Achievements of Autochthonous Wine Yeast Isolation and Selection in Romania—A Review
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Employed Techniques of Yeast Isolation, Identification and Selection
3.2. Yeast Biodiversity and Identification Results
| Genus | Species | Centre/Vineyard | References |
|---|---|---|---|
| Candida | C. colliculosa | Valea Călugărească, Dealu Mare | [46,47] |
| Recaș | [68] | ||
| C. famata | Valea Călugărească, Dealu Mare | [46,47] | |
| C. lusitaniae | Valea Călugărească, Dealu Mare | [46,47] | |
| C. magnoliae | Valea Călugărească, Dealu Mare | [46,47] | |
| Recaș | [68] | ||
| C. mycoderma | Cernavodă, Murfatlar | [52] | |
| Cotnari vineyard | [40] | ||
| C. pelliculosa | Valea Călugărească, Dealu Mare | [46,47] | |
| C. sphaerica | Valea Călugărească, Dealu Mare | [46,47] | |
| C. tropicalis | Recaș | [68] | |
| C. utilis | Valea Călugărească, Dealu Mare | [46,47] | |
| C. vini | Drăgășani, Tamburești | [50] | |
| Clavispora | C. lusitaniae | Valea Călugărească, Dealu Mare | [47] |
| Debaryomyces | D. hansenii | Valea Călugărească, Dealu Mare | [46,47] |
| Dekkera | D. anomala | Pietroasa vineyard | [53] |
| Geotrichum | G. penicillatum | Valea Călugărească, Dealu Mare | [47] |
| Hanseniaspora | H. uvarum | Recaș | [68] |
| Hansenula | H. anomala | Cernavodă, Murfatlar | [52] |
| Cotnari vineyard | [40] | ||
| Kloeckera | K. apiculata | Cernavodă, Murfatlar | [52] |
| Valea Călugărească, Dealu Mare | [46,47] | ||
| Drăgășani | [33] | ||
| Recaș | [68] | ||
| Lachancea | L. kluyveri | Cotnari vineyard | [40] |
| Metschnikowia | M. pulcherrima | Drăgășani | [50] |
| Pietroasa vineyard | [53] | ||
| Pichia | P. angusta | Recaș | [68] |
| P. anomala | Valea Călugărească, Dealu Mare | [46,47] | |
| P. fermentans | Cernavodă, Murfatlar | [52] | |
| Recaș | [68] | ||
| P. jadinii | Valea Călugărească, Dealu Mare | [46,47] | |
| P. kudriavzevii | Ilfov area | [63] | |
| P. membranaefaciens | Drăgășani, Tamburești | [50] | |
| Cotnari vineyard | [40] | ||
| P. ohmeri | Valea Călugărească, Dealu Mare | [47] | |
| Rhodotorula | R. glutinis | Valea Călugărească, Dealu Mare | [46,47] |
| Recaș | [68] | ||
| R. minuta | Valea Călugărească, Dealu Mare | [46,47] | |
| R. mucilaginosa | Cernavodă, Murfatlar | [52] | |
| Valea Călugărească, Dealu Mare | [47] | ||
| Recaș | [68] | ||
| Torulaspora | T. delbrueckii | Valea Călugărească, Dealu Mare | [46,47] |
| Torulopsis | T. stellata | Cernavodă, Murfatlar | [52] |
| Cotnari vineyard | [40] | ||
| Zygosaccharomyces | Z. bailii | Cotnari vineyard | [40] |
| Z. rouxii | Cotnari vineyard | [40] |
| Species | Centre/Vineyard | References |
|---|---|---|
| S. bayanus | Cernavodă, Murfatlar | [52] |
| Dealurile Bujorului | [43] | |
| Cotnari vineyard | [40] | |
| S. cerevisiae | Buzău vineyard | [49] |
| Pietroasa vineyard | [53] | |
| Recaș | [68] | |
| Valea Călugărească, Dealu Mare | [46] | |
| Cotnari vineyard | [40] | |
| S. chevalieri | Cotnari vineyard | [40] |
| S. ellipsoideus | Cernavodă, Murfatlar | [52] |
| Dealurile Bujorului | [43] | |
| Iași-Copou vineyard | [41] | |
| Cotnari vineyard | [40] | |
| S. florentinus | Cotnari vineyard | [40] |
| S. oviformis (synonym S. cerevisiae) | Cernavodă, Murfatlar | [52] |
| Dealurile Bujorului | [43] | |
| Cotnari vineyard | [40] | |
| S. uvarum | Cotnari vineyard | [40] |
3.3. Selected Yeast Properties and the Final Characteristics of Local Wines
| Grape Varieties | Vine Regions | References |
|---|---|---|
| Aligoté | Iași | [72] |
| Iași | [70] | |
| Dealu Bujorului | [69] | |
| Băbească gri | Dealu Bujorului | [69] |
| Cabernet sauvignon | Dealu Mare | [73] |
| Miniș-Măderat | [71] | |
| Dobra (Satu Mare) | [74] | |
| Cadarcă | Miniș-Măderat | [74] |
| Chardonnay | Iași | [55] |
| Feteasca albă | Dealu Bujorului | [69] |
| Iași | [55] | |
| Feteasca neagră | Miniș-Măderat | [74] |
| Panciu | [75] | |
| Ratești (Satu Mare) and Aliman (Constanța) | [74] | |
| Fetească regală | Dealu Bujorului | [69] |
| Iași | [70] | |
| Miniș-Măderat | [71] | |
| Frâncușă | Iași | [70] |
| Grasa de Cotnari | Iași | [70] |
| Italian riesling | Dealu Bujorului | [69] |
| Iași | [70] | |
| Merlot | Aliman (Constanța) | [74] |
| Muscat ottonel | Dealu Bujorului | [69] |
| Iași | [70,76,77] | |
| Neuburger | Iași | [70] |
| Pinot gris | Iași | [70] |
| Miniș-Măderat | [71] | |
| Pinot noir | Ratești (Satu Mare) | [74] |
| Rose traminer | Iași | [70] |
| Sarba | Dealu Bujorului | [69] |
| Sauvignon | Dealu Mare | [78] |
| Sauvignon blanc | Dealu Bujorului | [69] |
| Iași | [55,70] | |
| Tamaioasă românească | Iași | [70] |
| Traminer | Miniș-Măderat | [71] |
| Grape Varieties | Vine Region | Alcohol Vol. (%) | Residual Sugars (g/L) | Total Acidity (g/L) | Volatile Acidity (g/L) | References |
|---|---|---|---|---|---|---|
| Aligoté | Iași | 11.33 | * | 6.72 | 0.35 | [72] |
| Dealu Bujorului | 13.1 | nd | 5.5 | 0.37 | [69] | |
| Iași | 10.08 | 0.72 | 9.14 | 0.33 | [70] | |
| Băbească gri | Dealu Bujorului | 13.2 | 12.7 | 5.9 | 0.38 | [69] |
| Cabernet Sauvignon | Dealu Mare | 13.1 | 3.8 | 4.3 | 0.7 | [73] |
| Cadarcă | Miniș-Măderat | 15 | 10.05 | 5.5 | 0.43 | [71] |
| Dobra (Satu Mare) | 12 | * | 5.42 | 0.47 | [74] | |
| Miniș-Măderat | 13.25 | 2.44 | 5.55 | 0.32 | [71] | |
| Chardonnay | Iași | 12.4 | nd | 5.9 | 0.29 | [55] |
| Fetească albă | Dealu Bujorului | 13.5 | nd | 4 | 0.39 | [69] |
| Iași | 11.6 | 0.2 | 5.6 | 0.28 | [55] | |
| Fetească neagră | Miniș-Măderat | 13.97 | 3.48 | 5.93 | 0.42 | [71] |
| Panciu | 13.5 | 3.9 | 5.32 | 0.88 | [75] | |
| Ratești (Satu Mare) | 13.06 | * | 5.98 | 0.57 | [74] | |
| Aliman (Constanța) | 13.43 | * | 5.41 | 0.73 | [74] | |
| Fetească regală | Dealu Bujorului | 13.8 | 1.9 | 5.3 | 0.42 | [69] |
| Iași | 13.94 | 6.63 | 6.92 | 0.43 | [70] | |
| Miniș-Măderat | 13.39 | 3.9 | 5.7 | 0.53 | [71] | |
| Frâncușă | Iași | 11.87 | 0.63 | 8.54 | 0.41 | [70] |
| Grasa de Cotnari | Iași | 11.6 | 1.7 | 8.55 | 0.25 | [70] |
| Italian Riesling | Dealu Bujorului | 11 | 72 | 4.9 | 0.61 | [69] |
| Iași | 11.83 | 0.77 | 7.07 | 0.29 | [70] | |
| Merlot | Aliman (Ostrov) | 14.14 | * | 5.25 | 0.65 | [57] |
| Muscat ottonel | Dealu Bujorului | 11 | 30.7 | 4.4 | 0.54 | [69] |
| Iași | 12.2 | 1.34 | 6.43 | 0.33 | [70] | |
| Iași | 13.6 | 1.67 | 6.4 | 0.35 | [76] | |
| Sparkling Muscat ottonel | Iași | 10.3 | * | 6.2 | 0.33 | [77] |
| Neuburger | Iași | 12.44 | 10.63 | 7.71 | 0.45 | [70] |
| Pinot gris | Iași | 14.49 | 4.81 | 6.68 | 0.33 | [70] |
| Miniș-Măderat | 13.39 | 2.04 | 5.93 | 0.47 | [71] | |
| Pinot noir | Ratești (Dealurile Silvaniei) | 13.47 | * | 6.01 | 0.53 | [74] |
| Rose Traminer | Iași | 14.1 | 1.67 | 6.73 | 0.25 | [70] |
| Șarba | Dealu Bujorului | 14.1 | 23 | 5.8 | 0.54 | [69] |
| Sauvignon | Dealu Mare | 12.2 | 10 | 5.8 | 0.3 | [78] |
| 13 | 12 | 5.4 | 0.4 | |||
| 12.5 | 12 | 5.2 | 0.4 | |||
| Sauvignon blanc | Dealu Bujorului | 14.35 | 12 | 5.2 | 0.57 | [69] |
| Iași | 11.24 | 1.1 | 5.94 | 0.29 | [70] | |
| Iași | 11.9 | 0.9 | 5.95 | 0.2 | [55] | |
| Tămâioasă românească | Iași | 11.63 | 15.47 | 6.93 | 0.31 | [70] |
| Traminer | Miniș-Măderat | 12.3 | 50 | 5.9 | 0.47 | [71] |
3.4. New Selection Directions in the Terroir Concept Context
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mărăcineanu, L.; Giugea, N.; Perț, C.; Căpruciu, R. Tradition and Quality of Romanian Viticulture. Ann. Univ. Craiova 2018, 23, 139–143. [Google Scholar]
- Mart, M. Everything You Need to Know about Romanian Wine. Vincarta 2017. [Google Scholar]
- International Organisation of Vine and Wine. Country Reports of Romania. 2022. Available online: https://www.oiv.int/what-we-do/country-report?oiv; https://www.oiv.int/what-we-do/data-discovery-report?oiv (accessed on 18 November 2022).
- Law 164 of 24 June 2015 of Vines and Wine under the System of the Common Organization of the Wine Market. Article 17, Published in Official Journal of Romania no. 472 of 30 June 2015. Available online: https://legislatie.just.ro/Public/DetaliiDocument/169280 (accessed on 4 December 2022).
- National Institute of Statistics. Romanian Statistical Yearbook—Time Series (CD-ROM); NIS: Bucharest, Romania, 2021. [Google Scholar]
- Giugea, N.; Muntean, C.; Mărăcineanu, C.; Călugăru, V. Resurse Oenoturistice; Alma: Craiova, Romania, 2020; ISBN 978-606-567-398-4. [Google Scholar]
- Antoce, A.O.; Nămoloșanu, I.C.; Matei Rădoi, F. Comparative Study Regarding the Ethanol Resistance of Some Yeast Strains Isolated from Romanian Vineyards. Rom. Biotechnol. Lett. 2011, 16, 5981–5988. [Google Scholar]
- Pretorius, I.S. Tasting the Terroir of Wine Yeast Innovation. FEMS Yeast Res. 2020, 20, foz084. [Google Scholar] [CrossRef]
- Gonzalez, R.; Morales, P. Truth in wine yeast. Microb. Biotechnol. 2022, 15, 1339–1356. [Google Scholar] [CrossRef] [PubMed]
- Molinet, J.; Cubillos, F.A. Wild Yeast for the Future: Exploring the Use of Wild Strains for Wine and Beer Fermentation. Front. Genet. 2020, 11, 589350. [Google Scholar] [CrossRef]
- Erny, C.; Raoult, P.; Alais, A.; Butterlin, G.; Delobel, P.; Matei-Radoi, F.; Casaregola, S.; Legras, J.L. Ecological success of a group of Saccharomyces cerevisiae/Saccharomyces kudriavzevii hybrids in the northern european wine-making environment. Appl. Environ. Microbiol. 2012, 78, 3256–3265. [Google Scholar] [CrossRef]
- Pretorius, I.S. Tailoring wine yeast for the new millennium: Novel approaches to the ancient art of winemaking. Yeast 2000, 16, 675–729. [Google Scholar] [CrossRef]
- Grangeteau, C.; Roullier-Gall, C.; Rousseaux, S.; Gougeon, R.D.; Schmitt-Kopplin, P.; Alexandre, H.; Guilloux-Benatier, M. Wine microbiology is driven by vineyard and winery anthropogenic factors. Microb Biotechnol. 2017, 10, 354–370. [Google Scholar] [CrossRef]
- Tofalo, R.; Perpetuini, G.; Schirone, M.; Fasoli, G.; Aguzzi, I.; Corsetti, A.; Suzzi, G. Biogeographical characterisation of Saccharomyces cerevisiae wine yeast by molecular methods. Front. Microbiol. 2013, 4, 166. [Google Scholar] [CrossRef]
- Mas, A.; Padilla, B.; Esteve-Zarzoso, B.; Beltran, G.; Reguant, C.; Bordons, A. Taking Advantage of Natural Biodiversity for Wine Making: The WILDWINE Project. Agric. Agric. Sci. Procedia 2016, 8, 4–9. [Google Scholar] [CrossRef]
- Alessandria, V.; Marengo, F.; Englezos, V.; Gerbi, V.; Rantsiou, K.; Cocolin, L. Mycobiota of Barbera grapes from the Piedmont region from a single vintage year. Am. J. Enol. Vitic. 2015, 66, 244–250. [Google Scholar] [CrossRef]
- Costantini, A.; Vaudano, E.; Pulcini, L.; Boatti, L.; Gamalero, E.; Garcia-Moruno, E. Yeast Biodiversity in Vineyard during Grape Ripening: Comparison between Culture Dependent and NGS Analysis. Processes 2022, 10, 901. [Google Scholar] [CrossRef]
- Di Maio, S.; Polizzotto, G.; Di Gangi, E.; Foresta, G.; Genna, G.; Verzera, A.; Scacco, A.; Amore, G.; Oliva, D. Biodiversity of Indigenous Saccharomyces Populations from Old Wineries of South-Eastern Sicily (Italy): Preservation and Economic Potential. PLoS ONE 2012, 7, e30428. [Google Scholar] [CrossRef]
- Tofalo, R.; Perpetuini, G.; Fasoli, G.; Schirone, M.; Corsetti, A.; Suzzi, G. Biodiversity study of wine yeasts belonging to the “terroir” of Montepulciano d’Abruzzo “Colline Teramane” revealed Saccharomyces cerevisiae strains exhibiting atypical and unique 5.8S-ITS restriction patterns. Food Microbiol. 2014, 39, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; Arroyob, T.; Bañuelosa, M.A.; Blancoc, P.; Brionesd, A.; Cantorale, J.M.; Castrilloc, D.; Cordero-Buesoe, G.; del Fresnoa, J.M.; Escott, C.; et al. Wine yeast selection in the Iberian Peninsula: Saccharomyces and non-Saccharomyces as drivers of innovation in Spanish and Portuguese wine industries. Crit. Rev. Food Sci. Nutr. 2022, 10, 1–29. [Google Scholar] [CrossRef]
- Padilla, B.; Garcia- Fernández, D.; González, B.; Izidoro, I.; Esteve-Zarzoso, B.; Beltran, G.; Mas, A. Yeast Biodiversity from DOQ Priorat Uninoculated Fermentations. Front. Microbiol. 2016, 7, 930. [Google Scholar] [CrossRef]
- Castrillo, D.; Rabuñal, E.; Neira, N.; Blanco, P. Yeast diversity on grapes from Galicia, NW Spain: Biogeographical Patterns and the Influence of the Farming System. OENO One 2019, 53, 573–587. [Google Scholar] [CrossRef]
- De Celis, M.; Ruiz, J.; Martín-Santamaría, A.; Alonso, M.; Marquina, D.; Navascués, E.; Gómez-Flechoso, M.Á.; Belda, I.; Santos, A. Diversity of Saccharomyces cerevisiae yeasts associated to spontaneous and inoculated fermenting grapes from Spanish vineyards. Lett. Appl. Microbiol. 2019, 68, 580–588. [Google Scholar] [CrossRef]
- Koulougliotis, D.; Eriotou, E. Isolation and identification of endogenous yeast strains in grapes and must solids of Mavrodafni kefalonias and antioxidant activity of the produced red wine. Ferment Technol. 2016, 5, 1. [Google Scholar] [CrossRef]
- Kachalkin, A.V.; Abdullabekova, D.A.; Magomedova, E.S.; Magomedov, G.G.; Chernov, I.Y. Yeasts of the vineyards in Dagestan and other regions. Microbiology 2015, 84, 425–432. [Google Scholar] [CrossRef]
- Brîndușe, E.; Nechita, A.; Ion, M.; Pașa, R.; Fîciu, L.; Ciubucă, A. Valorificarea Biodiversității Drojdiilor Autohtone de Vinificație În Scopul Obținerii Vinurilor Cu Tipicitate de Areal Viticol; Editura PIM: Iași, Romania, 2022; ISBN 978-606-13-7088-7. [Google Scholar]
- Nițescu, M.A. Contribution de l’étude Des Levures Roumaines. Ph.D. Thesis, Faculte de Sciences, Universite Paris Cite, Paris, France, 1915. [Google Scholar]
- Ringhianu, J.; Septilici, G. Folosirea Drojdiilor Selecționate La I.A.S. Și Importanța Lor În Obținerea Vinurilor de Calitate. Rev. I.A.S. 1958, 1, 4–7. [Google Scholar]
- Septilici, G. Contribuții La Studiul Comparativ al Câtorva Specii de Drojdii; Institutul de Cercetări Horti-Viticole, Lucrări Științifice: Paris, France, 1961; Volume 4, pp. 39–47. [Google Scholar]
- Septilici, G.; Gherman, M. Elemente noi în pregătirea, livrarea și folosirea culturilor de drojdii în vinificație. Rev. Grădina Via Și Livada 1963, 3, 12–19. [Google Scholar]
- Septilici, G.; Gherman, M. Rolul drojdiilor specializate în obținerea vinurilor de calitate. Rev. Grădina Via Și Livada 1963, 7, 47–52. [Google Scholar]
- Septilici, G.; Sandu-Ville, G. Conducerea fermentației musturilor în toamne cu temperaturi scăzute prin folosirea drojdiilor criofile. Rev. Grădina Via Și Livada 1965, 11, 89–94. [Google Scholar]
- Gherman, M. Influența culturilor de drojdii în amestec asupra calității vinurilor. Rev. Grădina Via Și Livada 1963, 39, 58–67. [Google Scholar]
- Gherman, M. Drojdii de genuri și specii diferite pentru reglarea acidității. Rev. Grădina Via Și Livada 1965, 38, 72–75. [Google Scholar]
- Dănoaie, F. Cercetări Privind Fiziologia Drojdiilor Izolate Din Podgoria Târnave. Ph.D. Thesis, Babeș-Bolyai University, Cluj-Napoca, Romania, 1989. [Google Scholar]
- Stamate, C.; Țârdea, C.; Burnete, C. Isolation and Identification of Microflora on Grapes for the Varieties in Viticultural Centres Blaj, Târnave Vineyard. Horticultura 2006, 49, 537–542. [Google Scholar]
- Oprean, L. Biotechnological Characteristics of Some Saccharomyces Species Isolated from Wine Yeast Culture. Food Sci. Biotechnol. 2005, 14, 722–726. [Google Scholar]
- Sandu-Ville, G. Contribuții La Studiul Și Clasificarea Drojdiilor de Vin Din Microflora Vinicolă a Podgoriei Copou-Iași. Ph.D. Thesis, Institutul Agronomic Iași, Iași, Romania, 1974. [Google Scholar]
- Sandu-Ville, G.; Savin, C. Conducerea Fermentației Alcoolice a Musturilor Cu Ajutorul Levurilor Criofile. Cercet. Agron. În Mold. 1987, 2, 68–74. [Google Scholar]
- Viziteu, G.A.; Manoliu, A.; Andor, I. Data Concerning the Yeasts Microbiota from Cotnari Vineyard. Rom. Biotechnol. Lett. 2008, 13, 3673–3680. [Google Scholar]
- Vasile, A.; Cotea, V.V.; Măntăluță, A.; Pașa, R.; Savin, C. The Preliminary Selection of Isolated Yeast Strains from the Indigenous Flora of Iași Vineyard. Sci. Artic. UASVM Iași—Hortic. Ser. 2009, 52, 811–816. [Google Scholar]
- Nechita, A.; Savin, C.; Pașa, R.; Zamfir, C.I.; Codreanu, M. Isolation of New Types of Yeasts Strains from Indigenous Flora of Iași Vineyards. Sci. Artic. UASVM Iași—Hortic. Ser. 2014, 57, 177–182. [Google Scholar]
- Găgeanu, A.; Câmpeanu, G.; Diguță, C.; Matei, F. Isolation and Identification of Local Wine Yeast Strains from Dealurile Bujorului Vineyard. Sci. Bulletin. Ser. F. Biotechnol. 2012, XVI, 22–25. [Google Scholar]
- Kontek, A.; Kontek, A. Caracteristicile Morfofiziologice Și Tehnologice Ale Unei Tulpini de Saccharomyces carlsbergensis Izolată Din Vin. An. ICVV 1975, VI, 543–552. [Google Scholar]
- Kontek, A.; Kontek, A. Contribuții La Studiul Taxonomic al Drojdiilor Din Podgoria Dealu Mare. An. ICVV 1976, VII, 597–609. [Google Scholar]
- Matei Rădoi, F.; Brîndușe, E.; Nicolae, G.; Tudorache, A.; Teodorescu, R.I. Yeast Biodiversity Evolution over Decades in Dealu Mare—Valea Călugărească Vineyard. Rom. Biotechnol. Lett. 2011, 16, 113–120. [Google Scholar]
- Brîndușe, E.; Tudorache, A.; Fotescu, L. Influence of Ecological Culture System on the Dynamics and Biodiversity of Non-Saccharomyces Autochthonous Wine Yeasts. Sci. Artic. UASVM Iași—Hortic. Ser. 2012, 55, 331–336. [Google Scholar]
- Brîndușe, E.; Tudorache, A.; Fotescu, L. Selected Autochthonous Yeast Strains with Influence on Wine Quality. Sci. Artic. UASVM Iași—Hortic. Ser. 2012, 55, 365–370. [Google Scholar]
- Bărbulescu, I.D.; Begea, M.; Frîncu, M.; Tamaian, R. Identification of Yeasts Tested for Fermentation on Different Carbon Sources. Prog. Cryog. Isot. Sep. 2015, 18, 51–58. [Google Scholar]
- Dragomir Tutulescu, F.; Popa, A. Wine-Growing Areas in Oltenia (Romania) Major Natural Sources for the Isolation, Identification and Selection of Oenological Microorganisms. Not. Bot. Horti Agrobot. Cluj-Napoca 2009, 37, 139–144. [Google Scholar]
- Beleniuc, G. Contribuții La Studiul Drojdiilor Izolate Din Podgoria Murfatlar. Ph.D. Thesis, Al. I. Cuza University: Iași, Romania, 1996. [Google Scholar]
- Beleniuc, G. Researches Regarding the Taxonomic Identification of Spontaneous Microflora from Viti-Vinicol Cernavodă Center from Murfatlar Vineyard. Res. J. Agric. Sci. 2013, 45, 93–99. [Google Scholar]
- Dumitrache, C.; Frîncu, M.; Rădoi, T.A.; Bărbulescu, I.D.; Mihai, C.; Matei, F.; Tudor, V.; Teodorescu, R.I. Identification by PCR ITS-RFLP Technique of New Yeast Isolates from Pietroasa Vineyard. Sci. Bull. Ser. B Hortic. 2020, LXIV, 287–293. [Google Scholar]
- Dragomir Tutulescu, F.; Popa, A. Viticultural Areas from Oltenia—Romania Which Have at Disposal a Rich and Performant Microflora of Oenological Interest. Agricultura 2010, 75, 33–39. [Google Scholar]
- Vasile, A.; Pașa, R.; Savin, C. The Influence of New Yeast Strains from the Indigenous Flora of Iași Vineyard on the Alcoholic Fermentation Process. Sci. Artic. UASVM Iași—Hortic. Ser. 2010, 53, 459–464. [Google Scholar]
- Matei, F.; Găgeanu, A. Killer Profile of Wine Yeast Strains Isolated in Dealurile Bujorului Vineyard. Rom. Biotechnol. Lett. 2011, 16, 144–147. [Google Scholar]
- Antoce, A.O.; Nămoloșanu, I.C. A Rapid Method for Testing Yeast Resistance to Ethanol for the Selection of Strains Suitable for Winemaking. Rom. Biotechnol. Lett. 2011, 16, 5953–5962. [Google Scholar]
- Barnett, J.A.; Payne, R.W.; Yarrow, D. Yeasts: Characteristics and Identification, 1st ed.; Cambridge University Press: Cambridge, UK, 1983; ISBN 978-0-521-25296-6. [Google Scholar]
- Barnett, J.A. A History of Research on Yeasts 2: Louis Pasteur and His Contemporaries, 1850–1880. Yeast 2000, 16, 755–771. [Google Scholar] [CrossRef]
- Kreger-van Rij, N.J.W. (Ed.) The Yeasts: A Taxonomic Study, 3rd ed.; Elsevier Science: Groningen, The Netherlands, 1984; ISBN 978-0-444-80421-1. [Google Scholar]
- Delfini, C. Innovative Trends in Oenology and in the Selection of Yeasts and Malolactic Bacteria for Wine Industry. Riv. Vitic. E Enol. 1992, 45, 17–30. [Google Scholar]
- Corbu, V.; Csutak, O. Molecular and Physiological Diversity of Indigenous Yeasts Isolated from Spontaneously Fermented Wine Wort from Ilfov County, Romania. Microorganisms 2023, 11, 37. [Google Scholar] [CrossRef]
- Corbu, V.; Csutak, O. Biodiversity Studies on Pichia kudriavzevii from Romanian Spontaneous Fermented Products. AgroLife Sci. J. 2020, 9, 104–113. [Google Scholar]
- Gaspar, E.; Gyöngyvér, M.; Oprean, L.; Iancu, R. Identification and Isolation of Yeast Strains Saccharomyces bayanus from Native Sources Using Mollecular Methods. Ann. Rom. Soc. Cell Biol. 2011, 16, 286–291. [Google Scholar]
- Oprean, L. Molecular Identification of Selected Yeast Strains Isolated from Sebeș-Apold Vineyard, Apoldu de Jos Viticultural Area. Ann. Rom. Soc. Cell Biol. 2014, 19, 45–48. [Google Scholar]
- Kontek, A. Studiul Factorilor Care Condiționează Formarea Acidității Volatile a Vinurilor La Vinificarea Primară. Ph.D. Thesis, University of Craiova, Craiova, Romania, 1977. [Google Scholar]
- Lodder, J.; Kreger-van Rij, N.J.W. The Yeasts. A Taxonomic Study, 2nd ed.; North Holland Publishing Company: Amsterdam, The Netherlands, 1967. [Google Scholar]
- Lengyel, E.; Oprean, L.; Tița, O.; Păcală, M.L.; Iancu, R.; Stegăruș, D.; Ketney, O. Isolation and Molecular Identifications of Wine Yeast Strains from Autochthonous Aromatic and Semi Aromatic Varieties. Ann. Rom. Soc. Cell Biol. 2012, 17, 134–138. [Google Scholar]
- Bora, F.D.; Donici, A.; Oșlobanu, A.; Fițiu, A.; Babeș, A.C.; Bunea, C.I. Qualitative Assessment of the White Wine Varieties Grown in Dealu Bujorului Vineyard, Romania. Not. Bot. Horti Agrobot. 2016, 44, 593–602. [Google Scholar] [CrossRef]
- Colibaba, L.C.; Cotea, V.V.; Lacureanu, F.G.; Tudose-Sandu-Ville, S.; Rotaru, L.; Niculaua, M.; Luchian, C.E. Studies of Phenolic and Aromatic Profile of Busuioacă de Bohotin Wines. BIO Web Conf. 2015, 5, 02008. [Google Scholar] [CrossRef]
- Dobrei, A.G.; Dobrei, A.; Iordănescu, O.; Nistor, E.; Balla, G.; Mălăescu, M.; Drăgunescu, A. Research Concerning the Correlation of Soil with Wines Quality in Some Varieties of Wine Grapes in Miniș-Măderat Vineyards. J. Hortic. For. Biotechnol. 2015, 19, 98–105. [Google Scholar]
- Vararu, F.; Moreno-Garcia, J.; Moreno, J.; Niculaua, M.; Nechita, B.; Zamfir, C.; Colibaba, C.; Dumitru, G.D.; Cotea, V.V. Minor Volatile Compounds Profiles of “Aligoté” Wines Fermented with Different Yeast Strains. Not. Sci. Biol. 2015, 7, 123–128. [Google Scholar] [CrossRef]
- Vișan, L.; Dobrinoiu, R.; Dumbravă, M. The Chemical and Aromatic Composition in a Romanian Wine Cabernet Sauvignon. Rom. Biotechnol. Lett. 2012, 17, 6855–6861. [Google Scholar]
- Manolache, M.; Pop, T.I.; Babeș, A.C.; Farcaș, I.A.; Muncaciu, M.L.; Călugăr, A.; Gal, E. Volatile Composition of Some Red Wines from Romania Assessed by GC-MS. Stud. Univ. Babeș-Bolyai Chem. 2018, 63, 125–142. [Google Scholar] [CrossRef]
- Manolache, M.; Pop, T.I.; Babeș, A.C.; Farcaș, I.A.; Godoroja, M.; Călugăr, A.; Gal, E. Assessment of Volatile Compounds of Some Red Wine Samples from Republic of Moldova and Romania Using GC-MS Analysis. Agricultura 2018, 105, 40–47. [Google Scholar]
- Vararu, F.; Moreno-Garcia, J.; Niculaua, M.; Cotea, V.V.; Mayen, M.; Moreno, J. Fermentative Volatilome Modulation of Muscat Ottonel Wines by Using Yeast Starter Cultures. LWT—Food Sci. Technol. 2020, 129, 109575. [Google Scholar] [CrossRef]
- Focea, M.C.; Luchian, C.; Zamfir, C.; Niculaua, M.; Moroșanu, A.M.; Nistor, A.; Andrieș, T.; Lacureanu, G.; Cotea, V.V. Organoleptic Characteristics of Experimental Sparkling Wines. Sci. Artic. UASVM Iași—Hortic. Ser. 2017, 60, 221–226. [Google Scholar]
- Vișan, L.; Dobrinoiu, R. Studies on the Aroma of Sauvignon Wine. Sci. Bulletin. Ser. F. Biotechnol. 2013, 17, 127–131. [Google Scholar]
- Scanes, K.T.; Hohmann, S.; Prior, B.A. Glycerol Production by the Yeast Saccharomyces cerevisiae and Its Relevance to Wine: A Review. S. Afr. J. Enol. Vitic. 1998, 19, 17–24. [Google Scholar] [CrossRef]
- Tudose, I.; Savin, C.; Stoica, C. Selecția Și Testarea Potențialului Criofil al Unor Tulpini de Saccharomyces ellipsoideus Izolate Din Podgoria Iași, Centrul Viticol Copou. An. ICVV 2002, 17, 256–261. [Google Scholar]
- Kontek, A.; Kontek, A.; Rusea, V.; Sandu-Ville, G. Selecția Și Caracterizarea a Două Sușe de Levuri Cu Capacitate Alcooligenă Ridicată. An. ICVV 1991, 13, 279–292. [Google Scholar]
- Sandu-Ville, G.; Popescu, C. Conducerea Fermentației Alcoolice a Musturilor Prin Utilizarea de Drojdii Cu Caracter Nespumant. Cercet. Agron. În Mold. 1980, 1, 89–92. [Google Scholar]
- Liță (Mihai), C.; Antoce, A.O.; Nămoloșanu, I.C. Studiul Influenței Unor Tulpini de Drojdii Selecționate Asupra Fermentației Alcoolice. Lucr. Științifice USAMV Iași Ser. Hortic. 2006, 49, 701–706. [Google Scholar]
- Lengyel, E.; Panaitescu, M. The Management of Selected Yeast Strains in Qualifying Terpene Flavours in Wine. Manag. Sustain. Dev. 2017, 9, 27–30. [Google Scholar] [CrossRef]
- Knight, S.; Klaere, S.; Fedrizzi, B.; Goddard, M.R. Regional Microbial Signatures Positively Correlate with Differential Wine Phenotypes: Evidence for a Microbial Aspect to Terroir. Sci. Rep. 2015, 5, 14233. [Google Scholar] [CrossRef]
- Belda, I.; Zarraonaindia, I.; Perisin, M.; Palacios, A.; Acedo, A. From Vineyard Soil to Wine Fermentation: Microbiome Approximations to Explain the “terroir” Concept. Front. Microbiol. 2017, 8, 821. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, H. Wine Yeast Terroir: Separating the Wheat from the Chaff—For an Open Debate. Microorganisms 2020, 8, 787. [Google Scholar] [CrossRef]
- Francesca, N.; Gaglio, R.; Alfonzo, A.; Settani, L.; Corona, O.; Mazzei, P.; Romano, R.; Piccolo, A.; Moschetti, G. The Wine: Typicality or Mere Diversity? The Effect of Spontaneous Fermentations and Biotic Factors on the Characteristics of Wine. Agric. Agric. Sci. Procedia 2016, 8, 769–773. [Google Scholar] [CrossRef]
- Jackson, R.S. Wine Tasting: A Professional Handbook (Food Science and Technology), 2nd ed.; Academic Press: Cambridge, MA, USA, 2009; ISBN 978-0-12-374181-3. [Google Scholar]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not Your Ordinary Yeast: Non-Saccharomyces Yeasts in Wine Production Uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Carrau, F.; Boido, E.; Ramey, D. Chapter Three—Yeasts for Low Input Winemaking: Microbial Terroir and Flavor Differentiation. Adv. Appl. Microbiol. 2020, 111, 89–121. [Google Scholar] [CrossRef]
- Fleet, G.H. Wine Yeasts for the Future. FEMS Yeast Res. 2008, 8, 979–995. [Google Scholar] [CrossRef]
- Comitini, F.; Capece, A.; Ciani, M.; Romano, P. New Insights on the Use of Wine Yeasts. Curr. Opin. Food Sci. 2017, 13, 44–49. [Google Scholar] [CrossRef]
- Garcia, M.; Esteve-Zarzoso, B.; Arroyo, T. Non-Saccharomyces Yeasts: Biotechnological Role for Wine Production. In Grape and wine Biotechnology; IntechOpen: London, UK, 2016; pp. 249–271. ISBN 978-953-51-2693-5. [Google Scholar]
- Manzanares, P.; Valles, S.; Viana, F. Chapter 4—Non-Saccharomyces Yeasts in the Winemaking Process. In Molecular Wine Microbiology; Academic Press: Cambridge, MA, USA, 2011; pp. 85–109. ISBN 978-0-12-375021-1. [Google Scholar]
- Englezos, V.; Rantsiou, K.; Torchio, F.; Rolle, L.; Gerbi, V.; Cocolin, L. Exploitation of the Non-Saccharomyces Yeast Starmerella bacillaris (Synonym Candida zemplinina) in Wine Fermentation: Physiological and Molecular Characterization. Int. J. Food Microbiol. 2015, 199, 33–40. [Google Scholar] [CrossRef]
- Sgouros, G.; Chalvantzi, I.; Mallouchos, A.; Paraskevopoulos, Y.; Banilas, G.; Nisiotou, A. Biodiversity and Enological Potential of Non-Saccharomyces Yeasts from Nimean Vineyards. Fermentation 2018, 4, 32. [Google Scholar] [CrossRef]
- Benito, A.; Calderon, F.; Benito, S. The Influence of Non-Saccharomyces Species on Wine Fermentation Quality Parameters. Fermentation 2019, 5, 54. [Google Scholar] [CrossRef]
- Ciani, M.; Maccarelli, F. Oenological Properties of Non-Saccharomyces Yeasts Associated with Wine-Making. J. Microbiol. Biotechnol. 1998, 14, 199–203. [Google Scholar] [CrossRef]
- Belda, I.; Ruiz, J.; Esteban-Fernandez, A.; Navascues, E.; Marquina, D.; Santos, A.; Moreno-Arribas, M.V. Microbial Contribution to Wine Aroma and Its Intended Use for Wine Quality Improvement. Molecules 2017, 22, 189. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Banda, C.; Park, H.D. Effect of Inoculation Strategy of Non-Saccharomyces Yeasts on Fermentation Characteristics and Volatile Higher Alcohol and Esters in Campbell Early Wines. Aust. J. Grape Wine Res. 2019, 25, 384–395. [Google Scholar] [CrossRef]
- Rocker, J.; Strub, S.; Ebert, K.; Grossmann, M. Usage of Different Aerobic Non-Saccharomyces Yeasts and Experimental Condition as a Tool for Reducing the Ethanol Content in Wines. Eur. Food Res. Technol. 2016, 242, 2051–2070. [Google Scholar] [CrossRef]
- Maturano, Y.P.; Rodriguez Assaf, L.A.; Toro, M.E.; Nally, M.C.; Vallejo, M.; Castellanos de Figueroa, L.I.; Combina, M.; Vazquez, F. Multi-Enzyme Production by Pure and Mixed Cultures of Saccharomyces and Non-Saccharomyces Yeasts during Wine Fermentation. Int. J. Food Microbiol. 2012, 155, 43–50. [Google Scholar] [CrossRef]
- Capozzi, V.; Garofalo, C.; Chiriatti, M.A.; Grieco, F.; Spano, G. Microbial Terroir and Food Innovation: The Case of Yeast Biodiversity in Wine. Microbiol. Res. 2015, 181, 75–83. [Google Scholar] [CrossRef]
- Nechita, A.; Filimon, V.R.; Pașa, R.; Damian, D.; Zaldea, G.; Filimon, R.; Zaiț, M. Oenological Characterization of Some Yeast Strains Isolated from the Iași Vineyard Romania. Rom. J. Hortic. 2020, I, 141–148. [Google Scholar] [CrossRef]
- Lai, Y.T.; Hsieh, C.W.; Lo, Y.C.; Liou, B.K.; Lin, H.W.; Hou, C.Y.; Cheng, K.C. Isolation and Identification of Aroma-Producing Non-Saccharomyces Yeast Strains and the Enological Characteristic Comparison in Wine Making. LWT—Food Sci. Technol. 2022, 154, 112653. [Google Scholar] [CrossRef]
- Domizio, P.; Liu, Y.; Bisson, L.F.; Barile, D. Use of Non-Saccharomyces Wine Yeasts as Novel Sources of Mannoproteins in Wine. Food Microbiol. 2014, 43, 5–15. [Google Scholar] [CrossRef]
- Andorra, I.; Berradre, M.; Rozes, N.; Mas, A.; Guillamon, J.M.; Esteve-Zarzoso, B. Effect of Pure and Mixed Cultures of the Main Wine Yeast Species on Grape Must Fermentations. Eur. Food Res. Technol. 2010, 231, 215–224. [Google Scholar] [CrossRef]
- Magyar, I.; Toth, T. Comparative Evaluation of Some Oenological Properties in Wine Strains of Candida stellata, Candida zemplinina, Saccharomyces uvarum and Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 94–100. [Google Scholar] [CrossRef]
- Domizio, P.; Romani, C.; Lencioni, L.; Comitini, F.; Gobbi, M.; Mannazzu, I.; Ciani, M. Outlining a Future for Non-Saccharomyces Yeasts: Selection of Putative Spoilage Wine Strains to Be Used in Association with Saccharomyces cerevisiae for Grape Juice Fermentation. Int. J. Food Microbiol. 2011, 147, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Anfang, N.; Brajkovich, M.; Goddard, M.R. Co-Fermentation with Pichia kluyveri Increases Varietal Thiol Concentrations in Sauvignon Blanc. Aust. J. Grape Wine Res. 2009, 15, 1–8. [Google Scholar] [CrossRef]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected Non-Saccharomyces Wine Yeasts in Controlled Multistarter Fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Sadoudi, M.; Tourdot-Marechal, R.; Rousseaux, S.; Steyer, D.; Gallardo-Chacon, J.J.; Ballester, J.; Vichi, S.; Guerin-Schneider, R.; Caixach, J.; Alexandre, H. Yeast-Yeast Interactions Revealed by Aromatic Profile Analysis of Sauvignon Blanc Wine Fermented by Single of Co-Culture of Non-Saccharomyces and Saccharomyces Yeasts. Food Microbiol. 2012, 32, 243–253. [Google Scholar] [CrossRef]
- Benito, S.; Palomero, F.; Morata, A.; Calderon, F.; Palomero, D.; Suarez-Lepe, J.A. Selection of Appropriate Schizosaccharomyces Strains for Winemaking. Food Microbiol. 2014, 42, 218–224. [Google Scholar] [CrossRef]
- Dumitrescu, R. Romanians Prefer to Drink Local Wines. Available online: https://www.romania-insider.com/romanians-prefer-drink-local-wine-2023 (accessed on 3 February 2023).
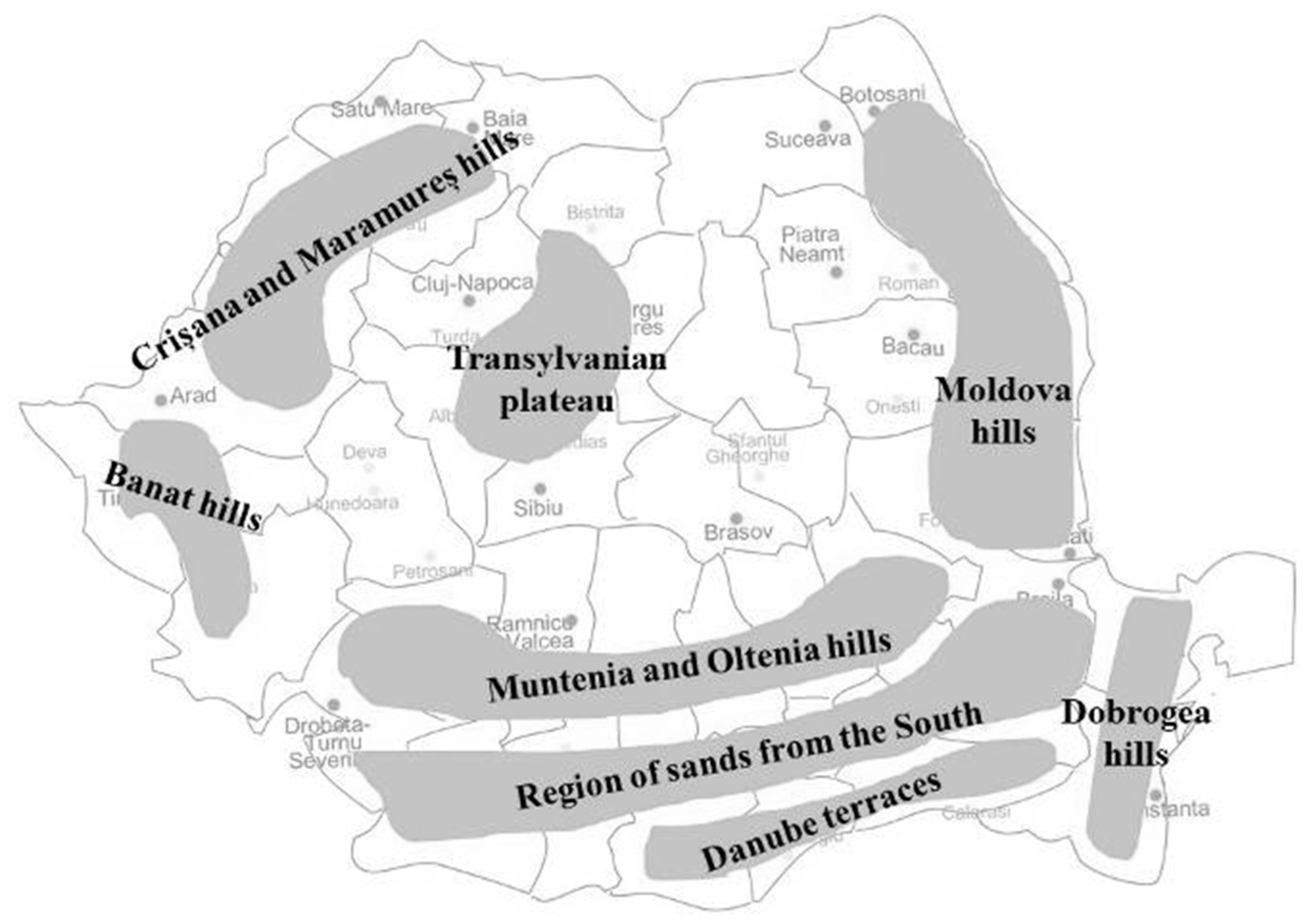
| Viticultural Area | Viticultural Region | Vineyards Denominations |
|---|---|---|
| Central area, inside the Carpathian arch | The Transylvanian plateau | Târnave, Alba, Sebeș-Apold, Lechința, Aiud |
| Peri-Carpathian hills | The hills of Moldova | Cotnari, Huși, Iași, Dealu Bujorului, Ivești, Nicorești, Panciu, Odobești, Cotești, Zeletin, Covurlui, Colinele Tutovei |
| The hills of Muntenia and Oltenia | Dealu Mare, Sâmburești, Ștefănești, Drăgășani, Dealurile Craiovei, Dealurile Buzăului, Podgoria Severinului, Plaiurile Drancei | |
| Banat | 6 independent centers | |
| Crișana and Maramureș | Diosig, Miniș-Măderat, Valea lui Mihai, Podgoria Silvaniei | |
| Danube Pontic area | The Dobrogea hills | Murfatlar, Sarica-Niculitel, Istria-Babadag |
| The Danube terraces | Ostrov, Greaca | |
| Region of sands and other favorable lands in the South of the country | Calafat, Sadova-Corabia, Podgoria Dacilor |
| Genus | Relevant Species | Initial Technological Significance | Real Biotechnological Role | References |
|---|---|---|---|---|
| Hanseniaspora/ Kloeckera | H. uvarum/ H. apiculata | Contamination /Spoilage | Higher alcohols, acetate, and ethyl esters production | [90,108] |
| Candida | C. stellata | Contamination | Glycerol production, fructophily | [109] |
| C. zemplinina/ Starmerella bacillaris | Contamination | Glycerol, succinic acid production; decrease of alcohol content | [94,98] | |
| Metschnikowia | M. pulcherrima | Contamination | Esters, terpenes, and thiols production, increase in aroma complexity | [91,98,107] |
| Pichia | P. anomala | Contamination /Spoilage | Increased production of volatile compounds, killer against Dekkera/Brettanomyces | [110] |
| P. kluyveri | 3-mercaptohexan-1-ol and 3-mercaptohexan-1-ol acetate production | [111] | ||
| Lachancea/ Kluyveromyces | L. thermotolerans | Contamination | Glycerol overproduction, reduction of volatile acidity | [112] |
| Torulaspora | T. delbrueckii | Spoilage | Succinic acid, polysaccharides production | [113] |
| Dekkera/ Brettanomyces | D. bruxellensis | Spoilage | Spoilage | [8,100] |
| Schizosaccharomyces | S. pombe | Spoilage | Malolactic deacidification; propanol and pyruvic acid production | [98,114] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rădoi-Encea, R.-Ș.; Pădureanu, V.; Diguță, C.F.; Ion, M.; Brîndușe, E.; Matei, F. Achievements of Autochthonous Wine Yeast Isolation and Selection in Romania—A Review. Fermentation 2023, 9, 407. https://doi.org/10.3390/fermentation9050407
Rădoi-Encea R-Ș, Pădureanu V, Diguță CF, Ion M, Brîndușe E, Matei F. Achievements of Autochthonous Wine Yeast Isolation and Selection in Romania—A Review. Fermentation. 2023; 9(5):407. https://doi.org/10.3390/fermentation9050407
Chicago/Turabian StyleRădoi-Encea, Raluca-Ștefania, Vasile Pădureanu, Camelia Filofteia Diguță, Marian Ion, Elena Brîndușe, and Florentina Matei. 2023. "Achievements of Autochthonous Wine Yeast Isolation and Selection in Romania—A Review" Fermentation 9, no. 5: 407. https://doi.org/10.3390/fermentation9050407
APA StyleRădoi-Encea, R.-Ș., Pădureanu, V., Diguță, C. F., Ion, M., Brîndușe, E., & Matei, F. (2023). Achievements of Autochthonous Wine Yeast Isolation and Selection in Romania—A Review. Fermentation, 9(5), 407. https://doi.org/10.3390/fermentation9050407







