Abstract
Anaerobic digestion (AD) is an environmentally friendly process for recovering low-carbon energy from the breakdown of organic substrates. In recent years, AD has undergone a major paradigm shift, and now the technology is not only considered as a “waste treatment” method and is instead viewed as a key enabler of the future “circular economy” with its potential for resource recovery (low-carbon energy, safe water, and nutrients). Currently, waste-derived biogas from AD is the most affordable and scalable source of renewable energy. Biomethane (upgraded biogas) can serve as a significant renewable and dispatchable energy source for combating the problem of global warming. Acidogenesis, an intermediate step of AD, can produce molecular hydrogen (H2) along with green chemicals/platform chemicals. The use of low-carbon hydrogen as a clean energy source is on the rise throughout the world, and is currently considered a potential alternative energy source that can contribute to the transition to a carbon-neutral future. In order to determine the future trade routes for hydrogen, nations are developing hydrogen policies, and various agreements. Hydrogen produced by biological routes has been found to be suitable due to its potential as a green energy source that is carbon neutral for the developing “Hydrogen Economy”. Recently, hydrogen blended with methane to a specific proportion and known as biohythane/hydrogen-enriched compressed natural gas (HCNG) has emerged as a promising clean fuel that can substantially contribute to an integrated net-zero energy system. This review provides an overview of the current state of fermentative hydrogen and methane production from biogenic waste/wastewater in a biorefinery approach and its utilization in the context of energy transition. The limitations and economic viability of the process, which are crucial challenges associated with biohydrogen/biomethane production, are discussed, along with its utilization.
1. Introduction
Rapid industrial development, unprecedented human population expansion and everyday activities have led to a buildup of greenhouse gases (GHGs) in the atmosphere, resulting in climate change. According to the Intergovernmental Panel on Climate Change’s (IPCC) 1.5 °C Global Warming Special Study, the earth must limit temperature increases to avoid damaging effects on the environment [1]. This goal is still reachable, but it demands the achievement of net-zero global emissions by 2050 and immediate, substantial, and long-term international climate action [2]. Therefore, in order to combat climate change and ensure environmental protection, it is essential to shift from the use of fossil fuels towards low-carbon, sustainable, and renewable energy sources as soon as possible [3]. After decades of study and industry initiatives, waste-to-energy (WTE) implementation is a practical solution for waste management with simultaneous energy generation since it offers a number of advantages [4]. WTE technologies/models enable us to maximize the value of waste/wastewater, which would otherwise be wasted [5]. Apart from these economic benefits, the environmental benefits include reduction in emissions of greenhouse gases. As concerns about climate change increase, the public and governments recognize the crucial role that waste-to-energy plants play in producing energy and safely processing waste, supporting the circular economy. The development of sustainable, efficient and low-carbon energy resources has attracted a lot of attention in recent decades. Offering a wide range of applications, WTE is an alternative to fossil fuels and is a renewable energy source [6]. Any process that generates energy (fire, power, or fuels) by using waste/wastewater as feedstock can be considered to be a WTE system [4].
Anaerobic digestion is a biochemical process that converts biogenic waste/wastewater to sustainable energy (biogas/bio-methane) and nutrients (biofertilizer) [7,8,9]. It has been found that 12 out of the 17 sustainable development goals (SDGs) are directly influenced and contributed to by biogas, as biogas can potentially boost renewable energy, reduce waste, improve waste management, significantly replace natural gas imports and generate employment [10]. Hydrogen (H2), a low-carbon fuel, has emerged as the most promising future energy carrier as it is renewable, has a high energy content (122 kJ/g, which is 2.75 times higher than fossil fuels) and produces only water upon consumption. Acidogenesis, an intermediate step in anaerobic digestion (hydrolysis, acidogenesis, acetogenesis and methanogenesis), produces biohydrogen (bioH2) along with volatile fatty acids (platform chemicals) from various feedstocks [11]. To minimize the environmental impact of fossil based hydrogen production, low-cost feedstock and renewable energy sources must be used [12,13]. Hydrogen can contribute to more than 20% of yearly global emissions reductions in 2050 [14], potentially decarbonizing several sectors, including steel, petrochemicals, fertilizers, heavy-duty mobility, maritime shipping and aviation, and aid with the development of flexible power [14,15,16]. Hydrogen has the potential to be a versatile and long-term power grid storage solution, can serve as a direct substitute for fossil fuels to be used in automobiles in replacement of gasoline or diesel, in homes to replace natural gas for heating, or to be used in place of fossil fuels at power plants and industries. One of the best examples is “green steel” manufacturing without the use of coal, which aims to considerably reduce the CO2 generation of steel manufacturing. Sweden, with a long tradition of high-quality steel, is presently investing in switching to green steel manufacturing, which may soon make Swedish steel the greenest in the world. The joint venture “HYBRIT“ has been initiated by SSAB, LKAB and Vattenfall, and aims to produce green steel by using hydrogen produced by low-carbon electricity [17]. Several major companies, such as Volvo Group, Volvo Cars and Mercedes Benz, recently announced partnerships with SSAB to enable carbon-neutral value chains for their steel products.
This review article discusses the potentials and possibilities of waste-derived gaseous fuels such as biohydrogen and biomethane production, their fusion to biohythane and their application in energy transition. The main focus is on biological routes for conversion of waste to gaseous fuels. The roles of individual microbial gaseous products in energy transition have been compared to fossil-based fuels. In particular, the opportunities and potentials of biogenic waste-derived hydrogen have been discussed, emphasizing the difficulties and opportunities of using biogenic hydrogen, when directly derived from the bioreactor, contains mixtures of gases that demand processing prior to its application.
There are different routes to produce hydrogen from renewable and non-renewable sources such as: (i) renewable energy-based hydrogen production which is considered as green, (ii) nuclear energy-based which is known as purple and (iii) blue, in which hydrogen is derived by using coal gasification and natural gas. Currently, natural gas (CH4) remains the main source of catalytic hydrogen production (about 70 M tons) and significantly contributes to the supply of three-quarters of the annual global hydrogen production. Even though steam methane reforming is a CO2-intensive process, this process is the most commonly employed industrial process for producing hydrogen [18].
Despite being an established technology, this process still has a number of drawbacks, such as the characteristics of the reactant and the reaction thermodynamics relating to high energy utilization and the manufacture of high energy. Reaction conditions are difficult, and have lower reaction efficiency and process stability [19]. In this method, steam and CH4 react to produce hydrogen-rich synthesis gas over a catalyst. High temperatures are required to attain a significant conversion level since the process is significantly endothermic [20,21]. Steam methane reforming (SMR) is often operated with a surplus of steam to prevent catalyst deactivation and pressure drop inside the reactor owing to carbon deposition [20]. According to the International Renewable Energy Agency (IRENA), the majority of annual global hydrogen production is utilized to refine petroleum and produce ammonia [22]. To make hydrogen a key player in low-carbon energy systems, its production must have low carbon emissions. Researchers are investigating several strategies to lower the cost of hydrogen production and reduce CO2 emissions. Low-carbon hydrogen production is not currently available at a reasonable cost (2.24–7.84 USD/kg H2) or with high efficiency; to reach this target, a complex multi-component system is now needed [23]. Currently, the cost of green hydrogen is too high for it to be made commercially available [16,24]. For example, the cost of on-site renewable hydrogen in the EU was 11 EUR/kg in 2020; this cost is expected to decrease to 7 EUR/kg in 2030 followed by 5 EUR/kg in 2050 [24]. Renewable electrolysis is still expensive and requires policy support to make it economically viable. If renewable hydrogen generation received a 3 EUR/kg hydrogen subsidy, this could be accomplished in 10 years [24]. Many factors influence the cost of renewable hydrogen such as feedstock, catalyst used, storage/transportation, distribution infrastructure and end-user equipment, which will need to be modified to make them acceptable for hydrogen transmission [16]. Electrolysis is presently regarded as the most practical approach for producing renewable hydrogen. However, installation of an electrolysis unit is costly and energy intensive [25].
On the other side, based on the economic aspects of a system that has an electrolyzer and a solar-powered electrical generating system, Yadav and Banerjee suggested that the cost of producing solar hydrogen using high-temperature steam electrolysis can be further decreased to 6–8 USD/kg if the cost of the components is reduced [26]. However, they used a parabolic trough solar collector (PTC) instead of photovoltaic cell (PV) for electricity generation through electrolyzer, where the electrolyzer was fed a solid oxide fuel cell with hydrogen. A significant amount of fresh water is needed to produce hydrogen, since water electrolysis needs around 9 kg of high-purity water to produce 1 kg of hydrogen. Using saltwater as a source of hydrogen will be an option because the world’s fresh water supplies are already limited and there is a lack of drinking water in many parts of the world. However, utilizing seawater to generate hydrogen has its own set of issues, such as the corrosion of anode material by chloride ions. Use of freshwater accounts for about 2% of the overall cost of the hydrogen production. As a result, additional research is required to identify water sources, boost productivity, and reduce the capital costs of electrolysis technology, which are seen to be the key issues with water electrolysis. To effectively produce oxygen and hydrogen from freshwater and saltwater, a two-electrode catalyst of nickel/molybdenum/nitrogen modified with a low quantity of iron and grown on nickel foam to produce hydrogen efficiently was recently developed that uses just one compound instead of two separate catalysts, decreasing the catalysts’ manufacturing cost [27].
2. Renewable Hydrogen
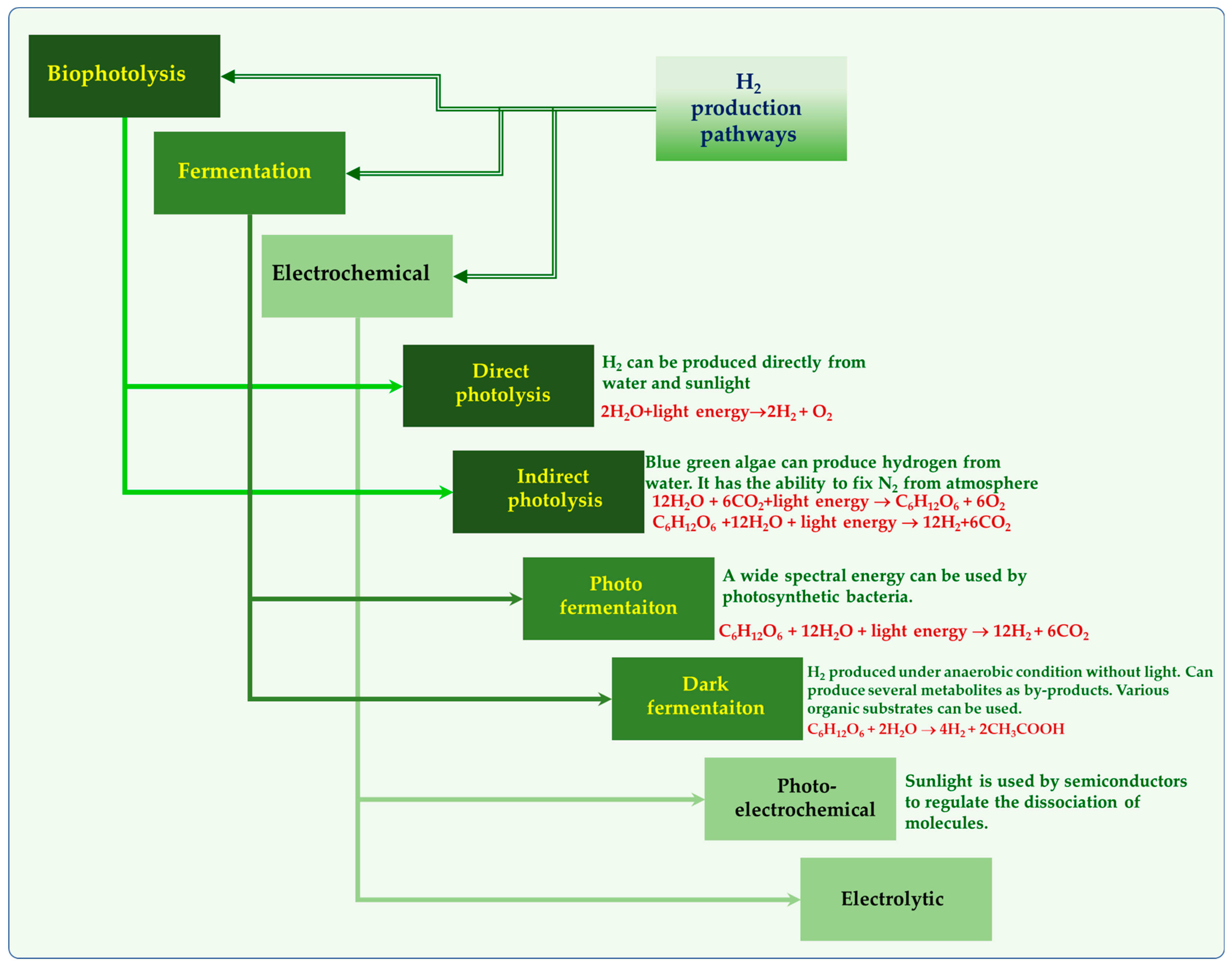
Renewable sources of hydrogen, solar, wind, hydro, geothermal, ocean thermal energy conversion, and biomass are the ideal options to replace fossil fuels (Figure 1). These renewable sources can be extremely important for the decarbonization of the major industries in order to achieve net zero CO2 emissions by 2050 [28]. Moreover, hydrogen can be considered renewable only if it is derived from renewable sources. Currently, the majority of the hydrogen (almost 90%) is derived through methane partial oxidation, coal gasification and natural gas reforming [13]. It is apparent that adopting renewable energy sources is the only way to reduce carbon emissions. Although it is crucial to phase out fossil fuels, the energy sources we choose to do it with are just as crucial.
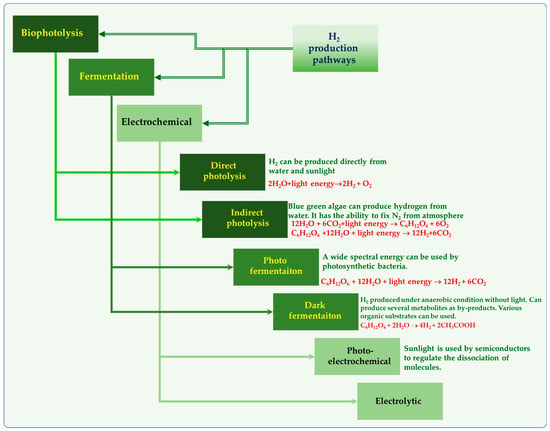
Figure 1.
Hydrogen production through various routes.
2.1. Biological Hydrogen
Hydrogen can be produced through bio-photolysis (direct and indirect), fermentation (dark and photo) and microbial electrolysis cells (MECs) [29]. Figure 1 represents different routes for producing renewable hydrogen. Specific microorganisms have the ability to break water molecules by using light energy to produce hydrogen, which is known as biophotolysis. Direct biophotolysis and indirect biophotolysis are the two categories of light-driven biophotolysis processes (Figure 1). Dark fermentation is a versatile biochemical process where a diverse range of microorganisms including aerobes, facultative anaerobes, and strict anaerobes, produce carboxylic acids (volatile fatty acids), short chain alcohols, acetone, H2, and CO2 from the degradation of organic substrates (such as starch, cellulose, etc.) under anoxic and anaerobic conditions [30,31,32].
On the other side, in photofermentation, using light energy, the purple non-sulfur photosynthetic bacteria (PNS) (Rhodobacter sp., Rhodobium sp., Rhodopseudomonas and Rhodospirillum) convert organics to H2 and CO2 under anaerobic conditions. Photo fermentative H2 production by PNS was first reported in the 1940s by Gest and Kamen [33]. Due to its ability to use solar energy and convert waste/wastewater to clean energy, photofermentation has received attention. Progressively, during the last two decades, many studies have been carried out employing different photosynthetic bacterial strains by using a range of substrates. For a long time, water photolysis has garnered a lot of interest and has been recognized as an environmentally friendly method for producing biological hydrogen. In 1939, Gafforn first documented H2 production by the green algae Scenedesmus obliquus [34]. Later in 1942, Gafforn and Rubin noticed that under anaerobic conditions, S. obliquus could absorb hydrogen and fix CO2 [35]. In contrast, molecular H2 can be produced under light conditions. Many studies have shown several green algae, including Chlamydomonas reinhardtii and Chlorellafiscal, etc, to be capable of producing hydrogen. Furthermore, the cyanobacteria’s (genera Nostoc and Anabaena) ability to produce hydrogen has been well explored. For the first time, Puente-Sánchez and coworkers have shown evidence that cyanobacteria can also live in deep continental subsurface and indicated that they obtain their energy by coupling hydrogen oxidation to different electron acceptors reduction [36]. Currently, many studies are trying to find new ways to boost the hydrogen enzyme’s tolerance to oxygen to enhance hydrogen production.
2.2. Integration of Dark and Photofermentation in a Biorefinery
Using a biorefinery is an attractive, sustainable option that has the potential to turn waste/wastewater into useful and safe ingredients for food, feed and fertilizers, alongside bio energy productions [4,37,38]. Integration of dark and photofermentation is suggested as a solution to the problem of limited hydrogen yield by dark fermentation, as the volatile fatty acids produced during dark fermentation cannot be completely oxidized. In a biorefinery format, integration can be performed in two stages (stage I and II), the first of which involves dark and photofermentation taking place in independent reactors under different conditions. Stage II, also described as a co-fermentation system, combines the processes of dark and photofermentation in one reactor. Compared to stage I, the co-fermentation process is considered to be more efficient for hydrogen production, due to the fact that it is more affordable and easier to adapt to environmental changes. The integration primarily avoids the addition of chemicals for pH adjustment since the consumption of in situ VFAs (volatile fatty acids) during photofermentation can counterbalance the acidification caused by the biosynthesis of acidogenic VFAs accumulated during dark fermentation. Secondly, since the acidogenic metabolites are consumed during photo fermentation, substrate inhibition or substrate toxicity can be reduced. Finally, hydrogen yield significantly increased along with reduction in VFAs. Several studies have been conducted evaluating biohydrogen production from various feedstocks employing various fermentation techniques. However, most of them focused on the optimization of influential parameters such as organic load, redox condition, nature of inoculum, light intensity, process temperature, etc. Recently “one-pot” integration of the dark and photofermentation approaches has received the greatest attention. Ghosh et al., 2020, evaluated single stage integrated dark (Clostridium acetobutylicum) and photofermentation (Rhodopseudomonas sp.) processes in a flat plate photobioreactor by employing a batch-repeated batch cycle strategy yielding a cumulative biohydrogen of 4.44 mol H2/mol glucose [39]. Theoretically, dark fermentation can produce 4 moles of H2 per mol of glucose, while photofermentation produce 8 mol H2/mol glucose. However, this never occurs in vivo [16,39,40]. Along with the production of VFAs as byproducts, a hydrogen yield of 2–3 mol H2 per mol glucose can be obtained experimentally through dark fermentation. Integration can offer an increase in hydrogen yield to 12 mol H2/mol glucose, which is roughly three and one and a half times greater than the maximum theoretical values individually attained in dark and photo fermentation, respectively [16,39,40]. The integration strategy not only improves biohydrogen yield, but also addresses the issue of optimal substrate utilization, making the process viable for energy generation with simultaneous waste remediation.
2.3. Microbial Electro-Hydrogenesis
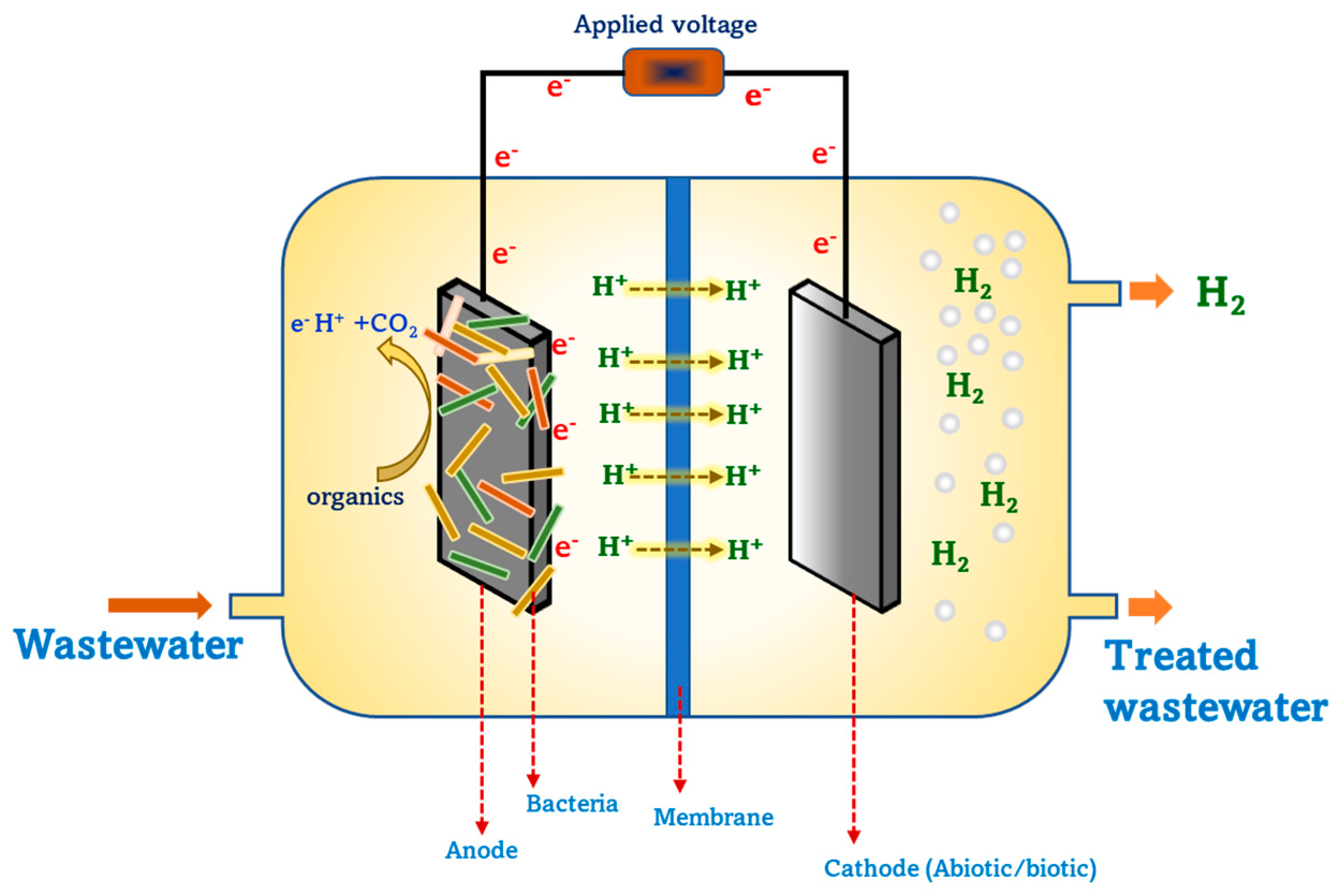
Biohydrogen production by microbial electrolysis cells (MECs) is being studied with much interest as it can utilize and transform the carbon present in waste into renewable energy or value-added products. MEC is also recognized as a sustainable technology for biohydrogen production from diverse wastewaters [41,42]. Several wastes/wastewaters such as dairy, distillery, sewage wastewaters, food waste, swine wastewaters, lignocellulosic biomass, etc., were utilized as substrates for biohydrogen and biomethane production in MEC [43]. MEC research spans across several fields connecting molecular biology, environmental engineering, microbiology, chemical engineering, physical chemistry, bioelectrochemistry, etc. MEC is a modified form of microbial fuel cell (MFC), wherein the cathode is not exposed to air/O2 but is instead placed inside of the cathode chamber, and voltage is applied to facilitate hydrogen gas production (Figure 2). At the anode, bacteria use the substrate and liberate reducing equivalents, while at the cathode, the hydrogen evolution reaction (HER) takes place whereby electrons and protons combine and form hydrogen. The voltage produced at the anode is about −0.3 V, while HER requires −0.41 V, thus necessitating an input voltage of around 0.1 V. However, an applied voltage of 0.25 to −0.8 V is required to meet HER for hydrogen production in an MEC. One significant advantage of hydrogen production from MEC is the lower energy absorption/utilization in comparison with water electrolysis [44,45]. The prime reason to opt for the MEC process over other water electrolysis processes is the possibility of high-purity hydrogen production, while the latter offers high production rates [46]. MEC is expected to become a low-energy and high product purity approach to produce hydrogen in an economically competitive way. The design and configuration of MEC highly impacts the rate of hydrogen yield as well as the current density [44]. MECs encompass a wide range of designs and configurations, ranging from single chambered membraneless to dual chambered with different membranes. Besides the general architecture, MECs are also designed and operated in other configurations such as tubular, rectangular shaped, cube shaped, etc [47,48]. MEC for biohydrogen production can be carried in both batch and continuous mode, which results in substantial yields [49]. From an economic viewpoint, single chambered membraneless MECs are relatively beneficial for attaining higher production rates [50]. Avoidance of membrane usage in single chambered MECs limits the resistance for current density that majorly occurs due to the membrane, thereby further enhancing the hydrogen yield. Research from the past several years illustrates the significance of MEC operations in a single chambered reactor in continuous mode to yield higher hydrogen production rates with simultaneous cost-effectiveness.
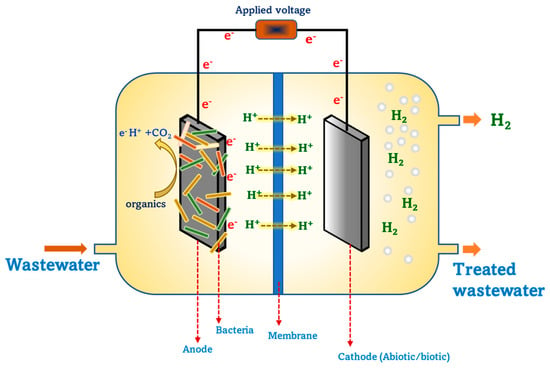
Figure 2.
Schematic representation of a double chambered MEC for biohydrogen production.
Several factors critically affect MEC performance in terms of product yield and substrate degradation. MEC is a bio-electrochemically catalyzed system that majorly relies on electroactive microbes as a biocatalyst for catalyzing electrochemical reactions at the interface of electrodes to produce biohydrogen/biomethane. With their complex metabolic machinery, microbes catalyze biochemical reactions through a series of enzymes at each step, thereby favoring the path towards the final product. Biohydrogen is the most studied product from MEC, while biomethane production, commonly referred as electro-methanogenesis, has also been studied with great interest in recent years. Electroactive microbes in these systems transfer electrons either via direct electron transfer or through shuttles and mediators on to the surface of electrodes. Another advantageous feature of microbial catalyzed electrochemical systems is biofilm formation, whereby bacteria colonize on the surface of an electrode that helps in enhanced electron transfer with minimal electron losses. The choice of biocatalyst typically determines the final desired product formation during a process, such as biohydrogen or biomethane, and pretreatment of the biocatalyst is a great option that facilitates in suppressing methanogenic bacteria, thereby promoting the growth of other bacteria capable of producing biohydrogen. Besides biocatalysts, other factors, such as electrode materials, applied voltage, pH, membrane, system architecture and temperature majorly affect the performance of MEC. Electrode material plays an important role in MECs by facilitating biofilm formation, providing an active redox catalytic area, electro-active interface for redox reactions, etc. An ideal electrode material for these systems needs to be low cost, durable, mechanically strong, biocompatible, and have amenable surface morphology for enhanced product yield and process performance. Anode and cathode distance, placement and type of material also impacts the performance of MECs. Depending on the type of substrate and operating conditions such as pH, the application of voltage to drive the redox reactions varies. Applied voltages of both positive and negative potentials were applied and studied in MEC for biohydrogen production. The rate of hydrogen varies in accordance with the applied voltage, which is further influenced with respect to microbiome community and operating conditions. pH also significantly affects bacteria metabolism, which in turn has an impact on overall biohydrogen yields and organic acid metabolite production. Apart from the aforementioned factors, the type of substrate also significantly contributes to the rate of product yield, as it influences bacteria metabolism. Nevertheless, techno-economic aspects such as capital costs, longevity, scalability and reproducibility are parameters of concern that need to be investigated further while progressing towards large scale applications.
3. Towards Large Scale Production and Utilization of Renewable Hydrogen
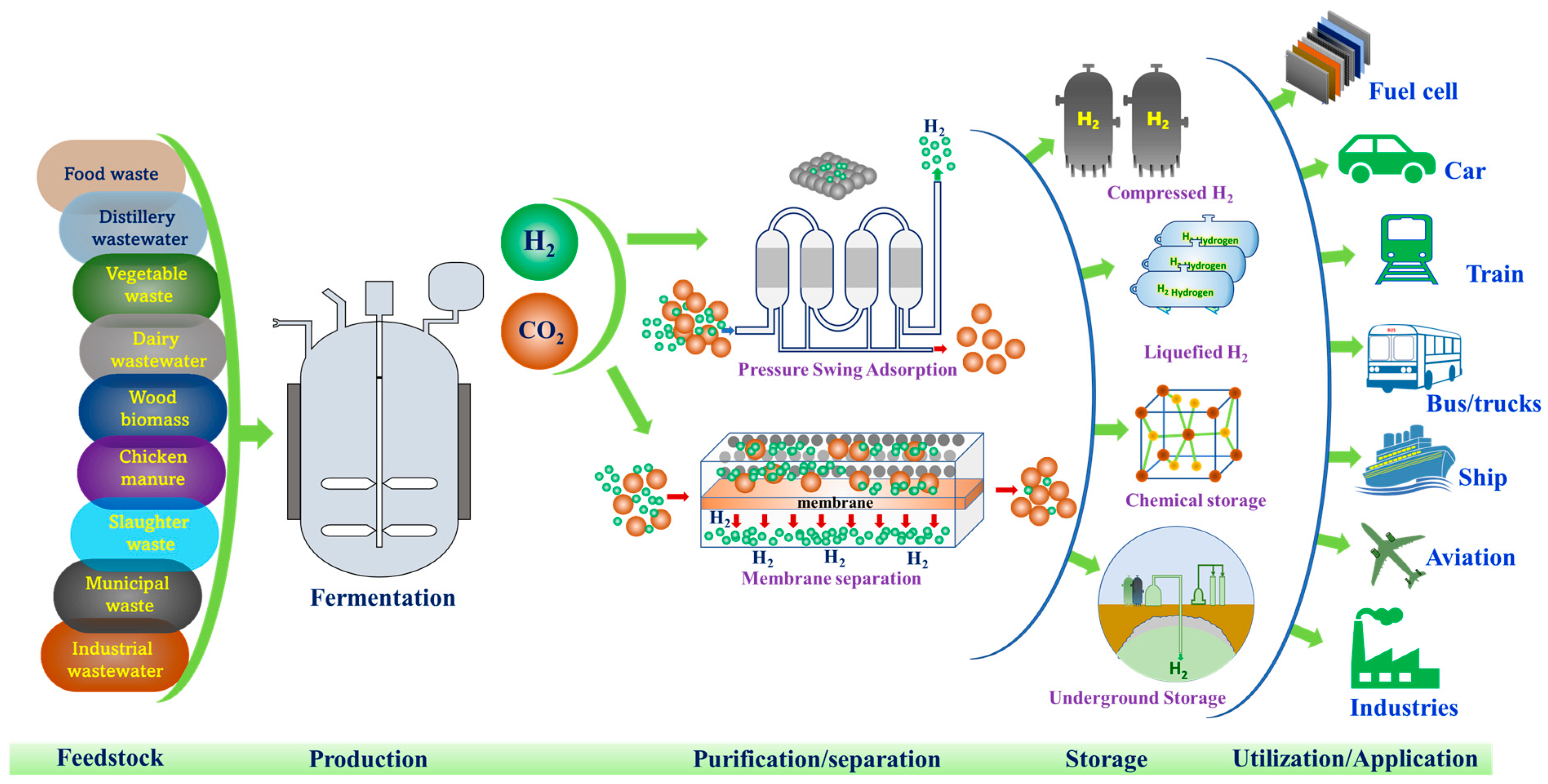
While there has been significant progress in hydrogen research over the past two decades from the lab to semi- and pilot scale, no large/full scale facility has been developed. Currently, research is moving towards the scale-up of biological hydrogen production supported by sustainability analysis. For hydrogen to become a pivotal player in the energy transition, its scale-up and commercialization is essential. Only a few studies are available on pilot-scale biohydrogen production. Balachander et al., 2020, demonstrated production of 76.2 m3 bio-H2 from a 10 m3 pilot plant by fermenting cane molasses and groundnut cake as a co-substrate with Enterobacter [51]. Elsewhere, about 54 m3 bio-H2 production was achieved from 400 kg COD of food waste from a pilot-scale (10 m3) reactor functioning at CSIR-IICT [13]. These facilities for bio-H2 are very promising for the production of clean, green and cheap energy in the future. Hydrogen production from organic wastes and residues holds the potential to become a way for economical and sustainable clean energy generation while simultaneously promoting sustainable waste management [52]. Additionally, the sustainability analysis demonstrated that by using waste and/or wastewater as a substrate rather than a fossil-based substrate, a reduction in greenhouse gas emissions can be achieved [13]. For large and commercial-scale production, biohydrogen research is required to demonstrate its economic and environmental benefits from various waste as feedstock, particularly at the pilot scale. Renewable hydrogen production must overcome challenges associated with process efficiency, separation of gas mixture, storage and transportation in order to sustain production and utilization, particularly at a large scale. On the other hand, its policy constitutes one of the factors that determines the adoption of renewable hydrogen in the system. Policy makers, energy and business leaders can play a significant role towards scaling the process for energy transition. For the development of a viable and sustainable hydrogen economy, purification of biohydrogen from the mixture of gases is crucial and needs more attention (Figure 3). Methods such as pressure swing adsorption and membrane separation can be used to separate and purify biohydrogen from the fermentation gas mixture [53,54]. One of the most popular methods is adsorption where the separation conditions such as temperature and pressure determines the efficiency of separation. The other method is absorption, where a solvent is employed making H2 more soluble. Towards utilization of biohydrogen after its production, it should be noted that H2 derived through biological routes does not differ from those of H2 produced from other processes and requires purification/separation and storage prior to its utilization. Purified biohydrogen can be stored by compressing under significant pressure tanks (200–500 bar) or in liquid form. However, compared to liquid storage, compressed gaseous storage is advantageous, easy to utilize and inexpensive, as the process of liquefaction (“Linde-Hampson” liquefaction cycle) involves compressing and exchanging heat (T = −253 °C) with the gaseous H2 after a series of interventions [55].
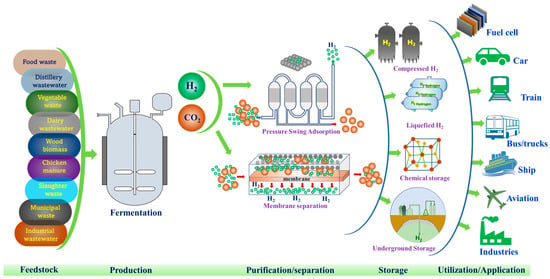
Figure 3.
Various stages involved from production to utilization of low-carbon hydrogen from biogenic waste/water as renewable feedstock.
On the other hand, hydrogen can also be stored in a solid form via metal hydrides (e.g., NaBH4, NaAlH4, LiBH4, AlH3, MgH2) or physisorption on a substrate with a large surface area. H2 can possibly be stored underground (e.g., in salt caverns, aquifers and depleted hydrocarbon deposits). This method involves pumping hydrogen into various deep geological formations and storing it there for days, weeks, or months. This method is considered safe because the storage deposits are so deep and oxygen-free that there is no risk to either humans or the environment. To reduce the emission of carbon dioxide and cost of hydrogen, Haghi et al. 2018, suggested underground hydrogen storage [56]. Underground hydrogen storage is a crucial technology for storing large amounts of hydrogen that aids in the industrial-scale adoption of a hydrogen economy (Figure 3).
Furthermore, H2 storage systems can allow our society to reduce our dependence on non-renewable energy sources, offer businesses (such as chemical plants and refineries) with an immediate supply of clean energy, and regulate energy prices during periods of peak demand. However, the success of the hydrogen economy also depends on the availability of adequate storage space because H2 has a significantly lower volumetric density (0.09 kg/m3) compared to natural gas (46 kg/m3) and is challenging to compress [57].
4. Biomethane: A Potential Solution for the Energy Crisis
With the advancements in science and technology, biogas from anaerobic digestion processes is now one of the top alternatives for global energy plans. Biogas is mainly composed of methane (CH4) and carbon dioxide (CO2), with traces of other gases. With lower carbon emissions, biomethane (bioCH4) has emerged as an alternative to natural gas. More importantly, the emergence of bioCH4 is due to its practical ability to substitute natural gas and diesel and to move towards decarbonizing difficult-to-abate sectors such as agriculture, industry and heavy transportation. Biomethane is a versatile resource that can be used for a variety of applications and can be produced from by-products and biogenic waste and wastewater by employing anaerobic digestion process (Table 1). Prior to use, the biogas needs to be upgraded to biomethane which can be performed by employing different technologies that are economically viable and long-lasting. Some of the widely used techniques include pressure swing adsorption, water scrubbing, membrane separation, chemical adsorption, cryogenic separation and biological conversion. The development of anaerobic digestion (AD) technology can potentially fight against climatic change, environmental deterioration and energy scarcity. However, development has not been consistent throughout the world because this technology is dependent on both the availability of feedstocks and the policies/regulations that support its production. The characteristics of the influent (substrate) are highly heterogeneous in nature and change over time. For instance, unexpected influxes of concentrated organic material to an AD might cause process upsets and significant disturbances resulting in lower biogas yield due to the dynamics of society and industry frequently resulting in transients. Currently, Europe has the highest number of biomethane producing units, followed by the United States, China and Canada [58]. Installation of biomethane plants increased by 40% in 2021 compared to the previous year, with Germany having the highest number, followed by France, Italy and Denmark. The plants will be responsible for 30–40% of Europe’s total gas consumption by 2050 [59]. About 18 gigawatts capacity biogas power production units operating globally; Germany, China, and the United States are the nations having the most installed capacity [60]. Italy is seeking for alternatives to Russian-supplied gas and the focus is on biogenic waste because it serves as a model for a circular bioeconomy along with an increase in recycling and resource recovery by recirculating the waste as resources [61]. The huge development of biomethane/biogas systems is due to its potential to meet energy demand. According to the International Energy Agency (IEA), about 20% of the world’s gas consumption can be satisfied by these technologies [60]. Biomethane mimics natural gas and can be easily stored, compressed (200 bar) or liquefied and injected into the existing natural gas infrastructure, and can provide economical energy. The demand for biomethane is expected to rise by nine-fold by 2040 [60]. Large-scale, commercial, and farm-based biogas plants are the primary focus of the European biogas sector. Additionally, anaerobic digestion of biogenic waste/wastewater accounts for 74% of biogas energy production and is dominated by biogas business [62]. In the EU, Sweden has the most advanced biomethane-based transportation system, using half of its biogas production for transportation [63]. The potential of biogas produced by AD in Stockholm was examined by Lönnqvist et al., 2015 [64]. Their findings suggested that Stockholm County’s vehicle gas demand can be met until 2020, but by 2030, it may only be able to supply half of it. However, it is estimated that by 2020 and 2030, the practical potential will only be 604 GWh and 689 GWh, respectively, necessitating the addition of more methane, which can be obtained from neighboring biogas, fossil gas, or woody biomass [64]. In Finland, biogas was found to be an effective transport fuel based on its cost-effectiveness (estimated from the perspective of the gas grid owner), which concurrently helped to reduce GHG and particle emissions [65]. Even though AD technology has already reached industrial/full scale, further research continues to improve biomethane yield along with recovering value-added products (ammonium) and treating waste/wastewater.

Table 1.
Energy potential of biohydrogen and biomethane derived from various feedstocks.
In addition to CO2, the elimination of trace pollutants such as H2S, xyloxanes, siloxanes and water are currently the focus of research. Biomethane containing organosilicon compounds known as siloxanes produce silica upon burning, which can accumulate on cold surfaces, damaging the combustion instruments [73,74]. To achieve pure biomethane, these impurities must be removed, which results in a product similar to purified natural gas. Biogas containing up to 60% biomethane (v/v) has a lower heating value (LHV) of roughly 5000–6000 kcal/N·m3. The LHV reaches 8000 kcal/N·m3 upon separation/purification of biomethane (96–97%), which is comparable to natural gas [73].
4.1. Hydrogen-Enriched Methane
The transportation sector has the highest dependency on fossil fuels, and in 2021 it was responsible for 37% of all end-use sector CO2 emissions. With the economic expansion of developing nations, this percentage is expected to rise even further. To combat global warming, it is crucial to decrease the use of fossil fuels in all sectors. This will result in lower emissions of CO2 and other GHGs. Currently, conventional fossil fuels are being utilized to drive a range of engines in the transportation and agriculture sectors [75]. As a substitute, compressed natural gas (CNG) is the ideal mixture for hybrid fuels in diesel or gasoline engines. The natural gas vehicle market is rapidly developing throughout the world, and many such vehicles operate on compressed natural gas (CNG). A tremendous growth in vehicles running on compressed natural gas has been witnessed in recent years. By utilizing CNG to power fuel-cell electric vehicles (e.g., cars or trucks) or as a feedstock for synthetic aviation and marine fuels, the low-carbon economy can replace the need for fossil fuels such as fossil-based gasoline and diesel. Recently, interest in CNG-based vehicles has grown in the US and Indian markets because they are more affordable, less combustible and deliver more energy [76,77].
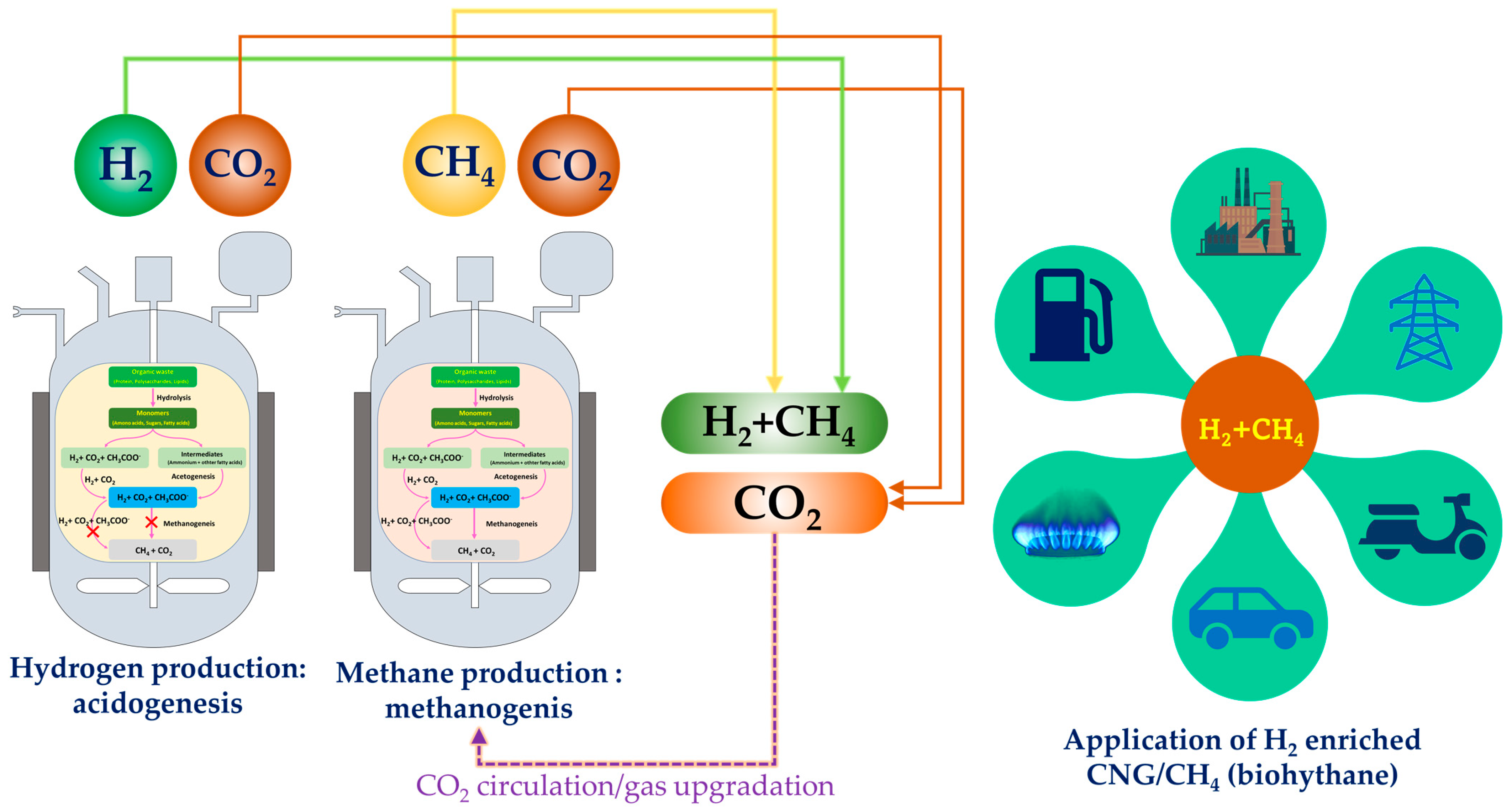
Mixing natural gas with hydrogen at certain ratios generates hydrogen-enriched compressed natural gas (HCNG) which enhances the combustion of natural gas and improves the lean burn capability of engines [78,79]. Biohythane/H2-enriched CNG is a mixture of hydrogen (10–30% v/v) and methane (70–90% v/v) and has the potential to be a future all-purpose energy source. Recently, a new phenomenon among environmental biotechnology processes with the potential to sustainably produce biohythane has emerged: acidogenesis in combination with methanogenesis. BioHCNG can potentially reduce the emissions of carbon monoxide (CO) by up to 50%, compared to methane [80]. Hythane is expected to travel a long way due to its economic and environmental benefits. This blend can be directly used in engines and thus can be commercialized as vehicle fuel [81]. Conversion of biogenic waste into hydrogen-enriched CNG (H2-CNG) can be an innovative approach to produce this fuel. Acidogenic fermentation combined with anaerobic digestion either in single or dual phase is a promising strategy to produce hydrogen and methane from biogenic waste/wastewater as a renewable feedstock towards bioH2-CNG/biohythane (Figure 4, Table 2). Currently, the hydrogen used in the blend is derived through steam reforming, which is an environmentally damaging process resulting in emissions of more than 10 kg of CO2/kgH2 [82]. To keep global warming at 1.5 °C, an environmentally friendly technique should be adopted to produce low-carbon hydrogen. In this direction, acidogenic dark fermentation—a versatile platform—can use high-strength organic substrates (waste/wastewater) to produce hydrogen and platform chemicals/volatile fatty acids [83]. In the past ten years, research on biohythane production from various biogenic wastes and wastewater has increased significantly. Many studies have explored biohythane production from various feedstocks such as food waste [84], distillery spent wash [85], coffee residues and sugarcane vinasse [86], co-digestion of rice straw and pig manure [87], swine manure [88], brewery spent grains [89], corn stalk [90], rubber sheet wastewater [91], birch sawdust [92] and algal biomass [93,94].
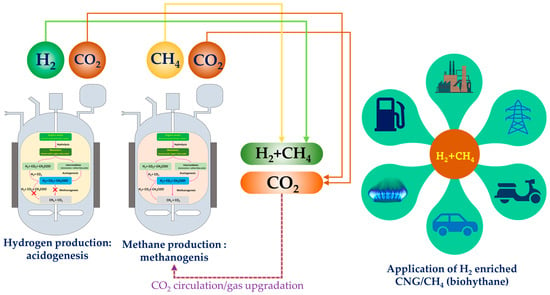
Figure 4.
Coupling acidogenesis and methanogenesis to derive bioH2 and bioCH4 towards bioH2CNG (biohythane) and its application in various sectors.

Table 2.
Energy of potential biohythane (bio-H-CNG) derived from various feedstock.
4.2. Hydrogen-Enriched CNG (HCNG)/Hythane as Fuel
Owning to its low cetane number, low molecular weight, and high vapor pressure, CNG has primarily been employed in spark ignition engines rather than compression ignition engines [78]. H2 addition to CNG is being highly recommended for its improved combustion qualities and because it can offset the undesirable properties (burning velocity, high ignition energy requirement, low quenching distance and low flame speed) and increase the lean limit operating of CNG [78]. The hydrogen in hythane considerably accelerates burning, lowers carbon emissions, widens the flame, and enhances combustion, aiding methane combustion in engines [78,100]. The Montreal Hythane Bus Project, a 1995 initiative, was the first to employ biohythane as a transportation fuel and demonstrated encouraging NOX reduction results when compared with regular CNG [103,104]. Similarly, the Beijing Hythane Bus Project in China demonstrated the utilization of biohythane [104]. Biohythane has the potential to replace fossil-based transportation fuel. The increased use of hythane as a fuel has been brought about by several factors, including (i) the blend either does not require any changes in vehicle layout, or only requires a minor modification to the engine; (ii) high combustion efficiency; (iii) low emission of CO2 and contribution to a clean environment [100,105,106]. In addition to this, biohythane has been utilized as a fuel in various systems. Meziane and Bentebbiche (2019) designed a combustor that can efficiently reduce NO and CO from a blend of natural gas and hydrogen in a micro gas turbine to 14% and 60%, respectively [107]. For the first time, Panagi et al. (2019) examined the use of biohythane as a fuel in solid oxide fuel cells to produce synthesis gas and power [108]. Dimopoulos et al., (2008) demonstrated that the use of hythane improved the performance of natural gas internal combustion engines and decreased well-to-wheel (WTW) emissions [109]. Evaluating the impact of various HCNG concentrations (0%, 20% and 40% volumetric H2), Hao et al. (2020) noticed that the combustion and stability of CNG engines was improved at 20%, indicating that hydrogen addition has some positive effects on emissions, such as a reduction in CO and CH4 emissions [110]. Interestingly, Tangoz et al. (2015) obtained a superior performance at 12.5 compression rates due to a reduction in NOx when using HCNG in comparison to other compression ratios despite the fact that H2 is known to cause NOx release [111]. The combustion and emission parameters of a thermal barrier-coated compression ignition engine when using biohythane derived from the anaerobic digestion of food waste, cow dung and sludge were experimentally examined and demonstrated a significant emission reduction of up to 62.6% compared to conventional diesel operation due to the lower carbon content [75]. Comparing the dual-fuel technique to the diesel-only mode, hydrocarbons and CO emissions were reduced by 16.2% and 29.1% respectively, displaying the potential of biohythane produced from biomass as a high-energy alternative fuel in engines [75]. Bhasker and Porpatham, 2017, observed that the wide flammability ranges of hydrogen were beneficial for ultra-lean combustion, extending the operational lean limit to an equivalence ratio of 0.42 (10% H2) compared to 0.50 of neat CNG operation [78]. In terms of emissions, H2 blending resulted in a drop in hydrocarbon emissions from 65 g/kWh to 6.9 g/kWh at an equivalent ratio of 0.5, as well as a drop in carbon monoxide and carbon dioxide emissions [78]. Biohythane/HCNG is currently emerging as a potential alternative, particularly for the transportation industry, and has the potentials to reduce emissions. However, achieving this reduction would depend on the implementation, commercialization and scale-up of these fuels, especially the transportation sector and policies to encourage a shift towards low-carbon travel options.
4.3. Techno-Economic and Life Cycle Assessment
Techno-economic analysis (TEA) represents a widely used methodology to evaluate economic and technical feasibility of any process that produces a product by considering the costs and benefits [112]. TEA links economic indicators with the technological parameters of bioprocesses and bioproduct systems [112,113]. For the cost–benefit analysis through TEA, two parameters/indicators, namely ‘capital expenditures (CAPEX)’ and ‘operating expenditures (OPEX)’, are often used. Factors such as cost of reactor/equipment, service facility, warehouse, piping, etc., contribute to CAPEX, while maintenance cost, transportation cost, overheads, labor, cost of input/raw material, utilities, etc., contribute to OPEX which are the key aspects that determine the techno-economic feasibility of a (bio)process. The benefits of the output and the cost of total production are generally considered for a long-term period of any production process, and the critical challenge lies in minimizing the CAPEX and OPEX with simultaneous focus on enhancing the yield of bioenergy carriers such as biohydrogen and biomethane that ultimately lead to profitability. Sarkar and Kumar demonstrated that the cost of hydrogen produced from bio-oil derived from fast pyrolysis of whole-tree-based biomass was 2.40 USD/kg which was relatively less expensive than the cost of the agricultural and forestry residuals [114]. Galera and Gutiérrez compared hydrogen and electricity production from glycerol through two different process, namely supercritical water reforming (SCWR) and autothermal supercritical water reforming (ASCWR). They found that the minimum selling price (MSP) of hydrogen was 5.36 USD/kg and 5.75 USD/kg for SCWR and ASCWR respectively [115]. The economic and environmental sustainability of a biorefining unit fermenting azolla biomass for biohydrogen along with other biochemicals was shown by TEA, which exhibited higher annual income than the annual operating cost with a payback time of 1.55 years [112]. Thus, biorefineries are considered sustainable and energy efficient; using zero waste discharge enables industries to manufacture products with lower carbon footprints [13,116]. TEA is an approach to estimate the sustainability aspects of a bioprocess and bioproduct/energy by viewing and evaluating from both the economic and technological perspectives. This approach also effectively assesses the feasibility of any bioprocess illustrating the long and short-term economic success. Nevertheless, TEA also poses certain limitations in obtaining results which might be ambiguous with several approximations, simplifications, and assumptions [117]. This limitation was suggested to be improved upon by combination with life cycle assessment (LCA) and exergy.
LCA is being considered as a sustainable indicator of predicting the impacts of any process/product on the environment in terms of carbon footprints, emissions, global warming, etc. [118]. Although most biological processes are embracing the concept of ‘carbon neutrality’, wherein the biogenic CO2 emitted is recycled/looped back for subsequent production of other products/energy, the concept still requires effort to become widely implemented. Biohydrogen production from organic carbon as discussed in this review also emits CO2, however, incorporation of other processes which can loop-in the emitted CO2 will eventually aid in negating the effects of carbon footprints on process efficiency [119]. By extracting bioenergy in the form of biological hydrogen and methane from food waste at the pilot scale, one study showed that a reduction in the overall impacts of non-renewable energy (−67.2 MJ primary) and global warming (−4.21 kg CO2 eq.) was achieved due to utilization of food waste as feedstock rather than conventional fossil-based feedstock [13]. For a bioenergy/bioproduct production process, the two pathways attributional LCA (ALCA) and consequential LCA (CLCA) can be considered, which define the life cycle of a process/product in a detailed manner. ALCA determines the environmental burdens of input and output flows such as materials and energy which aid the identification of environmental hotspots that pose unsustainability of bioproducts/bioenergy systems. While CLCA determines the changes in environmental burdens concerned with policies and decisions related to bioproducts/bioenergy systems, which acts as an early way of knowing the environmental concerns compared to other (fossil) counterparts. It must also be noted that in bio-based processes, although aiming to attain a single product, multiple products production will occur, and hence while performing LCA the environmental burdens need to be associated with the co-products as well which will give a detailed overview of the process. On the whole, LCA and TEA analysis are much-required sustainability indicators for knowing the environmental impacts of any bioenergy/bioprocess and how to reduce them.
5. Conclusions and Outlook
With the continuous increase in the pace of transitioning towards the production and utilization of renewable energy by replacing fossil resources, microbial gaseous fuels have gained momentum in concurrence with the intensified focus on climate change. Considering the importance of decarbonization and renewable energy generation, this review holistically presented the importance of waste-derived biohydrogen and biomethane as potential energy carriers. AD is a ready-to-use technology that is already generating biomethane from various biogenic waste/wastewater at scale, while green hydrogen has received unique attention towards long-term energy security and achieving climate mitigation objectives. Low-carbon hydrogen and biomethane produced by the fermentation process emerged as two primary possibilities for decarbonizing the gas supply, indicating that the future energy mix will be dominated by renewable energy sources. However, the challenges, particularly with scale-up/ commercialization and its successful utilization after separation, limit its value as a fuel. Although biogenic, the CO2 and other trace gases generated along with H2 and CH4 from both the acidogenic and methanogenic process need to be sustainably addressed to improve the calorific value of gases and broaden the range of application. Compared to the challenging physicochemical methods, a biological H2-based in situ biogas upgradation strategy can offer an economical way to utilize CO2 by converting it to methane and thus avoiding the cost of gas purification/catalysts. Despites decent energy efficiency as well as the lower environmental impact, current renewable hydrogen production and utilization is minimized due to the issues related to its storage, purity (to be injected in fuel cells), infrastructure, transportation, safety, and policies. With the development of research and technology, renewable hydrogen can play a significant role in the realization of a hydrogen-based economy. Furthermore, this review describes a holistic integration approach coupling acidogenesis and methanogenesis with extraction of H2 and CH4 towards hythane production/HCNG, which is an emerging future energy carrier exemplifying its benefits in replacing transportation fuel along with waste remediation making the whole process sustainable. Apart from the conventional routes of production, electro-hydrogenesis and electro-methanogenesis are emerging as promising future technologies for a wide range of applications in addition to sustainable electricity generation for decentralized usage. Finally, the economic impacts and environmental sustainability of low-carbon hydrogen demonstrated a stronger climate benefit and key opportunities for potential decarbonization of hard-to-abate sectors. Waste-derived biohydrogen and the other energy forms discussed in this current context offer great scope in attaining practicality at a larger scale by reconsidering fewer factors such as policies and regulations that would eventually favor the emergence of a circular green economy.
Author Contributions
O.S.: conceptualization, investigation, writing—original draft preparation; J.A.M.: Conceptualization, investigation, writing—original draft preparation; U.R.: Conceptualization, investigation, writing—review and editing; P.C.: Conceptualization, investigation, writing—review and editing; L.M.: Conceptualization, investigation, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AD | Anaerobic Digestion |
| AF | Acidogenic Fermentation |
| Bio-H-CNG | Biohydrogen Compressed Natural Gas |
| BSG | Brewery Spent Grains |
| CH4 | Methane |
| CNG | Compressed Natural Gas |
| CO | Carbon Monoxide |
| CO2 | Carbon dioxide |
| COD | Chemical Oxygen Demand |
| EU | European Union |
| FW | Food Waste |
| GDOC | Groundnut Deoiled Cake |
| GHG | Greenhouse Gases |
| HER | Hydrogen Evolution Reaction |
| IEA | International Energy Agency |
| IPCC | Intergovernmental Panel on Climate Change’s |
| IRENA | International Renewable Energy Agency |
| LCA | Life cycle assessment |
| LHV | Lower Heating Value |
| MEC | Microbial Electrolysis Cell |
| MSW | Municipal Solid Waste |
| NOx | Nitrogen Oxides |
| PNS | Purple Non-Sulfur Photosynthetic Bacteria |
| POME | Palm Oil Mill Effluent |
| PTC | Parabolic Trough Solar Collector |
| PV | Photovoltaic Cell |
| STP | Sewage Treatment Plant |
| SWW | Synthetic Wastewater |
| TEA | Techno-economic analysis |
| VFA | Volatile fatty acid |
| WTE | Waste-To-Energy |
References
- Masson-Delmotte, V.; Zhai, P.; Pörtner, H.-O.; Roberts, D.; Skea, J.; Shukla, P.R. Global Warming of 1.5 °C: IPCC Special Report on Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels in Context of Strengthening Response to Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Cambridge University Press: Cambridge, UK, 2022; ISBN 1009157949. [Google Scholar]
- Allen, M.; Antwi-Agyei, P.; Aragon-Durand, F.; Babiker, M.; Bertoldi, P.; Bind, M.; Brown, S.; Buckeridge, M.; Camilloni, I.; Cartwright, A. Technical Summary: Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2019. [Google Scholar]
- Riaz, S.; Park, S.-J. O, S-g-C3N4 nanotubes as photovoltaic boosters in quantum dot-sensitized all-weather solar cells: A synergistic approach for enhanced power conversion efficiency in dark-light conditions. Mater. Today Chem. 2022, 26, 101125. [Google Scholar] [CrossRef]
- Mohan, S.V.; Nikhil, G.; Chiranjeevi, P.; Reddy, C.N.; Rohit, M.; Kumar, A.N.; Sarkar, O. Waste biorefinery models towards sustainable circular bioeconomy: Critical review and future perspectives. Bioresour. Technol. 2016, 215, 2–12. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.; Li, Y.; Li, Q.; Li, P.; Luo, L.; Zhen, F.; Zheng, G.; Sun, Y. Low-Temperature Pretreatment of Biomass for Enhancing Biogas Production: A Review. Fermentation 2022, 8, 562. [Google Scholar] [CrossRef]
- Fazzino, F.; Pedullà, A.; Calabrò, P.S. Boosting the Circularity of Waste Management: Pretreated Mature Landfill Leachate Enhances the Anaerobic Digestion of Market Waste. Biofuel Res. J. 2023, 10, 1764–1773. [Google Scholar] [CrossRef]
- Lai, Y.H.; Lan, J.C.-W. Enhanced polyhydroxybutyrate production through incorporation of a hydrogen fuel cell and electro-fermentation system. Int. J. Hydrogen Energy 2020, 46, 16787–16800. [Google Scholar] [CrossRef]
- Matsakas, L.; Sarkar, O.; Jansson, S.; Rova, U.; Christakopoulos, P. A novel hybrid organosolv-steam explosion pretreatment and fractionation method delivers solids with superior thermophilic digestibility to methane. Bioresour. Technol. 2020, 316, 123973. [Google Scholar] [CrossRef]
- Mahmoud, A.; Zaghloul, M.S.; Hamza, R.A.; Elbeshbishy, E. Comparing VFA Composition, Biomethane Potential, and Methane Production Kinetics of Different Substrates for Anaerobic Fermentation and Digestion. Fermentation 2023, 9, 138. [Google Scholar] [CrossRef]
- Chodkowska-Miszczuk, J.; Martinat, S.; Cowell, R. Community tensions, participation, and local development: Factors affecting the spatial embeddedness of anaerobic digestion in Poland and the Czech Republic. Energy Res. Soc. Sci. 2019, 55, 134–145. [Google Scholar] [CrossRef]
- Nagarajan, S.; Jones, R.J.; Oram, L.; Massanet-Nicolau, J.; Guwy, A. Intensification of Acidogenic Fermentation for the Production of Biohydrogen and Volatile Fatty Acids—A Perspective. Fermentation 2022, 8, 325. [Google Scholar] [CrossRef]
- Valente, A.; Iribarren, D.; Dufour, J. Harmonised life-cycle global warming impact of renewable hydrogen. J. Clean. Prod. 2017, 149, 762–772. [Google Scholar] [CrossRef]
- Sarkar, O.; Katakojwala, R.; Mohan, S.V. Low-carbon hydrogen production from a waste-based biorefinery system and environmental sustainability assessment. Green Chem. 2021, 23, 561–574. [Google Scholar] [CrossRef]
- Sator, A.; Waardenburg, M.; Wilthaner, M. Five Charts on Hydrogen’s Role in a Net-Zero Future; McKinsey & Company: New York, NY, USA, 2022. [Google Scholar]
- Griffiths, S.; Sovacool, B.K.; Kim, J.; Bazilian, M.; Uratani, J.M. Industrial decarbonization via hydrogen: A critical and systematic review of developments, socio-technical systems and policy options. Energy Res. Soc. Sci. 2021, 80, 102208. [Google Scholar] [CrossRef]
- Dahiya, S.; Chatterjee, S.; Sarkar, O.; Mohan, S.V. Renewable hydrogen production by dark-fermentation: Current status, challenges and perspectives. Bioresour. Technol. 2020, 321, 124354. [Google Scholar] [CrossRef]
- Öhman, A.; Karakaya, E.; Urban, F. Enabling the transition to a fossil-free steel sector: The conditions for technology transfer for hydrogen-based steelmaking in Europe. Energy Res. Soc. Sci. 2021, 84, 102384. [Google Scholar] [CrossRef]
- Soltani, S.M.; Lahiri, A.; Bahzad, H.; Clough, P.; Gorbounov, M.; Yan, Y. Sorption-enhanced Steam Methane Reforming for Combined CO2 Capture and Hydrogen Production: A State-of-the-Art Review. Carbon Capture Sci. Technol. 2021, 1, 100003. [Google Scholar] [CrossRef]
- LeValley, T.L.; Richard, A.R.; Fan, M. The progress in water gas shift and steam reforming hydrogen production technologies—A review. Int. J. Hydrogen Energy 2014, 39, 16983–17000. [Google Scholar] [CrossRef]
- Carapellucci, R.; Giordano, L. Steam, dry and autothermal methane reforming for hydrogen production: A thermodynamic equilibrium analysis. J. Power Sources 2020, 469, 228391. [Google Scholar] [CrossRef]
- Schulz, L.A.; Kahle, L.C.; Delgado, K.H.; Schunk, S.A.; Jentys, A.; Deutschmann, O.; Lercher, J.A. On the coke deposition in dry reforming of methane at elevated pressures. Appl. Catal. A Gen. 2015, 504, 599–607. [Google Scholar] [CrossRef]
- IRENA. Hydrogen: A Renewable Energy Perspective; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2019. [Google Scholar]
- Yu, M.; Wang, K.; Vredenburg, H. Insights into low-carbon hydrogen production methods: Green, blue and aqua hydrogen. Int. J. Hydrogen Energy 2021, 46, 21261–21273. [Google Scholar] [CrossRef]
- Zhou, Y.; Searle, S. Cost of Renewable Hydrogen Produced Onsite at Hydrogen Refueling Stations in Europe; International Council on Clean Transportation: Washington, DC, USA, 2022. [Google Scholar]
- da Silva Veras, T.; Mozer, T.S.; da Silva César, A. Hydrogen: Trends, production and characterization of the main process worldwide. Int. J. Hydrogen Energy 2017, 42, 2018–2033. [Google Scholar] [CrossRef]
- Yadav, D.; Banerjee, R. Economic assessment of hydrogen production from solar driven high-temperature steam electrolysis process. J. Clean. Prod. 2018, 183, 1131–1155. [Google Scholar] [CrossRef]
- Ning, M.; Zhang, F.; Wu, L.; Xing, X.; Wang, D.; Song, S.; Zhou, Q.; Yu, L.; Bao, J.; Chen, S.; et al. Boosting efficient alkaline fresh water and seawater electrolysis via electrochemical reconstruction. Energy Environ. Sci. 2022, 15, 3945–3957. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.; Zhai, P.; Pörtner, H.O.; Roberts, D.; Skea, J.; Shukla, P.R.; Pirani, A.; Moufouma-Okia, W.; Péan, C.; Pidcock, R. Summary for Policymakers. Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2018. [Google Scholar]
- Quraishi, M.; Wani, K.; Pandit, S.; Gupta, P.K.; Rai, A.K.; Lahiri, D.; Jadhav, D.A.; Ray, R.R.; Jung, S.P.; Thakur, V.K.; et al. Valorisation of CO2 into Value-Added Products via Microbial Electrosynthesis (MES) and Electro-Fermentation Technology. Fermentation 2021, 7, 291. [Google Scholar] [CrossRef]
- Duarte, M.S.; Oliveira, J.V.; Pereira, C.; Carvalho, M.; Mesquita, D.P.; Alves, M.M. Volatile Fatty Acids (VFA) Production from Wastewaters with High Salinity—Influence of pH, Salinity and Reactor Configuration. Fermentation 2021, 7, 303. [Google Scholar] [CrossRef]
- Davila-Vazquez, G.; Arriaga, S.; Alatriste-Mondragón, F.; de León-Rodríguez, A.; Rosales-Colunga, L.M.; Razo-Flores, E. Fermentative biohydrogen production: Trends and perspectives. Rev. Environ. Sci. Bio./Technol. 2008, 7, 27–45. [Google Scholar] [CrossRef]
- Sarkar, O.; Mohan, S.V. Synergy of anoxic microenvironment and facultative anaerobes on acidogenic metabolism in a self-induced electrofermentation system. Bioresour. Technol. 2020, 313, 123604. [Google Scholar] [CrossRef]
- Gest, H.; Kamen, M.D. Photoproduction of Molecular Hydrogen by Rhodospirillum Rubrum. Science 1949, 109, 558–559. [Google Scholar] [CrossRef]
- Gaffron, H.; Rubin, J. Fermentative and photochemical production of hydrogen in algae. J. Gen. Physiol. 1942, 26, 219–240. [Google Scholar] [CrossRef]
- Greenbaum, E. Photosynthetic Hydrogen and Oxygen Production: Kinetic Studies. Science 1982, 215, 291–293. [Google Scholar] [CrossRef]
- Puente-Sánchez, F.; Arce-Rodríguez, A.; Oggerin, M.; García-Villadangos, M.; Moreno-Paz, M.; Blanco, Y.; Rodríguez, N.; Bird, L.; Lincoln, S.A.; Tornos, F.; et al. Viable cyanobacteria in the deep continental subsurface. Proc. Natl. Acad. Sci. USA 2018, 115, 10702–10707. [Google Scholar] [CrossRef]
- Riaz, S.; Rhee, K.Y.; Park, S.J. Polyhydroxyalkanoates (PHAs): Biopolymers for Biofuel and Biorefineries. Polymers 2021, 13, 253. [Google Scholar] [CrossRef] [PubMed]
- Karmee, S.K. Moving towards the Application of Biocatalysis in Food Waste Biorefinery. Fermentation 2023, 9, 73. [Google Scholar] [CrossRef]
- Ghosh, S.; Dutta, S.; Chowdhury, R. Ameliorated hydrogen production through integrated dark-photofermentation in a flat plate photobioreactor: Mathematical modelling and optimization of energy efficiency. Energy Convers. Manag. 2020, 226, 113549. [Google Scholar] [CrossRef]
- Dahiya, S.; Kumar, A.N.; Shanthi Sravan, J.; Chatterjee, S.; Sarkar, O.; Mohan, S.V. Food waste biorefinery: Sustainable strategy for circular bioeconomy. Bioresour. Technol. 2018, 248, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, A.K.; Mondal, P. Enhancing Biohydrogen Production from Sugar Industry Wastewater Using Ni, Ni–Co and Ni–Co–P Electrodeposits as Cathodes in Microbial Electrolysis Cells. Chemosphere 2022, 286, 131728. [Google Scholar] [CrossRef]
- Rikame, S.S.; Mungray, A.A.; Mungray, A.K. Modification of Anode Electrode in Microbial Fuel Cell for Electrochemical Recovery of Energy and Copper Metal. Electrochim. Acta 2018, 275, 8–17. [Google Scholar] [CrossRef]
- Kadier, A.; Simayi, Y.; Kalil, M.S.; Abdeshahian, P.; Hamid, A.A. A review of the substrates used in microbial electrolysis cells (MECs) for producing sustainable and clean hydrogen gas. Renew. Energy 2014, 71, 466–472. [Google Scholar] [CrossRef]
- Bora, A.; Mohanrasu, K.; Swetha, T.A.; Ananthi, V.; Sindhu, R.; Chi, N.T.L.; Pugazhendhi, A.; Arun, A.; Mathimani, T. Microbial electrolysis cell (MEC): Reactor configurations, recent advances and strategies in biohydrogen production. Fuel 2022, 328, 125269. [Google Scholar] [CrossRef]
- Hutchinson, A.J.; Tokash, J.C.; Logan, B.E. Analysis of carbon fiber brush loading in anodes on startup and performance of microbial fuel cells. J. Power Sources 2011, 196, 9213–9219. [Google Scholar] [CrossRef]
- Rousseau, R.; Etcheverry, L.; Roubaud, E.; Basséguy, R.; Délia, M.-L.; Bergel, A. Microbial electrolysis cell (MEC): Strengths, weaknesses and research needs from electrochemical engineering standpoint. Appl. Energy 2020, 257, 113938. [Google Scholar] [CrossRef]
- Cheng, S.; Logan, B.E. Sustainable and efficient biohydrogen production via electrohydrogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 18871–18873. [Google Scholar] [CrossRef]
- Rozendal, R.A.; Jeremiasse, A.W.; Hamelers, H.V.M.; Buisman, C.J.N. Hydrogen Production with a Microbial Biocathode. Environ. Sci. Technol. 2008, 42, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Tartakovsky, B.; Manuel, M.-F.; Wang, H.; Guiot, S. High rate membrane-less microbial electrolysis cell for continuous hydrogen production. Int. J. Hydrogen Energy 2009, 34, 672–677. [Google Scholar] [CrossRef]
- Khan, M.; Nizami, A.; Rehan, M.; Ouda, O.; Sultana, S.; Ismail, I.; Shahzad, K. Microbial electrolysis cells for hydrogen production and urban wastewater treatment: A case study of Saudi Arabia. Appl. Energy 2017, 185, 410–420. [Google Scholar] [CrossRef]
- Balachandar, G.; Varanasi, J.L.; Singh, V.; Singh, H.; Das, D. Biological hydrogen production via dark fermentation: A holistic approach from lab-scale to pilot-scale. Int. J. Hydrogen Energy 2020, 45, 5202–5215. [Google Scholar] [CrossRef]
- Lim, X. Turning Organic Waste into Hydrogen. ACS Central Sci. 2019, 5, 203–205. [Google Scholar] [CrossRef]
- Schorer, L.; Schmitz, S.; Weber, A. Membrane based purification of hydrogen system (MEMPHYS). Int. J. Hydrogen Energy 2019, 44, 12708–12714. [Google Scholar] [CrossRef]
- Shamsudin, I.; Abdullah, A.; Idris, I.; Gobi, S.; Othman, M. Hydrogen purification from binary syngas by PSA with pressure equalization using microporous palm kernel shell activated carbon. Fuel 2019, 253, 722–730. [Google Scholar] [CrossRef]
- Zarsazi, H.; Sadeghi, S.; Moghimi, M. Investigation of a Novel Hybrid LNG Waste Heat-/Wind-Driven Hydrogen Liquefaction System: Exergoeconomic Analysis and Multi-Criteria Optimization. J. Therm. Anal. Calorim. 2022, 1–17. [Google Scholar] [CrossRef]
- Haghi, E.; Raahemifar, K.; Fowler, M. Investigating the effect of renewable energy incentives and hydrogen storage on advantages of stakeholders in a microgrid. Energy Policy 2018, 113, 206–222. [Google Scholar] [CrossRef]
- McCay, M.H.; Shafiee, S. Hydrogen: An Energy Carrier. In Future Energy; Elsevier: Amsterdam, The Netherlands, 2020; pp. 475–493. [Google Scholar]
- Jain, S.; Newman, D.; Nzihou, A.; Dekker, H.; Le Feuvre, P.; Richter, H.; Gobe, F.; Morton, C.; Thompson, R. Global Potential of Biogas; The World Biogas Association: London, UK, 2019; pp. 1–56. [Google Scholar]
- EBA. New Report Highlights Biomethane Ramp-Up and Best Pathways for Full Renewable Gas Deployment; European Biogas Association: Brussels, Belgium, 2021. [Google Scholar]
- IEA. Outlook for Biogas and Biomethane: Prospects for Organic Growth; IEA: Paris, France, 2020. [Google Scholar]
- Morone, P.; Yilan, G.; Imbert, E. Using fuzzy cognitive maps to identify better policy strategies to valorize organic waste flows: An Italian case study. J. Clean. Prod. 2021, 319, 128722. [Google Scholar] [CrossRef]
- Scarlat, N.; Dallemand, J.-F.; Fahl, F. Biogas: Developments and perspectives in Europe. Renew. Energy 2018, 129, 457–472. [Google Scholar] [CrossRef]
- Kalinichenko, A.; Havrysh, V.; Perebyynis, V. Evaluation of Biogas Production and Usage Potential. Ecol. Chem. Eng. S 2016, 23, 387. [Google Scholar] [CrossRef]
- Lönnqvist, T.; Sanches-Pereira, A.; Sandberg, T. Biogas potential for sustainable transport—A Swedish regional case. J. Clean. Prod. 2015, 108, 1105–1114. [Google Scholar] [CrossRef]
- Uusitalo, V.; Soukka, R.; Horttanainen, M.; Niskanen, A.; Havukainen, J. Economics and greenhouse gas balance of biogas use systems in the Finnish transportation sector. Renew. Energy 2013, 51, 132–140. [Google Scholar] [CrossRef]
- Ayodele, T.; Alao, M.; Ogunjuyigbe, A.; Munda, J. Electricity generation prospective of hydrogen derived from biogas using food waste in south-western Nigeria. Biomass-Bioenergy 2019, 127, 105291. [Google Scholar] [CrossRef]
- Koroglu, E.O.; Ozdemir, O.K.; Ozkaya, B.; Demir, A. An integrated system development including PEM fuel cell/biogas purification during acidogenic biohydrogen production from dairy wastewater. Int. J. Hydrogen Energy 2019, 44, 17297–17303. [Google Scholar] [CrossRef]
- Nadaleti, W. Utilization of residues from rice parboiling industries in southern Brazil for biogas and hydrogen-syngas generation: Heat, electricity and energy planning. Renew. Energy 2018, 131, 55–72. [Google Scholar] [CrossRef]
- Hajizadeh, A.; Mohamadi-Baghmolaei, M.; Saady, N.M.C.; Zendehboudi, S. Hydrogen production from biomass through integration of anaerobic digestion and biogas dry reforming. Appl. Energy 2022, 309, 118442. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Heaven, S.; Banks, C. Energy potential from the anaerobic digestion of food waste in municipal solid waste stream of urban areas in Vietnam. Int. J. Energy Environ. Eng. 2014, 5, 365–374. [Google Scholar] [CrossRef]
- Rosa, A.; Chernicharo, C.; Lobato, L.; Silva, R.; Padilha, R.; Borges, J. Assessing the potential of renewable energy sources (biogas and sludge) in a full-scale UASB-based treatment plant. Renew. Energy 2018, 124, 21–26. [Google Scholar] [CrossRef]
- Mateus, S.; Carvalheira, M.; Cassidy, J.; Freitas, E.; Oehmen, A.; Reis, M.A. Two-stage anaerobic digestion system treating different seasonal fruit pulp wastes: Impact on biogas and hydrogen production and total energy recovery potential. Biomass-Bioenergy 2020, 141, 105694. [Google Scholar] [CrossRef]
- Tabatabaei, M.; Aghbashlo, M.; Valijanian, E.; Panahi, H.K.S.; Nizami, A.-S.; Ghanavati, H.; Sulaiman, A.; Mirmohamadsadeghi, S.; Karimi, K. A comprehensive review on recent biological innovations to improve biogas production, Part 2: Mainstream and downstream strategies. Renew. Energy 2020, 146, 1392–1407. [Google Scholar] [CrossRef]
- Levinsky, H. Why can’t we just burn hydrogen? Challenges when changing fuels in an existing infrastructure. Prog. Energy Combust. Sci. 2021, 84, 100907. [Google Scholar] [CrossRef]
- Deheri, C.; Acharya, S.K. Experimental Investigation of Biohythane Performance on Thermal Barrier-Coated Compression Ignition Engine. J. Energy Resour. Technol. 2022, 145, 11702. [Google Scholar] [CrossRef]
- Shamsapour, N.; Hajinezhad, A.; Noorollahi, Y. Developing a system dynamics approach for CNG vehicles for low-carbon urban transport: A case study. Int. J. Low-Carbon Technol. 2020, 16, 577–591. [Google Scholar] [CrossRef]
- Lähde, T.; Giechaskiel, B. Particle Number Emissions of Gasoline, Compressed Natural Gas (CNG) and Liquefied Petroleum Gas (LPG) Fueled Vehicles at Different Ambient Temperatures. Atmosphere 2021, 12, 893. [Google Scholar] [CrossRef]
- Bhasker, J.P.; Porpatham, E. Effects of compression ratio and hydrogen addition on lean combustion characteristics and emission formation in a Compressed Natural Gas fuelled spark ignition engine. Fuel 2017, 208, 260–270. [Google Scholar] [CrossRef]
- Mustafi, N.N.; Agarwal, A.K. Combustion and Emission Characteristics, and Emission Control of CNG Fueled Vehicles. In Alternative Fuels and Their Utilization Strategies in Internal Combustion Engines; Springer: Singapore, 2020; pp. 201–228. [Google Scholar]
- Sagar, S.; Agarwal, A.K. Knocking behavior and emission characteristics of a port fuel injected hydrogen-enriched compressed natural gas fueled spark ignition engine. Appl. Therm. Eng. 2018, 141, 42–50. [Google Scholar] [CrossRef]
- Zareei, J.; Alvarez, J.R.N.; Albuerne, Y.L.; Gámez, M.R.; Linzan, R.A. A Simulation Study of the Effect of HCNG Fuel and Injector Hole Number along with a Variation of Fuel Injection Pressure in a Gasoline Engine Converted from Port Injection to Direct Injection. Processes 2022, 10, 2389. [Google Scholar] [CrossRef]
- Tlili, O. Hydrogen Systems: What Contribution to the Energy System? Findings from Multiple Modelling Approaches; Université Paris-Saclay, Espace Technologique/Immeuble Discovery: Saint-Aubin, France, 2019; p. 294. [Google Scholar]
- Sukphun, P.; Sittijunda, S.; Reungsang, A. Volatile Fatty Acid Production from Organic Waste with the Emphasis on Membrane-Based Recovery. Fermentation 2021, 7, 159. [Google Scholar] [CrossRef]
- Sarkar, O.; Santhosh, J.; Dhar, A.; Mohan, S.V. Green hythane production from food waste: Integration of dark-fermentation and methanogenic process towards biogas up-gradation. Int. J. Hydrogen Energy 2021, 46, 18832–18843. [Google Scholar] [CrossRef]
- Pasupuleti, S.B.; Mohan, S.V. Single-stage fermentation process for high-value biohythane production with the treatment of distillery spent-wash. Bioresour. Technol. 2015, 189, 177–185. [Google Scholar] [CrossRef]
- Pinto, M.P.M.; Mudhoo, A.; Neves, T.D.A.; Berni, M.D.; Forster-Carneiro, T. Co–digestion of coffee residues and sugarcane vinasse for biohythane generation. J. Environ. Chem. Eng. 2018, 6, 146–155. [Google Scholar] [CrossRef]
- Liu, R.; Chen, X.; Zhang, K.; Han, Y.; Tong, Y.; Wang, J.; Xiao, B.; Liu, J. Effect of mixing ratio and total solids content on temperature-phased anaerobic codigestion of rice straw and pig manure: Biohythane production and microbial structure. Bioresour. Technol. 2022, 344, 126173. [Google Scholar] [CrossRef]
- Nguyen, T.-T.; Ta, D.-T.; Lin, C.-Y.; Chu, C.-Y.; Ta, T.-M. Biohythane production from swine manure and pineapple waste in a single-stage two-chamber digester using gel-entrapped anaerobic microorganisms. Int. J. Hydrogen Energy 2022, 47, 25245–25255. [Google Scholar] [CrossRef]
- Sarkar, O.; Rova, U.; Christakopoulos, P.; Matsakas, L. Influence of initial uncontrolled pH on acidogenic fermentation of brewery spent grains to biohydrogen and volatile fatty acids production: Optimization and scale-up. Bioresour. Technol. 2020, 319, 124233. [Google Scholar] [CrossRef]
- Li, J.; He, J.; Si, B.; Liu, Z.; Zhang, C.; Wang, Y.; Xing, X.-H. A pilot study of biohythane production from cornstalk via two-stage anaerobic fermentation. Int. J. Hydrogen Energy 2020, 45, 31719–31731. [Google Scholar] [CrossRef]
- Promnuan, K.; Higuchi, T.; Imai, T.; Kongjan, P.; Reungsang, A.; Sompong, O. Simultaneous biohythane production and sulfate removal from rubber sheet wastewater by two-stage anaerobic digestion. Int. J. Hydrogen Energy 2019, 45, 263–274. [Google Scholar] [CrossRef]
- Sarkar, O.; Rova, U.; Christakopoulos, P.; Matsakas, L. Organosolv pretreated birch sawdust for the production of green hydrogen and renewable chemicals in an integrated biorefinery approach. Bioresour. Technol. 2021, 344, 126164. [Google Scholar] [CrossRef]
- Chen, C.; Sun, C.; Xia, A.; Liao, Q.; Guo, X.; Huang, Y.; Fu, Q.; Zhu, X.; Zhu, X. Sustainable biohythane production from algal bloom biomass through two-stage fermentation: Impacts of the physicochemical characteristics and fermentation performance. Int. J. Hydrogen Energy 2020, 45, 34461–34472. [Google Scholar] [CrossRef]
- Jehlee, A.; Khongkliang, P.; Suksong, W.; Rodjaroen, S.; Waewsak, J.; Reungsang, A.; Sompong, O. Biohythane production from Chlorella sp. biomass by two-stage thermophilic solid-state anaerobic digestion. Int. J. Hydrogen Energy 2017, 42, 27792–27800. [Google Scholar] [CrossRef]
- Nguyen, M.-L.T.; Hung, P.-C.; Vo, T.-P.; Lay, C.-H.; Lin, C.-Y. Effect of food to microorganisms (F/M) ratio on biohythane production via single-stage dark fermentation. Int. J. Hydrogen Energy 2020, 46, 11313–11324. [Google Scholar] [CrossRef]
- Seengenyoung, J.; Mamimin, C.; Prasertsan, P.; Sompong, O. Pilot-scale of biohythane production from palm oil mill effluent by two-stage thermophilic anaerobic fermentation. Int. J. Hydrogen Energy 2019, 44, 3347–3355. [Google Scholar] [CrossRef]
- Ali, M.M.; Mustafa, A.M.; Zhang, X.; Lin, H.; Zhang, X.; Danhassan, U.A.; Zhou, X.; Sheng, K. Impacts of molybdate and ferric chloride on biohythane production through two-stage anaerobic digestion of sulfate-rich hydrolyzed tofu processing residue. Bioresour. Technol. 2022, 355, 127239. [Google Scholar] [CrossRef]
- Chang, H.; Wu, H.; Zhang, L.; Wu, W.; Zhang, C.; Zhong, N.; Zhong, D.; Xu, Y.; He, X.; Yang, J.; et al. Gradient electro-processing strategy for efficient conversion of harmful algal blooms to biohythane with mechanisms insight. Water Res. 2022, 222, 118929. [Google Scholar] [CrossRef]
- Lunprom, S.; Phanduang, O.; Salakkam, A.; Liao, Q.; Imai, T.; Reungsang, A. Bio-hythane production from residual biomass of Chlorella sp. biomass through a two-stage anaerobic digestion. Int. J. Hydrogen Energy 2018, 44, 3339–3346. [Google Scholar] [CrossRef]
- Santhosh, J.; Sarkar, O.; Mohan, S.V. Green Hydrogen-Compressed natural gas (bio-H-CNG) production from food waste: Organic load influence on hydrogen and methane fusion. Bioresour. Technol. 2021, 340, 125643. [Google Scholar] [CrossRef]
- Sarkar, O.; Mohan, S.V. Pre-aeration of food waste to augment acidogenic process at higher organic load: Valorizing biohydrogen, volatile fatty acids and biohythane. Bioresour. Technol. 2017, 242, 68–76. [Google Scholar] [CrossRef]
- Sarkar, O.; Katari, J.K.; Chatterjee, S.; Mohan, S.V. Salinity induced acidogenic fermentation of food waste regulates biohydrogen production and volatile fatty acids profile. Fuel 2020, 276, 117794. [Google Scholar] [CrossRef]
- Hans, M.; Kumar, S. Biohythane production in two-stage anaerobic digestion system. Int. J. Hydrogen Energy 2018, 44, 17363–17380. [Google Scholar] [CrossRef]
- Bolzonella, D.; Battista, F.; Cavinato, C.; Gottardo, M.; Micolucci, F.; Lyberatos, G.; Pavan, P. Recent developments in biohythane production from household food wastes: A review. Bioresour. Technol. 2018, 257, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Meena, R.A.A.; Banu, J.R.; Kannah, R.Y.; Yogalakshmi, K.; Kumar, G. Biohythane production from food processing wastes—Challenges and perspectives. Bioresour. Technol. 2020, 298, 122449. [Google Scholar] [CrossRef] [PubMed]
- Yeshanew, M.M.; Frunzo, L.; Pirozzi, F.; Lens, P.N.L.; Esposito, G. Production of biohythane from food waste via an integrated system of continuously stirred tank and anaerobic fixed bed reactors. Bioresour. Technol. 2016, 220, 312–322. [Google Scholar] [CrossRef]
- Meziane, S.; Bentebbiche, A. Numerical study of blended fuel natural gas-hydrogen combustion in rich/quench/lean combustor of a micro gas turbine. Int. J. Hydrogen Energy 2019, 44, 15610–15621. [Google Scholar] [CrossRef]
- Panagi, K.; Laycock, C.J.; Reed, J.P.; Guwy, A.J. Highly efficient coproduction of electrical power and synthesis gas from biohythane using solid oxide fuel cell technology. Appl. Energy 2019, 255, 113854. [Google Scholar] [CrossRef]
- Dimopoulos, P.; Bach, C.; Soltic, P.; Boulouchos, K. Hydrogen–natural gas blends fuelling passenger car engines: Combustion, emissions and well-to-wheels assessment. Int. J. Hydrogen Energy 2008, 33, 7224–7236. [Google Scholar] [CrossRef]
- Hao, D.; Mehra, R.K.; Luo, S.; Nie, Z.; Ren, X.; Fanhua, M. Experimental study of hydrogen-enriched compressed natural gas (HCNG) engine and application of support vector machine (SVM) on prediction of engine performance at specific condition. Int. J. Hydrogen Energy 2019, 45, 5309–5325. [Google Scholar] [CrossRef]
- Tangöz, S.; Akansu, S.O.; Kahraman, N.; Malkoç, Y. Effects of compression ratio on performance and emissions of a modified diesel engine fueled by HCNG. Int. J. Hydrogen Energy 2015, 40, 15374–15380. [Google Scholar] [CrossRef]
- Hemalatha, M.; Sarkar, O.; Mohan, S.V. Self-sustainable azolla-biorefinery platform for valorization of biobased products with circular-cascading design. Chem. Eng. J. 2019, 373, 1042–1053. [Google Scholar] [CrossRef]
- Thomassen, G.; Van Dael, M.; Van Passel, S.; You, F. How to assess the potential of emerging green technologies? Towards a prospective environmental and techno-economic assessment framework. Green Chem. 2019, 21, 4868–4886. [Google Scholar] [CrossRef]
- Sarkar, S.; Kumar, A. Large-scale biohydrogen production from bio-oil. Bioresour. Technol. 2010, 101, 7350–7361. [Google Scholar] [CrossRef] [PubMed]
- Galera, S.; Ortiz, F.G. Techno-economic assessment of hydrogen and power production from supercritical water reforming of glycerol. Fuel 2015, 144, 307–316. [Google Scholar] [CrossRef]
- Aghbashlo, M.; Hosseinzadeh-Bandbafha, H.; Shahbeik, H.; Tabatabaei, M. The role of sustainability assessment tools in realizing bioenergy and bioproduct systems. Biofuel Res. J. 2022, 9, 1697–1706. [Google Scholar] [CrossRef]
- Amid, S.; Aghbashlo, M.; Tabatabaei, M.; Karimi, K.; Nizami, A.-S.; Rehan, M.; Hosseinzadeh-Bandbafha, H.; Soufiyan, M.M.; Peng, W.; Lam, S.S. Exergetic, exergoeconomic, and exergoenvironmental aspects of an industrial-scale molasses-based ethanol production plant. Energy Convers. Manag. 2021, 227, 113637. [Google Scholar] [CrossRef]
- Gheewala, S.H. Life Cycle Assessment for Sustainability Assessment of Biofuels and Bioproducts. Biofuel Res. J. 2023, 10, 1810–1815. [Google Scholar] [CrossRef]
- Sarkar, O.; Rova, U.; Christakopoulos, P.; Matsakas, L. Green hydrogen and platform chemicals production from acidogenic conversion of brewery spent grains co-fermented with cheese whey wastewater: Adding value to acidogenic CO2. Sustain. Energy Fuels 2022, 6, 778–790. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).