Co-Fermentation of Chlorella vulgaris with Oleaginous Yeast in Starch Processing Effluent as a Carbon-Reducing Strategy for Wastewater Treatment and Biofuel Feedstock Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wastewater and Microbial Species
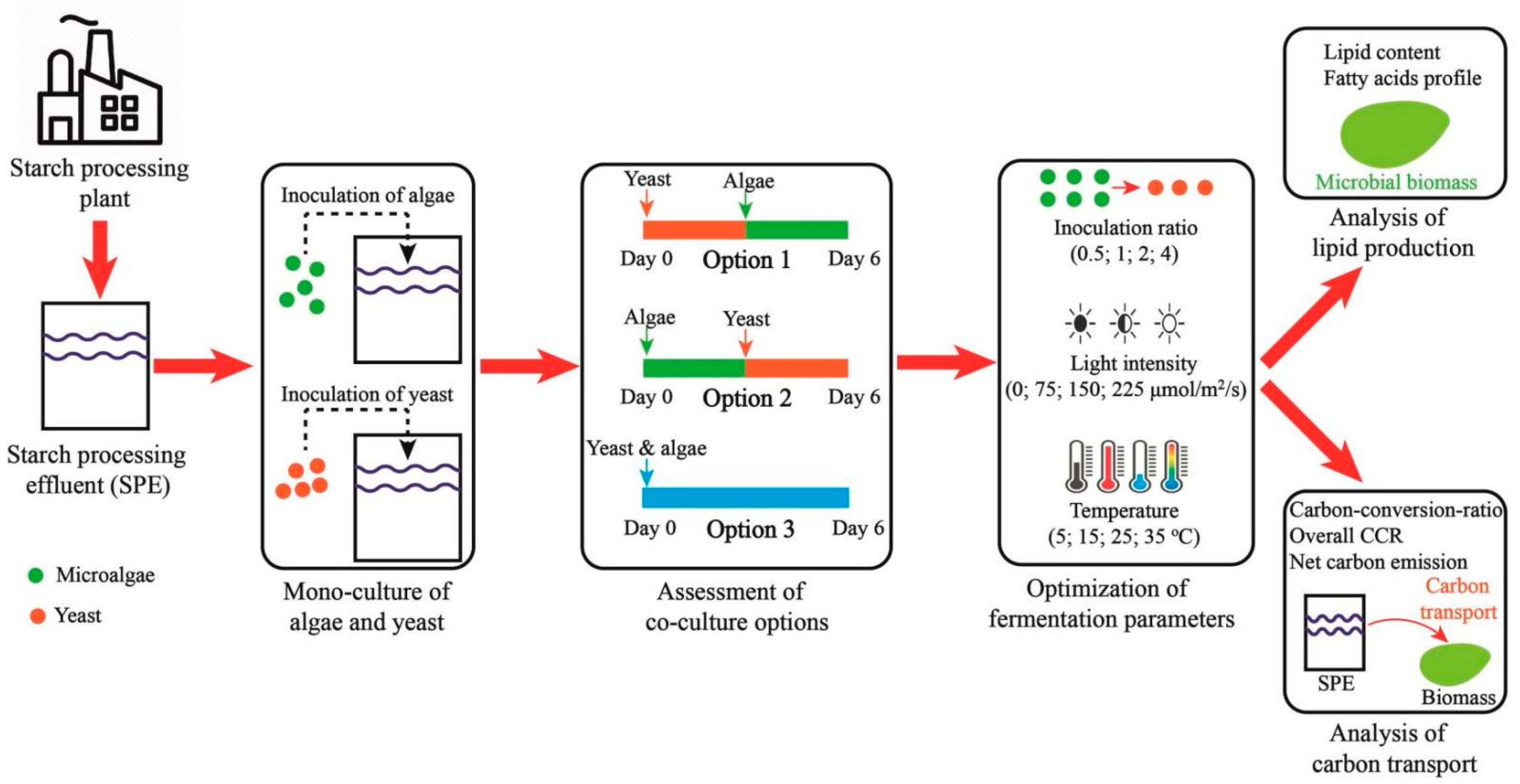
2.2. Experimental Design
2.3. Monoculture of Microorganism in the SPE
2.4. Assessment of Co-Culture Options
2.5. Optimization of Critical Fermentation Parameters
2.6. Parameters Analysis
2.6.1. Water Quality Analysis
2.6.2. Biomass Yield Measurement
2.6.3. Biomass Component Analysis
2.6.4. Estimation of Carbon Transport in the SPE-Based Fermentation
3. Results
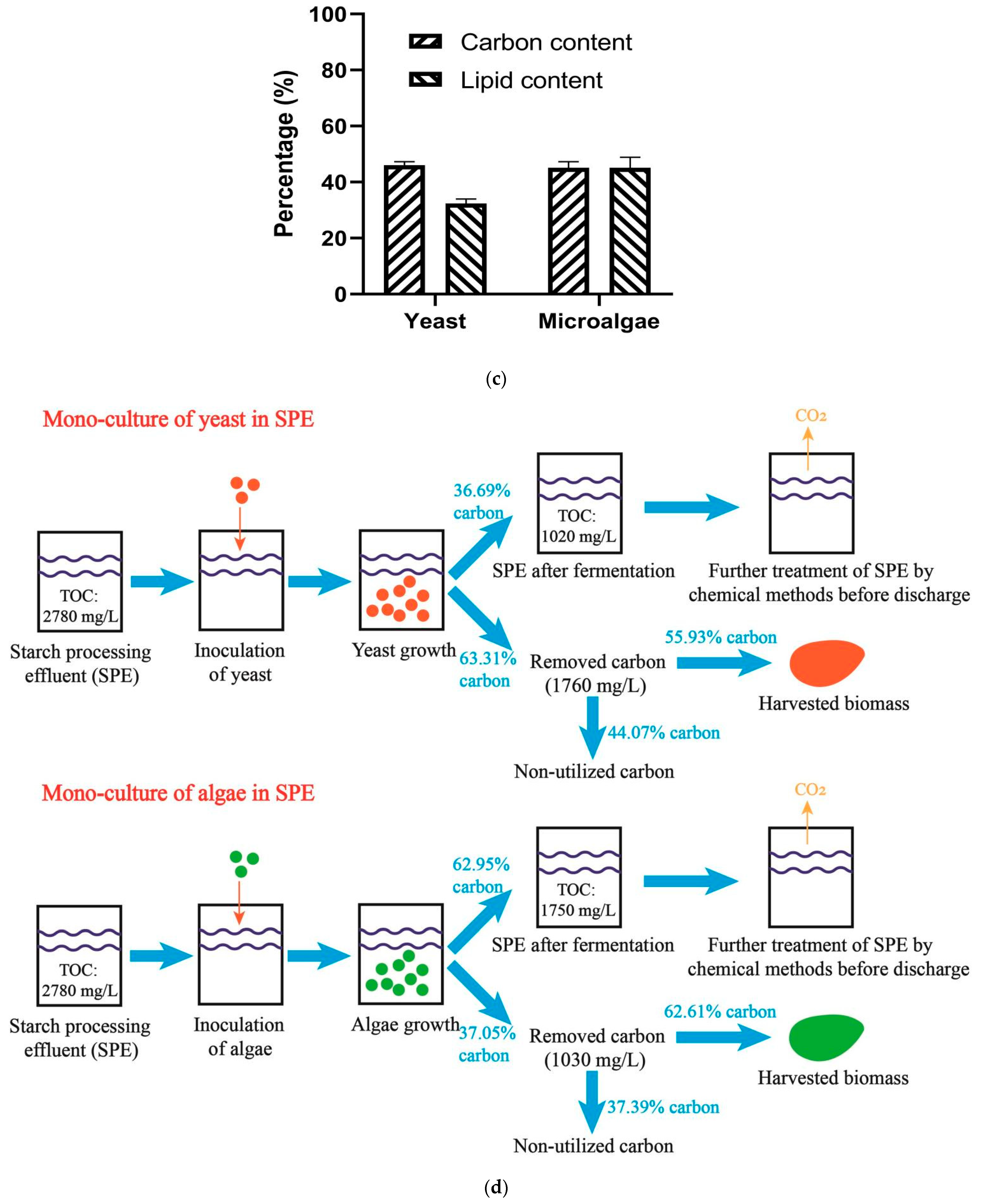
3.1. Monoculture of Yeast and Algae
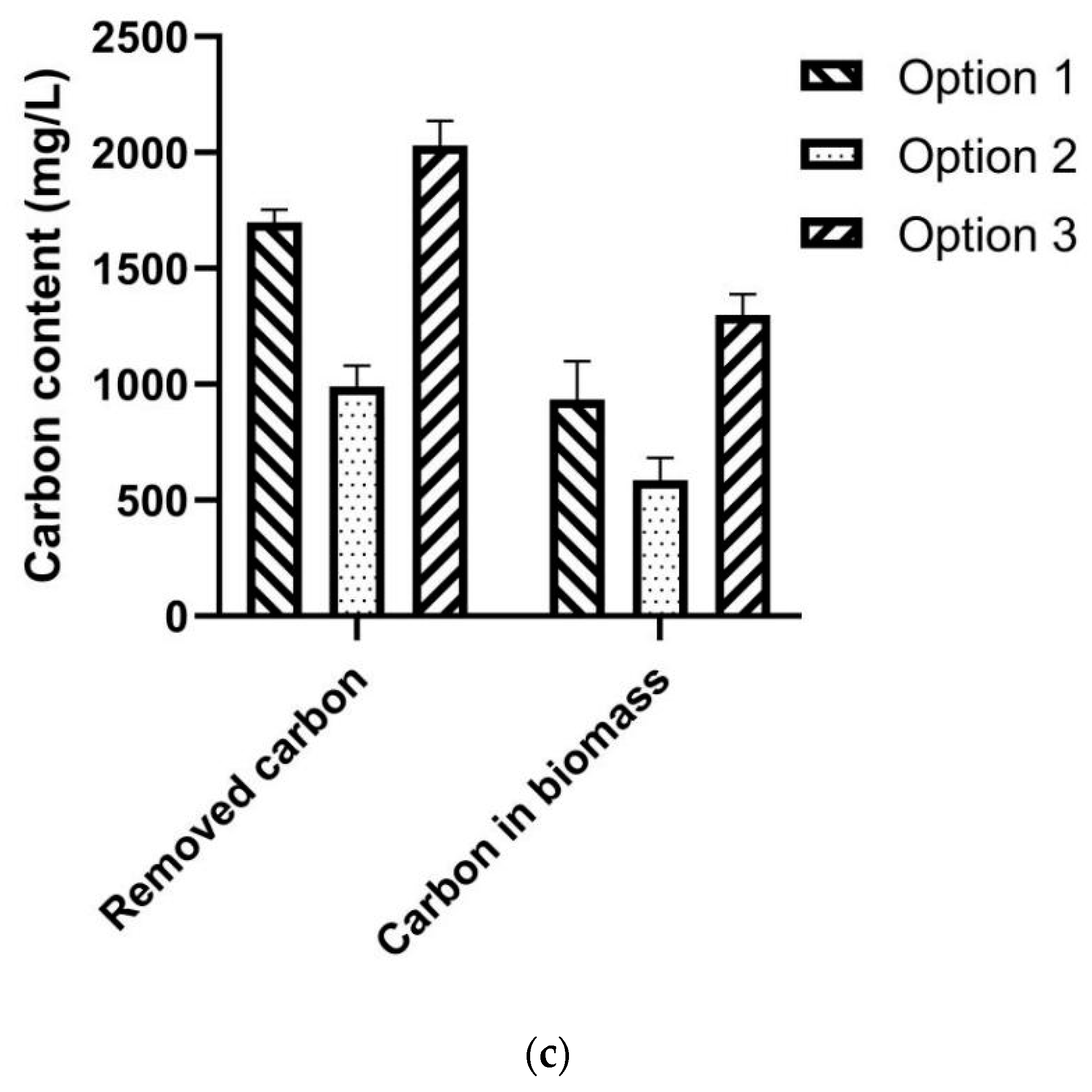
3.2. Assessment of Three Co-Culture Options
3.3. Evaluation of Critical Factors
3.3.1. Inoculation Ratio
3.3.2. Light Intensity
3.3.3. Temperature
3.4. Biomass for Biofuel Production
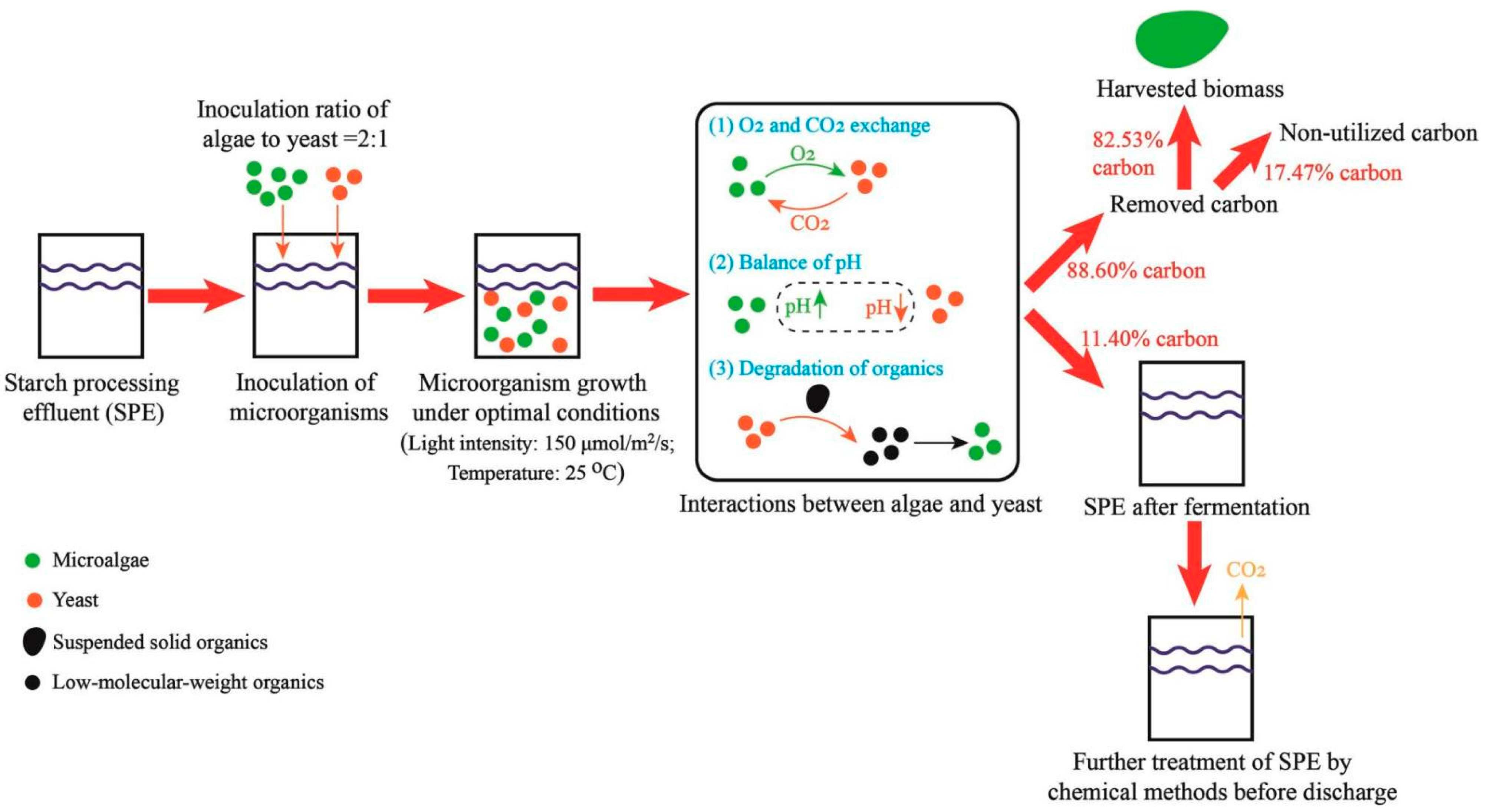
3.5. Assessment of Carbon Emission of the SPE-Based Fermentation
4. Discussion
4.1. Monoculture of Microorganism for the SPE-Based Fermentation
4.2. Interactions between Microalgae and Yeast in Co-Culture System
4.3. Utilization of Microbial Biomass for Biodiesel Production
4.4. Reduction of Carbon Emission of the SPE-Based Fermentation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.; Wu, X.; Zhong, Y.; Lu, Q.; Zhou, W. The 10th Asia-Pacific conference on algal biotechnology: Thoughts and comments. J. Clean. Prod. 2020, 264, 121626. [Google Scholar] [CrossRef]
- Kumar, M.; Sundaram, S.; Gnansounou, E.; Larroche, C.; Thakur, I.S. Carbon dioxide capture, storage and production of biofuel and biomaterials by bacteria: A review. Bioresour. Technol. 2018, 247, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Correa, D.F.; Beyer, H.L.; Fargione, J.E.; Hill, J.D.; Possingham, H.P.; Thomas-Hall, S.R.; Schenk, P.M. Towards the implementation of sustainable biofuel production systems. Renew. Sustain. Energy Rev. 2019, 107, 250–263. [Google Scholar] [CrossRef]
- Aurtherson, P.B.; Nalla, B.T.; Srinivasan, K.; Mehar, K.; Devarajan, Y. Biofuel production from novel Prunus domestica kernel oil: Process optimization technique. Biomass Convers. Bior. 2021, 1–7. [Google Scholar] [CrossRef]
- Alalwan, H.A.; Alminshid, A.H.; Aljaafari, H.A.S. Promising evolution of biofuel generations. Subject review. Renew. Energy Focus 2019, 28, 127–139. [Google Scholar] [CrossRef]
- Bonatsos, N.; Marazioti, C.; Moutousidi, E.; Anagnostou, A.; Koutinas, A.; Kookos, I.K. Techno-economic analysis and life cycle assessment of heterotrophic yeast-derived single cell oil production process. Fuel 2020, 264, 116839. [Google Scholar] [CrossRef]
- Spalvins, K.; Vamza, I.; Blumberga, D. Single cell oil production from waste biomass: Review of applicable industrial by-products. Environ. Clim. Technol. 2019, 23, 325–337. [Google Scholar] [CrossRef]
- Mhlongo, S.I.; Ezeokoli, O.T.; Roopnarain, A.; Ndaba, B.; Sekoai, P.T.; Habimana, O.; Pohl, C.H. The potential of single-cell oils derived from filamentous fungi as alternative feedstock sources for biodiesel production. Front. Microbiol. 2021, 12, 637381. [Google Scholar] [CrossRef]
- Ling, J.; Nip, S.; Cheok, W.L.; de Toledo, R.A.; Shim, H. Lipid production by a mixed culture of oleaginous yeast and microalga from distillery and domestic mixed wastewater. Bioresour. Technol. 2014, 173, 132–139. [Google Scholar] [CrossRef]
- Zhang, C.; Li, F.; Ho, S.-H.; Chen, W.-H.; Gunarathne, D.S.; Show, P.L. Oxidative torrefaction of microalga Nannochloropsis Oceanica activated by potassium carbonate for solid biofuel production. Environ. Res. 2022, 212, 113389. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Sources of microbial oils with emphasis to Mortierella (Umbelopsis) isabellina fungus. World J. Microbiol. Biotechn. 2019, 35, 63. [Google Scholar] [CrossRef] [PubMed]
- Lal, A.; Banerjee, S.; Das, D. Aspergillus sp. assisted bioflocculation of Chlorella MJ 11/11 for the production of biofuel from the algal-fungal co-pellet. Sep. Purif. Technol. 2021, 272, 118320. [Google Scholar] [CrossRef]
- Tossavainen, M.; Lahti, K.; Edelmann, M.; Eskola, R.; Lampi, A.-M.; Piironen, V.; Korvonen, P.; Ojala, A.; Romantschuk, M. Integrated utilization of microalgae cultured in aquaculture wastewater: Wastewater treatment and production of valuable fatty acids and tocopherols. J. Appl. Phycol. 2019, 31, 1753–1763. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Wu, B.; Pisutpaisal, N.; Wang, Y.W.; Ma, K.D.; Dai, L.C.; Qin, H.; Tan, F.-R.; Maeda, T.; Xu, Y. Bioenergy from dairy manure: Technologies, challenges and opportunities. Sci. Total Environ. 2021, 790, 148199. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Zhou, W.; Min, M.; Ma, X.; Chandra, C.; Doan, Y.T.T.; Ma, Y.; Zheng, H.; Cheng, S.; Griffith, R. Growing Chlorella sp. on meat processing wastewater for nutrient removal and biomass production. Bioresour. Technol. 2015, 198, 189–197. [Google Scholar] [CrossRef]
- Tan, X.B.; Zhao, X.C.; Yang, L.B. Strategies for enhanced biomass and lipid production by Chlorella pyrenoidosa culture in starch processing wastewater. J. Clean. Prod. 2019, 236, 117671. [Google Scholar] [CrossRef]
- Tung, T.Q.; Miyata, N.; Iwahori, K. Growth of Aspergillus oryzae during treatment of cassava starch processing wastewater with high content of suspended solids. J. Biosci. Bioeng. 2004, 97, 329–335. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, X.; Jin, M.; Chen, H.; Yi, F.; Wang, L.; Qiao, N.; Yu, D. Incorporating corn oil refining wastewater improves lipid accumulation and self-settling property of Trichosporon fermentans in corn starch wastewater. Sep. Purif. Technol. 2021, 275, 119250. [Google Scholar] [CrossRef]
- Hussain, F.; Shah, S.Z.; Ahmad, H.; Abubshait, S.A.; Abubshait, H.A.; Laref, A.; Manikandan, A.; Kusuma, H.S.; Iqbal, M. Microalgae an ecofriendly and sustainable wastewater treatment option: Biomass application in biofuel and bio-fertilizer production. A review. Renew. Sustain. Energy Rev. 2021, 137, 110603. [Google Scholar] [CrossRef]
- Lu, Q.; Zhou, W.; Min, M.; Ma, X.; Ma, Y.; Chen, P.; Zheng, H.; Doan, Y.T.T.; Liu, H.; Chen, C. Mitigating ammonia nitrogen deficiency in dairy wastewaters for algae cultivation. Bioresour. Technol. 2016, 201, 33–40. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, X.; Zhou, W.; Min, M.; Cheng, Y.; Chen, P.; Shi, J.; Wang, Q.; Liu, Y.; Ruan, R. Oil crop biomass residue-based media for enhanced algal lipid production. Appl. Biochem. Biotechnol. 2013, 171, 689–703. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, S.; Liao, K.; Lu, Q.; Zhou, W. Microalgae biotechnology as a promising pathway to eco-friendly aquaculture: A state-of-the-art review. J. Chem. Technol. Biotechnol. 2021, 96, 837–852. [Google Scholar] [CrossRef]
- Rane, D.V.; Pawar, P.P.; Odaneth, A.A.; Lali, A.M. Microbial oil production by the oleaginous red yeast, Rhodotorula glutinis NCIM 3168, using corncob hydrolysate. Biomass Convers. Bior. 2021, 13, 1987–1997. [Google Scholar] [CrossRef]
- Jeon, Y.C.; Cho, C.W.; Yun, Y.-S. Measurement of microalgal photosynthetic activity depending on light intensity and quality. Biochem. Eng. J. 2005, 27, 127–131. [Google Scholar] [CrossRef]
- Bazdar, E.; Roshandel, R.; Yaghmaei, S.; Mardanpour, M.M. The effect of different light intensities and light/dark regimes on the performance of photosynthetic microalgae microbial fuel cell. Bioresour. Technol. 2018, 261, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Abreu, A.P.; Morais, R.C.; Teixeira, J.A.; Nunes, J. A comparison between microalgal autotrophic growth and metabolite accumulation with heterotrophic, mixotrophic and photoheterotrophic cultivation modes. Renew. Sustain. Energy Rev. 2022, 159, 112247. [Google Scholar] [CrossRef]
- Adesanya, V.O.; Davey, M.P.; Scott, S.A.; Smith, A.G. Kinetic modelling of growth and storage molecule production in microalgae under mixotrophic and autotrophic conditions. Bioresour. Technol. 2014, 157, 293–304. [Google Scholar] [CrossRef]
- Ramos, M.J.; Fernández, C.M.; Casas, A.; Rodríguez, L.; Pérez, Á. Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour. Technol. 2009, 100, 261–268. [Google Scholar] [CrossRef]
- Yu, D.; Wang, X.; Fan, X.; Ren, H.; Hu, S.; Wang, L.; Shi, Y.; Liu, N.; Qiao, N. Refined soybean oil wastewater treatment and its utilization for lipid production by the oleaginous yeast Trichosporon fermentans. Biotechnol. Biofuels 2018, 11, 299. [Google Scholar] [CrossRef]
- Wu, Y.H.; Hu, H.Y.; Yu, Y.; Zhang, T.Y.; Zhu, S.F.; Zhuang, L.L.; Zhang, X.; Lu, Y. Microalgal species for sustainable biomass/lipid production using wastewater as resource: A review. Renew. Sustain. Energy Rev. 2014, 33, 675–688. [Google Scholar] [CrossRef]
- Chiu, S.Y.; Kao, C.Y.; Chen, T.Y.; Chang, Y.B.; Kuo, C.M.; Lin, C.S. Cultivation of microalgal Chlorella for biomass and lipid production using wastewater as nutrient resource. Bioresour. Technol. 2015, 184, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Guadalupe-Daqui, M.; Goodrich-Schneider, R.M.; Sarnoski, P.J.; Carriglio, J.C.; Sims, C.A.; Pearson, B.J.; MacIntosh, A.J. The effect of CO2 concentration on yeast fermentation: Rates, metabolic products, and yeast stress indicators. J. Ind. Microbiol. Biotechnol. 2023, 50, kuad001. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Yang, S.; Agboyibor, C.; Chen, D.; Zhang, A.; Niu, S. Light irradiation can regulate the growth characteristics and metabolites compositions of Rhodotorula mucilaginosa. J. Food Sci. Technol. 2019, 56, 5509–5517. [Google Scholar] [CrossRef]
- Liu, X.; Jia, B.; Sun, X.; Ai, J.; Wang, L.; Wang, C.; Zhao, F.; Zhan, J.; Huang, W. Effect of initial pH on growth characteristics and fermentation properties of Saccharomyces cerevisiae. J. Food Sci. 2015, 80, M800–M808. [Google Scholar] [CrossRef] [PubMed]
- Mafakher, L.; Mirbagheri, M.; Darvishi, F.; Nahvi, I.; Zarkesh-Esfahani, H.; Emtiazi, G. Isolation of lipase and citric acid producing yeasts from agro-industrial wastewater. New Biotechnol. 2010, 27, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Walls, L.E.; Velasquez-Orta, S.B.; Romero-Frasca, E.; Leary, P.; Noguez, I.Y.; Ledesma, M.T.O. Non-sterile heterotrophic cultivation of native wastewater yeast and microalgae for integrated municipal wastewater treatment and bioethanol production. Biochem. Eng. J. 2019, 151, 107319. [Google Scholar] [CrossRef]
- Motto, S.A.; Christwardana, M. Potency of Yeast–Microalgae Spirulina Collaboration in Microalgae-Microbial Fuel Cells for Cafeteria Wastewater Treatment; IOP Publishing: Bristol, UK, 2018; p. 012022. [Google Scholar]
- Lu, Q.; Ji, C.; Yan, Y.; Xiao, Y.; Li, J.; Leng, L.; Zhou, W. Application of a novel microalgae-film based air purifier to improve air quality through oxygen production and fine particulates removal. J. Chem. Technol. Biotechnol. 2019, 94, 1057–1063. [Google Scholar] [CrossRef]
- Yang, L.; Li, H.; Liu, T.; Zhong, Y.; Ji, C.; Lu, Q.; Fan, L.; Li, J.; Leng, L.; Li, K. Microalgae biotechnology as an attempt for bioregenerative life support systems: Problems and prospects. J. Chem. Technol. Biotechnol. 2019, 94, 3039–3048. [Google Scholar] [CrossRef]
- Zhang, Z.; Ji, H.; Gong, G.; Zhang, X.; Tan, T. Synergistic effects of oleaginous yeast Rhodotorula glutinis and microalga Chlorella vulgaris for enhancement 2005of biomass and lipid yields. Bioresour. Technol. 2014, 164, 93–99. [Google Scholar] [CrossRef]
- Moheimani, N.R.; Borowitzka, M.A. Limits to productivity of the alga Pleurochrysis carterae (Haptophyta) grown in outdoor raceway ponds. Biotechnol. Bioeng. 2007, 96, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Leng, Y.; Lu, Q.; Zhou, W. The application of microalgae biomass and bio-products as aquafeed for aquaculture. Algal Res. 2021, 60, 102541. [Google Scholar] [CrossRef]
- Arora, N.; Patel, A.; Mehtani, J.; Pruthi, P.A.; Pruthi, V.; Poluri, K.M. Co-culturing of oleaginous microalgae and yeast: Paradigm shift towards enhanced lipid productivity. Environ. Sci. Pollut. Res. 2019, 26, 16952–16973. [Google Scholar] [CrossRef] [PubMed]
- Zuccaro, G.; Steyer, J.P.; van Lis, R. The algal trophic mode affects the interaction and oil production of a synergistic microalga-yeast consortium. Bioresour. Technol. 2019, 273, 608–617. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.; Lu, Q.; Zhou, W. Toxicity alleviation for microalgae cultivation by cationic starch addition and ammonia stripping and study on the cost assessment. RSC Adv. 2019, 9, 38235–38245. [Google Scholar] [CrossRef]
- Fallahi, A.; Rezvani, F.; Asgharnejad, H.; Nazloo, E.K.; Hajinajaf, N.; Higgins, B. Interactions of microalgae-bacteria consortia for nutrient removal from wastewater: A review. Chemosphere 2021, 272, 129878. [Google Scholar] [CrossRef]
- Tienungoon, S.; Ratkowsky, D.A.; McMeekin, T.A.; Ross, T. Growth limits of Listeria monocytogenes as a function of temperature, pH, NaCl, and lactic acid. Appl. Environ. Microb. 2000, 66, 4979–4987. [Google Scholar] [CrossRef]
- Qiu, R.; Gao, S.; Lopez, P.A.; Ogden, K.L. Effects of pH on cell growth, lipid production and CO2 addition of microalgae Chlorella sorokiniana. Algal Res. 2017, 28, 192–199. [Google Scholar] [CrossRef]
- Kraft, E.; Oliveira Filho, L.C.I.D.; Carneiro, M.C.; Klauberg-Filho, O.; Baretta, C.R.D.M.; Baretta, D. Edaphic fauna affects soybean productivity under no-till system. Sci. Agric. 2020, 78, e20190137. [Google Scholar] [CrossRef]
- Vasistha, S.; Khanra, A.; Clifford, M.; Rai, M.P. Current advances in microalgae harvesting and lipid extraction processes for improved biodiesel production: A review. Renew. Sustain. Energy Rev. 2021, 137, 110498. [Google Scholar] [CrossRef]
- Giakoumis, E.G.; Sarakatsanis, C.K. A comparative assessment of biodiesel cetane number predictive correlations based on fatty acid composition. Energies 2019, 12, 422. [Google Scholar] [CrossRef]




| Parameter | Value Range | Parameter | Value Range |
|---|---|---|---|
| TOC (mg/L) | 2680–2820 | TAN (mg/L) | 118.4–137.5 |
| COD (mg/L) | 9210–10,880 | SS (g/L) | 0.58–0.61 |
| TN (mg/L) | 228–281 | pH | 4.6–4.9 |
| TP (mg/L) | 25.7–29.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Q.; Ma, C.; Guo, L.; Lu, Y.; Li, H. Co-Fermentation of Chlorella vulgaris with Oleaginous Yeast in Starch Processing Effluent as a Carbon-Reducing Strategy for Wastewater Treatment and Biofuel Feedstock Production. Fermentation 2023, 9, 476. https://doi.org/10.3390/fermentation9050476
Lu Q, Ma C, Guo L, Lu Y, Li H. Co-Fermentation of Chlorella vulgaris with Oleaginous Yeast in Starch Processing Effluent as a Carbon-Reducing Strategy for Wastewater Treatment and Biofuel Feedstock Production. Fermentation. 2023; 9(5):476. https://doi.org/10.3390/fermentation9050476
Chicago/Turabian StyleLu, Qian, Chunyang Ma, Lei Guo, Yujie Lu, and Huankai Li. 2023. "Co-Fermentation of Chlorella vulgaris with Oleaginous Yeast in Starch Processing Effluent as a Carbon-Reducing Strategy for Wastewater Treatment and Biofuel Feedstock Production" Fermentation 9, no. 5: 476. https://doi.org/10.3390/fermentation9050476
APA StyleLu, Q., Ma, C., Guo, L., Lu, Y., & Li, H. (2023). Co-Fermentation of Chlorella vulgaris with Oleaginous Yeast in Starch Processing Effluent as a Carbon-Reducing Strategy for Wastewater Treatment and Biofuel Feedstock Production. Fermentation, 9(5), 476. https://doi.org/10.3390/fermentation9050476







