Efficacy of Ultrasound-Assisted Lactic Acid Fermentation and Its Effect on the Nutritional and Sensory Quality of Novel Chickpea-Based Beverage
Abstract
:1. Introduction
2. Materials and Methods
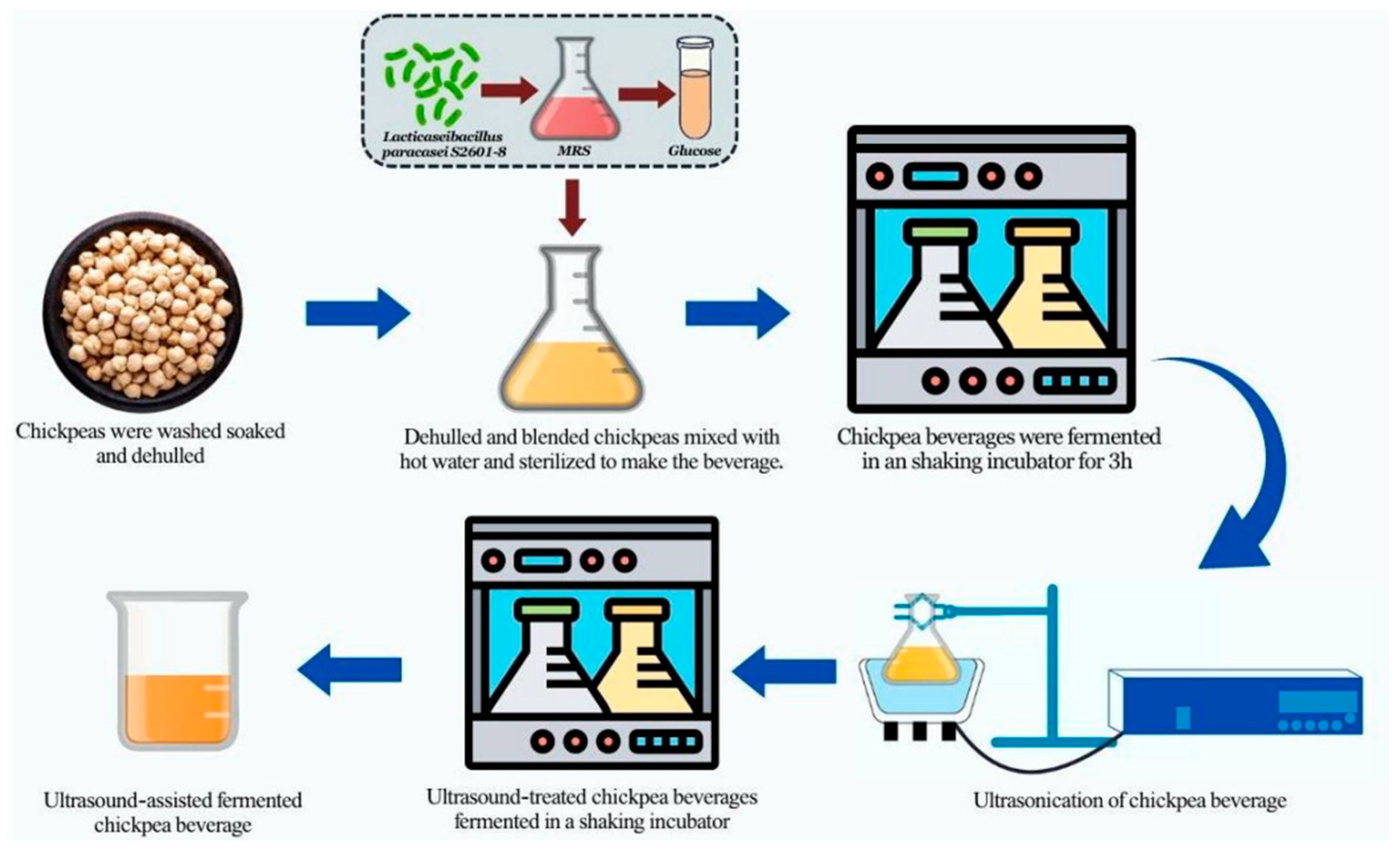
2.1. Formulation of Ultrasound-Assisted Chickpea Fermented Milk
2.1.1. Chickpea Milk Preparation
2.1.2. Ultrasound-Assisted Chickpea Milk Preparation
2.2. Proximate Analysis
- where; A = volume of 0.01 mol/L HCl titrated against sample;
- B = volume of 0.01 mol/L HCl titrated against blank =1.2 mL;
- W = weight of sample = 2 mL;
- V = total volume of sample digest = 50 mL;
- V1 = volume of sample digest distilled = 5 mL.
2.3. Effect of Ultrasound Treatment on Chickpea Beverage Fermentation
2.3.1. Scanning Electron Microscopy (SEM) Analysis
2.3.2. Atomic Force Microscopy (AFM) Analysis
2.3.3. Fourier Transform Infrared (FTIR) Spectroscopy
2.4. High-Performance Liquid Chromatography (HPLC) Analysis
2.4.1. Organic Acids
2.4.2. Phytochemicals
2.5. Microbiological Analysis of Chickpea Beverage Samples
2.6. Sensory Profile
2.6.1. Colour Determination
2.6.2. eNose Analysis
2.6.3. Consumer Sensory Analysis Tests
2.6.4. Statistical Analysis
3. Results
3.1. Proximate Analysis of Treated and Untreated Chickpea Beverages
3.2. Effect of Processing on Structural Qualities of Ultrasound-Assisted Fermentation on Chickpea Beverages
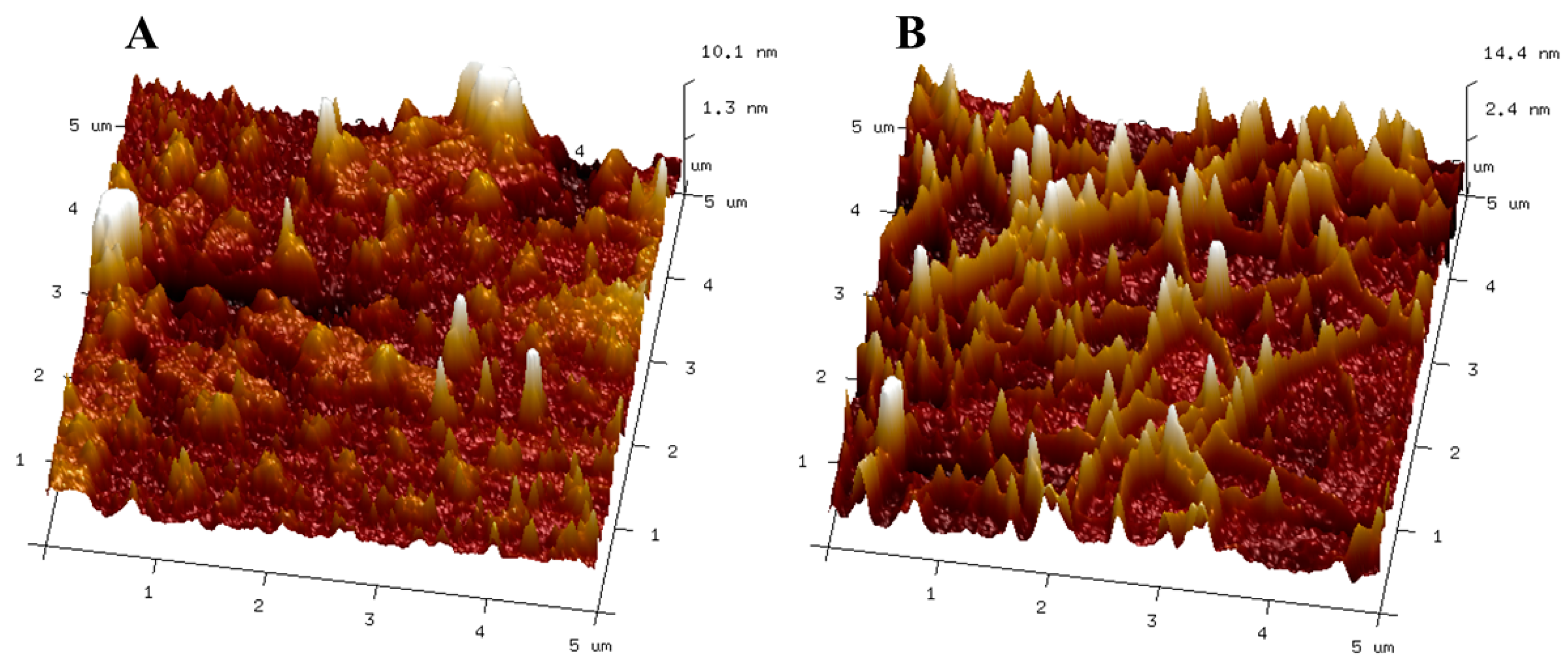
3.2.1. Influence of Ultrasonication on Structural Integrity via AFM
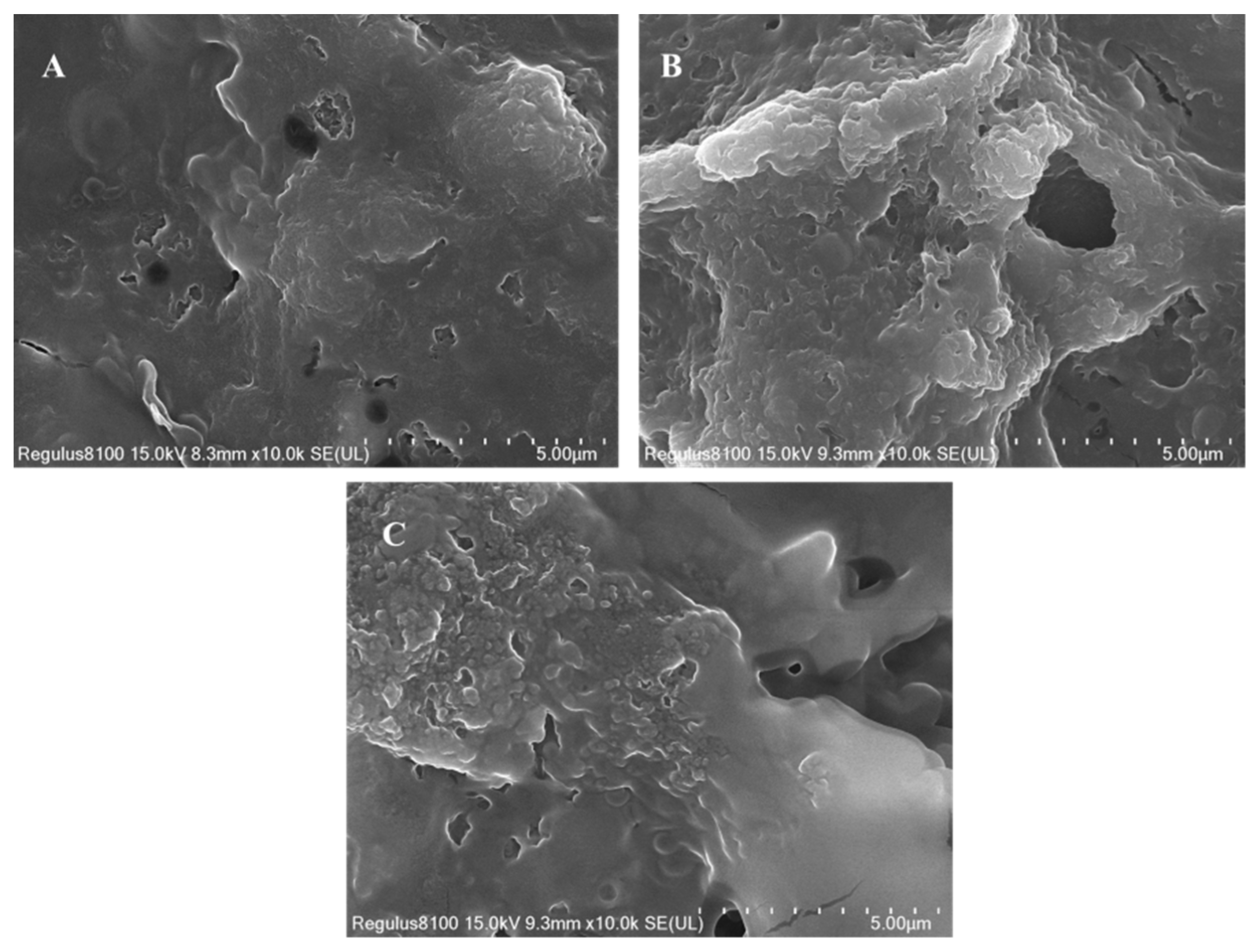
3.2.2. Influence of Ultrasonication on Structural Integrity via SEM
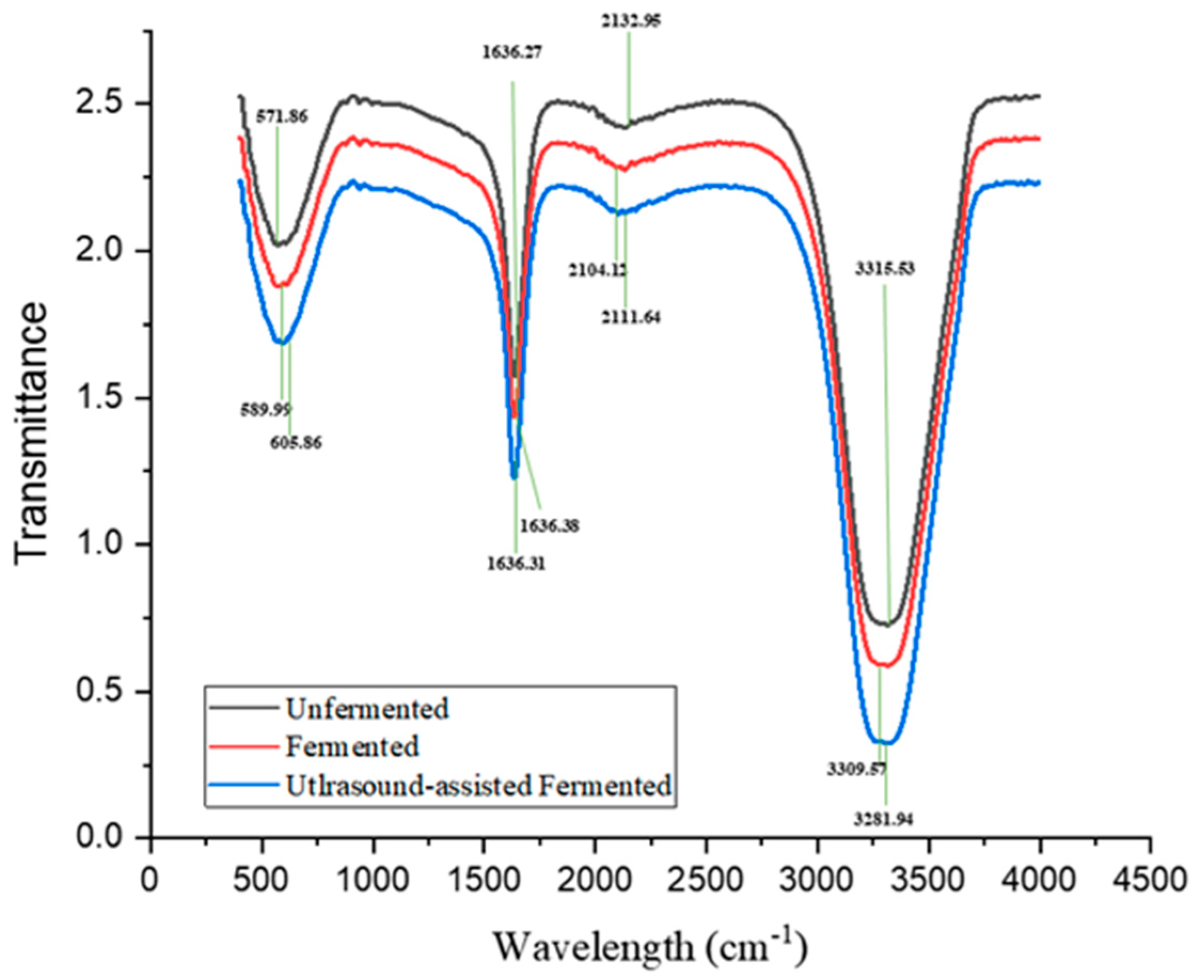
3.2.3. Influence of Ultrasonication on Structural Integrity via FTIR
3.3. High-Performance Liquid Chromatography (HPLC) Analysis
3.3.1. Organic Acids Content of Ultrasound-Assisted Chickpea Beverages
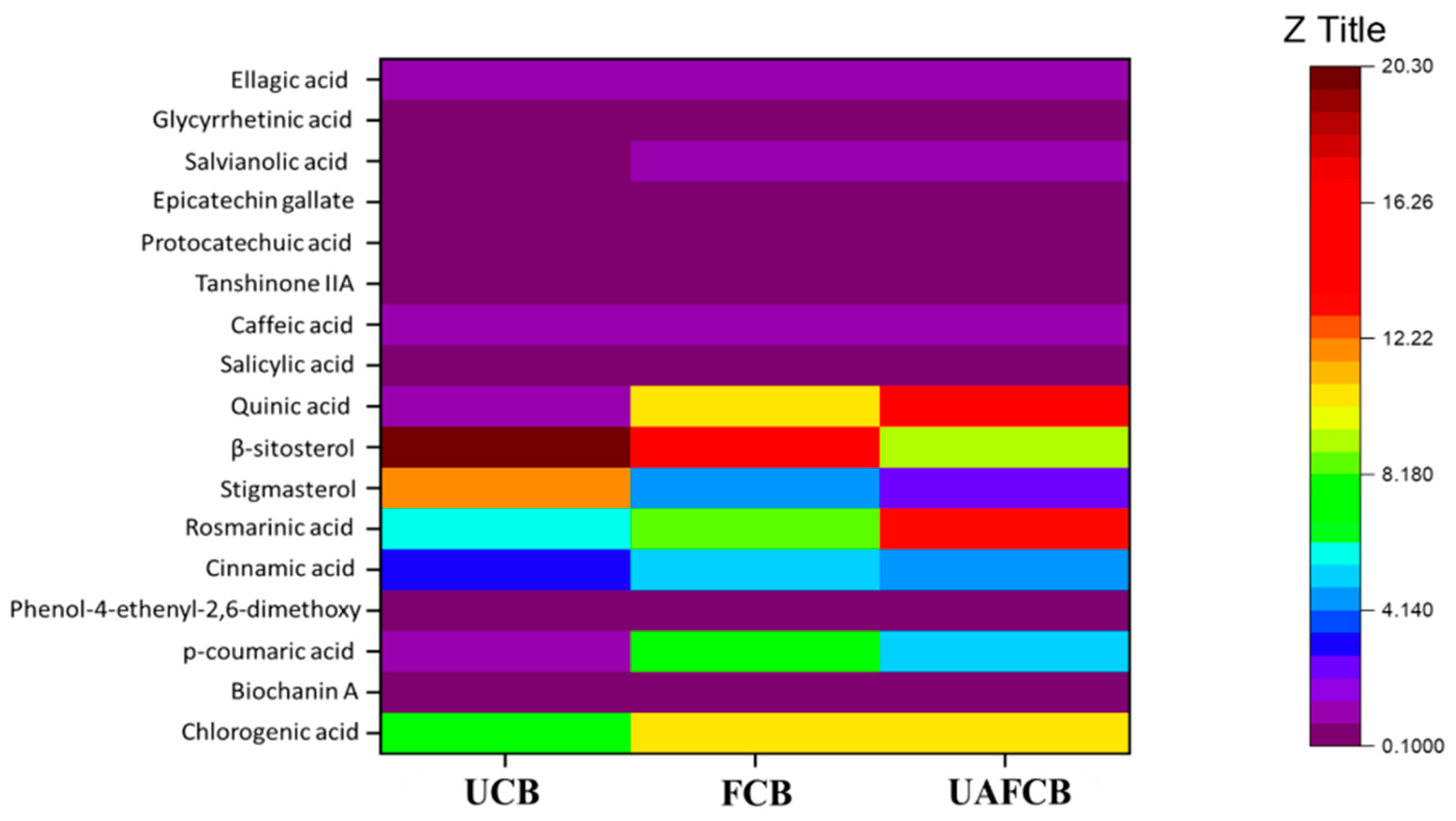
3.3.2. Phytochemicals of Ultrasound-Assisted Chickpea Beverages
3.4. Microbial Enumeration
3.5. Sensory Profile
3.5.1. Effect of Processing on Appearance and Colour UAF on Chickpea Beverages
3.5.2. eNose Analysis
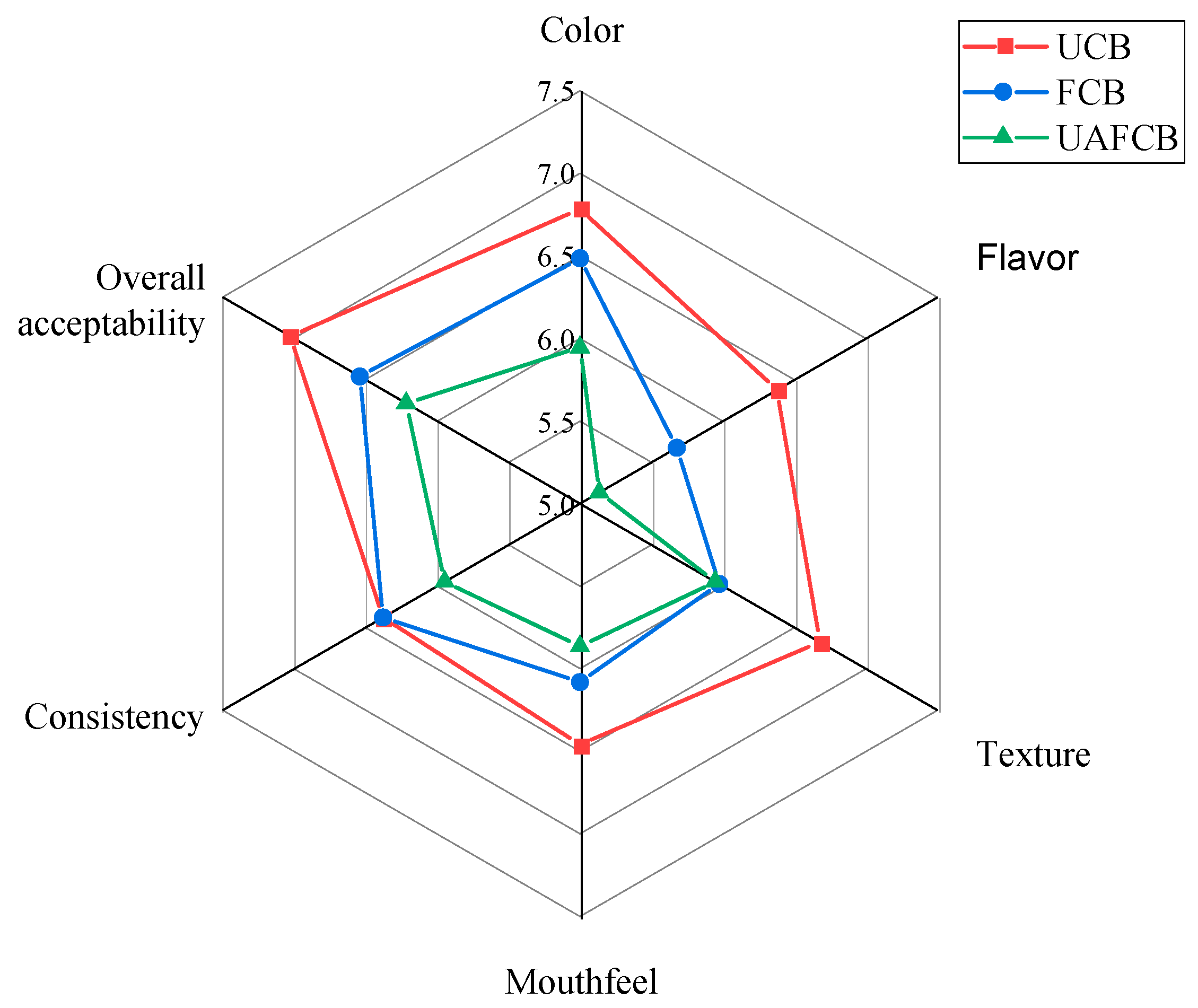
3.5.3. Consumer Acceptability Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Merga, B.; Haji, J. Economic Importance of Chickpea: Production, Value, and World Trade. Cogent Food Agric. 2019, 5, 1615718. [Google Scholar] [CrossRef]
- Wang, S.; Chelikani, V.; Serventi, L. Evaluation of Chickpea as Alternative to Soy in Plant-Based Beverages, Fresh and Fermented. LWT 2018, 97, 570–572. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef] [PubMed]
- Cencic, A.; Chingwaru, W. The Role of Functional Foods, Nutraceuticals, and Food Supplements in Intestinal Health. Nutrients 2010, 2, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Nazhand, A.; Souto, E.B.; Lucarini, M.; Souto, S.B.; Durazzo, A.; Santini, A. Ready to Use Therapeutical Beverages: Focus on Functional Beverages Containing Probiotics. Beverages 2020, 6, 26. [Google Scholar] [CrossRef]
- Mullins, A.P.; Arjmandi, B.H. Health Benefits of Plant-Based Nutrition: Focus on Beans in Cardiometabolic Diseases. Nutrients 2021, 13, 519. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Xie, B.; Sun, Z. Influence of Lactic Acid Bacteria Fermentation on Physicochemical Properties and Antioxidant Activity of Chickpea Yam Milk. J. Food Qual. 2021, 2021, 5523356. [Google Scholar] [CrossRef]
- Benali, A.; En-nahli, Y.; Elbaouchi, A.; Kabbour, M.R.; Kumar, S. Nutritional and Technological Optimization of Wheat-Chickpea- Milk Powder Composite Flour and Its Impact on Rheological and Sensorial Properties of Leavened Flat Bread. Foods 2021, 10, 1843. [Google Scholar] [CrossRef]
- Lopes, M.; Pierrepont, C.; Duarte, C.M.; Filipe, A.; Medronho, B.; Sousa, I. Legume Beverages from Chickpea and Lupin, as New Milk Alternatives. J. Ren. Nutr. 2020, 20, e7–e15. [Google Scholar] [CrossRef]
- Rincon, L.; Braz Assunção Botelho, R.; de Alencar, E.R. Development of Novel Plant-Based Milk Based on Chickpea and Coconut. LWT 2020, 128, 109479. [Google Scholar] [CrossRef]
- Sethi, S.; Tyagi, S.K.; Anurag, R.K. Plant-Based Milk Alternatives an Emerging Segment of Functional Beverages: A Review. J. Food Sci. Technol. 2016, 53, 3408–3423. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.E.; Shakappa, D. A Review of the Nutritional and Antinutritional Constituents of Chickpea (Cicer arietinum) and Its Health Benefits. Crop Pasture Sci. 2022, 73, 401–414. [Google Scholar] [CrossRef]
- Gupta, N.; Quazi, S.; Jha, S.K.; Siddiqi, M.K.; Verma, K.; Sharma, S.; Khan, R.H.; Bhagyawant, S.S. Chickpea Peptide: A Nutraceutical Molecule Corroborating Neurodegenerative and ACE-I Inhibition. Nutrients 2022, 14, 4824. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.; Roura, S.I.; Valle, C.E. Quality of Swiss Chard Produced by Conventional and Organic Methods. LWT-Food Sci. Technol. 2003, 36, 135–141. [Google Scholar] [CrossRef]
- Nkhata, S.G.; Ayua, E.; Kamau, E.H. Fermentation and Germination Improve Nutritional Value of Cereals and Legumes through Activation of Endogenous Enzymes. Food Sci. Nutr. 2018, 6, 2446–2458. [Google Scholar] [CrossRef]
- Popova, A.; Mihaylova, D. Antinutrients in Plant-Based Foods: A Review. Open Biotechnol. J. 2019, 13, 68–76. [Google Scholar] [CrossRef]
- Zhao, Y.S.; Eweys, A.S.; Zhang, J.Y.; Zhu, Y.; Bai, J.; Darwesh, O.M.; Zhang, H.B.; Xiao, X. Fermentation Affects the Antioxidant Activity of Plant-Based Food Material through the Release and Production of Bioactive Components. Antioxidants 2021, 10, 2004. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Thakur, K.; Feng, J.Y.; Cai, J.S.; Zhang, J.G.; Hu, F.; Wei, Z.J. B-Vitamin Enriched Fermented Soymilk: A Novel Strategy for Soy-Based Functional Foods Development. Trends Food Sci. Technol. 2020, 105, 43–55. [Google Scholar] [CrossRef]
- Ojha, K.S.; Mason, T.J.; O’Donnell, C.P.; Kerry, J.P.; Tiwari, B.K. Ultrasound Technology for Food Fermentation Applications. Ultrason. Sonochem. 2017, 34, 410–417. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Chakraborty, S. Optimization of Extraction Process for Legume-Based Synbiotic Beverages, Followed by Their Characterization and Impact on Antinutrients. Int. J. Gastron. Food Sci. 2022, 28, 100506. [Google Scholar] [CrossRef]
- Boeck, T.; Ispiryan, L.; Hoehnel, A.; Sahin, A.W.; Coffey, A.; Zannini, E.; Arendt, E.K. Lentil-Based Yogurt Alternatives Fermented with Multifunctional Strains of Lactic Acid. Foods 2022, 11, 2013. [Google Scholar] [CrossRef] [PubMed]
- Demarinis, C.; Verni, M.; Pinto, L.; Rizzello, C.G.; Baruzzi, F. Use of Selected Lactic Acid Bacteria for the Fermentation of Legume-Based Water Extracts. Foods 2022, 11, 3346. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.Y.; Liu, Y.; Xu, M.; Zhang, T.H.; Ren, H.; Liu, W.; Li, M.Y. Accelerated aging of grape pomace vinegar by using additives combined with physical methods. J. Food Process. Eng. 2020, 43, e13398. [Google Scholar] [CrossRef]
- Gao, X.; Liu, E.; Zhang, J.; Yang, L.; Huang, Q.; Chen, S.; Ma, H.; Ho, C.; Liao, L. Accelerating Aroma Formation of Raw Soy Sauce Using Low Intensity Sonication. Food Chem. 2020, 329, 127118. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Rombaut, N.; Sicaire, A.; Meullemiestre, A.; Abert-vian, M. Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Lopeda-correa, M.; Vald, B.E.; Osorio-tob, J.F. Ultrasound-Assisted Extraction of Phenolic Compounds from Adenaria Floribunda Stem: Economic Assessment. Foods 2022, 11, 2904. [Google Scholar] [CrossRef]
- Lai, J.; Xin, C.; Zhao, Y.; Feng, B.; He, C.; Dong, Y.; Fang, Y.; Wei, S. Optimization of Ultrasonic Assisted Extraction of Antioxidants from Black Soybean (Glycine max var) Sprouts Using Response Surface Methodology. Molecules 2013, 18, 1101–1110. [Google Scholar] [CrossRef]
- Carrillo-lopez, L.M.; Garcia-galicia, I.A.; Tirado-gallegos, J.M.; Sanchez-Vega, R.; Huerta-jimenez, M.; Ashokkumar, M.; Alarcon-rojo, A.D. Recent Advances in the Application of Ultrasound in Dairy Products: Effect on Functional, Physical, Chemical, Microbiological and Sensory Properties. Ultrason. Sonochem. 2021, 73, 105467. [Google Scholar] [CrossRef]
- Ma, X.; Li, T.; He, Y.; Chen, M.; Zhou, J.; Yin, L.; Ma, H. Preliminary Study on Ultrasonic Ageing Zhenjiang Vinegar Mechanism Based on Maillard Simulation System. J. Food Qual. 2020, 2020, 1087863. [Google Scholar] [CrossRef]
- Alves, L.D.L.; Donadel, J.Z.; Athayde, D.R.; Stefanello, M.; Klein, B.; Fagundes, M.B.; De Menezes, C.R.; Barin, J.S.; Cezar, P.; Campagnol, B.; et al. Effect of Ultrasound on Proteolysis and the Formation of Volatile Compounds in Dry Fermented Sausages. Ultrason. Sonochem. 2020, 67, 105161. [Google Scholar] [CrossRef]
- Yıkmıs, S.; Bozgeyik, E.; Simsek, M.A. Ultrasound Processing of Verjuice (Unripe Grape Juice) Vinegar: Effect on Bioactive Compounds, Sensory Properties, Microbiological Quality and Anticarcinogenic Activity. J. Food Sci. Technol. 2020, 57, 3445–3456. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Su, Y.; Zhang, Y.; Zhu, P.; Mei, Z.; Zhou, X. Potential Use of Ultrasound to Promote Fermentation, Maturation, and Properties of Fermented Foods: A Review. Food Chem. 2021, 357, 129805. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kristo, E.; Lapointe, G. Food Hydrocolloids The Effect of Apple Pomace on the Texture, Rheology and Microstructure of Set Type Yogurt. Food Hydrocoll. 2019, 91, 83–91. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Khanashyam, A.C.; Mundanat, A.S.; Shah, K.; Babu, K.S.; Thorakkattu, P.; Al-asmari, F.; Pandiselvam, R. Valorization of Fruit Waste for Bioactive Compounds and Their Applications in the Food Industry. Foods 2023, 12, 556. [Google Scholar] [CrossRef]
- Nemo, R.; Bacha, K. Microbial, Physicochemical and Proximate Analysis of Selected Ethiopian Traditional Fermented Beverages. LWT-Food Sci. Technol. 2020, 131, 109713. [Google Scholar] [CrossRef]
- Mefleh, M.; Faccia, M.; Natrella, G.; Caponio, F.; Summo, C.; De Angelis, D.; Pasqualone, A. Development and Chemical-Sensory Characterization of Chickpeas-Based Beverages Fermented with Selected Starters. Foods 2022, 11, 3578. [Google Scholar] [CrossRef]
- Akpabli-Tsigbe, N.D.K.; Ma, Y.; Ekumah, J.N.; Osabutey, J.; Hu, J.; Xu, M.; Johnson, N.A.N. Single-Frequency Ultrasonic Extraction of Bioactive Chlorogenic Acid from Heilong48 Soybean Variety: Parametric Optimization and Comprehensive Evaluation of Physicochemical and Bioactive Properties. Food Sci. Nutr. 2022, 10, 374–387. [Google Scholar] [CrossRef]
- Zhang, P.; Tang, F.; Cai, W.; Zhao, X. Evaluating the Effect of Lactic Acid Bacteria Fermentation on Quality, Aroma, and Metabolites of Chickpea Milk. Front. Nutr. 2022, 9, 1069714. [Google Scholar] [CrossRef]
- Oba, D.O.; Okunola, O.J.; Oranusi, S.U.; Okagbue, H.I. Data on Microbial and Physicochemical Assessment of Mixed Fruit Wine Produced from Physically Damaged Fruits. Data Br. 2018, 19, 678–686. [Google Scholar] [CrossRef]
- Poliseli-scopel, F.H.; Hernández-herrero, M.; Guamis, B.; Ferragut, V. Comparison of Ultra High Pressure Homogenization and Conventional Thermal Treatments on the Microbiological, Physical and Chemical Quality of Soymilk. LWT-Food Sci. Technol. 2012, 46, 42–48. [Google Scholar] [CrossRef]
- Lucia, M.; Rahayu, S.; Haerah, D.; Wahyuni, D. Detection of Staphylococcus Aureus and Streptococcus Agalactiae: Subclinical Mastitis Causes in Dairy Cow and Dairy Buffalo (Bubalus bubalis). Am. J. Biomed. Res. 2017, 5, 8–13. [Google Scholar] [CrossRef]
- Zheng, X.; Yan, Z.; Han, B.; Zwietering, M.H.; Samson, R.A.; Boekhout, T.; Nout, M.J.R. Complex Microbiota of a Chinese “Fen” Liquor Fermentation Starter (Fen-Daqu), Revealed by Culture-Dependent and Culture-Independent Methods. Food Microbiol. 2012, 31, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Boateng, I.D.; Yang, X.; Li, Y. Optimization of Infrared-Drying Parameters for Ginkgo biloba L. Seed and Evaluation of Product Quality and Bioactivity. Ind. Crop. Prod. 2020, 160, 113108. [Google Scholar] [CrossRef]
- Plesoianu, A.M.; Nour, V. Effect of Some Polysaccharide-Based Edible Coatings on Fresh White Button Mushroom (Agaricus bisporus) Quality during Cold Storage. Agriculture 2022, 12, 1491. [Google Scholar] [CrossRef]
- Monteiro, S.S.; Ribeiro, S.R.; Soquetta, M.B.; Pires, F.J.; Wagner, R.; Severo da Rosa, C. Evaluation of the Chemical, Sensory and Volatile Composition of Sapota-Do- Solimões Pulp at Di Ff Erent Ripening Stages. Food Res. Int. 2018, 109, 159–167. [Google Scholar] [CrossRef]
- Craine, E.B.; Murphy, K.M.; Bramwell, S.; Ross, C.F.; Fisk, S. Strategic Malting Barley Improvement for Craft Brewers through Consumer Sensory Evaluation of Malt and Beer. J. Food Sci. 2021, 86, 3628–3644. [Google Scholar] [CrossRef]
- Jere, A.D.; Mbachi, A.; Mlotha, V.; Thuy, U.; Phan, X.; Adhikari, K. Acceptability of Traditional Cooked Pumpkin Leaves Seasoned with Peanut Flour Processed from Blanched, Deskinned and Raw Peanuts of Different Varieties. Sci. Afr. 2020, 10, e00598. [Google Scholar] [CrossRef]
- Boateng, I.D.; Saalia, F.K.; Zhang, W.; Yang, X.; Li, Y. Non-Thermal Pretreatment Affects Ginkgo biloba L. Seed’s Product Qualities, Sensory, and Physicochemical Properties. J. Food Sci. 2022, 87, 94–111. [Google Scholar] [CrossRef]
- Capozzi, V.; Fragasso, M.; Romaniello, R.; Berbegal, C.; Russo, P.; Spano, G. Spontaneous Food Fermentations and Potential Risks for Human Health. Fermentation 2017, 3, 49. [Google Scholar] [CrossRef]
- Huang, G.; Chen, S.; Dai, C.; Sun, L.; Sun, W.; Tang, Y.; Xiong, F.; He, R.; Ma, H. Effects of Ultrasound on Microbial Growth and Enzyme Activity. Ultrason. Sonochem. 2017, 37, 144–149. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Potkule, J.; Punia, S.; Dhakane-lad, J.; Singh, S.; Dhumal, S.; Chandra, P.; Bhushan, B.; Anitha, T.; et al. Functional Characterization of Plant-Based Protein to Determine Its Quality for Food Applications. Food Hydrocoll. 2022, 123, 106986. [Google Scholar] [CrossRef]
- Larsen, L.R.; Van Der Weem, J.; Caspers-weiffenbach, R.; Schieber, A.; Weber, F. Effects of Ultrasound on the Enzymatic Degradation of Pectin. Ultrason. Sonochem. 2021, 72, 105465. [Google Scholar] [CrossRef]
- Kentish, S.; Ashokkumar, M. The Physical and Chemical Effects of Ultrasound. In Ultrasound Technologies for Food and Bioprocessing; Springer: New York, NY, USA, 2011; pp. 1–12. [Google Scholar] [CrossRef]
- Chemat, F.; Khan, M.K. Applications of Ultrasound in Food Technology: Processing, Preservation and Extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Zafar, T.A.; Aldughpassi, A.; Al-mussallam, A. Microstructure of Whole Wheat versus White Flour and Wheat- Chickpea Flour Blends and Dough: Impact on the Glycemic Response of Pan Bread. Int. J. Food Sci. 2020, 2020, 8834960. [Google Scholar] [CrossRef] [PubMed]
- Akpabli-Tsigbe, N.D.K.; Ma, Y.; Ekumah, J.N.; Osabutey, J.; Hu, J.; Xu, M.; Johnson, N.A.N. Novel Solid-State Fermentation Extraction of 5-O-Caffeoylquinic Acid from Heilong48 Soybean Using Lactobacillus Helviticus: Parametric Screening and Optimization. LWT 2021, 149, 111809. [Google Scholar] [CrossRef]
- Chandrapala, J.; Zisu, B.; Palmer, M.; Kentish, S.; Ashokkumar, M. Effects of Ultrasound on the Thermal and Structural Characteristics of Proteins in Reconstituted Whey Protein Concentrate. Ultrason. Sonochem. 2011, 18, 951–957. [Google Scholar] [CrossRef]
- Abesinghe, A.M.N.L.; Islam, N.; Vidanarachchi, J.K.; Prakash, S.; Silva, K.F.S.T.; Karim, M.A. Effects of Ultrasound on the Fermentation pro Fi Le of Fermented Milk Products Incorporated with Lactic Acid Bacteria. Int. Dairy J. 2019, 90, 1–14. [Google Scholar] [CrossRef]
- Gupta, M.; Abu-ghannam, N. Barley for Brewing: Characteristic Changes during Malting, Brewing and Applications of Its. Compr. Rev. Food Sci. Food Saf. 2010, 9, 318–328. [Google Scholar] [CrossRef]
- Ayyash, M.; Liu, S.-Q.; Al Mheiri, A.; Aldhaheri, M.; Raeisi, B.; Al-Nabulsi, A.; Osaili, T.; Olaimat, A. In Vitro Investigation of Health-Promoting Benefits of Fermented Camel Sausage by Novel Probiotic Lactobacillus plantarum: A Comparative Study with Beef Sausages Mutamed. LWT-Food Sci. Technol. 2018, 99, 346–354. [Google Scholar] [CrossRef]
- Ashokkumar, M.; Lee, J.; Kentish, S.; Grieser, F. Bubbles in an Acoustic Field: An Overview. Ultrason. Sonochem. 2007, 14, 470–475. [Google Scholar] [CrossRef]
- Fadlelmoula, A.; Pinho, D.; Carvalho, V.H.; Catarino, S.O.; Minas, G. Fourier Transform Infrared (FTIR) Spectroscopy to Analyse Human Blood over the Last 20 Years: A Review towards Lab-on-a-Chip Devices. Micromachines 2022, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Dıblan, S.; Kadiroğlu, P.; Aydemir, L.Y. FT-IR Spectroscopy Characterization and Chemometric Evaluation of Legumes Extracted with Different Solvents. Food Health 2018, 4, 80–88. [Google Scholar] [CrossRef]
- Bayu, A.; Nandiyanto, D.; Oktiani, R.; Ragadhita, R. How to Read and Interpret FTIR Spectroscope of Organic Material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Mi’skiewicz, K.; Rosicka-kaczmarek, J.; Nebesny, E. Effects of Chickpea Protein on Carbohydrate Reactivity in Acrylamide Formation in Low Humidity Model Systems. Foods 2020, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- Jukanti, A.K.; Gaur, P.M.; Gowda, C.L.L.; Chibbar, R.N. Nutritional Quality and Health Benefits of Chickpea (Cicer arietinum L.): A Review. Br. J. Nutr. 2012, 108, S11–S26. [Google Scholar] [CrossRef]
- Kadiroğlu, P.; Aydemir, L.Y.; Akcakaya, F.G. Prediction of Functional Properties of Registered Chickpea Samples Using FT-IR Spectroscopy and Chemometrics. LWT 2018, 93, 463–469. [Google Scholar] [CrossRef]
- Zhao, F.; Zhai, X.; Liu, X.; Lian, M.; Liang, G.; Cui, J.; Dong, H. Properties, and Enzymolysis of Walnut Protein Isolate. Molecules 2022, 27, 208. [Google Scholar] [CrossRef]
- Kang, S.; Zhang, J.; Guo, X.; Lei, Y.; Yang, M. Effects of Ultrasonic Treatment on the Structure, Functional Properties of Chickpea Protein Isolate and Its Digestibility. Foods 2022, 11, 880. [Google Scholar] [CrossRef]
- Adebo, O.A.; Medina-Meza, I.G. Impact of Fermentation on the Phenolic Compounds and Antioxidant Activity of Whole Cereal Grains. Molecules 2020, 25, 927. [Google Scholar] [CrossRef]
- Mengesha, Y.; Tebeje, A.; Tilahun, B. Review Article A Review on Factors Influencing the Fermentation Process of Teff (Eragrostis Teff) and Other Cereal-Based Ethiopian Injera. Int. J. Food Sci. 2022, 2022, 4419955. [Google Scholar] [CrossRef]
- Toker, C.; Karhan, M.; Ulger, S.; Departments, S.; Crops, F. Endogenous Organic Acid Variations in Different Chickpea (Cicer arietinum L) Genotypes Endogenous Organic Acid Variations in Different Chickpea (Cicer arietinum L.) Genotypes. Acta Agric. Scand. Sect. B-Plant Soil Sci. 2004, 54, 42–44. [Google Scholar] [CrossRef]
- Wang, H.; Tao, Y.; Li, Y.; Wu, S.; Li, D.; Liu, X.; Han, Y.; Manickam, S.; Loke, P. Application of Ultrasonication at Different Microbial Growth Stages during Apple Juice Fermentation by Lactobacillus Plantarum: Investigation on the Metabolic Response. Ultrason. Sonochem. 2021, 73, 105486. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.P.; Rosario, A.I.L.S.; Lelis, C.A.; Rekowsky, B.S.S.; Carvalho, A.P.A.; Rosário, D.K.A.; Elias, T.A.; Costa, M.P.; Foguel, D.; Conte-junior, C.A. Bioactive Compounds from Kefir and Their Potential Benefits on Health: A Systematic Review and Meta-Analysis. Oxid. Med. Cell. Longev. 2021, 2021, 9081738. [Google Scholar] [CrossRef] [PubMed]
- Suwannakham, S.; Yang, S. Enhanced Propionic Acid Fermentation by Propionibacterium Acidipropionici Mutant Obtained by Adaptation in a Fibrous-Bed Bioreactor. Biotechnol. Bioeng. 2005, 91, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Leitzmann, C. Characteristics and Health Benefits of Phytochemicals. Complement. Med. Res. 2016, 23, 69–74. [Google Scholar] [CrossRef]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial Fermentation and Its Role in Quality Improvement of Fermented Foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Engmann, F.N.; Ma, Y.; Tchabo, W.; Ma, H. Ultrasonication Treatment Effect on Anthocyanins, Color, Microorganisms and Enzyme Inactivation of Mulberry (Moraceae Nigra) Juice. J. Food Process. Preserv. 2015, 39, 854–862. [Google Scholar] [CrossRef]
- Ismail, B.B.; Yusuf, H.L.; Pu, Y.; Zhao, H.; Guo, M.; Liu, D. Ultrasound-Assisted Adsorption/Desorption for the Enrichment and Purification of Flavonoids from Baobab (Adansonia Digitata) Fruit Pulp. Ultrason. Sonochem. 2020, 65, 104980. [Google Scholar] [CrossRef]
- Bu, K.; Wito, I.; Mikucka, W.; Zieli, M. Valorization of Distillery Stillage by Polyphenol Recovery Using Microwave-Assisted, Ultrasound-Assisted and Conventional Extractions. J. Environ. Manag. 2022, 322, 116150. [Google Scholar] [CrossRef]
- Ojha, K.S.; Tiwari, B.K.; Donnell, C.P.O. Effect of Ultrasound Technology on Food and Nutritional Quality. Adv. Food Nutr. Res. 2018, 84, 207–240. [Google Scholar] [CrossRef]
- Jiménez-López, J.; Ruiz-Medina, A.; Ortega-Barrales, P.; Llorent-Martínez, E.J. Phytochemical pro Fi Le and Antioxidant Activity of Caper Berries (Capparis spinosa L.): Evaluation of the in Fl Uence of the Fermentation Process. Food Chem. 2018, 250, 54–59. [Google Scholar] [CrossRef]
- Weragama, D.; Weerasingha, V.; Jayasumana, L.; Adikari, J.; Vidanarachchi, J.K.; Priyashantha, H. The Physicochemical, Microbiological, and Organoleptic Properties and Antioxidant Activities of Cream Cheeses Fortified with Dried Curry Leaves (Murraya koenigii L.) Powder. Food Sci. Nutr. 2021, 9, 5774–5784. [Google Scholar] [CrossRef] [PubMed]
- Alp, D.; Bulantekin, Ö. The Microbiological Quality of Various Foods Dried by Applying Different Drying Methods: A Review. Eur. Food Res. Technol. 2021, 247, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Khairuzzaman; Chowdhury, F.M.; Zaman, S.; Al Mamun, A.; Bari, L. Food Safety Challenges towards Safe, Healthy, and Nutritious Street Foods in Bangladesh. Int. J. Food Sci. 2014, 2014, 483519. [Google Scholar] [CrossRef] [PubMed]
- Ferragut, V.; Hernández-herrero, M.; Teresa, M.; Borras-suarez, M.; González-linares, J. Ultra-High-Pressure Homogenization (UHPH) System for Producing High-Quality Vegetable-Based Beverages: Physicochemical, Microbiological, Nutritional and Toxicological Characteristics. J. Sci. Food Agric. 2014, 95, 953–961. [Google Scholar] [CrossRef]
- Cebri, G.; Condón, S.; Tecnología, P.M. Physiology of the Inactivation of Vegetative Bacteria by Thermal Treatments: Mode of Action, Influence of Environmental Factors and Inactivation Kinetics. Foods 2017, 6, 107. [Google Scholar] [CrossRef]
- Rachon, G.; Raleigh, C.P.; Pawlowsky, K. Heat Resistance of Yeast Ascospores and Their Utilisation for the Validation of Pasteurisation Processes for Beers. J. Inst. Brew. 2021, 127, 149–159. [Google Scholar] [CrossRef]
- Park, J.; Kim, M. Comparison of Dry Medium Culture Plates for Mesophilic Aerobic Bacteria in Milk, Ice Cream, Ham, and Codfish Fillet Products. Prev. Nutr. Food Sci. 2013, 18, 269–272. [Google Scholar] [CrossRef]
- Pothakos, V.; Samapundo, S.; Devlieghere, F. Total Mesophilic Counts Underestimate in Many Cases the Contamination Levels of Psychrotrophic Lactic Acid Bacteria (LAB) in Chilled-Stored Food Products at the End of Their Shelf-Life. YFMIC 2012, 32, 437–443. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in Food Systems: Significance and Emerging Strategies Towards Improved Viability and Delivery of Enhanced Beneficial Value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef]
- Wang, L.; Xu, B.; Wei, B.; Zeng, R. Low Frequency Ultrasound Pretreatment of Carrot Slices: E Ff Ect on the Moisture Migration and Quality Attributes by Intermediate-Wave Infrared Radiation Drying. Ultrason. Sonochem. 2018, 40, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Krishnaswamy, K.; Mustapha, A. Physical Properties of Complementary Food Powder Obtained from Upcycling of Greek Yogurt Acid Whey with Kodo and Proso Millets. J. Food Process. Eng. 2021, 44, e13878. [Google Scholar] [CrossRef]
- Tamanna, N.; Mahmood, N. Food Processing and Maillard Reaction Products: Effect on Human Health and Nutrition. Int. J. Food Sci. 2015, 2015, 526762. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Hu, Y.; Abiola, O.; Ma, H. Dual-Frequency Multi-Angle Ultrasonic Processing Technology and Its Real-Time Monitoring on Physicochemical Properties of Raw Soymilk and Soybean Protein. Ultrason. Sonochem. 2021, 80, 105803. [Google Scholar] [CrossRef]
- Derardja, A.E.; Pretzler, M.; Kampatsikas, I.; Radovic, M.; Fabisikova, A.; Zehl, M.; Barkat, M.; Rompel, A. Current Research in Food Science Polyphenol Oxidase and Enzymatic Browning in Apricot (Prunus armeniaca L.): Effect on Phenolic Composition and Deduction of Main Substrates. Curr. Res. Food Sci. 2022, 5, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kumar, A.; Jayachandran, L.E.; Srinivasa, P. Sequential Microwave—Ultrasound Assisted Extraction of Soymilk and Optimization of Extraction Process. LWT 2021, 151, 112220. [Google Scholar] [CrossRef]
- Zhang, Q.A.; Shen, Y.; Fan, X.H.; García Martín, J.F. Preliminary Study of the Effect of Ultrasound on Physicochemical Properties of Red Wine. CYTA-J. Food 2016, 14, 55–64. [Google Scholar] [CrossRef]
- Oladejo, A.O.; Ma, H.; Qu, W.; Zhou, C. Effects of Ultrasound on Mass Transfer Kinetics, Structure, Carotenoid and Vitamin C Content of Osmodehydrated Sweet Potato (Ipomea batatas). Food Bioprocess Technol. 2017, 10, 1162–1172. [Google Scholar] [CrossRef]
- Makarewicz, M. The Interactions between Polyphenols and Microorganisms, Especially Gut Microbiota. Antioxidants 2021, 10, 188. [Google Scholar] [CrossRef]
- Tyagi, H.; Daulton, E.; Bannaga, A.S.; Arasaradnam, R.P.; Covington, J.A. Non-Invasive Detection and Staging of Colorectal Cancer Using a Portable Electronic Nose. Sensors 2021, 21, 5440. [Google Scholar] [CrossRef]
- Baietto, M.; Wilson, A.D.; Bassi, D.; Ferrini, F. Evaluation of Three Electronic Noses for Detecting Incipient Wood Decay. Sensors 2010, 10, 1062–1092. [Google Scholar] [CrossRef] [PubMed]
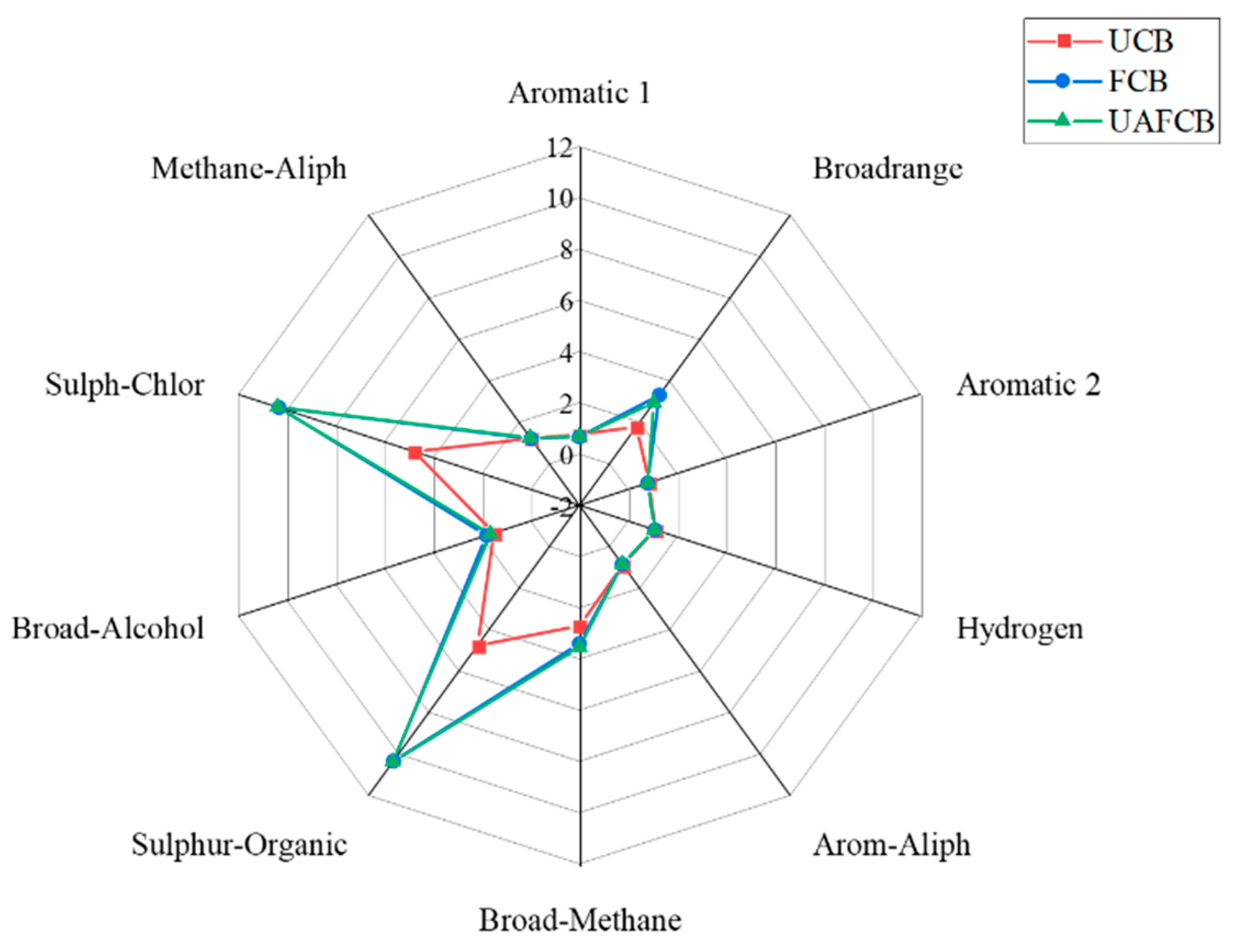
| Sensor Number | Sensor Name | Main Applications |
|---|---|---|
| S1 | W1C | Aroma component 1 |
| S2 | W5S | Broadrange (nitrogen oxide) |
| S3 | W3C | Aromatic component (ammonia) |
| S4 | W6S | Hydrogen |
| S5 | W5C | Aromatic hydrocarbons |
| S6 | W1S | Methane |
| S7 | W1W | Organic sulfide |
| S8 | W2S | Alcohols, aldehydes, and ketones |
| S9 | W2W | Aromatic sulfide |
| S10 | W3S | Alkanes |
| Proximate Content | UCB | FCB | UAFCB |
|---|---|---|---|
| Moisture | 85.16 ± 2.50 a | 80.63 ± 0.55 a | 81.12 ± 2.60 a |
| Solids | 14.84 ± 2.49 a | 19.37 ± 0.55 a | 18.88 ± 2.60 a |
| Ash | 0.32 ± 0.08 a | 0.38 ± 0.15 a | 0.39 ± 0.19 a |
| Proteins | 0.71 ± 0.01 a | 0.69 ± 0.01 b | 0.67 ± 0.005 c |
| Fats | 6 ± 1.00 a | 5.67 ± 2.08 a | 4.33 ± 1.53 a |
| Carbohydrates | 7.85 ± 0.20 a | 12.95 ± 0.32 b | 13.45 ± 1.45 b |
| Organic Acid | Target Concentration (mg/mL) | ||
|---|---|---|---|
| UCB | FCB | UAFCB | |
| Malic acid | 0.53 ± 3 × 10−5 a | 0.93 ± 2 × 10−5 b | 0.56 ± 2 × 10−5 c |
| Lactic Acid | 0.71 ± 2 × 10−5 a | 1.62 ± 1 × 10−5 b | 1.01 ± 3 × 10 −4 c |
| Acetic Acid | 0.09 ± 3 × 10−5 a | 0.36 ± 2 × 10−5 b | 0.21 ± 4 × 10−5 c |
| Citric acid | 0.15 ± 1 × 10−5 a | 0.05 ± 1 × 10−5 b | 0.03 ± 2 × 10−5 c |
| Propanoic acid | 1.79 ± 3 × 10−5 a | 5.56 ± 2 × 10−5 b | 3.50 ± 0.00344 c |
| Phytochemical | Target Concentration (µg/mL) | ||
|---|---|---|---|
| UCB | FCB | UAFCB | |
| Chlorogenic acid | 7.44 ± 0.04 a | 10.3 ± 0.05 b | 10.20 ± 0.05 b |
| Biochanin A | 0.10 ± 0.00 a | 0.205 ± 0.00 b | 0.21 ± 0.00 b |
| p-coumaric acid | 1.44 ± 0.02 a | 7.63 ± 0.05 b | 5.15 ± 0.03c |
| Phenol, 4-ethenyl-2,6-dimethoxy | 0.29 ± 0.00 a | 0.26 ± 0.00 b | 0.33 ±0.00 c |
| Cinnamic acid | 3.33 ± 0.03 a | 5.07 ± 0.04 b | 4.69 ± 0.04 c |
| Rosmarinic Acid | 5.58 ± 0.04 a | 8.7 ± 0.05 b | 13.20 ±0.05 c |
| Stigmasterol | 11.70 ± 0.05 a | 4.67 ± 0.04 b | 2.41 ± 0.03 c |
| β-sitosterol | 20.30 ± 0.05 a | 14.7 ± 0.05 b | 9.31 ± 0.04 c |
| Quinic acid | 0.80 ± 0.01 a | 10.70 ± 0.01 b | 14.10 ± 0.05 c |
| Salicylic acid | 0.69 ± 0.01 a | 0.70 ± 0.01 a | 0.68 ± 0.01 a |
| Caffeic acid | 0.86 ± 0.01 a | 0.84 ± 0.01 a | 0.85 ± 0.01 a |
| Tanshinone IIA | 0.11 ± 0.01 a | 0.11 ± 0.00 a | 0.11 ± 0.00 a |
| Protocatechuic acid | 0.60 ± 0.01 a | 0.52 ± 0.01 b | 0.63 ± 0.004 c |
| Epicatechin gallate | 0.44 ± 0.01 a | 0.43 ± 0.01 a | 0.44 ± 0.01 a |
| Salvianolic acid | 0.76 ± 0.01 a | 0.78 ± 0.01 b | 0.80 ± 0.005 c |
| Glycyrrhetinic acid | 0.11 ± 0.00 a | 0.11 ± 0.00 a | 0.11 ± 0.01 a |
| Ellagic acid | 0.90 ± 0.01 a | 1.00 ± 0.00 b | 1.29 ± 0.02 c |
| Total phytochemical content | 55.41 | 66.71 | 64.51 |
| Organism | UCB (log cfu mL−1) | FCB × 104 (log cfu mL−1) | UAFCB × 104 (log cfu mL−1) |
|---|---|---|---|
| Mesophilic bacteria | n.d | 2.26 ± 0.03 | 2.41 ± 0.02 |
| Staphylococcus aureus | n.d | n.d | n.d |
| Yeast and mould | n.d | n.d | n.d |
| Samples | L* | a* | b* | Chroma | Hue | Browning Index | ΔE |
|---|---|---|---|---|---|---|---|
| UCB | 31.35 ± 1.12 a | 0.34 ± 0.37 a | 5.98 ± 0.38 a | 36.23 ± 4.13 a | 86.61 ± 3.81 a | −12.01 ± 0.54 a | 0.05 ± 1.12 a |
| FCB | 33.00 ± 1.22 ab | −0.12 ± 0.26 a | 5.66 ± 0.40 a | 32.34 ± 4.61 a | 91.09 ± 2.64 a | −14.63 ± 0.06 b | 0.05 ± 1.22 a |
| UAFCB | 34.63 ± 0.71 bc | −0.29 ± 0.03 a | 6.15 ± 0.05 a | 38.11 ± 0.68 a | 91.09 ± 0.261 a | −14.45 ± 0.33 b | 0.05 ± 0.71 a |
| Sample | Colour | Flavour | Texture | Mouthfeel | Consistency | Overall Acceptability |
|---|---|---|---|---|---|---|
| UCB | 6.78 ± 1.65 | 6.38 ± 1.57 | 6.68 ± 1.53 | 6.46 ± 1.68 | 6.38 ± 1.52 | 7.03 ± 1.80 |
| FCB | 6.49 ± 1.41 | 5.68 ± 2.12 | 5.97 ± 1.91 | 6.08 ± 2.05 | 6.38 ± 1.77 | 6.54 ± 1.78 |
| UAFCB | 5.95 ± 1.36 | 5.14 ± 2.13 | 5.95 ± 1.77 | 5.86 ± 1.96 | 5.95 ± 1.71 | 6.22 ± 1.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, N.A.N.; Adade, S.Y.-S.S.; Ekumah, J.-N.; Li, Y.; Betchem, G.; Issaka, E.; Ma, Y. Efficacy of Ultrasound-Assisted Lactic Acid Fermentation and Its Effect on the Nutritional and Sensory Quality of Novel Chickpea-Based Beverage. Fermentation 2023, 9, 495. https://doi.org/10.3390/fermentation9060495
Johnson NAN, Adade SY-SS, Ekumah J-N, Li Y, Betchem G, Issaka E, Ma Y. Efficacy of Ultrasound-Assisted Lactic Acid Fermentation and Its Effect on the Nutritional and Sensory Quality of Novel Chickpea-Based Beverage. Fermentation. 2023; 9(6):495. https://doi.org/10.3390/fermentation9060495
Chicago/Turabian StyleJohnson, Nana Adwoa Nkuma, Selorm Yao-Say Solomon Adade, John-Nelson Ekumah, Yanshu Li, Garba Betchem, Eliasu Issaka, and Yongkun Ma. 2023. "Efficacy of Ultrasound-Assisted Lactic Acid Fermentation and Its Effect on the Nutritional and Sensory Quality of Novel Chickpea-Based Beverage" Fermentation 9, no. 6: 495. https://doi.org/10.3390/fermentation9060495
APA StyleJohnson, N. A. N., Adade, S. Y.-S. S., Ekumah, J.-N., Li, Y., Betchem, G., Issaka, E., & Ma, Y. (2023). Efficacy of Ultrasound-Assisted Lactic Acid Fermentation and Its Effect on the Nutritional and Sensory Quality of Novel Chickpea-Based Beverage. Fermentation, 9(6), 495. https://doi.org/10.3390/fermentation9060495






