Effect of Compound Additives on Nutritional Composition, Fermentation Quality, and Bacterial Community of High-Moisture Alfalfa Silage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Silage Preparation
2.3. Silage Chemical Composition Analysis
2.3.1. pH, Organic Acids, and Ammonia-N
2.3.2. Conventional Silage Quality Detection
2.4. Silage Bacterial Community Analysis
2.5. Statistical Analysis
3. Results
3.1. Chemical Composition and Bacterial Community of Fresh Alfalfa and Corn Flour
3.2. Effect of Compound Additives on Nutrient Composition of High-Moisture Alfalfa Silage
3.3. Effect of Compound Additives on the Nutrient Composition of High-Moisture Alfalfa Silage
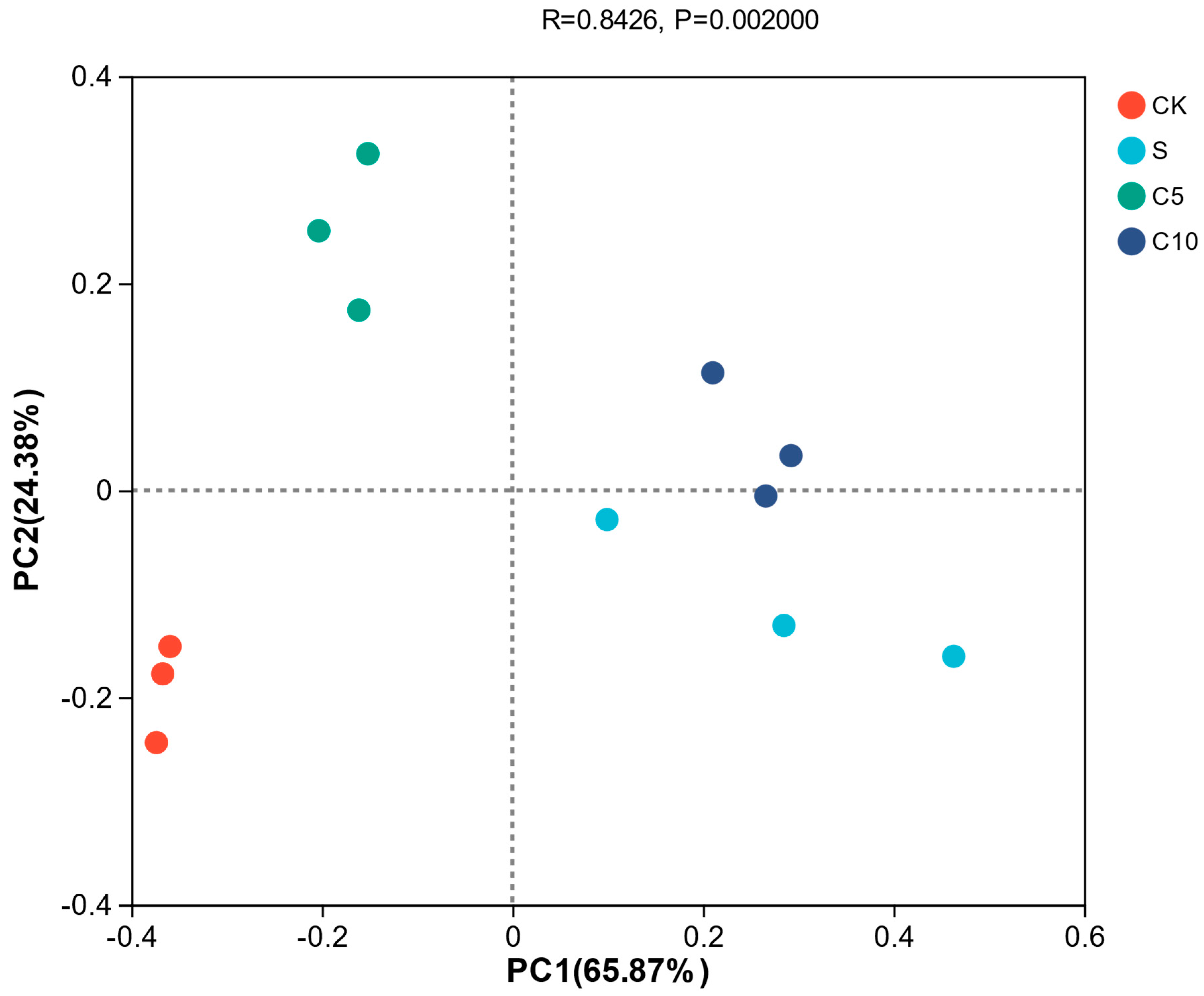
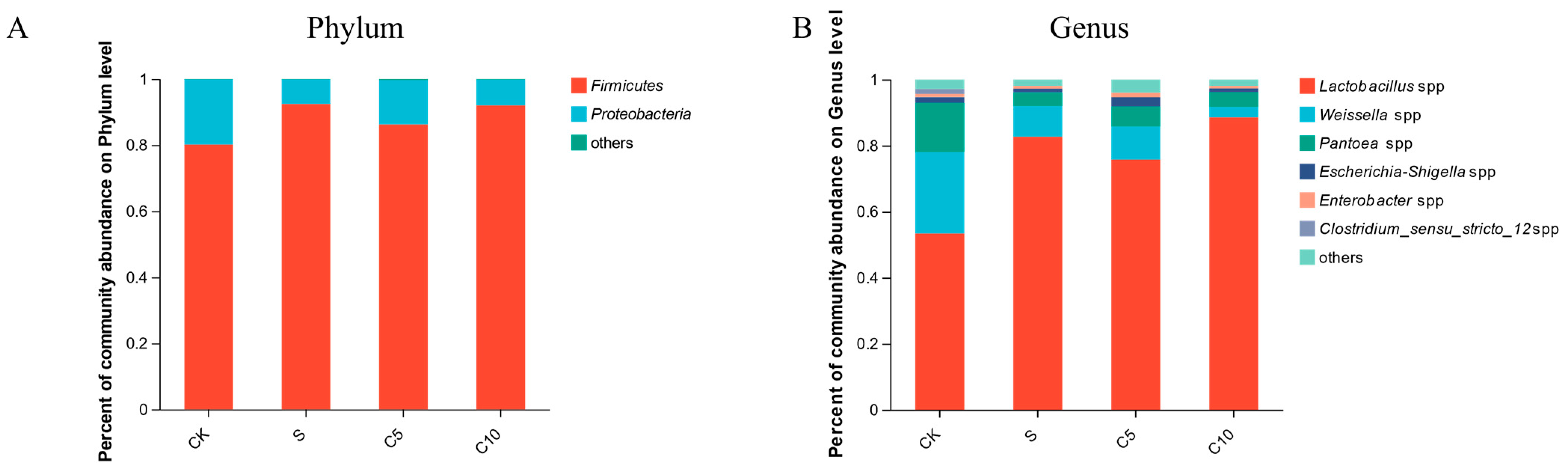
3.4. Effect of Compound Additives on Microbial Diversity of High-Moisture Alfalfa Silage
4. Discussion
4.1. Effect of Compound Additives on Nutrient Composition and Fermentation Quality of High-Moisture Alfalfa Silage
4.2. Effect of Compound Additives on Microbial Diversity of High-Moisture Alfalfa Silage
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Z.; Xie, N.; Li, H.; Feng, W.; Liu, Z.; Yang, F.; Yu, Z.; Zhi, J.; Liu, Z. Effects of Siloguard on Quality of High Moisture Alfalfa. J. Hebei Agric. Sci. 2018, 22, 53–57. [Google Scholar] [CrossRef]
- Li, R.; Jiang, D.; Zheng, M.; Tian, P.; Zheng, M.; Xu, C. Microbial community dynamics during alfalfa silage with or without clostridial fermentation. Sci. Rep. 2020, 10, 17782. [Google Scholar] [CrossRef]
- Mills, J.A.; Kung, L. The Effect of Delayed Ensiling and Application of a Propionic Acid-Based Additive on the Fermentation of Barley Silage1, 2. J. Dairy Sci. 2002, 85, 1969–1975. [Google Scholar] [CrossRef]
- Wang, M. Study of Mixed Silages Made from Sweet Soghum and Alfalfa on Fermentation Quality, Aerobic Stability an Microbial Changes. Master’s Thesis, Tarim University, Xinjiang, China, 2017. [Google Scholar]
- Rigó, E.; Zsédely, E.; Tóth, T.; Schmidt, J. Ensiling alfalfa with hydrolyzed corn meal additive and bacterial inoculant. Acta Agron. Óváriensis 2011, 53, 15–23. [Google Scholar]
- Namihira, T.; Shinzato, N.; Akamine, H.; Maekawa, H.; Matsui, T. Influence of nitrogen fertilization on tropical-grass silage assessed by ensiling process monitoring using chemical and microbial community analyses. J. Appl. Microbiol. 2010, 108, 1954–1965. [Google Scholar] [CrossRef]
- Broderick, G.A.; Kang, J.H. Automated Simultaneous Determination of Ammonia and Total Amino Acids in Ruminal Fluid and In Vitro Media1. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef]
- Wang, S.; Sun, Y.; Zhao, J.; Dong, Z.; Li, J.; Nazar, M.; Shao, T. Assessment of inoculating various epiphytic microbiota on fermentative profile and microbial community dynamics in sterile Italian ryegrass. J. Appl. Microbiol. 2020, 129, 509–520. [Google Scholar] [CrossRef]
- Lu, Y.; Cheng, Q.; Chen, L.; Sun, L.; Xiao, B.; Gou, W.; You, M.; Bai, S.; Li, P. Fermentation Quality and Bacterial Community Characteristics of Oat Silage at Different Altitudes. Acta Agrestia Sin. 2020, 28, 350–357. [Google Scholar]
- Hasan, M.T.; AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Artington, VA, USA, 1990. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Ni, K.; Wang, F.; Zhu, B.; Yang, J.; Zhou, G.; Pan, Y.; Tao, Y.; Zhong, J. Effects of lactic acid bacteria and molasses additives on the microbial community and fermentation quality of soybean silage. Bioresour. Technol. 2017, 238, 706–715. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Stackebrandt, E.; Goebel, B.M. Taxonomic Note: A Place for DNA-DNA Reassociation and 16S rRNA Sequence Analysis in the Present Species Definition in Bacteriology. Int. J. Syst. Evol. Microbiol. 1994, 44, 846–849. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Woolford, M.K. Microbiological screening of food preservatives, cold sterilants and specific antimicrobial agents as potential silage additives. J. Sci. Food Agric. 1975, 26, 226–237. [Google Scholar] [CrossRef]
- Jingling, H.U.A.; Yonggen, Z.; Defu, W.; Yuxin, M.E.N. Effects of lactic acid bacteria on quality of rice straw silage. J. Northeast. Agric. Univ. 2007, 38, 473–477. [Google Scholar]
- Sun, R.; Liu, Y.; Chen, T.; Lv, R.; Zhou, H.; Li, M.; Zi, X. Effect of Lactic Acid Bacteria and Molasses on the Silage Quality and Bacterial Diversity of Paper Mulberry. Acta Agrestia Sin. 2023, 31, 280–286. [Google Scholar]
- Weinberg, Z.G.; Muck, R.E. New trends and opportunities in the development and use of inoculants for silage. FEMS Microbiol. Rev. 1996, 19, 53–68. [Google Scholar] [CrossRef]
- Luo, R. Effect of Molasses Supplemental Amount on Alfalfa Silage Quality and Microbial Community. Master’s Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2021. [Google Scholar]
- Liu, Q.; Wang, Y.; Yang, S.; Yang, Y.; Li, C.; Guo, Y.; Lin, C. Effects of Different Additives on Medicago sativa Silage Quality and the Lactic Acid Bacterial Community. Acta Agrestia Sin. 2016, 24, 1119–1125. [Google Scholar]
- Wang, M.; Yang, Y.; Yu, Y.; Yu, Z. Interactions between additives and ensiling density on quality of Medicago sativa silage. Acta Prataculturae Sin. 2018, 27, 156–162. [Google Scholar]
- Tanizawa, Y.; Tohno, M.; Kaminuma, E.; Nakamura, Y.; Arita, M. Complete genome sequence and analysis of Lactobacillus hokkaidonensis LOOC260T, a psychrotrophic lactic acid bacterium isolated from silage. BMC Genom. 2015, 16, 240. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Yu, Z.; Zhou, H. Effects of lactic acid bacteria inoculants and enzymes on Roegneria turczaninovii silages. Pratacultural Sci. 2013, 30, 1439–1444. [Google Scholar]
- Zhang, Y.; Zi, X.; Li, M.; Zhou, H. Effects of organic acids on quality and nutrient composition of Stylosanthes guianensis silage. Chin. J. Anim. Nutr. 2016, 28, 1609–1614. [Google Scholar]
- Nsereko, V.L.; Smiley, B.K.; Rutherford, W.M.; Spielbauer, A.; Forrester, K.J.; Hettinger, G.H.; Harman, E.K.; Harman, B.R. Influence of inoculating forage with lactic acid bacterial strains that produce ferulate esterase on ensilage and ruminal degradation of fiber. Anim. Feed. Sci. Technol. 2008, 145, 122–135. [Google Scholar] [CrossRef]
- Li, D.; Zheng, Y.; Zhang, Y.; Yanli, L.; Yang, F. Screening and ldentification of Ferulic Acid Esterase Lactobacillus in Silage. J. Grassl. Forage Sci. 2018, 240, 20–21. [Google Scholar]
- Jing, P. Screening and Characterizing of Ferulic Acid Esterase-Producing Lactic Acid Bacteria and Their Application in Alfalfa Silage. Master’s Thesis, Lanzhou University, Lanzhou, China, 2017. [Google Scholar]
- Pang, H.; Qin, G.; Tan, Z.; Li, Z.; Wang, Y.; Cai, Y. Natural populations of lactic acid bacteria associated with silage fermentation as determined by phenotype, 16S ribosomal RNA and recA gene analysis. Syst. Appl. Microbiol. 2011, 34, 235–241. [Google Scholar] [CrossRef]
- Zhang, J.; Qiao, H.; Liu, Y. Effects of moisture and additives on feed quality of alfalfa silage. Pratacultural Sci. 2014, 31, 766–770. [Google Scholar]
- Zhou, X.; Huang, Q.; Wang, J.; Zhang, J.; Cao, Y. Effects of adding lactic acid bacteria and molasses on fermentation quality and in vitro dry matter disappearance rate of Rumex hanus by. silage with different moisture contents. Chin. J. Anim. Nutr. 2021, 33, 1594–1606. [Google Scholar] [CrossRef]
- Kung, L.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef]
- Kung, L.; Robinson, J.R.; Ranjit, N.K.; Chen, J.H.; Golt, C.M.; Pesek, J.D. Microbial Populations, Fermentation End-Products, and Aerobic Stability of Corn Silage Treated with Ammonia or a Propionic Acid-Based Preservative. J. Dairy Sci. 2000, 83, 1479–1486. [Google Scholar] [CrossRef] [PubMed]
- Pahlow, G.; Muck, R.; Driehuis, F.; Oude Elferink, S.; Spoelstra, S.F. Microbiology of Ensiling. In Silage Science and Technology, Agronomy 42; Buxton, D.R., Muck, R.E., Harrison, J.H., Eds.; American Society of Agronomy: Madison, WI, USA, 2003; Volume 42, pp. 31–93. [Google Scholar]
- Zhang, M.; Wang, Y.; Tan, Z.; Li, Z.; Li, Y.; Lv, H.; Zhang, B.; Jin, Q. Microorganism profile, fermentation quality and rumen digestibility in vitro of maize-stalk silages produced at different maturity stages. Crop Pasture Sci. 2017, 68, 225–233. [Google Scholar] [CrossRef]
- Feng, Q.; Shi, W.; Chen, S.; Degen, A.A.; Qi, Y.; Yang, F.; Zhou, J. Addition of Organic Acids and Lactobacillus acidophilus to the Leguminous Forage Chamaecrista rotundifolia Improved the Quality and Decreased Harmful Bacteria of the Silage. Animals 2022, 12, 2260. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.; Kon, M.; Bar-Yam, Y. A New Phylogenetic Diversity Measure Generalizing the Shannon Index and Its Application to Phyllostomid Bats. Am. Nat. 2009, 174, 236–243. [Google Scholar] [CrossRef]
- Ni, K.; Zhao, J.; Zhu, B.; Su, R.; Pan, Y.; Ma, J.; Zhou, G.; Tao, Y.; Liu, X.; Zhong, J. Assessing the fermentation quality and microbial community of the mixed silage of forage soybean with crop corn or sorghum. Bioresour. Technol. 2018, 265, 563–567. [Google Scholar] [CrossRef]
- Hu, W.; Schmidt, R.J.; McDonell, E.E.; Klingerman, C.M.; Kung, L. The effect of Lactobacillus buchneri 40788 or Lactobacillus plantarum MTD-1 on the fermentation and aerobic stability of corn silages ensiled at two dry matter contents. J. Dairy Sci. 2009, 92, 3907–3914. [Google Scholar] [CrossRef]
- Wang, F.; Dong, X.; Zhu, X.; Wang, W.; Hao, J. Effect of lactic acid bacteria additives on silage quality and feeding efficiency of high moisture whole plant maize. Heilongjiang Anim. Sci. Vet. Med. 2019, 18, 118–122+126. [Google Scholar] [CrossRef]
- Wang, C.; He, L.; Xing, Y.; Zhou, W.; Yang, F.; Chen, X.; Zhang, Q. Fermentation quality and microbial community of alfalfa and stylo silage mixed with Moringa oleifera leaves. Bioresour. Technol. 2019, 284, 240–247. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, Z.; Du, S.; Sun, L.; Bao, J.; Hao, J.; Ge, G. Lactobacillus plantarum and propionic acid improve the fermentation quality of high-moisture amaranth silage by altering the microbial community composition. Front. Microbiol. 2022, 13, 1066641. [Google Scholar] [CrossRef]
- Evans, N.J.; Brown, J.M.; Murray, R.D.; Getty, B.; Birtles, R.J.; Hart, C.A.; Carter, S.D. Characterization of novel bovine gastrointestinal tract Treponema isolates and comparison with bovine digital dermatitis treponemes. Appl. Environ. Microbiol. 2011, 77, 138–147. [Google Scholar] [CrossRef]
- Hou, Z.; Zheng, X.; Zhang, X.; Chen, Q.; Wu, D. Effects of urea supplementation on the nutritional quality and microbial community of alfalfa (Medicago sativa L.) silage. Arch. Microbiol. 2022, 204, 414. [Google Scholar] [CrossRef] [PubMed]
- Ogunade, I.M.; Jiang, Y.; Pech Cervantes, A.A.; Kim, D.H.; Oliveira, A.S.; Vyas, D.; Weinberg, Z.G.; Jeong, K.C.; Adesogan, A.T. Bacterial diversity and composition of alfalfa silage as analyzed by Illumina MiSeq sequencing: Effects of Escherichia coli O157:H7 and silage additives. J. Dairy Sci. 2018, 101, 2048–2059. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, H.; Yan, Y.; Zhang, G. Effects of rice bran and lactic acid bacteria preparation on fermentation quality and microbial diversity of Caragana korshinskii silage. Chin. J. Anim. Nutr. 2022, 34, 1800–1808. [Google Scholar] [CrossRef]
- Sun, W. Effect of Adding Different Types of Lactic Acid Bacteria on Mixed Silage of Hybrid Trees with Maize Flour. Master’s Thesis, Guizhou University, Guiyang, China, 2021. [Google Scholar]
- Jones, D.J.C. The Biochemistry of Silage (2nd edn), by p. McDonald, A.R. Henderson & S. J. E. Heron. 340 pp. Kingston, Kent: Chalcombe Publications (1991). £49.50 (UK) £55.00 (elsewhere) (hardback). ISBN 0 948617 22 5. J. Agric. Sci. 1991, 117, 386. [Google Scholar] [CrossRef]
- Tao, L.; Diao, Q. Effects of Ensiling and Natural Drying on Composition and Abundance of Bacterium of Corn Stalk Analyzed by MiSeq Sequencing Technology. Sci. Agric. Sin. 2015, S1, 94–103. [Google Scholar]
- Pan, J.; Yu, S.; Cai, J.; Li, K.; Ou, W.; Wang, Z. Effect of different biological probiotics on the quality of cassava root silage and the impact on microbial flora diversity. Pratacult. Sci. 2021, 38, 2301–2312. [Google Scholar]
- Xu, D. Mechanisms of Microbial and Metabolome on Fermentation of Corn Silage Effected by Climate Zones and Inoculations. Ph.D. Thesis, Lanzhou University, Lanzhou, China, 2021. [Google Scholar]
- Bao, A. Study on Biodiversity and Fermentation Characteristics of Lactic Acid Bacteria in Elymus nutans Silage Ensiled at Different Areas of the Qinghai-Tibetan Plateau. Master’s Thesis, Lanzhou University, Lanzhou, China, 2016. [Google Scholar]
- Muck, R. Silage microbiology and its control through additives. Rev. Bras. Zootec. Braz. J. Anim. Sci. 2010, 39, 183–191. [Google Scholar] [CrossRef]
- Rabelo, C.; Carvalho Basso, F.; McAllister, T.; Lage, J.; Gonçalves, G.; Lara, E.; Oliveira, A.; Berchielli, T.; Reis, R. Influence of Lactobacillus buchneri and forage: Concentrate ratio on the growth performance, fatty acid profile in longissimus muscle and meat quality of beef cattle. Can. J. Anim. Sci. 2016, 96, 4. [Google Scholar] [CrossRef]
- Cai, Y.; Benno, Y.; Ogawa, M.; Ohmomo, S.; Kumai, S.; Nakase, T. Influence of Lactobacillus spp. from an Inoculant and of Weissella and Leuconostoc spp. from Forage Crops on Silage Fermentation. Appl. Environ. Microbiol. 1998, 64, 2982–2987. [Google Scholar] [CrossRef]
- Graf, K.; Ulrich, A.; Idler, C.; Klocke, M. Bacterial community dynamics during ensiling of perennial ryegrass at two compaction levels monitored by terminal restriction fragment length polymorphism. J. Appl. Microbiol. 2016, 120, 1479–1491. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, J.; Dong, Z.; Li, J.; Kaka, N.A.; Shao, T. Sequencing and microbiota transplantation to determine the role of microbiota on the fermentation type of oat silage. Bioresour. Technol. 2020, 309, 123371. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.G.; Cheng, H.J.; Liu, D.; Wei, C.; An, W.J.; Wang, Y.F.; Sun, H.T.; Song, E.L. Treatment of Whole-Plant Corn Silage with Lactic Acid Bacteria and Organic Acid Enhances Quality by Elevating Acid Content, Reducing pH, and Inhibiting Undesirable Microorganisms. Front. Microbiol. 2020, 11, 593088. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Song, J.; Liu, Z.; Zhang, G.; Zhang, Y. Fermentation Quality and Microbial Community of Corn Stover or Rice Straw Silage Mixed with Soybean Curd Residue. Animals 2022, 12, 919. [Google Scholar] [CrossRef] [PubMed]
- Dunière, L.; Sindou, J.; Chaucheyras-Durand, F.; Chevallier, I.; Sergentet, D. Silage processing and strategies to prevent persistence of undesirable microorganisms. Anim. Feed. Sci. Technol. 2013, 182, 1–15. [Google Scholar] [CrossRef]
- Ávila, C.L.S.; Carvalho, B.F. Silage fermentation-updates focusing on the performance of micro-organisms. J. Appl. Microbiol. 2020, 128, 966–984. [Google Scholar] [CrossRef] [PubMed]
- Qian, D.-K.; Geng, Z.-Q.; Sun, T.; Dai, K.; Zhang, W.; Jianxiong Zeng, R.; Zhang, F. Caproate production from xylose by mesophilic mixed culture fermentation. Bioresour. Technol. 2020, 308, 123318. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.S.C.; Balkwill, D.L.; Drake, G.R.; Tanner, R.S. Clostridium carboxidivorans sp. nov., a solvent-producing clostridium isolated from an agricultural settling lagoon, and reclassification of the acetogen Clostridium scatologenes strain SL1 as Clostridium drakei sp. nov. Int. J. Syst. Evol. Microbiol. 2005, 55, 2085–2091. [Google Scholar] [CrossRef]



| Nutritional Composition | DM (FM%) | CP (DM%) | NDF (DM%) | ADF (DM%) | WSC (DM%) | Crude Ash (DM%) |
|---|---|---|---|---|---|---|
| Alfalfa | 22.04 | 19.94 | 43.81 | 31.66 | 4.96 | 9.75 |
| Corn flour | 90.2 | 9.08 | 21.60 | 6.17 | 27.89 | 1.06 |
| Item | CK | S | C5 | C10 | SEM | p-Value |
|---|---|---|---|---|---|---|
| DM | 21.30 ± 0.50 c | 21.69 ± 0.14 c | 26.92 ± 0.40 b | 30.79 ± 1.76 a | 1.21 | <0.01 |
| CP (DM%) | 16.17 ± 0.23 b | 16.80 ± 0.31 a | 15.78 ± 0.27 bc | 15.26 ± 0.38 c | 0.19 | <0.01 |
| WSC (DM%) | 1.46 ± 0.08 c | 4.37 ± 0.04 b | 4.28 ± 0.25 b | 4.74 ± 0.18 a | 0.40 | <0.01 |
| NDF (DM%) | 41.39 ± 3.00 a | 40.12 ± 1.30 a | 35.85 ± 1.28 b | 33.31 ± 2.32 b | 1.11 | <0.01 |
| ADF (DM%) | 34.15 ± 1.13 a | 30.68 ± 1.42 b | 26.05 ± 1.25 c | 23.22 ± 0.87 d | 1.30 | <0.01 |
| Item | CK | S | C5 | C10 | SEM | p-Value |
|---|---|---|---|---|---|---|
| pH | 5.63 ± 0.12 a | 5.27 ± 0.30 a | 4.83 ± 0.20 b | 4.48 ± 0.11 b | 0.14 | <0.01 |
| LA (DM%) | 2.44 ± 0.05 c | 3.29 ± 0.03 b | 3.41 ± 0.23 ab | 3.68 ± 0.24 a | 0.15 | <0.01 |
| AA (DM%) | 1.48 ± 0.04 a | 1.16 ± 0.00 b | 1.23 ± 0.08 b | 1.22 ± 0.02 b | 0.04 | 0.15 |
| PA (DM%) | ND | ND | ND | ND | - | - |
| BA (DM%) | 0.49 ± 0.02 a | 0.32 ± 0.01 b | ND | ND | - | - |
| NH3-N/TN (%) | 4.89 ± 0.25 a | 3.69 ± 0.15 b | 3.02 ± 0.11 c | 2.57 ± 0.06 d | 0.27 | <0.01 |
| LA/AA | 1.63 ± 0.08 b | 2.83 ± 0.03 a | 2.79 ± 0.29 a | 3.02 ± 0.25 a | 0.17 | <0.01 |
| Item | CK | S | C5 | C10 | SEM | p-Value |
|---|---|---|---|---|---|---|
| Shannon index | 2.81 ± 0.05 a | 1.65 ± 0.59 b | 2.42 ± 0.19 a | 1.74 ± 0.09 b | 0.16 | 0.12 |
| Simpson index | 0.09 ± 0.01 b | 0.40 ± 0.22 a | 0.19 ± 0.04 ab | 0.33 ± 0.03 a | 0.05 | 0.05 |
| Ace index | 61.62 ± 1.67 b | 59.71 ± 1.08 b | 65.37 ± 1.32 a | 56.70 ± 0.86 c | 1.00 | 0.61 |
| Chao index | 60.63 ± 2.29 b | 58.76 ± 1.80 bc | 64.62 ± 1.28 a | 56.45 ± 0.64 c | 0.99 | 0.19 |
| Good’s coverage | 0.9997 ± 0.0001 a | 0.9993 ± 0.0002 a | 0.9996 ± 0.0003 a | 0.9994 ± 0.0002 a | 0.0001 | 0.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, H.; Wang, H.; Bao, B.; Qu, H.; Wang, J.; Sun, L.; Liu, B.; Gao, F. Effect of Compound Additives on Nutritional Composition, Fermentation Quality, and Bacterial Community of High-Moisture Alfalfa Silage. Fermentation 2023, 9, 453. https://doi.org/10.3390/fermentation9050453
Jiang H, Wang H, Bao B, Qu H, Wang J, Sun L, Liu B, Gao F. Effect of Compound Additives on Nutritional Composition, Fermentation Quality, and Bacterial Community of High-Moisture Alfalfa Silage. Fermentation. 2023; 9(5):453. https://doi.org/10.3390/fermentation9050453
Chicago/Turabian StyleJiang, Heng, Haoran Wang, Buhe Bao, Hui Qu, Jiao Wang, Le Sun, Bin Liu, and Fengqin Gao. 2023. "Effect of Compound Additives on Nutritional Composition, Fermentation Quality, and Bacterial Community of High-Moisture Alfalfa Silage" Fermentation 9, no. 5: 453. https://doi.org/10.3390/fermentation9050453




