Green Fractionation and Structural Characterization of Lignin Nanoparticles via Carboxylic-Acid-Based Deep Eutectic Solvent (DES) Pretreatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
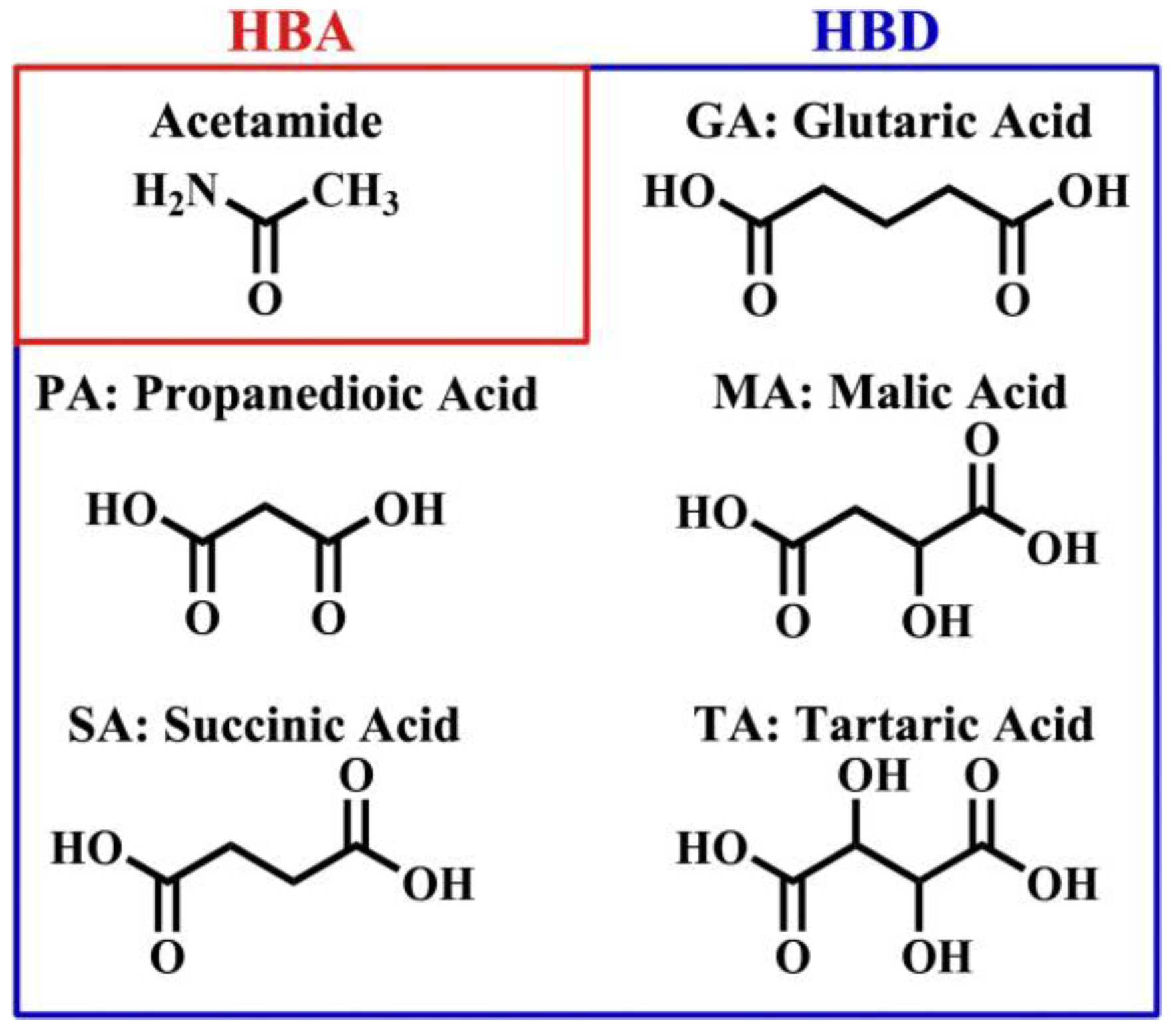
2.2. Preparation of Polycarboxylic-Acid-Based DES
2.3. Polycarboxylic-Acid-Based DES Pretreatment
2.4. Analysis Methods
3. Results and Discussion
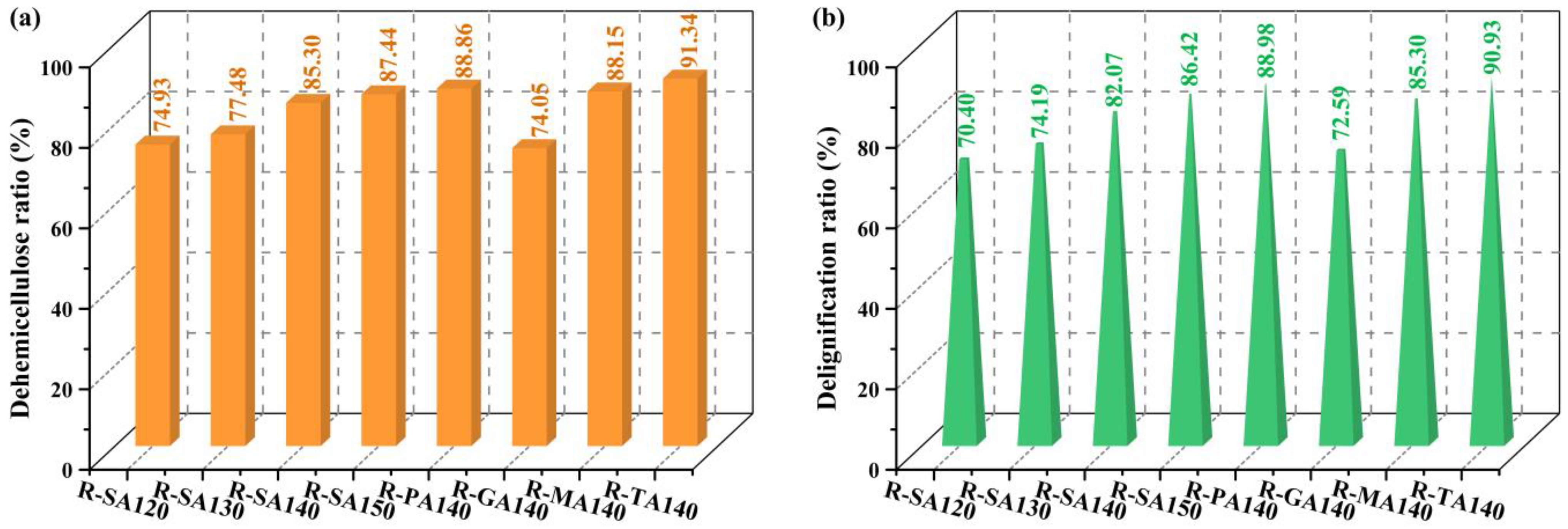
3.1. Pretreatment Performance Analysis
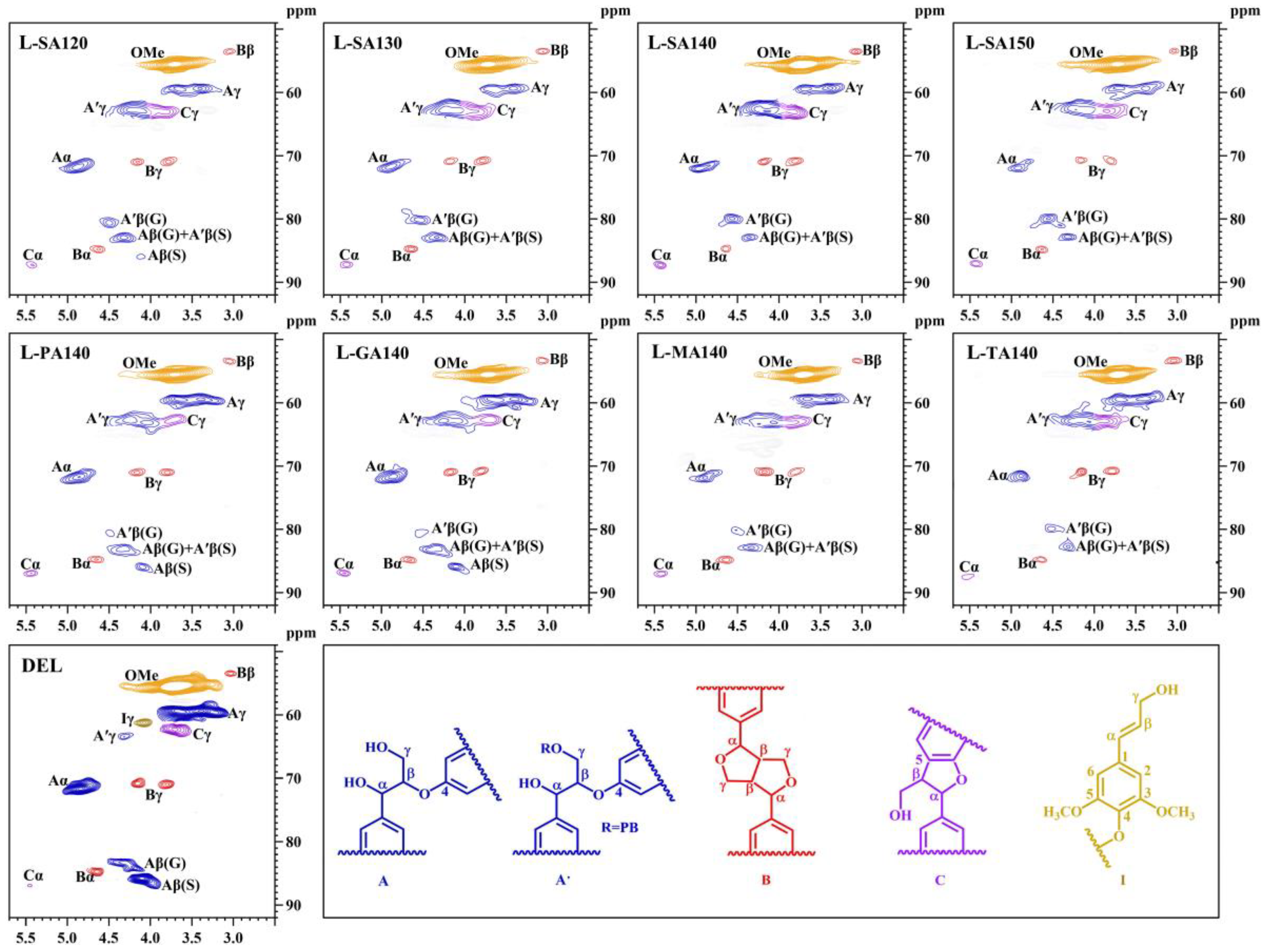
3.2. Structural Analysis of the DES Lignin Fractions
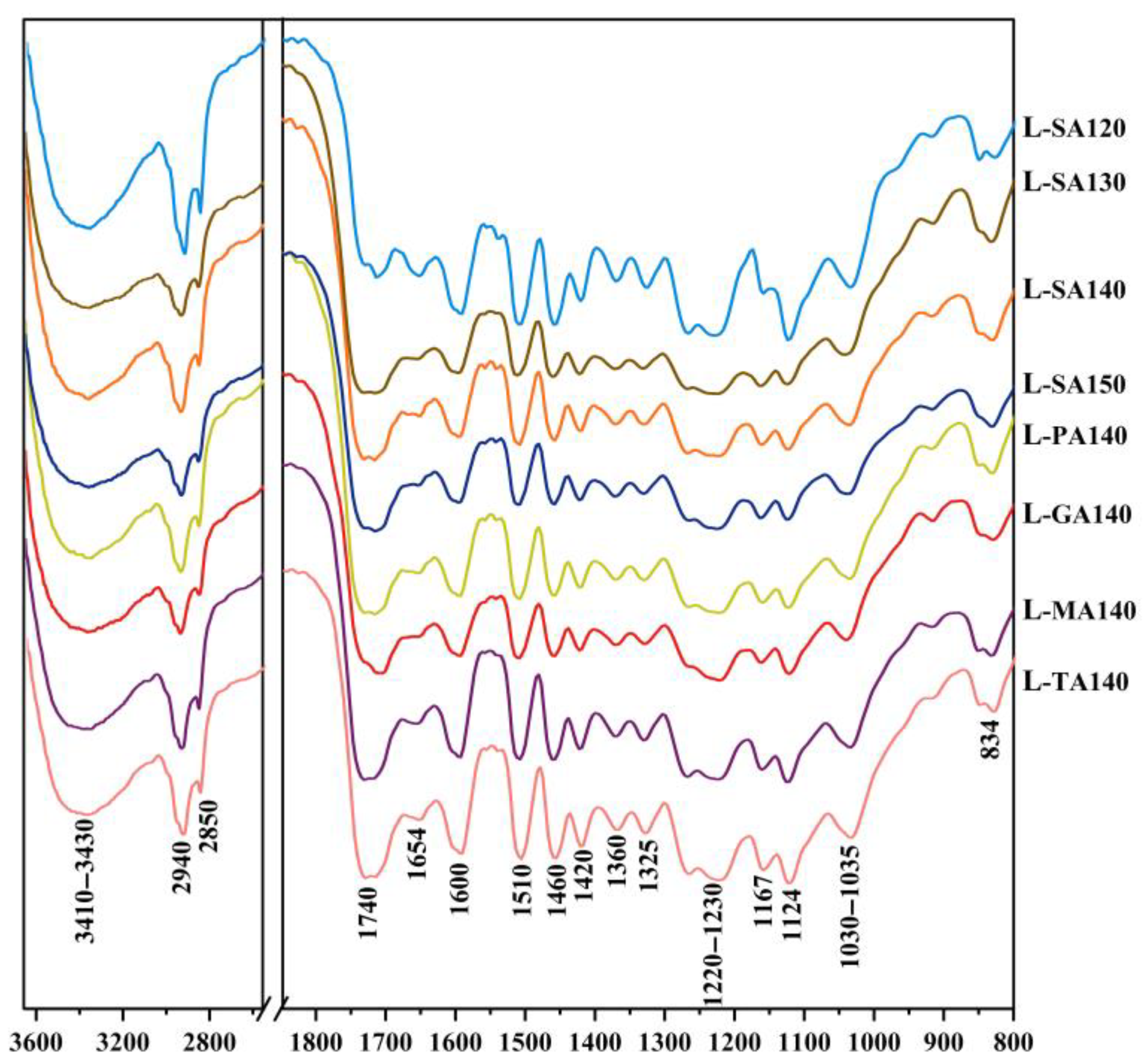
3.3. Molecular Weights and FT-IR Analysis of the DES Lignin Macromolecules
3.4. DPPH Antioxidant Activity and Morphology Analysis of the DES Lignin
3.5. Structural Changes of Lignin Macromolecule during the Polycarboxylic-Acid-Based DES Pretreatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liao, Y.; Koelewijn, S.-F.; Van den Bossche, G.; Van Aelst, J.; Van den Bosch, S.; Renders, T.; Navare, K.; Nicolaï, T.; Van Aelst, K.; Maesen, M. A Sustainable Wood Biorefinery for Low–Carbon Footprint Chemicals Production. Science 2020, 6484, 1385–1390. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.A.; Barnes, S.H.; Bowland, C.C.; Meek, K.M.; Littrell, K.C.; Keum, J.K.; Naskar, A.K. A Path for Lignin Valorization via Additive Manufacturing of High-Performance Sustainable Composites with Enhanced 3D Printability. Sci. Adv. 2018, 4, eaat4967. [Google Scholar] [CrossRef] [PubMed]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M. Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef] [PubMed]
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J.; Hallett, J.P.; Leak, D.J.; Liotta, C.L. The Path Forward for Biofuels and Biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, K. Lignocellulose: A Chewy Problem. Nature 2011, 474, S12–S14. [Google Scholar] [CrossRef] [PubMed]
- Schutyser, W.; Renders, A.T.; Van den Bosch, S.; Koelewijn, S.-F.; Beckham, G.T.; Sels, B.F. Chemicals from Lignin: An Interplay of Lignocellulose Fractionation, Depolymerisation, and Upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.G.; Meng, X.; Pu, Y.; Ragauskas, A.J. The Critical Role of Lignin in Lignocellulosic Biomass Conversion and Recent Pretreatment Strategies: A Comprehensive Review. Bioresour. Technol. 2020, 301, 122784. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhao, S.; Yang, S.; Ding, S.-Y. Lignin Plays a Negative Role in the Biochemical Process for Producing Lignocellulosic Biofuels. Curr. Opin. Biotechnol. 2014, 27, 38–45. [Google Scholar] [CrossRef]
- Wang, H.M.; Yuan, T.Q.; Song, G.Y.; Sun, R.C. Advanced and Versatile Lignin-Derived Biodegradable Composite Film Materials toward a Sustainable World. Green Chem. 2021, 23, 3790–3817. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Tang, X.; Zuo, M.; Li, Z.; Liu, H.; Xiong, C.; Zeng, X.; Sun, Y.; Hu, L.; Liu, S.; Lei, T. Green Processing of Lignocellulosic Biomass and Its Derivatives in Deep Eutectic Solvents. ChemSusChem 2017, 10, 2696–2706. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Reznicek, W.D.; Wan, C. Deep Eutectic Solvent Pretreatment Enabling Full Utilization of Switchgrass. Bioresour. Technol. 2018, 263, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, B.; Xia, Q.; Meng, J.; Chen, W.; Liu, S.; Wang, Q.; Liu, Y.; Li, J.; Yu, H. Efficient Cleavage of Strong Hydrogen Bonds in Cotton by Deep Eutectic Solvents and Facile Fabrication of Cellulose Nanocrystals in High Yields. ACS Sustain. Chem. Eng. 2017, 5, 7623–7631. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, W.; Xia, Q.; Guo, B.; Wang, Q.; Liu, S.; Liu, Y.; Li, J.; Yu, H. Efficient Cleavage of Lignin–Carbohydrate Complexes and Ultrafast Extraction of Lignin Oligomers from Wood Biomass by Microwave-Assisted Treatment with Deep Eutectic Solvent. ChemSusChem 2017, 10, 1692–1700. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, X.; Jiang, J.; Zhang, Y.; Bi, S.; Wang, H.-M. Revealing Structural and Functional Specificity of Lignin from Tobacco Stalk during Deep Eutectic Solvents Deconstruction Aiming to Targeted Valorization. Ind. Crops Prod. 2022, 180, 114696. [Google Scholar] [CrossRef]
- Chen, Z.; Ragauskas, A.; Wan, C. Lignin Extraction and Upgrading Using Deep Eutectic Solvents. Ind. Crops Prod. 2020, 147, 112241. [Google Scholar] [CrossRef]
- Ma, C.-Y.; Xu, L.-H.; Sun, Q.; Shen, X.-J.; Wen, J.-L.; Yuan, T.-Q. Tailored One-Pot Lignocellulose Fractionation to Maximize Biorefinery toward Controllable Producing Lignin Nanoparticles and Facilitating Enzymatic Hydrolysis. Chem. Eng. J. 2022, 450, 138315. [Google Scholar] [CrossRef]
- Meshitsuka, G.; Isogai, A. Chemical Structures of Cellulose, Hemicelluloses, and Lignin. In Chemical Modification of Lignocellulosic Materials; Routledge: London, UK, 2017; pp. 11–33. [Google Scholar]
- Zhu, X.; Peng, C.; Chen, H.; Chen, Q.; Zhao, Z.K.; Zheng, Q.; Xie, H. Opportunities of Ionic Liquids for Lignin Utilization from Biorefinery. ChemistrySelect 2018, 3, 7945–7962. [Google Scholar] [CrossRef]
- Calvo-Flores, F.G.; Dobado, J.A. Lignin as Renewable Raw Material. ChemSusChem 2010, 3, 1227–1235. [Google Scholar] [CrossRef]
- Ma, C.-Y.; Gao, X.; Peng, X.-P.; Gao, Y.-F.; Liu, J.; Wen, J.-L.; Yuan, T.-Q. Microwave-Assisted Deep Eutectic Solvents (DES) Pretreatment of Control and Transgenic Poplars for Boosting the Lignin Valorization and Cellulose Bioconversion. Ind. Crops Prod. 2021, 164, 113415. [Google Scholar] [CrossRef]
- Ma, C.-Y.; Peng, X.-P.; Sun, S.-L.; Wen, J.-L.; Yuan, T.-Q. Short-Time Deep Eutectic Solvents Pretreatment Enhanced Production of Fermentable Sugars and Tailored Lignin Nanoparticles from Abaca. Int. J. Biol. Macromol. 2021, 192, 417–425. [Google Scholar] [CrossRef]
- Ma, C.-Y.; Xu, L.-H.; Sun, Q.; Sun, S.-N.; Cao, X.-F.; Wen, J.-L.; Yuan, T.-Q. Ultrafast Alkaline Deep Eutectic Solvent Pretreatment for Enhancing Enzymatic Saccharification and Lignin Fractionation from Industrial Xylose Residue. Bioresour. Technol. 2022, 352, 127065. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Tang, S.; Zhang, W.; Tao, Y.; Lai, C.; Li, X.; Yong, Q. Unveiling the Structural Properties of Lignin–Carbohydrate Complexes in Bamboo Residues and Its Functionality as Antioxidants and Immunostimulants. ACS Sustain. Chem. Eng. 2018, 6, 12522–12531. [Google Scholar] [CrossRef]
- Meng, X.; Crestini, C.; Ben, H.; Hao, N.; Pu, Y.; Ragauskas, A.J.; Argyropoulos, D.S. Determination of Hydroxyl Groups in Biorefinery Resources via Quantitative 31P NMR Spectroscopy. Nat. Protoc. 2019, 14, 2627–2647. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Cao, S.; Ragauskas, A.J. Application of Quantitative 31P NMR in Biomass Lignin and Biofuel Precursors Characterization. Energy Environ. Sci. 2011, 4, 3154–3166. [Google Scholar] [CrossRef]
- Ma, C.-Y.; Sun, Q.; Zuo, C.; Xu, L.-H.; Sun, S.-N.; Wen, J.-L.; Yuan, T.-Q. Efficient Fractionation and Targeted Valorization of Industrial Xylose Residue by Synergistic and Mild Alkaline Deep Eutectic Solvent-hydrogen Peroxide Pretreatment. Fuel Process. Technol. 2023, 241, 107591. [Google Scholar] [CrossRef]
- Wen, J.-L.; Yuan, T.-Q.; Sun, S.-L.; Xu, F.; Sun, R.-C. Understanding the Chemical Transformations of Lignin during Ionic Liquid Pretreatment. Green Chem. 2014, 16, 181–190. [Google Scholar] [CrossRef]
- Wang, H.-M.; Wang, B.; Wen, J.-L.; Yuan, T.-Q.; Sun, R.-C. Structural Characteristics of Lignin Macromolecules from Different Eucalyptus Species. ACS Sustain. Chem. Eng. 2017, 5, 11618–11627. [Google Scholar] [CrossRef]
- Wen, J.-L.; Sun, Y.-C.; Xu, F.; Sun, R.-C. Fractional Isolation and Chemical Structure of Hemicellulosic Polymers Obtained from Bambusa Rigida Species. J. Agric. Food Chem. 2010, 58, 11372–11383. [Google Scholar] [CrossRef]
- Wen, J.-L.; Xue, B.-L.; Xu, F.; Sun, R.-C.; Pinkert, A. Unmasking the Structural Features and Property of Lignin from Bamboo. Ind. Crops Prod. 2013, 42, 332–343. [Google Scholar] [CrossRef]
- Shen, X.-J.; Wen, J.-L.; Mei, Q.-Q.; Chen, X.; Sun, D.; Yuan, T.-Q.; Sun, R.-C. Facile Fractionation of Lignocelluloses by Biomass-Derived Deep Eutectic Solvent (DES) Pretreatment for Cellulose Enzymatic Hydrolysis and Lignin Valorization. Green Chem. 2019, 21, 275–283. [Google Scholar] [CrossRef]
- Kim, H.; Ralph, J. A Gel-State 2D-NMR Method for Plant Cell Wall Profiling and Analysis: A Model Study with the Amorphous Cellulose and Xylan from Ball-Milled Cotton Linters. RSC Adv. 2014, 4, 7549–7560. [Google Scholar] [CrossRef]
- Kim, H.; Ralph, J.; Akiyama, T. Solution-State 2D NMR of Ball-Milled Plant Cell Wall Gels in DMSO-d6. BioEnergy Res. 2008, 1, 56–66. [Google Scholar] [CrossRef]
- Chen, X.; Cao, X.; Sun, S.; Yuan, T.; Wang, S.; Shi, Q.; Sun, R. Hydrothermal Acid Hydrolysis for Highly Efficient Separation of Lignin and Xylose from Pre-Hydrolysis Liquor of Kraft Pulping Process. Sep. Purif. Technol. 2019, 209, 741–747. [Google Scholar] [CrossRef]
- Saha, B.C.; Yoshida, T.; Cotta, M.A.; Sonomoto, K. Hydrothermal Pretreatment and Enzymatic Saccharification of Corn Stover for Efficient Ethanol Production. Ind. Crops Prod. 2013, 44, 367–372. [Google Scholar] [CrossRef]
- Trajano, H.L.; Engle, N.L.; Foston, M.; Ragauskas, A.J.; Tschaplinski, T.J.; Wyman, C.E. The Fate of Lignin during Hydrothermal Pretreatment. Biotechnol. Biofuels 2013, 6, 110. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhao, X.; Zheng, Y.; Lin, W.; Lai, C.; Yong, Q.; Ragauskas, A.J.; Meng, X. Revealing the Mechanism of Surfactant-Promoted Enzymatic Hydrolysis of Dilute Acid Pretreated Bamboo. Bioresour. Technol. 2022, 360, 127524. [Google Scholar] [CrossRef]
- Li, C.; Knierim, B.; Manisseri, C.; Arora, R.; Scheller, H.V.; Auer, M.; Vogel, K.P.; Simmons, B.A.; Singh, S. Comparison of Dilute Acid and Ionic Liquid Pretreatment of Switchgrass: Biomass Recalcitrance, Delignification and Enzymatic Saccharification. Bioresour. Technol. 2010, 101, 4900–4906. [Google Scholar] [CrossRef]
- Wang, H.-M.; Ma, C.-Y.; Li, H.-Y.; Chen, T.-Y.; Wen, J.-L.; Cao, X.-F.; Wang, X.-L.; Yuan, T.-Q.; Sun, R.-C. Structural Variations of Lignin Macromolecules from Early Growth Stages of Poplar Cell Walls. ACS Sustain. Chem. Eng. 2019, 8, 1813–1822. [Google Scholar] [CrossRef]
- Yang, Y.-T.; Qin, M.-K.; Sun, Q.; Gao, Y.-F.; Ma, C.-Y.; Wen, J.-L. Structural Elucidation and Targeted Valorization of Poplar Lignin from the Synergistic Hydrothermal-Deep Eutectic Solvent Pretreatment. Int. J. Biol. Macromol. 2022, 209, 1882–1892. [Google Scholar] [CrossRef]
- Ma, C.-Y.; Wang, H.-M.; Wen, J.-L.; Shi, Q.; Wang, S.-F.; Yuan, T.-Q.; Sun, R.-C. Structural Elucidation of Lignin Macromolecule from Abaca during Alkaline Hydrogen Peroxide Delignification. Int. J. Biol. Macromol. 2020, 144, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Kadla, J.F.; Ehara, K.; Gilkes, N.; Saddler, J.N. Organosolv Ethanol Lignin from Hybrid Poplar as a Radical Scavenger: Relationship between Lignin Structure, Extraction Conditions, and Antioxidant Activity. J. Agric. Food Chem. 2006, 54, 5806–5813. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Dong, H.; Zhang, Z.; Bian, H.; Yong, Q. Procuring the Nano-Scale Lignin in Prehydrolyzate as Ingredient to Prepare Cellulose Nanofibril Composite Film with Multiple Functions. Cellulose 2020, 27, 9355–9370. [Google Scholar] [CrossRef]





| Sample | Solid Yield | Xyl a | Glu | AL | KL | Total |
|---|---|---|---|---|---|---|
| Control | - b | 19.30 ± 0.17 | 48.59 ± 0.78 | 2.05 ± 0.06 | 24.56 ± 0.21 | 94.50 ± 1.64 |
| R-SA120 | 70.41 ± 0.23 | 6.88 ± 0.14 | 67.76 ± 0.81 | 1.38 ± 0.02 | 9.81 ± 0.02 | 85.83 ± 0.99 |
| R-SA130 | 62.41 ± 0.21 | 6.95 ± 0.17 | 71.31 ± 0.81 | 1.29 ± 0.03 | 9.70 ± 0.05 | 89.26 ± 1.07 |
| R-SA140 | 59.42 ± 0.24 | 4.78 ± 0.15 | 79.84 ± 0.74 | 1.07 ± 0.01 | 6.97 ± 0.03 | 92.65 ± 0.96 |
| R-SA150 | 51.48 ± 0.19 | 4.72 ± 0.11 | 79.38 ± 0.76 | 0.81 ± 0.01 | 6.23 ± 0.02 | 91.15 ± 0.93 |
| R-PA140 | 52.26 ± 0.13 | 4.11 ± 0.14 | 81.08 ± 0.73 | 0.64 ± 0.03 | 4.97 ± 0.02 | 90.80 ± 0.98 |
| R-GA140 | 63.45 ± 0.15 | 7.90 ± 0.19 | 75.27 ± 0.80 | 1.84 ± 0.02 | 9.67 ± 0.06 | 94.68 ± 1.07 |
| R-MA140 | 58.02 ± 0.22 | 3.94 ± 0.18 | 78.56 ± 0.78 | 1.40 ± 0.03 | 5.33 ± 0.17 | 89.23 ± 1.16 |
| R-TA140 | 47.64 ± 0.19 | 3.51 ± 0.16 | 85.45 ± 0.68 | 0.76 ± 0.02 | 4.30 ± 0.01 | 94.03 ± 0.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, M.-K.; Zuo, C.; Yang, Y.-T.; Liu, Y.-H.; Ma, C.-Y.; Wen, J.-L. Green Fractionation and Structural Characterization of Lignin Nanoparticles via Carboxylic-Acid-Based Deep Eutectic Solvent (DES) Pretreatment. Fermentation 2023, 9, 491. https://doi.org/10.3390/fermentation9050491
Qin M-K, Zuo C, Yang Y-T, Liu Y-H, Ma C-Y, Wen J-L. Green Fractionation and Structural Characterization of Lignin Nanoparticles via Carboxylic-Acid-Based Deep Eutectic Solvent (DES) Pretreatment. Fermentation. 2023; 9(5):491. https://doi.org/10.3390/fermentation9050491
Chicago/Turabian StyleQin, Meng-Kai, Cheng Zuo, Yi-Ting Yang, Yi-Hui Liu, Cheng-Ye Ma, and Jia-Long Wen. 2023. "Green Fractionation and Structural Characterization of Lignin Nanoparticles via Carboxylic-Acid-Based Deep Eutectic Solvent (DES) Pretreatment" Fermentation 9, no. 5: 491. https://doi.org/10.3390/fermentation9050491
APA StyleQin, M.-K., Zuo, C., Yang, Y.-T., Liu, Y.-H., Ma, C.-Y., & Wen, J.-L. (2023). Green Fractionation and Structural Characterization of Lignin Nanoparticles via Carboxylic-Acid-Based Deep Eutectic Solvent (DES) Pretreatment. Fermentation, 9(5), 491. https://doi.org/10.3390/fermentation9050491






