Development of a Microbial-Assisted Process for Enhanced Astaxanthin Recovery from Crab Exoskeleton Waste
Abstract
1. Introduction
2. Materials and Methods
2.1. Microoganisms and Stock Cultures
2.2. Crab Waste Preparation
2.3. Chemical Extraction of Astaxanthin
2.4. Microbial-Assisted Extraction of Astaxanthin
2.5. Effect of Pretreatment Conditions on Astaxanthin Recovery
2.6. Enzymatic Activity
2.7. 13C-NMR and HPLC Analysis
2.8. Antioxidant Activity of Astaxanthin
2.9. Anti-Inflammatory Activity of Astaxanthin
2.10. Statistical Analysis
3. Results
3.1. Astaxanthin Recovery
3.2. Effect of Pretreatment Conditions on Astaxanthin Recovery
3.3. Enzymatic Activity
3.4. 13C-NMR and HPLC Analysis
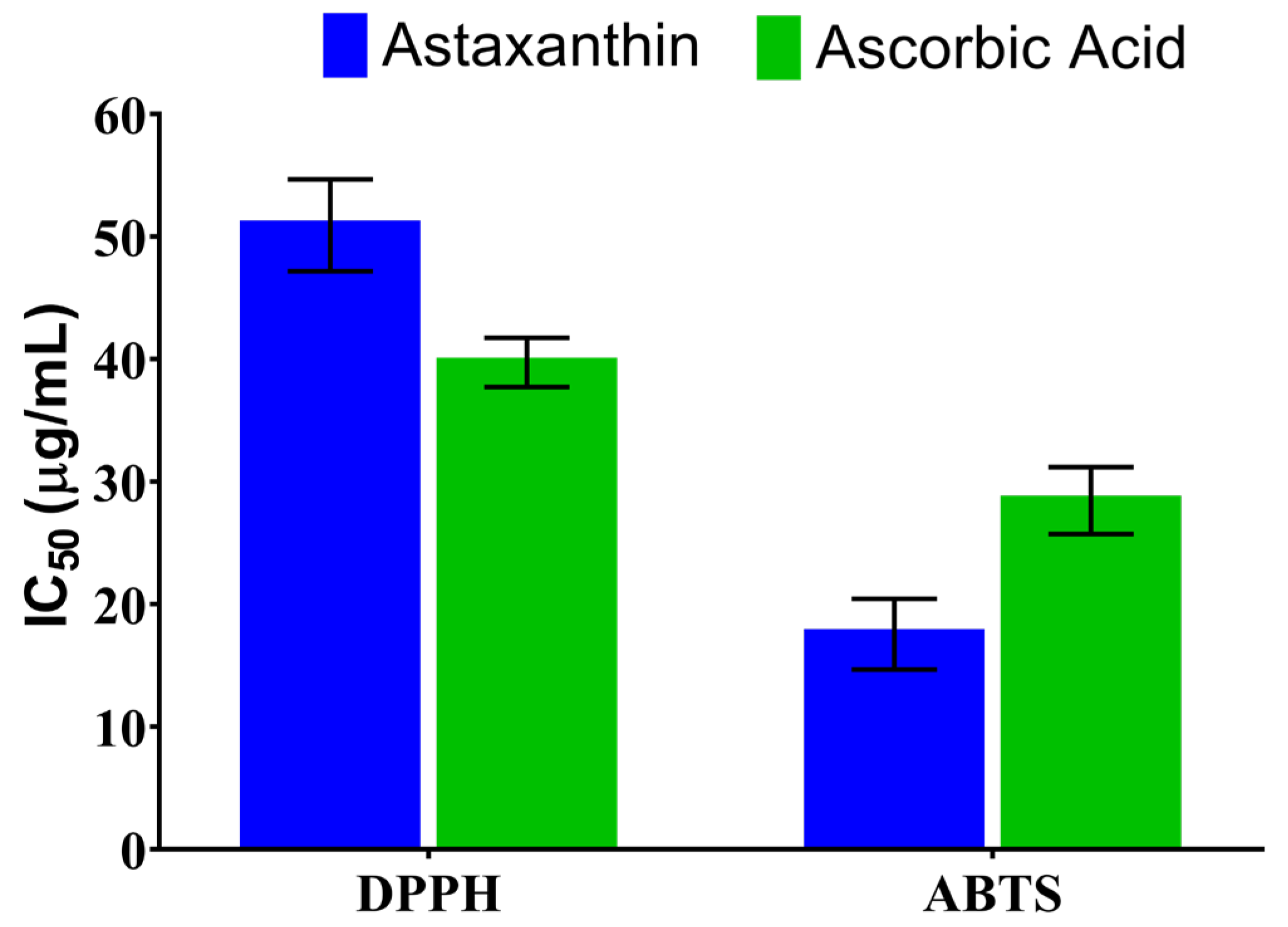
3.5. Antioxidant Activity
3.6. Anti-Inflammatory Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aneesh, P.A.; Ajeeshkumar, K.K.; Lekshmi, R.G.K.; Anandan, R.; Ravishankar, C.N.; Mathew, S. Bioactivities of Astaxanthin from Natural Sources, Augmenting Its Biomedical Potential: A Review. Trends Food Sci. Technol. 2022, 125, 81–90. [Google Scholar] [CrossRef]
- Siahaan, E.A.; Pangestuti, R.; Pratama, I.S.; Putra, Y.; Kim, S.K. Beneficial Effects of Astaxanthin in Cosmeceuticals with Focus on Emerging Market Trends. Glob. Perspect. Astaxanthin Ind. Prod. Food Health Pharm. Appl. 2021, 557–568. [Google Scholar] [CrossRef]
- Grand View Research Astaxanthin Market Size, Share, Growth & Trends Report 2030. Available online: https://www.grandviewresearch.com/industry-analysis/global-astaxanthin-market (accessed on 30 March 2023).
- Han, S.I.; Chang, S.H.; Lee, C.; Jeon, M.S.; Heo, Y.M.; Kim, S.; Choi, Y.E. Astaxanthin Biosynthesis Promotion with PH Shock in the Green Microalga, Haematococcus Lacustris. Bioresour. Technol. 2020, 314, 123725. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.P.M.; Souza, A.C.R.; Vasconcelos, A.R.; Prado, P.S.; Name, J.J. Antioxidant and Anti-Inflammatory Mechanisms of Action of Astaxanthin in Cardiovascular Diseases (Review). Int. J. Mol. Med. 2021, 47, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Khodir, S.A.; Sweed, E.; Gadallah, M.; Shabaan, A. Astaxanthin Attenuates Cardiovascular Dysfunction Associated with Deoxycorticosterone Acetate-Salt-Induced Hypertension in Rats. Clin. Exp. Hypertens. 2022, 44, 382–395. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.N.; Patil, S.; Barkate, H. Protective Effects of Astaxanthin on Skin: Recent Scientific Evidence, Possible Mechanisms, and Potential Indications. J. Cosmet. Derm. 2020, 19, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Lima, S.G.M.; Freire, M.C.L.C.; da Silva Oliveira, V.; Solisio, C.; Converti, A.; de Lima, Á.A.N. Astaxanthin Delivery Systems for Skin Application: A Review. Mar. Drugs 2021, 19, 511. [Google Scholar] [CrossRef]
- Ito, N.; Seki, S.; Ueda, F. The Protective Role of Astaxanthin for UV-Induced Skin Deterioration in Healthy People—A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2018, 10, 817. [Google Scholar] [CrossRef]
- Zhuge, F.; Ni, Y.; Wan, C.; Liu, F.; Fu, Z. Anti-Diabetic Effects of Astaxanthin on an STZ-Induced Diabetic Model in Rats. Endocr. J. 2021, 68, 451–459. [Google Scholar] [CrossRef]
- Gowd, V.; Xiao, J.; Wang, M.; Chen, F.; Cheng, K.W. Multi-Mechanistic Antidiabetic Potential of Astaxanthin: An Update on Preclinical and Clinical Evidence. Mol. Nutr. Food Res. 2021, 65, 2100252. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, J.J.; Lee, B.J.; Joo, M.K.; Chun, H.J.; Lee, S.W.; Bak, Y.T. Astaxanthin Inhibits Proliferation of Human Gastric Cancer Cell Lines by Interrupting Cell Cycle Progression. Gut Liver 2016, 10, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Faraone, I.; Sinisgalli, C.; Ostuni, A.; Armentano, M.F.; Carmosino, M.; Milella, L.; Russo, D.; Labanca, F.; Khan, H. Astaxanthin Anticancer Effects Are Mediated through Multiple Molecular Mechanisms: A Systematic Review. Pharm. Res 2020, 155, 104689. [Google Scholar] [CrossRef]
- Radice, R.P.; Limongi, A.R.; Viviano, E.; Padula, M.C.; Martelli, G.; Bermano, G. Effects of Astaxanthin in Animal Models of Obesity-Associated Diseases: A Systematic Review and Meta-Analysis. Free Radic. Biol. Med. 2021, 171, 156–168. [Google Scholar] [CrossRef]
- Wang, M.; Ma, H.; Guan, S.; Luo, T.; Zhao, C.; Cai, G.; Zheng, Y.; Jia, X.; Di, J.; Li, R.; et al. Astaxanthin from Haematococcus Pluvialis Alleviates Obesity by Modulating Lipid Metabolism and Gut Microbiota in Mice Fed a High-Fat Diet. Food Funct. 2021, 12, 9719–9738. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.X.; Xiong, F.; Pak, S.; Liang Ooi, S. Astaxanthin and Its Effects in Inflammatory Responses and Inflammation-Associated Diseases: Recent Advances and Future Directions. Molecules 2020, 25, 5342. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.E.; Shin, C.Y.; Han, S.H.; Kwon, K.J. Astaxanthin Suppresses PM2.5-Induced Neuroinflammation by Regulating Akt Phosphorylation in BV-2 Microglial Cells. Int. J. Mol. Sci. 2020, 21, 7227. [Google Scholar] [CrossRef]
- Liu, N.; Zeng, L.; Zhang, Y.M.; Pan, W.; Lai, H. Astaxanthin Alleviates Pathological Brain Aging through the Upregulation of Hippocampal Synaptic Proteins. Neural Regen. Res. 2021, 16, 1062–1067. [Google Scholar] [CrossRef]
- Sorrenti, V.; Davinelli, S.; Scapagnini, G.; Willcox, B.J.; Allsopp, R.C.; Willcox, D.C. Astaxanthin as a Putative Geroprotector: Molecular Basis and Focus on Brain Aging. Mar. Drugs 2020, 18, 351. [Google Scholar] [CrossRef]
- Bassijeh, A.; Ansari, S.; Hosseini, S.M.H. Astaxanthin Encapsulation in Multilayer Emulsions Stabilized by Complex Coacervates of Whey Protein Isolate and Persian Gum and Its Use as a Natural Colorant in a Model Beverage. Food Res. Int. 2020, 137, 109689. [Google Scholar] [CrossRef]
- Schmitt, I.; Meyer, F.; Krahn, I.; Henke, N.A.; Peters-Wendisch, P.; Wendisch, V.F. From Aquaculture to Aquaculture: Production of the Fish Feed Additive Astaxanthin by Corynebacterium Glutamicum Using Aquaculture Sidestream. Molecules 2023, 28, 1996. [Google Scholar] [CrossRef]
- Elbahnaswy, S.; Elshopakey, G.E. Recent Progress in Practical Applications of a Potential Carotenoid Astaxanthin in Aquaculture Industry: A Review. Fish Physiol. Biochem. 2023, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.C.; Li, X.Y.; Wang, C.C.; Yang, J.Y.; Xue, C.H.; Zhang, T.T.; Wang, Y.M. Free Astaxanthin-Rich Diets Enhanced Astaxanthin Accumulation in Egg Yolks Compared to Esterified Astaxanthin-Rich Diets. Food Chem. 2023, 405, 134872. [Google Scholar] [CrossRef]
- Zajac, G.; Machalska, E.; Kaczor, A.; Kessler, J.; Bouř, P.; Baranska, M. Structure of Supramolecular Astaxanthin Aggregates Revealed by Molecular Dynamics and Electronic Circular Dichroism Spectroscopy. Phys. Chem. Chem. Phys. 2018, 20, 18038–18046. [Google Scholar] [CrossRef]
- Budriesi, R.; Micucci, M.; Daglia, M.; Corazza, I.; Biotti, G.; Mattioli, L.B. Chemical Features and Biological Effects of Astaxanthin Extracted from Haematococcus Pluvialis Flotow: Focus on Gastrointestinal System. Biol. Life Sci. Forum 2022, 12, 31. [Google Scholar] [CrossRef]
- Ambati, R.R.; Moi, P.S.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xie, J.; Zhou, L.; Zhang, J.; Chen, Z.; Xiao, J.; Cao, Y.; Xiao, H. Recent Advances in Health Benefits and Bioavailability of Dietary Astaxanthin and Its Isomers. Food Chem. 2023, 404, 134605. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, E. Let Astaxanthin Be Thy Medicine. PharmaNutrition 2015, 3, 115–122. [Google Scholar] [CrossRef]
- Higuera-Ciapara, I.; Félix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A Review of Its Chemistry and Applications. Crit. Rev. Food Sci. Nutr. 2007, 46, 185–196. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R.; Kumari, A.; Panwar, A. Astaxanthin: A Super Antioxidant from Microalgae and Its Therapeutic Potential. J. Basic Microbiol. 2022, 62, 1064–1082. [Google Scholar] [CrossRef]
- Capelli, B.; Bagchi, D.; Cysewski, G.R. Synthetic Astaxanthin Is Significantly Inferior to Algal-Based Astaxanthin as an Antioxidant and May Not Be Suitable as a Human Nutraceutical Supplement. Nutrafoods 2014, 12, 145–152. [Google Scholar] [CrossRef]
- Patel, A.K.; Tambat, V.S.; Chen, C.W.; Chauhan, A.S.; Kumar, P.; Vadrale, A.P.; Huang, C.Y.; Di Dong, C.; Singhania, R.R. Recent Advancements in Astaxanthin Production from Microalgae: A Review. Bioresour. Technol. 2022, 364, 128030. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T. Carotenoids in Marine Animals. Mar. Drugs 2011, 9, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Li, X.; Wang, J.; Wang, C.; Yang, M.; Zheng, P. Effects of Dietary Astaxanthin (AX) Supplementation on Pigmentation, Antioxidant Capacity and Nutritional Value of Swimming Crab, Portunus Trituberculatus. Aquaculture 2018, 490, 169–177. [Google Scholar] [CrossRef]
- Jagruthi, C.; Yogeshwari, G.; Anbazahan, S.M.; Shanthi Mari, L.S.; Arockiaraj, J.; Mariappan, P.; Learnal Sudhakar, G.R.; Balasundaram, C.; Harikrishnan, R. Effect of Dietary Astaxanthin against Aeromonas Hydrophila Infection in Common Carp, Cyprinus Carpio. Fish Shellfish Immunol. 2014, 41, 674–680. [Google Scholar] [CrossRef]
- de Carvalho, C.C.C.R.; Caramujo, M.J. Carotenoids in Aquatic Ecosystems and Aquaculture: A Colorful Business with Implications for Human Health. Front. Mar. Sci. 2017, 4, 93. [Google Scholar] [CrossRef]
- Routray, W.; Dave, D.; Cheema, S.K.; Ramakrishnan, V.V.; Pohling, J. Biorefinery Approach and Environment-Friendly Extraction for Sustainable Production of Astaxanthin from Marine Wastes. Crit. Rev. Biotechnol. 2019, 39, 469–488. [Google Scholar] [CrossRef]
- Bradić, B.; Novak, U.; Likozar, B. Crustacean Shell Bio-Refining to Chitin by Natural Deep Eutectic Solvents. Green Process. Synth. 2020, 9, 13–25. [Google Scholar] [CrossRef]
- Franco-Zavaleta, M.E.; Jiménez-Pichardo, R.; Tomasini-Campocosio, A.; Guerrero-Legarreta, I. Astaxanthin Extraction from Shrimp Wastes and Its Stability in 2 Model Systems. J. Food Sci. 2010, 75, C394–C399. [Google Scholar] [CrossRef]
- Ahmadkelayeh, S.; Cheema, S.K.; Hawboldt, K. Evaluation of Conventional Solvent Processes for Lipid and Astaxanthin Extraction from Shrimp Processing By-Products. Chem. Eng. Commun. 2022, 210, 398–411. [Google Scholar] [CrossRef]
- Hu, J.; Lu, W.; Lv, M.; Wang, Y.; Ding, R.; Wang, L. Extraction and Purification of Astaxanthin from Shrimp Shells and the Effects of Different Treatments on Its Content. Rev. Bras. Farmacogn. 2019, 29, 24–29. [Google Scholar] [CrossRef]
- Nunes, A.N.; Roda, A.; Gouveia, L.F.; Fernández, N.; Bronze, M.R.; Matias, A.A. Astaxanthin Extraction from Marine Crustacean Waste Streams: An Integrate Approach between Microwaves and Supercritical Fluids. ACS Sustain. Chem. Eng. 2021, 9, 3050–3059. [Google Scholar] [CrossRef]
- Hülsey, M.J. Shell Biorefinery: A Comprehensive Introduction. Green Energy Environ. 2018, 3, 318–327. [Google Scholar] [CrossRef]
- Chandra Roy, V.; Ho, T.C.; Lee, H.J.; Park, J.S.; Nam, S.Y.; Lee, H.; Getachew, A.T.; Chun, B.S. Extraction of Astaxanthin Using Ultrasound-Assisted Natural Deep Eutectic Solvents from Shrimp Wastes and Its Application in Bioactive Films. J. Clean. Prod. 2021, 284, 125417. [Google Scholar] [CrossRef]
- Sharayei, P.; Azarpazhooh, E.; Zomorodi, S.; Einafshar, S.; Ramaswamy, H.S. Optimization of Ultrasonic-Assisted Extraction of Astaxanthin from Green Tiger (Penaeus semisulcatus) Shrimp Shell. Ultrason. Sonochem. 2021, 76, 105666. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, W.; Ramaswamy, H.S.; Yu, Y.; Zhu, S.; Wang, J.; Li, H. High Pressure Extraction of Astaxanthin from Shrimp Waste (Penaeus vannamei Boone): Effect on Yield and Antioxidant Activity. J. Food Process Eng. 2017, 40, e12353. [Google Scholar] [CrossRef]
- De Aguiar Saldanha Pinheiro, A.C.; Martí-Quijal, F.J.; Barba, F.J.; Benítez-González, A.M.; Meléndez-Martínez, A.J.; Castagnini, J.M.; Tappi, S.; Rocculi, P. Pulsed Electric Fields (PEF) and Accelerated Solvent Extraction (ASE) for Valorization of Red (Aristeus antennatus) and Camarote (Melicertus kerathurus) Shrimp Side Streams: Antioxidant and HPLC Evaluation of the Carotenoid Astaxanthin Recovery. Antioxidants 2023, 12, 406. [Google Scholar] [CrossRef]
- Cheong, J.Y.; Muskhazli, M.; Nor Azwady, A.A.; Ahmad, S.A.; Adli, A.A. Three Dimensional Optimisation for the Enhancement of Astaxanthin Recovery from Shrimp Shell Wastes by Aeromonas Hydrophila. Biocatal. Agric. Biotechnol. 2020, 27, 101649. [Google Scholar] [CrossRef]
- Hamdi, S.; Elsayed, N.; Algayar, M.; Ishak, V.; Ahmed, M.; Ahmed, S.; Kamal, M.; El-Ghany, M.A. Eco-Friendly Methods for Recycling of Crayfish “Procambarus Clarkii” by-Product for Astaxanthin Extraction and Quantification. Egypt. J. Aquat. Biol. Fish. 2022, 26, 239–251. [Google Scholar] [CrossRef]
- Antunes, S.A.; Ferreira, S.R.S.; Hense, H.; Albino Antunes-Valcareggi, S. Enzymatic Hydrolysis of Blue Crab (Callinectes sapidus) Waste Processing to Obtain Chitin, Protein, and Astaxanthin-Enriched Extract. Int. J. Environ. Agric. Res. 2017, 3, 81–92. [Google Scholar]
- Deng, J.J.; Mao, H.H.; Fang, W.; Li, Z.Q.; Shi, D.; Li, Z.W.; Zhou, T.; Luo, X.C. Enzymatic Conversion and Recovery of Protein, Chitin, and Astaxanthin from Shrimp Shell Waste. J. Clean. Prod. 2020, 271, 122655. [Google Scholar] [CrossRef]
- Hsu, S.C.; Lockwood, J.L. Powdered Chitin Agar as a Selective Medium for Enumeration of Actinomycetes in Water and Soil. Appl. Microbiol. 1975, 29, 422–426. [Google Scholar] [CrossRef]
- Farahat, M.G. Enhancement of β-Cyclodextrin Production and Fabrication of Edible Antimicrobial Films Incorporated with Clove Essential Oil/β-Cyclodextrin Inclusion Complex. Microbiol. Biotechnol. Lett. 2020, 48, 12–23. [Google Scholar] [CrossRef]
- Hamdi, S.A.H.; Ghonaim, G.M.; El Sayed, R.R.; Rodríguez-Couto, S.; Abd El-Ghany, M.N. Bioprocess of Astaxanthin Extraction from Shrimp Waste via the Common Microorganisms Saccharomyces Cerevisiae and Lactobacillus Acidophilus in Comparison to the Chemical Method. Biomass Convers. Biorefin. 2022, 1–7. [Google Scholar] [CrossRef]
- Akeed, Y.; Atrash, F.; Naffaa, W. Partial Purification and Characterization of Chitinase Produced by Bacillus Licheniformis B307. Heliyon 2020, 6, e03858. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, S.; Iqbal, S.; Ahmed, I.; Janjua, H.A. Production, Optimization, and Partial Purification of Alkali-Thermotolerant Proteases from Newly Isolated Bacillus Subtilis S1 and Bacillus Amyloliquefaciens KSM12. Processes 2022, 10, 1050. [Google Scholar] [CrossRef]
- Ghori, M.I.; Iqbal, M.J.; Hameed, A. Characterization of a Novel Lipase from Bacillus sp. Isolated from Tannery Wastes. Braz. J. Microbiol. 2011, 42, 22–29. [Google Scholar] [CrossRef]
- Hamdi, S.; Elsayed, N.; Algayar, M.; Ishak, V.; Ahmed, M.; Ahmed, S.; Kamal, M.; Abd El-Ghany, M. Biological Extraction, HPLC Quantification and Medical Applications of Astaxanthin Extracted from Crawfish “Procambarus clarkii” Exoskeleton By-Product. Biology 2022, 11, 1215. [Google Scholar] [CrossRef]
- Bauer, A.; Minceva, M. Direct Extraction of Astaxanthin from the Microalgae Haematococcus Pluvialis Using Liquid–Liquid Chromatography. RSC Adv. 2019, 9, 22779–22789. [Google Scholar] [CrossRef]
- Farahat, M.G. Enhanced Anti-Oxidant Activity of Neoagarooligosaccharides Produced by β-Agarase Derived from Aquimarina Agarilytica NI125. Nov. Res. Microbiol. J. 2019, 3, 511–525. [Google Scholar] [CrossRef]
- González-Palma, I.; Escalona-Buendía, H.B.; Ponce-Alquicira, E.; Téllez-Téllez, M.; Gupta, V.K.; Díaz-Godínez, G.; Soriano-Santos, J. Evaluation of the Antioxidant Activity of Aqueous and Methanol Extracts of Pleurotus Ostreatus in Different Growth Stages. Front. Microbiol. 2016, 7, 1099. [Google Scholar] [CrossRef]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for Antioxidant Assays for Food Components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef]
- Huang, W.C.; Zhao, D.; Xue, C.; Mao, X. An Efficient Method for Chitin Production from Crab Shells by a Natural Deep Eutectic Solvent. Mar. Life Sci. Technol. 2022, 4, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Raabe, D.; Sachs, C.; Romano, P. The Crustacean Exoskeleton as an Example of a Structurally and Mechanically Graded Biological Nanocomposite Material. Acta Mater. 2005, 53, 4281–4292. [Google Scholar] [CrossRef]
- Jung, S.; Woo, C.; Fugaban, J.I.I.; Vazquez Bucheli, J.E.; Holzapfel, W.H.; Todorov, S.D. Bacteriocinogenic Potential of Bacillus Amyloliquefaciens Isolated from Kimchi, a Traditional Korean Fermented Cabbage. Probiotics Antimicrob. Proteins 2021, 13, 1195–1212. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-J.; Ahn, B.-Y. Isolation and Identification of Bacillus Amyloliquefaciens BY01 with High Productivity of Menaquinone for Cheonggukjang Production. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 783–789. [Google Scholar] [CrossRef]
- Medeiros, S.; Xie, J.; Dyce, P.W.; Cai, H.Y.; DeLange, K.; Zhang, H.; Li, J. Isolation of Bacteria from Fermented Food and Grass Carp Intestine and Their Efficiencies in Improving Nutrient Value of Soybean Meal in Solid State Fermentation. J. Anim. Sci. Biotechnol. 2018, 9, 29. [Google Scholar] [CrossRef]
- Du, H.; Yao, W.; Kulyar, M.F.-A.; Ding, Y.; Zhu, H.; Pan, H.; Li, K.; Bhutta, Z.A.; Liu, S.; Li, J. Effects of Bacillus Amyloliquefaciens TL106 Isolated from Tibetan Pigs on Probiotic Potential and Intestinal Microbes in Weaned Piglets. Microbiol. Spectr. 2022, 10, e01205-21. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Su, W.; Ying, Z.; Chen, Y.; Zhang, L.; Lu, Z.; Wang, T. Effects of Dietary Bacillus Amyloliquefaciens Supplementation on Growth Performance, Intestinal Morphology, Inflammatory Response, and Microbiota of Intra-Uterine Growth Retarded Weanling Piglets. J. Anim. Sci. Biotechnol. 2018, 9, 22. [Google Scholar] [CrossRef]
- Dabiré, Y.; Somda, N.S.; Somda, M.K.; Compaoré, C.B.; Mogmenga, I.; Ezeogu, L.I.; Traoré, A.S.; Ugwuanyi, J.O.; Dicko, M.H. Assessment of Probiotic and Technological Properties of Bacillus Spp. Isolated from Burkinabe Soumbala. BMC Microbiol. 2022, 22, 228. [Google Scholar] [CrossRef]
- Kamel, Z.; Mohamed, N.M.; Farahat, M.G. Optimization of Culture Conditions for Production of B-Galactosidase by Bacillus Megaterium NM56 Isolated from Raw Milk. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 366–376. [Google Scholar]
- Brutscher, L.M.; Borgmeier, C.; Garvey, S.M.; Spears, J.L. Preclinical Safety Assessment of Bacillus Subtilis BS50 for Probiotic and Food Applications. Microorganisms 2022, 10, 1038. [Google Scholar] [CrossRef]
- Gamiz-Hernandez, A.P.; Angelova, I.N.; Send, R.; Sundholm, D.; Kaila, V.R.I. Protein-Induced Color Shift of Carotenoids in β-Crustacyanin. Angew. Chem. Int. Ed. 2015, 54, 11564–11566. [Google Scholar] [CrossRef]
- Nekvapil, F.; Pinzaru, S.C.; Barbu–Tudoran, L.; Suciu, M.; Glamuzina, B.; Tamaș, T.; Chiș, V. Color-Specific Porosity in Double Pigmented Natural 3d-Nanoarchitectures of Blue Crab Shell. Sci. Rep. 2020, 10, 3019. [Google Scholar] [CrossRef] [PubMed]
- Cianci, M.; Rizkallah, P.J.; Olczak, A.; Raftery, J.; Chayen, N.E.; Zagalsky, P.F.; Helliwell, J.R. The Molecular Basis of the Coloration Mechanism in Lobster Shell: β-Crustacyanin at 3.2-Å Resolution. Proc. Natl. Acad. Sci. USA 2002, 99, 9795–9800. [Google Scholar] [CrossRef] [PubMed]
- Christensson, N.; Žídek, K.; Magdaong, N.C.M.; Lafountain, A.M.; Frank, H.A.; Zigmantas, D. Origin of the Bathochromic Shift of Astaxanthin in Lobster Protein: 2D Electronic Spectroscopy Investigation of β-Crustacyanin. J. Phys. Chem. B 2013, 117, 11209–11219. [Google Scholar] [CrossRef]
- Ngalimat, M.S.; Yahaya, R.S.R.; Baharudin, M.M.A.A.; Yaminudin, S.M.; Karim, M.; Ahmad, S.A.; Sabri, S. A Review on the Biotechnological Applications of the Operational Group Bacillus Amyloliquefaciens. Microorganisms 2021, 9, 614. [Google Scholar] [CrossRef]
- Wang, S.L.; Shih, I.L.; Liang, T.W.; Wang, C.H. Purification and Characterization of Two Antifungal Chitinases Extracellularly Produced by Bacillus Amyloliquefaciens V656 in a Shrimp and Crab Shell Powder Medium. J. Agric. Food Chem. 2002, 50, 2241–2248. [Google Scholar] [CrossRef]
- Abdel-Salam, M.S.; Ameen, H.H.; Soliman, G.M.; Elkelany, U.S.; Asar, A.M. Improving the Nematicidal Potential of Bacillus Amyloliquefaciens and Lysinibacillus Sphaericus against the Root-Knot Nematode Meloidogyne Incognita Using Protoplast Fusion Technique. Egypt. J. Biol. Pest Control 2018, 28, 31. [Google Scholar] [CrossRef]
- Xie, F.; Feng, F.; Liu, D.; Quan, S.; Liu, L.; Zhang, X.; Chen, G. Bacillus Amyloliquefaciens 35 M Can Exclusively Produce and Secrete Proteases When Cultured in Soybean-Meal-Based Medium. Colloids Surf. B Biointerfaces 2022, 209, 112188. [Google Scholar] [CrossRef]
- Mushtaq, H.; Jehangir, A.; Ganai, S.A.; Farooq, S.; Ganai, B.A.; Nazir, R. Biochemical Characterization and Functional Analysis of Heat Stable High Potential Protease of Bacillus Amyloliquefaciens Strain HM48 from Soils of Dachigam National Park in Kashmir Himalaya. Biomolecules 2021, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- Pandey, G.R.; Shrestha, A.; Karki, T.B.; Neupane, S.; Ojha, S.; Koirala, P.; Timilsina, P.M. Screening and Identification of Thermotolerant and Osmotolerant Bacillus Amyloliquefaciens BKHE Isolated from Kinema of Eastern Nepal for Alkaline Protease Production. Int. J. Microbiol. 2022, 2022, 6831092. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Ye, C.; Liu, Y.; Huang, K.; Jiang, X.; Zou, D.; Li, L.; Han, W.; Wei, X. Genetic Engineering for Enhanced Production of a Novel Alkaline Protease BSP-1 in Bacillus Amyloliquefaciens. Front. Bioeng. Biotechnol. 2022, 10, 1565. [Google Scholar] [CrossRef] [PubMed]
- El-Bialy, H.A.A.; Abd El-Khalek, H.H. A Comparative Study on Astaxanthin Recovery from Shrimp Wastes Using Lactic Fermentation and Green Solvents:An Applied Model on Minced Tilapia. J. Radiat. Res. Appl. Sci. 2020, 13, 594–605. [Google Scholar] [CrossRef]
- Khanafari, A.; Saberi, A.; Azar, M.; Vosooghi, G.; Jamili, S.; Sabbaghzadeh, B. Extraction of Astaxanthin Esters from Shrimp Waste by Chemical and Microbial Methods. J. Envrion. Health Sci. Eng. 2007, 4, 93–98. [Google Scholar]
- Lin, X.; Bo, H.; Gu, J.; Yi, X.; Zhang, P.; Liu, R.; Li, H.; Sun, G.; Lin, C.H. Astaxanthin, a Carotenoid Antioxidant, Pretreatment Alleviates Cognitive Deficits in Aircraft Noised Mice by Attenuating Inflammatory and Oxidative Damage to the Gut, Heart and Hippocampus. Biomed. Pharmacother. 2022, 148, 112777. [Google Scholar] [CrossRef]
- Masoudi, A.; Jorjani, M.; Alizadeh, M.; Mirzamohammadi, S.; Mohammadi, M. Anti-Inflammatory and Antioxidant Effects of Astaxanthin Following Spinal Cord Injury in a Rat Animal Model. Brain Res. Bull. 2021, 177, 324–331. [Google Scholar] [CrossRef]
- Zheng, X.; Huang, Q. Assessment of the Antioxidant Activities of Representative Optical and Geometric Isomers of Astaxanthin against Singlet Oxygen in Solution by a Spectroscopic Approach. Food Chem. 2022, 395, 133584. [Google Scholar] [CrossRef]
- Parkes, R.; Barone, M.E.; Herbert, H.; Gillespie, E.; Touzet, N. Antioxidant Activity and Carotenoid Content Responses of Three Haematococcus Sp. (Chlorophyta) Strains Exposed to Multiple Stressors. Appl. Biochem. Biotechnol. 2022, 194, 4492–4510. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Baker, J.S.; Davison, G.W.; Xu, S.; Zhou, Y.; Bao, X. Astaxanthin Promotes Mitochondrial Biogenesis and Antioxidant Capacity in Chronic High-Intensity Interval Training. Eur. J. Nutr. 2023, 62, 1453–1466. [Google Scholar] [CrossRef]
- Chung, B.Y.; Park, S.H.; Yun, S.Y.; Yu, D.S.; Lee, Y.B. Astaxanthin Protects Ultraviolet B-Induced Oxidative Stress and Apoptosis in Human Keratinocytes via Intrinsic Apoptotic Pathway. Ann. Derm. 2022, 34, 125–131. [Google Scholar] [CrossRef]
- Chae, S.Y.; Park, R.; Hong, S.W. Surface-Mediated High Antioxidant and Anti-Inflammatory Effects of Astaxanthin-Loaded Ultrathin Graphene Oxide Film That Inhibits the Overproduction of Intracellular Reactive Oxygen Species. Biomater. Res. 2022, 26, 30. [Google Scholar] [CrossRef]
- Rizzardi, N.; Pezzolesi, L.; Samorì, C.; Senese, F.; Zalambani, C.; Pitacco, W.; Calonghi, N.; Bergamini, C.; Prata, C.; Fato, R. Natural Astaxanthin Is a Green Antioxidant Able to Counteract Lipid Peroxidation and Ferroptotic Cell Death. Int. J. Mol. Sci. 2022, 23, 15137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Luo, Y.; Gu, R.; Jiang, Z. Astaxanthin Alleviates Inflammatory Response in Neonatal Necrotizing Enterocolitis Rats by Regulating NOD2/TLR4 Pathway. Gastroenterol. Res. Pract. 2023, 2023, 6078308. [Google Scholar] [CrossRef]
- Kohandel, Z.; Farkhondeh, T.; Aschner, M.; Pourbagher-Shahri, A.M.; Samarghandian, S. Anti-Inflammatory Action of Astaxanthin and Its Use in the Treatment of Various Diseases. Biomed. Pharmacother. 2022, 145, 112179. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Hamada, C.; Wakabayashi, K.; Kanda, R.; Kaneko, K.; Horikoshi, S.; Tomino, Y.; Suzuki, Y. Scavenging of Reactive Oxygen Species by Astaxanthin Inhibits Epithelial–Mesenchymal Transition in High Glucose-Stimulated Mesothelial Cells. PLoS ONE 2017, 12, e0184332. [Google Scholar] [CrossRef]
- Speranza, L.; Pesce, M.; Patruno, A.; Franceschelli, S.; De Lutiis, M.A.; Grilli, A.; Felaco, M. Astaxanthin Treatment Reduced Oxidative Induced Pro-Inflammatory Cytokines Secretion in U937: SHP-1 as a Novel Biological Target. Mar. Drugs 2012, 10, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L.; Chen, L.; et al. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Silva, A.M.S.; Ribeiro, D.; Silva, V.L.M.; Fernandes, E. Bis-Chalcones: A Review of Synthetic Methodologies and Anti-Inflammatory Effects. Eur. J. Med. Chem. 2023, 252, 115280. [Google Scholar] [CrossRef]
- Li, D.Y.; Xue, M.Y.; Geng, Z.R.; Chen, P.Y. The Suppressive Effects of Bursopentine (BP5) on Oxidative Stress and NF-ĸB Activation in Lipopolysaccharide-Activated Murine Peritoneal Macrophages. Cell. Physiol. Biochem. 2012, 29, 9–20. [Google Scholar] [CrossRef]
- Brown, G.C. Regulation of Mitochondrial Respiration by Nitric Oxide Inhibition of Cytochrome c Oxidase. Biochim. Biophys. Acta BBA Bioenerg. 2001, 1504, 46–57. [Google Scholar] [CrossRef]
- Popko, K.; Gorska, E.; Stelmaszczyk-Emmel, A.; Plywaczewski, R.; Stoklosa, A.; Gorecka, D.; Pyrzak, B.; Demkow, U. Proinflammatory Cytokines IL-6 and TNF-α and the Development of Inflammation in Obese Subjects. Eur. J. Med. Res. 2010, 15, 120–122. [Google Scholar] [CrossRef] [PubMed]




| Enzyme | Enzyme Activity (U/mL) | |
|---|---|---|
| B. amyloliquefaciens CPFD8 | S. cerevisiae 006-001 | |
| Chitinase | 18.6 ± 2.1 | - |
| Protease | 127.6 ± 19.2 (a) | 44.8 ± 5.4 (b) |
| Lipase | 7.2 ± 1.9 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd El-Ghany, M.N.; Hamdi, S.A.; Elbaz, R.M.; Aloufi, A.S.; El Sayed, R.R.; Ghonaim, G.M.; Farahat, M.G. Development of a Microbial-Assisted Process for Enhanced Astaxanthin Recovery from Crab Exoskeleton Waste. Fermentation 2023, 9, 505. https://doi.org/10.3390/fermentation9060505
Abd El-Ghany MN, Hamdi SA, Elbaz RM, Aloufi AS, El Sayed RR, Ghonaim GM, Farahat MG. Development of a Microbial-Assisted Process for Enhanced Astaxanthin Recovery from Crab Exoskeleton Waste. Fermentation. 2023; 9(6):505. https://doi.org/10.3390/fermentation9060505
Chicago/Turabian StyleAbd El-Ghany, Mohamed N., Salwa A. Hamdi, Reham M. Elbaz, Abeer S. Aloufi, Rana R. El Sayed, Ghadeer M. Ghonaim, and Mohamed G. Farahat. 2023. "Development of a Microbial-Assisted Process for Enhanced Astaxanthin Recovery from Crab Exoskeleton Waste" Fermentation 9, no. 6: 505. https://doi.org/10.3390/fermentation9060505
APA StyleAbd El-Ghany, M. N., Hamdi, S. A., Elbaz, R. M., Aloufi, A. S., El Sayed, R. R., Ghonaim, G. M., & Farahat, M. G. (2023). Development of a Microbial-Assisted Process for Enhanced Astaxanthin Recovery from Crab Exoskeleton Waste. Fermentation, 9(6), 505. https://doi.org/10.3390/fermentation9060505









