Optimization Conditions for Ethanol Production from Sweet Sorghum Juice by Thermotolerant Yeast Saccharomyces cerevisiae: Using a Statistical Experimental Design
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Culture Mediums
2.2. Plant Material
2.3. Microorganism and Inoculum Preparation
2.4. Fermentation Conditions
2.5. Screening of the Fermentation Factors Affecting Ethanol Production by S. cerevisiae DBKKUY-53 at 37 °C
2.6. Optimization Conditions for Ethanol Production from SSJ by S. cerevisiae DBKKUY-53 at 37 °C
2.7. Analytical Methods
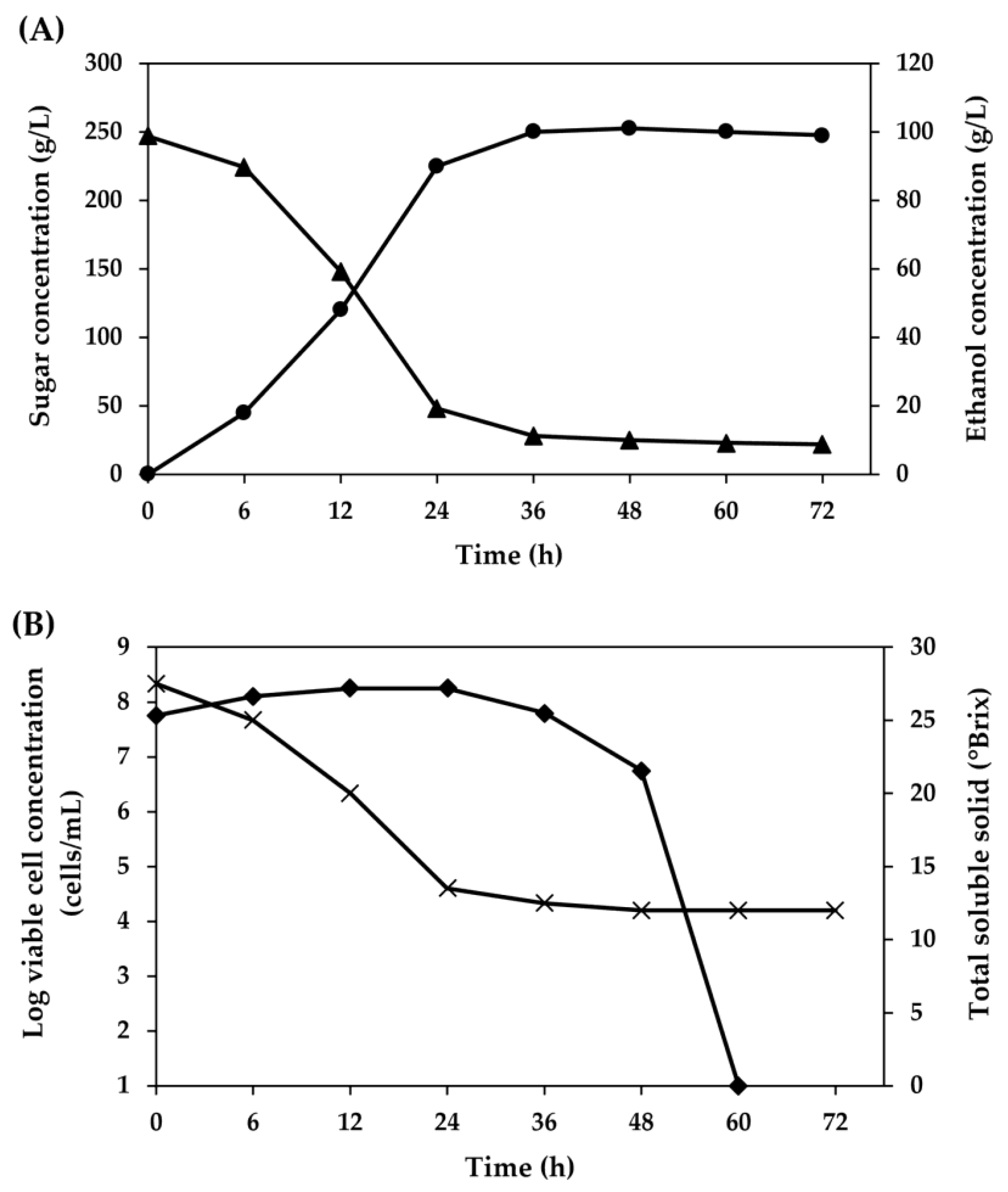
3. Results and Discussion
3.1. Screening of the Fermentation Factors Affecting Ethanol Production from SSJ at 37 °C by Thermotolerant S. cerevisiae DBKKUY-53 Using PBD
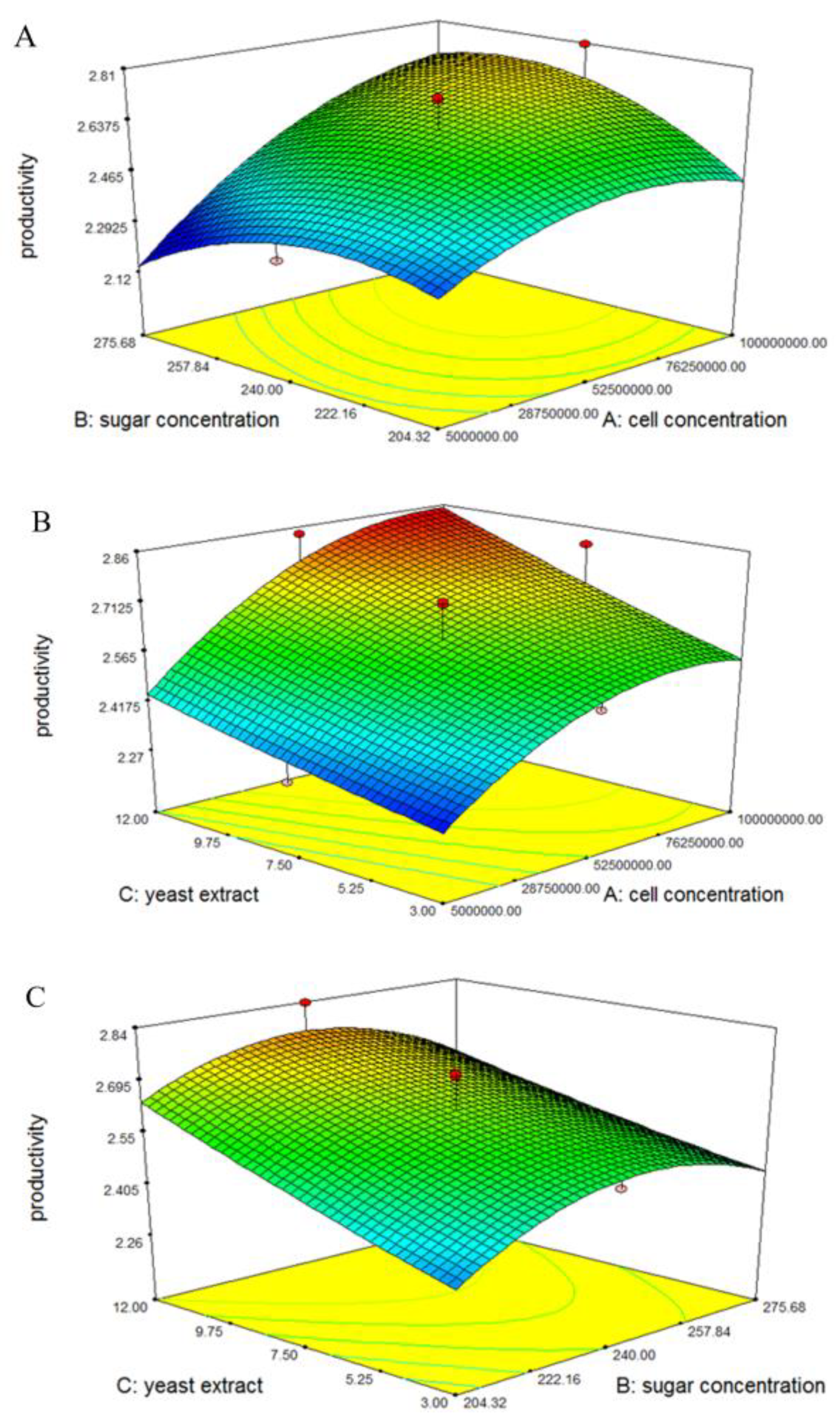
3.2. Optimization of Fermentation Conditions for Ethanol Production from SSJ at 37 °C by Thermotolerant S. cerevisiae DBKKUY-53 Using CCD
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manzanares, P. The role of biorefinering research in the development of a modern bioeconomy. Acta Innov. 2020, 37, 47–56. [Google Scholar] [CrossRef]
- Silveira, M.H.L.; Morais, A.R.C.; Lopes, A.M.D.C.; Olekszyszen, D.N.; Bogel-Łukasik, R.; Andreaus, J.; Ramos, L.P. Current pretreatment technologies for the development of cellulosic ethanol and biorefineries. ChemSusChem 2015, 8, 3366–3390. [Google Scholar] [CrossRef] [PubMed]
- Eiadpum, A.; Limtong, S.; Phisalaphong, M. High-temperature ethanol fermentation by immobilized coculture of Kluyveromyces marxianus and Saccharomyces cerevisiae. J. Biosci. Bioeng. 2012, 3, 325–329. [Google Scholar] [CrossRef]
- da Costa Lopes, A.M.; Lins, R.M.G.; Rebelo, R.A.; Lukasik, R.M. Biorefinery approach for lignocellulosic biomass valorisation with an acidic ionic liquid. Green Chem. 2018, 20, 4043–4057. [Google Scholar] [CrossRef]
- Girio, F.M.; Fonseca, C.; Carvalheiro, F.; Duarte, L.C.; Marques, S.; Bogel-Lukasik, R. Hemicelluloses for fuel ethanol: A review. Bioresour. Technol. 2010, 101, 4775–4800. [Google Scholar] [CrossRef]
- Jaisil, P.; Putto, C. Analysis of feedstocks for ethanol production in Thailand. Alternative Energy 2010, 5, 30–34. [Google Scholar]
- Laopaiboon, L.; Thanonkeo, P.; Jaisil, P.; Laopaiboon, P. Ethanol production from sweet sorghum juice in batch and fed-batch fermentations by Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2007, 23, 1497–1501. [Google Scholar] [CrossRef]
- Prasad, S.; Singh, A.; Jain, N.; Joshi, H.C. Ethanol production from sweet sorghum syrup for utilization as automotive fuel in India. Energy Fuels 2007, 21, 2415–2420. [Google Scholar] [CrossRef]
- Wu, X.; Staggenborg, S.; Propheter, J.L.; Rooney, W.L.; Yu, J.; Wang, D. Features of sweet sorghum juice and their performance in ethanol fermentation. Ind. Crop. Prod. 2010, 31, 164–170. [Google Scholar] [CrossRef]
- Limtong, S.; Sringiew, C.; Yongmanitchai, W. Production of fuel ethanol at high temperature from sugar cane juice by a newly isolated Kluyveromyces marxianus. Bioresour. Technol. 2007, 98, 3367–3374. [Google Scholar] [CrossRef]
- Nuanpeng, S.; Thanonkeo, S.; Yamada, M.; Thanonkeo, P. Ethanol production from sweet sorghum juice at high temperatures using a newly isolated thermotolerant yeast Saccharomyces cerevisiae DNKKU Y-53. Energies 2016, 9, 253. [Google Scholar] [CrossRef]
- Charoensopharat, K.; Thanonkeo, P.; Thanonkeo, S.; Yamada, M. Ethanol production from Jerusalem artichoke tubers at high temperature by newly isolated thermotolerant inulin-utilizing yeast Kluyveromyces marxianus using consolidated bioprocessing. Antonie Leeuwenhoek 2015, 108, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Pilap, W.; Thanonkeo, S.; Klanrit, P.; Thanonkeo, P. The potential of the newly isolated thermotolerant Kluyveromyces marxianus for high-temperature ethanol production using sweet sorghum juice. 3 Biotech 2018, 8, 126. [Google Scholar] [CrossRef] [PubMed]
- Chamnipa, N.; Thanonkeo, S.; Klanrit, P.; Thanonkeo, P. The potential of the newly isolated thermotolerant yeast Pichia kudriavzevii RZ8-1 for high-temperature ethanol production. Braz. J. Microbiol. 2018, 49, 378–391. [Google Scholar] [CrossRef]
- Phong, H.X.; Klanrit, P.; Dung, N.T.P.; Yamada, M.; Thanonkeo, P. Isolation and characterization of thermotolerant yeasts for the production of second-generation bioethanol. Ann. Microbiol. 2019, 69, 765–776. [Google Scholar] [CrossRef]
- Avchar, R.; Lanjekar, V.; Kshirsagar, P.; Dhakephalkar, P.K.; Dagar, S.S.; Baghela, A. Buffalo rumen harbours diverse thermotolerant yeasts capable of producing second-generation bioethanol from lignocellulosic biomass. Renew. Energ. 2021, 173, 795–807. [Google Scholar] [CrossRef]
- Aouine, M.; Elalami, D.; Koraichi, S.I.; Haggoud, A.; Barakat, A. Exploring natural fermented foods as a source for new efficient thermotolerant yeasts for the production of second-generation bioethanol. Energies 2022, 15, 4954. [Google Scholar] [CrossRef]
- Techaparin, A.; Thanonkeo, P.; Klanrit, P. High-temperature ethanol production using thermotolerant yeast newly isolated from Greater Mekong Subregion. Braz. J. Microbiol. 2017, 48, 461–475. [Google Scholar] [CrossRef]
- Phong, H.X.; Klanrit, P.; Dung, N.T.P.; Thanonkeo, S.; Yamada, M.; Thanonkeo, P. High-temperature ethanol fermentation from pineapple waste hydrolysate and gene expression analysis of thermotolerant yeast Saccharomyces cerevisiae. Sci. Rep. 2022, 12, 13965. [Google Scholar] [CrossRef]
- Nguyen, P.V.; Nguyen, K.H.V.; Nguyen, N.L.; Ho, X.T.T.; Truong, P.H.; Nguyen, K.C.T. Lychee-derived, thermotolerant yeasts for second-generation bioethanol production. Fermentation 2022, 8, 515. [Google Scholar] [CrossRef]
- Pilap, W.; Thanonkeo, S.; Klanrit, P.; Thanonkeo, P. The potential of multistress tolerant yeast, Saccharomycodes ludwigii, for second-generation bioethanol production. Sci. Rep. 2022, 12, 22062. [Google Scholar] [CrossRef]
- Wang, Y.X.; Lu, Z.X. Optimization of processing parameters for the mycelial growth and extracellular polysaccharide production by Boletus sp. ACCC50328. Process Biochem. 2005, 40, 1043–1051. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Nuanpeng, S.; Thanonkeo, S.; Klanrit, P.; Thanonkeo, P. Ethanol production from sweet sorghum by Saccharomyces cerevisiae DBKKUY-53 immobilized on alginate-loofah matrices. Braz. J. Microbiol. 2018, 49, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.L.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandão, G.C.; da Silva, E.G.P.; Portugal, L.A.; dos Reis, P.S.; Souza, A.S.; et al. Box-Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef]
- Singh, A.; Tuteja, S.; Singh, N.; Bishnoi, N.R. Enhanced saccharification of rice straw and hull by microwave-alkali pretreatment and lignocellulolytic enzyme production. Bioresour. Technol. 2011, 102, 1773–1782. [Google Scholar] [CrossRef]
- Plackett, R.L.; Burman, J.P. The design of optimum multifactorial experiments. Biometrika 1946, 33, 305–325. [Google Scholar] [CrossRef]
- Pornthap, T.; Sudarat, T.; Kannikar, C.; Mamoru, Y.; Thanonkeo, P.; Charoensuk, K.; Yamada, M. Ethanol production from Jerusalem artichoke (Helianthus tuberosus L.) by Zymomonas mobilis TISTR548. Afr. J. Biotechnol. 2011, 10, 10691–10697. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, X.; Tan, T. Optimization of media conditions for the production of ethanol from sweet sorghum juice by immobilized Saccharomyces cerevisiae. Biomass Bioenergy 2009, 33, 521–526. [Google Scholar] [CrossRef]
- Laopaiboon, L.; Nuanpeng, S.; Srinophakun, P.; Klanrit, P.; Laopaiboon, P. Ethanol production from sweet sorghum juice using very high gravity technology: Effects of carbon and nitrogen supplementations. Bioresour. Technol. 2009, 100, 4176–4182. [Google Scholar] [CrossRef]
- Pereira, F.; Guimarães, P.M.R.; Teixeira, J.A.; Domingues, L. Optimization of low-cost medium for very high gravity ethanol fermentations by Saccharomyces cerevisiae using statistical experimental designs. Bioresour. Technol. 2010, 101, 7856–7863. [Google Scholar] [CrossRef]
- Zoecklein, B.U.; Fugelsang, K.C.; Gump, B.H.; Nury, F.S. Wine Analysis and Production; Chapman & Hall: New York, NY, USA, 1995. [Google Scholar]
- Mecozzi, M. Estimation of total carbohydrate amount in environmental samples by the phenol-sulphuric acid method assisted by multivariate calibration. Chemom. Intell. Lab. Syst. 2005, 79, 84–90. [Google Scholar] [CrossRef]
- Tofighi, A.; Assadi, M.M.; Asadirad, M.H.A.; Karizi, S.Z. Bio-ethanol production by a novel autochthonous thermo-tolerant yeast isolated from wastewater. J. Environ. Health Sci. Eng. 2014, 12, 107. [Google Scholar] [CrossRef] [PubMed]
- Phutela, U.G.; Kaur, J. Process optimization for ethanol production from sweet sorghum juice using Saccharomyces cerevisiae strain NRRL Y-2034 by response surface methodology. Sugar Tech 2014, 16, 411–421. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, B.; Liang, X. Enhanced ethanol production from stalk juice of sweet sorghum by response surface methodology. Afr. J. Biotechnol. 2012, 11, 6117–6122. [Google Scholar]
- Bai, F.W.; Chen, L.J.; Zhang, Z.; Anderson, W.A.; Moo-Young, M. Continuous ethanol production and evaluation of yeast cell lysis and viability loss under very high gravity medium conditions. J. Biotechnol. 2004, 110, 287–293. [Google Scholar] [CrossRef]
- Ozmihci, S.; Kargi, F. Comparison of yeast strains for batch ethanol fermentation of cheese-whey powder (CWP) solution. Lett. Appl. Microbiol. 2007, 44, 602–606. [Google Scholar] [CrossRef]
- Li, Z.; Wang, D.; Shi, Y.C. Effects of nitrogen source on ethanol production in very high gravity fermentation of corn starch. J. Taiwan Inst. Chem. Eng. 2017, 70, 229–235. [Google Scholar] [CrossRef]
- Deesuth, O.; Laopaiboon, P.; Klanrit, P.; Laopaiboon, L. Improvement of ethanol production from sweet sorghum juice under high gravity and very high gravity conditions: Effects of nutrient supplementation and aeration. Ind. Crops Prod. 2015, 74, 95–102. [Google Scholar] [CrossRef]

| Code | Independent Factor | Unit | Low Level (−1) | High Level (+1) |
|---|---|---|---|---|
| A | Sugar concentration | g/L | 180 | 250 |
| B | pH | 4.5 | 5.5 | |
| C | Cell concentration | Cells/mL | 1 × 106 | 1 × 108 |
| D | Ammonium nitrate | g/L | 0.0 | 1.5 |
| E | Urea | g/L | 0.0 | 1.5 |
| F | Yeast extract | g/L | 0.0 | 9.0 |
| G | Diammonium phosphate | g/L | 0.0 | 1.5 |
| H | Magnesium sulfate | g/L | 0.5 | 3.0 |
| Code | Independent Factor | Unit | Levels | ||||
|---|---|---|---|---|---|---|---|
| −1.68 | −1.00 | 0 | +1.00 | +1.68 | |||
| A | Cell concentration | Cells/mL | 5.00 × 106 | 2.43 × 107 | 5.25 × 107 | 8.07 × 107 | 1.00 × 108 |
| B | Sugar concentration | g/L | 180 | 204 | 240 | 276 | 300 |
| C | Yeast extract | g/L | 3.0 | 4.8 | 7.5 | 10.2 | 12.0 |
| Run | Independent Factor and Level | P (g/L) | Qp (g/L/h) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | Model | Actual | Model | Actual | |
| 1 | −1 | −1 | +1 | −1 | +1 | +1 | −1 | +1 | 75.07 | 75.12 ± 2.01 | 3.17 | 3.10 ± 0.04 |
| 2 | −1 | +1 | −1 | +1 | +1 | −1 | +1 | +1 | 64.41 | 65.54 ± 1.68 | 1.26 | 1.35 ± 0.01 |
| 3 | +1 | −1 | −1 | −1 | +1 | −1 | +1 | +1 | 71.93 | 70.81 ± 2.01 | 1.55 | 1.46 ± 0.02 |
| 4 | +1 | −1 | +1 | +1 | +1 | −1 | −1 | −1 | 85.37 | 87.64 ± 0.62 | 2.44 | 2.43 ± 0.01 |
| 5 | +1 | +1 | −1 | −1 | −1 | +1 | −1 | +1 | 80.31 | 82.55 ± 2.32 | 1.66 | 1.66 ± 0.09 |
| 6 | +1 | +1 | −1 | +1 | +1 | +1 | −1 | −1 | 84.83 | 82.57 ± 0.42 | 1.70 | 1.72 ± 0.00 |
| 7 | −1 | +1 | +1 | +1 | −1 | −1 | −1 | +1 | 73.33 | 72.22 ± 0.98 | 2.11 | 2.02 ± 0.01 |
| 8 | −1 | +1 | +1 | −1 | +1 | +1 | +1 | −1 | 80.03 | 79.98 ± 1.48 | 3.23 | 3.31 ± 0.03 |
| 9 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | 60.75 | 59.56 ± 2.21 | 1.04 | 1.22 ± 0.03 |
| 10 | +1 | −1 | +1 | +1 | −1 | +1 | +1 | +1 | 92.15 | 90.96 ± 2.40 | 3.57 | 3.75 ± 0.05 |
| 11 | +1 | +1 | +1 | −1 | −1 | −1 | +1 | −1 | 85.81 | 85.88 ± 0.33 | 2.46 | 2.38 ± 0.00 |
| 12 | −1 | −1 | −1 | +1 | −1 | +1 | +1 | −1 | 72.05 | 73.26 ± 0.39 | 2.21 | 2.03 ± 0.01 |
| Factor | Sum Square | df | Mean Square | F Value | p Value Prob > F | Remark |
|---|---|---|---|---|---|---|
| Ethanol concentration (P, g/L) | ||||||
| A | 465.39 | 1 | 465.39 | 59.78 | 0.0045 | Significant |
| B | 10.78 | 1 | 10.78 | 1.38 | 0.3241 | |
| C | 275.72 | 1 | 275.72 | 35.42 | 0.0095 | Significant |
| D | 27.88 | 1 | 27.88 | 3.58 | 0.1548 | |
| E | 0.64 | 1 | 0.64 | 0.08 | 0.7935 | |
| F | 152.57 | 1 | 152.57 | 19.60 | 0.0214 | Significant |
| G | 3.82 | 1 | 3.82 | 0.49 | 0.5339 | |
| H | 11.40 | 1 | 11.40 | 1.46 | 0.3129 | |
| Ethanol productivity (Qp, g/L/h) | ||||||
| A | 0.012 | 1 | 0.012 | 0.25 | 0.6530 | |
| B | 0.20 | 1 | 0.20 | 4.30 | 0.1298 | |
| C | 4.76 | 1 | 4.76 | 101.44 | 0.0021 | Significant |
| D | 0.0024 | 1 | 0.0024 | 0.05 | 0.8347 | |
| E | 0.0081 | 1 | 0.0081 | 0.17 | 0.7065 | |
| F | 1.85 | 1 | 1.85 | 39.52 | 0.0081 | Significant |
| G | 0.39 | 1 | 0.39 | 8.25 | 0.0639 | |
| H | 0.0055 | 1 | 0.0055 | 0.12 | 0.7555 | |
| Run | Independent Factor | P (g/L) | Qp (g/L/h) | Yp/s (g/g) | ||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | Model | Actual | Model | Actual | ||
| 1 | 8.07 × 107 | 204 | 4.8 | 85.84 | 84.12 ± 2.03 | 2.38 | 2.34 ± 0.03 | 0.46 ± 0.02 |
| 2 | 5.25 × 107 | 240 | 7.5 | 94.07 | 92.33 ± 1.89 | 2.61 | 2.56 ± 0.02 | 0.44 ± 0.01 |
| 3 | 8.07 × 107 | 276 | 10.2 | 98.09 | 94.53 ± 1.60 | 2.72 | 2.63 ± 0.01 | 0.41 ± 0.01 |
| 4 | 5.25 × 107 | 240 | 7.5 | 94.07 | 90.47 ± 2.01 | 2.61 | 2.51 ± 0.04 | 0.43 ± 0.03 |
| 5 | 8.07 × 107 | 204 | 10.2 | 92.93 | 90.80 ± 0.68 | 2.58 | 2.52 ± 0.00 | 0.48 ± 0.00 |
| 6 | 5.25 × 107 | 240 | 7.5 | 94.07 | 97.75 ± 0.98 | 2.61 | 2.72 ± 0.01 | 0.47 ± 0.01 |
| 7 | 2.43 × 107 | 276 | 10.2 | 85.35 | 85.61 ± 1.67 | 2.37 | 2.38 ± 0.02 | 0.43 ± 0.02 |
| 8 | 2.43 × 107 | 204 | 10.2 | 89.35 | 88.69 ± 2.42 | 2.48 | 2.46 ± 0.01 | 0.45 ± 0.01 |
| 9 | 8.07 × 107 | 276 | 4.8 | 93.26 | 92.42 ± 1.20 | 2.59 | 2.57 ± 0.01 | 0.41 ± 0.01 |
| 10 | 5.00 × 106 | 240 | 7.5 | 84.47 | 82.40 ± 0.42 | 2.35 | 2.29 ± 0.00 | 0.40 ± 0.00 |
| 11 | 5.25 × 107 | 180 | 7.5 | 80.60 | 81.42 ± 0.98 | 2.24 | 2.26 ± 0.01 | 0.47 ± 0.01 |
| 12 | 5.25 × 107 | 240 | 7.5 | 94.07 | 97.41 ± 2.30 | 2.61 | 2.71 ± 0.03 | 0.48 ± 0.02 |
| 13 | 2.43 × 107 | 276 | 4.8 | 82.29 | 82.99 ± 0.62 | 2.28 | 2.31 ± 0.01 | 0.35 ± 0.00 |
| 14 | 5.25 × 107 | 240 | 3.0 | 90.72 | 89.91 ± 1.68 | 2.52 | 2.50 ± 0.01 | 0.42 ± 0.01 |
| 15 | 1.00 × 108 | 240 | 7.5 | 96.82 | 101.03 ± 1.04 | 2.68 | 2.81 ± 0.02 | 0.47 ± 0.01 |
| 16 | 5.25 × 107 | 240 | 7.5 | 94.07 | 92.08 ± 0.63 | 2.61 | 2.56 ± 0.01 | 0.44 ± 0.01 |
| 17 | 5.25 × 107 | 240 | 12.0 | 99.23 | 102.14 ± 2.41 | 2.75 | 2.84 ± 0.03 | 0.49 ± 0.03 |
| 18 | 5.25 × 107 | 300 | 7.5 | 83.09 | 84.39 ± 1.74 | 2.30 | 2.34 ± 0.02 | 0.39 ± 0.01 |
| 19 | 2.43 × 107 | 204 | 4.8 | 84.02 | 86.01 ± 0.68 | 2.33 | 2.39 ± 0.01 | 0.45 ± 0.01 |
| Factor | Sum Square | df | Mean Square | F Value | p Value Prob > F | Remark |
|---|---|---|---|---|---|---|
| Model | 638.91 | 9 | 70.99 | 6.16 | 0.0044 | Significant |
| A | 182.28 | 1 | 182.28 | 15.82 | 0.0026 | Significant |
| B | 8.73 | 1 | 8.73 | 0.76 | 0.4046 | |
| C | 87.85 | 1 | 87.85 | 7.62 | 0.0201 | Significant |
| AB | 41.10 | 1 | 41.10 | 3.57 | 0.0883 | |
| AC | 1.52 | 1 | 1.52 | 0.13 | 0.7243 | |
| BC | 2.69 | 1 | 2.69 | 0.23 | 0.6391 | |
| A2 | 28.69 | 1 | 28.69 | 2.49 | 0.1456 | |
| B2 | 295.11 | 1 | 295.11 | 25.61 | 0.0005 | Significant |
| C2 | 0.18 | 1 | 0.18 | 0.02 | 0.9022 | |
| Residual | 115.22 | 10 | 11.52 | |||
| Lack of fit | 58.88 | 5 | 11.78 | 1.05 | 0.4813 | Not significant |
| Pure Error | 56.34 | 5 | 11.27 | |||
| Cor Total | 754.13 | 19 | ||||
| R2 | 0.85 |
| Factor | Sum Square | df | Mean Square | F Value | p Value Prob > F | Remark |
|---|---|---|---|---|---|---|
| Model | 0.490 | 9 | 0.055 | 6.16 | 0.0044 | Significant |
| A | 0.140 | 1 | 0.140 | 15.81 | 0.0026 | Significant |
| B | 0.0067 | 1 | 0.0067 | 0.76 | 0.4046 | |
| C | 0.068 | 1 | 0.068 | 7.62 | 0.0201 | Significant |
| AB | 0.032 | 1 | 0.032 | 3.57 | 0.0882 | |
| AC | 0.0012 | 1 | 0.0012 | 0.13 | 0.7238 | |
| BC | 0.0021 | 1 | 0.0021 | 0.23 | 0.6391 | |
| A2 | 0.022 | 1 | 0.022 | 2.49 | 0.1457 | |
| B2 | 0.230 | 1 | 0.230 | 25.61 | 0.0005 | Significant |
| C2 | 0.0001 | 1 | 0.0001 | 0.02 | 0.9023 | |
| Residual | 0.089 | 10 | 0.0089 | |||
| Lack of fit | 0.045 | 5 | 0.0091 | 1.05 | 0.4811 | Not significant |
| Pure Error | 0.043 | 5 | 0.0086 | |||
| Cor Total | 0.580 | 19 | ||||
| R2 | 0.850 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuanpeng, S.; Thanonkeo, S.; Klanrit, P.; Yamada, M.; Thanonkeo, P. Optimization Conditions for Ethanol Production from Sweet Sorghum Juice by Thermotolerant Yeast Saccharomyces cerevisiae: Using a Statistical Experimental Design. Fermentation 2023, 9, 450. https://doi.org/10.3390/fermentation9050450
Nuanpeng S, Thanonkeo S, Klanrit P, Yamada M, Thanonkeo P. Optimization Conditions for Ethanol Production from Sweet Sorghum Juice by Thermotolerant Yeast Saccharomyces cerevisiae: Using a Statistical Experimental Design. Fermentation. 2023; 9(5):450. https://doi.org/10.3390/fermentation9050450
Chicago/Turabian StyleNuanpeng, Sunan, Sudarat Thanonkeo, Preekamol Klanrit, Mamoru Yamada, and Pornthap Thanonkeo. 2023. "Optimization Conditions for Ethanol Production from Sweet Sorghum Juice by Thermotolerant Yeast Saccharomyces cerevisiae: Using a Statistical Experimental Design" Fermentation 9, no. 5: 450. https://doi.org/10.3390/fermentation9050450






