Effect of Flaxseed Mucilage on the Probiotic, Antioxidant, and Structural-Mechanical Properties of the Different Lactobacillus Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains, Mediums, Flaxseed Mucilage
2.2. Incubation of LAB with Flaxseed Mucilage, LAB Enumeration, and Preparation of the Cell-Free Supernatants
2.3. General Probiotic Properties In Vitro
2.3.1. Cell Resistance to 3 and 7% NaCl, Acid, Bile Salts
2.3.2. Cell Resistance to Simulated Gastrointestinal Tract (GIT) Conditions
2.3.3. Antibiotic Resistance
2.4. Bacterial Surface Properties
2.5. Titratable Acidity and Total Phenolic Compounds (TPCs)
2.6. Antioxidant Activity of Cell-Free Supernatants of MRS and MWNM
2.6.1. Evaluation of Radical-Scavenging Ability (RSA) by 2,2-Di-phenyl-1-picrylhydrazyl (DPPH) Assay
2.6.2. Ferric-Reducing Antioxidant Power Assay (FRAP)
2.6.3. HO Free Radical Scavenging Ability
2.6.4. Fe-Chelating Activity
2.7. In Vitro Lipase Inhibition Assay
2.8. In Vitro α-Glucosidase Inhibition Assay
2.9. Scanning Probe Microscopy (SPM)
2.10. Statistical Analysis
3. Results
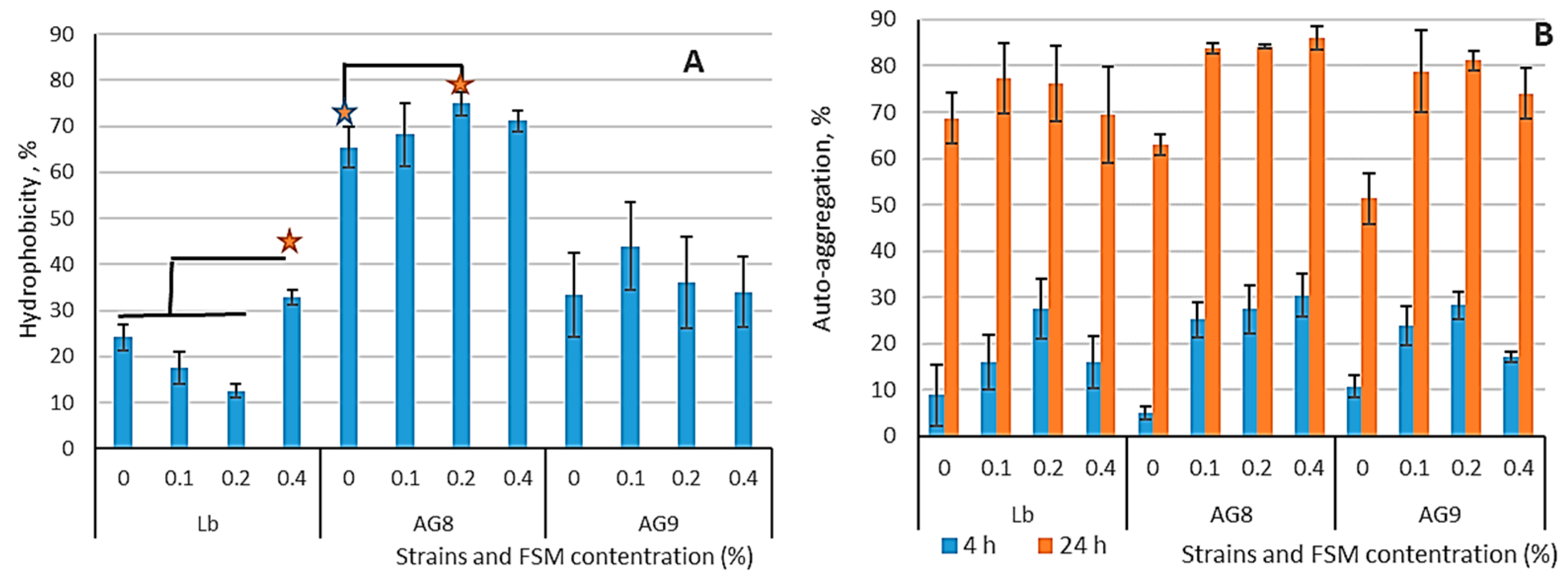
3.1. Hydrophobicity and Auto-Aggregation of LABs Cells
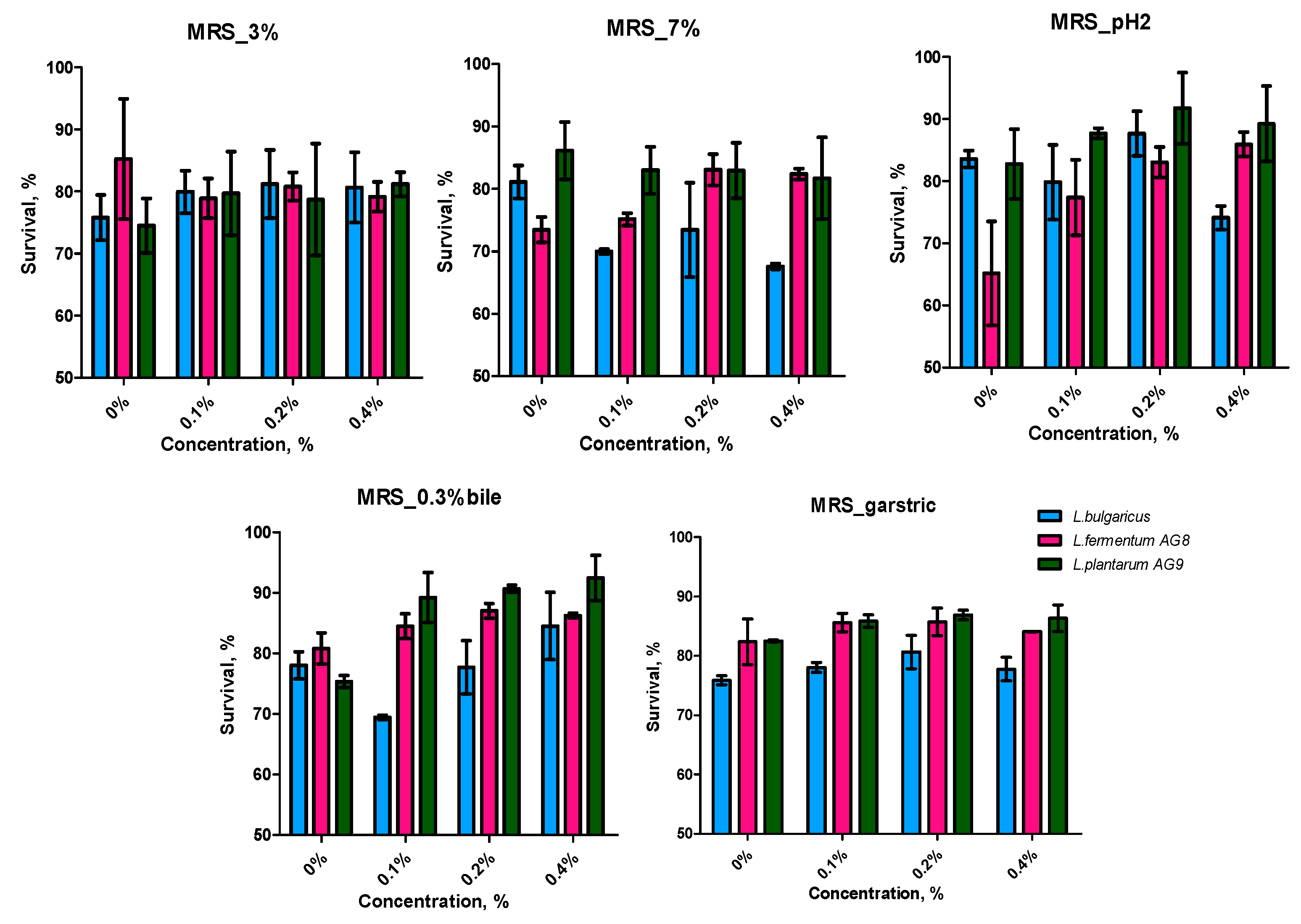
3.2. Characteristics of Probiotic Properties of LAB at Cultuvation on a MRS
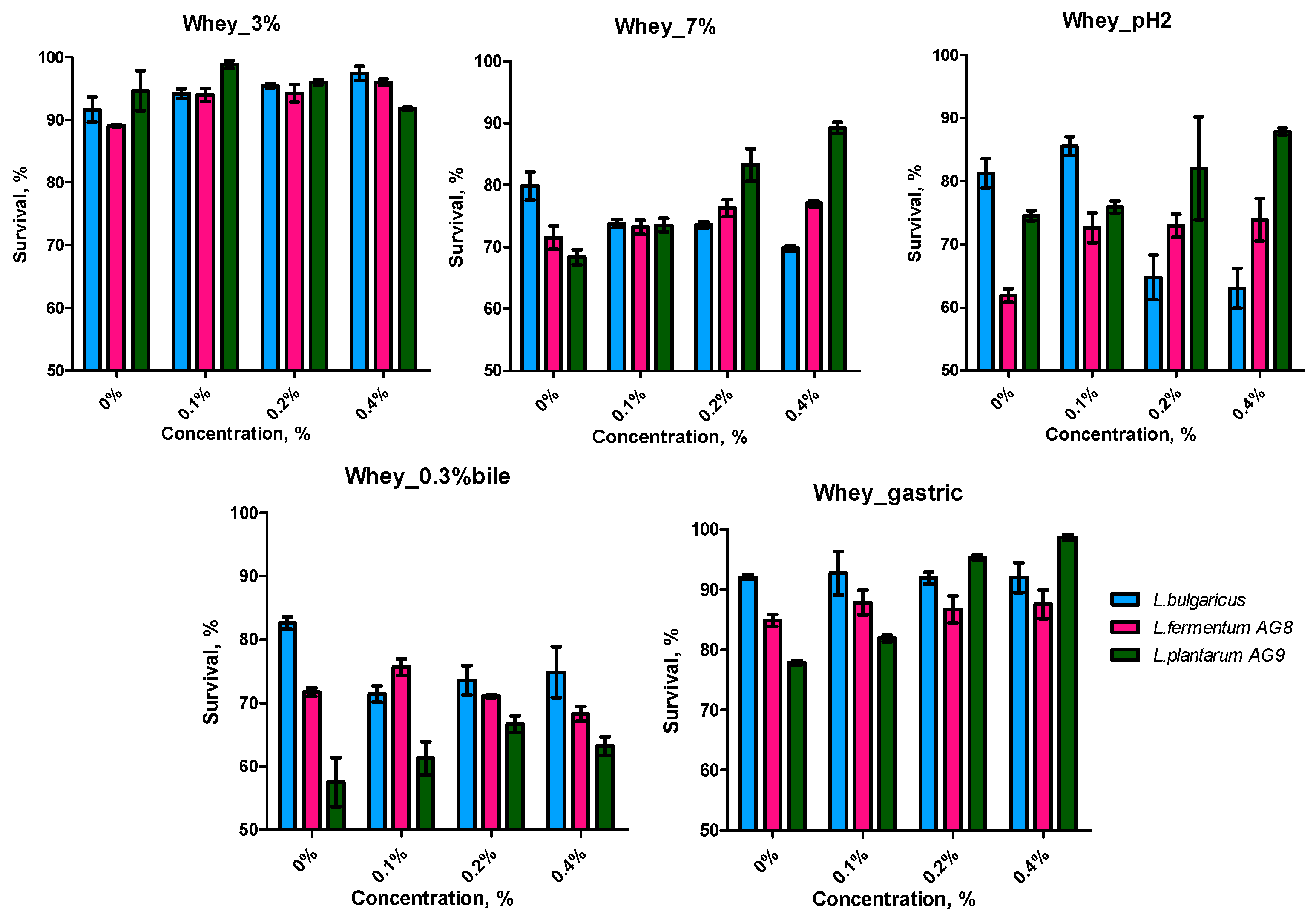
3.3. Characteristics of Probiotic Properties of LAB during Cultuvation on a Milk Whey Broth
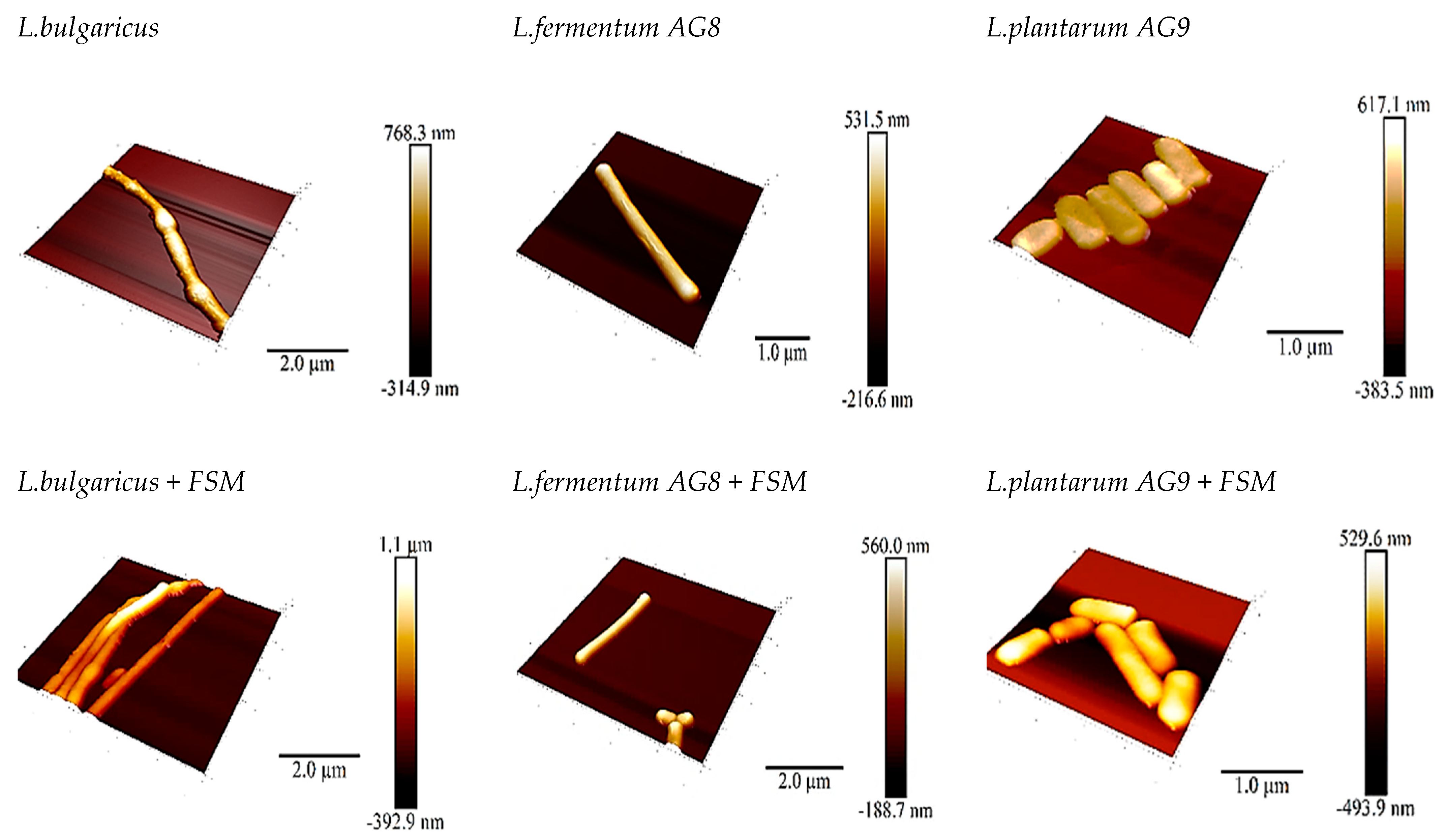
3.4. Scanning Probe Microscopy (SPM)
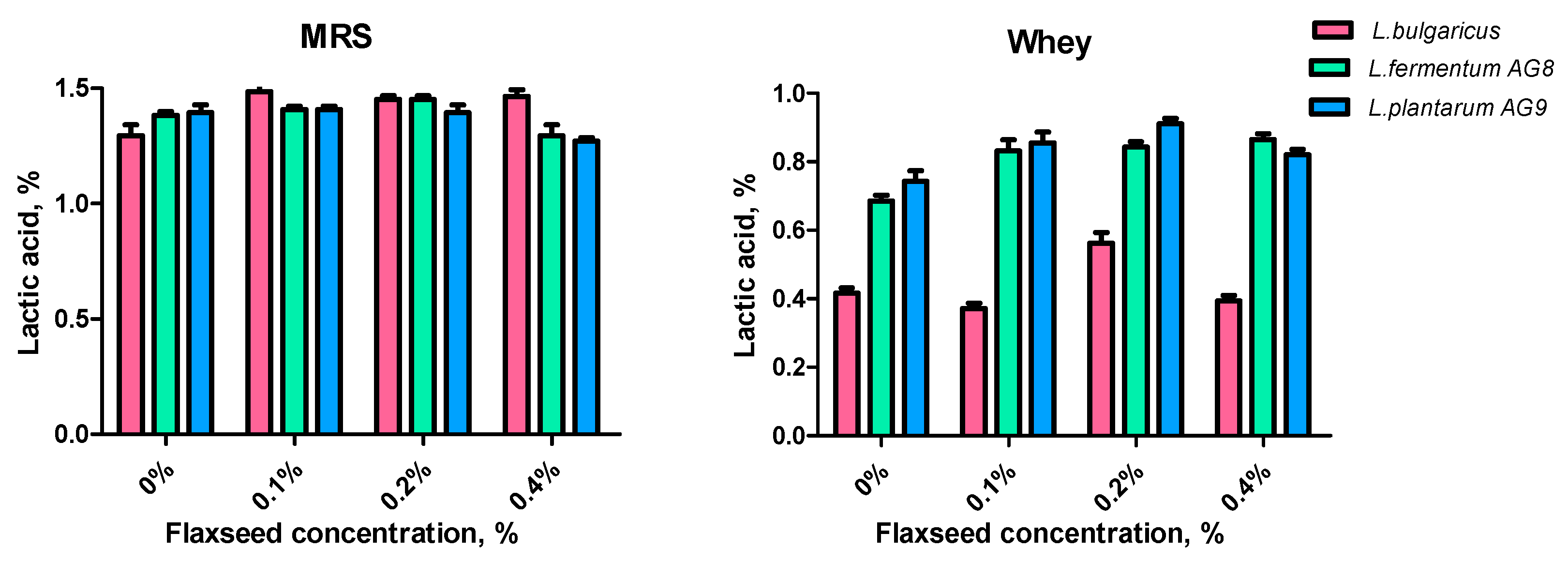
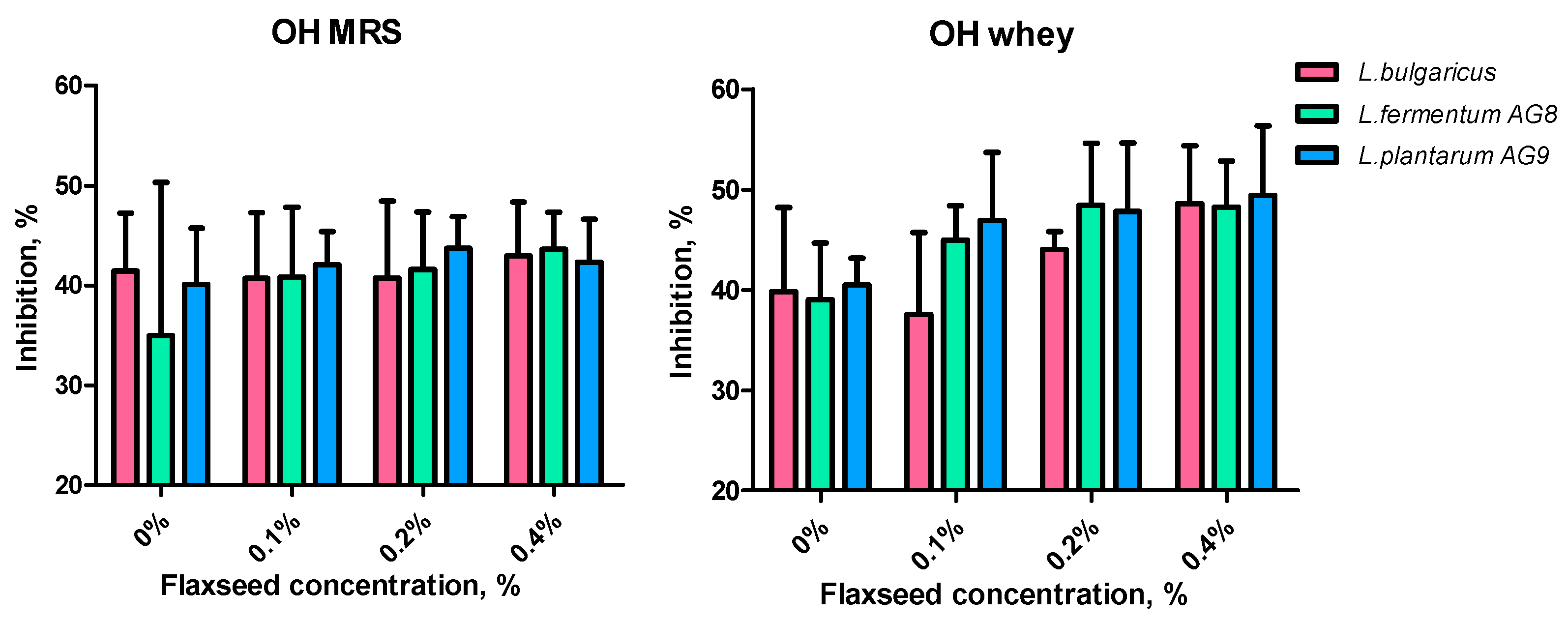
3.5. Effect of FSM on Lactic Acid Synthesis and Antioxidant Properties
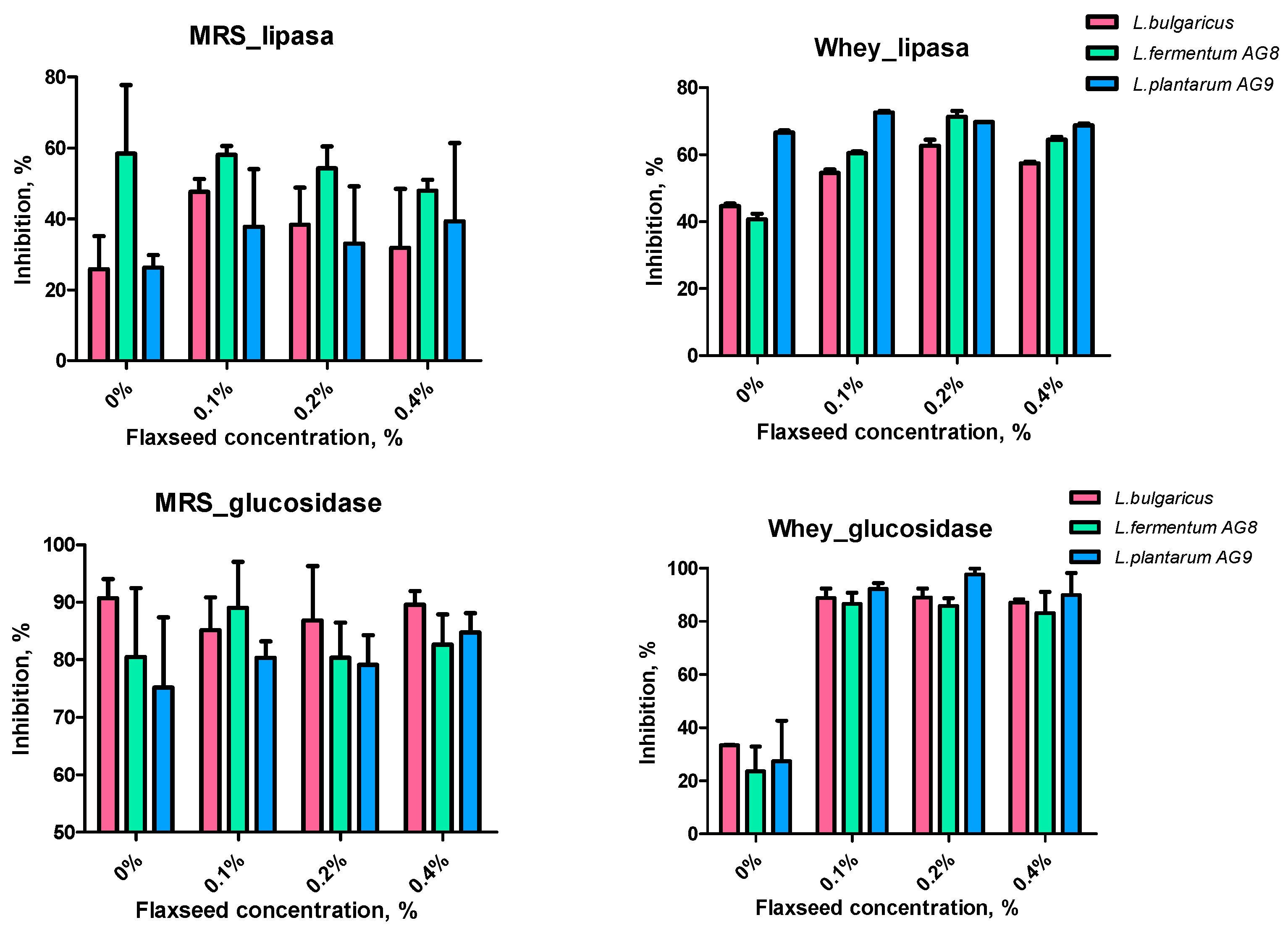
3.6. Effect of FSM on Enzyme Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Q.; Gänzle, M.G. Host-adapted lactobacilli in food fermentations: Impact of metabolic traits of host adapted lactobacilli on food quality and human health. Curr. Opin. Food Sci. 2020, 31, 71–80. [Google Scholar] [CrossRef]
- Gavrilova, E.; Anisimova, E.; Gabdelkhadieva, A.; Nikitina, E.; Vafina, A.; Yarullina, D.; Bogachev, M.; Kayumov, A. Newly isolated lactic acid bacteria from silage targeting biofilms of foodborne pathogens during milk fermentation. BMC Microbiol. 2019, 19, 248. [Google Scholar] [CrossRef] [PubMed]
- Nikitina, E.; Petrova, T.; Vafina, A.; Ezhkova, A.; Yahia, M.N.; Kayumov, A. Textural and functional properties of skimmed and whole milk fermented by novel Lactiplantibacillus plantarum AG10 strain isolated from silage. Fermentation 2022, 8, 290. [Google Scholar] [CrossRef]
- Holzapfel, W.H.; Shulinger, U. Introduction to pre- and probiotics. Food Res. Int. 2002, 35, 125–129. [Google Scholar] [CrossRef]
- Charteris, W.P.; Kelly, P.M.; Morelli, L.; Collins, J.K. Development and application of an in vitro methodology to determine the transit tolerance of potentially probiotic Lactobacillus and Bifidobacterium species in the upper human gastrointestinal tract. J. Appl. Microbiol. 1998, 84, 759–768. [Google Scholar] [CrossRef]
- Conway, P.L.; Gorbach, S.L.; Goldin, B.R. Survival of lactic acid bacteria in the human stomach and adhesion to intestinal cells. J. Dairy Sci. 1987, 70, 1–12. [Google Scholar] [CrossRef]
- Champomier Vergès, M.-C.; Zuñiga, M.; Morel-Deville, F.; Peréz-Martínez, G.; Zagorec, M.; Ehrlich, S.D. Relationships between arginine degradation, pH and survival in Lactobacillus sakei. FEMS Microbiol. Lett. 1999, 180, 297–304. [Google Scholar] [CrossRef]
- Capozzi, V.; Arena, M.P.; Russo, P.; Spano, G.; Fiocco, D. Stressors and food environment: Toward strategies to improve robustness and stress tolerance in probiotics. In Probiotics, Prebiotics, and Synbiotics; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2016; Chapter 16; pp. 245–256. [Google Scholar]
- Peredo, A.G.; Beristain, C.I.; Pascual, L.A.; Azuara, E.; Jimenez, M. The effect of prebiotics on the viability of encapsulated probiotic bacteria. LWT-Food Sci. Technol. 2016, 73, 191–196. [Google Scholar] [CrossRef]
- Mueller, M.; Cavarkapa, A.; Unger, F.M.; Viernstein, H.; Praznik, W. Prebiotic potential of neutral oligo- and polysaccharides from seed mucilage of Hyptis suaveolens. Food Chem. 2017, 221, 508–514. [Google Scholar] [CrossRef]
- Gannasin, S.P.; Mustafa, S.; Adzahan, N.M.; Muhammad, K. In vitro prebiotic activities of tamarillo (Solanum betaceum Cav.) hydrocolloids. J. Funct. Foods 2015, 19, 10–19. [Google Scholar] [CrossRef]
- Bustamante, M.; Villarroel, M.; Rubilar, M.; Shene, C. Lactobacillus acidophilus La-05 encapsulated by spray drying: Effect of mucilage and protein from flaxseed (Linum usitatissimum L.). LWT-Food Sci. Technol. 2015, 62, 1162–1168. [Google Scholar] [CrossRef]
- Bustamante, M.; Oomah, B.D.; Rubilar, M.; Shene, C. Effective Lactobacillus plantarum and Bifidobacterium infantis encapsulation with chia seed (Salvia hispanica L.) and flaxseed (Linum usitatissimum L.) mucilage and soluble protein by spray drying. Food Chem. 2017, 216, 97–105. [Google Scholar] [CrossRef]
- Muralikrishna, G.; Salimath, P.V.; Tharanathan, R.N. Structural features of an arabinoxylan and a rhamno-galacturonan derived from linseed mucilage. Carbohydr. Res. 1987, 161, 265–271. [Google Scholar] [CrossRef]
- Cui, W.; Mazza, G.; Biliaderis, C.G. Chemical structure, molecular size distribution and rheological properties of flaxseed gum. J. Agric. Food Chem. 1994, 42, 1891–1895. [Google Scholar] [CrossRef]
- Naran, R.; Chen, G.; Carpita, N.C. Novel rhamnogalacturonan I and arabinoxylan polysaccharides of flax seed mucilage. Plant Physiol. 2008, 148, 132–141. [Google Scholar] [CrossRef]
- Ding, H.H.; Qian, K.Y.; Goff, H.D.; Wang, Q.; Cui, S.W. Structural and conformational characterization of arabinoxylans from flaxseed mucilage. Food Chem. 2018, 254, 266–271. [Google Scholar] [CrossRef]
- Western, T.L. The sticky tale of seed coat mucilages: Production, genetics, and role in seed germination and dis-persal. Seed Sci. Res. 2011, 22, 1–25. [Google Scholar] [CrossRef]
- Qian, K.Y.; Cui, S.W.; Wu, Y.; Goff, H.D. Flaxseed gum from flaxseed hulls: Extraction, fractionation, and characterization. Food Hydrocoll. 2012, 28, 275–283. [Google Scholar] [CrossRef]
- Čukelj, N.; Novotni, D.; Sarajlija, H.; Drakula, S.; Voučko, B.; Ćurić, D. Flaxseed and multigrain mixtures in the development of functional biscuits. LWT 2017, 86, 85–92. [Google Scholar] [CrossRef]
- Wang, Y.; Fofana, B.; Roy, M.; Ghose, K.; Yao, X.H.; Nixon, M.S.; Nair, S.; Nyomba, G.B.L. Flaxseed lignan secoisolariciresinol diglucoside improves insulin sensitivity through upregulation of GLUT4 expression in diet-induced obese mice. J. Funct. Foods 2015, 18, 1–9. [Google Scholar] [CrossRef]
- Baba, W.N.; Jan, K.; Punoo, H.A.; Wani, T.A.; Dar, M.M.; Masoodi, F.A. Techno-functional properties of yoghurts fortified with walnut and flaxseed oil emulsions inguar gum. LWT 2018, 92, 242–249. [Google Scholar] [CrossRef]
- Veeramani, C.; Alsaif, M.A.; Al-Numair, K.S. Herbacetin, A flaxseed flavonoid, ameliorates high percent dietary fat induced insulin resistance and lipid accumulation through the regulation of hepatic lipid metabolizing and lipid-regulating enzymes. Chem. Biol. Interact. 2018, 288, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Courtois, J. Oligosaccharides from land plants and algae: Production and applications in therapeutics and biotechnology. Curr. Opin. Microbiol. 2009, 12, 261–273. [Google Scholar] [CrossRef] [PubMed]
- HadiNezhad, M.; Duc, C.; Han, N.; Farah, H. Flaxseed soluble dietary fibre enhances lactic acid bacterial survival and growth in kefir and possesses high antioxidant capacity. J. Food Res. 2013, 2, 152–163. [Google Scholar] [CrossRef]
- Alhssan, E.; Ercan, S.S.; Bozkurt, H. Effect of flaxseed mucilage and gum arabic on probiotic survival and quality of kefir during cold storage. Foods 2023, 12, 662. [Google Scholar] [CrossRef]
- Bali, V.; Panesar, P.S.; Bera, M.B.; Panesar, R. Fructo-oligosaccharides: Production, purification and potential applications. Crit. Rev. Food Sci. Nutr. 2015, 55, 1475–1490. [Google Scholar] [CrossRef]
- Liu, J.; Shim, Y.Y.; Shen, J.; Wang, Y.; Ghosh, S.; Reaney, M.J.T. Variation of composition and functional properties of gum from six Canadian flaxseed (Linum usitatissimum L.) cultivars. Int. J. Food Sci. Technol. 2016, 51, 2313–2326. [Google Scholar] [CrossRef]
- Fedeniuk, R.W.; Biliaderis, C.G. Composition and physicochemical properties of linseed (Linum usitatissimum L.) mucilage. J. Agric. Food Chem. 1994, 42, 240–247. [Google Scholar] [CrossRef]
- Chen, H.H.; Xu, S.Y.; Wang, Z. Gelation properties of flaxseed gum. J. Food Eng. 2006, 77, 295–303. [Google Scholar] [CrossRef]
- Khalloufi, S.; Corredig, M.; Goff, H.D.; Alexander, M. Flaxseed gums and their adsorption on whey protein-stabilized oil-in-wateremulsions. Food Hydrocoll. 2009, 23, 611–618. [Google Scholar] [CrossRef]
- Basiri, S.; Haidary, N.; Shekarforoush, S.S.; Niakousari, M. Flaxseed mucilage: A natural stabilizer in stirred yogurt. Carbohydr. Polym. J. 2018, 187, 59–65. [Google Scholar] [CrossRef]
- Jin, L.Z.; Ho, Y.W.; Abdullah, N.; Jalaludin, S. Acid and bile tolerance of Lactobacillus isolated from chicken intestine. Lett. Appl. Microbiol. 1998, 27, 183–185. [Google Scholar] [CrossRef]
- Ronka, E.; Malinen, E.; Saarela, M.; Rinta-Koski, M.; Aarnikunnas, J.; Palva, A. Probiotic and milk technological properties of Lactobacillus brevis. Int. J. Food Microbiol. 2003, 83, 63–70. [Google Scholar] [CrossRef]
- Nikitina, E.; Petrova, T.; Sungatullina, A.; Kharina, M.; Mikshina, P.; Gavrilova, E.; Kayumov, A. The profile of exopolysaccharides produced by various Lactobacillus species from silage during not-fat milk fermentation. Fermentation 2023, 9, 197. [Google Scholar] [CrossRef]
- Bao, Y.; Zhang, Y.; Zhang, Y.; Liu, Y.; Wang, S.; Dong, X.; Wang, Y.; Zhang, H. Screening of potential probiotic properties of Lactobacillus fermentum isolated from traditional dairy products. Food Control. 2010, 21, 695–701. [Google Scholar] [CrossRef]
- Qin, S.; Huang, Z.; Wang, Y.; Pei, L.; Shen, Y. Probiotic potential of Lactobacillus isolated from horses and its therapeutic effect on DSS-induced colitis in mice. Microb. Pathog. 2022, 165, 105216. [Google Scholar] [CrossRef]
- Bruslik, N.L.; Akhatova, D.R.; Toimentseva, A.A.; Abdulkhakov, S.R.; Ilyinskaya, O.N.; Yarullina, D.R. Estimation of probiotic lactobacilli drug resistance. Antibiot. Khimioterapiia 2015, 60, 6–13. [Google Scholar]
- Maldonado, N.C.; de Ruiz, C.S.; Otero, M.C.; Sesma, F.; Nader-Macías, M.E. Lactic acid bacteria isolated from young calve—Characterization and potential as probiotics. Res. Vet. Sci. 2012, 92, 342–349. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Nikitina, E.V.; Yurtaeva, T.A.; Tsyganov, M.S.; Ezhkova, G.O. Physico-chemical and antioxidant properties of skimmed varenets (Slavic baked milk yogurt) mixed with enzyme-modified potato starches. Curr. Res. Nutr. Food Sci. J. 2021, 9, 88–99. [Google Scholar] [CrossRef]
- Muniandy, P.; Shori, A.B.; Baba, A.S. Influence of green, white and black tea addition on the antioxidant activity of probiotic yogurt during refrigerated storage. Food Pack Shelf Life 2016, 8, 1–8. [Google Scholar] [CrossRef]
- Al-Yousef, H.M.; Alqahtani, A.S.; Hassan, W.H.B.; Alzoubi, A.; Abdelaziz, S. Chemical profile, in vitro antioxidant, pancreatic lipase, and alpha-amylase inhibition assays of the aqueous extract of elettaria cardamomum L. fruits. J. Chem. 2021, 2021, 5583001. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, Y.; Cheng, Y.; Wang, Y. Rapid screening and identification of α-glucosidase inhibitors from mulberry leaves using enzyme-immobilized magnetic beads coupled with HPLC/MS and NMR. Biomed. Chromatogr. 2013, 27, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, A.C.; Isolauri, E.; Salminen, S.J. The role of the intestinal microflora for the development of the immune system in early childhood. Eur. J. Nutr. 2002, 41, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Leahy, S.C.; Higgins, D.G.; Fitzgerald, G.F.; Van Sinderen, D. Getting better with bifidobacteria. J. Appl. Microbiol. 2005, 98, 1303–1315. [Google Scholar] [CrossRef]
- Gueimonde, M.; Noriega, L.; Margolles, A.; De Los Reyes-Gavilan, C.G.; Salminen, S. Ability of Bifidobacterium strains with acquired resistance to bile to adhere to human intestinal mucus. Internat. J. Food Microbiol. 2005, 101, 341–346. [Google Scholar] [CrossRef]
- Vlková, E.; Rada, V.; Smehilová, M.; Killer, J. Auto-aggregation and co-aggregation ability in bifidobacteria and clostridia. Folia Microbiol. 2008, 53, 263–269. [Google Scholar] [CrossRef]
- Qin, X.-S.; Gao, Q.-Y.; Luo, Z.-G. Enhancing the storage and gastrointestinal passage viability of probiotic powder (Lactobacillus plantarum) through encapsulation with pickering high internal phase emulsions stabilized with WPI-EGCG covalent conjugate nanoparticles. Food Hydrocoll. 2021, 116, 106658. [Google Scholar] [CrossRef]
- Ta, L.P.; Bujna, E.; Antal, O.; Ladányi, M.; Juhász, R.; Szécsi, A.; Kun, S.; Sudheer, S.; Gupta, V.K.; Nguyen, Q.D. Effects of various polysaccharides (alginate, carrageenan, gums, chitosan) and their combination with prebiotic saccharides (resistant starch, lactosucrose, lactulose) on the encapsulation of probiotic bacteria Lactobacillus casei 01 strain. Int. J. Biol. Macromol. 2021, 183, 1136–1144. [Google Scholar] [CrossRef]
- Pastell, H.; Westermann, P.; Meyer, A.S.; Tuomainen, P.; Tenkanen, M. In vitro fermentation of arabinoxylan-derived carbohydrates by Bifidobacteria and mixed fecal microbiota. J. Agric. Food Chem. 2009, 57, 8598–8606. [Google Scholar] [CrossRef]
- Sørensen, H.R.; Pedersen, S.; Meyer, A.S. Optimization of reaction conditions for enzymatic viscosity reduction and hydrolysis of wheat arabinoxylan in an industrial ethanol fermentation residue. Biotechnol. Prog. 2006, 22, 505–513. [Google Scholar] [CrossRef]
- Marquez, A.; Andrada, E.; Russo, M.; Bolondi, M.L.; Fabersani, E.; Medina, R.; Gauffin-Cano, P. Characterization of autochthonous lactobacilli from goat dairy products with probiotic potential for metabolic diseases. Heliyon 2022, 8, e10462. [Google Scholar] [CrossRef]
- Panwar, H.D.; Calderwood, I.R.; Grant, S.; Grover, B.D. Green Lactobacillus strains isolated from infant faeces possess potent inhibitory activity against intestinal alpha- and beta-glucosidases suggesting anti-diabetic potential. Eur. J. Nutr. 2014, 53, 1465–1474. [Google Scholar] [CrossRef]
- Sabikhi, L.; Babu, R.; Thompkinson, D.K.; Kapila, S. Resistance of microencapsulated Lactobacillus acidophilus LA1 to processing treatments and simulated gut conditions. Food Bioprocess Technol. 2010, 3, 586–593. [Google Scholar] [CrossRef]
- Parente, E.; Ciocia, F.; Ricciardi, A.; Zotta, T.; Felis, G.E.; Torriani, S. Diversity of stress tolerance in Lactobacillus plantarum, Lactobacillus pentosus and Lactobacillus paraplantarum: A multivariate screening study. Int. J. Food Microbiol. 2010, 144, 270–279. [Google Scholar] [CrossRef]
- Zotta, T.; Guidone, A.; RIanniello, G.; Ricciardi, A.; Parente, E. Aerobic metabolism and oxidative stress tolerance in the Lactobacillus plantarum group. World J. Microbiol. Biotechnol. 2013, 29, 1713–1722. [Google Scholar]
- Zotta, T.; Ricciardi, A.; Ianniello, R.G.; Parente, E.; Reale, A.; Rossi, F.; Iacumin, L.; Comi, G.; Coppola, R. Assessment of aerobic and respiratory growth in the Lactobacillus casei group. PLoS ONE 2014, 9, e99189. [Google Scholar] [CrossRef]
- Guilbaud, M.; Zagorec, M.; Chaillou, S.; Champomier-Vergès, M. Intraspecies diversity of Lactobacillus sakei response to oxidative stress and variability of strain performance in mixed strains challenges. Food Microbiol. 2012, 29, 197–204. [Google Scholar] [CrossRef]
- Lavari, L.; Ianniello, R.; Páez, R.; Zotta, T.; Cuatrin, A.; Reinheimer, J.; Parente, E.; Vinderola, G. Growth of Lactobacillus rhamnosus 64 in whey permeate and study of the effect of mild stresses on survival to spray drying. LWT-Food Sci. Technol. 2015, 63, 322–330. [Google Scholar] [CrossRef]
- Abozed, S.S.; El-kalyoubi, A.; Abdelrashid, A.; Salama, M.F. Total phenolic contents and antioxidant activities of various solvent extracts from whole wheat and bran. Ann. Agric. Sci. 2014, 59, 63–67. [Google Scholar] [CrossRef]
- Oliveira, A.M.F.; Pinheiro, L.S.; Pereira, C.K.S.; Matias, W.N.; Gomes, R.A.; Chaves, O.S.; de Souza, M.D.F.V.; de Almeida, R.N.; de Assis, T.S. Total phenolic content and antioxidant activity of some Malvaceae family species. Antioxidants 2012, 1, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Piluzza, G.; Bullitta, S. Correlations between phenolic content and antioxidant properties in twenty-four plant species of traditional ethnoveterinary use in the Mediterranean area. Pharm. Biol. 2011, 49, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Vieira, J.M.; Mantovani, R.A.; Raposo, M.F.J.; Coimbra, M.A.; Vicente, A.A.; Cunha, R.L. Effect of extraction temperature on rheological behavior and antioxidant capacity of flaxseed gum. Carbohydr. Polym. 2019, 213, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Cremonini, E.; Bettaieb, A.; Haj, F.J.; Fraga, C.G.; Oteiza, P.I. (−)-Epicatechin improves insulin sensitivity in high fat diet-fed mice. Arch. Biochem. Biophys. 2016, 559, 13–21. [Google Scholar] [CrossRef]
- Liang, S.; Liao, W.; Ma, X.; Li, X.; Wang, Y. H2O2 oxidative preparation, characterization and antiradical activity of a novel oligosaccharide derived from flaxseed gum. Food Chem. 2017, 230, 135–144. [Google Scholar] [CrossRef]
- Diez-Ozaeta, I.; Astiazaran, O.J. Fermented foods: An update on evidence-based health benefits and future perspectives. Food Res. Int. 2022, 156, 111133. [Google Scholar] [CrossRef]
- Champagne, C.P.; Raymond, Y.; Guertin, N.; Martoni, C.J.; Jones, M.L.; Mainville, I.; Arcand, Y. Impact of a yogurt matrix and cell microencapsulation on the survival of Lactobacillus reuteri in three in vitro gastric digestion procedures. Benef. Microbes 2015, 6, 753–763. [Google Scholar] [CrossRef]
- Shinde, T.; Sun-Waterhouse, D.; Brooks, J. Co-extrusion encapsulation of probiotic Lactobacillus acidophilus alone or together with apple skin polyphenols: An aqueous and value-added delivery system using alginate. Food Bioprocess Technol. 2014, 7, 1581–1596. [Google Scholar] [CrossRef]



| Antibiotic | FSM Concentration, % | Ceftriaxone | Cefoxitin | Amoxicillin | Clindamycin | Erythromycin | Streptomycin |
|---|---|---|---|---|---|---|---|
| Strains | 30 mcg | 30 mcg | 25 mcg | 2 mcg | 15 mcg | 300 mcg | |
| L. bulgarucus | 0 | 9.5 ± 1.3 | 6 ± 1.8 | 14.8 ± 1.3 | 10.5 ± 2.4 | 8.8 ± 2.1 | 1.9 ± 0.6 |
| 0.1 | 7.5 ± 1 | 4 ± 0 | 13.5 ± 1.3 | 10.5 ± 1.3 | 9 ± 2.4 | 1.9 ± 1 | |
| 0.2 | 7.3 ± 1 | 5 ± 0 | 11.8 ± 1.3 | 9.8 ± 2.2 | 9 ± 1.8 | 1.5 ± 0.9 | |
| 0.4 | 9.3 ± 2.2 | 5 ± 0 | 9.8 ± 0.5 | 11.3 ± 3.3 | 8.5 ± 1.7 | 1.4 ± 0.8 | |
| L. fermentum AG8 | 0 | 11 ± 2.9 | 4 ± 0 | 10 ± 1.2 | 13.5 ± 2.4 | 11 ± 1.8 | 2.3 ± 1 |
| 0.1 | 9.7 ± 2.8 | 5.5 ± 0.7 * | 10.8 ± 1 | 12.3 ± 1.5 | 8.8 ± 3.9 | 2.9 ± 1 | |
| 0.2 | 8.8 ± 1.5 | 7.5 ± 0.7 * | 11 ± 1.8 | 11.3 ± 0.5 | 12 ± 1.8 | 3.5 ± 0.6 | |
| 0.4 | 9 ± 1.2 | 2.5 ± 0.7 | 11.5 ± 2.4 | 12.3 ± 2.2 | 11 ± 1.4 | 2.5 ± 1.3 | |
| L. plantarum AG9 | 0 | 8 ± 0.8 | 2.1 ± 0.1 | 12 ± 1.8 | 7.8 ± 1.7 | 12.5 ± 1.3 | 1.6 ± 0.5 |
| 0.1 | 6.5 ± 1.3 | 0.1 ± 0.1 * | 11 ± 1.8 | 6 ± 2.2 | 12 ± 0.8 | 1 ± 0.7 | |
| 0.2 | 6 ± 2.2 | 0.1 ± 0.1 * | 11.8 ± 0.5 | 4 ± 1.6 | 12.5 ± 1.7 | 1.1 ± 0.6 | |
| 0.4 | 7 ± 1.4 | 0.1 ± 0.1 * | 14.3 ± 1.7 | 7 ± 2.9 | 13.5 ± 2.4 | 1.3 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sungatullina, A.; Petrova, T.; Kharina, M.; Mikshina, P.; Nikitina, E. Effect of Flaxseed Mucilage on the Probiotic, Antioxidant, and Structural-Mechanical Properties of the Different Lactobacillus Cells. Fermentation 2023, 9, 486. https://doi.org/10.3390/fermentation9050486
Sungatullina A, Petrova T, Kharina M, Mikshina P, Nikitina E. Effect of Flaxseed Mucilage on the Probiotic, Antioxidant, and Structural-Mechanical Properties of the Different Lactobacillus Cells. Fermentation. 2023; 9(5):486. https://doi.org/10.3390/fermentation9050486
Chicago/Turabian StyleSungatullina, Alya, Tatyana Petrova, Maria Kharina, Polina Mikshina, and Elena Nikitina. 2023. "Effect of Flaxseed Mucilage on the Probiotic, Antioxidant, and Structural-Mechanical Properties of the Different Lactobacillus Cells" Fermentation 9, no. 5: 486. https://doi.org/10.3390/fermentation9050486
APA StyleSungatullina, A., Petrova, T., Kharina, M., Mikshina, P., & Nikitina, E. (2023). Effect of Flaxseed Mucilage on the Probiotic, Antioxidant, and Structural-Mechanical Properties of the Different Lactobacillus Cells. Fermentation, 9(5), 486. https://doi.org/10.3390/fermentation9050486






