Degradability of Vicia ervilia Grain Using In Situ and CNCPS Methods, and Model-Based Analysis of Its Ruminal Degradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Methods of Preparation and Processing of Samples
2.2. Chemical Analysis
2.3. In Situ Ruminal Procedure
2.4. Crude Protein Fractionation
2.5. Calculations and Statistical Analysis
3. Results
3.1. Chemical Analysis
3.2. In Situ DM and CP Disappearance
3.3. Determine DM Degradability Parameters Using Digestive Models
3.4. Determine CP Degradability Parameters Using Digestive Models
3.5. Crude Protein Fractions of Treatments
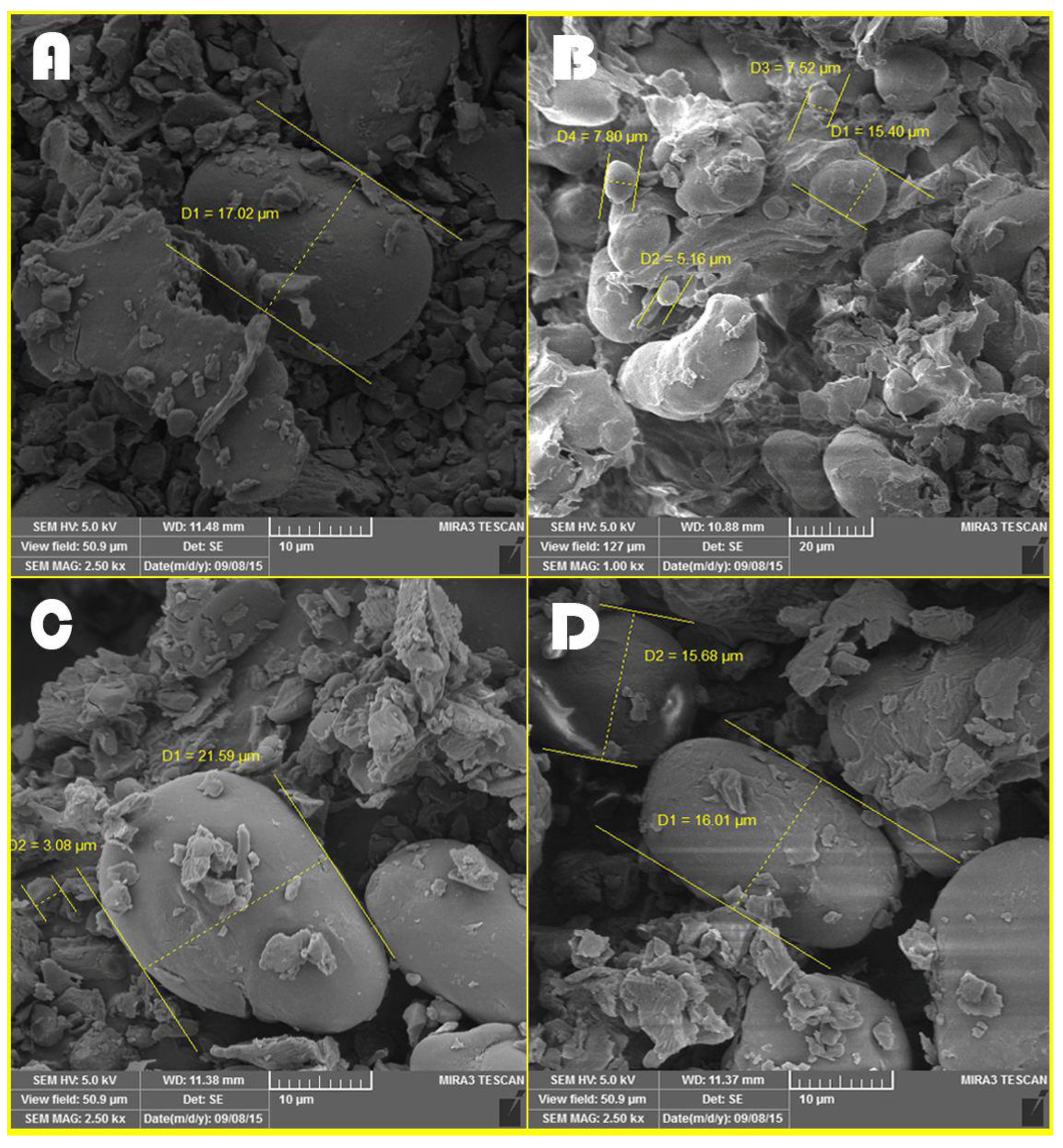
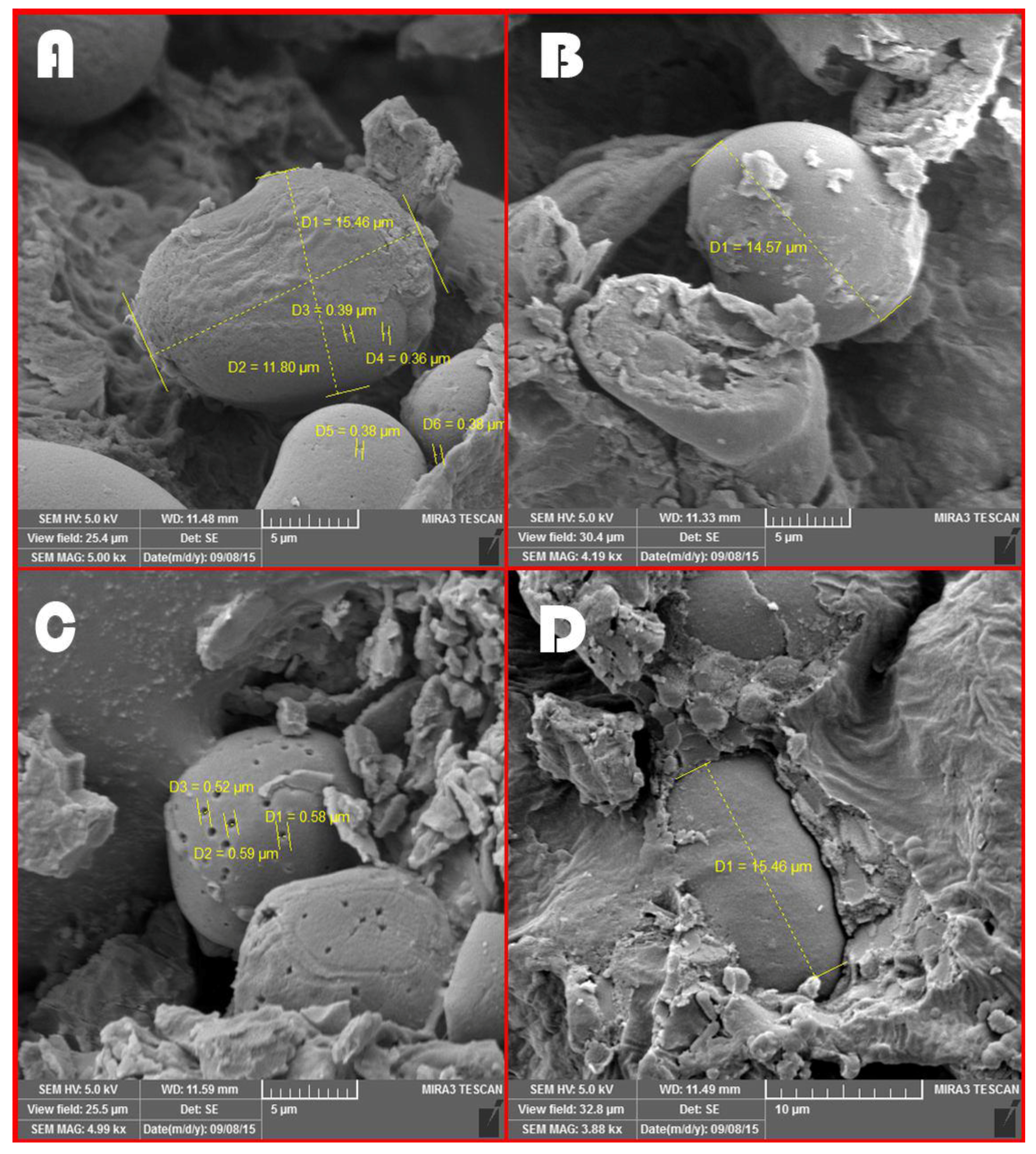
3.6. Microscopic Images Taken from Different Treatments before the Ruminal Incubation Time
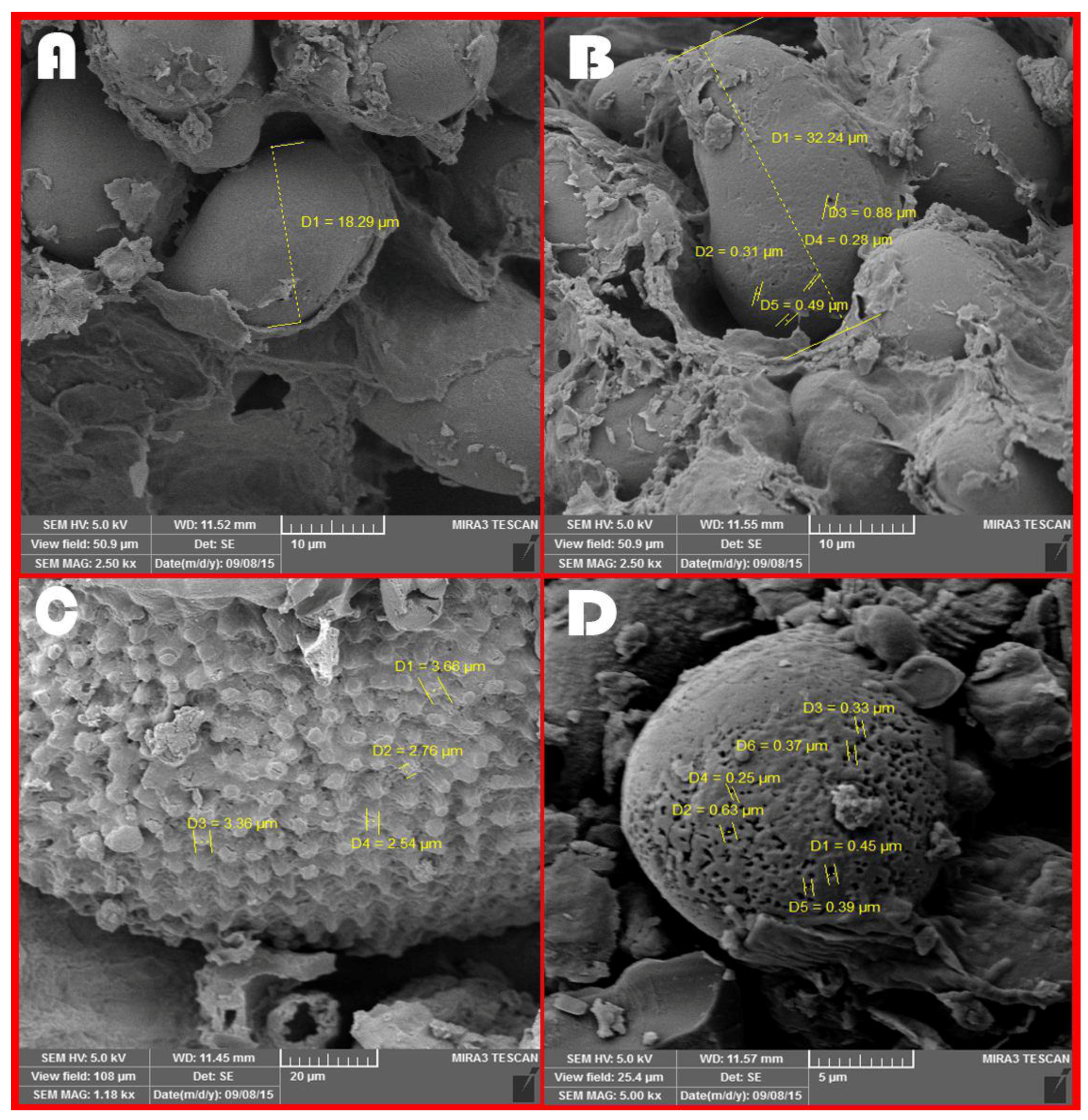
3.7. Microscopic Images Taken from Different Treatments at 48 h of Ruminal Incubation Time
4. Discussion
4.1. Chemical Composition
4.2. In Situ Dry Matter and Crude Protein Disappearance
4.3. Determine DM Degradability Parameters Using Digestive Models
4.4. Determine CP Degradability Parameters Using Digestive Models
4.5. Crude Protein Fractions of Treatments
4.6. Microscopic Images Taken from Different Treatments before the Ruminal Incubation Time
4.7. Microscopic Images Taken from Different Treatments at 48 h of Ruminal Incubation Time
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farran, M.T.; Dakessian, P.B.; Darwish, A.H.; Uwayjan, M.G.; Dbouk, H.K.; Sleiman, F.T.; Ashkarian, V.M. Performance of broilers and production and egg quality parameters of laying hens fed 60% raw or treated common Vetch (Vicia sativa) Seeds. Poult. Sci. 2001, 80, 203–208. [Google Scholar] [CrossRef]
- Aguilera, J.F.; Bustos, M.; Molina, E. The degradability of legume seed meals in the rumen: Effect of heat treatment. Anim. Feed Sci. Technol. 1992, 36, 101–112. [Google Scholar] [CrossRef]
- Liener, I.E. (Ed.) Toxic Constituents of Plant Foodsluffs, 2nd ed.; Academic Press: New York, NY, USA, 1980; 502p. [Google Scholar]
- National Research Council: Nutrient Requirements of Dairy Cattle; National Academies Press: Washington, DC, USA, 2001.
- McNiven, M.A.; Hamilton, R.M.G.; Robinson, P.H.; De Leeuiwe, J.W. Effect of flame roasting on the nutritional quality of common cereal grains for ruminants and non-ruminants. Anim. Feed Sci. Technol. 1994, 47, 31–40. [Google Scholar] [CrossRef]
- Fiems, L.O.; Cottyn, B.G.; Boucque, C.V.; Vanacker, J.M.; Buysse, F.X. Effect of grain processing on in Sacco digestibility and gigestibility and degradability in the rumen. Arch. Anim. Nut. 1990, 40, 713–721. [Google Scholar]
- Palangi, V.; Macit, M.; Bayat, A.R. Mathematical models describing disappearance of Lucerne hay in the rumen using the nylon bag technique. S. Afr. J. Anim. Sci. 2020, 50, 719–725. [Google Scholar] [CrossRef]
- Eslampeivand, A.; Taghizadeh, A.; Safamehr, A.; Palangi, V.; Paya, H.; Shirmohammadi, S.; Abachi, S. Nutritive value assessment of orange pulp ensiled with urea using gas production and nylon bag techniques. Biomass Conv. Bioref. 2022. [Google Scholar] [CrossRef]
- Huhtanen, P.; Ahvenjärvi, S. Problems in determining metabolisable protein value of dairy cow diets and the impact on protein feeding. Animal 2022, 16, 100539. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International; AOAC International: Rockville, MD, USA, 2005. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Sniffen, C.J.; Connor, J.D.O.; Van Soest, P.J.; Fox, D.G.; Russell, J.B. A net carbohydrate and protein system for evaluating cattle diets: 11. Carbohydrate and protein availability. J. Anim. Sci. 1992, 70, 3562. [Google Scholar] [CrossRef]
- Licitra, G.; Hernandez, T.M.; Van Soest, P.J. Standardization of procedures for nitrogen fractionation of ruminant feeds. Feed Sci. Technol. 1996, 57, 347–358. [Google Scholar] [CrossRef]
- Ørskov, E.R.; McDonald, L. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci. 1979, 92, 499–503. [Google Scholar] [CrossRef]
- Dhanoa, M.S.; France, J.; Siddons, R.C.; Lopez, S.; Buchanan, S.J.G. A non-linear compartmental model to describe for age degradation kinetics during incubation in polyester bags in the rumen. Br. J. Nut. 1995, 73, 3–15. [Google Scholar] [CrossRef] [PubMed]
- France, J.; Thornley, J.H.M.; Lopez, S.; Siddons, R.C.; Dhanoa, M.S.; Van Soest, P.J.; Gill, M. On the two-compartment model for estimating extent of feed degradation in the rumen. J. Theor. Biol. 1990, 146, 269–287. [Google Scholar] [CrossRef] [PubMed]
- SAS Institute Inc. SAS/CONNECT® 9.4 User’s Guide, 4th ed.; SAS Institute Inc.: Cary, NC, USA, 2018. [Google Scholar]
- Yu, P.; Goelema, J.O.; Holmes, H.G.; Tamminga, S. Influence of pressure toasting on rumen degradation characteristics of lactating dairy cows. In Proceedings of the 8th AAAP Animal Science Congress, Chiba, Japan, 13–18 October 1996; pp. 694–695. [Google Scholar]
- Palangi, V.; Macit, M. In situ crude protein and dry matter ruminal degradability of heat-treated barley. Rev. Méd. Vét. 2019, 170, 123–128. [Google Scholar]
- Taghizadeh, A.; Safamehr, A.; Palangi, V.; Mehmannavaz, Y. The determination of metabolizable protein of some feedstuffs used in ruminant. Res. J. Biol. Sci. 2008, 3, 804–806. [Google Scholar]
- Fathi Nasri, M.H.; Danesh Mesgaran, M.; France, J.; Cant, J.P.; Kebreab, E. Evaluation of Models to Describe Ruminal Degradation Kinetics from In Situ Ruminal Incubation of Whole Soybeans. J. Dairy Sci. 2006, 89, 3087–3095. [Google Scholar] [CrossRef]
- Ljøkjel, K.; Harstad, O.M.; Prestløkken, E.; Skrede, A. In situ digestibility of protein in barley grain and peas in dairy cows: Influence of heat treatment and glucose addition. Anim. Feed Sci. Technol. 2003, 87, 87–104. [Google Scholar] [CrossRef]
- Stern, M.D.; Santos, K.A.; Satter, L.D. Protein degradation on rumen and amino acids absorption in the small intestine of lactating dairy cattle fed heat-treated whole soybeans. J. Dairy Sci. 1985, 68, 45–56. [Google Scholar] [CrossRef]
- Waltz, D.M.; Stern, M.D. Evaluation methods for protecting soybean protein from degradation by rumen bacteria. Anim. Feed Sci. Technol. 1989, 25, 11–122. [Google Scholar] [CrossRef]
- Iommelli, P.; Zicarelli, F.; Musco, N.; Sarubbi, F.; Grossi, M.; Lotito, D.; Tudisco, R. Effect of cereals and legumes processing on in situ rumen protein degradability: A review. Fermentation 2022, 8, 363. [Google Scholar] [CrossRef]
- Paya, H.; Taghizadeh, A.; Janamohamadi, H.; Moghadam, G.A. Ruminal dry matter and crude protein degradability of some tropical (Iranian) feeds used in ruminant diets estimated using the in situ and in vitro techniques. J. Biol. Sci. 2008, 3, 720–725. [Google Scholar]
- Engstrom, D.F.; Mathison, G.W.; Goonewardene, L.A. Effect of_-glucan, starch and fiber content and steam vs. dry rolling of barley grain on its degradability and utilization by steers. Anim. Feed Sci. Technol. 1992, 37, 33–46. [Google Scholar] [CrossRef]
- Burakowska, K.; Górka, P.; Kent-Dennis, C.; Kowalski, Z.M.; Laarveld, B.; Penner, G.B. Effect of heat-treated canola meal and glycerol inclusion on performance and gastrointestinal development of Holstein calves. J. Dairy Sci. 2020, 103, 7998–8019. [Google Scholar] [CrossRef]
- Vahidi, M.F.; Gharechahi, J.; Behmanesh, M.; Ding, X.Z.; Han, J.L.; Salekdeh, G.H. Diversity of microbes colonizing forages of varying lignocellulose properties in the sheep rumen. PeerJ 2021, 9, e10463. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.; Andres, S. Rumen Degradability of some feed legume seeds. Anim. Res. 2003, 52, 17–25. [Google Scholar] [CrossRef]
- Haese, E.; Titze, N.; Rodehutscord, M. In situ ruminal disappearance of crude protein and phytate from differently processed rapeseed meals in dairy cows. J. Sci. Food Agric. 2022, 102, 2805–2812. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, J.E. Nitrogen metabolism in sheep. 2. A comparision between rumen degradability of nitrogen and organic matter in Sacco and in vivo in sheep fed rations with hay. Barley and various protein supplement. Swed. J. Agric. Res. 1984, 14, 37–43. [Google Scholar]
- Palangi, V. Identification of ruminal fermentation curves of some legume forages using particle swarm optimization. Animals 2023, 13, 1339. [Google Scholar] [CrossRef]
- McMeniman, N.P.; Armstrong, D.G. The flow of amino acids into the small intestine of cattle when fed heated and unheated beans (Vicia faba). J. Agric. Sci. 1979, 93, 181–188. [Google Scholar] [CrossRef]
- Sadeghi, A.A.; Shawrang, P. Effects of microwave irradiation on ruminal protein and starch degradation of corn grain. Anim. Feed Sci. Technol. 2006, 127, 113–123. [Google Scholar] [CrossRef]
- Arieli, A.; Ben-Moshe, A.; Zamwel, S.; Tagari, H. In situ evaluatuion of ruminal and intestinal digestibility of heat-treated whole cottonseeds. J. Dairy Sci. 1989, 72, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Polan, C.E.; Stieve, D.E.; Garrett, J.L. Protein preservation and ruminal degradation of ensiled forage treated with heat, formic acid, ammonia, or microbial inoculant. J. Dairy Sci. 1998, 81, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Golshan, S.; Pirmohammadi, R.; Khalilvandi-Behroozyar, H. Microwave irradiation of whole soybeans in ruminant nutrition: Protein and carbohydrate metabolism in vitro and in situ. Vet. Res. Forum 2019, 10, 343. [Google Scholar] [PubMed]
- Li, Y.; Cheng, Y.; Zhang, Z.; Wang, Y.; Mintah, B.K.; Dabbour, M.; Ma, H. Modification of rapeseed protein by ultrasound-assisted pH shift treatment: Ultrasonic mode and frequency screening, changes in protein solubility and structural characteristics. Ultrason. Sonochemistry 2020, 69, 105240. [Google Scholar] [CrossRef]
- Tang, S.Q.; Du, Q.H.; Fu, Z. Ultrasonic treatment on physicochemical properties of water-soluble protein from Moringa oleifera seed. Ultrason. Sonochemistry 2021, 71, 105357. [Google Scholar] [CrossRef]
- Erickson, P.S.; Kalscheur, K.F. Nutrition and feeding of dairy cattle. In Animal Agriculture; Academic Press: New York, NY, USA, 2020; pp. 157–180. [Google Scholar]
- Ren, Y.; Quilliam, C.; Weber, L.P.; Warkentin, T.D.; Tulbek, M.C.; Ai, Y. Effects of pulse crop types and extrusion parameters on the physicochemical properties, in vitro and in vivo starch digestibility of pet foods. Cereal Chem. 2022, 99, 625–639. [Google Scholar] [CrossRef]
- McDonough, C.M.; Anderson, B.J.; Rooney, L.W. Structural characteristics of steam-flaked sorghum. Cereal Chem. 1997, 74, 542–547. [Google Scholar] [CrossRef]
- Ezeogu, L.I.; Duodu, K.G.; Taylor, J.R.N. Effects of endosperm texture and cooking conditions on the in vitro starch digestibility of sorghum and maize flours. J. Cereal Sci. 2005, 42, 33–44. [Google Scholar] [CrossRef]
- Srakaew, W.; Wachirapakorn, C.; Cherdthong, A.; Wongnen, C. Ruminal Degradability and Bypass Nutrients of Alkaline or Steam-Treated Cassava Chip and Corn Grain. Tropic. Anim. Sci. J. 2021, 44, 451–461. [Google Scholar] [CrossRef]
- Kokić, B.; Dokić, L.; Pezo, L.; Jovanović, R.; Spasevski, N.; Kojić, J.; Hadnađev, M. Physicochemical Changes of Heat-Treated Corn Grain Used in Ruminant Nutrition. Animals 2022, 12, 2234. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, S.; Ai, L. Physical barrier effects of dietary fibers on lowering starch digestibility. Curr. Opin. Food Sci. 2022, 48, 100940. [Google Scholar] [CrossRef]
| Ørskov and McDonald without lag time [14] | |
| Ørskov and McDonald with lag time [14] | |
| France without lag time [16] | |
| Dhanoa without lag time [15] | |
| Dhanoa with lag time [15] |
| Treatments | SEM n = 3 | ||||
|---|---|---|---|---|---|
| Item | Control | Steam Flaking | Roasting | Microwave | |
| DM | 94.97 b | 89.74 c | 97.84 a | 96.63 ab | 0.32 |
| CP | 20.89 ab | 20.82 b | 21.62 a | 21.37 ab | 0.12 |
| EE | 4.94 | 3.86 | 5.29 | 5.9 | 1.07 |
| CA | 2.65 | 2.24 | 2.16 | 2.8 | 0.07 |
| NDF | 13.37 c | 14.3 c | 18.47 ab | 20.67 a | 0.94 |
| ADF | 10.44 b | 11.06 a | 11.35 a | 9.5 b | 0.53 |
| DM Disappearance | CP Disappearance | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Incubation Time (h) | Control | Steam Flaking | Microwave | Roasting | SEM n = 4 | Control | Steam Flaking | Microwave | Roasting | SEM n = 4 |
| 0 | 14.92 a | 14.74 a | 11.83 ab | 7.7 b | 0.759 | 12.25 a | 14.19 a | 12.12 a | 9.35 b | 0.36 |
| 2 | 28.88 b | 33.46 a | 24.76 bc | 21.74 c | 0.661 | 21.66 b | 25.23 a | 17.9 c | 15.5 c | 0.4 |
| 4 | 35.36 b | 35.4 b | 39.8 a | 25.53 c | 0.61 | 28.13 b | 31.67 a | 27.12 b | 22.87 c | 0.5 |
| 8 | 44.59 | 45.67 | 44.28 | 39.92 | 0.909 | 35.1 b | 38.17 a | 34.36 b | 25.56 c | 0.36 |
| 12 | 72.72 a | 52.59 b | 44.96 b | 53.48 b | 1.805 | 41.02 b | 44.53 a | 39.78 b | 32.58 c | 0.46 |
| 24 | 82.22 | 60.18 | 60.77 | 60.77 | 3.34 | 45.99 ab | 47.39 a | 45.23 ab | 42.98 b | 0.55 |
| 36 | 92.95 a | 96.88 b | 84.56 b | 75.95 c | 0.51 | 63.43 ab | 64.35 a | 61.07 ab | 59.36 b | 0.64 |
| 48 | 95.44 b | 99.38 a | 87.29 c | 79.11 d | 0.462 | 85.2 a | 90.32 a | 85.09 c | 73.83 b | 1.32 |
| Model | a | b | c | l | d | k | SSM | CSST | R-Square | Explanation |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | ||||||||||
| 1 | 14.98 | 83.48 | 0.0743 | 20,191.8 | 21,169.6 | 95.38 | ||||
| 2 | 14.87 | 83.49 | 0.074 | 10−8 | 20,191.8 | 21,169.6 | 95.38 | † | ||
| 3 | 15.97 | 80.06 | 0.1001 | 0.4378 | 20,203.1 | 21,169.6 | 95.43 | |||
| 4 | 15.43 | 82.49 | 0.079 | −0.0138 | 20193.6 | 21,169.6 | 95.39 | |||
| 5 | 14.08 | 77.86 | 0.1465 | 0.4171 | −0.192 | 19,957.6 | 21,169.6 | 94.27 | † | |
| Microwave | ||||||||||
| 1 | 18.05 | 76.14 | 0.47 | 14,137.7 | 15,156.6 | 93.28 | ||||
| 2 | 14.48 | 79.72 | 0.47 | −0.963 | 14,137.7 | 15,156.6 | 93.28 | † | ||
| 3 | 16.98 | 81.44 | 0.26 | 4.588 | 14,184.2 | 15,156.6 | 93.58 | |||
| 4 | 13.98 | 81.5 | 0.0 31 | 0.0804 | 14,329.8 | 15,156.6 | 94.55 | |||
| 5 | 12.93 | 71.12 | 0.129 | 0.485 | −0.18 | 13,262.8 | 15,156.6 | 87.51 | † | |
| Steam flaking | ||||||||||
| 1 | 20.48 | 79.52 | 0.48 | 80,278.5 | 80,966 | 99.15 | ||||
| 2 | 17.71 | 81.39 | 0.48 | −0.594 | 80,281.4 | 80,966 | 99.16 | |||
| 3 | 21.43 | 78.57 | 0.51 | 0.744 | 80,273.9 | 80,966 | 99.14 | |||
| 4 | 17.57 | 82.43 | 0.04 | −0.04 | 80,340.8 | 80,966 | 99.22 | |||
| 5 | 25 | 75 | 0.06 | 0.442 | −0.08 | 80,023.9 | 80,966 | 98.84 | † | |
| Roasting | ||||||||||
| 1 | 9.8 | 70.36 | 0.069 | 14,115.8 | 14,753.2 | 95.68 | ||||
| 2 | 5.64 | 74.54 | 0.069 | −0.829 | 6.152 | 14,115.8 | 14,753.2 | 95.68 | † | |
| 3 | 9.08 | 75.73 | 0.033 | 14,148.2 | 14,753.2 | 95.90 | ||||
| 4 | 7.53 | 77.67 | 0.042 | 0.073 | 14,160.2 | 14,753.2 | 95.98 | |||
| 5 | 7.7 | 81.81 | 0.025 | 0.2881 | 0.0139 | 14,163.7 | 14,753.2 | 96.00 |
| Model | a | b | c | l | d | k | SSM | CSST | R-Square | Explanation |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | ||||||||||
| 1 | 12.5 | 88 | 0.028 | −1.736 | 17,453.1 | 17,722.7 | 98.47 | |||
| 2 | 12.5 | 88 | 0.025 | 17,495.1 | 17,722.7 | 98.71 | ||||
| 3 | 12.5 | 87.50 | 0.024 | 1.527 | 17,495.1 | 17,722.7 | 98.49 | |||
| 4 | 12 | 42.59 | 0.2 | 0.04 | 17,497.1 | 17,722.7 | 98.72 | |||
| 5 | 11.55 | 87 | 0.027 | 0.0005 | 0.024 | 17,441.1 | 17,722.7 | 98.41 | † | |
| Microwave | ||||||||||
| 1 | 15 | 84 | 0.026 | 16,733.1 | 16,981.5 | 98.53 | ||||
| 2 | 14 | 85 | 0.024 | −0.793 | 16,725.8 | 16,981.5 | 98.49 | † | ||
| 3 | 12 | 87 | 0.027 | 1.009 | 16,719.1 | 16,981.5 | 98.45 | |||
| 4 | 12 | 88 | 0.02 | 0.027 | 16,737.5 | 16,981.5 | 98.56 | |||
| 5 | 11 | 87 | 0.027 | 0.0189 | 0.0225 | 16,677.9 | 16,981.5 | 98.12 | † | |
| Steam flaking | ||||||||||
| 1 | 19.78 | 80.14 | 0.027 | 19,457 | 19,825.2 | 98.14 | † | |||
| 2 | 19.42 | 85 | 0.025 | −0.0316 | 19,457.2 | 19,825.2 | 98.14 | † | ||
| 3 | 14 | 66.77 | 0.041 | 1.798 | 19,313.6 | 19,825.2 | 97.41 | † | ||
| 4 | 12 | 88 | 0.018 | 0.0638 | 19,461.2 | 19,825.2 | 98.16 | |||
| 5 | 11.99 | 87 | 0.03 | 0.0017 | 0.0299 | 19,374.5 | 19,825.2 | 97.72 | † | |
| Roasting | ||||||||||
| 1 | 11.36 | 88.64 | 0.022 | 13,322.5 | 13,387.2 | 99.51 | ||||
| 2 | 10.98 | 85 | 0.023 | −0.1472 | 13,317.3 | 13,387.2 | 99.47 | † | ||
| 3 | 11.36 | 70 | 0.079 | 80.49 | 13,242.0 | 13,387.2 | 98.91 | † | ||
| 4 | 11.36 | 89 | 0.0217 | 12,429.9 | 13,387.2 | 92.84 | † | |||
| 5 | 12 | 87 | 0.02 | 0.002 | 0.001 | 13,285.9 | 13,387.2 | 99.24 | † |
| Treatments | SEM | ||||
|---|---|---|---|---|---|
| Item | Control | Steam Flaking | Roasting | Microwave | |
| A | 4.97 ab | 6.58 a | 5.14 ab | 3.95 b | 0.25 |
| B1 | 12.68 | 13.67 | 12.00 | 14.3 | 0.38 |
| B2 | 60.3 a | 56.52 ab | 56.7 ab | 55.17 b | 0.63 |
| B3 | 18.78 b | 20.78 ab | 22.24 a | 22.58 a | 0.36 |
| C | 3.27 b | 2.45 b | 3.93 a | 4.00 a | 0.19 |
| Feeds | Average Diameter of Starch Granules at 0 h | Average Digestion Channels of Starch Granules at 48 h of Incubation | Comparison of Digestion Channels with Diameter of Starch Granules |
|---|---|---|---|
| Control | 8.97 | 0.37 | 0.04 |
| Steam flaking | 17.02 | 0.49 | 0.03 |
| Roasting | 15.85 | 0.4 | 0.02 |
| Microwave | 12.34 | 0.57 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taghavi, M.; Taghizadeh, A.; Mehmannavaz, Y.; Hoseinkhani, A.; Mohammadzadeh, H.; Macit, M.; Palangi, V.; Lackner, M. Degradability of Vicia ervilia Grain Using In Situ and CNCPS Methods, and Model-Based Analysis of Its Ruminal Degradation. Fermentation 2023, 9, 419. https://doi.org/10.3390/fermentation9050419
Taghavi M, Taghizadeh A, Mehmannavaz Y, Hoseinkhani A, Mohammadzadeh H, Macit M, Palangi V, Lackner M. Degradability of Vicia ervilia Grain Using In Situ and CNCPS Methods, and Model-Based Analysis of Its Ruminal Degradation. Fermentation. 2023; 9(5):419. https://doi.org/10.3390/fermentation9050419
Chicago/Turabian StyleTaghavi, Marziyeh, Akbar Taghizadeh, Yousef Mehmannavaz, Ali Hoseinkhani, Hamid Mohammadzadeh, Muhlis Macit, Valiollah Palangi, and Maximilian Lackner. 2023. "Degradability of Vicia ervilia Grain Using In Situ and CNCPS Methods, and Model-Based Analysis of Its Ruminal Degradation" Fermentation 9, no. 5: 419. https://doi.org/10.3390/fermentation9050419
APA StyleTaghavi, M., Taghizadeh, A., Mehmannavaz, Y., Hoseinkhani, A., Mohammadzadeh, H., Macit, M., Palangi, V., & Lackner, M. (2023). Degradability of Vicia ervilia Grain Using In Situ and CNCPS Methods, and Model-Based Analysis of Its Ruminal Degradation. Fermentation, 9(5), 419. https://doi.org/10.3390/fermentation9050419








