying Wang, Xuejuan Deng, Guohua Liu, Yves Beckers, and Huiyi Cai. 2023. "Improving the Nutritional Value of Plant Protein Sources as Poultry Feed through Solid-State Fermentation with a Special Focus on Peanut Meal—Advances and Perspectives" Fermentation 9, no. 4: 364. https://doi.org/10.3390/fermentation9040364
APA StyleLi, C., Li, S., Zhu, Y., Chen, S., Wang, X., Deng, X., Liu, G., Beckers, Y., & Cai, H. (2023). Improving the Nutritional Value of Plant Protein Sources as Poultry Feed through Solid-State Fermentation with a Special Focus on Peanut Meal—Advances and Perspectives. Fermentation, 9(4), 364. https://doi.org/10.3390/fermentation9040364








