In Vitro Fermentation Characteristics of Pine Needles (Pinus densiflora) as Feed Additive
Abstract
1. Introduction
2. Materials and Methods
2.1. Pine Needles and Microorganisms for Fermentation
2.2. Fermentation of Pine Needles and Changes in Viable Cell Count and pH
2.3. Antibacterial Activity Using Agar Well-Diffusion Assay
2.4. Determination of Total Phenols and Flavonoids
2.5. Antioxidant Assays
2.6. Superoxide Radical-Scavenging Assay
2.7. Enzymatic Activities
2.8. Statistical Analysis
3. Results
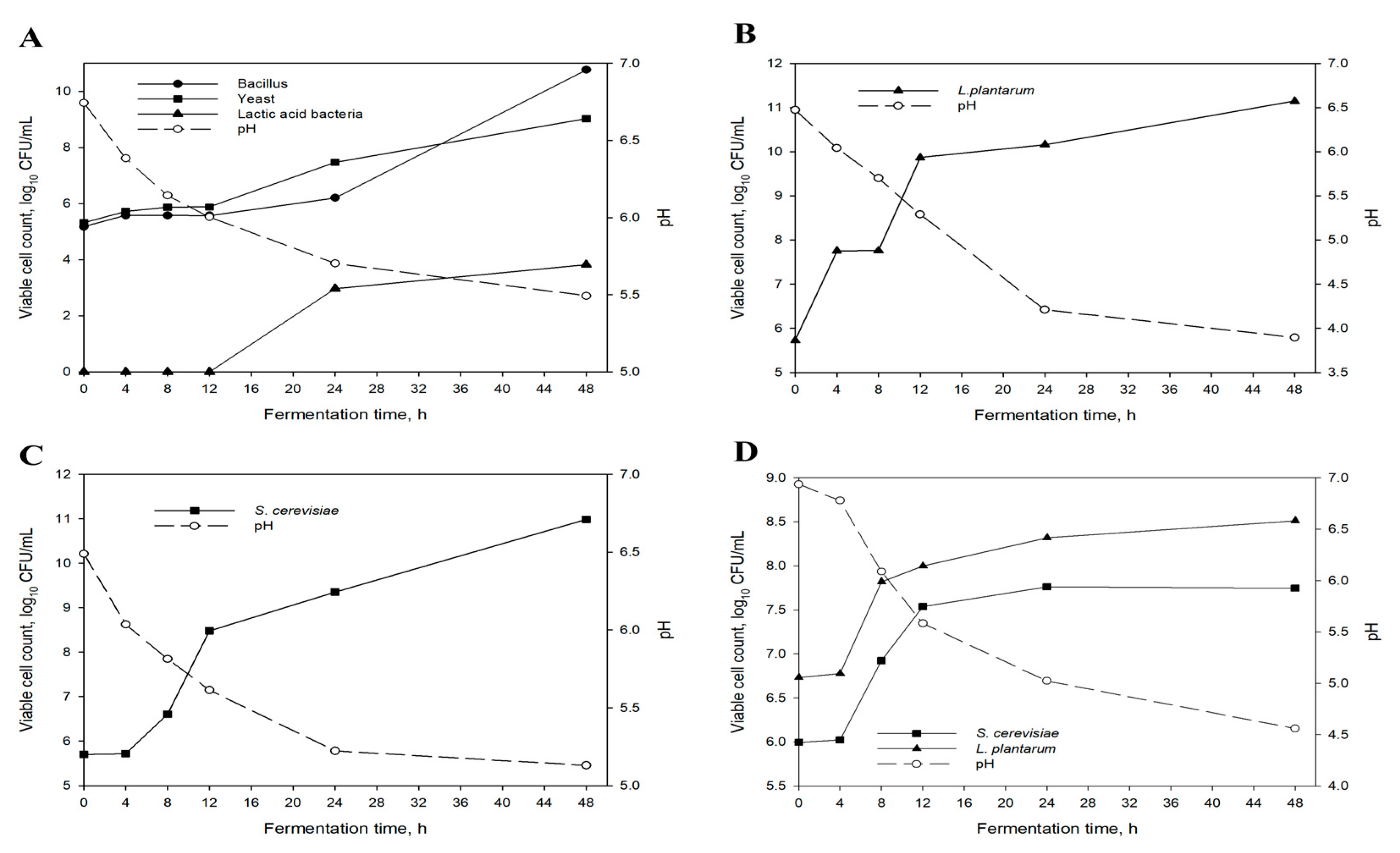
3.1. Microbial Population and pH Changes of Fermented Pine Needles
3.2. Changes in the Bioactivities of Pine Needles following the Fermentation
3.2.1. Antibacterial Activity
3.2.2. Total Polyphenols and Flavonoids
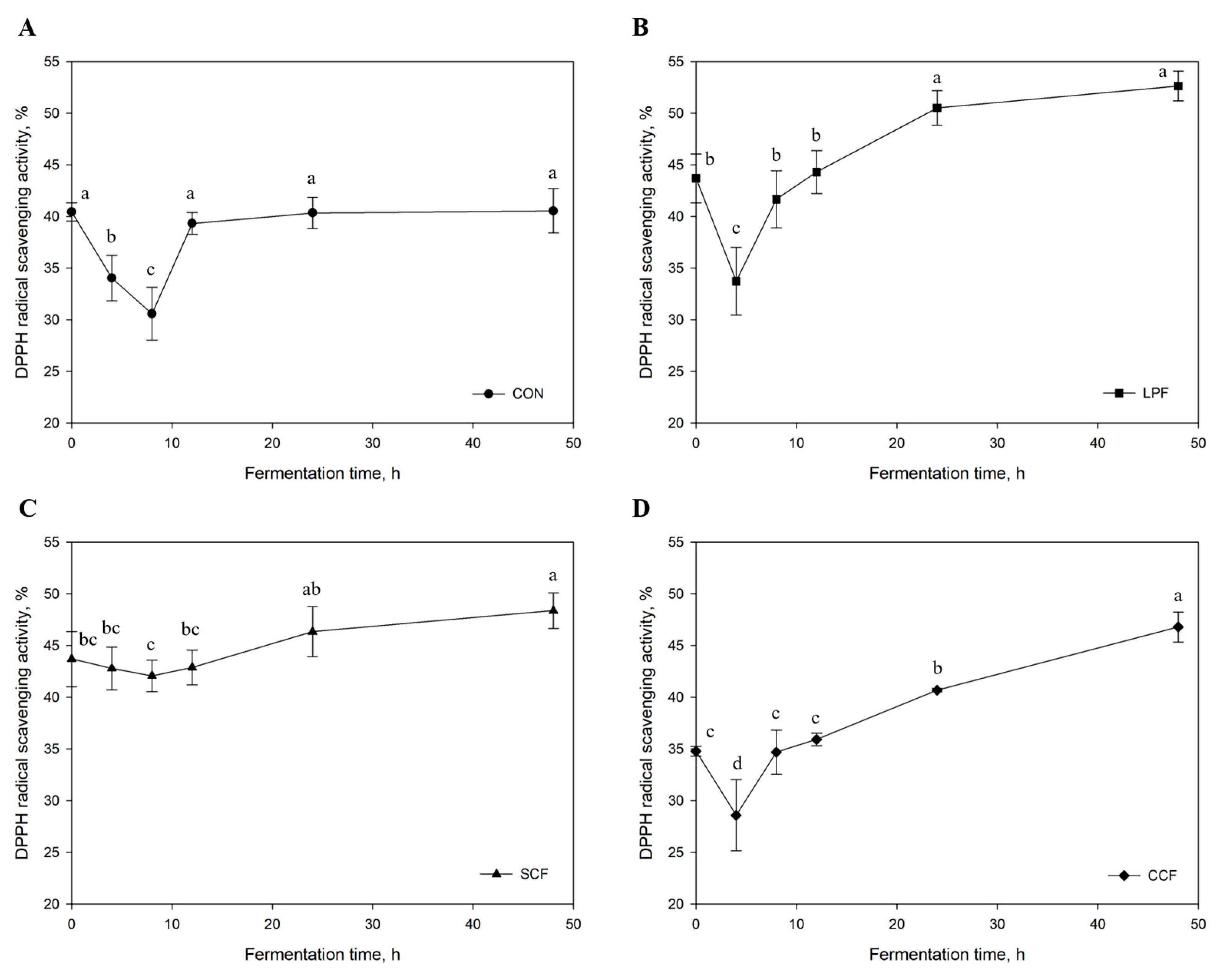
3.2.3. Antioxidant Activity
3.2.4. Antioxidant Activity of Porcine Aortic Endothelial Cell
3.3. Enzymatic Activities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ashayerizadeh, A.; Dabiri, N.; Mirzadeh, K.H.; Ghorbani, M.R. Effect of dietary supplementation of probiotic and prebiotic on growth indices and serum biochemical parameters of broiler chickens. JCAB 2011, 5, 152–156. [Google Scholar]
- Upadhayay, U.P.P.D.D.; Vishwa, P.C.V. Growth promoters and novel feed additives improving poultry production and health, bioactive principles and beneficial applications: The trends and advances-a review. Int. J. Pharmacol. 2014, 10, 129–159. [Google Scholar]
- Alagawany, M.; Elnesr, S.S.; Farag, M.R.; Tiwari, R.; Yatoo, M.I.; Karthik, K.; Michalak, I.; Dhama, K. Nutritional significance of amino acids, vitamins and minerals as nutraceuticals in poultry production and health–a comprehensive review. Vet Q. 2021, 41, 1–29. [Google Scholar] [CrossRef]
- Ayalew, H.; Zhang, H.; Wang, J.; Wu, S.; Qiu, K.; Qi, G.; Tekeste, A.; Wassie, T.; Chanie, D. Potential feed additives as antibiotic alternatives in broiler production. Front. Vet. Sci. 2022, 9, 916473. [Google Scholar] [CrossRef] [PubMed]
- Terciolo, C.; Dapoigny, M.; Andre, F. Beneficial effects of Saccharomyces boulardii CNCM I-745 on clinical disorders associated with intestinal barrier disruption. Clin. Exp. Gastroenterol. 2019, 12, 67. [Google Scholar] [CrossRef]
- Lambo, M.T.; Chang, X.; Liu, D. The recent trend in the use of multistrain probiotics in livestock production: An overview. Animals 2021, 11, 2805. [Google Scholar] [CrossRef]
- Ding, S.; Yan, W.; Ma, Y.; Fang, J. The impact of probiotics on gut health via alternation of immune status of monogastric animals. Anim. Nutr. 2021, 7, 24–30. [Google Scholar] [CrossRef]
- Wang, Y.; Cho, J.H.; Chen, Y.J.; Yoo, J.S.; Huang, Y.; Kim, H.J.; Kim, I.H. The effect of probiotic BioPlus 2B® on growth performance, dry matter and nitrogen digestibility and slurry noxious gas emission in growing pigs. Livest. Sci. 2009, 120, 35–42. [Google Scholar] [CrossRef]
- Veizaj-Delia, E.; Piu, T.; Lekaj, P.; Tafaj, M. Using combined probiotic to improve growth performance of weaned piglets on extensive farm conditions. Livest. Sci. 2010, 134, 249–251. [Google Scholar] [CrossRef]
- Kelsey, A.J.; Colpoys, J.D. Effects of dietary probiotics on beef cattle performance and stress. J. Vet. Behav. 2018, 27, 8–14. [Google Scholar] [CrossRef]
- He, T.; Long, S.; Mahfuz, S.; Wu, D.; Wang, X.; Wei, X.; Piao, X. Effects of probiotics as antibiotics substitutes on growth performance, serum biochemical parameters, intestinal morphology, and barrier function of broilers. Animals 2019, 9, 985. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Hamidon, F.; Rajangan, C.; Soh, K.P.; Gan, C.Y.; Lim, T.S.; Abdullah, W.N.W.; Liong, M.T. Application of probiotics for the production of safe and high-quality poultry meat. Korean J. Food Sci. Anim. Resour. 2016, 36, 567. [Google Scholar] [CrossRef]
- Al-Shawi, S.G.; Dang, D.S.; Yousif, A.Y.; Al-Younis, Z.K.; Najm, T.A.; Matarneh, S.K. The potential use of probiotics to improve animal health, efficiency, and meat quality: A Review. Agriculture 2020, 10, 452. [Google Scholar] [CrossRef]
- Collado, M.C.; Grześkowiak, Ł.; Salminen, S. Probiotic strains and their combination inhibit in vitro adhesion of pathogens to pig intestinal mucosa. Curr. Microbiol. 2007, 55, 260–265. [Google Scholar] [CrossRef]
- Shiou, S.R.; Yu, Y.; Guo, Y.; He, S.M.; Mziray-Andrew, C.H.; Hoenig, J.; Sun, J.; Petrof, E.O.; Claud, E.C. Synergistic protection of combined probiotic conditioned media against neonatal necrotizing enterocolitis-like intestinal injury. PLoS ONE 2013, 8, e65108. [Google Scholar] [CrossRef] [PubMed]
- Kwoji, I.D.; Aiyegoro, O.A.; Okpeku, M.; Adeleke, M.A. Multi-Strain probiotics: Synergy among isolates enhances biological activities. Biology 2021, 10, 322. [Google Scholar] [CrossRef]
- Ouwehand, A.C.; Invernici, M.M.; Furlaneto, F.A.; Messora, M.R. Effectiveness of multi-strain versus single-strain probiotics: Current status and recommendations for the future. J. Clin. Gastroenterol. 2018, 52, S35–S40. [Google Scholar] [CrossRef]
- Dziedziński, M.; Kobus-Cisowska, J.; Stachowiak, B. Pinus species as prospective reserves of bioactive compounds with potential use in functional food—Current state of knowledge. Plants 2021, 10, 1306. [Google Scholar] [CrossRef]
- Kim, S.J.; Park, S.Y.; Lee, J.; Chang, M.; Chung, Y.; Lee, T.K. Biochemical compositions and biological activities of extracts from 3 species of Korean pine needles. J. Food Nutr. Res. 2017, 5, 31–36. [Google Scholar]
- Kim, J.Y.; Kim, S.C.; Kim, B.R. Microfibril angle characteristics of Korean pine trees from depending on provinces. J. Korean Wood Sci. Technol. 2020, 48, 569–576. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. Bioactive compounds involved in the formation of the sparse understory vegetation in pine forests. Curr. Org. Chem. 2021, 25, 1731–1738. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Zanuso, E.; Genisheva, Z.; Rocha, C.M.; Teixeira, J.A. Green and sustainable valorization of bioactive phenolic compounds from pinus by-products. Molecules 2020, 25, 2931. [Google Scholar] [CrossRef] [PubMed]
- Nantapo, C.W.T.; Marume, U. Exploring the Potential of Myrothamnus flabellifolius Welw.(Resurrection Tree) as a Phytogenic Feed Additive in Animal Nutrition. Animals 2022, 12, 1973. [Google Scholar] [CrossRef]
- Lee, W.D.; Kothari, D.; Moon, S.G.; Kim, J.; Kim, K.I.; Ga, G.W.; Kim, Y.G.; Kim, S.K. Evaluation of Non-Fermented and Fermented Chinese Chive Juice as an Alternative to Antibiotic Growth Promoters of Broilers. Animals 2022, 12, 2742. [Google Scholar] [CrossRef] [PubMed]
- Kothari, D.; Lee, W.D.; Jung, E.S.; Niu, K.M.; Lee, C.H.; Kim, S.K. Controlled fermentation using autochthonous Lactobacillus plantarum improves antimicrobial potential of Chinese chives against poultry pathogens. Antibiotics 2020, 9, 386. [Google Scholar] [CrossRef]
- Park, J.H.; Song, T.H.; Kim, I. Egg production, egg quality, and cecal microbial populations of layersfed diets supplemented with fermented phytogenic feed additive. Turk. J. Vet. Anim. Sci. 2016, 40, 660–666. [Google Scholar] [CrossRef]
- Shan, C.H.; Guo, J.; Sun, X.; Li, N.; Yang, X.; Gao, Y.; Qiu, D.; Li, X.; Wang, Y.; Feng, M.; et al. Effects of fermented Chinese herbal medicines on milk performance and immune function in late-lactation cows under heat stress conditions. Anim. Sci. J. 2018, 96, 4444–4457. [Google Scholar] [CrossRef]
- Yin, J.; Kim, H.S.; Kim, Y.M.; Kim, I.H. Effects of dietary fermented red ginseng marc and red ginseng extract on growth performance, nutrient digestibility, blood profile, fecal microbial, and noxious gas emission in weanling pigs. J. Appl. Anim. Res. 2018, 46, 1084–1089. [Google Scholar] [CrossRef]
- Kothari, D.; Oh, J.S.; Kim, J.H.; Lee, W.D.; Kim, S.K. Effect of dietary supplementation of fermented pine needle extract on productive performance, egg quality, and serum lipid parameters in laying hens. Animals 2021, 11, 1475. [Google Scholar] [CrossRef]
- Osono, T.; Hirose, D. Colonization and lignin decomposition of pine needle litter by Lophodermium pinastri. For. Pathol. 2011, 41, 156–162. [Google Scholar] [CrossRef]
- Kwak, C.S.; Moon, S.C.; Lee, M.S. Antioxidant, antimutagenic, and antitumor effects of pine needles (Pinus densiflora). Nutr. Cancer 2006, 56, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.H.; Kothari, D.; Jung, H.I.; Lim, J.M.; Kim, W.L.; Kwon, H.C.; Han, S.G.; Seo, S.M.; Choi, Y.K.; Kim, S.K. Noni juice-fortified yogurt mitigates dextran sodium sulfate-induced colitis in mice through the modulation of inflammatory cytokines. J. Funct. Foods 2021, 86, 104652. [Google Scholar] [CrossRef]
- Niu, K.M.; Kothari, D.; Lee, W.D.; Lim, J.M.; Khosravi, S.; Lee, S.M.; Lee, B.J.; Kim, K.W.; Han, H.S.; Kim, S.K. Autochthonous Bacillus licheniformis: Probiotic potential and survival ability in low-fishmeal extruded pellet aquafeed. Microbiologyopen 2019, 8, e00767. [Google Scholar] [CrossRef]
- Park, E.H.; Bae, W.Y.; Eom, S.J.; Kim, K.T.; Paik, H.D. Improved antioxidative and cytotoxic activities of chamomile (Matricaria chamomilla) florets fermented by Lactobacillus plantarum KCCM 11613P. J. Zhejiang Univ. Sci. B 2017, 18, 152. [Google Scholar] [CrossRef]
- Gerardi, C.; Tristezza, M.; Giordano, L.; Rampino, P.; Perrotta, C.; Baruzzi, F.; Capozzi, V.; Mita, G.; Grieco, F. Exploitation of Prunus mahaleb fruit by fermentation with selected strains of Lactobacillus plantarum and Saccharomyces cerevisiae. Food Microbiol. 2019, 84, 103262. [Google Scholar] [CrossRef]
- Delgado, A.; Brito, D.; Fevereiro, P.; Peres, C.; Marques, J.F. Antimicrobial activity of L. plantarum, isolated from a traditional lactic acid fermentation of table olives. Lait 2001, 81, 203–215. [Google Scholar] [CrossRef]
- Peng, W.; Meng, D.; Yue, T.; Wang, Z.; Gao, Z. Effect of the apple cultivar on cloudy apple juice fermented by a mixture of Lactobacillus acidophilus, Lactobacillus plantarum, and Lactobacillus fermentum. Food Chem. 2021, 340, 127922. [Google Scholar] [CrossRef]
- Casey, E.; Sedlak, M.; Ho, N.W.; Mosier, N.S. Effect of acetic acid and pH on the cofermentation of glucose and xylose to ethanol by a genetically engineered strain of Saccharomyces cerevisiae. FEMS Yeast Res. 2010, 10, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Afonso, C.; Correia, A.P.; Freitas, M.V.; Mouga, T.; Baptista, T. In vitro evaluation of the antibacterial and antioxidant activities of extracts of Gracilaria gracilis with a view into its potential use as an additive in fish feed. Appl. Sci. 2021, 11, 6642. [Google Scholar] [CrossRef]
- Neath, C.; Portocarero, N.; Jones, C. In vitro susceptibility of swine pathogens to feed additives and active ingredients with potential as antibiotic replacements. J. Appl. Microbiol. 2022, 132, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Kothari, D.; Lee, W.D.; Niu, K.M.; Kim, S.K. The genus Allium as poultry feed additive: A review. Animals 2019, 9, 1032. [Google Scholar] [CrossRef]
- Niu, K.M.; Kothari, D.; Lee, W.D.; Cho, S.; Wu, X.; Kim, S.K. Optimization of Chinese chive juice as a functional feed additive. Appl. Sci. 2020, 10, 6194. [Google Scholar] [CrossRef]
- Kim, Y.S.; Shin, D.H. Volatile components and antibacterial effects of pine needle (Pinus densiflora S. and Z.) extracts. Food microbiol. 2005, 22, 37–45. [Google Scholar] [CrossRef]
- Lee, J.; Kang, H.K.; Cheong, H.; Park, Y. A novel antimicrobial peptides from pine needles of Pinus densiflora Sieb. et Zucc. against foodborne bacteria. Front. Microbiol. 2021, 12, 662462. [Google Scholar] [CrossRef]
- Jeon, Y.H.; Seo, J.E.; Kim, J.H.; Lee, Y.J.; Choi, S.W. Quantitative changes of flavonol glycosides from pine needles by cultivar, harvest season, and thermal process. Prev. Nutr. Food Sci. 2021, 26, 100. [Google Scholar] [CrossRef] [PubMed]
- Settle, T.; Leonard, S.S.; Falkenstein, E.; Fix, N.; Van Dyke, K.; Klandorf, H. Effects of a phytogenic feed additive versus an antibiotic feed additive on oxidative stress in broiler chicks and a possible mechanism determined by electron spin resonance. Int. J. Poult. Sci. 2014, 13, 62. [Google Scholar] [CrossRef]
- Rather, S.A.; Masoodi, F.A.; Akhter, R.; Rather, J.A.; Shiekh, K.A. Advances in use of natural antioxidants as food additives for improving the oxidative stability of meat products. MJFT 2016, 1, 10–17. [Google Scholar]
- Kothari, D.; Lee, W.D.; Kim, S.K. Allium flavonols: Health benefits, molecular targets, and bioavailability. Antioxidants 2020, 9, 888. [Google Scholar] [CrossRef]
- Kovács, D.; Karancsi, Z.; Farkas, O.; Jerzsele, Á. Antioxidant activity of flavonoids in LPS-treated IPEC-J2 porcine intestinal epithelial cells and their antibacterial effect against bacteria of swine origin. Antioxidants 2020, 9, 1267. [Google Scholar] [CrossRef]
- Mousavi, S.H.; Sadeghian Motahar, S.F.; Salami, M.; Kavousi, K.; Sheykh Abdollahzadeh Mamaghani, A.; Ariaeenejad, S.; Salekdeh, G.H. In vitro bioprocessing of corn as poultry feed additive by the influence of carbohydrate hydrolyzing metagenome derived enzyme cocktail. Sci. Rep. 2022, 12, 405. [Google Scholar] [CrossRef]
- Kaschubek, T.; Mayer, E.; Rzesnik, S.; Grenier, B.; Bachinger, D.; Schieder, C.; König, J.; Teichmann, K. Effects of phytogenic feed additives on cellular oxidative stress and inflammatory reactions in intestinal porcine epithelial cells. J. Anim. Sci. 2018, 96, 3657–3669. [Google Scholar] [CrossRef] [PubMed]
- Juan, M.Y.; Chou, C.C. Enhancement of antioxidant activity, total phenolic and flavonoid content of black soybeans by solid state fermentation with Bacillus subtilis BCRC 14715. Food microbiol. 2010, 27, 586–591. [Google Scholar] [CrossRef]
- Mohammed, S.; Manan, F.A. Analysis of total phenolics, tannins and flavonoids from Moringa oleifera seed extract. J. Chem. Pharm. Res. 2015, 7, 132–135. [Google Scholar]
- Dulf, F.V.; Vodnar, D.C.; Socaciu, C. Effects of solid-state fermentation with two filamentous fungi on the total phenolic contents, flavonoids, antioxidant activities and lipid fractions of plum fruit (Prunus domestica L.) by-products. Food Chem. 2016, 209, 27–36. [Google Scholar] [CrossRef]
- Liu, W.; Dun, M.; Liu, X.; Zhang, G.; Ling, J. Effects on total phenolic and flavonoid content, antioxidant properties, and angiotensin I-converting enzyme inhibitory activity of beans by solid-state fermentation with Cordyceps militaris. Int. J. Food Prop. 2022, 25, 477–491. [Google Scholar] [CrossRef]
- Kuo, H.C.; Kwong, H.K.; Chen, H.Y.; Hsu, H.Y.; Yu, S.H.; Hsieh, C.W.; Lin, H.W.; Chu, Y.L.; Cheng, K.C. Enhanced antioxidant activity of Chenopodium formosanum Koidz. by lactic acid bacteria: Optimization of fermentation conditions. PLoS ONE 2021, 16, e0249250. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.J.; Lee, S.Y.; Kim, Y.C.; Choi, I.; Kim, G.B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Aziz, S.; Germano, T.A.; Thiers, K.L.L.; Batista, M.C.; de Souza Miranda, R.; Arnholdt-Schmitt, B.; Costa, J.H. Transcriptome analyses in a selected gene set indicate alternative oxidase (AOX) and early enhanced fermentation as critical for salinity tolerance in rice. Plants 2022, 11, 2145. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, P.; You, S.; Zhao, D.; An, Q.; Wang, D.; Zhang, J.; Li, M.; Wang, C. Anti-Inflammatory Effects of Opuntia Milpa Alta Polysaccharides Fermented by Lactic Acid Bacteria in Human Keratinocyte HaCaT Cells. Chem. Biodivers. 2022, 19, e202100923. [Google Scholar] [CrossRef]
- Kim, J.; Choung, S.Y. Pinus densiflora bark extract prevents selenite-induced cataract formation in the lens of Sprague Dawley rat pups. Mol. Vis. 2017, 23, 638. [Google Scholar]
- Tang, W.; Xing, Z.; Li, C.; Wang, J.; Wang, Y. Molecular mechanisms and in vitro antioxidant effects of Lactobacillus plantarum MA2. Food Chem. 2017, 221, 1642–1649. [Google Scholar] [CrossRef] [PubMed]
- Kthiri, A.; Hidouri, S.; Wiem, T.; Jeridi, R.; Sheehan, D.; Landouls, A. Biochemical and biomolecular effects induced by a static magnetic field in Saccharomyces cerevisiae: Evidence for oxidative stress. PLoS ONE 2019, 14, e0209843. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Park, H.M.; Kang, C.H. Antioxidant Effect via Bioconversion of Isoflavonoid in Astragalus membranaceus Fermented by Lactiplantibacillus plantarum MG5276 In Vitro and In Vivo. Fermentation 2022, 8, 34. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Dadar, M.; Ringø, E. Modulation of nutrient digestibility and digestive enzyme activities in aquatic animals: The functional feed additives scenario. Aquac. Res. 2017, 48, 3987–4000. [Google Scholar] [CrossRef]
- Ibrahim, D.; Moustafa, A.; Shahin, S.E.; Sherief, W.R.; Abdallah, K.; Farag, M.F.; Nassan, M.A.; Ibrahim, S.M. Impact of fermented or enzymatically fermented dried olive pomace on growth, expression of digestive enzyme and glucose transporter genes, oxidative stability of frozen meat, and economic efficiency of broiler chickens. Front. Vet. Sci. 2021, 8, 644325. [Google Scholar] [CrossRef]
- Omemu, A.M.; Oyewole, O.B.; Bankole, M.O. Significance of yeasts in the fermentation of maize for ogi production. Food Microbiol. 2007, 24, 571–576. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, Y.B.; Zhang, X.R.; Chen, C.; Xiang, H.; Xie, Q. Monitoring of the bacterial and fungal biodiversity and dynamics during Massa Medicata Fermentata fermentation. Appl. Microbiol. Biotechnol. 2013, 97, 9647–9655. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, X.Y.; Qin, C.Q.; Li, T.T.; Liu, W.H.; Ren, D.F. Fermentation of rose residue by Lactiplantibacillus plantarum B7 and Bacillus subtilis natto promotes polyphenol content and beneficial bioactivity. J. Biosci. Bioeng. 2022, 134, 501–507. [Google Scholar] [CrossRef]


| Items | Amount |
|---|---|
| Moisture, % | 14.2 ± 0.120 |
| Crude protein, % | 27.0 ± 0.056 |
| Crude fat, % | 4.56 ± 0.141 |
| Crude fiber, % | 11.8 ± 0.134 |
| Crude ash, % | 14.8 ± 0.084 |
| Calcium, % | 0.715 ± 0.007 |
| Phosphorus, % | 0.835 ± 0.021 |
| Acid detergent fiber, % | 15.0 ± 0.176 |
| Neutral detergent fiber, % | 19.2 ± 0.268 |
| Potassium, ppm | 46,591 ± 23.2 |
| Magnesium, ppm | 2092 ± 14.8 |
| Sodium, ppm | 362 ± 18.6 |
| Iron, ppm | 1080 ± 11.1 |
| Sulfur, ppm | 1329 ± 48.4 |
| Pathogen Bacteria | Diameter of Inbihition Zone (mm) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON 1 | LPF | SCF | CCF | |||||||||||||
| 24 h | 48 h | SEM 2 | p-Value | 24 h | 48 h | SEM | p-Value | 24 h | 48 h | SEM | p-Value | 24 h | 48 h | SEM | p-Value | |
| Listeria monocytogenes SK728 | 8.67 | 8.33 | 0.223 | 0.519 | 10.00 b | 13.00 a | 0.764 | 0.021 | 9.33 | 9.67 | 0.224 | 0.519 | 10.00 b | 12.00 a | 0.516 | <0.05 |
| Clostridium perfringens SK870 | ND 3 | ND | - | - | 7.00 | 9.00 | 0.683 | 0.158 | ND | ND | - | - | 6.33 b | 8.33 a | 0.494 | <0.05 |
| Enterotoxigenic E. coli SK871 | ND | ND | - | - | ND | ND | - | - | ND | ND | - | - | ND | ND | - | - |
| Burkholderia contaminans SK875 | ND | ND | - | - | 7.67 | 9.00 | 0.558 | 0.275 | ND | ND | - | - | ND | ND | - | - |
| Haemopillus parasuis SK890 | ND | ND | - | - | 6.33 | 8.00 | 0.477 | 0.067 | 6.67 | 6.33 | 0.342 | 0.678 | 6.33 b | 8.00 a | 0.401 | <0.05 |
| Staphylococcus aureus SK2027 | ND | ND | - | - | 6.67 b | 8.00 a | 0.333 | <0.05 | ND | ND | - | - | 7.00 b | 8.00 a | 0.224 | <0.05 |
| Haemophilus somnus SK2047 | ND | ND | - | - | 12.67 b | 17.33 a | 1.065 | <0.05 | ND | ND | - | - | 9.33 b | 14.67 a | 1.265 | <0.05 |
| Salmonella gallinarum SK3359 | ND | ND | - | - | ND | ND | - | - | ND | ND | - | - | ND | ND | - | - |
| E. coli SK4230 | ND | ND | - | - | ND | ND | - | - | ND | ND | - | - | ND | ND | - | - |
| Pantoea agglomerans SK4295 | ND | ND | - | - | 8.33 b | 10.67 a | 0.619 | <0.05 | ND | ND | - | - | ND | ND | - | - |
| Items 1 | Fermentation Time (h) | SEM 2 | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 4 | 8 | 12 | 24 | 48 | |||
| Total polyphenol contents, mg/mL | ||||||||
| CON | 2.64 cC | 2.19 dC | 1.96 eD | 2.87 aB | 2.79 abB | 2.71 bcB | 0.081 | <0.05 |
| LPF | 2.85 bcB | 2.02 dD | 2.83 cB | 2.94 abAB | 2.94 abA | 3.01 aA | 0.083 | <0.05 |
| SCF | 3.00 aA | 2.84 cA | 2.91 bA | 2.99 abA | 2.95 abA | 3.03 aA | 0.018 | <0.05 |
| CCF | 2.52 D | 2.40 B | 2.38 C | 2.45 C | 2.48 C | 2.45 C | 0.018 | <0.05 |
| SEM | 0.058 | 0.094 | 0.117 | 0.066 | 0.058 | 0.074 | ||
| p-value | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | ||
| Total flavonoid contents, mg/mL | ||||||||
| CON | 7.05 bB | 5.99 cC | 5.29 dC | 8.59 aAB | 8.40 aA | 7.20 bA | 0.293 | <0.05 |
| LPF | 7.91 bA | 5.46 cD | 8.32 aA | 8.35 aB | 7.94 bB | 7.67 bA | 0.245 | <0.05 |
| SCF | 8.36 bA | 7.96 cA | 8.32 bA | 8.89 aA | 8.11 bcB | 7.60 dA | 0.101 | <0.05 |
| CCF | 6.67 aB | 6.45 abB | 6.24 bB | 6.64 abC | 6.50 abC | 6.25 bB | 0.057 | <0.05 |
| SEM | 0.217 | 0.282 | 0.401 | 0.268 | 0.222 | 0.192 | ||
| p-value | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | ||
| Items 1 | Fermentation Time (h) | Enzymatic Activity (mm) | |||
|---|---|---|---|---|---|
| Amylase | Cellulase | Protease | Lipase | ||
| CON | 24 | ND 2 | 9.00 ± 0.00 | 8.00 ± 0.00 | 8.33 ± 0.58 |
| 48 | 10.33 ± 0.58 | 9.00 ± 1.00 | 11.33 ± 1.53 | ||
| LPF | 24 | ND | 8.67 ± 1.15 | 8.00 ± 0.00 | ND |
| 48 | 9.67 ± 0.58 | 8.33 ± 0.58 | |||
| SCF | 24 | 18.00 ± 0.00 | ND | 8.67 ± 0.58 | ND |
| 48 | 18.33 ± 0.58 | 9.00 ± 0.00 | |||
| CCF | 24 | 12.67 ± 0.58 | 10.00 ± 0.58 | 8.33 ± 0.58 | ND |
| 48 | 12.33 ± 0.58 | 8.67 ± 0.58 | 9.00 ± 0.00 | ||
| p-value | Product | <0.05 | <0.05 | 0.163 | <0.05 |
| Fermentation time | 1.000 | 0.273 | <0.05 | <0.05 | |
| Product × Fermentation time | 0.468 | <0.05 | 0.618 | <0.05 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, Y.-A.; Lee, W.-D.; Kim, J.; Kim, S.; Choi, M.-G.; On, J.-Y.; Jeon, S.-W.; Han, S.-G.; Kim, S.-K. In Vitro Fermentation Characteristics of Pine Needles (Pinus densiflora) as Feed Additive. Fermentation 2023, 9, 415. https://doi.org/10.3390/fermentation9050415
Hwang Y-A, Lee W-D, Kim J, Kim S, Choi M-G, On J-Y, Jeon S-W, Han S-G, Kim S-K. In Vitro Fermentation Characteristics of Pine Needles (Pinus densiflora) as Feed Additive. Fermentation. 2023; 9(5):415. https://doi.org/10.3390/fermentation9050415
Chicago/Turabian StyleHwang, Young-A, Woo-Do Lee, Juhyeon Kim, Solhee Kim, Min-Gyung Choi, Jeong-Yeon On, Sang-Woo Jeon, Sung-Gu Han, and Soo-Ki Kim. 2023. "In Vitro Fermentation Characteristics of Pine Needles (Pinus densiflora) as Feed Additive" Fermentation 9, no. 5: 415. https://doi.org/10.3390/fermentation9050415
APA StyleHwang, Y.-A., Lee, W.-D., Kim, J., Kim, S., Choi, M.-G., On, J.-Y., Jeon, S.-W., Han, S.-G., & Kim, S.-K. (2023). In Vitro Fermentation Characteristics of Pine Needles (Pinus densiflora) as Feed Additive. Fermentation, 9(5), 415. https://doi.org/10.3390/fermentation9050415





